Medicine Group 2025 February 15;6(2):139-152. doi: 10.37871/jbres2066.
Analysis of the Predictive Value of CA125 Elimination Rate Constant (KELIM) for Outcomes of Interval Debulking Surgery and Prognosis in High-Grade Serous Ovarian Cancer
Chaorong Zeng and Zhuoying Hu*
- KELIM score
- Cut-off value
- Ovarian cancer
- Interval debulking surgery
- Survival
Abstract
Objective: To determine the cutoff value of the KELIM score in patients with High-Grade Serous Ovarian Cancer (HGSOC) undergoing Neoadjuvant Chemotherapy (NACT) followed by Interval Debulking Surgery (IDS), and to better assess the significance of the KELIM score as a prognostic and predictive indicator for HGSOC.
Methods: A total of 124 patients with HGSOC who underwent NACT+IDS were included. The Receiver Operating Characteristic (ROC) curve and Youden index were used to determine the cutoff value of the KELIM score for predicting complete cytoreductive surgery (R0 resection) in HGSOC patients undergoing NACT. Logistic regression analysis was conducted to identify factors predicting complete cytoreductive surgery, and Cox regression analysis was used to assess the correlation between the KELIM score and recurrence and mortality in HGSOC. Based on the cutoff value of the KELIM score, patients were divided into two groups, and differences in clinic pathological parameters between the groups were compared.
Results: The optimal cutoff value of the KELIM score for predicting R0 resection was 1.25. The KELIM score was identified as an independent risk factor for both R0 resection and recurrence in patients with HGSOC.
Conclusion: A KELIM score cutoff of 1.25 is optimal for predicting complete cytoreductive surgery and recurrence in patients with high-grade serous ovarian cancer.
Background
Among malignancies of the female reproductive tract, ovarian cancer ranks second in incidence after cervical and endometrial cancers, but holds the highest mortality rate. High-Grade Serous Carcinoma (HGSC) is the most prevalent subtype of ovarian cancer with a poor prognosis. Due to the lack of early typical symptoms and effective screening methods, approximately 75% of patients with HGSC are diagnosed at an advanced stage [1]. For patients with stage III-IV ovarian cancer who have extensive metastasis, poor general condition, and multiple comorbidities, Primary Debulking Surgery (PDS) often fails to achieve satisfactory cytoreduction and increases the incidence of complications and mortality. Neoadjuvant Chemotherapy (NACT) followed by Interval Debulking Surgery (IDS) can improve the R0 resection rate and has become one of the initial treatment modalities for Advanced Ovarian Cancer (AOC).
Residual tumor burden after IDS is one of the most important prognostic factors affecting survival [2]. Patients who achieve R0 resection generally have longer relapse-free survival and overall survival compared to those who undergo incomplete cytoreductive surgery. Currently, imaging examination are commonly performed after neoadjuvant chemotherapy and before IDS to assess chemotherapy sensitivity and the likelihood of complete cytoreduction. However, the assessment of peritoneal micrometastases through traditional imaging is challenging, and the Response Evaluation Criteria in Solid Tumors (RECIST) is not suitable for evaluating tumor response in such patients [3]. Laparoscopy also plays a role in the diagnosis and treatment of ovarian cancer and the assessment of residual tumor burden after surgery. However, it is an invasive procedure, and there are currently controversies surrounding this technique due to issues such as iatrogenic tumor rupture and dissemination, tumor metastasis or implantation at the abdominal wall puncture site, and the impact of the CO2 pneumoperitoneum environment on the biological behavior of ovarian cancer. Some literature has even reported that despite laparoscopy predicting complete cytoreduction in some patients undergoing neoadjuvant chemotherapy, patients still underwent suboptimal surgery [4].
CA125 (Carbohydrate Antigen 125) is the most commonly tested serum tumor marker in the diagnosis, treatment, and follow-up of ovarian cancer. Changes in serum CA125 levels during treatment are correlated with the prognosis and survival of ovarian cancer patients, serving as a valuable, simple, and economical additional tool for early assessment of tumor response to chemotherapy and prognosis in clinical trials. In recent years, numerous prognostic indicators based on serum CA125 have been proposed, such as the CA-125 nadir, half-life value, and GCIG CA125 response. However, these have not been widely adopted due to varying cutoff values and controversial research findings. Mathematical modeling of CA-125 dynamics during chemotherapy by researchers such as YOU may represent a promising strategy. Utilizing data from the CALYPSO Phase III trial, a model dynamic parameter related to CA-125 clearance, namely the modeled CA125 ELIMination rate constant K (KELIM), was reported to have strong prognostic value in terms of progression-free survival for patients with recurrent ovarian cancer treated with a carboplatin-based chemotherapy regimen [5]. The reproducible predictive value of modeled dynamic parameters for other serum tumor markers, such as Prostate-Specific Antigen (PSA), human Chorionic Gonadotropin (hCG), Alpha-Fetoprotein (AFP), and Circulating Tumor Cells (CTCs), has been reported in previous work [6-9].
KELIM represents the clearance rate of CA125 during systemic treatment, which is independent of renal function and correlates with tumor burden status, effectively reflecting the sensitivity of tumors to chemotherapy. A higher KELIM value indicates a faster tumor elimination rate and better chemotherapy efficacy, thus leading to a higher rate of complete surgical resection. Therefore, KELIM serves as a potentially useful tool for preoperative assessment of tumor resectability. Researchers validated the feasibility of KELIM in predicting complete cytoreductive surgery, as well as Progression-Free Survival (PFS) and Overall Survival (OS) of patients, through data from the CHIVA Phase II trial [10] and neoadjuvant chemotherapy patients in the ICON-8 trial [11]. Studies by the YOU team also found that patients with good KELIM scores, regardless of whether they received neoadjuvant chemotherapy or standard chemotherapy, had a doubled probability of long progression-free survivorship (LPS) >5 years [12] and a 3.5-fold higher probability of Long-Term Disease-Free (LDF) survival ≥5 years [13]. Research from the Netherlands cancer registry [14] and real-world data from three other countries [15-17] also demonstrated the predictive value of the KELIM score for satisfactory Interval Debulking Surgery (IDS).
Most of the existing research on KELIM originates from the you team, with the study population primarily consisting of European ethnicity. Relatively few studies have been conducted in Asia, and the use of KELIM to guide clinical practice still lacks strong persuasive power. The purpose of this study is to explore the predictive value of KELIM for complete cytoreductive surgery and prognosis using real clinical data from an Asian ovarian cancer population.
Methods
Study subjects
Patients diagnosed with primary high-grade serous ovarian cancer at the First Affiliated Hospital of Chongqing Medical University between May 2015 and September 2022 were selected. Inclusion criteria were: 1. Patients with primary high-grade serous ovarian cancer who underwent Neoadjuvant Chemotherapy (NACT) followed by Interval Debulking Surgery (IDS); 2. Confirmed diagnosis of high-grade serous ovarian cancer by postoperative pathological examination; 3. Underwent standard chemotherapy after surgery; 4. The system had at least two CA125 values and corresponding test dates within 100 days after the first chemotherapy; 5. Regular follow-up. Exclusion criteria were: 1. Concurrent with other malignant tumors; 2. Incomplete clinicopathological data; 3. Loss to follow-up.
