Medicine Group. 2024 July 20;5(7):797-816. doi: 10.37871/jbres1958.
Noninvasive Vital Signs Monitoring in the Neonatal Intensive Care Unit
Chengyang Qian, Junye Li and Michelle Khine*
Abstract
Patients in the Neonatal Intensive Care Unit (NICU) have high mortality and morbidity rates due to their low birth weight or being preterm. Due to their vulnerability, neonates require close monitoring so that timely treatment can be performed. However, current, state-of-the-art technologies for continuous vital sign monitoring in the NICU are not optimal due to the use of wires and adhesives. Wires impede the natural movement of neonates, disallow intimate kangaroo care, and impose inconvenience on daily caretaking. The use of adhesives can damage neonates’ skin during removal. To address these problems, many wearable systems or contactless setups have been proposed for their respective vital signs. In this review, an overview of these newer technologies, along with their working principles, is discussed.
Introduction
About 2.3 million babies died worldwide within 28 days after their births in 2021; most of them were either born with low birth weight or preterm [1,2]. Those born preterm or low birth weight have significantly higher risks of neonatal morbidity and mortality due to immature organ function or external pathogens [3,4]. These infants are often admitted into the neonatal intensive care unit (NICU) after birth so that their vital signs can be closely monitored for timely treatment [5]. Most commonly monitored vital signs include Heart Rate (HR), Blood Pressure (BP), Respiration Rate (RR), blood Oxygen level (SpO2), and Temperature (T) [6,7]. These vital signs can be used for the prediction and diagnosis of conditions that are lethal to neonates; for example, abnormal HR can indicate a potential infection and irregular RR can imply hypoxemia [6,8].
However, current setups for vital sign monitoring are not optimal (Figure 1). Wires and catheters impede the natural movement of neonates, impose inconvenience on daily caretaking, and, most importantly, discourage kangaroo care, a type of intimate physical bonding between parents and infants that is known to improve short-term physiological stability and impart long-term health benefits [9,10]. Some invasive equipment, such as the arterial catheter, not only causes infection but also leads to psychosomatic problems and mental disorders in neonates due to their painful installation [5,11]. Noninvasive transducers and electrodes require adhesives to attach onto the neonate’s skin firmly. Since neonates have thinner skin and weaker skin structure, the removal of those electrodes or transducers usually damages neonates’ skin and promotes infection [12].
Recent progress in wearable electronics has the potential to revolutionize vital sign monitoring in the NICU. The fabrication of point-of-care sensors and diagnostics on soft and flexible substrates minimize the stiffness mismatch with the skin, leading to more comfortable wearability of those devices [13-15]. The soft and flexible substrates allow for conformal contact with skin and thus, obtain high-resolution signals with less motion artifacts [16]. Combined with built-in batteries, wireless transmission units, and corresponding circuit boards, these sensors can operate without wires and provide real-time monitoring of vital signs. Typically used elastomer substrates, such as Polydimethylsiloxane (PDMS) and Ecoflex, are ideal interfaces with neonate’s skin since they adhere onto the skin via Van Der Waals force, effectively eliminating the need for adhesives [17,18]. In addition to wearable electronics, contactless methods, such as radar, thermal imaging, and optics, can also bypass the shortcomings in traditional vital sign monitoring setups in the NICU [19-21].
In this review, we will explain the clinical utility of monitoring each vital sign and discuss the current gold standard for each vital sign along with their respective drawbacks. A survey of burgeoning technologies, along with their working principles and current state of development, is then discussed. Finally, we explore potential improvements and recommendations to help facilitate their clinical adoption.
Heart Rate (HR) monitoring in neonates
Electrocardiography (ECG) is an important bio potential in the NICU since it is not only a useful technique for measuring HR but also effective at examining the rhythm of heartbeats [22]. In neonates, ECG is an effective method for screening congenital heart disease and long QT syndrome, a symptom that is known to cause 10% of sudden infant death syndrome [23,24]. Despite being a noninvasive method, ECG typically uses adhesive-to-skin attachment and conductive gel for better skin-electrode contact. However, ECG gel is known to cause irritation, and the adhesives often cause skin damage during removal [12,25].
ECG-based HR monitoring method using novel electrodes: Many methods have already been proposed to circumvent the need for adhesives and gels. Kim YS, et al. [26] fabricated electrodes on Ecoflex that can adhere onto skins via Van Der Waals interaction. By mixing the Ecoflex Gel with Ecoflex 0030 for the substrate material, the device demonstrated excellent adhesion and high conformality with skin, effectively eliminating the need for adhesives and gels. Chung HU, et al. [27] used carbon black in PDMS to replace wet conductive gel. Textile-based electrodes are also desirable due to their operation without gel and application using only pressure where no adhesive is needed. Yapici MK, et al. [28] fabricated electrodes by dipping plain textiles into a graphene oxide solution, while Chen W, et al. [29] achieved textile electrodes by coating or printing conductive material onto regular textiles. Compared with the electrodes mentioned above, capacitive electrodes are non-contact sensors that utilize capacitive coupling to detect ECG, and they can usually be integrated into clothes and used as dielectric for contact with skin [30-34]. Tang Y, et al. [30] further improved the capacitive electrodes such that common motion artifacts could be eliminated.
Other innovative HR monitoring methods: Besides the ECG-based method, other methods also exist for HR monitoring. Although these methods avoid the use of adhesives and gels, they will not be able to derive extra information compared with the ECG-based method. More importantly, the devices used will occupy additional spaces on neonates whose bodies are already small. For example, ECG can be used to obtain respiration rate and derive blood pressure information with the help of PPG, respectively. The mechanisms will be elaborated in the subsequent sections in detail. Despite the infrequent use of these HR monitoring methods, they might be applicable in other contexts.
HR can be obtained by directly listening to the palpation of the heart using regular stethoscopes or digital stethoscopes that specifically detect the frequency range of heartbeats [35-37]. However, this method requires constantly placing the stethoscope in the right place which not only makes it hard to obtain continuous HR information but also competes with the more versatile ECG devices for the same body area. Perhaps, the commercialized Eko digital stethoscope could avoid the competition for the same body area since it combines the function of ECG and regular stethoscope [38]. Eko digital stethoscope can even transfer the captured information wirelessly and therefore, has the potential for continuous monitoring if it can be firmly attached.
Doppler ultrasound emits high-frequency sound waves and their difference with the reflected waves to assess HR, but it requires the use of gel for optimal contact and holding the device in place, as cumbersome as the stethoscope [39]. Electrical velocimetry measures the impedance changes associated with cardiac cycles and uses that change to infer HR, but it requires one more electrode to operate compared with common ECG [40,41]. Laser Doppler points a single-point laser beam toward neonates’ chests to detect the small movements due to heartbeats and can be even further exploited for respiration rate derivation [42].
