Medicine Group 2025 February 18;6(2):153-156. doi: 10.37871/jbres2067.
Tissue Analysis of the Vocal Folds Cellular and Biochemical Aspects
Mette Pedersen*
Abstract
The vocal folds are pivotal in human communication, functioning as the primary tone generator of the body. This study investigates advancements in the evaluation of vocal fold functionality through voice-related biomarkers with clinically validated methods, such as basic acoustic measures, Maximum Phonation Time (MPT), the Voice Handicap Index (VHI), and the GRBAS scale. They provide structured frameworks for assessing vocal fold states. Research on genetic regulation of fundamental frequency highlights the significance of integrating genomic and biochemical data to understand vocal fold function and development. Despite advancements in the understanding of many tissue components like elastin and fibrinogen, clinical applications for pathologies of the vocal folds such as multi-handicap syndromes and neurodegenerative disorders remain limited. Regular biopsy changes the function of the vocal folds and cannot be carried out in vivo.
Environmental factors, such as radiation exposure, impact vocal folds and are badly understood. Studies on pubertal individuals from Chornobyl revealed reduced pitch range and intensity. Optical Coherence Tomography (OCT) has emerged as an optical biopsy. It is a tool for visualizing tissue and eventual abnormalities, offering significant potential in evaluating hormonal tissue effects, and biochemical properties in defined in vivo situations. Biochemical understanding of medical treatments for tissue-specific abnormalities, such as genetic and hormonal imbalances, are underexplored.
This article underscores the necessity of an interdisciplinary approach to vocal fold understanding, integrating biochemical research with clinical practice, that includes among others OCT and defined voice-related biomarkers. This integration is crucial for addressing gaps in diagnostics and treatment, ultimately improving outcomes in managing e.g. hormonal, genetic, neurodegenerative, and environmentally induced voice disorders.
Introduction
The vocal folds are a critical component of communication, serving as the tone generator of the human body with movement on average of 50/2000 Hz and 40/120 db. Considerable research has been conducted on normal and pathological conditions, in a consensus paper outlining clinically applicable background methods for evaluating voice-related biomarkers [1]. These methods encompass both acoustic and airflow measures. Evaluating vocal fold tissue function requires a comprehensive approach that also includes both subjective self-assessments and expert listener evaluations [2,3]. Suggested biomarkers include basic acoustic measurements of fundamental frequency, jitter, shimmer, and harmonics-to-noise ratio, Maximum Phonation Time (MPT), the Voice Handicap Index (VHI), and the GRBAS test [1,4]. These tools provide a framework for clinically defining the background for tissue-related situations and phenomena of the vocal folds, marking a significant advancement in this field with a possibility for a better understanding of the tissue and its function including treatment for disorders related to the vocal folds.
Perspectives
Research into normal vocal folds has highlighted several critical areas for advancing the "state of the art." These include the study of cells, glands, elastin, fibrinogen, and extracellular phenomena [5]. Despite extensive research in these areas, clinical applications for vocal fold conditions remain sparse [1]. Genetic studies reveal the importance of understanding fundamental frequency regulation and its linkage to genomic profiles [6,7]. To achieve this, it is necessary to analyze how genes regulate vocal fold function at a biochemical level which is not known.
