Medicine Group 2024 December 29;5(12):1602-1615. doi: 10.37871/jbres2047.
Current Treatments for Parkinson's Disease
Ayse Aksoy, Duygu Deniz Usta and Atiye Seda Yar Saglam*
- Parkinson's disease
- Dopamine
- Treatment approaches
Abstract
Parkinson's Disease (PD) is the second most frequently observed slowly progressive neurodegenerative disease after Alzheimer's. Although dopamine replacement remains an essential component of treatment, the point at which dyskinetic movements and motor fluctuations begin may demand a number of different approaches, both medical and surgical, delivered within a multidisciplinary framework. Significant new approaches to dopamine replacement are emerging. One of the most challenging aspects of treating the disease is the management of various non-motor symptoms, including anxiety, depression, constipation, bladder dysfunction, and sleep disorders. Innovative strategies are urgently required to combat these symptoms, which have a significant negative impact on quality of life. This review presents the latest therapeutic approaches that support the optimal treatment of both the non-motor and motor symptoms of PD.
Introduction
Parkinson's Disease (PD), a neurodegenerative disorder which involves, among many other factors, the onset and progressive loss of dopaminergic neurons of the substantia nigra pars compacta by protein aggregates; these are primarily Lewy bodies composed of a-synuclein [1]. PD is the second most frequently observed neurodegenerative disease worldwide. The incidence of PD is substantial, and it has a major impact on society. According to 2016 data, approximately 6.1 million people live with PD globally [2].
Motor dysfunctions like tremors, rigidity, bradykinesia, and postural instability constitute the main symptoms of PD. In addition to these, PD patients frequently experience associated non-motor symptoms. These can consist of autonomic dysfunction, such as orthostatic hypotension, as well as psychiatric issues, such as depression and anxiety and depression. PD can thus be seen as a systemic disease, rather than simply as a disorder involving the central nervous system. Non-Motor Symptoms (NMS) of PD are increasingly recognized as key components of the disease, often emerging before motor symptoms and significantly impacting quality of life [3,4]. Cognitive impairments, which can range from mild deficits to full-blown dementia, affect a substantial proportion of patients and are associated with widespread cortical pathology [5,6]. Depression and anxiety are common, affecting up to 40% of patients, likely due to dysregulation of serotonergic and dopaminergic systems [7,8]. Autonomic dysfunctions, such as constipation and orthostatic hypotension, are frequently linked to Lewy body pathology in peripheral autonomic neurons [9,10]. Sleep disturbances, including Rapid Eye Movement (REM) sleep behavior disorder, may serve as early indicators of PD [11,12]. Together, these non-motor symptoms highlight the complex, multifaceted nature of PD, extending well beyond the motor impairments traditionally associated with the disease [13,14].
Environmental factors are also considered crucial, as exposure to specific chemicals and pesticides may lead to symptoms similar to those of PD. In addition to environmental factors, more than 200 genes that are associated with PD have been identified. In short, PD is a complicated genetic disease in which genetic factors, environmental aspects, and aging all combine [15]. Therefore, multiple parameters should be considered when treating PD. Despite the availability of several medications, the disease is still incurable, and symptoms are only partially controlled, with severe side effects occurring. Although many years have been spent researching and investing in the development of new drug molecules and treatments, no specific therapies to prevent or slow down how the disease progresses have yet been clinically approved. However, many experimental treatments have demonstrated success in preclinical animal models and clinical trials are currently taking place to investigate these further. This review highlights recent preclinical studies.
Treatment of PD requires careful consideration of a number of factors, including the patient's symptoms and signs, age, stage of the disease, degree of functional disability, and level of physical activity and productivity. Almost all available treatments are symptomatic and do not reverse the natural course of the disease (Table 1).
| Table 1: Therapeutic strategies for Parkinson’s disease: Mechanisms, side effects, and recommendations. | ||||
| Treatment | Target Symptoms | Mechanism of Action | Side Effects | Recommendations |
| Levodopa (L-Dopa) | Motor symptoms | Converts to dopamine in the brain to replenish deficient levels [16]. | Dyskinesia, motor fluctuations, nausea, hypotension [17]. | Often used as first-line therapy; requires careful monitoring over long-term use. |
| Dopamine Agonists | Motor and selected non-motor symptoms | Directly stimulate dopamine receptors [18]. | Hallucinations, impulsive behaviors, peripheral edema, excessive sleepiness [19]. | Recommended for younger patients; older patients require monitoring for neuropsychiatric effects. |
| MAO-B Inhibitors | Mild motor symptoms | Inhibit dopamine breakdown by blocking monoamine oxidase-B [20]. | Headache, nausea, insomnia [21]. | Commonly used in early stages or as adjunct therapy with levodopa. |
| COMT Inhibitors | Motor fluctuations | Prolong dopamine activity by inhibiting catechol-O-methyltransferase [22]. | Diarrhea, liver toxicity, dyskinesia [23]. | Typically combined with levodopa to enhance its effects. |
| Amantadine | Dyskinesia and motor symptoms | Antagonizes NMDA receptors to modulate glutamate [24]. | Hallucinations, skin discoloration, peripheral edema [25]. | Effective for controlling dyskinesia; used as an adjunct therapy. |
| Anticholinergics | Tremor | Reduce acetylcholine activity in the brain [26]. | Cognitive impairment, dry mouth, blurred vision, constipation [27]. | Suitable for younger patients with predominant tremor; caution advised for older individuals. |
| Deep Brain Stimulation (DBS) | Advanced motor symptoms resistant to medication | Stimulates specific brain regions such as the subthalamic nucleus [28]. | Surgical risks, device complications, infection [29]. | Effective in advanced stages; patient selection is crucial for success. |
| Photothermal-Based Treatments | Motor symptoms and localized brain regions | Use of photothermal agents to target and reduce aggregated alpha-synuclein proteins through localized heat [30]. | Potential tissue damage, thermal sensitivity, off-target effects [30]. | Currently experimental; may provide non-invasive options for localized treatment. |
| Nucleic Acid-Based Treatments | Genetic contributors to Parkinson’s disease | Delivery of RNA interference (RNAi) or antisense oligonucleotides to modulate disease-causing genes [31]. | Immune reactions, off-target gene suppression, delivery challenges [31]. | Promising for genetic forms of Parkinson’s; further clinical trials needed. |
| Cellular Treatments | Progressive motor and non-motor symptoms | Transplantation of dopaminergic neurons or stem cells to restore dopamine production [32]. | Immune rejection, tumor formation, graft-induced dyskinesia [32]. | Effective in preclinical models; patient safety and graft functionality are key concerns. |