In addition to directly monitoring heart palpation, pulsatile blood flow in the artery or tissue can also be equivalently used for HR assessment. Pulsatile blood flow can be detected using mechanical-based sensors via their deflections when placed above artery pulse points or PPG-based sensors via measuring blood volume change in local tissues. Many of them play crucial roles in not only HR detection but also continuous blood pressure monitoring. To keep this section concise, their working principles are further elaborated in detail in section 2.2.
Continuous Blood Pressure (BP) monitoring in neonates
BP is one of the most important vital signs to monitor because it is a direct indication of cardiovascular health and proper organ perfusion [43]. Due to their immature cardiovascular systems, premature or critically ill neonates with abnormal BP are not rare [44,45]. In the first 20 hours of their lives, 20 % of premature neonates are diagnosed with hypotension (low BP) [46]. Hypotension is typically accompanied by shock, a symptom defined by improper tissue and organ perfusion [45]. Neonates with sustained hypotension are at double the risk of having intraventricular hemorrhages, a complication that can cause long-term neurological damage [47]. Compared with hypotension, hypertension (high BP) cases are only seen in 2-3% of NICU admissions, and therefore, its onset is frequently neglected due to its rarity [45,48]. Neonatal hypertension can cause left ventricular hypertrophy, vascular injury, encephalopathy, and hypertensive retinopathy [49]. Hypertension during the neonatal period can even lead to chronic cardiovascular and renal disease beyond infancy [49,50]. As such, BP should be monitored closely due to its potential short-term and long-term effects on neonates.
Although many methods are used clinically for neonate blood pressure measurement, they each have significant limitations and drawbacks. A sphygmomanometer is one of the most widely used BP measurement methods for neonates [44]. It is an oscillometer method that involves cuff inflation and deflation to determine systolic and diastolic BP, respectively [44,51]. Despite being non-invasive, this method can cause discomfort for neonates due to the application of high pressure; it is also more prone to low accuracy and user error [44,52-54]. According to Dionne N, et al. [52] a cuff whose width is half of arm circumference will provide the most accurate BP results, but the cuffs in NICU usually don’t fit well enough to provide accurate BP. Additionally, sphygmomanometers only offer intermittent measurements which cannot provide continuous hemodynamic information so that timely treatment can be performed. The current gold standard for continuous BP monitoring in neonates involves using an indwelling intra-arterial catheter. In this method, a catheter with a built-in pressure sensor is inserted into the umbilical or peripheral artery for direct pressure monitoring [44,51-53]. Due to its invasiveness, complications such as thrombosis and infection can occur, and the risks of those complications are especially high for neonates due to their vulnerabilities [55]. In contrast, current state-of-the-art wearable devices can provide not only noninvasive but also continuous BP information.
Mechanical-based wearable BP device: It is well-known that arterial pulse wave is closely related to BP waveform and therefore, accurate capture of arterial pulse wave can be used to infer BP values [56,57]. Multiple sensing modalities were developed on flexible substrates for better wearability, and among them, the most common modalities are capacitive, piezoresistive, and piezoelectric [54,58-62]. Upon placement on top of the artery, these sensors experience periodic deflection due to arterial pulse and convert mechanical deflection into changes in capacitance, resistance, and voltage, respectively. Those changes in electrical signals are equivalent to arterial pulse waveform and can be converted to continuous BP via further signal processing.
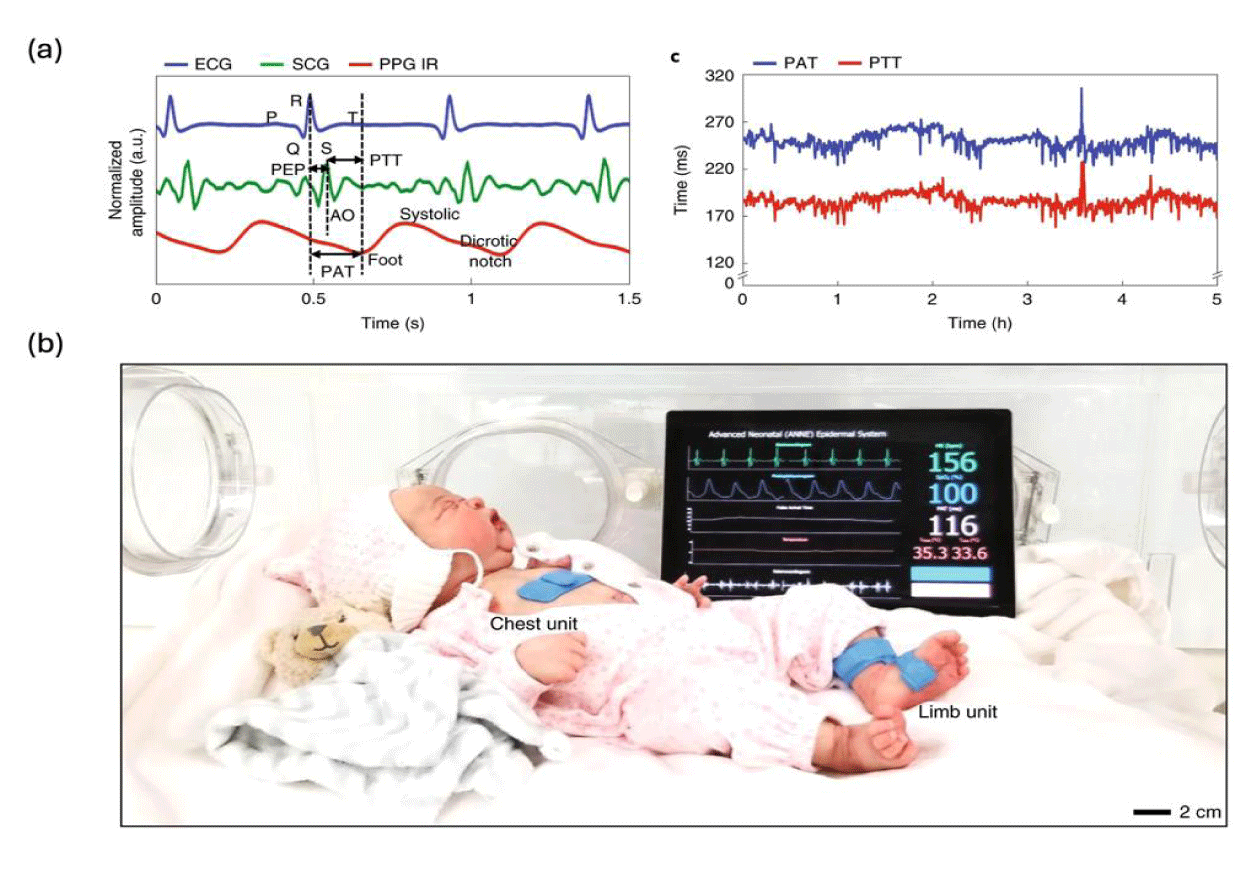
Among all the published works, Rao A, et al. [54] clinically demonstrated that capacitance-based wearable BP sensors can be used on neonates. In their work, a highly sensitive capacitive sensor was achieved using a micro-pyramid as a dielectric and used to collect pulse waveform from the wrist/foot of neonates. Using artificial neural network techniques, an algorithm was trained and could be successfully used to infer blood pressure information from pulse waveform. Despite the lack of clinical validation, other pressure sensors can still be potentially used on neonates since they used either PDMS or Ecoflex as their substrates. Qian C, et al. [58] utilized iontronic dielectric and micro-ridge to enhance their capacitive sensor sensitivity. Using an initial calibration and a 4th-order Butterworth band-pass filter (cutoff frequency of 0.5 and 10 Hz), sustained beat-to-beat BP could be derived. Abiri A, et al. [63] designed an algorithm based on a novel intra-beat biomarker, diastolic transit time (the time span between the systolic peak and the next diastolic trough). In this work, blood pressure change associated with diastolic transit time was quantified and then used to convert the capacitive signal into absolute blood pressure. The algorithm was used to process the hemodynamic waveform obtained from a capacitive pressure sensor and it demonstrated better results than other widely studied algorithms [61,63]. Compared with their respective ground truth, all the works presented above gave systolic BP, diastolic BP, and mean BP that have mean biases less than 5 mmHg and standard deviation less than 8 mmHg, meaning they are interchangeable with an arterial catheter and meet AAMI standard [64,65]. The performances of all the devices mentioned above are summarized in table 1.
