Medicine Group 2025 April 09;6(4):312-319. doi: 10.37871/jbres2086.
Synergy of Ergothioneine and Tyrosine-Arginine Peptide to boost β-Endorphin Generation to Alleviate Stress-Induced Dermatological Disorders
Fangru Jiang and Zhe Liu*
- Ergothioneine
- Skin health
- β-endorphin
- Bioactive
Abstract
Objective: This study aimed to investigate the synergistic effects of Ergothioneine (EGT) and Tyrosine-Arginine Peptide (TAP) in promoting β-endorphin production in the skin, aiming to alleviate stress-induced dermatological disorders.
Method: An optimal ratio between EGT and TAP for β-endorphin stimulation was ascertained through cell experiments using human dermal fibroblasts and immortalized human keratinocytes. These cells were treated with cortisol to induce stress-related conditions. β-endorphin content was measured following treatment with various EGT-TAP ratios. Additional gene expression analysis was conducted to observe the regulatory effects of the combination on key skin health indicators, using specific primers and qPCR methods.
Results: The combination of EGT and TAP displayed a significant synergistic effect on β-endorphin production, with the optimal ratio identified as 9:1. This ratio resulted in a 175.75% and 190.20% increase in β-endorphin generation compared to individual EGT and TAP treatments, respectively. The EGT-TAP combination also mitigated cortisol-induced disruptions in gene expression, positively affecting the expression of extracellular matrix proteins and mitigating the negative effects of stress on skin cell models.
Conclusion: The combined administration of EGT and TAP at a 9:1 ratio significantly boosts β-endorphin levels and beneficially regulates gene expression under stress-induced conditions in skin cells. This synergy presents a novel and effective approach to enhance skin health and counteract the detrimental impacts of stress, offering a promising avenue for the development of advanced dermatological treatments.
Introduction
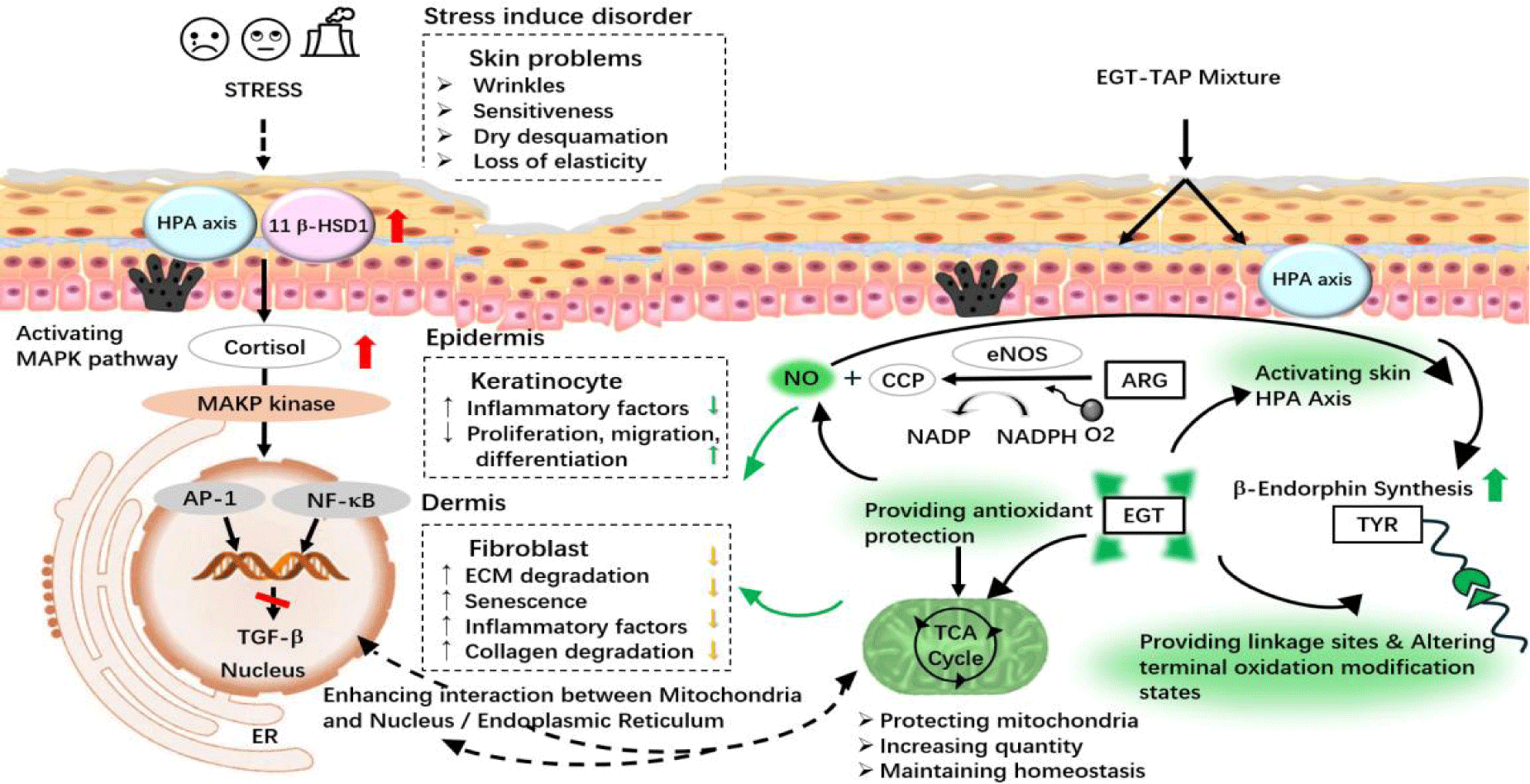
Stressors, including psychological strain, negative emotions, and noxious pollutants, can precipitate dermatological disorders or exacerbate skin diseases [1,2]. Research by Padgett DA, et al. [3] indicated that stress may lead to dysregulation of the Hypothalamic-Pituitary-Adrenal (HPA) axis, resulting in a disruption of stress hormones and an elevation of cortisol levels, which in turn triggers the release of various cytokines and chemokines. Studies by Choe [4] demonstrated that psychological stress can increase the activity of 11β-HSD1, facilitating the conversion of cortisone to cortisol. Elevated levels of cortisol can heighten various skin immune responses [5], stimulate the expression of IL-1β, TNF-α, and IL-10 [6], and compromise the skin's physical and microbiotic barrier [7]. Prolonged systemic cortisol excess inhibits wound healing [8] and disrupts the formation of the fibroblast matrix [9]. Moreover, excessive cortisol secretion alters TGF-β expression, affecting fibroblast proliferation, migration, and differentiation [10]. Schoepe [11] found a negative correlation between cortisol levels and various Extracellular Matrix (ECM) proteins, such as Collagen I, III, and elastin.
