Medicine Group 2025 January 31;6(1):107-116. doi: 10.37871/jbres2061.
Evaluation of Screening Program for Hepatitis C Virus (HCV) At AST Pesaro-Urbino: First Year Results
Raffaele La Porta1*, Antonio Vitiello2, Annalisa Belli3, Davide Sisti3, Andrea Zovi2, Francesca Bruscolini1, Saverio Guerra1, Sara Carbonari1, Sara Di Benedetto1, Tania Rodini3, Simone Barocci1 and Stefano Amatori3
2Ministry of Health, Directorate-General for Health Prevention, Rome, Italy
3Department of Biomolecular Sciences, University of Urbino Carlo Bo, Urbino, Italy
- 1969-1989 birth cohorts
- HCV
- Hepatitis C virus
- Anti-HCV antibody
- HCV-RNA
- Screenin
Abstract
Background: Hepatitis C virus (HCV) infection is the leading cause of chronic liver disease worldwide. In 2016, the World Health Organization (WHO) launched a global-screening initiative aimed at eliminating HCV as a public health threat by 2030. In Italy, the national screening program targeted all people born between 1969 and 1989. This study reports on an HCV screening program conducted within the population served by AST Pesaro-Urbino of the Marche region, central Italy.
Methods: This prospective study was conducted within the AST Pesaro-Urbino, following the launch of an HCV screening campaign in July 2023. The program targeted all individuals born between 1969 and 1989. Between August 2023 and July 2024, all subjects were submitted to anti-HCV tests, and those testing positive were subsequently tested for HCV-RNA test.
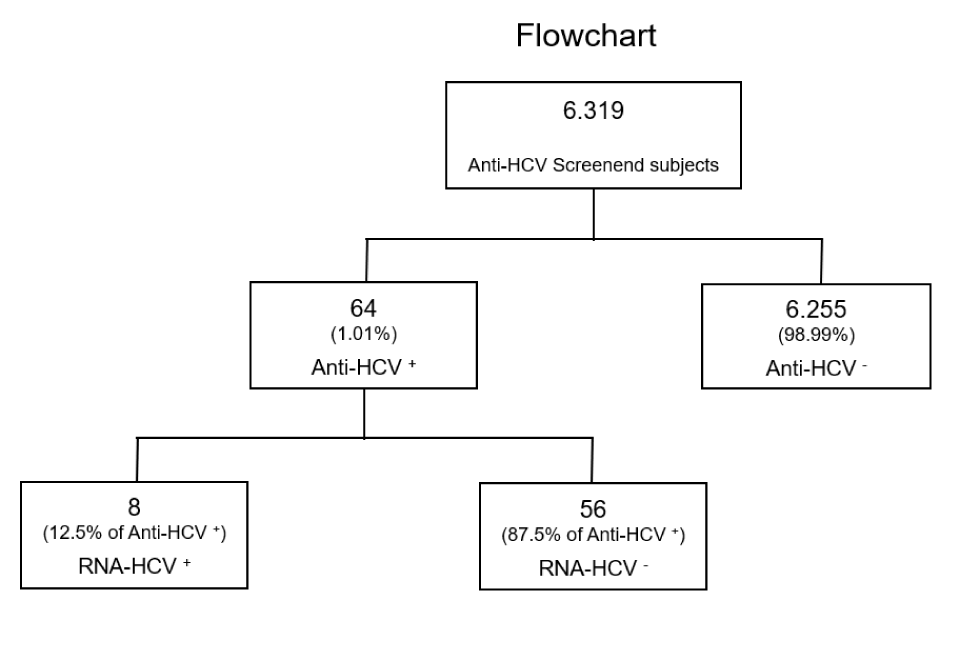
Results: Six blood collection centers participated in the HCV screening campaign. A total of 12.4% of the target population born between 1969 and 1989 was screened, corresponding to 6,319 individuals, of whom 2,579 (40.8%) were males and 3,740 (59.2%) were females. Among those screened, 64 individuals (1.0%; 35 males and 29 females) tested positive for anti-HCV antibodies. Of these, 8 individuals (0.13%; 5 males and 3 females) were confirmed to be HCV-RNA positive. This screening program allowed us to obtain a prevalence of viremic infections of 0.13%.
Conclusion: The prevalence of active HCV infection in the sample tested from 1969-1989 present in the territory of AST Pesaro-Urbino is remarkably low, at 0,13%; prevalence either represents true population value or could be due at a biased sample.