| Table 1: All the novel methods for noninvasive and continuous BP monitoring and their performance compared with their respective ground truth. Abbreviations: SBP (Systolic Blood Pressure); DBP (Diastolic Blood Pressure); MAP (Mean Arterial Pressure); MB (Mean Bias); SD (Standard Deviation). | ||||||
| Authors | Device Used | Processing Methods | SBP MB ± SD (mmHg) | DBP MB ± SD (mmHg) | MAP MB ± SD (mmHg) | Ground truth |
| Rao A, et al. [54] | Capacitive Sensor | Artificial neural network | -0.6 ± 9.2 | -0.4 7.9 | -0.6 ± 7.8 | Arterial catheter |
| Qian C, et al. [58] | Capacitive Sensor | Band-pass filter | 0.48 ± 5.57 | 1.43 ± 3.66 | 1.59 ± 2.96 | Caretaker |
| Abiri A, et al. [63] | Capacitive Sensor | Diastolic transit time | 0.05 ± 3.07 | -0.21 ± 2.47 | -0.12 ± 2.35 | Arterial catheter |
| Chung HU, et al. [27] | ECG + PPG/ SCG + PPG | PAT/PPT | 1.6±7.99 / -0.04±7.86 | / | / | Arterial catheter |
| Li J, et al. [66] | Piezoelectric Sensor | PTT + Extreme gradient boosting | -0.05±4.61 | 0.11+3.68 | / | BioPAC |
| AAMI [64] | / | / | 5 ± 8 | 5 ± 9 | 5 ± 8 | Arterial catheter |
Multi-Signal-Based Wearable BP Device: Photoplethysmography (PPG) uses a photoelectric technique to detect the change in blood volume [67]. The detected change in blood volume produces waveforms that are morphologically similar to the BP waveform and therefore, the PPG signal can be potentially calibrated into BP information [68-73]. However, PPG signals usually have poor quality due to the effect of ambient light, skin tone, and low-frequency baseline wandering [63]. In addition, PPG signals require heavy pre-processing (moving average filters, frequency filters, and noise-reduction techniques) to be usable [67,68,74-77]. Most importantly, PPG needs high applanation pressure to obtain high-resolution waveforms with proper morphology, and high applanation pressure is known to cause gangrene in neonates [78-80]. In summary, PPG alone cannot be used in neonates for Continuous Noninvasive Blood Pressure (CNBP) monitoring.
Despite its limitations by itself, PPG can be used for CNBP with the help of ECG or another PPG. To avoid the high applanation pressure, many proposed to analyze PPG signals’ temporal dimension to obtain Pulse Wave Velocity (PWV) and use PWV for BP estimation [63,79,80]. PWV is defined as the travel speed of pressure pulse within the artery and its relationship with BP is defined by the Moens-Kortweg equation [81,82]. Proposed by Poon C, et al. and Chen W, et al. [83-85] Pulse Transit Time (PTT) and Pulse Arrival Time (PAT) are the most commonly used methods to obtain PWV. In the PTT method, two synchronized PPG devices are placed at two different artery sites and PWV is calculated using their separation distance and the travel time of the pressure pulse [84]. In the PAT method, an ECG device is used to replace one of the PPG devices in the PTT method and the same method is used for PWV calculation [83,85]. Their respective derivations are shown in figure 2a. Chung HU, et al. [18,27] fabricated ECG and PPG devices on soft substrates and used the PAT method to obtain continuous blood pressure on neonates (Figure 2b). The PAT and PTT can also be used on mechanical-based wearable BP devices. Li J, et al. [66] fabricated a device with two piezoelectric sensors placed on two different arterial sites and created a machine-learning model that was trained using PTT and other waveform features. Both works gave resulting BPs that met the AAMI standard, and their respective performances are also included in table 1.
Respiration Rate (RR) monitoring in neonates
RR is an important vital signal because it not only indicates a well-functional ventilatory system but also can be used for the prediction, diagnosis, and management of respiratory and non-respiratory diseases [86]. RR needs to be closely monitored in neonates since their vulnerability and immature brain mechanisms that regulate respiratory rhythms are known to cause irregular breathing patterns [86-88]. Irregular RR usually precedes cardiopulmonary arrest and sudden infant death syndrome, two acute diseases that are known to cause sudden death in neonates [88-91]. Respiratory distress syndrome and apnea, two respiratory diseases that respectively cause chronic lung disease and neurodevelopmental impairment, are frequently seen in neonates [92-94]. Their symptoms are rapid breathing and pause in respiration and therefore, monitoring of RR can be used for their diagnosis. Due to its predictive and diagnostic values, RR is closely monitored in the NICU.
There are many ways to monitor respiration rates in neonates, but they all have their respective drawbacks. Thermistor detects respiration rate using a built-in resistor that changes its resistance when heated up by warm breath. However, it is placed in front of the neonate’s nares and thus, can cause apneas since it obstructs the upper respiratory airway [21,95]. Capnography tracks CO2 change incurred from periodic exhalation and uses it for RR monitoring [86,96]. Yet, capnography has low accuracy due to air leaks in endotracheal tubes and low CO2 production in neonates [97]. Chest impedance detects RR via monitoring chest impedance change incurred by period air filling during regular breath, but it can be confounded by blood volume change in the heart [98]. Belt-based sensors monitor RR by detecting chest expansion, but they are known to cause discomfort when worn for long times [19,99]. Although PPG can be used to derive RR using various signal processing techniques, it still needs to use high-resolution waveforms where high applanation pressure is required [100-111]. In neonates, clip-on PPG and high applanation pressure are known to cause gangrene [78].
