Medicine Group 2024 December 26;5(12):1597-1601. doi: 10.37871/jbres2046.
Omitting Axillary Dissection in Breast Cancer with Sentinel-Node Metastases. Extending Axillary Surgical De-Escalation
Gilles Houvenaeghel1*, Monique Cohen2 and Alexandre de Nonneville3
2Department of Surgical Oncology, Institut Paoli-Calmettes, CRCM, Marseille, France
3Department of Medical Oncology, CNRS, INSERM, Institut Paoli-Calmettes, CRCM, Marseille, France
Introduction
Axillary stadification for patients with early breast cancer without clinical adenopathy is performed using Sentinel Lymph Node Biopsy (SLNB) for up-front surgery since results of randomized trials comparing ALND versus no ALND for patients with non-involved Sentinel Node (SN) [1]. SLNB has been validated for tumors less than 3 cm, then less than 5 cm [2]. Current data from trials that included a few tumors larger than 5 cm [3-5] and the results of cohort analysis make it possible to consider SLNB for tumors larger than 5 cm. In the French cohort analysis of 28,077 patients, the false negative rate of sentinel lymph node biopsy of tumors 5 cm or larger was not different from that of smaller tumors: the false negative rates were 3.31%, 3.64%, 4.07% and 2.94% for pT1, pT2 < 30 mm, pT2 ≥ 30 mm and pT3 tumors respectively [6].
Since results of ACOSOG Z0011randomized trial [7], omission of complementary ALND had progressively increased for patients with breast conservative treatment with all criteria of this trial for up-front surgery, particularly since results with 10-years follow-up. However, due to some initial bias of this trial and no another trial that included patients with SN macro-metastases, non-inferiority of omission of cALND remain discussed in the absence of high-level scientific evidence. Moreover, ACOSOG Z0011randomized trial had included patients with SN micro-metastases and patients with SN macro-metastases, without planed analysis for these two SN metastases sizes.
Discussion about ALND Omission in Breast Cancer with Sentinel Node Metastases
Omission of completion ALND (cALND) was non-inferior to cALND in the SENOMAC-trial [3], in a larger population than the ACOSOG trial [7], including patients with breast conservative surgery or mastectomy and 1-2 Sentinel-Node (SN) macrometastases: 920 mastectomies and 870 patients with extracapsular-extension, 147 cT3, 561 Grade 3. Preoperative ultrasonography of the axilla was mandatory. Patients with suspicious but non-palpable axillary lymph nodes on ultrasonography were eligible for inclusion, even if metastasis was confirmed by fine-needle aspiration. Non-sentinel-node-metastases rates were 31.3% and 51.3% among patients with 1 or 2 sentinel-node-macrometastases, with pN2 (4 to 9 metastases) in 7 patients (0.5%) and 116 patients (9.9%), in the Sentinel-Node Biopsy (SLNB) only group and in the cALND group, and pN3 (≥ 10 metastases) in 35 patients (3.0%) in the cALND group.
Clinical non-inferiority was defined as 5-year Overall Survival (OS) that was not worse by more than 2.5 percentage points when cALND was omitted. After a median follow-up of 46.8 months, the estimated 5-year Recurrence-Free Survival (RFS), a secondary endpoint, was 88.7% in the cALND group and 89.7% in the SLNB-only group. This trial provides robust evidence that omitting cALND is safe for patients with clinically node-negative T1, T2, or T3 Breast Cancer (BC) and one or two sentinel-node macrometastases who received adjuvant systemic treatment and radiation therapy in accordance with national guidelines.