| Physical Therapy | Motor and balance issues | Enhances mobility, posture, and gait [33]. | No significant side effects . | Recommended as a complementary intervention at all disease stages. |
| Exercise and Diet | Overall symptoms | Improves muscle strength, flexibility, and energy levels [34]. | No significant side effects. | Critical for improving quality of life and managing overall health. |
| Psychological Therapy | Non-motor symptoms (e.g., depression, anxiety) | Addresses emotional and cognitive challenges through structured interventions [35]. | No significant side effects. | Plays an essential role in managing non-motor symptoms. |
Pharmacological treatment
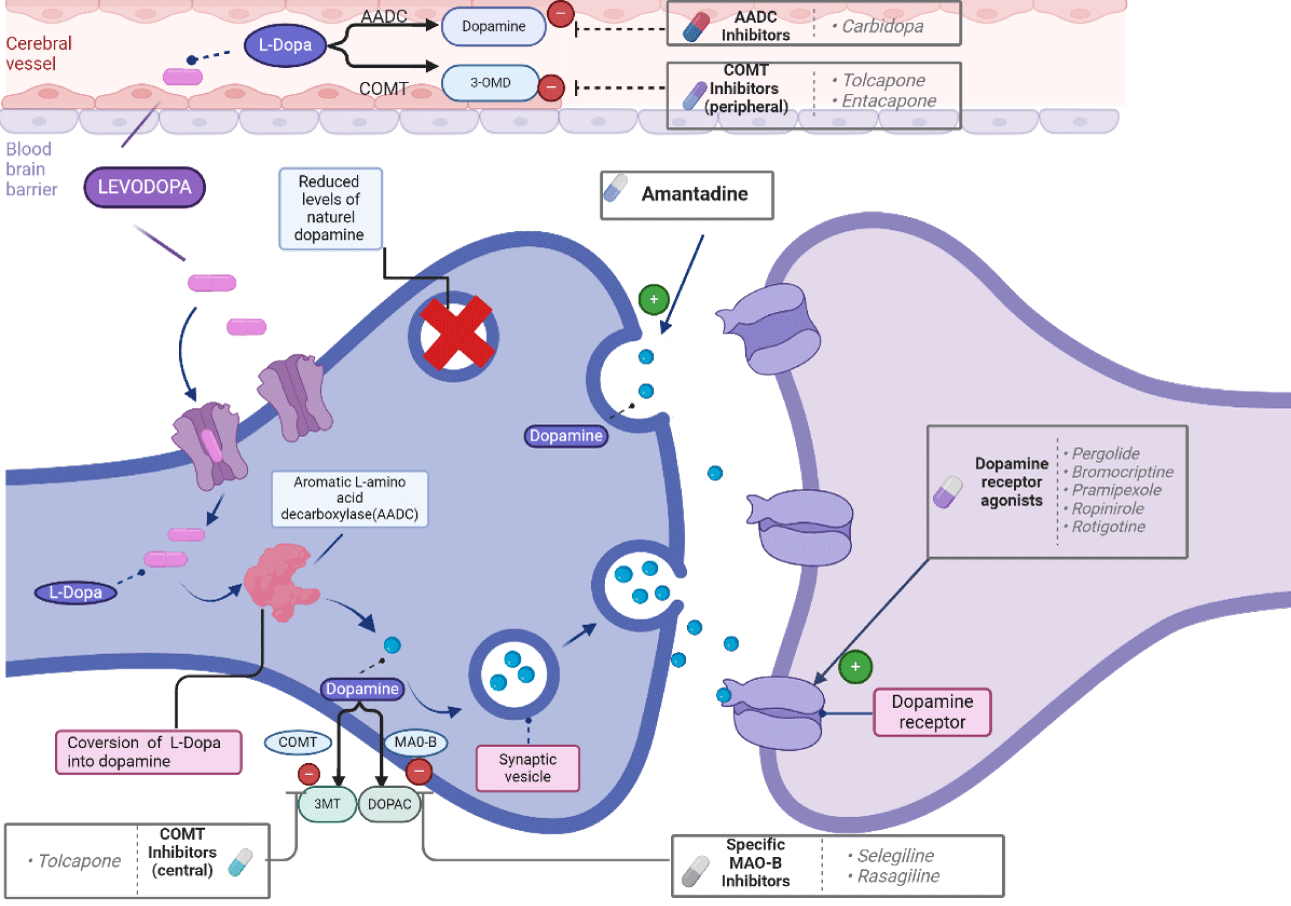
The main drugs used for the treatment of motor symptoms in PD are levodopa, Dopamine Receptor Agonists (DA), Monoamine Oxidase Type B (MAO B) inhibitors, anticholinergic agents, amantadine, Catechol-O-Methyl Transferase (COMT) inhibitors. Levodopa or L-Dopa is a dopamine precursor found in the amino acid structure and is the most effective drug for the treatment of movement disorders caused by PD. Carbidopa cannot cross the blood brain barrier and reduces the peripheral side effects of Levodopa to a certain extent by blocking the peripheral metabolism of L-dopa to dopamine. COMT inhibitors are used primarily to help with “wearing off” — changes in the ability to move as the effect of levodopa becomes short-lived. MAO B inhibitors are drug molecules that show their effect by inhibiting monoamine oxidase B, the enzyme responsible for dopamine destruction in the brain. DA are drug molecules that act by stimulating dopamine receptors (Figure 1) [36,37].
Levodopa (L-dopa; 3,4-dihydroxy-L-phenylalanine) is converted into dopamine in the brain. It is to date the most successful medication used to control the motor symptoms of PD. Because the neurotransmitter dopamine is not able to cross the blood-brain barrier, its precursor levodopa is used to treat PD and is the most effective antiparkinsonian agent [38]. Levodopa has been the main medication employed for antiparkinsonian treatment since it was introduced in the latter half of the 1960s, and no other drug or surgical intervention is more effective [39]. L-dopa rapidly and effectively controls PD, particularly bradykinesia and rigidity. L-dopa, which exerts its symptomatic effect as a result of its conversion in the brain to dopamine, has several complications, including nausea, hypotension, drowsiness, hallucinations, impulse control disorders, dystonia, and dyskinesia. These limiting complications are due to the intermittent stimulation of striatal dopamine receptors [40]. Therefore, it is used in combination with other drugs to prevent its conversion to dopamine in the periphery. Carbidopa, a dopa decarboxylase inhibitor, is commonly used in combination with L-dopa to prevent its conversion to dopamine in the periphery [41]. As a peripheral decarboxylase inhibitor that cannot cross the blood-brain barrier, carbidopa reduces the peripheral conversion of L-dopa to dopamine. This enhances the availability of L-dopa to cross the blood-brain barrier, thereby increasing its central efficacy. Consequently, the required dose of L-dopa to achieve therapeutic effects can be reduced, which may also mitigate peripheral side effects to some extent [42].
DA are pharmacological agents designed to treat various conditions, including PD, restless legs syndrome, galactorrhea, prolactinoma, amenorrhea, and specific cases of acromegaly [43]. These agents mimic dopamine's effects in the brain. Examples include ropinirole, rotigotine, and pramipexole. DA represent the most significant group of drugs for PD treatment after L-dopa, providing symptomatic relief by stimulating postsynaptic D1-D3 dopamine receptors without affecting dopamine metabolism [44]. They cause fewer motor complications than L-dopa and are often used as first-line monotherapy, particularly in younger patients with early-onset PD, to delay the initiation of L-dopa therapy [42]. Monoamine Oxidases (MAOs) are enzymes that catalyze the oxidation of monoamines, including dopamine. There are two isoforms of MAO: MAO-A, predominantly found in the small intestine, and MAO-B, primarily located in the brain [45,46]. MAO inhibitors with specificity and selectivity for MAO-B prolong dopamine activity in the striatum by inhibiting the breakdown of both exogenous and endogenous dopamine. As a result, these inhibitors can be used as monotherapy in the early stages of PD or as adjunctive therapy in levodopa-treated patients experiencing motor complications [47].
The balance between acetylcholine and dopamine neurotransmitters is crucial for the coordinated functioning of striated muscles. Agents such as trihexyphenidyl and benztropine can help manage tremors and muscle stiffness. In patients with PD, a reduction in dopamine levels and a relative increase in acetylcholine contribute to symptoms such as muscle stiffness and tremors. Anticholinergic drugs, which decrease cholinergic activity, were among the first medications developed for PD treatment and are now widely used, either alone or in combination with other drugs. However, their use should be avoided in elderly and cognitively impaired patients due to the risk of confusion [48].
Amantadine, an antiviral agent initially developed for the treatment of influenza, was serendipitously found to improve the symptoms of PD. It is used to manage rigidity, resting tremors, and, in some cases, fatigue, offering short-term symptomatic relief for patients. Additionally, amantadine has been shown to reduce the risk of levodopa-induced dyskinesia by enabling a reduction in the required dose of levodopa [48].
COMT is an enzyme that catalyzes the methylation of catechol substrates, first identified by Axelrod and Tomchick in 1958 [49]. COMT inhibitors extend the duration of levodopa’s effects by preventing its breakdown. COMT substrates include various catechols, such as catecholamines, their hydroxylated metabolites, catechol estrogens, ascorbic acid, and medicinal compounds [50]. Additionally, COMT metabolizes L-dopa in the periphery to form 3-O-methyldopa. By inhibiting this process, COMT inhibitors increase the amount of L-dopa that reaches the brain. These inhibitors must always be used in combination with L-dopa, as they are ineffective as monotherapy. Currently used COMT inhibitors include tolcapone, entacapone, and nitecapone [42].
Tolcapone is effective in the central nervous system due to its ability to cross the blood-brain barrier, whereas entacapone and nitecapone act only peripherally. The use of COMT inhibitors has significantly improved levodopa treatment for advanced stages of PD. However, the currently available inhibitors have certain limitations, and safety concerns regarding some of them restrict their clinical use and effectiveness. Further research is needed to develop COMT inhibitors that are both safer and more effective for the treatment of PD [42].
Gene-based treatment approaches
Evidence from multiple components of genetic risk and genes that cause disease has confirmed that these are involved in the pathophysiology of PD [51]. Several loci (EIF4G1, PARK 13, PARK 15, and PARK 1) and risk components (HLA, GAK, MAP, BST1, PARK 16, GBA, and LRRK2, SNCA) have been identified in linkage analyses and association studies [52].