Abbreviations
COVID-19: Coronavirus Disease-19; DAA: Direct-Acting Antivirals; HCV: Hepatitis C Virus; SD: Standard Deviation; WHO; World Health Organization
Introduction
Hepatitis C Virus (HCV) infection is a leading cause of chronic liver disease worldwide, with significant public health implications [1,2]. If left pharmacologically untreated, the infection can lead in 20% of cases to the development of cirrhosis, hepatocellular carcinoma, and even death [3]. Despite its severity, HCV remains undiagnosed due to its asymptomatic nature, with most individuals becoming aware of the infection only through screening [4,5]. Worldwide, an estimated 1% of the population, approximately 71 million individuals, live with chronic HCV infection and 1.75 million new infections occur annually [6,7]. In 2016, the World Health Organization (WHO) launched a global screening campaign to eliminate HCV as a public health threat by 2030, setting ambitious goals, including a 65% reduction in HCV-related mortality and a 90% increase in diagnosis [8]. Italy has an estimated chronic infection rate of 1% [9] but, to date, only few studies have explored the epidemiology of HCV in the general Italian population [10,11], mainly because focusing on cases of acute viral hepatitis [12] makes it difficult to have a correct estimate of infection. Research conducted on specific cohorts suggest an HCV.RNA positivity prevalence of approximately 1% [13,14]. In 2019, the Italian government allocated 71.5 million euros to promote nationwide HCV-screening programs to eliminate hepatitis C virus infection and targeting individuals born between 1969 and 1989, as well as all individuals from AS and prisons, thus adhering HCV elimination program according to WHO global elimination objectives [15]. Legislative measures, including Decree Law 162 of Dec. 30, 2019, implemented by the “Decreto Milleproroghe” of Dec. 28, 2020 and the State-Regions Conference of Dec. 17, 2020, were enacted to formalize and implement this interventions in order to enhance the campaign for the global elimination of this infection by 2030. The screening project was finally implemented by the Ministry of Health Decree of May 14, 2021 [16]. HCV hepatitis today can be treated with the new Direct-Acting Antiviral drugs (DAAs), allowing the eradication of the infection in a large number of patients (90%) with a low rate of side effects [17,18]. Therefore, due to the availability of these new drugs, there has been an increase in HCV awareness campaigns, resulting in an increase in the number of antiviral treatments in infected people. Therefore, a national screening program on the 1969-1989 birth cohort was launched in 2021, although it experienced a significant delay in implementation across the country due to the COVID-19 pandemic. An effective screening program primarily aims to identify individuals who are unaware of the infection and serve as reservoir for HCV-transmission. Screening plays a critical role in facilitating early detection, initiating patients to drug treatment, and interrupting the viral transmission cycle, thereby preventing new infections. Due to the 2019 Coronavirus pandemic (COVID-19), the allocation of funds was not transferred to the regions until 2021, and the first screening programs did not start until 2022. In July 2023, the screening campaign promoted by the Ministry of Health and joined by the Marche Region, for the elimination of HCV started in all Territorial Health Authorities. The study conducted in the Pesaro-Urbino AST of the Marche Region showed a low prevalence (0.13%) of active HCV infection among previously untested subjects, underscoring the necessity of continued efforts to optimize screening strategies in the region.
Materials and Methods
Screening centers
In July 2023, Marche Region activated the HCV screening campaign aimed at people born between 1969 and 1989. Patients were subjected to anti-HCV tests and, those with positive anti-HCV, were subjected to HCV-RNA test. Patients screened within AS and prisons are included in this analysis.
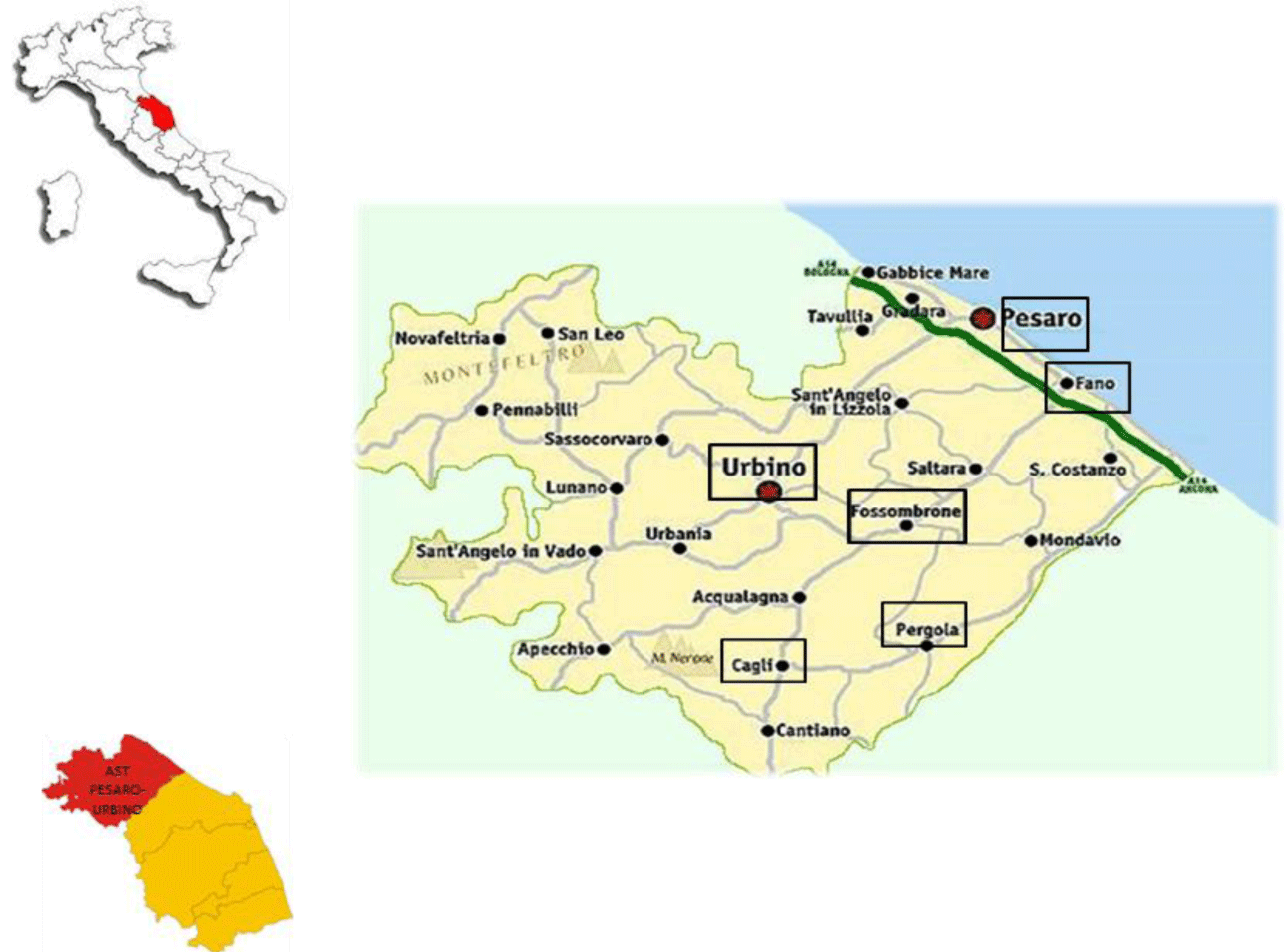
Six different centres were identified for the collection of anti-HCV samples (and consequently for the collection of HCV-RNA samples as well). The collection centres are reported in figure 1. These six collection centres are located in different towns: Ospedale di Comunità Celli (Cagli), Presidio Ospedaliero Santa Croce (Fano), Ospedale Civile (Fossombrone), Presidio Ospedaliero San Salvatore (Pesaro), Ospedale Santi Donnino e Carlo (Pergola), and Ospedale Santa Maria della Misericordia (Urbino). A total of 6319 samples were collected.