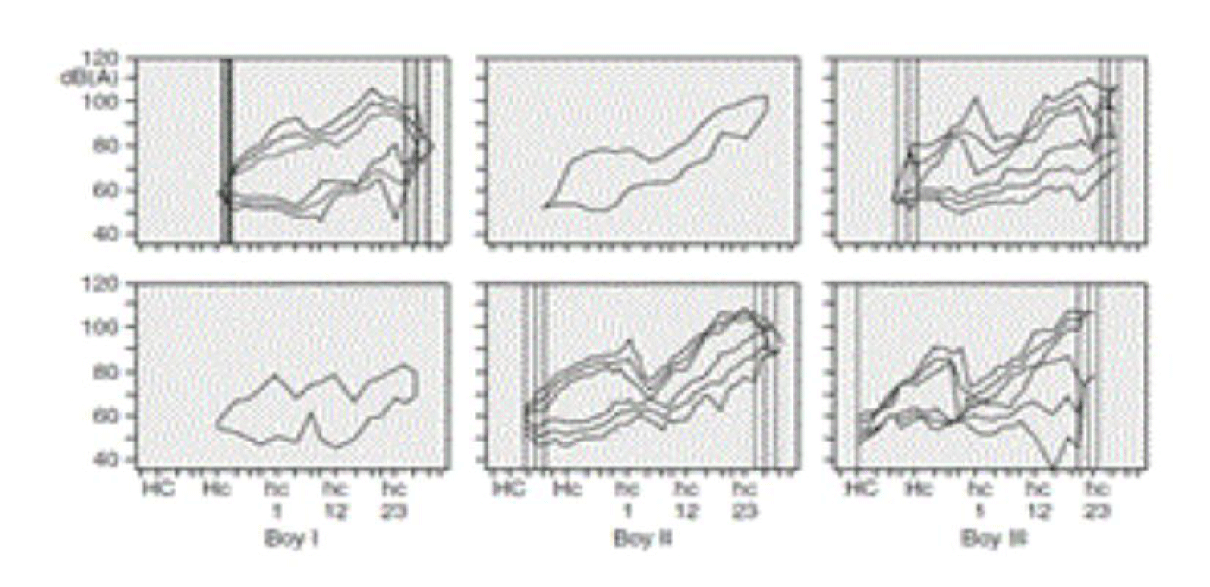
Hormonal regulation of vocal fold development is another essential area of investigation e.g. during puberty. Existing research on hormone-related tissue is fragmented and lacks systematic exploration. Hormonal effects, determined by concentrations in the tissue and receptor activity, require a better biochemical understanding to advance clinical treatments, particularly in the context of gender-related hormonal effects (Figure 1).
Perspectives in pathology
In pathological conditions, research has focused on the regulation of components of vocal fold tissue, such as fibrinogen and elastin. However, these insights have not yet been translated into many clinical applications, particularly not for genetic and neurodegenerative conditions [8]. The reason is among others the difficulty of taking biopsies of the vocal fold in vivo without changing the tissue and the function. Nonetheless, there is increasing focus on voice-related biomarkers and genetic-related pathologies, but not specifically on vocal-fold genetic-related pathologies [9-11]. Although voice-related biomarkers have been introduced, their biochemical foundations remain insufficiently understood. Addressing these limitations is imperative for improving clinical outcomes.
Differentiating results of brain trauma provides a compelling example of the need for enhanced biochemical understanding. Central brain injuries often result in unnecessary vocal fold insufficiency, which could be better understood and managed with focused research. Studies of motor deficiencies in young patients have demonstrated that some vocal fold regulation was left, and some voice aspects could be restored through training, as evidenced by voice range profiles [12,13]. Similarly, environmental provocations, such as radiation exposure, highlight the need for further exploration. Research on pubertal children in Chornobyl revealed reduced vocal fold movement and diminished pitch range and intensity, emphasizing the importance of understanding tissue distortions caused by environmental factors [14]. There is scarce literature on medical treatment for patients after cancer-radiation therapy of the vocal folds, the effect of training is not well documented [15]. In many other fields, OCT does make clinicians able to solve tissue-related pathologies. This possibility is to be further developed for vocal folds.
Perspectives of methodologies
Optical Coherence Tomography (OCT) has emerged as a transformative tool for analyzing vocal fold tissue. OCT enables the visualization in vivo of capillary changes, glands, edema, and infection- or allergy-related cells, providing a means to evaluate abnormalities with precision [5,16-18]. This is also the case for understanding vocal-fold movements on a cellular level [19]. A broader application of OCT with a biochemical approach could significantly impact the clinical assessment of both normal and pathological vocal folds. This is not possible with regular in vivo biopsies since the vocal folds function is thereby changed, or even destroyed.