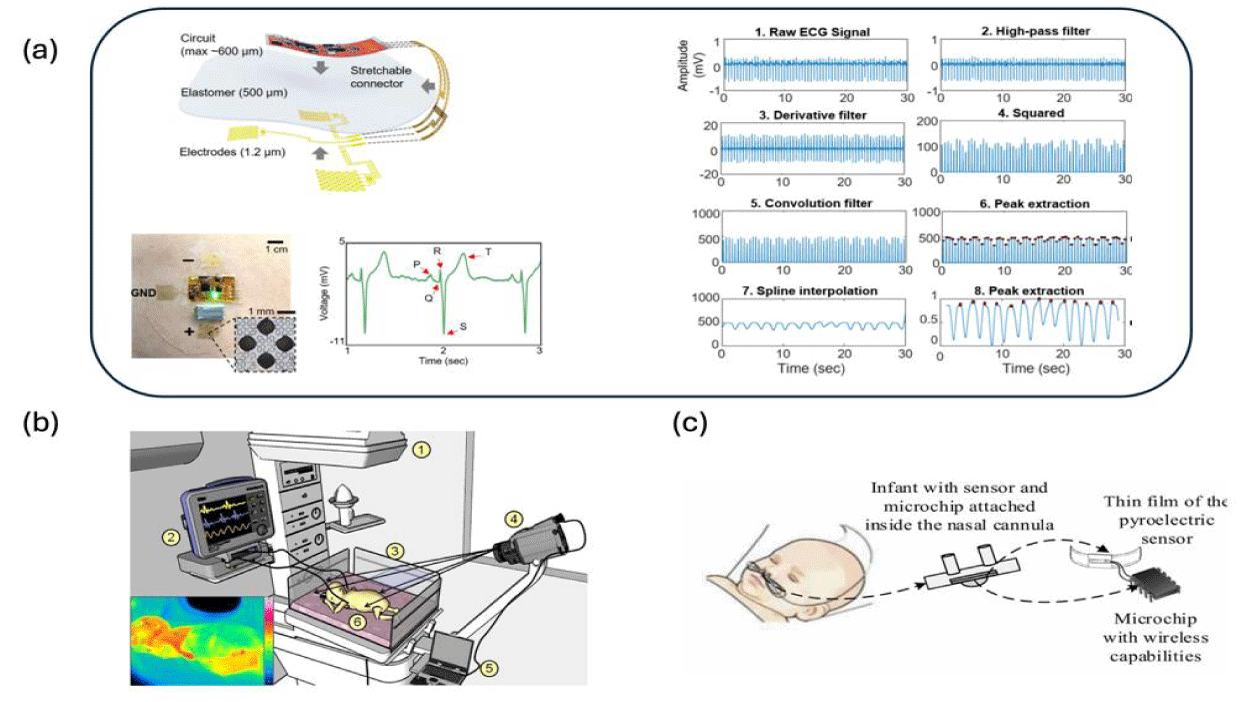
ECG-Based RR Sensor: RR can be derived from ECG using either heart rate change caused by respiration (RSA method), or R-peak amplitude change due to regular chest movements during breathing (EDR method) [112]. However, traditional ECG requires cable to connect to the electrode, imposing challenges on daily caretaking and contact between parents and their babies [9]. Additionally, the uses of gel and adhesive on the ECG electrode are known to cause skin irritation if worn for long times and damage to neonates’ skin during removal, respectively [113,114]. To avoid these problems, Chung HU, et al. and Kim YS, et al. [27,115] designed wireless systems that could operate without any cable; PDMS and Ecoflex were used as substrates so that their devices could interact with skin via Van Der Waals force without using adhesive or gel. They used the EDR method and the RSA method to obtain RR, but these two methods can be used interchangeably if ECG signals are obtained. The design of Kim YS, et al. [26] is shown in figure 3a, along with a demonstration of their EDR method.
Contactless RR Sensor: Despite their more complicated setup, contactless methods avoid the discomfort associated with contact-based devices. They are usually radar-based, optical-based, and thermal-imaging-based, with the generic setup shown in figure 3b [116].
Radar-based devices track chest expansion via the Doppler effect and convert the detected expansion into RR [19,117-120]. Dong S, et al. [19] designed a radar-based IoT system and demonstrated the system was capable of monitoring RR in patients with apnea during their sleep. Their measured RRs had high correlations with ground truth for both Rapid Eye Movement (REM) sleep and non-REM sleep. Gleichauf J, et al. [119] proposed to use a 3D camera to enhance their radar sensor and validated their device with a neonatal simulator. Their system demonstrated precise RR monitoring when subjects’ RR was within the range of 20-60 breaths per minute. Beltrao G, et al. [120] performed RR measurements on neonates using radar and processed the signal via the time-frequency decomposition method. In their studies, neonates in prone position were shown to have the most accurate RR results.
Using optical-based methods, movements of certain body parts are filmed and analyzed to derive RR [20,114,121-125]. Rossol SL, et al. [125] analyzed the footage of neonates using a novel algorithm that detects micromotion and stationarity. The resulting RRs and those obtained from the ECG-based method had a correlation value of 0.948. Sun Y, et al. [114] obtained RR from videos of neonates that were analyzed using the deep flow-based method. The obtained RRs had a correlation value of 0.74 with those obtained from chest impedance.
Thermal-imaging-based methods detect temperature change induced by respiration and can be used to infer RR [21,126-128]. Abbas AK, et al. [21] analyzed the real-time infrared thermography around neonates’ nostrils and used it to derive RR. They successfully detected a 0.3 to 0.5 oC temperature difference between the inhalation and exhalation phases of neonates. Pereira CB, et al. [128] utilized infrared thermography and developed an algorithm that did not target any region of interest on the body. They validated their method on 8 neonates and obtained a root-mean-square error of 4.15 ± 1.44 breaths/minute. In addition to these methods that use one thermal camera, multi-camera systems were also proposed to enhance the accuracy of RR monitoring. Maurya L, et al. [127] proposed to use both visible and thermal images for more accurate RR extraction and their method gave an average error of 1.5 breath/ minute on neonates. Lorato I, et al. [126] used multiple infrared cameras on neonates and obtained a mean absolute error of 2.07 breaths/minute.
Airflow-based RR sensor: Airflow due to breathing can be monitored and used to approximate RR [129]. Piezoelectric and capacitive sensors were made to detect the mechanical disturbance caused by airflow [130,131]. The warm air in the airflow can also cause pyroelectric material to generate a transient voltage that can be used for RR sensing (Figure 3c)[132]. Their form factors can be further optimized via microfabrication techniques to avoid obstructive apnea associated with regular thermistors.