In luminal breast cancer, adjuvant CDK4/6 inhibitors (eg, abemaciclib) improve invasive Disease-Free Survival (DFS). For patients with cT1-2, grade 1-2 tumors, and one or two sentinel lymph node metastases, cALND remains the only prognostic tool capable of identifying ≥ 4 nodal metastases (pN2–3), which is the only criterion for recommending adjuvant abemaciclib in this situation. Authors aimed to weigh the numbers needed to diagnose by cALND to identify patients with pN2–3 against: 1) the number needed to treat for avoiding one invasive DFS event at 5 years after completing 2 years of adjuvant abemaciclib and 2) the number needed to harm regarding severe or very severe patient-reported arm morbidity at 1 year and arm lymphoedema at 5 years [8]. Among 1,705 patients, 802 (47%) had a cALND and 903 (53%) had a SLNB only. Among 1,342 patients who responded to questionnaires, after a median follow-up of 45∙2 months (IQR 25∙6-59∙8), patient-reported severe or very severe impairment of physical arm function was reported in 84 (13%) of 634 patients who had cALND versus 30 (4%) of 708 who had SLNB only (p < 0∙0001). Eleven patients undergoing cALND resulted in one patient with severe or very severe impairment of physical arm function. Five (1%) of 903 patients in the SLNB alone and 101 (13%) of 802 patients in the cALND group had ≥ 4 nodal metastases, an absolute difference of 12%. Eight patients needed to undergo cALND to identify one candidate for adjuvant abemaciclib by monarchE criteria4. The published 5-year invasive DFS rates from cohort 1 in the monarchE trial are 83.2% (95% CI 81·5-84·7) in the abemaciclib plus endocrine therapy group and 75∙3% (95% CI 73·4–77·2) in the endocrine therapy alone group, resulting in an absolute risk reduction of 7∙9% [9]. Thirteen patients must be treated with abemaciclib to avoid one invasive DFS event at 5 years. Thus, to avoid one invasive DFS event at 5 years with adjuvant abemaciclib, cALND would need to be performed in 104 patients and would result in nine patients having severe or very severe impairment of physical arm function 1 year after surgery.
As a method to potentially identify an indication for abemaciclib, and subsequently avoid invasive DFS events at 5 years with 2 years of adjuvant abemaciclib, cALND carries a substantial risk of severe or very severe arm morbidity and so cALND should be discouraged for this purpose.
Therefore, a potential survival effect of abemaciclib in the group that received cALND in the SENOMAC [3], Z0011 [10], and AMAROS [11] trials could not be evaluated. The proportion of patients with pN2–3 status in the cALND groups was 13·7% in ACOSOG Z0011 [7], 12∙9% in AMAROS [11], and 12.9% in SENOMAC [3], However, no information on the number of patients with grade 3 tumors among these patients in Z0011 and AMAROS is available, which would represent an indication for abemaciclib independent of cALND.
Non-sentinel-node metastases rates in the event of 1 or 2 sentinel macro metastases were 27.3% and 21.2% in the ACOSOG Z0011 and SERC trials [5,11], respectively, and in the event of sentinel micro metastases were 7.6% and 13% in IBCSG 23-01 and AATRM trials [12,13]. The SERC (NCT01717131) non-inferiority phase-3 trial comparing no cALND with cALND, randomized 2,216 patients, including patients with mastectomy and more than two sentinel-node-metastases, focusing on DFS as primary endpoint [14]. Non-sentinel-node metastases rates were 14.5%, 19.3%, and 35.2% for patients without adjuvant chemotherapy, with chemotherapy before cALND and chemotherapy after cALND, respectively [14]. Higher positive-non-sentinel-node rate was significantly associated with chemotherapy after cALND and > 2 involved sentinel nodes. Despite this relatively high rate of non-sentinel node metastases, omission of completion ALND was not associated with a high rate of axillary recurrence due to the effect of tangential radiotherapy fields in the case of conservative treatment and due to the effect of adjuvant systemic treatments [3,7,15] Post-mastectomy-radiotherapy gives at Berg level 1 a dose at least equivalent to the one brought by post-breast-conservative-surgery radiotherapy [16].
The vast majority of patients treated by up-front surgery have endocrine receptors-positive and Her2-negative tumors which present a risk of events which persist after 5 or 7 years. A median follow-up of more than 5 or 7 years is therefore required before concluding that omission of completion ALND is non-inferior in the event of sentinel lymph node involvement. Moreover, when more than two SN present a macro-metastasis, additional ALND is currently recommended. The results of the SERC trial [15] with randomization of additional axillary dissection, including patients with more than two sentinel lymph nodes invaded by a macro metastasis should provide an answer on the non-inferiority of abstaining from additional axillary dissection. SERC final results should be available in the coming months; and may further corroborate and extend the results of the RFS secondary endpoint from the SENOMAC trial.
In a French cohort (NCT02869607; median follow-up 63 months), 5-year Recurrence-Free Survival (RFS) for 2,381 patients with cALND was 88.3%, Vs 82.2% for 127 patients without cALND (p = 0.013). In multivariate analysis, independent factors associated with RFS were age ≥ 75 years, pT > 20mm, grade-3, lympho-vascular-invasion, no endocrine-therapy, SN-number-harvested < 2, and absence of radiotherapy, but neither mastectomy versus breast-conserving surgery nor cALND Vs no cALND showed significant impact.