Gene therapy is a method of regulating genes in human cells or preventing diseases [53]. A more complex definition would include the replacing, silencing, or modifying of a faulty gene with a beneficial gene [54]. Non-replicating viral vectors and multiple serotypes of recombinant Adeno-Associated Virus (AAV) are involved with this molecular engineering method [55].
Alpha-Synuclein (AS) is a protein, has a crucial function in the pathogenesis of PD, because mutations and duplications of the AS gene (SNCA) locus give rise to familial PD [56,57]. Aggregated AS is a prime constituent of Lewy bodies and a mark of AS-associated PD and most idiopathic cases; it is also possible for it to be secreted into the extracellular space and "spread" to anatomically connected brain regions trans-synaptically [58].
Therapeutics that are specifically targeted to limit the accumulation of AS may prevent or reduce the speed of the neurodegenerative processes seen both in PD and different synucleinopathies. The small molecule alpha-synuclein misfolding inhibitor, NPT200-11 significantly reduces AS aggregation. Its impact on AS neuropathology have been assessed using human alpha-synuclein expression in animal models and have been shown to reduce alpha-synuclein pathology in the cortex, reduce the neuroinflammation associated with this (astrogliosis), normalize levels of striatal Dopamine Transporter (DAT), and improve motor function [59].
Anle18b (MODAG GmbH) is an AS aggregation inhibitor now in development for synucleinopathy multisystem atrophy which could potentially be used in PD; it is currently undergoing a Phase 1 safety and tolerability trial (NCT04208152) [60].
The process of neurodegeneration activates the tyrosine kinase Abl. A study has revealed that lentiviral expression of a-synuclein in mouse substantia nigra causes the activation of Abl (phosphorylation), and that lentiviral Abl expression increases the levels of a-synuclein in PD brains, consistent with Abl activation. Administering the tyrosine kinase inhibitor nilotinib reduces Abl activity and leads to better autophagic clearance of a-synuclein in transgenic and lentiviral gene transfer models. According to the data, it may be possible to use nilotinib therapeutically to degrade a-synuclein in both PD and other a-synucleinopathies [61,62].
Soon after LRRK2 (the leucine-rich repeat kinase 2) gene at the PARK8 locus on chromosome 12 had been cloned and identified , it was found that disease-associated mutant forms of the protein could cause CNS neuron death [63]. In the last decade, considerable efforts have been made to develop potent and selective small-molecule inhibitors of LRRK2 and to conduct preclinical tests in various PD models [64]. Administration of an LRRK2 kinase inhibitor in a mouse model of synucleinopathy reduced a-synuclein aggregation through enhancing the interaction of a-synuclein with the pathway of lysosomal degradation. These findings indicate that LRRK2-mediated RAB35 phosphorylation could be a therapeutic target in terms of altering the progression of disease [65].
b-Glucocerebrosidase is an enzyme with glucosylceramidase activity and heterogeneous mutations in the GBA1 gene encoding the enzyme is one of the major genetic causes of PD [66,67]. This mutation increases the risk of PD by 20 times [68]. In phenotypical terms, it is virtually impossible to distinguish between IPD and GBA-PD apart from the acceleration of the progress of motor and non-motor symptoms in those with GBA-PD [69,70]. GCase deficiency can give rise to AS accumulation through oligomer stabilization, which can lead to even more decrease in GCase activity, giving the effect of a bidirectional positive feedback loop [71]. This "toxic" relationship has been developed both in vitro and in vivo, and the most favored hypothesis is that GCase deficiency causes first lysosomal dysfunction and then proteinopathy in synucleinopathies [72]. These results strengthen the view that the restoration of normal levels of GCase enzyme activity may limit the speed at which PD progresses in patients with GBA1 mutations. In mouse models it has been shown that using PR001, an AAV9 vector-based gene therapy developed in order to deliver a functional GBA1 gene to the brain, may slow down or halt the disease. At present, PR001 is being clinically trialed in Parkinson's patients who have GBA1 mutations [73]. Another study investigated a-synuclein metabolism in LIMP-2-deficient mice, as it is closely linked to the expression of Lysosomal Integral Membrane Protein Type 2 (LIMP-2). In an in vivo study, mice exhibited a dose-dependent a-synuclein phenotype, which included severe neurological deficits as well as premature death. A significant reduction in GCase activity in LIMP-2-deficient brains brought about inflammation, lipid storage, impaired autophagic/lysosomal function, a-synuclein accumulation, mediating neurotoxicity of Dopaminergic (DA) neurons, and apoptotic cell death. Heterologous expression of LIMP-2 increased the speed at which overexpressed a-synuclein was cleared, possibly through an increase in the activity of lysosomal GCase. In human PD midbrain DA neurons which survived, there was an increase in levels of LIMP-2, perhaps as compensation for the lack of lysosomal GCase. The manipulation of LIMP-2 expression to increase the amount of lysosomal GCase activity may thus show promise in treating synucleinopathies in a strategic manner [74].
While it is true that gene therapy has not as yet led to a cure for PD, growing evidence supports the idea that this treatment modality is a vital avenue to explore in the future.
Photothermal-based treatments
Phototherapies are currently undergoing a rapid evolution; they are therapeutic modalities which use various wavelengths of light to cause photothermal or photochemical alterations within a specifically targeted tissue [75]. The most frequently encountered phototherapies are Photodynamic Therapy (PDT) and Photothermal Therapy (PTT), which deploy light and exogenous or endogenous absorbers in order to generate cytotoxic Reactive Oxygen Species (ROS) and to elevate the local temperature, respectively [76].
PTT typically uses light that is Near-Infrared (NIR) to increase the temperature of tissue and bring about localized photocoagulation. PTT uses a light power that is relatively high to achieve subcoagulative (43-55°C) or coagulative (55-100°C) temperatures that will lead to rapid cell death through damage to the cell membrane and protein denaturation. Recent research has provided confirmation that low-temperature photothermal treatment (LTPTT, 41-43°C) with an 808 nm NIR laser enhances BBB permeability, increases the accumulation of drugs in the brain, and demonstrates good therapeutic outcomes in neurodegenerative diseases [77,78]. Furthermore, LTPTT is able to improve the cell membrane’s permeability, which results in a important increase in cellular uptake [79,80]. Moreover, using an NIR laser to irradiate cells with internal photothermal Nanoparticles (NPs) is able to bring about endo/lysosomal cavitation, which may facilitate the escape of gene drugs from endo/lysosomes to prevent enzymolysis [80]. Therefore, combining low-temperature photothermal techniques and nanotechnology may be a basis for gene therapy in PD.
One primary limiting factors in treating PD is the blood-brain barrier. Therefore, developing therapeutic agents that are able to cross this barrier is crucial. In one recent study, MgOp@PPLP nanoparticles containing MgO nanoparticles as substrate, polydopamine-coated anti-SNCA plasmid, and polyethylene glycol, lactoferrin, and puerarin were used to improve the hydrophilicity, brain targeting and antioxidant properties of the particles, respectively. In vitro and in vivo models, MgOp@PPLP was shown to have good neuroprotective effects. Therefore, the MgOp@PPLP nanoplatform with good biocompatibility is, when combined with Non-Invasive Near-Infrared (NIR) radiation, is an ideal way of combatting neurodegenerative diseases [81].
In another study, a novel 2D graphdiyne (GDY)-based nano platform was used to deliver minoxycycline [82], a semisynthetic tetracycline-derived antibiotic, a drug candidate for PD treatment known to show, across the blood-brain barrier, anti-apoptotic, antioxidant, and anti-inflammatory effects. GDY nanosheets loaded with minoxycycline have been shown to trigger the release of more than 30 percent of the drug molecule by near-infrared irradiation. The GDY nano platform, capable of Photothermal (PT) conversion and has no significant in vitro toxicity, has been demonstrated the blood-brain barrier in animal and cellular models. The behavioral defects of PD mice can be corrected by restoring the number of dopaminergic neurons to normal levels after chemical synergistic treatment by PT and GDY. Nanosheet-mediated PT treatment shows therapeutic results comparable to L-DOPA, which is a commercially available PD drug. The GDI-based delivery system shows promise as a platform on which chemical drugs that target neurodegenerative diseases, in particular PD, can be loaded [83].