Study data and methods
Study data: Patient general clinical characteristics (Basic information, FIGO stage, menstrual status, body mass index, family history of cancer, CA125 levels and time points within 100 days after the start of chemotherapy, and genetic testing results), pathological features (Lymph node metastasis status), and treatment characteristics (Surgical approach, number of neoadjuvant chemotherapy cycles, maintenance treatment regimen, platinum sensitivity, and whether there was recurrence or death) were collected from the electronic medical record system. Follow-up dates were recorded up to December 31, 2023. Patient prognosis was assessed through telephone follow-up, with records of recurrence date, time of death, and cause of death.
The grading of residual cancer foci after surgery is classified as R0 (no gross residual disease), R1 (the longest diameter of the largest gross residual lesion ≤ 1 cm), and R2 (the longest diameter of the largest gross residual lesion > 1 cm) [18]. Complete cytoreductive surgery is defined as R0, while incomplete cytoreductive surgery includes R1 and R2.
The definitions of progression or recurrence refer to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [19], as well as the CA125 evaluation criteria established by the GCIG [20]. Overall Survival (OS) and Progression-Free Survival (PFS) are calculated from the date of first treatment to the date of death (or last follow-up for surviving patients) and from the date of first treatment to the date of recurrence (or last follow-up for those without recurrence), respectively.
The KELIM value for each patient was calculated using the online tool developed by You B, et al. [10] at the following website: https://www.biomarker-kinetics.org/CA-125-neo. To do this, the three chemotherapy dates and corresponding CA-125 levels within 100 days during the neoadjuvant chemotherapy period for each patient were entered. If there were fewer than three CA125 values within the 100 days of neoadjuvant chemotherapy, the baseline CA-125 value was used for calculation.
Statistical methods: Categorical variables are presented as counts and percentages. Continuous variables are described using means with standard deviations or medians with interquartile ranges. The chi-square test is used to assess differences in categorical variables between the R0 and non-R0 groups, as well as between the KELIM < 1.25 and KELIM ≥ 1.25 groups. The t-test and Wilcoxon rank sum test are employed to evaluate differences in continuous variables across these same groups. The primary outcome of interest is the outcome of interval debulking surgery, while secondary outcomes include Overall Survival (OS), Progression-Free Survival (PFS), and Platinum-Free Interval (PFI). Univariate and multivariate analyses of interval debulking surgery outcomes are conducted using logistic regression models. OS, PFS, and PFI rates are calculated using the Kaplan-Meier method, with group differences compared using the Log-rank test. Cox regression models are applied for univariate and multivariate analyses of OS and PFS. Statistical analyses are performed using SPSS version 26.0. All p-values are two-sided, and statistical significance is considered at p < 0.05.
Results
Clinicopathological characteristics of patients
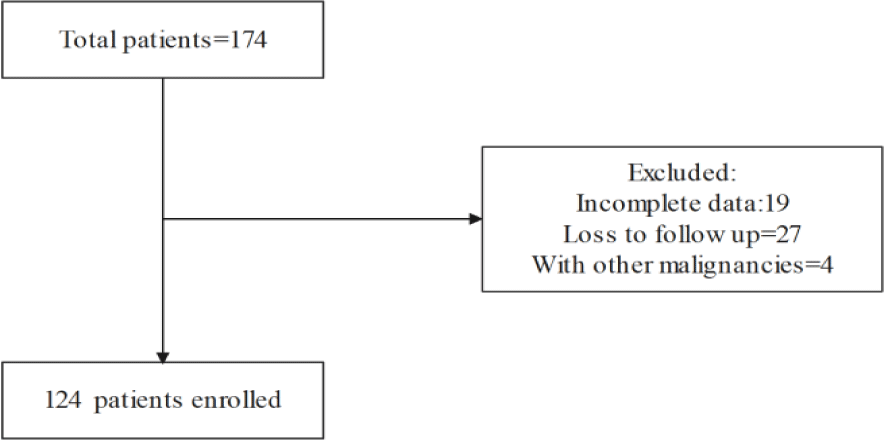
A total of 174 patients undergoing NACT were screened. Among them, 27 patients were lost to follow-up, 4 patients had other concurrent malignancies, and 19 patients had incomplete clinical data. Ultimately, 124 patients were included in the study (Figure 1).
The demographic parameters of the study population are shown in table 1. Among the 124 patients, the average age at diagnosis was 56.56 years, with 86 patients being postmenopausal and 32 having a family history of cancer. According to the FIGO staging, 85 patients (68.5%) were in Stage III and 39 (31.5%) were in Stage IV. Of the 124 patients, 53 underwent lymph node biopsy or dissection, and postoperative pathological examination revealed that 19 patients (15.3%) had lymph node metastasis. Fifty-three patients (42.7%) achieved R0 resection after Interval Debulking Surgery (IDS). Among the 71 patients who underwent incomplete cytoreductive surgery, 34 only achieved R2 resection due to large residual lesions on the diaphragm or small intestine. The majority of patients (79.9%) received 2-3 cycles of neoadjuvant chemotherapy before surgery, while a minority (20.1%) received more than 3 cycles. In terms of previous treatment, 36 patients (29.0%) were treated with bevacizumab, and 60 (48.4%) were treated with PARP inhibitors. Among the 53 patients who underwent genetic testing, 23 (18.5%) had BRCA gene mutations. The mean KELIM score was 1.18. A total of 23 patients (18.5%) had a platinum-free interval of less than 6 months. One hundred patients experienced recurrence, and 58 patients died.