In benign laryngeal disorders, there remains no evidence of effective tissue-level medical treatments, including e.g. the use of antibiotics, antihistamines, hormones, corticosteroids, androgens, estrogens, and many others. However with OCT documentation in vivo, without regular biopsy, is possible [20]. Biochemical measurements facilitated by OCT are essential for addressing this gap. For instance, reflux—a common disorder affecting the posterior vocal folds—also remains poorly understood in terms of its tissue effects, including the effects of acid and enzyme-induced damage. Further research is necessary to address these deficiencies.
Discussion and Conclusion
The aforementioned aspects emphasize the need to integrate biochemical perspectives into vocal fold research. Advances in tools such as consensus on voice-related biomarkers, and OCT provide opportunities to precisely define and measure the biochemical abnormalities in vocal fold tissue in a specific defined situation also making a comparison of studies better in the future. A deeper understanding of the biochemical underpinnings of vocal fold function is critical for improving diagnostic accuracy and developing better evidence-based targeted therapies. These advancements hold the potential to address a wide range of disorders, including hormonal imbalances, genetic anomalies, neurodegenerative conditions, and environmental provocations.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Lechien JR, Geneid A, Bohlender JE, Cantarella G, Avellaneda JC, Desuter G, Sjogren EV, Finck C, Hans S, Hess M, Oguz H, Remacle MJ, Schneider-Stickler B, Tedla M, Schindler A, Vilaseca I, Zabrodsky M, Dikkers FG, Crevier-Buchman L. Consensus for voice quality assessment in clinical practice: guidelines of the European Laryngological Society and Union of the European Phoniatricians. Eur Arch Otorhinolaryngol. 2023 Dec;280(12):5459-5473. doi: 10.1007/s00405-023-08211-6. Epub 2023 Sep 14. PMID: 37707614.
- Jacobson B, Johnson FA, Grywalski C, Silbergleit A, Jacobson G, Benninger MS, Newman CW. The Voice Handicap Index (VHI): Development and validation. AJSLP. 1997;6(3):66-70 doi: 10.1044/1058-0360.0603.66.
- De Bodt MS, Wuyts FL, Van de Heyning PH, Croux C. Test-retest study of the GRBAS scale: influence of experience and professional background on perceptual rating of voice quality. J Voice. 1997 Mar;11(1):74-80. doi: 10.1016/s0892-1997(97)80026-4. PMID: 9075179.
- Pedersen M. Artificial intelligence for screening voice disorders: Aspects of risk factors. AJMCRR. 2025;4(2):1-8. doi: 10.58372/2835-6276.1254.
- Wei W, Choi WJ, Men S, Song S, Wang R. Wide-field and long-ranging-depth optical coherence tomography microangiography of human oral mucosa (Conference Presentation). Proceedings of SPIE. 2018. doi: 10.1117/12.2290685.
- Gisladottir RS, Helgason A, Halldorsson BV, Helgason H, Borsky M, Chien YR, Gudnason J, Gudjonsson SA, Moisik S, Dediu D, Thorleifsson G, Tragante V, Bustamante M, Jonsdottir GA, Stefansdottir L, Rutsdottir G, Magnusson SH, Hardarson M, Ferkingstad E, Halldorsson GH, Rognvaldsson S, Skuladottir A, Ivarsdottir EV, Norddahl G, Thorgeirsson G, Jonsdottir I, Ulfarsson MO, Holm H, Stefansson H, Thorsteinsdottir U, Gudbjartsson DF, Sulem P, Stefansson K. Sequence variants affecting voice pitch in humans. Sci Adv. 2023 Jun 9;9(23):eabq2969. doi: 10.1126/sciadv.abq2969. Epub 2023 Jun 9. PMID: 37294764; PMCID: PMC10256171.
- Pedersen M. Normal development of voice. 2024. doi: 10.1007/978-3-031-42391-8.