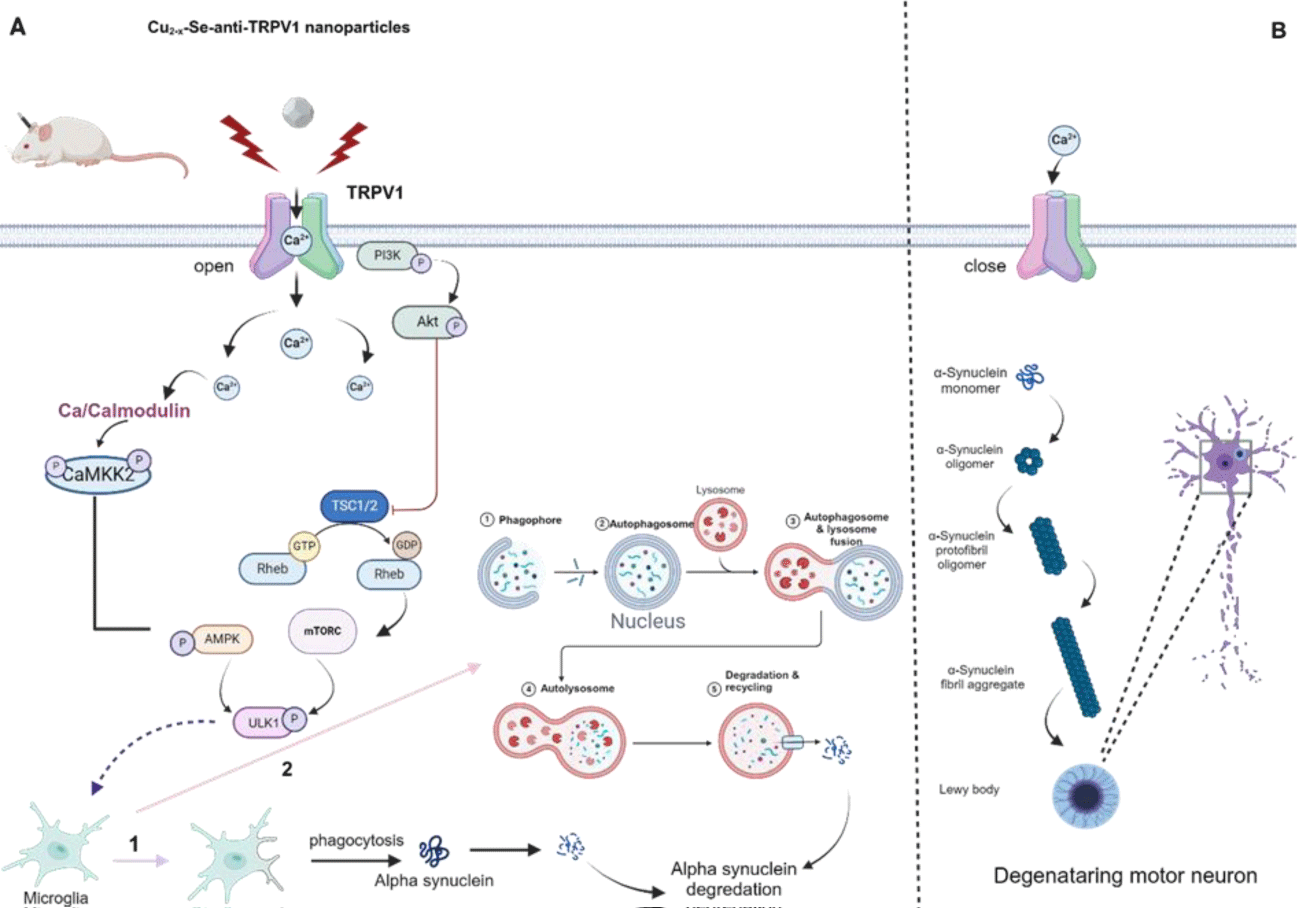
Yuan J, et al. [84], designed a Cu2-xSe-anti-TRPV1 nanoparticle (CS- in NPS) with the aim of targeting microglia and inducing Ca2+ influx to activate ATG5 and Ca2+/ by opening surface Transient Receptor Potential Vanilloid 1 (TRPV1) channels through second Near-Infrared (NIR-II) laser irradiation. (A) Cu2- xSe-anti-TRPV1 nanoparticle opens TRPV1 channels in a controlled manner, allowing Ca to pass into the cell. Intracellular Ca entry leads to alpha synuclein degradation, promoting microglial autophagy and apoptosis through both the calmodulin/Ca complex and the mTORC1/AMPK pathway. (B) Since there is no Ca passage into the cell due to the closed TRPV1 channel, Lewy bodies are formed as a result of alpha synuclein aggregation and this causes degeneration in motor neurons (Figure 2). The CaMKK2/AMPK/mTOR pathway promotes degradation of a-syn and phagocytosis. It was found that CS-AT NPs were delivered efficiently through focused ultrasound into the striatum of PD mice with high TRPV1 receptor expression, and the athletic performance of PD mice treated with CS-AT NPs and NIR-II irradiation was significantly better as a result of enhanced autophagy and phagocytotic clearance of a-syn by microglia. Tyrosine hydroxylase enzyme, glial fibrillary acidic protein, ionized calcium-binding adaptor protein 1, and pSer129-α-syn (p-α-syn) of the PD mice were found to be restored to the near normal levels of healthy mice. As a result of this study, it was demonstrated that the rationally designed photothermal nanoagent enhanced the therapeutic efficacy of PD by increasing microglial phagocytosis and autophagy to degrade α-synuclein through the controlled opening of Transient Receptor Potential Vanilloid 1 (TRPV1) channels.
Nucleic acid based-treatments
RNAs play a role in key processes during the progression of disease and are viewed as strong diagnostic biomarkers and therapeutic targets [85].In particular, oligonucleotide therapeutics are emerging as a promising new class of drugs for specifically targeting coding or non-coding RNA molecules in ways that aim to revolutionize how various diseases are treated [86].
Recent research has demonstrated that nucleic acid-based therapies are effective in the treatment of neurological diseases and have thus increased the possibility that new molecular therapies will be developed for PD [87]. Many small nucleic acid particles, such as miRNA, siRNA, shRNA, and plasmid DNA, are used to silence specific gene regions that cause many diseases, particularly PD [86].
Approximately 20-25 nucleotides long, miRNAs are non-coding RNAs. That non-coding RNA regulate gene expression after transcription by binding to their target mRNA’s 3' Untranslated Region (UTR) [88]. In a healthy human brain, miRNAs control cellular mRNA levels [89]. miRNAs have a pathogenic role that contributes to the basic causes of PD through their abnormal production while also emerging as an essential therapeutic agent in treatment [88]. In particular, it is possible to transfer miRNAs associated with microglia-derived extracellular vesicles from cell to cell and to regulate target genes so that the functions of recipient cells are modulated [89]. Zhu Y, et al. [90]. Targeted extracellular vesicles containing the PRAK inhibitor GLPG0259 miRNAs that were derived from microglia treated with monomeric α-synuclein into cells. As a result of the study, extracellular vesicles that were derived from monomeric α-synuclein-treated microglia were shown to reduce neuroinflammation by promoting anti-inflammatory microglia through the delivery of PRAK-targeting miRNAs to recipient microglia [91].
Small interfering RNAs (siRNAs) have double chain and are short regulatory RNA molecules that are able to silence post-transcriptional genes and, in some cases, the transcriptional level. siRNA therapy is a promising tool for treating neurological disorders such as PD. In one study, anti-SNCA siRNA was administered to the brain (substantia nigra) of a monkey model, and a decrease in α-syn mRNA protein levels was observed. No tissue-specific or systemic toxicity was determined in these monkeys. Systemic toxicity was written into them, demonstrating the safety and viability of using siRNA [92]. Kim YC, et al. [93], employed a Viral vector (AAV vector) with α-syn siRNA in a mouse model. The vector was well tolerated in mouse models of PD and there was a reduction of α-syn mRNA and protein.
While RNAi-based therapies offer several advantages, there are also persistent issues related to competition with cellular RNAi components and how to effectively deliver them in vivo. While recent research and studies in numerous animal models confirm that most effects that are offtarget are not dangerous, a number of other issues need to be resolved before RNAi-based drugs can be used in a clinical setting. Indeed, several RNAi-based human clinical trials are already underway [94]. The hope is that this technology will have a variety of practical uses in terms of treating neurodegenerative diseases, particularly PD.
Cellular treatments
Stem cells can form in any human body tissue and thus have great potential with regard to future therapies involving tissue regeneration and repair. To meet the definition of a "stem cell," cells must have two key features. First, stem cells have to be capable of unlimited self-renewal so that their progeny are exactly like the original cell. This is also the case with cancer cells, although such cells divide in an uncontrollable manner, while the division of stem cells is very regulated. The supplementary necessary for stem cells is thus that they must be able to produce a specific type of cell that can become part of a healthy animal [95].
