Medicine Group 2025 January 31;6(1):107-116. doi: 10.37871/jbres2062.
Non-Specific Interstitial Pneumonia in a Woman with Osteoporosis Treated with Denosumab
Estela Cristina Prieto Maillo*, Ana Gabriela Salazar Palacios, Ana Maria Martin Varillas, Jose Luis Fernandez Sanchez, Sergio Cadenas Menendez and Amparo Rosa Sanchez Serrano
- Non-Specific Interstitial Pneumonia (NSIP)
- Denosumab
- Osteoporosis
Abstract
Denosumab is an antiresorptive monoclonal antibody widely used in the treatment of osteoporosis.
A rare and potentially life-threatening adverse reaction likely associated with denosumab treatment is described, presenting as the first case report in the English literature of non-specific interstitial pneumonia (NSIP), to the best of our knowledge. The importance of clinically recognising drug-induced manifestations is evidenced in this case, where early withdrawal of the drug is usually associated with a clinical-prognostic improvement.
Introduction
Denosumab is an antiresorptive monoclonal antibody used in the treatment of osteoporosis. An exceptional case of Non-Specific Interstitial Pneumonia (NSIP) attributed to denosumab is described.
Case Description
A 68-year-old woman, with no history of exposure or consumption of toxic substances, was referred to the Pneumology Department due to progressive moderate exertional dyspnea, dry cough and intermittent vespertine fever since January 2023.
Her medical history includes osteoporosis and several vertebral crushes under follow-up by Rheumatology since 2011, which prescribed treatment with denosumab (60 mg, semiannual) in October 2022. Concurrently, three months after starting this treatment, she developed recurrent cystitis, dry oral and esophageal mucosa, gastric discomfort, asthenia and generalized pain.
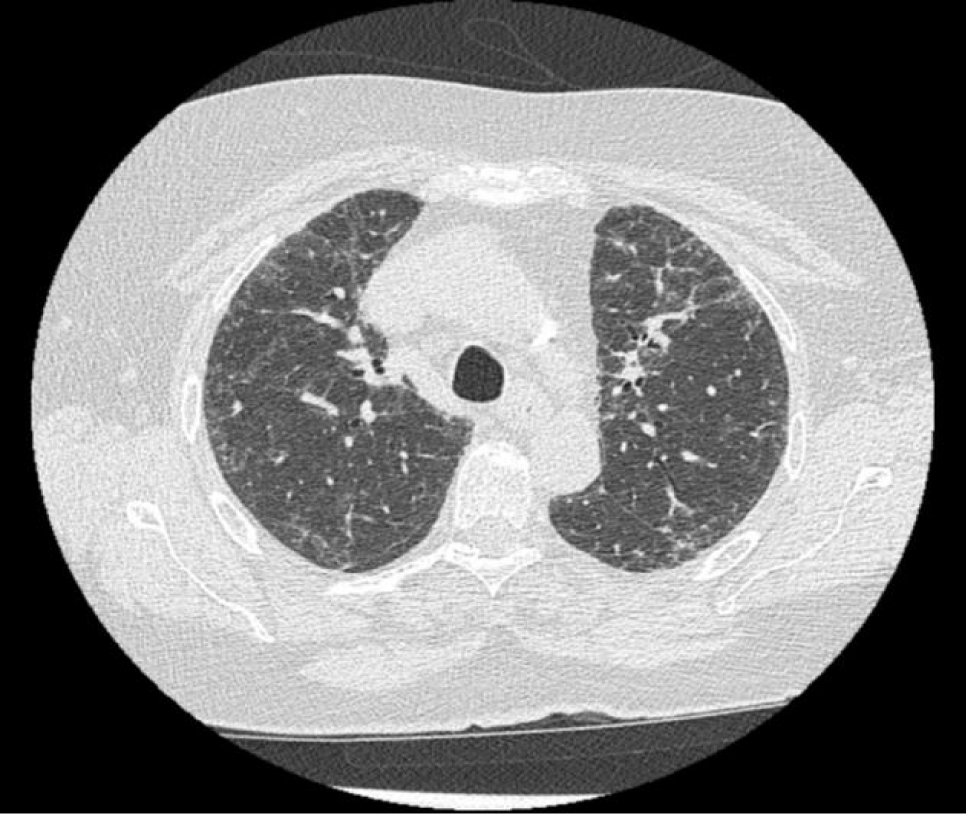
Auscultation showed high-pitched bibasilar inspiratory crackles. There was no alteration of gas exchange while breathing room air at rest. The chest radiograph revealed diffuse reticular-nodular pattern, predominantly in the lung bases. Pulmonary function tests showed a decrease in DLCO (diffusion capacity of carbon monoxide corrected by hemoglobin) of 3.14 mmol/min/kPa (58%). A chest high-resolution CT (computed tomography) scan displayed areas of increased density in symmetrical and central peribronchovascular ‘ground glass’ with reticular opacities and bronchiolectasis, findings compatible with interstitial lung diseases suggestive of NSIP (non-specific interstitial pneumonia) (Figure 1).