Tidal Volume (TV) extrapolation from existing RR sensors: TV is defined as air volume being exchanged during regular breathing cycles [133]. Although TV is not strictly a vital sign, it is usually closely used with RR for better identification of common abnormalities such as hypoventilation, apnea, bradypnea, and tachypnea [133]. For neonates, TV measurement is important as clinicians need to ensure enough oxygenation is delivered during active ventilation [134]. Typically, clinicians use chest expansion to estimate TV, but it is inaccurate [135]. The schemes used above for RR sensing can be further extrapolated for TV evaluation. Soliman MM, et al. [136] demonstrated that ECG-derived respiratory signals and seismocardiogram-derived respiratory signals can be used to train machine learning models and predict TV, despite the Pearson correlation coefficient being only 0.61. Laufer B, et al. [137] demonstrated contactless camera setup could be used for motion capture and upper-body circumference derivation. They demonstrated a high correlation between upper body circumferences and spirometer volumes, the gold standard for measuring TV. Mechanical-based sensors can also be used for TV sensing. Chu M, et al. [138] proposed to use piezoresistive strain sensors on the ribcage and abdomen to monitor their expansion. The changes in strains were demonstrated to be highly correlated to readings from the spirometer and therefore, can be used to infer TV. Besides piezoresistive sensors, piezoelectric, triboelectric, and capacitive sensors can all conceptually be used for the same purpose if their applanations can be engineered so that they don’t require a bulky belt and adhesives to wear [139-141].
Blood oxygen level (SpO2) monitoring in neonates
In preterm neonates, their SpO2 needs to be closely monitored so that it can be kept at an optimal range, from 89% to 95% [142]. Hypoxia can occur when SpO2 falls too low, causing tissue and brain damage [143,144]. On the other hand, high SpO2 leads to hyperoxia and excessive reactive oxygen species generation, which can cause tissue damage through reaction with lipids, DNAs, proteins, etc [145,146]. SpO2 can be measured using an invasive arterial line that draws blood at regular intervals and analyzes the oxygen content inside [147]. PPG (or pulse oximetry) usually tracks deoxyhemoglobin and oxyhemoglobin at 600 nm and 940 nm wavelength, respectively, and determines blood oxygen levels noninvasively by calculating their respective concentration [148]. PPGs are usually clipped onto the peripheral arteries such as fingers and toes, but neonates have low perfusion in their peripheral arteries and therefore, the measurement can be inaccurate [149,150]. Additionally, PPG’s clip-on scheme can cause gangrene [78,151].
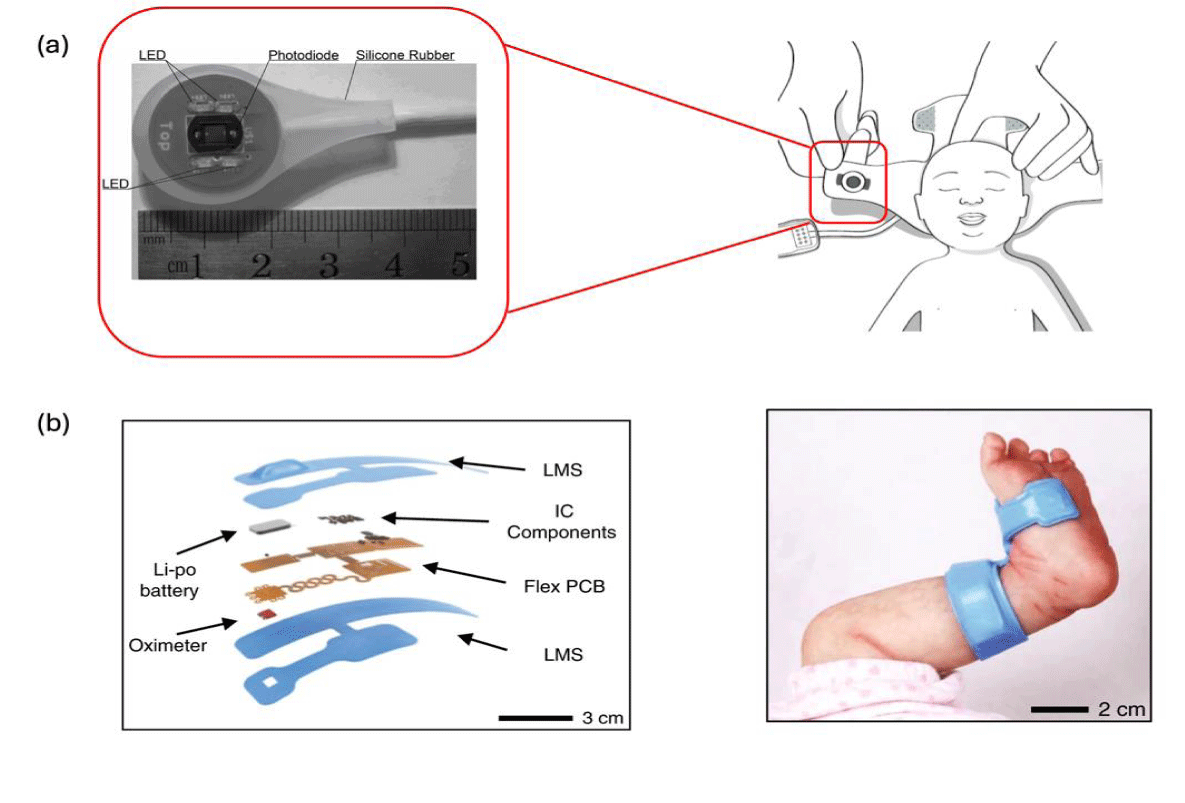
To take advantage of PPG’s noninvasive approach, avoid tissue damage, and circumvent the inaccurate measurement from peripheral arteries, new PPG devices were developed to measure from proximal locations. Compared with peripheral locations where perfusion is not only inherently weaker but is also further reduced by conditions such as infection and use of inotropes, proximal locations, such as the brain, preserve blood flow due to their relative importance [152-156]. In fact, many studies have proven that PPG signals taken from foreheads via reflectance are as accurate as those that are taken from peripheral digits via transmission [157-159]. Although many published works only showed how their proposed PPG device could track heart rate, they have the same potential for tracking SpO2. Hayes-Gill BR, et al. [160]. designed a reflectance-based PPG in a silicone cover for more comfortable wearability and could be used for SpO2 monitoring on a neonate’s forehead (Figure 4a) They further designed a T-shaped band so that their device could be easily mounted onto foreheads [161]. Other cap-based mounting methods were also proposed by Stockwell SJ, et al. and Proenca M, et al. [157,162]. Proenca M, et al. [157] demonstrated their design had a mean error of 0% and a 95% confidence interval of 3.8%. Weteringen W, et al. [163] added optical transcutaneous oxygen tension and electrochemical transcutaneous carbon dioxide measurement techniques on top of existing reflectance-based PPG. The additional functions allowed better measurement validity for long-term monitoring of SpO2. Their measured oxygen saturation had a high correlation (r = 0.979) with arterially sample oxygen saturation.