β-endorphin, an endogenous μ-opioid receptor ligand secreted by cells, not only directly influences the migration, differentiation, and cytokine production modes of keratinocytes in the skin [12] but also mitigate stress-induced skin problems. Slominski AT, et al. [13,14] suggested that promoting the production of β-endorphin in the skin could counteract skin problems and damage triggered by environmental stressors. The underlying mechanisms may involve initiating homeostatic responses, neutralizing harmful stimuli, and stimulating immune responses to preserve skin integrity. Research by Dieamant [15] further corroborates that an increase in β-endorphin can modulate cytokine release induced by UV radiation, hence reducing trans-epidermal water loss, alleviating skin dryness, and enhancing skin comfort. Additionally, studies by Kim [16] found that β-endorphin can counteract skin damage caused by UVB radiation.
This work aims to boost the generation of β-endorphin in the skin by Ergothioneine (EGT) and Tyrosine-Arginine peptide (TAP) to counteract stress-induced dermatological disorders. Cell experiments were employed to ascertain the optimal ratio between EGT and TAP. Following this, a cortisol-induced stress cell model was used to examine the regulatory effects of the composition.
Material and Experiment
Material
The materials of Ergothioneine (EGT) and Tyrosine-Arginine Peptide (TAP) were obtained from Bloomage Biotech Co., Ltd., with a purity exceeding 99%. Human cortisol and β-endorphin were chemical reagents with a purity of ≥ 96%, supplied by Shanghai Aladdin Biochemical Technology Co., Ltd. Human dermal fibroblasts (HDF, from Guangdong Biocell Biotechnology Co., Ltd.) and immortalized human keratinocytes (HaCat, from Wuhan Sunncell Biotechnology Co., Ltd.) were employed for cellular assays. The RNA-Quick Purification Kit (from Shanghai Yishan Biotechnology Co., Ltd.) and Hifair III 1st Strand cDNA Synthesis SuperMix for qPCR (from Yeasen Biotechnology Shanghai Co., Ltd.) were utilized to evaluate the gene expression of Filaggrin (FLG), Loricrin (LOR), Collagen I, Collagen III, and Matrix Metalloproteinase-9 (MMP-9). In addition, an endorphin kit (from Elabscience Biotechnology Co., Ltd.) and a hyaluronan kit (from Shanghai Enzyme-linked Biotechnology Co., Ltd.) were utilized to measure the levels of β-endorphin and Hyaluronic Acid (HA), respectively.
Cell experiments
Ratio optimization: EGT and TAP were mixed in varying ratios to identify the optimal formulation for stimulating the production of β-endorphin. As illustrated in table 1, from cases R-III to VII, the ratio of EGT to TAP varied from 40:1 to 1:9. Subsequently, at a fixed ratio of 9:1, the total concentration of EGT and TAP was adjusted in cases R-VIII to XI. Furthermore, the individual components of EGT and TAP were evaluated separately for comparison, specifically in cases R-I and II.
| Table 1: Test conditions of formula optimization of EGT and TAP. | ||||
| Case | Total concentration of the formula in the media (µg mL-1) | Weight Ratio of EGT: TAP | EGT concentration in the medium (µg mL-1) | TAP concentration in the medium (µg mL-1) |
| R-BC | NA | NA | NA | NA |
| R-NC | NA | NA | NA | NA |
| R-I | 60 | NA | 60 | 0 |
| R-II | 60 | 0 | 0 | 60 |
| R-III | 60 | 40:1 | 58.54 | 1.46 |
| R-IV | 60 | 20:1 | 57.14 | 2.86 |
| R-V | 60 | 9:1 | 54 | 6 |
| R-VI | 60 | 1:1 | 30 | 30 |
| R-VII | 60 | 1:9 | 6 | 54 |
| R-VIII | 55.5 | 9:1 | 50 | 5.5 |
| R-IX | 150 | 9:1 | 135 | 15 |
| R-X | 300 | 9:1 | 270 | 30 |
| R-XI | 600 | 9:1 | 540 | 60 |
In the experiments, HaCaT cells were treated by cortisol to establish a stress cell model. The procedure involved incubating the cells in a medium containing 100 µM cortisol at 37°C and 5% CO2 for 24 hours. Following this incubation period, the medium was refreshed, and the formulas outlined in table 1 were added to the culture medium. The cells were then incubated for an additional 24 hours under identical conditions (37°C, 5% CO2). After the incubation, the cells were washed with D-Hanks buffer and subsequently digested with a trypsin solution for 3 minutes. Finally, the samples were centrifuged at 2500 rpm for 5 minutes, and the cells were collected. To facilitate lysis, the cells underwent repeated freeze-thaw cycles, followed by another centrifugation at 2500 rpm for 10 minutes. The supernatant was then analyzed for β-endorphin content. Each case was repeated six times to obtain an average value.