Validation was achieved for cALND omission in BC with involved SN by micro-metastases with BCS or mastectomy [13]. For patients treated with up-front mastectomy and SN isolated tumor cells (ITC: pN0(i+) sn) or micrometastases (pN1mi sn), evidence for cALND omission remains limited due to underrepresentation in the IBCSG 23-01 trial [13]. In the SENOMIC trial [17], patients with SN micrometastases underwent BCS or mastectomy without cALND, yet the risk of involved non-sentinel nodes remains significant, exposing patients to undertreatment. Adjuvant chemotherapy and Post-Mastectomy Radiotherapy (PMRT) with Regional Nodal Irradiation (RNI) are often not indicated for these patients, unlike those with NSN involvement where these treatments are typically recommended. In the French cohort, for patients treated by mastectomy with sentinel node isolated tumor cells or micrometastases, the impact of cALND omission on survival in BC patients was evaluated. Among 554 BC, the non-SN involvement rate was 13.2%. With a median follow-up of 66.46 months, multivariate analysis showed cALND omission was significantly associated with OS (HR: 2.583, p = 0.043), DFS (HR: 2.538, p = 0.008), and metastasis-free survival (HR: 2.756, p = 0.014). For 161 patients aged ≤ 50 years with ER-positive/Her2-negative, OS and Breast Cancer-Specific Survival (BCSS) were notably impacted by cALND omission (OS HR: 103.47, p = 0.004; BCSS HR: 50.874, p = 0.035). These findings suggest the potential negative prognostic impact of cALND omission in patients with SN micrometastases or ITC. Further randomized trials are needed.
Conclusion
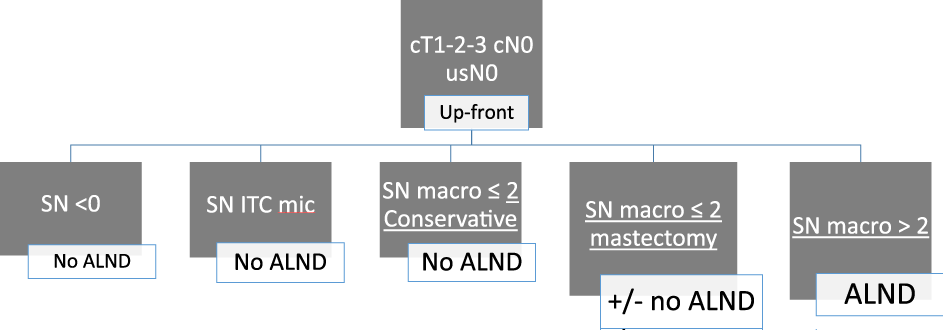
Omission of cALND in patients with clinically node-negative cT1-2-3 BC and 1-2 sentinel-node macro-metastases who received adjuvantfig systemic treatment and radiation therapy seems safe considering the first results of SENOMAC trial (Figure 1).
Further randomized trials results are needed, particularly for patients treated by mastectomy, and particularly without post mastectomy radiotherapy and or without adjuvant chemotherapy.
Systematic cALND should be discouraged when its sole purpose is to identify eligibility for abemaciclib in patients with 1-2 sentinel-node macrometastases.
References
- Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, Weaver DL, Miller BJ, Jalovec LM, Frazier TG, Noyes RD, Robidoux A, Scarth HM, Mammolito DM, McCready DR, Mamounas EP, Costantino JP, Wolmark N; National Surgical Adjuvant Breast and Bowel Project. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007 Oct;8(10):881-8. doi: 10.1016/S1470-2045(07)70278-4. PMID: 17851130.
- Houvenaeghel G, Quilichini O, Cohen M, Reyal F, Classe JM, Mazouni C, Giard S, Carrabin N, Charitansky H, Darai E, Hudry D, Azuar P, Villet R, Gimbergues P, Tunon-DE-Lara C, Lambaudie E. Sentinel lymph node biopsy validation for large tumors. Int J Surg. 2017 Dec;48:275-280. doi: 10.1016/j.ijsu.2017.10.077. Epub 2017 Nov 24. PMID: 29175020.
- de Boniface J, Filtenborg Tvedskov T, Rydén L, Szulkin R, Reimer T, Kühn T, Kontos M, Gentilini OD, Olofsson Bagge R, Sund M, Lundstedt D, Appelgren M, Ahlgren J, Norenstedt S, Celebioglu F, Sackey H, Scheel Andersen I, Hoyer U, Nyman PF, Vikhe Patil E, Wieslander E, Dahl Nissen H, Alkner S, Andersson Y, Offersen BV, Bergkvist L, Frisell J, Christiansen P; SENOMAC Trialists’ Group; SENOMAC Trialists' Group. Omitting Axillary Dissection in Breast Cancer with Sentinel-Node Metastases. N Engl J Med. 2024 Apr 4;390(13):1163-1175. doi: 10.1056/NEJMoa2313487. PMID: 38598571.