| Table 1: Clinical demographic characteristics of patients. | |||||
| Variables | Overall population N = 124 | R0 N = 53(42.7%) | Non-R0 N = 71(57.3%) | Wald | p-value |
| Age (Mean ± SD) | 56.56 ± 8.69 | 56.36 ± 8.68 | 56.70 ± 8.76 | t = -218 | 0.828 |
| Menopause (n, %) | x2 = 1.630 | 0.202 | |||
| Yes | 86(69.4%) | 40(75.5%) | 46(64.8%) | ||
| No | 38(30.6%) | 13(24.5%) | 25(35.2%) | ||
| Family tumor history (n, %) | x2 = 0.301 | 0.583 | |||
| Yes | 32(25.8%) | 15(28.3%) | 17(23.9%) | ||
| No | 92(74.2%) | 38(71.7%) | 54(76.1%) | ||
| BMI(kg/m2) | 23.35 ± 3.18 | 23.46 ± 3.12 | 23.27 ± 3.24 | t = 0.319 | 0.75 |
| FIGO Stage | x2 = 4.913 | 0.027 | |||
| Stage III | 85(68.5%) | 42(79.2%) | 43(60.6%) | ||
| StageIV | 39(31.5%) | 11(20.8%) | 28(39.4%) | ||
| CA125 at diagnosis (U/mL) [Median(range)] | 1856.10(828.70-3575.95) | 1774.5(915.45-3471.75) | 1939.2(800.35-3600.25) | Z = -0.66 | 0.948 |
| HE4 at diagnosis (pmol/L) [Median(range)] | 605.00(318.50-1194.00) | 482.00(317.75-899.25) | 736.00(315.50-1513.00) | Z = -2.046 | 0.042 |
| Lymph node metastasis | x2 = 0.062 | 0.970 | |||
| Yes | 19(15.3%) | 8(15.1%) | 11(15.5%) | ||
| No | 34(27.4%) | 14(26.4%) | 20(28.2%) | ||
| BRCA mutation (n, %) | x2 = 1.670 | 0.434 | |||
| Yes | 23(18.5%) | 12(22.6%) | 11(15.5%) | ||
| No | 30(24.2%) | 14(26.4%) | 16(22.5%) | ||
| Surgical procedure (n, %) | x2 = 5.598 | 0.061 | |||
| Laparoscope | 85(68.5%) | 42(79.2%) | 43(60.6%) | ||
| Laparotomy | 37(29.8%) | 11(20.8%) | 26(36.6%) | ||
| Conversion to laparotomy | 2(1.6%) | 0(0.0%) | 2(2.8%) | ||
| Cycles of NACT (n, %) | x2 = 6.278 | 0.179 | |||
| 2 | 25(20.2%) | 6(11.3%) | 19(26.8%) | ||
| 3 | 74(59.7%) | 33(62.3%) | 41(57.7%) | ||
| 4 | 20(16.1%) | 12(22.6%) | 8(11.3%) | ||
| 5 | 2(1.6%) | 1(1.9%) | 1(1.4%) | ||
| 6 | 3(2.4%) | 1(1.9%) | 2(2.8%) | ||
| PARP-inhibitor maintenance (n, %) | x2 = 0.055 | 0.815 | |||
| Yes | 60(48.4%) | 25(47.2%) | 35(49.3%) | ||
| No | 64(51.6%) | 28(52.8%) | 36(50.7%) | ||
| Bevacizumab Maintenance | x2 = 0.060 | 0.806 | |||
| Yes | 36(29.0%) | 16(30.2%) | 20(28.2%) | ||
| No | 88(71.0%) | 37(69.8%) | 51(71.8%) | ||
| Recurrence (n, %) | x2 = 12.654 | 0.000 | |||
| Yes | 100(80.6%) | 35(66.0%) | 65(91.5%) | ||
| No | 24(19.4%) | 18(34.0%) | 6(8.5%) | ||
| Death (n, %) | x2 = 3.037 | 0.081 | |||
| Yes | 58(46.8%) | 20(37.7%) | 38(53.5%) | ||
| No | 66(53.2%) | 33(62.3%) | 33(46.5%) | ||
| PFI (n, %) | x2 = 3.201 | 0.074 | |||
| <6months | 23(18.5%) | 6(11.3%) | 17(23.9%) | ||
| ≥6months | 101(81.5%) | 47(88.7%) | 54(76.1%) | ||
| KELIM (Mean ± SD) | 1.18 ± 0.43 | 1.324 ± 0.411 | 1.076 ± 0.422 | t = 3.27 | 0.001 |
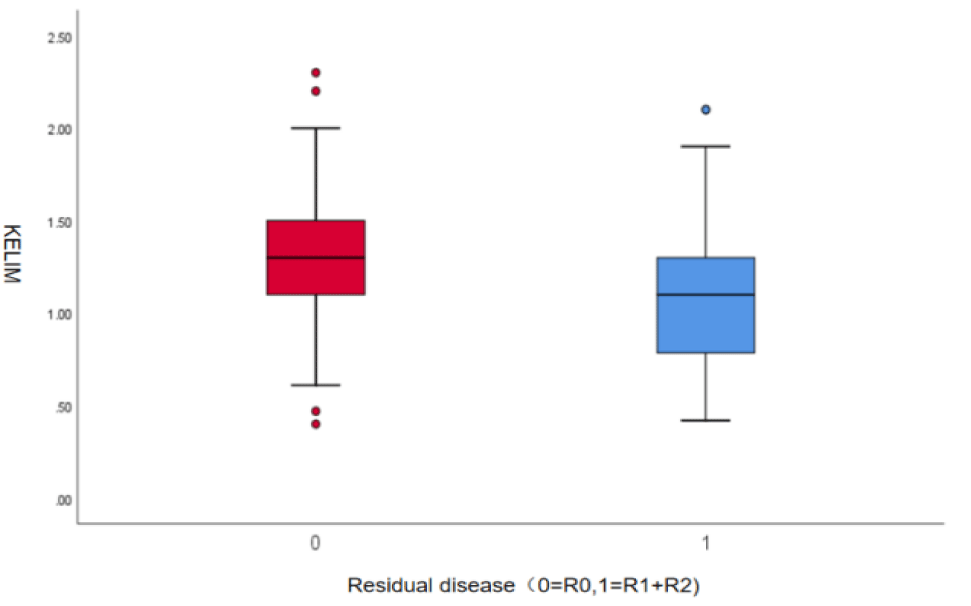
There were no statistically significant differences in age, BMI, menstrual status, family history of cancer, baseline CA125 levels, lymph node metastasis, BRCA mutation status, surgical approach, number of Neoadjuvant Chemotherapy (NACT) cycles, maintenance therapy with PARP inhibitors, maintenance therapy with bevacizumab, platinum-free interval, or mortality between patients with R0 cytoreduction and those with non-R0 cytoreduction (p > 0.05). However, it is noteworthy that the proportion of platinum-resistant patients was significantly lower in the R0 group compared to the non-R0 group [6 (11.3%) vs. 17 (23.9%)]. There were statistically significant differences in FIGO stage, baseline HE4 levels, recurrence, and KELIM scores between the R0 and non-R0 groups (p < 0.05). In the non-R0 group, patients had a lower average KELIM score of 1.076, while patients who underwent complete cytoreductive surgery had a higher average KELIM score of 1.324 (p = 0.001, table 1). The distribution of KELIM scores between the two groups is shown in a box plot (Figure 2).
Relationship between KELIM score and complete cytoreductive surgery
Optimal cut-off value of KELIM score for predicting R0 resection: The predictors of complete cytoreductive surgery were determined by the ROC curve (Table 2). Based on these analyses, the best predictor of incomplete cytoreductive surgery was KELIM, with an AUC of 0.680 (95% CI, 0.585-0.776) (Figure 3). The maximum Youden index was 0.336, with a sensitivity of 0.732 and a specificity of 0.604. According to the Youden index, the cut-off point for the KELIM score to predict complete cytoreductive surgery was 1.25.
| Table 2: Receiver Operator Curves (ROCs) for possible predictors of complete cytoreductive surgery. | |||
| AUC | p-value | 95%CI | |
| Age (year) | 0.471 | 0.594 | 0.363 - 0.579 |
| BMI(kg/m2) | 0.548 | 0.384 | 0.440 - 0.655 |
| FIGO Stage | 0.599 | 0.070 | 0.495 - 0.704 |
| CA125 at diagnosis (U/mL) | 0.509 | 0.872 | 0.401 - 0.617 |
| Cycles of NACT (n, %) | 0.606 | 0.053 | 0.502 - 0.710 |
| HE4 at diagnosis (pmol/L) | 0.610 | 0.041 | 0.508 - 0.712 |
| KELIM | 0.680 | 0.001 | 0.585 - 0.776 |
Logistic univariate and multivariate analysis of factors influencing r0 resection: As shown in table 3, logistic univariate analysis revealed that both the FIGO stage and KELIM score were statistically significant predictors of R0 resection. However, upon multivariate analysis, the KELIM score was confirmed as an independent predictor of achieving complete resection (OR=4.247; 95% CI, 1.857-9.716, p = 0.001).