It's worth noting that the blank control (R-BC) was not exposed to either cortisol or any formulas, while the negative control (R-NC) was treated with cortisol alone and no formulas.
Regulation of gene expressions: A series of gene expressions in HDF and HaCat cells were investigated. Firstly, HDF and HaCat were subjected to the same procedure described in the Section above to establish a stress cell model. Subsequently, the formulas were introduced to the media to regulate gene expressions. As listed in table 2, EGT-TAP combinations were applied in the cases of HDFS-3 & 4 and HaCat-3 & 4. For comparison, β-endorphin was added in the cases of HDFS-1 & 2 and HaCat-1 & 2. After adding the formulas, the cells were cultured for an additional 48 hours, after which the relative gene expressions were measured. Each case was performed six times to ensure the accuracy of the results.
| Table 2: Test conditions of gene expression regulation. | ||
| Case | Formula in the medium | Gene expression |
| HaCat-BC | NA | HA FLG LOR |
| HaCat-NC | NA | |
| HaCat-1 | 0.025 pg mL-1 β-endorphin | |
| HaCat-2 | 0.040 pg mL-1 β-endorphin | |
| HaCat-3 | 150 µg mL-1 mixture of EGT & TAP (9:1, wt/wt) | |
| HaCat-4 | 600 µg mL-1 mixture of EGT & TAP (9:1, wt/wt) | |
| HDFS-BC | NA | COL I COL III MMP-9 |
| HDFS-NC | NA | |
| HDFS-1 | 0.025 pg mL-1 β-endorphin | |
| HDFS-2 | 0.040 pg mL-1 β-endorphin | |
| HDFS-3 | 150 µg mL-1 mixture of EGT & TAP (9:1, wt/wt) | |
| HDFS-4 | 600 µg mL-1 mixture of EGT & TAP (9:1, wt/wt) | |
The cases of HDFS-BC and HaCat-BC served as blank controls, where the cells were not exposed to either cortisol or any formulas. On the other hand, the cases of HDFS-NC and HaCat-NC served as negative controls, where the cells were treated only with cortisol and no formulas.
Data analysis
The statistical analysis was derived from the mean and standard deviation of six replicates per sample in this investigation. A paired double-tailed t-test (α = 0.05) was performed to analyze differences between the results within the same experiment. Groups that share the same letter are considered to exhibit no significant difference, for example, “a” vs., “a” or “a” vs., “ab”; conversely, groups with completely different letters demonstrate a significant difference, such as “a” vs., “b”.
Results
Optimal ratio to boost β-endorphin generation
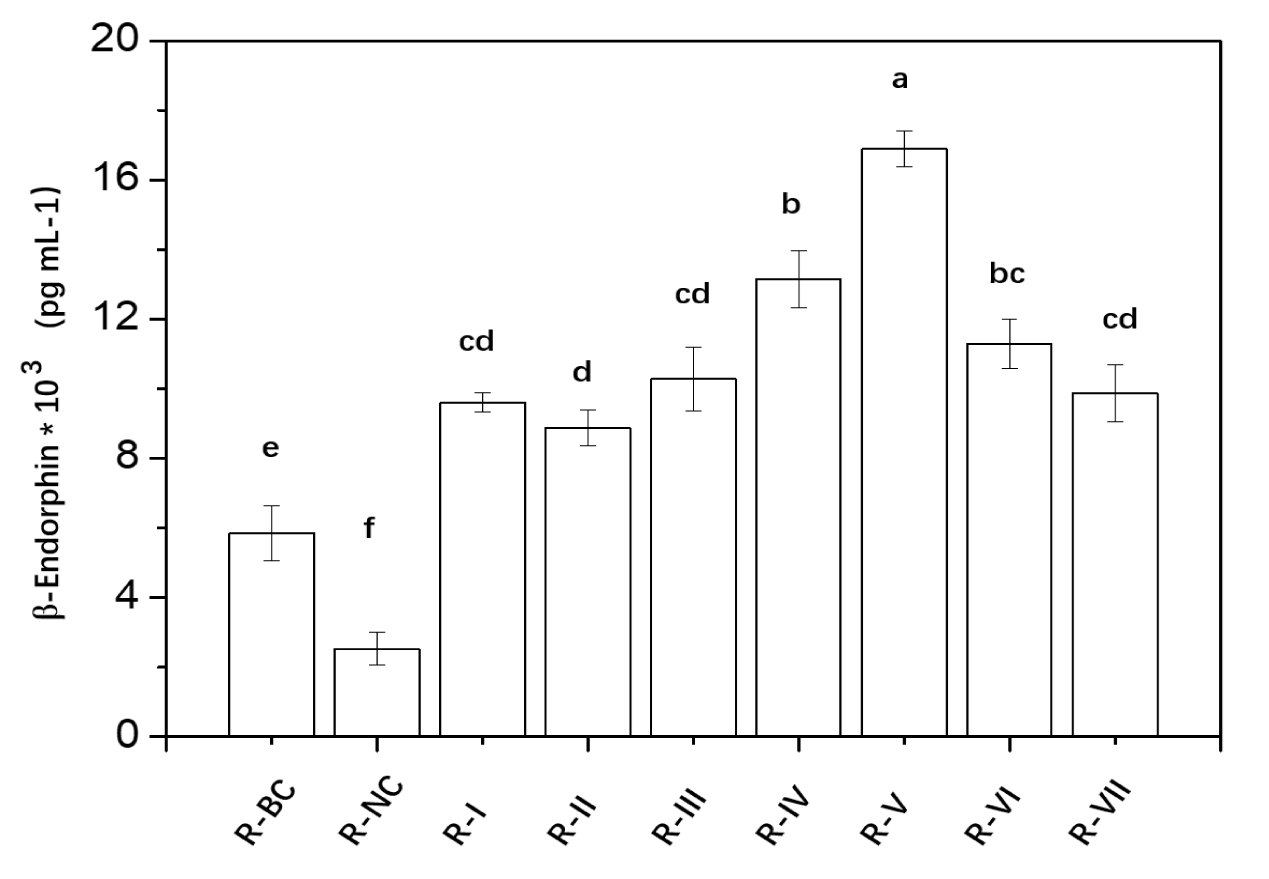