- Tinterri C, Canavese G, Gatzemeier W, Barbieri E, Bottini A, Sagona A, Caraceni G, Testori A, Di Maria Grimaldi S, Dani C, Boni L, Bruzzi P, Fernandes B, Scorsetti M, Zambelli A, Gentile D; SINODAR-ONE Collaborative Group. Sentinel lymph node biopsy versus axillary lymph node dissection in breast cancer patients undergoing mastectomy with one to two metastatic sentinel lymph nodes: sub-analysis of the SINODAR-ONE multicentre randomized clinical trial and reopening of enrolment. Br J Surg. 2023 Aug 11;110(9):1143-1152. doi: 10.1093/bjs/znad215. PMID: 37471574; PMCID: PMC10492188.
- Houvenaeghel G, Cohen M, Raro P, De Troyer J, Gimbergues P, Tunon de Lara C, Ceccato V, Vaini-Cowen V, Faure-Virelizier C, Marchal F, Gauthier T, Jouve E, Theret P, Regis C, Gabelle P, Pernaut J, Del Piano F, D'Halluin G, Lantheaume S, Darai E, Beedassy B, Dhainaut-Speyer C, Martin X, Girard S, Villet R, Monrigal E, Hoyek T, Le Brun JF, Colombo PE, Tallet A, Boher JM; SERC trial group. Sentinel node involvement with or without completion axillary lymph node dissection: treatment and pathologic results of randomized SERC trial. NPJ Breast Cancer. 2021 Oct 8;7(1):133. doi: 10.1038/s41523-021-00336-3. PMID: 34625562; PMCID: PMC8501060.
- Houvenaeghel G, Cohen M, Sabiani L, Martino M, Heinemann M, Chauvet MP. Sentinel lymph node biopsy validation for pt3 breast cancer. Int J Surg.
- Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, Ollila DW, Hansen NM, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, Hunt KK, Morrow M. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA. 2017 Sep 12;318(10):918-926. doi: 10.1001/jama.2017.11470. PMID: 28898379; PMCID: PMC5672806.
- de Boniface J, Appelgren M, Szulkin R, Alkner S, Andersson Y, Bergkvist L, Frisell J, Gentilini OD, Kontos M, Kühn T, Lundstedt D, Offersen BV, Olofsson Bagge R, Reimer T, Sund M, Christiansen P, Rydén L, Filtenborg Tvedskov T; SENOMAC Trialists’ Group. Completion axillary lymph node dissection for the identification of pN2-3 status as an indication for adjuvant CDK4/6 inhibitor treatment: a post-hoc analysis of the randomised, phase 3 SENOMAC trial. Lancet Oncol. 2024 Sep;25(9):1222-1230. doi: 10.1016/S1470-2045(24)00350-4. Epub 2024 Aug 6. PMID: 39121881.
- Rastogi P, O'Shaughnessy J, Martin M, Boyle F, Cortes J, Rugo HS, Goetz MP, Hamilton EP, Huang CS, Senkus E, Tryakin A, Cicin I, Testa L, Neven P, Huober J, Shao Z, Wei R, André V, Munoz M, San Antonio B, Shahir A, Harbeck N, Johnston S. Adjuvant Abemaciclib Plus Endocrine Therapy for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative, High-Risk Early Breast Cancer: Results From a Preplanned monarchE Overall Survival Interim Analysis, Including 5-Year Efficacy Outcomes. J Clin Oncol. 2024 Mar 20;42(9):987-993. doi: 10.1200/JCO.23.01994. Epub 2024 Jan 9. Erratum in: J Clin Oncol. 2024 Jun 10;42(17):2111. doi: 10.1200/JCO.24.00711. Erratum in: J Clin Oncol. 2025 Jan;43(1):113. doi: 10.1200/JCO-24-02469. PMID: 38194616; PMCID: PMC10950161.
- Houvenaeghel G, Heinemann M, Classe JM, Bouteille C, Gimbergues P, Azuar AS, Martino M, Tallet A, Cohen M, de Nonneville A. Omission of Completion Axillary Lymph Node Dissection for Patients with Breast Cancer Treated by Upfront Mastectomy and Sentinel Node Isolated Tumor Cells or Micrometastases. Cancers (Basel). 2024 Jul 26;16(15):2666. doi: 10.3390/cancers16152666. PMID: 39123393; PMCID: PMC11312260.