| Table 3: Univariate and multivariate analysis for predicting r0 resection. | ||||||
| Variables | Univariate analysis | Multivariate analysis | ||||
| HR(95% CI) | Wald | p-value | HR(95% CI) | Wald | p-value | |
| FIGO Stage | 0.402(0.178-0.910) | 4.776 | 0.029 | 0.449(0.182-1.110) | 3.006 | 0.083 |
| KELIM | 4.170(1.949-0.513) | 13.529 | 0.000 | 4.247(1.857-9.716) | 11.733 | 0.001 |
| Age (year) | 1.003(0.968-1.040) | 0.036 | 0.849 | |||
| BMI(kg/m2) | 1.019(0.910-1.140) | 0.104 | 0.748 | |||
| CA125 at diagnosis (U/mL) | 1.000(1.000-1.000) | 0.139 | 0.710 | |||
| HE4 at diagnosis (pmol/L) | 0.999(0.999-1.000) | 5.357 | 0.021 | 0.999(0.999-1.000) | 5.254 | 0.022 |
| Cycles of NACT | 1.448(0.936-2.368) | 2.819 | 0.093 | |||
The relationship between KELIM and patients' Progression-Free Survival (PFS) and Overall Survival (OS)
Using a univariate Cox regression model, clinicopathological factors that may influence the recurrence of high-grade serous carcinoma were analyzed. Factors with a P-value greater than 0.05 were excluded from the multivariate analysis, including BMI (kg/m²) (HR=1.017, p = 0.561), FIGO stage status (HR=1.017, p = 0.109), postoperative residual tumor (HR=0.813, p = 0.327), and baseline CA125 level (U/ml) (HR=1.000, p = 0.301). Factors with a p-value less than 0.05, including age, KELIM score, and baseline HE4 level, were further included in the multivariate Cox regression analysis. Ultimately, two factors were identified as independent predictors of recurrence in HGSOC, including the KELIM score (HR=0.547, p = 0.013) and baseline HE4 level (HR=1.000, p = 0.024). Age did not demonstrate statistical significance in the multivariate analysis (HR=0.977, p = 0.051) (Table 4).
| Table 4: Univariate and multivariate analysis of factors predicting recurrence in patients with high-grade serous ovarian cancer. | ||||||
| Variables | Univariate analysis | Multivariate analysis | ||||
| HR(95% CI) | Wald | p-value | HR(95% CI) | Wald | p-value | |
| Age (year) | 0.974(0.953-0.996) | 5.472 | 0.019 | 0.977(0.955-1.000) | 5.082 | 0.051 |
| BMI (kg/m2) | 1.017(0.961-1.075) | 0.338 | 0.561 | |||
| FIGO Stage | 1.418(0.925-2.174) | 2.567 | 0.109 | |||
| KELIM | 0.593(0.390-0.903) | 5.944 | 0.015 | 0.574(0.370-0.890) | 6.138 | 0.013 |
| Residual disease | 0.813(0.537-1.230) | 0.962 | 0.327 | |||
| CA125 at diagnosis (U/mL) | 1.000(1.000-1.000) | 1.068 | 0.301 | |||
| HE4 at diagnosis (pmol/L) | 1.000(1.000-1.000) | 4.153 | 0.042 | 1.000(1.000-1.000) | 5.082 | 0.024 |
Using a univariate Cox regression model, clinicopathological factors that may influence mortality in patients with high-grade serous ovarian cancer were analyzed. It was found that the P-values for all the factors considered were greater than 0.05. FIGO stage, KELIM score, postoperative residual tumor, PARP inhibitor therapy, and bevacizumab therapy were selected for further inclusion in a multivariate Cox regression analysis. However, no statistically significant factors were identified. Nevertheless, the p-value for the KELIM score was the closest to 0.05 (HR=0.589, p = 0.075) (Table 5).
| Table 5: Univariate and multivariate analysis of factors predicting mortality in high-grade serous ovarian cancer. | ||||||
| Variables | Univariate analysis | Multivariate analysis | ||||
| HR(95% CI) | Wald | p-value | HR(95% CI) | Wald | p-value | |
| Age (year) | 1.006(0.983-1.030) | 0.281 | 0.596 | |||
| BMI (kg/m2) | 0.959(0.878-1.047) | 0.877 | 0.349 | |||
| FIGO Stage | 1.040(0.604-1.790) | 0.020 | 0.887 | 1.044(0.573-1.902) | 0.020 | 0.888 |
| KELIM | 0.585(0.331-1.031) | 3.436 | 0.064 | 0.589(0.329-1.054) | 3.175 | 0.075 |
| Residual disease | 0.932(0.542-1.604) | 0.064 | 0.800 | 0.979(0.547-1.752) | 0.005 | 0.943 |
| CA125 at diagnosis (U/mL) | 1.000(1.000-1.000) | 0.001 | 0.971 | |||
| HE4 at diagnosis (pmol/L) | 1.000(1.000-1.000) | 0.541 | 0.462 | |||
| Bevacizumab maintenance | 0.799(0.429-1.486) | 0.504 | 0.478 | 0.991(0.510-1.924) | 0.001 | 0.979 |
| PARP-inhibitor maintenance | 0.709(0.417-1.207) | 1.606 | 0.205 | 0.696(0.388-1.250) | 1.469 | 0.225 |
Based on the cut-off value of KELIM, patients with a KELIM score <1.25 were defined as the low KELIM group, and those with a KELIM score ≥1.25 were defined as the high KELIM group. Follow-up of 124 patients revealed that patients in the high KELIM score group had significantly longer Progression-Free Survival (PFS), Overall Survival (OS), and Platinum-Free Interval (PFI) compared to those in the low KELIM score group. The mean PFS for patients in the KELIM <1.25 group was 23 months, while the mean PFS for patients in the KELIM ≥1.25 group was 31 months (X²=6.371, p = 0.012) (Figure 4). The mean OS for the KELIM <1.25 group was also lower than that of the KELIM ≥1.25 group, but the difference was not statistically significant (57 months vs. 68 months, X²=3.553, p = 0.059) (Figure 5).
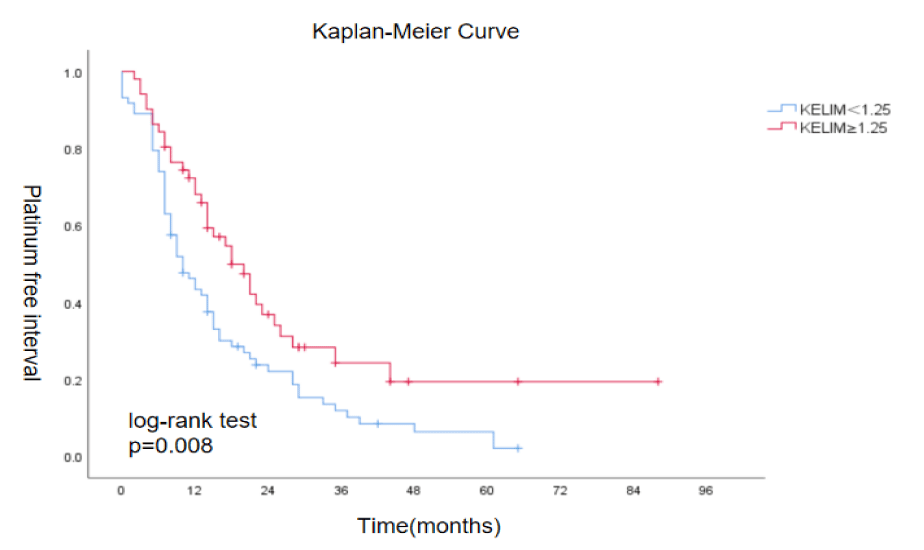
When plotting the curve between KELIM and Platinum-Free Interval (PFI), it was found that patients with a higher KELIM score also had a significantly longer PFI compared to those with a lower KELIM score. The mean PFI for the KELIM <1.25 group was 16 months, while the mean PFI for the KELIM ≥ 1.25 group was 30 months. The difference between the two groups was statistically significant (X²=7.053, p = 0.008) (Figure 6).