The promise of stem cell therapies is that they will be able to treat cancer and degenerative diseases and repair damaged tissues where therapeutic options are presently limited or unavailable. This potential has been recognized for many years, and the development of induced Pluripotent Stem Cells (iPSCs) has expanded the field of stem cells, giving rise to further innnovations and greater knowledge [96].
Several stem cell types have been investigated in terms of their potential use in PD therapy. Mesenchymal Stem Cells (MSCs) are stem cells in the adult state, are found in the connective tissues of cells and are potentiated by their capacity for differentiation. Further GDNF treatment led to an increase in the proportion of DAergic neurons. Subsequently, multi-lineage, differentiating, stress-resistant (Muse) cells with stage-specific embryonic antigen-3 (SSEA-3) were discovered [97]. Using Muse cells to treat CNS disorders is another line of inquiry. Intranasal delivery of MSCs may also be an attractive clinical option [98,99]. However, there has a yet been no successful data involving MSCs for patients with PD [100].
Cell replacement therapy that employs dopamine neurons derived from human Pluripotent Stem Cell (hPSC) derived may have significant promise. It offers an innovative regenerative strategy that builds on a long history involving fetal tissue grafts and captures the potential of hPSCs to function as a standardized and scalable cell source. The progress made to establish protocols for direct differentiation from hPSCs into midbrain Dopamine (mDA) neurons has been a catalyst in developing cell-based therapies for PD. As a result, various groups have been able to derive clinical-grade mDA neuron precursors, with well-understood clinical manufacturing practices leading towards clinical testing in patients with PD [101].
In recent decades, swift progress in stem cell technology, which has included the creation of robust manufacturing processes and differentiation protocols, has made easier the design of first-generation hPSC-derived DA neuron technologies that will eventually be used in the clinical trials in humans [102].
Neurotrophic factors
The word neurotrophin was formed by combining the words ‘neuron’ meaning nerve cell and ‘trophe’ meaning nutrition in Greek. Neurotrophins (NTs) are a family of polypeptidestructured growth factors that affect the survival and function of neurons and control synaptic function and synaptic plasticity [103].
Neurotrophins form a class of neurotrophic factors including neurokines and ligands of the Glial Cell-Derived Neurotrophic Factor (GDNF) family. Neurons express GDNF and, interestingly, are derived from a single neuronal subpopulation. Studies and emerging evidence have shown that neurotrophins may contribute to the pathogenesis of PD. Lewy body formation may lead to a modulation of GDNF and BDNF levels, which result in decreased BDNF expression and altered neuronal BDNF transport [104,105]. Also, a number of postmortem studies since 1999 have shown that levels of BDNF are reduced in PD patients’ SNc and striatal cell bodies [106].
Conclusion
Results from recent clinical trials have provided insight into both new treatments for PD and ways to make known therapies more effective. New treatments and formulations, such as extended-release levodopa/carbidopa and sublingual apomorphine, offer additional tools for managing motor symptoms, especially when fluctuations become challenging. These advancements highlight the ongoing effort to enhance the clinical management of individuals with PD and their quality of life.
Although several medical and surgical treatments have proven successful for motor symptoms, non-motor symptoms, for example cognitive impairment, anxiety, and hypotension cannot be treated alone. A severe clinical need exists for specific treatments targeted at these symptoms. Addressing these symptoms often requires a multifaceted approach, including pharmacological and non-pharmacological interventions. Research focused both on developing targeted therapies for non-motor symptoms and minimizing the side effects of existing treatments, is crucial for improving the overall management of PD.
Acknowledgement
The author received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors declare no conflict of interest.
Cedit Author Statement
Ayşe Aksoy: Study design, writing the article, data collection.
Duygu Deniz Usta: Study design, critical review of the article.
Atiye Seda Yar Sağlam: Study design, critical review of the article, conceptualization.
All authors read and agreed with the final version of the manuscript.
References
- Paccosi E, Proietti-De-Santis L. Parkinson's Disease: From Genetics and Epigenetics to Treatment, a miRNA-Based Strategy. Int J Mol Sci. 2023 May 31;24(11):9547. doi: 10.3390/ijms24119547. PMID: 37298496; PMCID: PMC10253466.
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019 May;18(5):459-480. doi: 10.1016/S1474-4422(18)30499-X. Epub 2019 Mar 14. PMID: 30879893; PMCID: PMC6459001.
- Postuma RB. Early markers of Parkinson's disease progression. Movement Disorders. 2023;38(3):451-460.
- Schapira AHV. Advances in Parkinson's disease pathogenesis. Nature Reviews Neurology. 2022;18(7):395-409.
- Aarsland D. Cognitive dysfunction in Parkinson's disease. The Lancet Neurology. 2022;21(10):845-856.
- Emre M. Dementia and Parkinson's disease: Clinical insights. Journal of Neurology. 2023;270(5):1234-1242.
- Chaudhuri KR. Non-motor symptoms in Parkinson’s disease: Diagnosis and management. Parkinsonism & Related Disorders. 2021;82:74:85.
- Rutten S. Anxiety and depression in Parkinson's disease. Journal of Clinical Psychiatry. 2023;84(2):12345.
- Jost WH. Autonomic dysfunction in Parkinson’s disease. Clinical Autonomic Research. 2022;32(1):15-24.
- Poewe W. Lewy body pathology and clinical manifestations. Journal of Parkinson’s disease. 2023;13(3):401-412.
- Schrag A. REM sleep behavior disorder as a prodromal symptom of Parkinson’s disease. Sleep Medicine Reviews. 2023;69:101567.
- Bloem BR. Postural instability and falls in Parkinson's disease. The Journal of Geriatric Neurology. 2023;14(5):233-243.
- Goetz CG. Motor and non-motor symptom overlap in Parkinson’s disease. Neurology. 2022;99(4):547-559.
- Lees AJ. Resting tremor as a diagnostic marker in Parkinson's disease. Parkinson’s Disease & Related Disorders. 2023;17(2):97-105.
- Funayama M, Hasegawa K, Kowa H, Saito M, Tsuji S, Obata F. A new locus for Parkinson's disease (PARK8) maps to chromosome 12p11.2-q13.1. Ann Neurol. 2002 Mar;51(3):296-301. doi: 10.1002/ana.10113. PMID: 11891824.
- Smith A, Johnson R. Levodopa and its role in managing Parkinson’s disease. Neurology Advances. 2023;34(3):145-156.
- Brown J, Carter R. Motor fluctuations and their management in Parkinson’s disease. Parkinson’s Research Journal. 2022;8(2):234-245.
- Miller J, White P. Dopamine agonists in Parkinson’s therapy: Efficacy and side effects. Movement Disorders Today. 2021;11(4):89-97.
- Taylor R, Adams T. Neuropsychiatric effects of dopamine agonists in Parkinson’s disease. Psychiatric Neurology Reports. 2020;9(3):134-145.
- Green S, Carter R. MAO-B inhibitors: A review of their role in Parkinson’s treatment. Neuropharmacology Review. 2019;0(5):345-358.
- Carter R. Long-term use of MAO-B inhibitors in Parkinson’s disease. Neurological Advances. 2018;5(2):67-78.
- Evans R, Brown K. COMT inhibitors in Parkinson’s disease therapy. Neurotherapeutics. 2017;14(1):56-67.
- Martin T, Davis L. Managing side effects of COMT inhibitors in Parkinson’s disease. Movement Disorders Journal. 2016;12(6):456-468.
- Roberts T, Wilson P. Amantadine’s role in managing dyskinesias in Parkinson’s disease. Neurotherapeutics. 2015;10(2):234-243.
- Davis L. Safety considerations for amantadine use in Parkinson’s disease. Therapeutic Advances in Neurology. 2014;7(3):145-152.
- Wilson P, Thomas J. Anticholinergic treatments in Parkinson’s disease: A double-edged sword. Journal of Neurological Therapies. 2013;8(4):145-157.
- Adams R, Green P. Cellular therapies for Parkinson’s disease. Stem Cell Research and Therapy. 2021;12(4):234-245.
- Miller J, Taylor R. Challenges in cellular therapy for neurodegenerative disorders. Frontiers in Neuroscience. 2020;14:345-358.
- Chen T. Photothermal strategies for neurodegenerative diseases. Nano Today. 2023;45:101290.
- Zhang L. Assessing the safety of photothermal therapies in animal models. Nanomedicine. 2022;18(5):345-358.