The results of β-endorphin generation under different conditions were illustrated in figure 1. A comparison between the negative and blank control indicates that cortisol stimulation indeed leads to a decrease in β-endorphin levels. From cases R-1 and R-2, it is evident that both EGT and TAP components, when administered independently, can promote β-endorphin secretion, with levels significantly higher than the blank control. Interestingly, while EGT is recognized for an antioxidant properties, reports on its efficacy in promoting β-endorphin synthesis were scarce. The mechanisms by which EGT may enhance β-endorphin secretion could be attributed to several factors: (1) EGT potentially regulates the activation of the HPA axis surrounding keratinocytes [17], thereby influencing the synthesis of β-endorphin through alterations in 5-HT and GABA levels; (2) EGT acts on the Atm/Atr-p53 signaling pathway, triggering the synthesis of pro-opiomelanocortin (POMC), which is subsequently processed by enzymes like PC1/3 and PC2 in the Golgi apparatus into peptide fragments, including ACTH and β-endorphin [18]; (3) As a sulfur-containing amino acid derivative, EGT functions as a small molecule thiol component, providing not only antioxidant properties but also the ability to mend protein functional alterations induced by nitrogen oxidative stress via S-nitrosylation [19-21].
The EGT-TAP combination exhibits varying efficacy in enhancing β-endorphin secretion at different ratios. At the ratios of 40:1, 1:1, and 1:9, the EGT-TAP combination does not demonstrate superior effects compared to the individual administrations of EGT and TAP. However, a significant synergistic effect is observed within the 20:1 to 9:1 range. Remarkably, at a 9:1 ratio, the combination yields the greatest synergistic effect, increasing β-endorphin generation by 175.75% and 190.20% as compared to the individual EGT and TAP administrations, respectively.
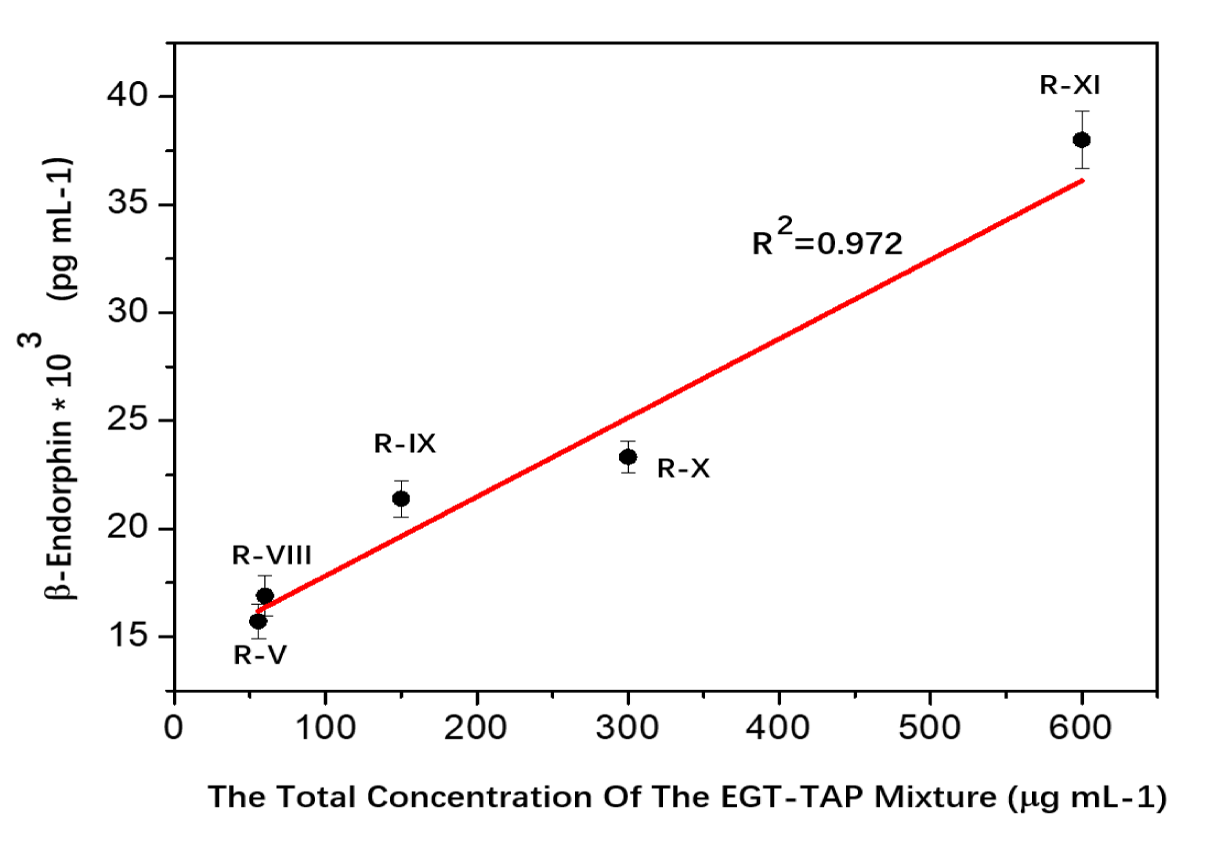
The dose-effect of EGT-TAP on β-endorphin generation was illustrated in figure 2. At the specific ratio of 9:1, the total concentration of the EGT-TAP mixture was adjusted from 55.5 to 600 µg mL-1, i.e. cases R-V and R-VIII ~ XI. The results reveal a linear association between β-endorphin secretion and the concentration of the EGT-TAP combination (R² = 0.972), as depicted in Eq-1.