Comparison of clinicopathological parameters between patient groups based on the cut-off value of KELIM score
Among the 124 patients who received neoadjuvant chemotherapy, 73 had a KELIM score <1.25 and 51 had a KELIM score ≥1.25. The clinicopathological characteristics of the two groups are shown in table 6. There were statistically significant differences in BRCA mutation status, postoperative residual tumor, and surgical approach between the two groups. Among the patients with a KELIM score <1.25, 25 underwent genetic testing, with 13 having BRCA mutations. Among those with a KELIM score ≥1.25, 28 underwent genetic testing, with 10 having BRCA mutations. The proportion of patients undergoing laparoscopic surgery was significantly higher in the KELIM ≥1.25 group compared to the KELIM <1.25 group [41 (80.4%) vs. 44 (60.3%), p = 0.045], and there were no cases of conversion to laparotomy in the KELIM ≥1.25 group. There were no statistically significant differences between the two groups in terms of age, BMI, menstrual status, family history of cancer, baseline CA125 level, baseline HE4 level, FIGO stage, lymph node metastasis, PARP inhibitor maintenance therapy, bevacizumab maintenance therapy, and platinum-free interval. However, it is noteworthy that the proportion of platinum-resistant patients was lower in the KELIM ≥1.25 group compared to the KELIM <1.25 group [8 (15.7%) vs. 15 (20.5%)] (Table 6).
| Table 6: Comparison of clinicopathological parameters between patients with high and low kelim scores [n = 124; x ± s; n, %; md (p25, p75)]. | ||||
| Variables | KELIM < 1.25 (n = 73) | KELIM ≥ 1.25 (n = 51) | Wald | p-value |
| Age (Mean ± SD) | 55.77 ± 11.15 | 56.73 ± 8.17 | t = -5.23 | 0.602 |
| Menopause (n, %) | x2 = 1.083 | 0.298 | ||
| Yes | 48(65.8%) | 38(74.5%) | ||
| No | 25(34.2%) | 13(25.5%) | ||
| BMI(kg/m2) | 23.15 ± 3.09 | 23.64 ± 3.30 | t = -8.30 | 0.408 |
| Family tumor history (n, %) | x2 = 1.402 | 0.236 | ||
| Yes | 16(21.9%) | 16(31.4%) | ||
| No | 57(78.1%) | 35(68.6%) | ||
| CA125 at diagnosis (U/mL) [Median(range)] | 1505.35(718.875,3256.95) | 1864.100(1041.550,5246.650) | Z = -1.818 | 0.069 |
| HE4 at diagnosis (pmol/L) [Median(range)] | 642.000(258.250,1305.725) | 671.000(407.000,1019.500) | Z = -4.446 | 0.665 |
| Lymph node metastasis | x2 = 1.212 | 0.546 | ||
| Yes | 13(17.8%) | 6(11.8%) | ||
| No | 18(24.7%) | 16(31.4%) | ||
| BRCA mutation (n, %) | x2 = 6.702 | 0.035 | ||
| Yes | 13(17.8%) | 10(19.6%) | ||
| No | 12(16.4%) | 18(35.3%) | ||
| FIGO Stage | x2 = 2.522 | 0.112 | ||
| Stage III | 46(63.0%) | 39(76.5%) | ||
| Stage IV | 27(37.0%) | 12(23.5%) | ||
| Surgical procedure (n, %) | x2 = 6.209 | 0.045 | ||
| Laparotomy | 27(37.0%) | 10(19.6%) | ||
| Laparoscope | 44(60.3%) | 41(80.4%) | ||
| Conversion to laparotomy | 2(2.7%) | 0(0.0%) | ||
| Residual disease | x2 = 14.164 | 0.000 | ||
| R0 | 21(28.8%) | 32(62.7%) | ||
| Non-R0 | 52(71.2%) | 19(37.3%) | ||
| PARP-inhibitor maintenance (n, %) | x2 = 0.014 | 0.906 | ||
| Yes | 35(47.9%) | 25(49.0%) | ||
| No | 38(52.1%) | 26(51.0%) | ||
| Bevacizumab Maintenance (n, %) | x2 = 0.778 | 0.378 | ||
| Yes | 19(26.0%) | 17(33.3%) | ||
| No | 54(74.0%) | 34(66.7%) | ||
| PFI < 6 months (n, %) | 15(20.5%) | 8(15.7%) | x2 = 0.470 | 0.493 |
Discussion
The surgical outcomes and prognosis of Interval Debulking Surgery (IDS) following Neoadjuvant Chemotherapy (NACT) are closely related [21]. The survival benefit of optimal cytoreductive surgery has been well-established, but there is currently no consensus on preoperative methods to predict the achievability of optimal cytoreduction in patients requiring IDS. The KELIM scoring system, developed using modern methods based on artificial intelligence and mathematical modeling, longitudinally describes the kinetic changes of serum tumor marker CA125 during treatment. A retrospective study from the CALYPSO trial involving 895 patients with platinum-sensitive recurrent ovarian cancer first demonstrated the independent prognostic value of the KELIM score in predicting Progression-Free Survival (PFS) [5]. Subsequently, Wilbaux M, et al. [6] first confirmed the correlation between KELIM and tumor size reduction after chemotherapy. In this study, using 124 patients with High-Grade Serous Ovarian Cancer (HGSOC) who received NACT, we confirmed that the KELIM score is an independent prognostic factor for predicting R0 resection. The mean KELIM score was higher in the R0 group compared to the non-R0 group (1.324 vs. 1.076), and the optimal cut-off value for predicting R0 resection using the ROC curve and Youden index was found to be 1.25. Ducoulombier S, et al. [22] retrospectively analyzed 54 patients with ovarian cancer who received NACT and completed IDS, showing that the KELIM score was an independent predictor of optimal cytoreductive surgery (achieving R0 or R1) (OR=0.18; 95% CI=0.04-0.69; p = 0.02). Cheng's analysis of 133 patients in the NACT population also showed that the median KELIM score was higher in patients who achieved R0 resection compared to the non-R0 group (1.20 vs. 0.71, p < 0.001). However, their study indicated a cut-off value of 0.925 for KELIM in predicting R0 resection, with patients with a KELIM score ≥0.925 having longer PFS and OS [23]. Additionally, You B, et al. [10] used data from the CHIVA II trial to show that patients who achieved R0 surgery had significantly higher KELIM scores compared to those who did not (1.04 vs. 0.54). You B, et al. [10] also discussed the relationship between KELIM and platinum-resistant recurrence in their paper. For patients with different surgical outcomes (R0 and non-R0), the platinum-resistant recurrence rate did not differ significantly when the KELIM score was sufficiently high, suggesting that patients with higher KELIM scores (KELIM >1.2) may not have their prognosis severely affected by the outcome of cytoreductive surgery. However, achieving complete cytoreductive surgery is more critical for patients with lower KELIM scores. Previous studies have reported that 15-20% of patients with advanced ovarian cancer do not respond well to chemotherapy [24]. By using the KELIM score, these patients who do not respond well to chemotherapy can be identified, and timely surgical treatment can be performed to achieve better prognosis. For patients with lower KELIM scores, striving for R0 resection is important.