- Pedersen M, Dinnesen A, Mahmood S. Genetic background of voice disorders and genetic perspectives in voice treatment. In: Phoniatrics I, fundamentals, voice disorders, disorders of language and hearing development. 2019. p.225-230.
- Calà F, Frassineti L, Sforza E, Onesimo R, D'Alatri L, Manfredi C, Lanata A, Zampino G. Artificial Intelligence Procedure for the Screening of Genetic Syndromes Based on Voice Characteristics. Bioengineering (Basel). 2023 Nov 29;10(12):1375. doi: 10.3390/bioengineering10121375. PMID: 38135966; PMCID: PMC10741055.
- Pelka F, Ensthaler M, Wendler O, Kniesburges S, Schützenberger A, Semmler M. Mechanical Parameters Based on High-Speed Videoendoscopy of the Vocal Folds in Patients With Ectodermal Dysplasia. J Voice. 2023 Mar 25:S0892-1997(23)00084-X. doi: 10.1016/j.jvoice.2023.02.027. Epub ahead of print. PMID: 36973131.
- Frassineti L, Calà F, Sforza E, Onesimo R, Leoni C, Lanatà A, Zampino G, Manfredi C. Quantitative acoustical analysis of genetic syndromes in the number listing task. Biomedical Signal Processing and Control. 2023;85(4):104887. doi: 10.1016/j.bspc.2023.104887.
- Pedersen MF. Stimmfunktion vor und nach behandlung von hirngeschädigten. Mit Stroboskopie, Phonetographie und Luftstromanalyse durchgefürht. Sprache, Stimme, Gehöhr. 1995.
- Pedersen M, Christensen AL. A study of phonetograms in young brain-damaged people. Proc XXIInd Congress of the International Association of Logopedics and Phoniatrics. 1992.
- Krushevskaja I, Pedersen M. A pilot study of radiated voices in Chernobyl. Proc XXIIth Congress of the International Association of Logopedics and Phoniatrics. 1992.
- Villari CR, Courey MS. Management of Dysphonia After Radiation Therapy. Otolaryngol Clin North Am. 2015 Aug;48(4):601-9. doi: 10.1016/j.otc.2015.04.006. Epub 2015 Jun 17. PMID: 26092762.
- Israelsen NM, Jensen M, Jønsson AO, Pedersen M. Ultrahigh resolution optical coherence tomography for detecting tissue abnormalities of the oral and laryngeal mucosa: A preliminary study. MAVEBA Proceedings. 2019;195-197.
- Thiboutot J, Yuan W, Park HC, Li D, Loube J, Mitzner W, Yarmus L, Li X, Brown RH. Visualization and Validation of The Microstructures in The Airway Wall in vivo Using Diffractive Optical Coherence Tomography. Acad Radiol. 2022 Nov;29(11):1623-1630. doi: 10.1016/j.acra.2022.01.008. Epub 2022 Mar 10. PMID: 35282990; PMCID: PMC9463401.
- Leichtle A, Penxova Z, Kempin T, Leffers D, Ahrens M, König P, Brinkmann R, Hüttmann G, Bruchhage KL, Schulz-Hildebrandt H. Dynamic microscopic optical coherence tomography as a new diagnostic tool for otitis media. Photonics. 2023;10(6):685. doi: 10.3390/photonics10060685.
- Sharma GK, Chen LY, Chou L, Badger C, Hong E, Rangarajan S, Chang TH, Armstrong WB, Verma SP, Chen Z, Ramalingam R, Wong BJ. Surface kinematic and depth-resolved analysis of human vocal folds in vivo during phonation using optical coherence tomography. J Biomed Opt. 2021 Aug;26(8):086005. doi: 10.1117/1.JBO.26.8.086005. PMID: 34414705; PMCID: PMC8374544.
- Meller A, Shakhova M, Rilkin Y, Novozhilov A, Kirillin M, Shakhov A. Optical coherence tomography in diagnosing inflammatory diseases of ENT. Photonics and Lasers in Medicine. 2014;3(4). doi: 10.1515/plm-2014-0025.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.