C β-Endorphin: *103 = 0.039 *C Mixture + 14.058 (Eq1)
C β-Endorphin: concentration of β-endorphin, pg mL-1
C mixture: concentration of the EGT-TAP mixture (9:1 ratio), μg mL-1
Regulation of gene expressions
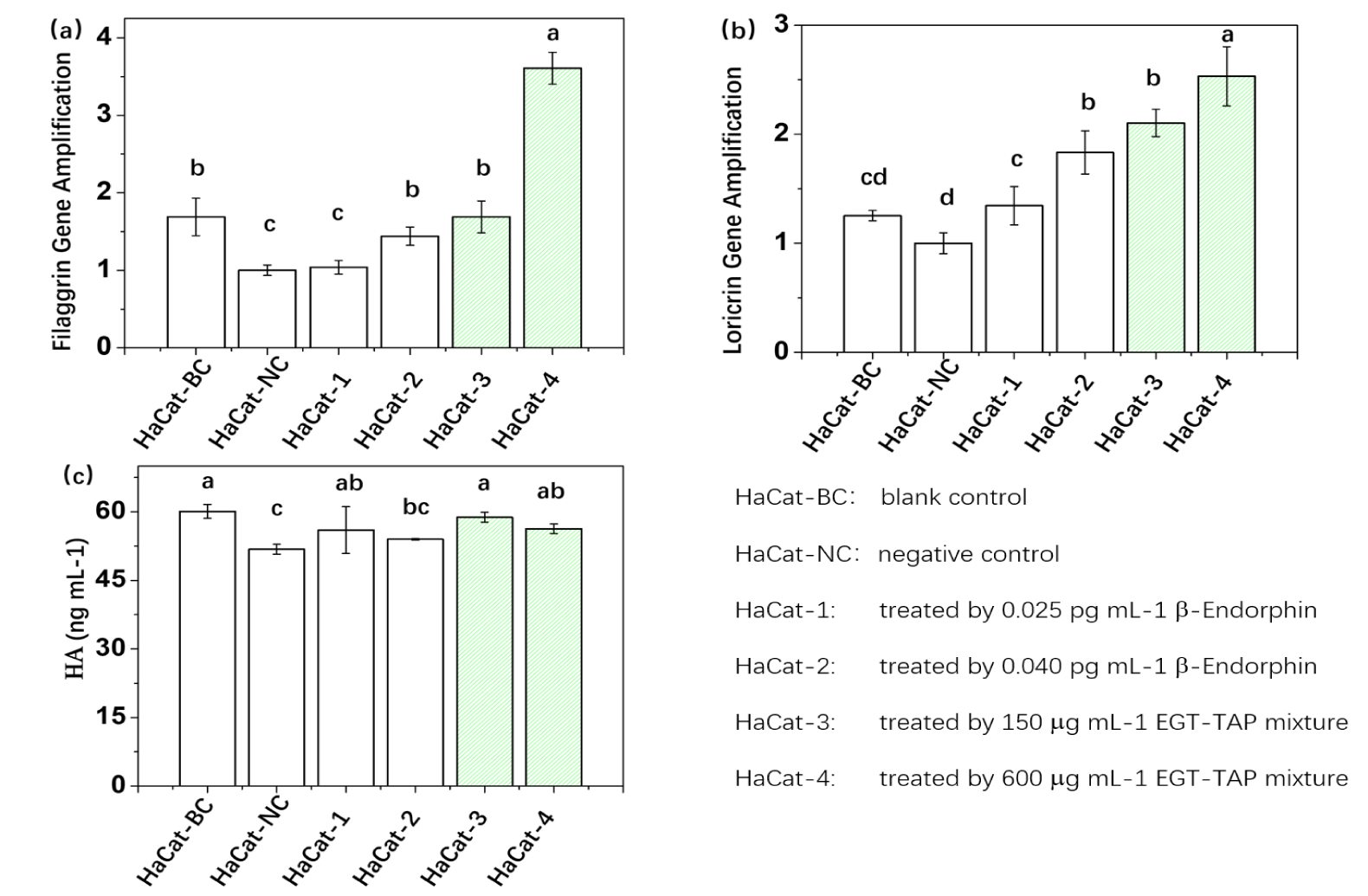
Figures 3,4 depicts the gene expression results in stress cell models subjected to various formula treatments. Each comparison includes: Blank control, Negative control, direct administration of β-endorphin, and administration of the EGT-TAP mixture. As confirmed from optimal ratio results, β-endorphin levels reached 0.021 and 0.038 pg mL-1 upon treatment with EGT-TAP mixture at concentrations of 150 µg mL-1 and 600 µg mL-1, respectively. These levels were analogous to those achieved with the direct administration of β-endorphin within this experiment.
Figure 3 (a)-(c) display the results of HaCat gene expressions. Compared with the negative control, the direct administration of β-endorphin upregulated the expressions of FLG, LOR, and HA. Furthermore, the gene expression levels of FLG and LOR were observed to increase concomitantly with the elevation of β-endorphin dosage. Intriguingly, the EGT-TAP mixture administration demonstrated a significantly superior effect in upregulation gene expressions compared to direct β-endorphin administration. For instance, the expression level of FLG in HaCat-4 was 150.69% higher than that in HaCat-2, while for LOR, there was an increase of 38.07%. However, for HA, although the EGT-TAP mixture exhibited a promotional effect relative to the negative control, its performance did not exceed that of the direct β-endorphin administration.