Furthermore, this study also confirmed the prognostic value of the KELIM score. Univariate analysis revealed that the KELIM score is a risk factor for recurrence in patients with High-Grade Serous Ovarian Cancer (HGSC). Further multivariate analysis found that the KELIM score is an independent prognostic factor for recurrence in these patients, in addition to the level of HE4. However, in both univariate and multivariate analyses of disease-specific mortality, the KELIM score did not demonstrate prognostic value due to sample size limitations. This does not negate the prognostic value of the KELIM score. A retrospective analysis of 217 ovarian cancer patients receiving Neoadjuvant Chemotherapy (NACT) found that, compared to patients with a KELIM score ≥1, those with a KELIM score <1 who underwent NACT for advanced HGSC were more likely to experience platinum-resistant disease, worse Progression-Free Survival (PFS), and worse Overall Survival (OS) [17] . Recently, a retrospective cohort study of 232 patients [25] and a national cancer registry database study of 1582 patients [14] also demonstrated the good predictive value of the KELIM score, aligning with our findings except for overall survival. A meta-analysis by Kim R, et al. [26] of CA 125-related kinetic parameters, including the KELIM score, GCIG CA125 response, and CA125 nadir, showed that the KELIM score had greater clinical relevance than the GCIG CA125 response and CA125 nadir. In both newly diagnosed and recurrent ovarian cancer patients, a good KELIM score was associated with significantly improved PFS. Therefore, further prospective, large-sample cohort studies are needed to explore the clinical application value of the KELIM score, which is an economical, convenient, and useful prognostic tool.
Meanwhile, our study results indicate that the HE4 level at diagnosis is an independent risk factor for predicting surgical outcomes and recurrence in patients (p < 0.05). The Area under the Curve (AUC) for predicting complete resection using HE4 is 0.610 (95% CI: 0.508-0.712; p = 0.041), suggesting that HE4 may be a useful additional strategy for predicting surgical outcomes. However, this differs from previous research conclusions. Vallius T, et al. [27] showed that the HE4 value at diagnosis was not associated with surgical outcomes, but the HE4 level before interval debulking surgery was predictive of surgical outcomes. Therefore, larger prospective studies are needed to confirm this finding. Additionally, the use of HE4 for follow-up in ovarian cancer patients has its limitations: most patients with epithelial ovarian cancer are over 50 years old, and HE4 levels are affected by age, with higher values observed in older individuals. HE4 is also significantly influenced by renal function and urinary tract infections. Currently, HE4 is detected using ELISA, and the results of each batch are affected by the standard, requiring frequent quality control calibration [28,29]. Therefore, the reliability of HE4 results during multiple chemotherapy cycles is limited, and its value for follow-up is restricted.
Patients were grouped based on the critical value of the KELIM score, and differences in various influencing factors between the groups were compared. The results showed that there were statistical differences in postoperative residual tumor, surgical approach, and BRCA mutation status between the two groups. Patients with a good KELIM score had a higher chance of achieving complete cytoreductive surgery compared to those with a poor KELIM score (62.7% vs. 28.8%, p < 0.001), and patients with higher chemotherapy sensitivity had a greater opportunity for R0 resection. Additionally, a higher proportion of laparoscopic surgeries were performed in the group with a KELIM score ≥1.25, with no conversions to laparotomy, indicating that patients with better chemotherapy sensitivity are more likely to undergo minimally invasive surgery. In our study, a larger proportion of patients had a KELIM score <1.25, which represents a high-risk group for platinum resistance, recurrence, and death. Our data showed that compared to patients with a KELIM score ≥1.25, those in the low KELIM score group had a higher proportion of platinum resistance (20.5% vs. 15.7%). Most patients in both groups had a platinum-free interval of >6 months, indicating that the majority of the overall patient population was platinum-sensitive during the first course of platinum-based chemotherapy. This is generally consistent with the data summarized by Colomban in the AGO-OVAR 9, AGO-OVAR 7, and ICON 7 trials, which showed that in patients with a good KELIM score, the risk of subsequent platinum-resistant recurrence was low regardless of disease stage (overall <20%). Their study also indicated that in patients with a poor KELIM score, the risk of subsequent platinum-resistant recurrence largely depends on disease stage [30]. In our study, 53 patients (42.7%) underwent BRCA gene testing, and a total of 23 patients had BRCA mutations, accounting for about 43.4%. This is generally consistent with previous studies reporting that approximately 50% of high-grade serous ovarian cancers have defects in the homologous recombination repair pathway [31]. Analysis showed that patients in the higher KELIM score group had a higher rate of BRCA mutations. However, since BRCA testing has only been conducted in our center in recent years, and some patients did not undergo BRCA testing due to financial constraints, our sample size was relatively small, limiting the generalization of our conclusions.
Finally, by plotting Kaplan-Meier (K-M) curves, it was found that patients with a KELIM score <1.25 had significantly worse progression-free survival, overall survival, and platinum-free interval compared to those with a KELIM score ≥1.25, with statistical significance observed for all differences. This indicates that the prognostic management of patients with high-grade serous ovarian cancer who have a lower KELIM score is crucial. However, not all patients in the low KELIM score group experience poor prognosis, and the KELIM score should not be the sole consideration when determining adjuvant treatment plans, maintenance treatment strategies, and follow-up schedules. Currently, the number of postoperative chemotherapy cycles, targeted therapies, and PARP inhibitor maintenance treatment for patients with high-grade serous ovarian cancer still primarily depend on clinicopathological factors such as age, FIGO stage, postoperative residual tumor burden, vascular invasion, lymph node metastasis, and genetic status.
In summary, our study validates KELIM score as a prognostic factor for predicting postoperative residual tumor and survival in patients with high-grade serous ovarian cancer, despite certain limitations in this research. The treatment regimens adopted among the study population were not entirely uniform. In addition to postoperative adjuvant chemotherapy, some individuals also used maintenance therapies such as maintenance chemotherapy, PARP inhibitors, and bevacizumab, leading to heterogeneity within the sample. Furthermore, as this was a single-center study, the number of samples we studied was limited, and a smaller sample size may result in greater fluctuations and uncertainties in the ROC curve.
Meanwhile There are several potential risks associated with the clinical application of KELIM: 1) The calculation of KELIM relies on longitudinal CA-125 dynamic change data within the first 100 days after the initiation of chemotherapy. If these data are incomplete or contain errors, it will directly affect the accuracy of the KELIM value and the reliability of the prediction results. 2) Currently, there is no unified standard for selecting the cutoff value of KELIM, which may lead to discrepancies in prediction results among different studies. 3) Research on KELIM primarily relies on retrospective data, with relatively few prospective studies. The evidence supporting the generalization of research conclusions is insufficient. Therefore, clinicians should use KELIM cautiously, taking into consideration the specific condition of the patient to analyze prognosis and formulate treatment and follow-up plans.
Conclusion
In this retrospective analysis, the optimal cutoff value for the KELIM score was established through the ROC curve and Youden index, and its prognostic predictive capability was validated for patients with high-grade serous ovarian cancer undergoing the NACT+IDS regimen. The study demonstrated that the KELIM score is an independent predictor of recurrence in patients with high-grade serous ovarian cancer. A KELIM score <1.25 was significantly associated with patient recurrence, while patients with a KELIM score ≥1.25 had longer progression-free survival and platinum-free interval. However, our study did not observe a predictive correlation between the KELIM score and disease-specific mortality. Due to the limitations of being a single-center retrospective study, with some patients lost to follow-up and a limited sample size, further research is needed to explore the application value of the KELIM score.
Declaration of Competing Interest
No funding was received to assist with the preparation of this manuscript.