Screening procedures
All people in the specific age group received a letter with all the necessary information and a proposed screening appointment, which involves a venous blood draw. Specific informed consent was provided authorizing the use of the clinical information, and participant data was fully anonymized through appropriate pseudonymization. Therefore, it is not possible to trace back to the identity of individual subjects, whose information was limited to gender, age, anti-HCV test results, and, if applicable, HCV RNA test results. Therefore, it is not possible to trace back to the identity of individual subjects, whose information was limited to gender, age, anti-HCV test results, and, if applicable, HCV RNA test results. Two blood tubes were taken from each person; serological testing was performed on the first and molecular testing on the second, if necessary. In case of anti-HCV positivity, patients were tested for HCV-RNA. Patients with positive HCV-RNA were sent to the referring specialist center to complete the diagnostic process, including an evaluation of the antiviral treatment. Outpatient blood collection centers located outside hospitals were also involved. All serum samples for anti-HCV screening were analyzed at the Hospital of Urbino, while samples HCV-RNA samples were analyzed at the Hospital of Pesaro, Italy. For each patient, two test tubes were taken, the first for anti-HCV test, the second for probable HCV-RNA test. Liaison XL Murex HCV Ab (DiaSorin SpA, Saluggia, Italy) was used for the anti-HCV testing by an indirect CLIA immunoassay. This assay is based on two recombinant antigens (core and NS4) specific for HCV that are used for coating magnetic particles (solid phase), and a third ready-to-use aqueous HCV antigen (biotinylated NS3). The samples were analyzed on Liaison XL (DiaSorin SpA, Saluggia, Italy). Quality control was performed before any analytical investigation. For HCV-RNA testing, a PCR assay was used and the samples were analyzed on Cobas 6800 (Roche Diagnostics Ltd, Burgess Hill, UK). Cobas 6800 HCV Sterilin round-base polystyrene (LP4) tubes (Thermo Fisher Scientific, Hemel Hempstead, UK) or centrifuged BD Vacutainer EDTA whole blood bottles (Becton Dickinson, Oxford, UK) were loaded directly onto the sample supply module of the Cobas 6800 instrument. All data related to the screening test results were entered into a Microsoft Excel platform after fully anonymizing each subject [19,20].
Statistical analysis
Categorical variables were reported as frequencies (percentages) and continuous variables (age) as mean ± SD. Categorical variables were compared using the Chi-square. The Chi-square test was used to investigate potential differences in the distribution of positive and negative anti-HCV outcomes across the various collection centres. It was also employed to assess the distribution of sex across different age ranges and the distribution of outcomes (positive or negative for anti-HCV) within the considered age ranges.The Fischer exact tests were also applied, to investigate the distribution of RNA-HCV outcomes, as the standard Chi-square test could not be used for this purpose due to the presence of at least one expected frequency < 5. To test the distribution of positive cases in relation to the population size of each province and, separately, in relation to the population size within the screening age range, a Chi-square goodness-of-fit test for proportions was used. Cramer’s V as Effect size value was also computed.
Moreover, considering the sensibility (93.18%) and the specificity (99.35%) [21] of the screening test and the prevalence observed, a positive predictive value was obtained as follows:
Legend: Pr = Prevalence; Sens = Sensibility; Spec = Specificity.
All tests considered were two-sided at a fixed significance level of 0.05. Data analyses were performed using Microsoft Excel and R studio software.
Results
Descriptive characteristics
The HCV screening program herein described involved six blood collection centers in AST Pesaro-Urbino. The screening program, that started at August 1, 2023 and finished at July 31, 2024, involved 6,319 subjects, 2,579 males (40,8%) and 3,740 (59,2%) females. Although very close to the threshold of significance, the statistical analysis did not reveal statistically significant differences in the distribution of males and females across the various age ranges χ2(3) (p) = 7.29 (p = 0.06). By comparing the expected frequencies with the observed frequencies, a higher participation of females in the screening program emerges across all age groups. These results are showed in table 1.
| Table 1: Demographic characteristics of screening participants. | ||||
| M n (%) | F n (%) | Anti-HCV+ n | RNA-HCV+ n | |
| Total | 2579 (40.8%) | 3740 (59.2%) | 64 | 8 |
| 1969-1974 | 1232 (47.8%) | 1671 (44.7%) | 32 | 5 |
| 1975-1979 | 625 (24.2%) | 932 (24.9%) | 2 | 0 |
| 1980-1984 | 417 (16.17%) | 681 (18.2%) | 9 | 3 |
| 1985-1989 | 305 (11.8%) | 456 (12.1%) | 3 | 0 |
Regarding the distribution of anti-HCV test positivity between males and females, there is a higher prevalence of positivity among male subjects, 56.3% males versus 43.7% females’ positivity. These results are reported in table 2, while in figure 2 is shown the results flowchart.