Serological and auto-immune panel were negative. The patient declined invasive diagnostic tests.
Given that the patient reported a symptomatic spectrum compatible and temporally framed with the start of treatment with denosumab, a severe adverse reaction with symptoms limiting daily life was assumed, so the drug was discontinued, and corticosteroid treatment was started, to evaluate the response.
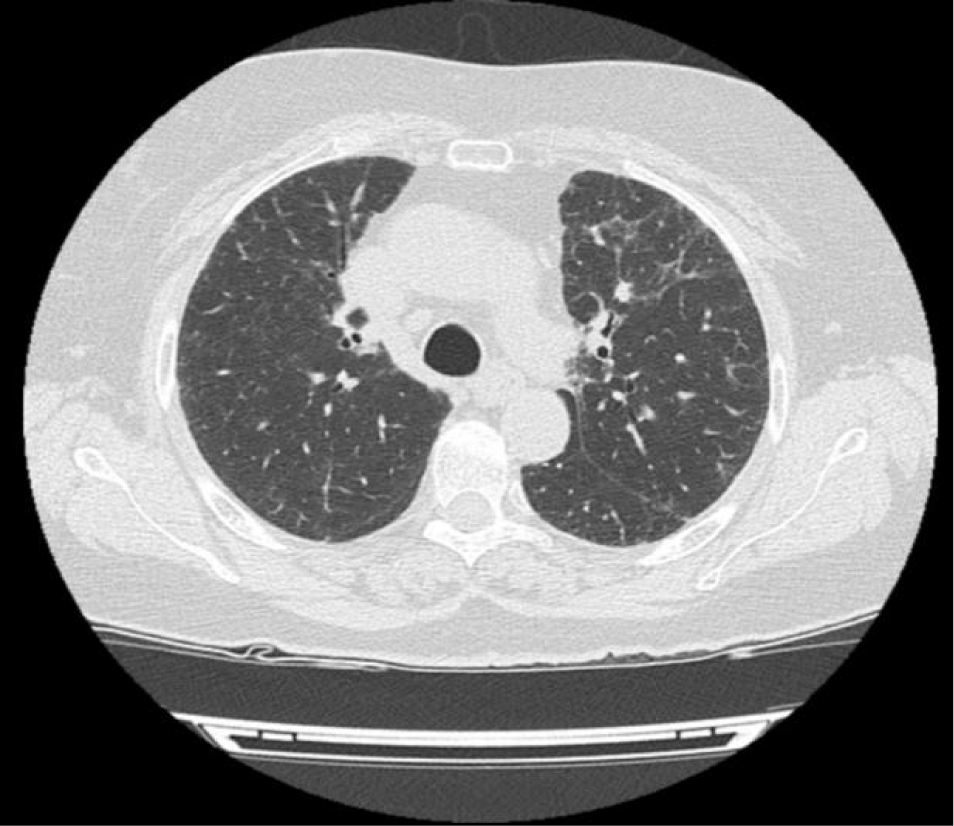
After three months the patient showed clinical (of the various symptoms) and functional, improvement with a significant increase in DLCOc 5.04 mmol/min/kPa (87.7%). The CT scan showed an evident decreased ‘ground glass’ opacities (Figure 2).
Discussion
Denosumab is a human monoclonal antibody used in the treatment of osteoporosis to inhibit the growth and function of osteoclasts. It binds to the Receptor Activator of Nuclear Factor Kappa-B Ligand (RANKL) and prevents binding to the receptor activator of nuclear factor kappa-B (RANK). Both are expressed in the lung, allowing degradation of the extracellular matrix [1,2].
Drug-induced Interstitial Lung Disease (ILD) is complex to diagnose due to the long list of drugs involved, and diagnosis is usually by exclusion [3].