Despite being relatively peripheral and therefore less optimal, palm, sole, ankle and wrist can also be used for obtaining SpO2 [150]. Chung HU, et al. [27] designed PPG on a flexible circuit board that could bend and conform with body curvature so that high-quality signals could be obtained (Figure 4b). They installed their device on neonates’ feet and obtained PPG signals from their soles. Compared with the gold standard, the result had a respective mean bias and root mean square accuracy of 0.11% and 2.98%, meeting the FDA’s standard of less than 3.5% root mean square accuracy.
Temperature
Like the blood oxygen level, neonates’ temperatures also need to be closely monitored so that they can be kept at an optimal range, between 36.5 and 37.5 °C [164]. High temperature can be an indication of fever, caused by inflammatory response against sepsis [165]. The symptom of low temperature is called hypothermia, a comorbidity that rarely causes direct death but does encourage the development of hypoglycemia, respiratory distress syndrome, jaundice, and metabolic acidosis [166,167]. Core and skin temperatures are the two most frequently used metrics to evaluate neonates’ temperature [168]. Core temperatures are usually taken by inserting a probe into the rectum, nasopharynx, esophagus, or tympanic membrane [169,170]. Among all these locations, temperature taken at the rectum and esophagus are considered the gold standards for core temperature [169,171]. However, the rectal method can cause defecation, distress, cross-contamination, and perforation; the esophageal method can be inaccurate for neonates since they have minimal thermal insulation between the esophagus and trachea [169,171]. In addition, both methods are at least perceived as invasive. As such, they are not suitable for continuous neonate temperature monitoring. Many have proposed using pacifiers with built-in temperature so that neonates’ temperature can be measured while sucking them [172,173]. However, many neonates failed to adequately suck the pacifiers.
Compared with core temperature, skin temperature is less indicative of body condition due to accuracy incurred from fluctuation in ambient temperature, local blood perfusion, and location of measurement sites [174]. However, its measurement is noninvasive and more convenient. The recommended body locations for taking skin temperature are foreheads, abdominals, and axillae, using regular probe thermometers, infrared thermometers, or liquid crystal thermometers [169,175]. Due to their easy accessibility and simple sensing scheme, many wearable devices have been designed for skin temperature sensing. Choi J, et al. [176] integrated a liquid crystal temperature sensor onto a soft substrate and the liquid crystal displayed different colors at different temperatures. Jeon HS, et al. [177] designed a thermochromic membrane by mixing a thermochromic pigment and thermoplastic polymer and the membrane could sense different temperatures through changes in color intensity. Kumar A, et al. [178] created thermo-resistive material by embedding gold-coated silver nanowires in poly-ethylene glycol-polyurethane copolymer and screen-printed them onto a flexible substrate to form a temperature sensor. Similar wearable thermistors were also designed using different materials and substrates [179-182]. Although not specifically mentioned in any of the above works, all sensors were most likely detecting skin temperature changes under relatively constant and moderate ambient temperature, and like skin temperature, ambient temperature should equally contribute to the changes in material properties of these thermistors. As such, more experiments need to be performed under more dynamic temperatures.
Many efforts have been made to minimize the discrepancy between skin and temperature so that they can be used interchangeably. The most promising method is to use two thermistors, one positioned close to the skin and one positioned far away from the skin [183-186]. The purpose is to obtain the heat gradient between the two sensors and use the gradient as an extra parameter for core temperature estimation. The zero heat flux, the most commonly used method, insulates the skin locally and heats up the outer thermistor so that it has the same temperature as the thermistor contacting the skin. This effectively creates a region of zero heat gradient between the core and skin surface, making skin and core temperature equivalent [185]. Using this method, Atallah L, et al. [186] successfully obtained neonatal brain temperature that agreed with esophageal temperature. However, under relatively high temperatures, the outer thermistor might be heated to a temperature that is higher than that of the inner thermistor, making it impossible to create zero heat flux. Extra heat insulation material or creating a vacuum environment to protect the outer thermistor from the surroundings might be helpful to mitigate the effect of higher ambient temperature. The same approach might also be applicable to the thermistors mentioned in the previous paragraph.
Conclusion
Despite the enormous progress on the wearable device and contactless setup, there seems to be a lack of trust on using them as standalone systems for vital sign monitoring in the NICU. In 2017, only 5 devices (about 9% of total devices) were approved by the FDA for use on infants and neonates [187]. Aside from the limited availability, the devices are also known to be less technologically sophisticated compared with their adult counterparts [188]. As such, the use of adult devices on neonates is recognized and permitted in the US [189]. Despite the agreement amongst physicians that off-label use of adult devices is not ideal, there are many obstacles to developing pediatric-specific devices [189] From a clinical perspective, besides the obvious size difference, neonates and adults are neurodevelopmentally, physiologically, and epidemiologically different. These differences cause the same diseases in adults to manifest differently in neonates and therefore, medical devices should be designed differently, whether it is for diagnostic or treatment purposes [190]. Due to scarcity of the subjects, it is hard to recruit enough patients to generate enough data for clinical verification purposes and additionally, there are also ethical concerns on how and when to test medical devices on subjects who are critically ill [191]. Finally, not enough financial resources are allocated towards neonatal health research and medical device development due to the smaller market size for pediatric medical devices and the lower reimbursement rate for pediatric care [192-194].
All the devices mentioned above still have drawbacks, despite significant progress and innovation thus far. While the neonatal PPG devices dislplay novel applanation methods, they still do not address the inherent melanin issue for patients with darker skin tones. Darker skins absorb more light and therefore, their SpO2s are usually erroneously overestimated [195,196]. As such, a separate calibration method that accommodates both light and dark skin color should be developed so that SpO2 can be gauged more accurately. For contactless monitoring methods, specific Regions of Interest (ROI) are usually targeted for the measurement of relevant vital signs. However, body movement can cause ROI to move and therefore result in detecting irrelevant signals. Perhaps algorithms can be developed to actively track ROIs. Mechanical-based BP sensors are easily affected by motion since motion causes applanation pressure to change and consequently, the amplitude of the waveform will change too. The change in amplitude will lead to wrong BP values. Hence, a recalibration method might be needed after the detection of significant movement. For the temperature sensors, their accuracy might benefit from adding proper heat insulation so that environmental temperature can be decoupled. Finally, for ECG devices used for HR, BP, and RR monitoring, their designs and applanation methods avoid the use of adhesive and gels and can function wirelessly. Nevertheless, both new ECGs and other wearable sensors mentioned above need batteries to operate, imposing obstacles on wearability and their continuous monitoring capability. Perhaps, these wearable sensing systems can eventually adopt wireless power transfer systems or wireless passive sensing modalities so that they can operate without any built-in batteries [197-199].