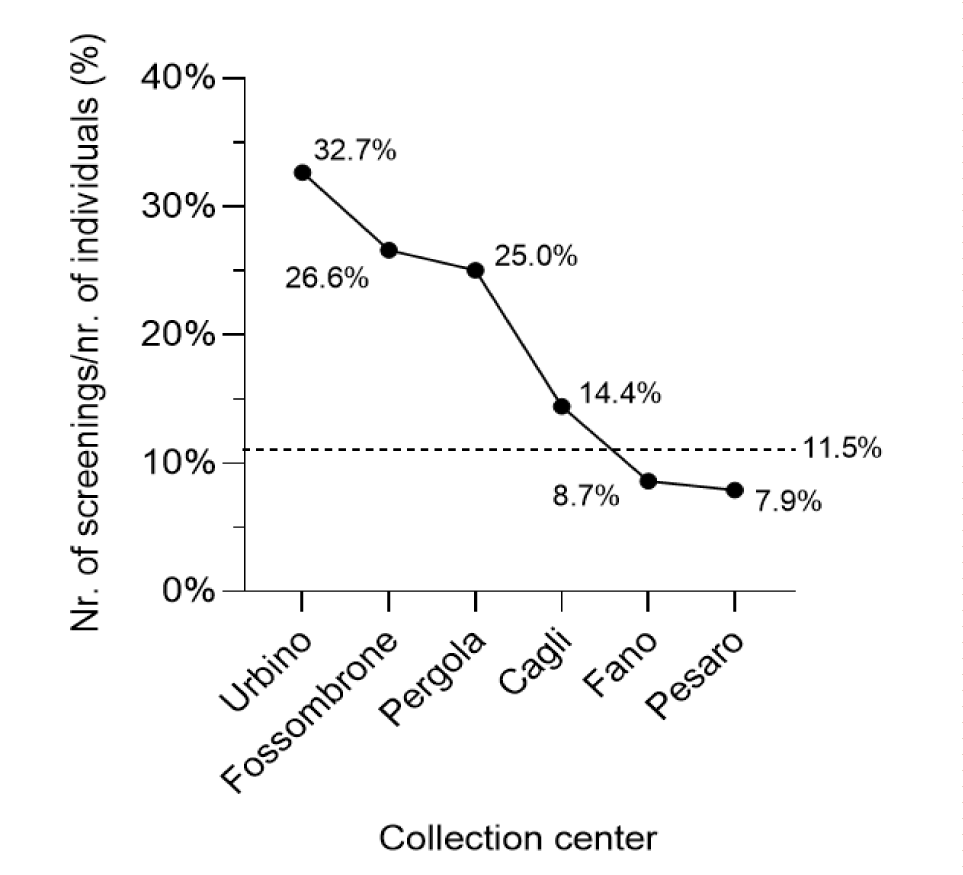
In addition, to test the distribution of positive cases in relation to the population size of each province in screening age, a Chi-square goodness-of-fit test for proportions was used. Figure 3 shows the proportion of subjects who participated in the screening as a function of the screening-age population of each city relative to the considered collection centre.
A Chi-Square goodness-of-fitting test, used to evaluate the proportion of adherence for each municipality, was extremely significant (p < 0.001) with a Cramer’s V = 0.65, indicates a large effect size. This result suggests a non-uniform distribution of the screening’s participants in terms of age, with varying adherence to the screening program, as shown in figure 3.
Screening program results
Of the 54,828 total inhabitants in age of screening (birth year 1969-1989) of the six cities involved in the screening, only 6,319 individuals participated in the screening campaign, resulting in an adherence rate of 11.52%. Of the 6,319 subjects tested, 64 subjects were positive to the screening test for anti-HCV (Tables 2,3). Although more females participated in the screening program compared to males, a higher percentage of positivity in anti-HCV was observed among males (males’ positivity 56.3% vs females’ positivity 43.7%). However, statistical analysis revealed no significant differences in the distribution of positive cases across the age ranges χ2(5) (p) = 2,50 (p = 0.77); Cramer’s V = 0.02. The same test was used to assess the distribution of positivity across different age groups, yielding non-significant results; χ2(3) (p) = 4.69 (p = 0.19), Cramer’s V = 0.03.
| Table 2: Gender-specific positivity rate. | ||
| Anti-HCV+ * n (%) | RNA-HCV+ n (%) | |
| Total | 64 | 8 |
| Males | 36 (56.3%) | 5 (62.5%) |
| Females | 28 (43.7%) | 3 (37.5%) |
| Table 3: Distribution of Screening tests and positive outcomes in collecting centres. | |||||||
| Total | Cagli | Fano | Fossombrone | Pesaro | Pergola | Urbino | |
| Overall population | 192172 | 7951 | 59963 | 9077 | 95580 | 5762 | 13839 |
| 1969-1989 population | 54828 | 2212 | 17522 | 2606 | 27097 | 1549 | 3842 |
| Screening tests (n) | 6319 | 319 | 115 | 693 | 2149 | 388 | 1255 |
| Positive anti-HCV (PPV) n (%*) | 64 (1%) | 4 (1,2%) | 12 (0,8%) | 10 (1,4%) | 23 (1,1%) | 4 (1,0%) | 11 (0,9%) |
| Positive HCV-RNA n (%*) | 8 (0.12%) | 0 (0%) | 0 (0%) | 1 (0.016%) | 4 (0.063%) | 2 (0.032) | 1 (0.016%) |
| *Percentage of positive anti-HCV and positive HCV-RNA are calculated on the total number of screenings for each centre. | |||||||
Among the 64 subject who resulted positive in anti-HCV test, only 8 (5 males and 3 females) were confirmed to be HCV-RNA positive. 8 of 64 (12.5%) subjects were HCV-RNA positive. Given the low number of positive cases, a Fisher's exact test was used to evaluate the distribution of positive outcomes in the HCV-RNA test relating to screening centre. The analysis did not reveal any statistically significant differences (p = 0.20).