Author Contributions
Zhuoying Hu contributed to the study conception and design. Material preparation, data collection and analysis were performed by Chaorong Zeng. The first draft of the manuscript was written by Chaorong Zeng and all authors commented on previous versions of the manuscript. All authors read and approved.
Ethics Approval
Ethics approval was obtained from the institutional review board of the First Affiliated Hospital of Chongqing Medical University (Approval number: K2024-157-01 on March 15, 2024) to ensure adherence to the ethical standards outlined in the Declaration of Helsinki. Written informed consent was obtained from all the participants prior to the enrollment of this study.
Declaration of Data Availability
The data that support the findings of this study are available from related files.
References
- Makar AP, Tropé CG, Tummers P, Denys H, Vandecasteele K. Advanced Ovarian Cancer: Primary or Interval Debulking? Five Categories of Patients in View of the Results of Randomized Trials and Tumor Biology: Primary Debulking Surgery and Interval Debulking Surgery for Advanced Ovarian Cancer. Oncologist. 2016 Jun;21(6):745-54. doi: 10.1634/theoncologist.2015-0239. Epub 2016 Mar 23. PMID: 27009938; PMCID: PMC4912357.
- Horowitz NS, Miller A, Rungruang B, Richard SD, Rodriguez N, Bookman MA, Hamilton CA, Krivak TC, Maxwell GL. Does aggressive surgery improve outcomes? Interaction between preoperative disease burden and complex surgery in patients with advanced-stage ovarian cancer: an analysis of GOG 182. J Clin Oncol. 2015 Mar 10;33(8):937-43. doi: 10.1200/JCO.2014.56.3106. Epub 2015 Feb 9. PMID: 25667285; PMCID: PMC4348639.
- Rutten MJ, van de Vrie R, Bruining A, Spijkerboer AM, Mol BW, Kenter GG, Buist MR. Predicting surgical outcome in patients with International Federation of Gynecology and Obstetrics stage III or IV ovarian cancer using computed tomography: a systematic review of prediction models. Int J Gynecol Cancer. 2015 Mar;25(3):407-15. doi: 10.1097/IGC.0000000000000368. PMID: 25695545.
- van de Vrie R, Rutten MJ, Asseler JD, Leeflang MM, Kenter GG, Mol BWJ, Buist M. Laparoscopy for diagnosing resectability of disease in women with advanced ovarian cancer. Cochrane Database Syst Rev. 2019 Mar 23;3(3):CD009786. doi: 10.1002/14651858.CD009786.pub3. PMID: 30907434; PMCID: PMC6432174.
- You B, Colomban O, Heywood M, Lee C, Davy M, Reed N, Pignata S, Varsellona N, Emons G, Rehman K, Steffensen KD, Reinthaller A, Pujade-Lauraine E, Oza A. The strong prognostic value of KELIM, a model-based parameter from CA 125 kinetics in ovarian cancer: data from CALYPSO trial (a GINECO-GCIG study). Gynecol Oncol. 2013 Aug;130(2):289-94. doi: 10.1016/j.ygyno.2013.05.013. Epub 2013 May 18. PMID: 23694718.
- Wilbaux M, Hénin E, Oza A, Colomban O, Pujade-Lauraine E, Freyer G, Tod M, You B. Prediction of tumour response induced by chemotherapy using modelling of CA-125 kinetics in recurrent ovarian cancer patients. Br J Cancer. 2014 Mar 18;110(6):1517-24. doi: 10.1038/bjc.2014.75. Epub 2014 Feb 20. PMID: 24556626; PMCID: PMC3960627.
- You B, Harvey R, Henin E, Mitchell H, Golfier F, Savage PM, Tod M, Wilbaux M, Freyer G, Seckl MJ. Early prediction of treatment resistance in low-risk gestational trophoblastic neoplasia using population kinetic modelling of hCG measurements. Br J Cancer. 2013 May 14;108(9):1810-6. doi: 10.1038/bjc.2013.123. Epub 2013 Apr 16. PMID: 23591194; PMCID: PMC3664307.
- Wilbaux M, Hénin E, Oza A, Colomban O, Pujade-Lauraine E, Freyer G, Tod M, You B. Dynamic modeling in ovarian cancer: an original approach linking early changes in modeled longitudinal CA-125 kinetics and survival to help decisions in early drug development. Gynecol Oncol. 2014 Jun;133(3):460-6. doi: 10.1016/j.ygyno.2014.04.003. Epub 2014 Apr 12. PMID: 24726614.
- You B, Colomban O, Tod M, Ray Coquard I, Lortholary A, Hardy-Bessard AC, Du Bois A, Huober J, Meier W, Kurzeder C, Pfisterer J. The predictive value of the CA-125 modeled kinetic parameter KELIM is validated in 3 independent datasets Annals of Oncology. 2016;27(6):296-312. doi:10.1093/annonc/mdw374.4.
- You B, Robelin P, Tod M, Louvet C, Lotz JP, Abadie-Lacourtoisie S, Fabbro M, Desauw C, Bonichon-Lamichhane N, Kurtz JE, Follana P, Leheurteur M, Piano FD, Ferron G, De Rauglaudre G, Ray-Coquard I, Combe P, Chevalier-Place A, Joly F, Leary A, Pujade-Lauraine E, Freyer G, Colomban O. CA-125 ELIMination Rate Constant K (KELIM) Is a Marker of Chemosensitivity in Patients with Ovarian Cancer: Results from the Phase II CHIVA Trial. Clin Cancer Res. 2020 Sep 1;26(17):4625-4632. doi: 10.1158/1078-0432.CCR-20-0054. Epub 2020 Mar 24. PMID: 32209570.
- Colomban O, Clamp A, Cook A, McNeish IA, You B. Benefit From Fractionated Dose-Dense Chemotherapy in Patients With Poor Prognostic Ovarian Cancer: ICON-8 Trial. JCO Clin Cancer Inform. 2023 Apr;7:e2200188. doi: 10.1200/CCI.22.00188. PMID: 37075255; PMCID: PMC10281428.
- You B, Van Wagensveld L, Tod M, Gabe SS, Roy K, Du Bois A, Selle F, Timothy P, Pfisterer J, Florence J, Cook A, Kaminsky-Forrett MC, Wollschlaeger K, Alain L, Oliver T, Alexandra L, Freyer G, Maaike van der Aa, Olivier C. The impact of chemosensitivity assessed by modeled CA-125 KELlM on the likelihood of long progression-free survivorship(PS)after 1st line treatment in ovarian cancer:an analysis of 4450 patients. Annals of Oncology. 2020;31:S616. doi: 10.1016/j.annonc.2020.08.954.
- You B, Van Wagensveld L, Tod M, Sonke GS, Horlings HM, Kruitwagen RFPM, Du Bois A, Selle F, Perren T, Pfisterer J, Joly F, Cook A, Kaminsky MC, Wollschlaeger K, Lortholary A, Tome O, Leary A, Freyer G, Van Der Aa M, Colomban O. Low probability of disease cure in advanced ovarian carcinomas before the PARP inhibitor era. Br J Cancer. 2022 Jul;127(1):79-83. doi: 10.1038/s41416-022-01732-7. Epub 2022 Mar 31. PMID: 35361918; PMCID: PMC9276767.
- van Wagensveld L, Colomban O, van der Aa MA, Freyer G, Sonke GS, Kruitwagen RFPM, You B. Confirmation of the utility of the CA-125 elimination rate (KELIM) as an indicator of the chemosensitivity in advanced-stage ovarian cancer in a "real-life setting". J Gynecol Oncol. 2024 May;35(3):e34. doi: 10.3802/jgo.2024.35.e34. Epub 2024 Jan 8. PMID: 38216134; PMCID: PMC11107274.