To overcome these obstacles and technical difficulties, congress passed legislation to provide funding for the pediatric device consortia in 2007 [200]. Since 2009, $ 37 million has been awarded to different consortia across 4 funding cycles to support the development, production, and distribution of pediatric medical devices [201]. Afterall, those devices’ shortcomings can only be exposed when they are widely in use for long terms so that engineers can make improvements in the necessary places and make them more reliable. Minimally, these new devices or setups should be used along with their respective gold standards so that their performance can be directly compared. Additionally, the collected data and the recording from gold standards can also be used for training artificial intelligence models so that more accurate predictions can be made on potentially existing conditions. If these systems can be clinically validated and widely used, they will minimize painful procedures for neonates, allow for more robust and accurate continuous monitoring, encourage more intimate infant-parent contact, and most importantly, revolutionize caretaking in the NICU.
Conflict of Interest
MK is a co-founder and has equity interest in Vena Vitals, Makani Science, and Novoheart, companies that may potentially benefit from the research results. These relationships have been reviewed and approved by the University of California, Irvine in accordance with its conflict-of-interest policies.
References
- Lawn JE, Blencowe H, Oza S, You D, Lee AC, Waiswa P, Lalli M, Bhutta Z, Barros AJ, Christian P, Mathers C, Cousens SN; Lancet Every Newborn Study Group. Every Newborn: progress, priorities, and potential beyond survival. Lancet. 2014 Jul 12;384(9938):189-205. doi: 10.1016/S0140-6736(14)60496-7. Epub 2014 May 19. Erratum in: Lancet. 2014 Jul 12;384(9938):132. PMID: 24853593.
- Suárez-Idueta L, Blencowe H, Okwaraji YB, Yargawa J, Bradley E, Gordon A, Flenady V, Paixao ES, Barreto ML, Lisonkova S, Wen Q, Velebil P, Jírová J, Horváth-Puhó E, Sørensen HT, Sakkeus L, Abuladze L, Yunis KA, Al Bizri A, Barranco A, Broeders L, van Dijk AE, Alyafei F, Olukade TO, Razaz N, Söderling J, Smith LK, Draper ES, Lowry E, Rowland N, Wood R, Monteath K, Pereyra I, Pravia G, Ohuma EO, Lawn JE; National Vulnerable Newborn Mortality Collaborative Group and Vulnerable Newborn Measurement Core Group. Neonatal mortality risk for vulnerable newborn types in 15 countries using 125.5 million nationwide birth outcome records, 2000-2020. BJOG. 2023 May 8. doi: 10.1111/1471-0528.17506. Epub ahead of print. PMID: 37156244.
- Hsu JF, Chang YF, Cheng HJ, Yang C, Lin CY, Chu SM, Huang HR, Chiang MC, Wang HC, Tsai MH. Machine Learning Approaches to Predict In-Hospital Mortality among Neonates with Clinically Suspected Sepsis in the Neonatal Intensive Care Unit. J Pers Med. 2021 Jul 22;11(8):695. doi: 10.3390/jpm11080695. PMID: 34442338; PMCID: PMC8400295.
- Dol J, Hughes B, Bonet M, Dorey R, Dorling J, Grant A, Langlois EV, Monaghan J, Ollivier R, Parker R, Roos N, Scott H, Shin HD, Curran J. Timing of neonatal mortality and severe morbidity during the postnatal period: a systematic review. JBI Evid Synth. 2023 Jan 1;21(1):98-199. doi: 10.11124/JBIES-21-00479. PMID: 36300916; PMCID: PMC9794155.
- Khanam FT, Perera AG, Al-Naji A, Gibson K, Chahl J. Non-Contact Automatic Vital Signs Monitoring of Infants in a Neonatal Intensive Care Unit Based on Neural Networks. J Imaging. 2021 Jul 23;7(8):122. doi: 10.3390/jimaging7080122. PMID: 34460758; PMCID: PMC8404938.
- Khanam FT, Chahl LA, Chahl JS, Al-Naji A, Perera AG, Wang D, Lee YH, Ogunwa TT, Teague S, Nguyen TXB, McIntyre TD, Pegoli SP, Tao Y, McGuire JL, Huynh J, Chahl J. Noncontact Sensing of Contagion. J Imaging. 2021 Feb 5;7(2):28. doi: 10.3390/jimaging7020028. PMID: 34460627; PMCID: PMC8321279.
- Khanam FTZ, Al-Naji A, Chahl J. Remote monitoring of vital signs in diverse non-clinical and clinical scenarios using computer vision systems: A review. Applied Sciences. 2019;9(20):4474. doi: 10.3390/app9204474.
- Cretikos MA, Bellomo R, Hillman K, Chen J, Finfer S, Flabouris A. Respiratory rate: the neglected vital sign. Med J Aust. 2008 Jun 2;188(11):657-9. doi: 10.5694/j.1326-5377.2008.tb01825.x. PMID: 18513176.
- Bonner O, Beardsall K, Crilly N, Lasenby J. 'There were more wires than him': the potential for wireless patient monitoring in neonatal intensive care. BMJ Innov. 2017 Feb;3(1):12-18. doi: 10.1136/bmjinnov-2016-000145. Epub 2017 Jan 4. PMID: 28250963; PMCID: PMC5293857.
- Kwak SS, Yoo S, Avila R, Chung HU, Jeong H, Liu C, Vogl JL, Kim J, Yoon HJ, Park Y, Ryu H, Lee G, Kim J, Koo J, Oh YS, Kim S, Xu S, Zhao Z, Xie Z, Huang Y, Rogers JA. Skin-Integrated Devices with Soft, Holey Architectures for Wireless Physiological Monitoring, With Applications in the Neonatal Intensive Care Unit. Adv Mater. 2021 Nov;33(44):e2103974. doi: 10.1002/adma.202103974. Epub 2021 Sep 12. PMID: 34510572.
- Lee JH. Catheter-related bloodstream infections in neonatal intensive care units. Korean J Pediatr. 2011 Sep;54(9):363-7. doi: 10.3345/kjp.2011.54.9.363. Epub 2011 Sep 30. PMID: 22232628; PMCID: PMC3250601.
- Lund C. Medical adhesives in the NICU. Newborn and Infant Nursing Reviews. 2014;14(4):160-165. doi: 10.1053/j.nainr.2014.10.001.
- Ates HC, Nguyen PQ, Gonzalez-Macia L, Morales-Narváez E, Güder F, Collins JJ, Dincer C. End-to-end design of wearable sensors. Nat Rev Mater. 2022;7(11):887-907. doi: 10.1038/s41578-022-00460-x. Epub 2022 Jul 22. PMID: 35910814; PMCID: PMC9306444.