The screening program revealed a prevalence of 0.13% for viral HCV. With these prevalence data, the positive and negative predictive values were calculated, given the known sensitivity and specificity of the tool used. The resulting positive predictive value (PV+) is 0.157, while the negative predictive value (PV-) is 0.999. The PV+ value indicates a high number of false positives, suggesting that among all individuals who tested positive, only 15.57% are actually infected with HCV. On the other side, 99.99% of all individuals who tested negative, were truly negative.
Discussion
The WHO global initiative to eradicate Hepatitis C Virus infection (HCV) has led to the implementation of screening strategies to identify and manage infected individuals. Laboratory tests play an important role in identifying HCV-reactive cases and reducing HCV infection rates and hepatitis-related mortality [22]. The advent of new DAA drugs has revolutionized the treatment of HCV infection leading to cure rates above 90% [23,24]. This is of course dependent on early diagnosis, which can only be made by screening tests. This study can therefore certainly contribute to the implementation of increasingly targeted and specific screening strategies that could also be applied to many other viral infections [25]. Despite all this, the study has several limitations. The first limitation is that it concerns a small cohort. However, this is only the first interim report of a regional opportunistic screening program that is still ongoing and that will include new centres in the forthcoming months. A second limitation is that the study enrolled only people born between 1969 and 1989, limiting the comparison of epidemiological data with other studies which include larger population cohorts. The recruiting strategy was indeed based on the indications from the Italian Ministry of Health. The choice is based on analyzes that evaluate the benefits of this screening, compared to the resources necessary for its implementation. This model, indeed, was previously suggested to be most cost-effective, also when compared to models only directed at at-risk populations or other birth-cohorts. This advantage is justified by a set of factors, including the spread of hepatitis C in this segment of the population and the share of hepatitis not yet diagnosed and which it is estimated could remain silent for a long time [26]. Another limitation of the study is that only demographic data (gender and age) were available. The overall number of demographic and clinical variables captured by the database is still limited and the rates of linkage to care and treatment outcomes are not yet available. Nevertheless, our study allows to evaluate the epidemiological aspect of HCV in a small demographic reality like that of the AST Pesaro-Urbino, contributing to a complete understanding of the prevalence of the disease. The study shows that the active HCV infection evidenced by HCV-positive RNA is much lower than those reported in the literature [27,28]. This study reports the results of the screening campaign conducted in the Pesaro Urbino AST in the Marche region of Italy to identify people unaware of HCV infection. The function of the screening is to identify individuals in the so-called "shadow world" who are unaware of their positivity. This is the first study of its kind conducted in the Marche Region. In this work, the results obtained after 1 year of screening from August 2023 to July 2024 on only the population born between 1969 and 1989 were considered. The data obtained indicate a low prevalence of infection in the screened population of approximately 0.13% that is in line with recent data from a northern Italian region (Lombardy, 0.1%) [29]. Previously, only two studies targeted people owning to the 1969–1989 birth cohort and reported rates of active HCV infection of 0.05% and 0.07%, respectively [30,31]. Other studies conducted in people of any age reported prevalence of active HCV infection of 0.5% and 0.7% in hospitalized subjects [32,33], and of 0.07% in those undergoing anti-SARS-CoV-2 testing or vaccination [34]. Thus, these studies shows a very low prevalence of HCV-infected individuals compared with studies conducted before 2019 [35]; it is reasonable to hypothesize that this discrepancy is due to the use of the new DAA-based therapies. Furthermore, our data indicate that the prevalence of HCV infection is very low in individuals born between 1969 and 1989. This finding is not unexpected since it is in line with previous Italian studies [36] and also confirmed by recent investigations. The higher prevalence in males and older age groups is also in line with these studies and suggests that screening programs should prioritize these populations. Therefore, the hypothesis of extending screening to the population born before 1969 is very plausible. Our study shows that the anti-HCV positive subjects were HCV RNA negative (87.5%). This result, however, is in line with other reports, as the rates of undetectable HCV-RNA values in anti-HCV-positive subjects identified by opportunistic screening programs in recent studies range from 64% to 82% [32]. To prevent HCV infection, it is necessary to reduce the risk of exposure to the virus in the population most at risk. Therefore, it is important to promote awareness and screening programs to identify those unaware of the infection. The lack of an HCV vaccine makes screening campaigns with serologic and molecular testing very important. Therefore, the continuous collaboration among health care providers, researchers, and policy makers is fundamental to refine and implement new strategies that will contribute to the eradication of HCV infection. It is emphasized that the screening program revealed a relatively low prevalence of 0.13%: this discrepancy is unlikely to be attributed to the patient selection process in our study, as the screening was conducted among individuals accessing healthcare facilities. It is plausible, however, that the observed prevalence might have been further diluted if the screening had encompassed the entire general population, including individuals who do not utilize healthcare centres.
The Italian evidence regarding the cost-effectiveness profile of HCV screening and DAA treatment produced from the Italian SSN perspective supports an evidence-based health policy for HCV elimination in Italy. The evidence on cost benefits of treating patients diagnosed by screening is important for the ongoing central and regional decision-making process. Several studies have shown that HCV-related disease inflicts an enormous economic and clinical burden due to HCV-related extrahepatic comorbidities. Early eradication of HCV could reduce these burdens. In addition to being a fundamental policy aspect for HCV elimination, screening is also reported to be cost effective in Italy [26].