- Bartels SAL, Donker M, Poncet C, Sauvé N, Straver ME, van de Velde CJH, Mansel RE, Blanken C, Orzalesi L, Klinkenbijl JHG, van der Mijle HCJ, Nieuwenhuijzen GAP, Veltkamp SC, van Dalen T, Marinelli A, Rijna H, Snoj M, Bundred NJ, Merkus JWS, Belkacemi Y, Petignat P, Schinagl DAX, Coens C, van Tienhoven G, van Duijnhoven F, Rutgers EJT. Radiotherapy or Surgery of the Axilla After a Positive Sentinel Node in Breast Cancer: 10-Year Results of the Randomized Controlled EORTC 10981-22023 AMAROS Trial. J Clin Oncol. 2023 Apr 20;41(12):2159-2165. doi: 10.1200/JCO.22.01565. Epub 2022 Nov 16. PMID: 36383926.
- Solá, M.; Alberro, J.A.; Fraile, M.; Santesteban, P.; Ramos, M.; Fabregas, R.; Moral, A.; Ballester, B.; Vidal, S. Complete axillary lymph node dissection versus clinical follow-up in breast cancer patients with sentinel node micrometastasis: Final results from the multicenter clinical trial AATRM 048/13/2000. Ann. Surg. Oncol. 2013, 20, 120–127.
- Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, Baratella P, Chifu C, Sargenti M, Intra M, Gentilini O, Mastropasqua MG, Mazzarol G, Massarut S, Garbay JR, Zgajnar J, Galatius H, Recalcati A, Littlejohn D, Bamert M, Colleoni M, Price KN, Regan MM, Goldhirsch A, Coates AS, Gelber RD, Veronesi U; International Breast Cancer Study Group Trial 23-01 investigators. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013 Apr;14(4):297-305. doi: 10.1016/S1470-2045(13)70035-4. Epub 2013 Mar 11. Erratum in: Lancet Oncol. 2013 Jun;14(7):e254. PMID: 23491275; PMCID: PMC3935346.
- Houvenaeghel G, Cohen M, Raro P, De Troyer J, Gimbergues P, Tunon de Lara C, Ceccato V, Vaini-Cowen V, Faure-Virelizier C, Marchal F, Gauthier T, Jouve E, Theret P, Regis C, Gabelle P, Pernaut J, Del Piano F, D'Halluin G, Lantheaume S, Darai E, Beedassy B, Dhainaut-Speyer C, Martin X, Girard S, Villet R, Monrigal E, Hoyek T, Le Brun JF, Colombo PE, Tallet A, Boher JM; SERC trial group. Sentinel node involvement with or without completion axillary lymph node dissection: treatment and pathologic results of randomized SERC trial. NPJ Breast Cancer. 2021 Oct 8;7(1):133. doi: 10.1038/s41523-021-00336-3. PMID: 34625562; PMCID: PMC8501060.
- Houvenaeghel G, de Nonneville A, Cohen M, Classe JM, Reyal F, Mazouni C, Chopin N, Martinez A, Daraï E, Coutant C, Colombo PE, Gimbergues P, Chauvet MP, Azuar AS, Rouzier R, de Lara CT, Muracciole X, Agostini A, Gonçalves A, Lambaudie E. Isolated ipsilateral local recurrence of breast cancer: predictive factors and prognostic impact. Breast Cancer Res Treat. 2019 Jan;173(1):111-122. doi: 10.1007/s10549-018-4944-2. Epub 2018 Sep 20. PMID: 30238274.
- Nicolas C, Petit C, Tallet A, Boher JM, Varela Cagetti L, Favrel V, Gonzague Casabianca L, Guenole M, Mailleux H, Darreon J, Bannier M, Cohen M, Sabiani L, Tallet C, Teyssandier C, Gonçalves A, De Nonneville A, Lopez Almeida L, Coste N, Tyran M, Houvenaeghel G. Does Breast Surgery Type Alter Incidental Axillary Irradiation? A Dosimetric Analysis of the "Sentinel Envahi et Randomisation du Curage" SERC Trial. Cancers (Basel). 2024 Mar 19;16(6):1198. doi: 10.3390/cancers16061198. PMID: 38539532; PMCID: PMC10969031.
- Andersson Y, Bergkvist L, Frisell J, de Boniface J. Omitting completion axillary lymph node dissection after detection of sentinel node micrometastases in breast cancer: first results from the prospective SENOMIC trial. Br J Surg. 2021 Sep 27;108(9):1105-1111. doi: 10.1093/bjs/znab141. PMID: 34010418.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.