In susceptible subjects, the most common adverse effects of denosumab are back, flank and extremity pain, myalgia, hypercholesterolaemia, diarrhoea, gastro-esophageal reflux and epigastric pain, cystitis and recurrent urinary and respiratory infections, stomatitis/mucositis, dry skin and mucous membranes, asthenia and dyspnea, some of which were also reported by the patient. Interstitial lung disease associated to denosumab is an exceptional adverse reaction. The Pneumotox website (www.pneumotox.com) incorporate denosumab as a causative agent of ILD, describing only one case of hypersensitivity pneumonitis and other one case of organised pneumonia secondary to this treatment, in two women [4,5]. However, no cases of non-specific interstitial pneumonia have been reported.
In a study published in 2018 [6], the time-to-onset of drug-induced interstitial lung disease after administration of monoclonal antibodies was analysed, being more than 2 months the median time-to-onset of ILD for denosumab, consistent with the time sequence of our case.
The history of drug exposure, the absence of more likely alternative diagnoses and the clinical-radiological improvement after withdrawal of denosumab, as well as the good response to corticosteroid therapy, reinforce the hypothesis of a causal relationship. In addition, a score of 6 on the Naranjo probability scale determines as probable the relationship of this adverse effect (NSIP) with this specific drug.
Denosumab, by inhibiting the interaction between RANKL and its receptor RANK on alveolar macrophages, could plausibly decrease extracellular matrix degradation. We hypothesize that this mechanism, along with the patient's age [3] and a possible individual susceptibility (family history of a maternal aunt who died from supposed Idiopathic Pulmonary Fibrosis (IPF)), could contribute to the development of ILD.
In the clinical practice, these findings highlight the importance of exhaustive medical history, reviewing concomitant treatments and the temporal evolution of symtoms. This allows the search for a causal agent of the interstitial lung disease, whose control will enhance both the clinical and prognosis.
In conclusion, diffuse interstitial lung disease encompasses a heterogeneous group of pathologies that, in general, present a progressive clinical and functional evolution, as well as a poor prognosis. The described adverse effects of monoclonal antibodies such as denosumab should be taken into account, assessing the risk-benefit of their indication in each patient. This case highlights the importance of clinically recognising drug-induced manifestations, since early withdrawal of the drug is usually associated with a clinical-prognostic improvement.
References
- Lewiecki EM. New and emerging concepts in the use of denosumab for the treatment of osteoporosis. Ther Adv Musculoskelet Dis. 2018 Oct 22;10(11):209-223. doi: 10.1177/1759720X18805759. PMID: 30386439; PMCID: PMC6204627.
- Liu W, Zhang X. Receptor activator of nuclear factor-κB ligand (RANKL)/RANK/osteoprotegerin system in bone and other tissues (review). Mol Med Rep. 2015 May;11(5):3212-8. doi: 10.3892/mmr.2015.3152. Epub 2015 Jan 7. PMID: 25572286.
- Skeoch S, Weatherley N, Swift AJ, Oldroyd A, Johns C, Hayton C, Giollo A, Wild JM, Waterton JC, Buch M, Linton K, Bruce IN, Leonard C, Bianchi S, Chaudhuri N. Drug-Induced Interstitial Lung Disease: A Systematic Review. J Clin Med. 2018 Oct 15;7(10):356. doi: 10.3390/jcm7100356. PMID: 30326612; PMCID: PMC6209877.
- Ruiz AC, Carrascosa MF, Concha ST, Gil AH, Rivero JG. Interstitial Lung Disease in a Patient Treated with Denosumab. Eur J Case Rep Intern Med. 2019 Jul 3;6(7):001131. doi: 10.12890/2019_001131. PMID: 31410352; PMCID: PMC6663050.
- Mori Y, Izumiyama T, Mori N, Aizawa T. Interstitial lung disease in a woman with rheumatoid arthritis treated with denosumab: A case report. Mod Rheumatol Case Rep. 2022 Jun 24;6(2):155-159. doi: 10.1093/mrcr/rxab046. PMID: 34791403.
- Komada F, Nakayama Y, Takara K. [Analysis of Time-to-onset and Onset-pattern of Interstitial Lung Disease after the Administration of Monoclonal Antibody Agents]. Yakugaku Zasshi. 2018;138(12):1587-1594. Japanese. doi: 10.1248/yakushi.18-00094. PMID: 30504674.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.