- Brown J, Smith A. Advances in nucleic acid therapies for neurodegenerative diseases. Molecular Therapy. 2020;15(3):456-468.
- Harris L. The impact of exercise on Parkinson’s disease symptoms. Movement Science Journal. 2007;19(2):112-119.
- Johnson T, Adams M. Physical therapy in neurodegenerative conditions. Rehabilitation Research Reviews. 2008;10(2):89-102.
- Taylor R, Adams T. Cognitive and emotional interventions in Parkinson’s disease. Psychology Today. 2005;15(3):34-45.
- Green S. Psychotherapy as a complementary treatment for Parkinson’s disease. Journal of Neuropsychology. 2004;12(1):56-67.
- Kulisevsky J. Pharmacological management of Parkinson's disease motor symptoms: update and recommendations from an expert. Rev Neurol. 2022 Oct 31;75(s04):S1-S10. English, Spanish. doi: 10.33588/rn.75s04.2022217. PMID: 36342310; PMCID: PMC10281635.
- Aminoff MJ. Pharmacologic management of Parkinsonism and other movement disorders. In: Katzung BG, editor. Basic and Clinical Pharmacology. 14th ed. New York; McGraw-Hill Education: 2017.
- LeWitt PA. Levodopa therapy for Parkinson's disease: Pharmacokinetics and pharmacodynamics. Mov Disord. 2015 Jan;30(1):64-72. doi: 10.1002/mds.26082. Epub 2014 Dec 1. PMID: 25449210.
- Olanow CW, Stocchi F. Levodopa: A new look at an old friend. Mov Disord. 2018 Jul;33(6):859-866. doi: 10.1002/mds.27216. Epub 2017 Nov 27. PMID: 29178365.
- Lane EL. L-DOPA for Parkinson's disease-a bittersweet pill. Eur J Neurosci. 2019 Feb;49(3):384-398. doi: 10.1111/ejn.14119. Epub 2018 Sep 16. PMID: 30118169.
- Dhall R, Kreitzman DL. Advances in levodopa therapy for Parkinson disease: Review of RYTARY (carbidopa and levodopa) clinical efficacy and safety. Neurology. 2016 Apr 5;86(14 Suppl 1):S13-24. doi: 10.1212/WNL.0000000000002510. Epub 2016 Apr 4. PMID: 27044646.
- Çakmur R. Parkinson hastalığı ve medikal tedavisi. Klinik Gelişim. 2010;1:53-60.
- Woitalla D, Buhmann C, Hilker-Roggendorf R, Höglinger G, Koschel J, Müller T, Weise D. Role of dopamine agonists in Parkinson's disease therapy. J Neural Transm (Vienna). 2023 Jun;130(6):863-873. doi: 10.1007/s00702-023-02647-0. Epub 2023 May 11. Erratum in: J Neural Transm (Vienna). 2024 Sep;131(9):1145. doi: 10.1007/s00702-023-02695-6. PMID: 37165120.
- Choi SG, Tittle T, Garcia-Prada D, Kordower JH, Melki R, Killinger BA. Alpha-synuclein aggregates are phosphatase resistant. Acta Neuropathol Commun. 2024 May 31;12(1):84. doi: 10.1186/s40478-024-01785-0. PMID: 38822421; PMCID: PMC11141014.
- Dezsi L, Vecsei L. Monoamine Oxidase B Inhibitors in Parkinson's Disease. CNS Neurol Disord Drug Targets. 2017;16(4):425-439. doi: 10.2174/1871527316666170124165222. PMID: 28124620.
- Saura Marti J, Kettler R, Da Prada M, Richards JG. Molecular neuroanatomy of MAO-A and MAO-B. J Neural Transm Suppl. 1990;32:49-53. doi: 10.1007/978-3-7091-9113-2_5. PMID: 2089112.
- Fernandez HH, Chen JJ. Monoamine oxidase-B inhibition in the treatment of Parkinson's disease. Pharmacotherapy. 2007 Dec;27(12 Pt 2):174S-185S. doi: 10.1592/phco.27.12part2.174S. PMID: 18041937.
- Zahoor I, Shafi A, Haq E. Pharmacological Treatment of Parkinson’s Disease. In: Stoker TB, Greenland JC, editors. Parkinson’s Disease: Pathogenesis and Clinical Aspects [Internet]. Brisbane (AU): Codon Publications; 2018 Dec 21. Chapter 7. PMID: 30702845.
- Guldberg HC, Marsden CA. Catechol-O-methyl transferase: pharmacological aspects and physiological role. Pharmacol Rev. 1975 Jun;27(2):135-206. PMID: 1103160.
- Bonifácio MJ, Palma PN, Almeida L, Soares-da-Silva P. Catechol-O-methyltransferase and its inhibitors in Parkinson's disease. CNS Drug Rev. 2007 Fall;13(3):352-79. doi: 10.1111/j.1527-3458.2007.00020.x. PMID: 17894650; PMCID: PMC6494163.
- Bandres-Ciga S, Diez-Fairen M, Kim JJ, Singleton AB. Genetics of Parkinson's disease: An introspection of its journey towards precision medicine. Neurobiol Dis. 2020 Apr;137:104782. doi: 10.1016/j.nbd.2020.104782. Epub 2020 Jan 25. PMID: 31991247; PMCID: PMC7064061.
- Soto-Ortolaza AI, Heckman MG, Labbé C, Serie DJ, Puschmann A, Rayaprolu S, Strongosky A, Boczarska-Jedynak M, Opala G, Krygowska-Wajs A, Barcikowska M, Czyzewski K, Lynch T, Uitti RJ, Wszolek ZK, Ross OA. GWAS risk factors in Parkinson's disease: LRRK2 coding variation and genetic interaction with PARK16. Am J Neurodegener Dis. 2013 Nov 29;2(4):287-99. PMID: 24319646; PMCID: PMC3852568.
- Axelsen TM, Woldbye DPD. Gene Therapy for Parkinson's Disease, An Update. J Parkinsons Dis. 2018;8(2):195-215. doi: 10.3233/JPD-181331. PMID: 29710735; PMCID: PMC6027861.
- Dumbhare O, Gaurkar SS. A Review of Genetic and Gene Therapy for Parkinson's Disease. Cureus. 2023 Feb 5;15(2):e34657. doi: 10.7759/cureus.34657. PMID: 36909056; PMCID: PMC9991874.
- Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, Brodsky MA, Burchiel K, Kelly P, Dalvi A, Scott B, Stacy M, Turner D, Wooten VG, Elias WJ, Laws ER, Dhawan V, Stoessl AJ, Matcham J, Coffey RJ, Traub M. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol. 2006 Mar;59(3):459-66. doi: 10.1002/ana.20737. Erratum in: Ann Neurol. 2006 Dec;60(6):747. PMID: 16429411.
- Dorsey ER, Bloem BR. The Parkinson Pandemic-A Call to Action. JAMA Neurol. 2018 Jan 1;75(1):9-10. doi: 10.1001/jamaneurol.2017.3299. PMID: 29131880.
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997 Jun 27;276(5321):2045-7. doi: 10.1126/science.276.5321.2045. PMID: 9197268.
- Postuma RB, Aarsland D, Barone P, Burn DJ, Hawkes CH, Oertel W, Ziemssen T. Identifying prodromal Parkinson's disease: pre-motor disorders in Parkinson's disease. Mov Disord. 2012 Apr 15;27(5):617-26. doi: 10.1002/mds.24996. PMID: 22508280.
- Price DL, Koike MA, Khan A, Wrasidlo W, Rockenstein E, Masliah E, Bonhaus D. The small molecule alpha-synuclein misfolding inhibitor, NPT200-11, produces multiple benefits in an animal model of Parkinson's disease. Sci Rep. 2018 Nov 1;8(1):16165. doi: 10.1038/s41598-018-34490-9. PMID: 30385782; PMCID: PMC6212487.
- Polissidis A, Xilouri M, Stefanis L. The role of alpha-synuclein in the pathogenesis of Parkinson's disease: Molecular mechanisms and therapeutic strategies. J Neurochem. 2020;152(6):649-661.
- Pagan F, Hebron M, Valadez EH, Torres-Yaghi Y, Huang X, Mills RR, Wilmarth BM, Howard H, Dunn C, Carlson A, Lawler A, Rogers SL, Falconer RA, Ahn J, Li Z, Moussa C. Nilotinib Effects in Parkinson's disease and Dementia with Lewy bodies. J Parkinsons Dis. 2016 Jul 11;6(3):503-17. doi: 10.3233/JPD-160867. PMID: 27434297; PMCID: PMC5008228.