The active offer of screening is an important milestone that requires regional governance that manages the processes’ complexity integrating well-organized interdisciplinary paths between territorial and hospital specialized medicine. Each region must identify the objectives and strategies to define the present and future steps and reach HCV elimination goal by 2030.
As already stated, this is only the first interim report of a part of the screening program which is still ongoing, and which will involve others regional centers in the future. Implementation of such programs at regional and national level might help to identify HCV positive patients unaware of their status, to improve the cascade of care and, thus, to reach the WHO elimination goal. The association of different screening plans, as well as age-bracket extension, may be useful to make the screening process more widespread and faster in the future. By integrating our data with a broader epidemiological context, we will be able to refine our regional and national strategies, to better target the populations most at risk and move closer to the goal of eradicating HCV.
Author Contributions
Raffaele La Porta: conceptualization; methodology; data collection; writing – original draft; data management.
Antonio Vitiello: conceptualization; methodology; supervision
Annalisa Belli: conceptualization; methodology; writing – review and editing.
Davide Sisti: conceptualization; methodology; writing – review and editing.
Andrea Zovi: conceptualization; methodology; supervision
Francesca Bruscolini: data collection
Saverio Guerra: data collection
Sara Carbonari: data collection
Sara Di Benedetto: data collection
Tania Rodini: data collection
Simone Barocci: conceptualization; methodology
Stefano Amatori: conceptualization; methodology; writing – review and editing.
Acknowledgment
We thank all the laboratory personnel for their technical support.
Funding
This research received no external funding.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could appear to have influenced the work reported in this paper.
References
- D'souza S, Lau KC, Coffin CS, Patel TR. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J Gastroenterol. 2020 Oct 14;26(38):5759-5783. doi: 10.3748/wjg.v26.i38.5759. PMID: 33132633; PMCID: PMC7579760.
- Guntipalli P, Pakala R, Kumari Gara S, Ahmed F, Bhatnagar A, Endaya Coronel MK, Razzack AA, Solimando AG, Thompson A, Andrews K, Enebong Nya G, Ahmad S, Ranaldo R, Cozzolongo R, Shahini E. Worldwide prevalence, genotype distribution and management of hepatitis C. Acta Gastroenterol Belg. 2021 Oct-Dec;84(4):637-656. doi: 10.51821/84.4.015. PMID: 34965046.
- Martinez MA, Franco S. Therapy Implications of Hepatitis C Virus Genetic Diversity. Viruses. 2020 Dec 29;13(1):41. doi: 10.3390/v13010041. PMID: 33383891; PMCID: PMC7824680.
- Mohamed EA, Giama NH, Abdalla AO, Shaleh HM, Oseini AM, Ali HA, Ahmed F, Taha W, Ahmed Mohammed H, Cvinar J, Waaeys IA, Ali H, Allotey LK, Ali AO, Mohamed SA, Harmsen WS, Ahmmad EM, Bajwa NA, Afgarshe MD, Shire AM, Balls-Berry JE, Roberts LR. High prevalence of chronic viral hepatitis B and C in Minnesota Somalis contributes to rising hepatocellular carcinoma incidence. World J Gastroenterol. 2022 Sep 21;28(35):5217-5229. doi: 10.3748/wjg.v28.i35.5217. PMID: 36188718; PMCID: PMC9516675.
- Lazarus JV, Picchio CA, Colombo M. Hepatocellular Carcinoma Prevention in the Era of Hepatitis C Elimination. Int J Mol Sci. 2023 Sep 21;24(18):14404. doi: 10.3390/ijms241814404. PMID: 37762706; PMCID: PMC10531569.
- Polaris Observatory HCV Collaborators. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol. 2022 May;7(5):396-415. doi: 10.1016/S2468-1253(21)00472-6. Epub 2022 Feb 16. PMID: 35180382.
- Spada E, Marcantonio C, Vescio MF, Marascio N, Villano U, Pisani G, Tritarelli E, Bruni R, Barreca GS, Torti C, Matera G, Liberto MC, Focà A, Pezzotti P, Ciccaglione AR; Viral Hepatitis ISS-UNICZ study group. Changing epidemiology of hepatitis C in Italy: a population-based survey in a historically high endemic area. Minerva Med. 2023 Apr;114(2):191-202. doi: 10.23736/S0026-4806.21.07280-3. Epub 2021 Apr 29. PMID: 33913660.
- D'Ambrosio R, Anolli MP, Pugliese N, Masetti C, Aghemo A, Lampertico P. Prevalence of HCV infection in Europe in the DAA era: Review. Liver Int. 2024 Jul;44(7):1548-1563. doi: 10.1111/liv.15981. Epub 2024 May 28. PMID: 38804727.
- Andriulli A, Stroffolini T, Mariano A, Valvano MR, Grattagliano I, Ippolito AM, Grossi A, Brancaccio G, Coco C, Russello M, Smedile A, Petrini E, Martini S, Gaeta GB, Rizzetto M. Declining prevalence and increasing awareness of HCV infection in Italy: A population-based survey in five metropolitan areas. Eur J Intern Med. 2018 Jul;53:79-84. doi: 10.1016/j.ejim.2018.02.015. Epub 2018 Feb 21. PMID: 29475770.
- Stasi C, Silvestri C, Voller F. Update on Hepatitis C Epidemiology: Unaware and Untreated Infected Population Could Be the Key to Elimination. SN Compr Clin Med. 2020;2(12):2808-2815. doi: 10.1007/s42399-020-00588-3. Epub 2020 Oct 18. PMID: 33103061; PMCID: PMC7568689.