- Zouzoulas D, Tsolakidis D, Tzitzis P, Sofianou I, Chatzistamatiou K, Theodoulidis V, Topalidou M, Timotheadou E, Grimbizis G. The Use of CA-125 KELIM to Identify Which Patients Can Achieve Complete Cytoreduction after Neoadjuvant Chemotherapy in High-Grade Serous Advanced Ovarian Cancer. Cancers (Basel). 2024 Mar 24;16(7):1266. doi: 10.3390/cancers16071266. PMID: 38610943; PMCID: PMC11010898.
- Sepulveda ZV, Marin-jimenez JA, Ortiz l,et al. KELlM during neoadjuvant chemotherapy as predictor of interval debulking surgery and treatment response in advanced ovarian cancer. Int J Gynecol Cancer. 2023;33.
- Piedimonte S, Kim R, Bernardini MQ, Atenafu EG, Clark M, Lheureux S, May T. Validation of the KELIM score as a predictor of response to neoadjuvant treatment in patients with advanced high grade serous ovarian cancer. Gynecol Oncol. 2022 Dec;167(3):417-422. doi: 10.1016/j.ygyno.2022.10.014. Epub 2022 Oct 27. PMID: 37191644.
- Kuroki L, Guntupalli SR. Treatment of epithelial ovarian cancer. BMJ. 2020 Nov 9;371:m3773. doi: 10.1136/bmj.m3773. PMID: 33168565.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009 Jan;45(2):228-47. doi: 10.1016/j.ejca.2008.10.026. PMID: 19097774.
- Stuart GC, Kitchener H, Bacon M, duBois A, Friedlander M, Ledermann J, Marth C, Thigpen T, Trimble E; participants of 4th Ovarian Cancer Consensus Conference (OCCC); Gynecologic Cancer Intergroup. 2010 Gynecologic Cancer InterGroup (GCIG) consensus statement on clinical trials in ovarian cancer: report from the Fourth Ovarian Cancer Consensus Conference. Int J Gynecol Cancer. 2011 May;21(4):750-5. doi: 10.1097/IGC.0b013e31821b2568. PMID: 21543936.
- Vincent L, Jankowski C, Ouldamer L, Ballester M, Bendifallah S, Bolze PA, Akladios C, Costaz H, Lavoué V, Canlorbe G, Collinet P, Touboul C, Huchon C, Bricou A, Dridi S, Padéano MM, Bengrine L, Arnould L, Coutant C; Groupe de Recherche FRANCOGYN. Prognostic factors of overall survival for patients with FIGO stage IIIc or IVa ovarian cancer treated with neo-adjuvant chemotherapy followed by interval debulking surgery: A multicenter cohort analysis from the FRANCOGYN study group. Eur J Surg Oncol. 2020 Sep;46(9):1689-1696. doi: 10.1016/j.ejso.2020.04.029. Epub 2020 Apr 24. PMID: 32417154.
- Ducoulombier S, Golfier F, Colomban O, Benayoun D, Bolze PA, Tod M, You B. Modeling CA-125 During Neoadjuvant Chemotherapy for Predicting Optimal Cytoreduction and Relapse Risk in Ovarian Cancer. Anticancer Res. 2017 Dec;37(12):6879-6886. doi: 10.21873/anticanres.12150. PMID: 29187468.
- Li C, Cui Q, Wang X, Yao S, Tu H, Chen M. CA-125 elimination rate constant K (KELIM) as a promising predictor of complete cytoreduction after neoadjuvant chemotherapy in advanced ovarian cancer patients: a retrospective study from two Chinese hospitals. BMC Cancer. 2024 May 20;24(1):609. doi: 10.1186/s12885-024-12252-3. PMID: 38769484; PMCID: PMC11107035.
- Liu YL, Filippova OT, Zhou Q, Iasonos A, Chi DS, Zivanovic O, Sonoda Y, Gardner GJ, Broach VA, O'Cearbhaill RE, Konner JA, Aghajanian C, Long Roche K, Tew WP. Characteristics and survival of ovarian cancer patients treated with neoadjuvant chemotherapy but not undergoing interval debulking surgery. J Gynecol Oncol. 2020 Jan;31(1):e17. doi: 10.3802/jgo.2020.31.e17. PMID: 31833259; PMCID: PMC6918896.
- Bouvarel B, Colomban O, Frenel JS, Loaec C, Bourgin C, Berton D, Freyer G, You B, Classe JM. Clinical impact of CA-125 ELIMination rate constant K (KELIM) on surgical strategy in advanced serous ovarian cancer patients. Int J Gynecol Cancer. 2024 Apr 1;34(4):574-580. doi: 10.1136/ijgc-2023-004872. PMID: 38242546.
- Kim JH, Cho HW, Park EY, Han KH, Kim ET, Lee JK, Park SY, Armbrust R, Fotopoulou C, Lim MC. Prognostic value of CA125 kinetics, half-life, and nadir in the treatment of epithelial ovarian cancer: a systematic review and meta-analysis. Int J Gynecol Cancer. 2023 Dec 4;33(12):1913-1920. doi: 10.1136/ijgc-2023-004825. PMID: 37949486.
- Vallius T, Hynninen J, Auranen A, Carpén O, Matomäki J, Oksa S, Virtanen J, Grénman S. Serum HE4 and CA125 as predictors of response and outcome during neoadjuvant chemotherapy of advanced high-grade serous ovarian cancer. Tumour Biol. 2014 Dec;35(12):12389-95. doi: 10.1007/s13277-014-2553-1. Epub 2014 Sep 5. PMID: 25190018.
- Dayyani F, Uhlig S, Colson B, Simon K, Rolny V, Morgenstern D, Schlumbrecht M. Diagnostic Performance of Risk of Ovarian Malignancy Algorithm Against CA125 and HE4 in Connection With Ovarian Cancer: A Meta-analysis. Int J Gynecol Cancer. 2016 Nov;26(9):1586-1593. doi: 10.1097/IGC.0000000000000804. PMID: 27540691.
- Chudecka-Głaz A, Cymbaluk-Płoska A, Wężowska M, Menkiszak J. Could HE4 level measurements during first-line chemotherapy predict response to treatment among ovarian cancer patients? PLoS One. 2018 Mar 27;13(3):e0194270. doi: 10.1371/journal.pone.0194270. PMID: 29584739; PMCID: PMC5870956.
- Colomban O, Tod M, Leary A, Ray-Coquard IL, Lortholary A, Hardy-Bessard AC, Pfisterer J, Du Bois A, Kurzeder C, Burges A,Peron J, Freyer G, You B. Early prediction of the platinum-resistant relapse risk using the CA125 modeled kinetic parameter KELIM: A pooled analysis of AGO-OVAR 7 & 9; ICON 7 (AGO/GINECO/ MRC CTU/GCIG trials)[J]. Annals of Oncology. 2019;30(419). doi: 10.1093/annonc/mdz250.035.
- Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D'Andrea AD. Homologous Recombination Deficiency: Exploiting the Fundamental Vulnerability of Ovarian Cancer. Cancer Discov. 2015 Nov;5(11):1137-54. doi: 10.1158/2159-8290.CD-15-0714. Epub 2015 Oct 13. PMID: 26463832; PMCID: PMC4631624.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.