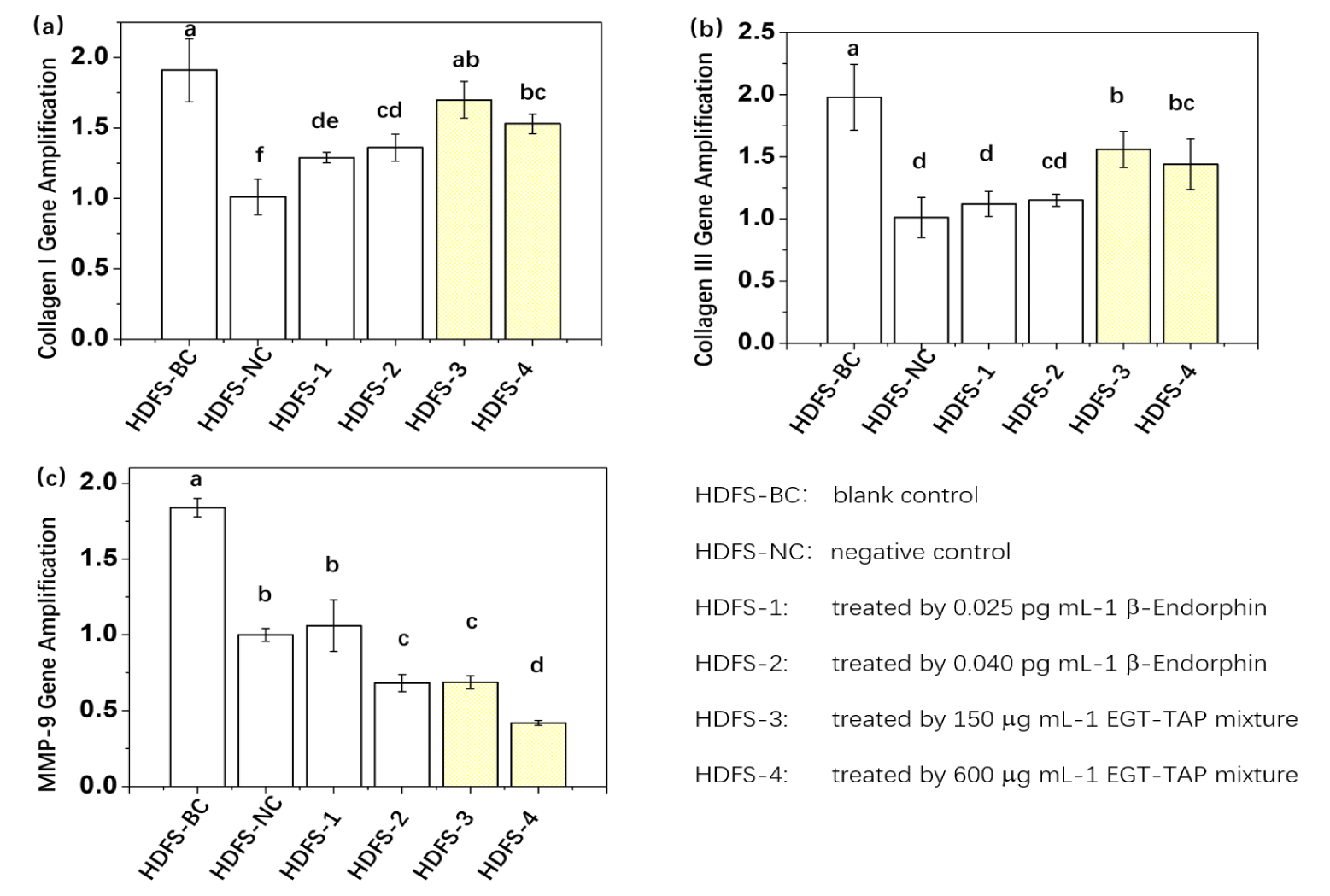
The experimental design for fibroblast testing paralleled that of keratinocytes, with results statistically analyzed and illustrated in figure 4. For the regulation of HDF gene expression, the effects observed with direct β-endorphin administration and EGT-TAP administration did not follow an entirely consistent trend, as depicted in figure 4 (a)-(c). The direct β-endorphin administration group exhibited an upregulation of Collagen I and a downregulation of MMP-9 at elevated dosage levels, but showed no significant effect on Collagen III. Conversely, the EGT-TAP administration group positively regulated the expression of Collagen I, Collagen III, and MMP-9, with both dosage levels producing significant effects.
Discussion
The synergistic enhancement of β-endorphin production by EGT and TAP can be elucidated by the following mechanisms, as depicted in figure 5. TAP provides the substrate required for the production of β-endorphin, while EGT offers the essential structural sites for Tyrosine (TYR), facilitating peptide chain formation and structural organization. Moreover, the antioxidant properties of EGT diminish the potential of oxidative modifications during β-endorphin synthesis [22]. Additionally, EGT can protect Nitric Oxide (NO) stimulated by Arginine (ARG) [23], thereby encouraging an elevation in β-endorphin levels [24].
Furthermore, the amalgamation of EGT and TAP demonstrates a regulatory impact on gene expression that surpasses the effect of an equivalent dose of β-endorphin alone. This indicates that the combination not only promotes the production of β-endorphin but also manages other physiological pathways within the cell, most notably mitochondrial health. Under stressful conditions, mitochondria, functioning as hormone sensors, can be negatively affected by surplus cortisol, leading to mitochondrial dysfunction and morphological alterations [25]. EGT is capable of neutralizing ROS within mitochondria to minimize its leakage, thereby reducing the downstream inflammatory response instigated by immune stress [26]. Simultaneously, EGT amplifies electron transfer capabilities, fostering mitochondrial oxidative phosphorylation for increased ATP production, and regulates mitochondrial respiration [27,28], as well as augmenting the count of mitochondria. Moreover, EGT helps to maintain mitochondrial membrane potential, stabilizes membrane structure, and prevents membrane rupture, ensuring regular mitochondrial metabolism, proteostasis, and morphology [29]. Consequently, mitochondria can interact with the endoplasmic reticulum, ameliorating the cellular environment by alleviating endoplasmic reticulum stress [30], and maintaining communication with the cell nucleus [31], thereby regulating cellular homeostasis and metabolic adaptation to support standard cellular operations.