- Mennini FS, Marcellusi A, Andreoni M, Gasbarrini A, Salomone S, Craxì A. Health policy model: long-term predictive results associated with the management of hepatitis C virus-induced diseases in Italy. Clinicoecon Outcomes Res. 2014 Jun 19;6:303-10. doi: 10.2147/CEOR.S62092. PMID: 24971024; PMCID: PMC4069043.
- Mele A, Ferrigno L, Romanò L, Alfonsi V, D'Angelo F, Crateri S, Tosti ME; Gruppo di collaborazione SEIEVA. Aggiornamento sull’epidemiologia dell’epatite A in Italia negli ultimi cinque anni. Dati dalla sorveglianza SEIEVA 2015-2019 [An update on the epidemiology of hepatitis A in Italy 2015-2019. Data from the surveillance of acute viral hepatitis SEIEVA]. Epidemiol Prev. 2021 May-Jun;45(3):173-180. Italian. doi: 10.19191/EP21.3.P173.062. PMID: 34212698.
- Kondili LA, Andreoni M, Aghemo A, Mastroianni CM, Merolla R, Gallinaro V, Craxì A. Prevalence of hepatitis C virus estimates of undiagnosed individuals in different Italian regions: a mathematical modelling approach by route of transmission and fibrosis progression with results up to January 2021. New Microbiol. 2022 Dec;45(4):249-259. Epub 2022 May 25. PMID: 36066213.
- Stasi C, Silvestri C, Berni R, Rossana Brunetto M, Zignego AL, Orsini C, Milani S, Ricciardi L, De Luca A, Blanc P, Nencioni C, Aquilini D, Bartoloni A, Bresci G, Marchi S, Filipponi F, Colombatto P, Forte P, Galli A, Luchi S, Chigiotti S, Nerli A, Corti G, Sacco R, Carrai P, Ricchiuti A, Giusti M, Almi P, Cozzi A, Carloppi S, Laffi G, Voller F, Cipriani F. Epidemiological, demographic and clinical data on chronic viral hepatitis C in Tuscany. Curr Med Res Opin. 2019 Apr;35(4):661-666. doi: 10.1080/03007995.2018.1482264. Epub 2018 Jun 26. PMID: 29847179.
- Rossetti B, Bai F, Tavelli A, Galli M, Antinori A, Castelli F, Pellizzer G, Cozzi-Lepri A, Bonora S, Monforte AD, Puoti M, De Luca A; ICONA Foundation study group. Evolution of the prevalence of hepatitis C virus infection and hepatitis C virus genotype distribution in human immunodeficiency virus-infected patients in Italy between 1997 and 2015. Clin Microbiol Infect. 2018 Apr;24(4):422-427. doi: 10.1016/j.cmi.2017.07.021. Epub 2017 Jul 29. PMID: 28765078.
- https://www.gazzettaufficiale.it/eli/id/2020/02/29/20G00021/sg (accessed on 24 November 2023).
- Songtanin B, Nugent K. Burden, Outcome, and Comorbidities of Extrahepatic Manifestations in Hepatitis C Virus Infection. Biology (Basel). 2022 Dec 22;12(1):23. doi: 10.3390/biology12010023. PMID: 36671716; PMCID: PMC9855523.
- Morris MD, McDonell C, Luetkemeyer AF, Thawley R, McKinney J, Price JC. Community-Based Point-of-Diagnosis Hepatitis C Treatment for Marginalized Populations: A Nonrandomized Controlled Trial. JAMA Netw Open. 2023 Oct 2;6(10):e2338792. doi: 10.1001/jamanetworkopen.2023.38792. PMID: 37862013; PMCID: PMC10589813.
- Krawczyk A, Hintze C, Ackermann J, Goitowski B, Trippler M, Grüner N, Neumann-Fraune M, Verheyen J, Fiedler M. Clinical performance of the novel DiaSorin LIAISON(®) XL murex: HBsAg Quant, HCV-Ab, HIV-Ab/Ag assays. J Clin Virol. 2014 Jan;59(1):44-9. doi: 10.1016/j.jcv.2013.10.009. Epub 2013 Nov 7. PMID: 24268764.
- Guidelines on hepatitis B and C testing. WHO guidelines approved by the guidelines review committee. World Health Organization (WHO). 2017.
- Romano C, Tortorella O, Dalla Mora L, Di Stasio D, Sellitto A, Adinolfi LE, Marrone A. Prevalence and Outcome of Serum Autoantibodies in Chronic Hepatitis C Patients Undergoing Direct-Acting Antiviral Treatment. Front Immunol. 2022 Apr 11;13:882064. doi: 10.3389/fimmu.2022.882064. PMID: 35479086; PMCID: PMC9038215.
- Di Stasio D, Lucchese A, Romano A, Adinolfi LE, Serpico R, Marrone A. The clinical impact of direct-acting antiviral treatment on patients affected by hepatitis C virus-related oral lichen planus: a cohort study. Clin Oral Investig. 2022 Aug;26(8):5409-5417. doi: 10.1007/s00784-022-04507-9. Epub 2022 Apr 27. PMID: 35477818; PMCID: PMC9381460.
- Pradat P, Virlogeux V, Trépo E. Epidemiology and Elimination of HCV-Related Liver Disease. Viruses. 2018 Oct 6;10(10):545. doi: 10.3390/v10100545. PMID: 30301201; PMCID: PMC6213504.