- Simuni T, Fiske B, Merchant K, Coffey C, Klingner E, Caspell-Garcia C, Lafontant DE, Matthews H, Wyse RK, Brundin P. Nilotinib in patients with advanced Parkinsons disease: A randomized phase 2A study (NILO-PD). medRxiv. 2020.
- Smith WW, Pei Z, Jiang H, Moore DJ, Liang Y, West AB, Dawson VL, Dawson TM, Ross CA. Leucine-rich repeat kinase 2 (LRRK2) interacts with parkin, and mutant LRRK2 induces neuronal degeneration. Proc Natl Acad Sci U S A. 2005 Dec 20;102(51):18676-81. doi: 10.1073/pnas.0508052102. Epub 2005 Dec 13. PMID: 16352719; PMCID: PMC1317945.
- Zhao Y, Dzamko N. Recent Developments in LRRK2-Targeted Therapy for Parkinson's Disease. Drugs. 2019 Jul;79(10):1037-1051. doi: 10.1007/s40265-019-01139-4. PMID: 31161537.
- Bae EJ, Kim DK, Kim C, Mante M, Adame A, Rockenstein E, Ulusoy A, Klinkenberg M, Jeong GR, Bae JR, Lee C, Lee HJ, Lee BD, Di Monte DA, Masliah E, Lee SJ. LRRK2 kinase regulates α-synuclein propagation via RAB35 phosphorylation. Nat Commun. 2018 Aug 27;9(1):3465. doi: 10.1038/s41467-018-05958-z. PMID: 30150626; PMCID: PMC6110743.
- Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, Chen CM, Clark LN, Condroyer C, De Marco EV, Dürr A, Eblan MJ, Fahn S, Farrer MJ, Fung HC, Gan-Or Z, Gasser T, Gershoni-Baruch R, Giladi N, Griffith A, Gurevich T, Januario C, Kropp P, Lang AE, Lee-Chen GJ, Lesage S, Marder K, Mata IF, Mirelman A, Mitsui J, Mizuta I, Nicoletti G, Oliveira C, Ottman R, Orr-Urtreger A, Pereira LV, Quattrone A, Rogaeva E, Rolfs A, Rosenbaum H, Rozenberg R, Samii A, Samaddar T, Schulte C, Sharma M, Singleton A, Spitz M, Tan EK, Tayebi N, Toda T, Troiano AR, Tsuji S, Wittstock M, Wolfsberg TG, Wu YR, Zabetian CP, Zhao Y, Ziegler SG. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N Engl J Med. 2009 Oct 22;361(17):1651-61. doi: 10.1056/NEJMoa0901281. PMID: 19846850; PMCID: PMC2856322.
- Sidransky E, Lopez G. The link between the GBA gene and parkinsonism. Lancet Neurol. 2012 Nov;11(11):986-98. doi: 10.1016/S1474-4422(12)70190-4. PMID: 23079555; PMCID: PMC4141416.
- Neumann J, Bras J, Deas E, O'Sullivan SS, Parkkinen L, Lachmann RH, Li A, Holton J, Guerreiro R, Paudel R, Segarane B, Singleton A, Lees A, Hardy J, Houlden H, Revesz T, Wood NW. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson's disease. Brain. 2009 Jul;132(Pt 7):1783-94. doi: 10.1093/brain/awp044. Epub 2009 Mar 13. PMID: 19286695; PMCID: PMC2702833.
- Cilia R, Tunesi S, Marotta G, Cereda E, Siri C, Tesei S, Zecchinelli AL, Canesi M, Mariani CB, Meucci N, Sacilotto G, Zini M, Barichella M, Magnani C, Duga S, Asselta R, Soldà G, Seresini A, Seia M, Pezzoli G, Goldwurm S. Survival and dementia in GBA-associated Parkinson's disease: The mutation matters. Ann Neurol. 2016 Nov;80(5):662-673. doi: 10.1002/ana.24777. Epub 2016 Oct 3. PMID: 27632223.
- Koros C, Simitsi A, Stefanis L. Genetics of Parkinson's Disease: Genotype-Phenotype Correlations. Int Rev Neurobiol. 2017;132:197-231. doi: 10.1016/bs.irn.2017.01.009. Epub 2017 Mar 1. PMID: 28554408.
- Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA, Sidransky E, Grabowski GA, Krainc D. Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011 Jul 8;146(1):37-52. doi: 10.1016/j.cell.2011.06.001. Epub 2011 Jun 23. PMID: 21700325; PMCID: PMC3132082.
- Sardi SP, Viel C, Clarke J, Treleaven CM, Richards AM, Park H, Olszewski MA, Dodge JC, Marshall J, Makino E, Wang B, Sidman RL, Cheng SH, Shihabuddin LS. Glucosylceramide synthase inhibition alleviates aberrations in synucleinopathy models. Proc Natl Acad Sci U S A. 2017 Mar 7;114(10):2699-2704. doi: 10.1073/pnas.1616152114. Epub 2017 Feb 21. PMID: 28223512; PMCID: PMC5347608.
- Abeliovich A, Hefti F, Sevigny J. Gene Therapy for Parkinson's Disease Associated with GBA1 Mutations. J Parkinsons Dis. 2021;11(s2):S183-S188. doi: 10.3233/JPD-212739. PMID: 34151863; PMCID: PMC8543272.
- Rothaug M, Zunke F, Mazzulli JR, Schweizer M, Altmeppen H, Lüllmann-Rauch R, Kallemeijn WW, Gaspar P, Aerts JM, Glatzel M, Saftig P, Krainc D, Schwake M, Blanz J. LIMP-2 expression is critical for β-glucocerebrosidase activity and α-synuclein clearance. Proc Natl Acad Sci U S A. 2014 Oct 28;111(43):15573-8. doi: 10.1073/pnas.1405700111. Epub 2014 Oct 14. PMID: 25316793; PMCID: PMC4217458.
- Wilson BC, Weersink RA. The Yin and Yang of PDT and PTT. Photochem Photobiol. 2020 Mar;96(2):219-231. doi: 10.1111/php.13184. Epub 2019 Dec 30. PMID: 31769516.
- Li N, Sun W, Wang Y, Dong C, Yang J, Sun W, et al. Photothermal and chemodynamic combination therapy via a nanoplatform of pH-responsive biomimetic copper peroxide for cancer. ACS Nano. 2020;14(10):13105–13117.
- Gong L, Zhang X, Ge K, Yin Y, Machuki JO, Yang Y, Shi H, Geng D, Gao F. Carbon nitride-based nanocaptor: An intelligent nanosystem with metal ions chelating effect for enhanced magnetic targeting phototherapy of Alzheimer's disease. Biomaterials. 2021 Jan;267:120483. doi: 10.1016/j.biomaterials.2020.120483. Epub 2020 Oct 26. PMID: 33129186.
- Zhou H, Gong Y, Liu Y, Huang A, Zhu X, Liu J, Yuan G, Zhang L, Wei JA, Liu J. Intelligently thermoresponsive flower-like hollow nano-ruthenium system for sustained release of nerve growth factor to inhibit hyperphosphorylation of tau and neuronal damage for the treatment of Alzheimer's disease. Biomaterials. 2020 Apr;237:119822. doi: 10.1016/j.biomaterials.2020.119822. Epub 2020 Jan 24. PMID: 32035322.
- Feng L, Yang X, Shi X, Tan X, Peng R, Wang J, Liu Z. Polyethylene glycol and polyethylenimine dual-functionalized nano-graphene oxide for photothermally enhanced gene delivery. Small. 2013 Jun 10;9(11):1989-97. doi: 10.1002/smll.201202538. Epub 2013 Jan 6. PMID: 23292791.
- Hsieh TY, Huang WC, Kang YD, Chu CY, Liao WL, Chen YY, Chen SY. Neurotensin-Conjugated Reduced Graphene Oxide with Multi-Stage Near-Infrared-Triggered Synergic Targeted Neuron Gene Transfection In Vitro and In Vivo for Neurodegenerative Disease Therapy. Adv Healthc Mater. 2016 Dec;5(23):3016-3026. doi: 10.1002/adhm.201600647. Epub 2016 Nov 2. PMID: 27805786.
- Gao Y, Cheng Y, Chen J, Lin D, Liu C, Zhang LK, Yin L, Yang R, Guan YQ. NIR-Assisted MgO-Based Polydopamine Nanoparticles for Targeted Treatment of Parkinson's Disease through the Blood-Brain Barrier. Adv Healthc Mater. 2022 Dec;11(23):e2201655. doi: 10.1002/adhm.202201655. Epub 2022 Oct 13. PMID: 36153843..