Conclusion
This work revealed that a synergistic blend of EGT and TAP significantly escalates β-endorphin production in skin cells, proposing a new approach to treat stress-induced skin disorders. The optimal EGT-TAP ratio (9:1) markedly enhances the β-endorphin level and surpasses the performance of the compounds when utilized individually. This synergy also positively regulates gene expressions related to skin integrity and inflammation.
In essence, EGT-TAP combination presents a multifunctional and effective treatment strategy for reinforcing skin health and combating the deleterious effects of stress on the skin, laying the groundwork for advanced dermatological therapies.
Ethical Statement
In conducting the research detailed within our manuscript, we, the authors, affirm that the experimental protocols and procedures employed throughout this study have been assessed and determined to be exempt from ethical concerns.
Non-Involvement of Human Subjects: Our study did not involve human participants, their data, or biological material.
Non-Involvement of Animal Subjects: No animal subjects were used at any point during our research.
Authors’ Contribution
F. J. carried out the experiments and analyzed the data. F. J. and Z. L., wrote the manuscript
References
- Hall JM, Cruser D, Podawiltz A, Mummert DI, Jones H, Mummert ME. Psychological Stress and the Cutaneous Immune Response: Roles of the HPA Axis and the Sympathetic Nervous System in Atopic Dermatitis and Psoriasis. Dermatol Res Pract. 2012;2012:403908. doi: 10.1155/2012/403908. Epub 2012 Aug 30. PMID: 22969795; PMCID: PMC3437281.
- Maarouf M, Maarouf CL, Yosipovitch G, Shi VY. The impact of stress on epidermal barrier function: an evidence-based review. Br J Dermatol. 2019 Dec;181(6):1129-1137. doi: 10.1111/bjd.17605. Epub 2019 Mar 18. PMID: 30614527.
- Padgett DA, Marucha PT, Sheridan JF. Restraint stress slows cutaneous wound healing in mice. Brain Behav Immun. 1998 Mar;12(1):64-73. doi: 10.1006/brbi.1997.0512. PMID: 9570862.
- Choe SJ, Kim D, Kim EJ, Ahn JS, Choi EJ, Son ED, Lee TR, Choi EH. Psychological Stress Deteriorates Skin Barrier Function by Activating 11β-Hydroxysteroid Dehydrogenase 1 and the HPA Axis. Sci Rep. 2018 Apr 20;8(1):6334. doi: 10.1038/s41598-018-24653-z. PMID: 29679067; PMCID: PMC5910426.
- Pondeljak N, Lugović-Mihić L. Stress-induced Interaction of Skin Immune Cells, Hormones, and Neurotransmitters. Clin Ther. 2020 May;42(5):757-770. doi: 10.1016/j.clinthera.2020.03.008. Epub 2020 Apr 7. PMID: 32276734.
- Christian LM, Graham JE, Padgett DA, Glaser R, Kiecolt-Glaser JK. Stress and wound healing. Neuroimmunomodulation. 2006;13(5-6):337-46. doi: 10.1159/000104862. Epub 2007 Aug 6. PMID: 17709956; PMCID: PMC2792763.
- Jiang B, Cui L, Zi Y, Jia Y, He C. Skin surface lipid differences in sensitive skin caused by psychological stress and distinguished by support vector machine. J Cosmet Dermatol. 2019 Aug;18(4):1121-1127. doi: 10.1111/jocd.12793. Epub 2018 Oct 2. PMID: 30280473.
- Wang AS, Armstrong EJ, Armstrong AW. Corticosteroids and wound healing: clinical considerations in the perioperative period. Am J Surg. 2013 Sep;206(3):410-7. doi: 10.1016/j.amjsurg.2012.11.018. Epub 2013 Jun 4. PMID: 23759697.
- Ebrecht M, Hextall J, Kirtley LG, Taylor A, Dyson M, Weinman J. Perceived stress and cortisol levels predict speed of wound healing in healthy male adults. Psychoneuroendocrinology. 2004 Jul;29(6):798-809. doi: 10.1016/S0306-4530(03)00144-6. PMID: 15110929.
- Kondo T, Ishida Y. Molecular pathology of wound healing. Forensic Sci Int. 2010 Dec 15;203(1-3):93-8. doi: 10.1016/j.forsciint.2010.07.004. Epub 2010 Aug 23. PMID: 20739128.
- Schoepe S, Schäcke H, May E, Asadullah K. Glucocorticoid therapy-induced skin atrophy. Exp Dermatol. 2006 Jun;15(6):406-20. doi: 10.1111/j.0906-6705.2006.00435.x. PMID: 16689857.
- Bigliardi-Qi M, Sumanovski LT, Büchner S, Rufli T, Bigliardi PL. Mu-opiate receptor and Beta-endorphin expression in nerve endings and keratinocytes in human skin. Dermatology. 2004;209(3):183-9. doi: 10.1159/000079887. PMID: 15459530.
- Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. Key role of CRF in the skin stress response system. Endocr Rev. 2013 Dec;34(6):827-84. doi: 10.1210/er.2012-1092. Epub 2013 Aug 12. PMID: 23939821; PMCID: PMC3857130.
- Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000 Jul;80(3):979-1020. doi: 10.1152/physrev.2000.80.3.979. PMID: 10893429.
- Dieamant Gde C, Velazquez Pereda Mdel C, Eberlin S, Nogueira C, Werka RM, Queiroz ML. Neuroimmunomodulatory compound for sensitive skin care: in vitro and clinical assessment. J Cosmet Dermatol. 2008 Jun;7(2):112-9. doi: 10.1111/j.1473-2165.2008.00373.x. PMID: 18482014.
- Kim HS, Kim HJ, Hong YD, Son ED, Cho SY. β-endorphin suppresses ultraviolet B irradiation-induced epidermal barrier damage by regulating inflammation-dependent mTORC1 signaling. Sci Rep. 2023 Dec 15;13(1):22357. doi: 10.1038/s41598-023-49886-5. PMID: 38102220; PMCID: PMC10724221.
- Huang JH, Li Y, Zhang S, Zou Y, Zheng QW, Lin JF, Guo LQ. Amelioration effect of water extract from Ganoderma resinaceum FQ23 solid-state fermentation fungal substance with high-yield ergothioneine on anxiety-like insomnia mice. Food Funct. 2022 Dec 13;13(24):12925-12937. doi: 10.1039/d2fo01847k. PMID: 36445290.
- Yardman-Frank JM, Fisher DE. Skin pigmentation and its control: From ultraviolet radiation to stem cells. Exp Dermatol. 2021 Apr;30(4):560-571. doi: 10.1111/exd.14260. Epub 2020 Dec 24. PMID: 33320376; PMCID: PMC8218595.
- Brigelius-Flohé R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta. 2013 May;1830(5):3289-303. doi: 10.1016/j.bbagen.2012.11.020. Epub 2012 Nov 29. PMID: 23201771.
- Mieyal JJ, Chock PB. Posttranslational modification of cysteine in redox signaling and oxidative stress: Focus on s-glutathionylation. Antioxid Redox Signal. 2012 Mar 15;16(6):471-5. doi: 10.1089/ars.2011.4454. Epub 2012 Jan 4. PMID: 22136616; PMCID: PMC3270050.
- Allen EM, Mieyal JJ. Protein-thiol oxidation and cell death: regulatory role of glutaredoxins. Antioxid Redox Signal. 2012 Dec 15;17(12):1748-63. doi: 10.1089/ars.2012.4644. Epub 2012 Jun 5. PMID: 22530666; PMCID: PMC3474186.
- Wu S, Szilagyi A, Zhang Y. Improving protein structure prediction using multiple sequence-based contact predictions. Structure. 2011 Aug 10;19(8):1182-91. doi: 10.1016/j.str.2011.05.004. PMID: 21827953; PMCID: PMC3154634.
- Albers I, Zernickel E, Stern M, Broja M, Busch HL, Heiss C, Grotheer V, Windolf J, Suschek CV. Blue light (λ=453 nm) nitric oxide dependently induces β-endorphin production of human skin keratinocytes in-vitro and increases systemic β-endorphin levels in humans in-vivo. Free Radic Biol Med. 2019 Dec;145:78-86. doi: 10.1016/j.freeradbiomed.2019.09.022. Epub 2019 Sep 22. PMID: 31553937.
- Rajapakse NW, Mattson DL. Role of L-arginine in nitric oxide production in health and hypertension. Clin Exp Pharmacol Physiol. 2009 Mar;36(3):249-55. doi: 10.1111/j.1440-1681.2008.05123.x. Epub 2008 Nov 28. PMID: 19076168.
- Liu X, Zhang X, Zhao L, Long J, Feng Z, Su J, Gao F, Liu J. Mitochondria as a sensor, a central hub and a biological clock in psychological stress-accelerated aging. Ageing Res Rev. 2024 Jan;93:102145. doi: 10.1016/j.arr.2023.102145. Epub 2023 Nov 28. PMID: 38030089.
- Cheah IK, Halliwell B. Ergothioneine; antioxidant potential, physiological function and role in disease. Biochim Biophys Acta. 2012 May;1822(5):784-93. doi: 10.1016/j.bbadis.2011.09.017. Epub 2011 Oct 4. PMID: 22001064.
- Singh B, Schoeb TR, Bajpai P, Slominski A, Singh KK. Reversing wrinkled skin and hair loss in mice by restoring mitochondrial function. Cell Death Dis. 2018 Jul 20;9(7):735. doi: 10.1038/s41419-018-0765-9. PMID: 30026579; PMCID: PMC6053453.
- Moretti-Horten DN, Peselj C, Taskin AA, Myketin L, Schulte U, Einsle O, Drepper F, Luzarowski M, Vögtle FN. Synchronized assembly of the oxidative phosphorylation system controls mitochondrial respiration in yeast. Dev Cell. 2024 Apr 22;59(8):1043-1057.e8. doi: 10.1016/j.devcel.2024.02.011. Epub 2024 Mar 19. PMID: 38508182.
- D'Onofrio N, Martino E, Balestrieri A, Mele L, Cautela D, Castaldo D, Balestrieri ML. Diet-derived ergothioneine induces necroptosis in colorectal cancer cells by activating the SIRT3/MLKL pathway. FEBS Lett. 2022 May;596(10):1313-1329. doi: 10.1002/1873-3468.14310. Epub 2022 Feb 14. PMID: 35122251.
- Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011 Nov 25;334(6059):1081-6. doi: 10.1126/science.1209038. PMID: 22116877.
- Zhu D, Li X, Tian Y. Mitochondrial-to-nuclear communication in aging: an epigenetic perspective. Trends Biochem Sci. 2022 Aug;47(8):645-659. doi: 10.1016/j.tibs.2022.03.008. Epub 2022 Apr 6. PMID: 35397926.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.