- Andriulli A, Stroffolini T, Mariano A, Valvano MR, Grattagliano I, Ippolito AM, Grossi A, Brancaccio G, Coco C, Russello M, Smedile A, Petrini E, Martini S, Gaeta GB, Rizzetto M. Declining prevalence and increasing awareness of HCV infection in Italy: A population-based survey in five metropolitan areas. Eur J Intern Med. 2018 Jul;53:79-84. doi: 10.1016/j.ejim.2018.02.015. Epub 2018 Feb 21. PMID: 29475770.
- Kondili LA, Andreoni M, Alberti A, Lobello S, Babudieri S, Roscini AS, Merolla R, Marrocco W, Craxì A. Estimated prevalence of undiagnosed HCV infected individuals in Italy: A mathematical model by route of transmission and fibrosis progression. Epidemics. 2021 Mar;34:100442. doi: 10.1016/j.epidem.2021.100442. Epub 2021 Feb 11. PMID: 33607538.
- Kondili LA, Gamkrelidze I, Blach S, Marcellusi A, Galli M, Petta S, Puoti M, Vella S, Razavi H, Craxi A, Mennini FS; PITER collaborating group. Optimization of hepatitis C virus screening strategies by birth cohort in Italy. Liver Int. 2020 Jul;40(7):1545-1555. doi: 10.1111/liv.14408. Epub 2020 Apr 2. PMID: 32078234; PMCID: PMC7384106.
- Messina V, Pisaturo M, Alessio L, Russo A, Tripaldelli E, Petruzziello A, Annecchiarico A, Romano MR, Maggi P, Coppola N. Hepatitis C virus (HCV) micro-elimination in the hospital setting: The results of the HCV Caserta hospital project. J Infect Public Health. 2022 May;15(5):562-565. doi: 10.1016/j.jiph.2022.04.003. Epub 2022 Apr 12. PMID: 35461078.
- Huang Y, Pan H, Gao Q, Lv P, Xu X, Zhao Z. The role of a two-assay serological testing strategy for anti-HCV screening in low-prevalence populations. Sci Rep. 2021 Apr 22;11(1):8689. doi: 10.1038/s41598-021-88138-2. PMID: 33888806; PMCID: PMC8062551.
- D'Ambrosio R, Piccinelli S, Beccalli B, Spinetti A, Puoti M, Fagiuoli S, Magni CF, Vavassori A, Sacchi P, Castaldi S, Bombardieri G, Farina C, Buoro S, Amorosi A, Corradin M, Cereda D, Lampertico P; Regional HCV Working Group. A territory-wide opportunistic, hospital-based HCV screening in the general population from northern Italy: The 1969-1989 birth-cohort. Liver Int. 2023 Dec;43(12):2645-2656. doi: 10.1111/liv.15727. Epub 2023 Sep 16. PMID: 37715524.
- D'Ambrosio R, Rizzardini G, Puoti M, Fagiuoli S, Anolli MP, Gabiati C, D'Amico F, Pasulo L, Restelli U, Colombo M, Lampertico P. Implementation of HCV screening in the 1969-1989 birth-cohort undergoing COVID-19 vaccination. Liver Int. 2022 May;42(5):1012-1016. doi: 10.1111/liv.15216. Epub 2022 Mar 12. PMID: 35220667; PMCID: PMC9115160.
- Viganò M, Cerini F, Ridolfo S, Rumi MG. Hepatitis C virus screening in the 1969-1989 birth cohort: Is not enough! Liver Int. 2022 Aug;42(8):1915. doi: 10.1111/liv.15316. Epub 2022 Jun 6. PMID: 35596923.
- Dettori S, Russo C, Mora S, Giacomini M, Taramasso L, Dentone C, Vena A, Bassetti M, Di Biagio A. Prevalence of Viral Hepatitis in Unselected, Consecutively Enrolled Patients Hospitalised for SARS-CoV-2. J Community Health. 2022 Oct;47(5):800-805. doi: 10.1007/s10900-022-01111-6. Epub 2022 Jun 21. PMID: 35729474; PMCID: PMC9211782.
- Rosato V, Kondili LA, Nevola R, Perillo P, Mastrocinque D, Aghemo A, Claar E. Elimination of Hepatitis C in Southern Italy: A Model of HCV Screening and Linkage to Care among Hospitalized Patients at Different Hospital Divisions. Viruses. 2022 May 19;14(5):1096. doi: 10.3390/v14051096. PMID: 35632837; PMCID: PMC9143022.
- Torre P, Annunziata M, Sciorio R, Coppola C, Masarone M, Persico M. Hepatitis C screening during SARS-CoV-2 testing or vaccination. Experience in an area of southern Italy in the province of Salerno. Liver Int. 2022 Jun;42(6):1467-1469. doi: 10.1111/liv.15273. Epub 2022 Apr 23. PMID: 35395130; PMCID: PMC9115238.
- Morisco F, Loperto I, Stroffolini T, Lombardo FL, Cossiga V, Guarino M, De Feo A, Caporaso N. Prevalence and risk factors of HCV infection in a metropolitan area in southern Italy: Tail of a cohort infected in past decades. J Med Virol. 2017 Feb;89(2):291-297. doi: 10.1002/jmv.24635. Epub 2016 Jul 27. PMID: 27431017.
- Citarella A, Cammarota S, Bernardi FF, Coppola C, D'Antò M, Fogliasecca M, Giusto E, Masarone M, Salomone Megna A, Sellitto C, Servodio R, Smaldone M, Staiano L, Trama U, Conti V, Persico M. Screening, Linkage to Care and Treatment of Hepatitis C Infection in Primary Care Setting in the South of Italy. Life (Basel). 2020 Dec 18;10(12):359. doi: 10.3390/life10120359. PMID: 33352991; PMCID: PMC7766029.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.