- Cankaya S, Cankaya B, Kilic U, Kilic E, Yulug B. The therapeutic role of minocycline in Parkinson's disease. Drugs Context. 2019 Mar 6;8:212553. doi: 10.7573/dic.212553. PMID: 30873213; PMCID: PMC6408180.
- Liu Y, Xu M, Chen Q, Guan G, Hu W, Zhao X, Qiao M, Hu H, Liang Y, Zhu H, Chen D. Gold nanorods/mesoporous silica-based nanocomposite as theranostic agents for targeting near-infrared imaging and photothermal therapy induced with laser. Int J Nanomedicine. 2015 Jul 28;10:4747-61. doi: 10.2147/IJN.S82940. PMID: 26251596; PMCID: PMC4524460.
- Yuan J, Liu H, Zhang H, Wang T, Zheng Q, Li Z. Controlled Activation of TRPV1 Channels on Microglia to Boost Their Autophagy for Clearance of Alpha-Synuclein and Enhance Therapy of Parkinson's Disease. Adv Mater. 2022 Mar;34(11):e2108435. doi: 10.1002/adma.202108435. Epub 2022 Feb 6. PMID: 35023596.
- Zhao M, Wang R, Yang K, Jiang Y, Peng Y, Li Y, Zhang Z, Ding J, Shi S. Nucleic acid nanoassembly-enhanced RNA therapeutics and diagnosis. Acta Pharm Sin B. 2023 Mar;13(3):916-941. doi: 10.1016/j.apsb.2022.10.019. Epub 2022 Oct 27. PMID: 36970219; PMCID: PMC10031267.
- Herkt M, Thum T. Pharmacokinetics and Proceedings in Clinical Application of Nucleic Acid Therapeutics. Mol Ther. 2021 Feb 3;29(2):521-539. doi: 10.1016/j.ymthe.2020.11.008. Epub 2020 Nov 12. PMID: 33188937; PMCID: PMC7854291.
- Pandey SK, Singh RK. Recent developments in nucleic acid-based therapies for Parkinson's disease: Current status, clinical potential, and future strategies. Front Pharmacol. 2022 Oct 20;13:986668. doi: 10.3389/fphar.2022.986668. PMID: 36339626; PMCID: PMC9632735.
- O'Brien J, Hayder H, Zayed Y, Peng C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol (Lausanne). 2018 Aug 3;9:402. doi: 10.3389/fendo.2018.00402. PMID: 30123182; PMCID: PMC6085463.
- Catalanotto C, Cogoni C, Zardo G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int J Mol Sci. 2016 Oct 13;17(10):1712. doi: 10.3390/ijms17101712. PMID: 27754357; PMCID: PMC5085744.
- Zhu Y, Zhao G, Li Q. Extracellular vesicles derived from microglia treated with monomeric α-synuclein ameliorate neuroinflammation by delivering miRNAs targeting PRAK. Neurosci Lett. 2022;818:137562.
- Li N, Huang Y, Wu Y, Wang Q, Ji P. Extracellular vesicles derived from monomeric α-synuclein-treated microglia ameliorate neuroinflammation by delivery of miRNAs targeting PRAK. Neurosci Lett. 2024 Jan 1;818:137562. doi: 10.1016/j.neulet.2023.137562. Epub 2023 Nov 19. PMID: 37984486.
- McCormack AL, Mak SK, Henderson JM, Bumcrot D, Farrer MJ, Di Monte DA. Alpha-synuclein suppression by targeted small interfering RNA in the primate substantia nigra. PLoS One. 2010 Aug 11;5(8):e12122. doi: 10.1371/journal.pone.0012122. PMID: 20711464; PMCID: PMC2920329.
- Kim YC, Miller A, Lins LC, Han SW, Keiser MS, Boudreau RL, Davidson BL, Narayanan NS. RNA Interference of Human α-Synuclein in Mouse. Front Neurol. 2017 Jan 31;8:13. doi: 10.3389/fneur.2017.00013. PMID: 28197125; PMCID: PMC5281542.
- Amiri A, Barreto G, Sathyapalan T, Sahebkar A. siRNA Therapeutics: Future Promise for Neurodegenerative Diseases. Curr Neuropharmacol. 2021;19(11):1896-1911. doi: 10.2174/1570159X19666210402104054. PMID: 33797386; PMCID: PMC9185778.
- Biehl JK, Russell B. Introduction to stem cell therapy. J Cardiovasc Nurs. 2009 Mar-Apr;24(2):98-103; quiz 104-5. doi: 10.1097/JCN.0b013e318197a6a5. PMID: 19242274; PMCID: PMC4104807.
- Herberts CA, Kwa MS, Hermsen HP. Risk factors in the development of stem cell therapy. J Transl Med. 2011 Mar 22;9:29. doi: 10.1186/1479-5876-9-29. PMID: 21418664; PMCID: PMC3070641.
- Kuroda Y, Wakao S, Kitada M, Murakami T, Nojima M, Dezawa M. Isolation, culture and evaluation of multilineage-differentiating stress-enduring (Muse) cells. Nat Protoc. 2013;8(7):1391-415. doi: 10.1038/nprot.2013.076. Epub 2013 Jun 20. PMID: 23787896.
- Danielyan L, Beer-Hammer S, Stolzing A, Schäfer R, Siegel G, Fabian C, Kahle P, Biedermann T, Lourhmati A, Buadze M, Novakovic A, Proksch B, Gleiter CH, Frey WH, Schwab M. Intranasal delivery of bone marrow-derived mesenchymal stem cells, macrophages, and microglia to the brain in mouse models of Alzheimer's and Parkinson's disease. Cell Transplant. 2014;23 Suppl 1:S123-39. doi: 10.3727/096368914X684970. Epub 2014 Oct 9. PMID: 25302802..
- Salama M, Sobh M, Emam M, Abdalla A, Sabry D, El-Gamal M, Lotfy A, El-Husseiny M, Sobh M, Shalash A, Mohamed WM. Effect of intranasal stem cell administration on the nigrostriatal system in a mouse model of Parkinson's disease. Exp Ther Med. 2017 Mar;13(3):976-982. doi: 10.3892/etm.2017.4073. Epub 2017 Jan 20. PMID: 28450929; PMCID: PMC5403256.
- Yasuhara T, Kameda M, Sasaki T, Tajiri N, Date I. Cell Therapy for Parkinson's Disease. Cell Transplant. 2017 Sep;26(9):1551-1559. doi: 10.1177/0963689717735411. PMID: 29113472; PMCID: PMC5680961.
- Kim TW, Koo SY, Studer L. Pluripotent Stem Cell Therapies for Parkinson Disease: Present Challenges and Future Opportunities. Front Cell Dev Biol. 2020 Aug 6;8:729. doi: 10.3389/fcell.2020.00729. PMID: 32903681; PMCID: PMC7438741.
- Parmar M, Grealish S, Henchcliffe C. The future of stem cell therapies for Parkinson disease. Nat Rev Neurosci. 2020 Feb;21(2):103-115. doi: 10.1038/s41583-019-0257-7. Epub 2020 Jan 6. PMID: 31907406.
- Duan Y, Gong K, Xu S, Zhang F, Meng X, Han J. Regulation of cholesterol homeostasis in health and diseases:from mechanisms to targeted therapeutics. Sig Transduct Target Ther. 2022;7:265.
- Miller KM, Patterson JR, Kochmanski J, Kemp CJ, Stoll AC, Onyekpe CU, Cole-Strauss A, Steece-Collier K, Howe JW, Luk KC, Sortwell CE. Striatal Afferent BDNF Is Disrupted by Synucleinopathy and Partially Restored by STN DBS. J Neurosci. 2021 Mar 3;41(9):2039-2052. doi: 10.1523/JNEUROSCI.1952-20.2020. Epub 2021 Jan 20. PMID: 33472823; PMCID: PMC7939095.
- Pramanik S, Sulistio YA, Heese K. Neurotrophin Signaling and Stem Cells-Implications for Neurodegenerative Diseases and Stem Cell Therapy. Mol Neurobiol. 2017 Nov;54(9):7401-7459. doi: 10.1007/s12035-016-0214-7. Epub 2016 Nov 5. PMID: 27815842.
- Nagatsu T, Sawada M. Biochemistry of postmortem brains in Parkinson's disease: historical overview and future prospects. J Neural Transm Suppl. 2007;(72):113-20. doi: 10.1007/978-3-211-73574-9_14. PMID: 17982884.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.