- Roy S, Arshad F, Eissa S, Safavieh M, Alattas SG, Ahmed MU, Zourob M. Recent developments towards portable point-of-care diagnostic devices for pathogen detection. Sensors and Diagnostics. Royal Society of Chemistry. 2022;1(1):87-105. doi: 10.1039/d1sd00017a.
- Heikenfeld J, Jajack A, Rogers J, Gutruf P, Tian L, Pan T, Li R, Khine M, Kim J, Wang J, Kim J. Wearable sensors: modalities, challenges, and prospects. Lab Chip. 2018 Jan 16;18(2):217-248. doi: 10.1039/c7lc00914c. PMID: 29182185; PMCID: PMC5771841.
- Kenry, Yeo JC, Lim CT. Emerging flexible and wearable physical sensing platforms for healthcare and biomedical applications. Microsyst Nanoeng. 2016 Sep 26;2:16043. doi: 10.1038/micronano.2016.43. PMID: 31057833; PMCID: PMC6444731.
- Xue Y, Zhang J, Chen X, Zhang J, Chen G, Zhang K, Lin J, Guo C, Liu J. Trigger-detachable hydrogel adhesives for bioelectronic interfaces. Adv Funct Mater. 2021;31(47). doi: 10.1002/adfm.202106446.
- Chung HU, Kim BH, Lee JY, Lee J, Xie Z, Ibler EM, Lee K, Banks A, Jeong JY, Kim J, Ogle C, Grande D, Yu Y, Jang H, Assem P, Ryu D, Kwak JW, Namkoong M, Park JB, Lee Y, Kim DH, Ryu A, Jeong J, You K, Ji B, Liu Z, Huo Q, Feng X, Deng Y, Xu Y, Jang KI, Kim J, Zhang Y, Ghaffari R, Rand CM, Schau M, Hamvas A, Weese-Mayer DE, Huang Y, Lee SM, Lee CH, Shanbhag NR, Paller AS, Xu S, Rogers JA. Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science. 2019 Mar 1;363(6430):eaau0780. doi: 10.1126/science.aau0780. PMID: 30819934; PMCID: PMC6510306.
- Dong S, Wen L, Li Y, Lu J, Zhang Z, Yuan C, Gu C. Remote respiratory variables tracking with biomedical radar-based iot system during sleep. IEEE Internet Things J. 2024;99:1-1. doi: 10.1109/JIOT.2024.3367932.
- Nakajim K, Matsumoto Y, Tamura T. Development of real-time image sequence analysis for evaluating posture change and respiratory rate of a subject in bed. Physiol Meas. 2001 Aug;22(3):N21-8. doi: 10.1088/0967-3334/22/3/401. PMID: 11556682.
- Abbas AK, Heimann K, Jergus K, Orlikowsky T, Leonhardt S. Neonatal non-contact respiratory monitoring based on real-time infrared thermography. Biomed Eng Online. 2011 Oct 20;10:93. doi: 10.1186/1475-925X-10-93. PMID: 22243660; PMCID: PMC3258209.
- Kaplan Berkaya S, Uysal AK, Sora Gunal E, Ergin S, Gunal S, Gulmezoglu MB. A survey on ECG analysis. Biomed Signal Process Control. 2018;43:216-235. doi: 10.1016/j.bspc.2018.03.003.
- Stramba-Badiale M, Karnad DR, Goulene KM, Panicker GK, Dagradi F, Spazzolini C, Kothari S, Lokhandwala YY, Schwartz PJ. For neonatal ECG screening there is no reason to relinquish old Bazett's correction. Eur Heart J. 2018 Aug 14;39(31):2888-2895. doi: 10.1093/eurheartj/ehy284. PMID: 29860404.
- Schwartz PJ, Garson A Jr, Paul T, Stramba-Badiale M, Vetter VL, Wren C; European Society of Cardiology. Guidelines for the interpretation of the neonatal electrocardiogram. A task force of the European Society of Cardiology. Eur Heart J. 2002 Sep;23(17):1329-44. doi: 10.1053/euhj.2002.3274. PMID: 12269267.
- Hoffmann KP, Ruff R. Flexible dry surface-electrodes for ECG long-term monitoring. Annu Int Conf IEEE Eng Med Biol Soc. 2007;2007:5740-3. doi: 10.1109/IEMBS.2007.4353650. PMID: 18003316.
- Kim YS, Mahmood M, Lee Y, Kim NK, Kwon S, Herbert R, Kim D, Cho HC, Yeo WH. All-in-One, Wireless, Stretchable Hybrid Electronics for Smart, Connected, and Ambulatory Physiological Monitoring. Adv Sci (Weinh). 2019 Jul 24;6(17):1900939. doi: 10.1002/advs.201900939. PMID: 31508289; PMCID: PMC6724359.
- Chung HU, Rwei AY, Hourlier-Fargette A, Xu S, Lee K, Dunne EC, Xie Z, Liu C, Carlini A, Kim DH, Ryu D, Kulikova E, Cao J, Odland IC, Fields KB, Hopkins B, Banks A, Ogle C, Grande D, Park JB, Kim J, Irie M, Jang H, Lee J, Park Y, Kim J, Jo HH, Hahm H, Avila R, Xu Y, Namkoong M, Kwak JW, Suen E, Paulus MA, Kim RJ, Parsons BV, Human KA, Kim SS, Patel M, Reuther W, Kim HS, Lee SH, Leedle JD, Yun Y, Rigali S, Son T, Jung I, Arafa H, Soundararajan VR, Ollech A, Shukla A, Bradley A, Schau M, Rand CM, Marsillio LE, Harris ZL, Huang Y, Hamvas A, Paller AS, Weese-Mayer DE, Lee JY, Rogers JA. Skin-interfaced biosensors for advanced wireless physiological monitoring in neonatal and pediatric intensive-care units. Nat Med. 2020 Mar;26(3):418-429. doi: 10.1038/s41591-020-0792-9. Epub 2020 Mar 11. PMID: 32161411; PMCID: PMC7315772.
- Yapici MK, Alkhidir T, Samad YA, Liao K. Graphene-clad textile electrodes for electrocardiogram monitoring. Sens Actuators B Chem. 2015;221:1469-1474. doi: 10.1016/j.snb.2015.07.111.
- Chen W, Oetomo SB, Feijs L, Bouwstra S, Ayoola I, Dols S. Design of an integrated sensor platform for vital sign monitoring of newborn infants at Neonatal Intensive Care Units. J Healthc Eng. 2010;1(4):535-554. doi: 10.1260/2040-2295.1.4.535.
- Tang Y, Chang R, Zhang L, Yan F, Ma H, Bu X. Electrode humidification design for artifact reduction in capacitive ECG measurements. Sensors. 2020;20(12):1-17. doi: 10.3390/s20123449.
- Ueno A, Akabane Y, Kato T, Hoshino H, Kataoka S, Ishiyama Y. Capacitive sensing of electrocardiographic potential through cloth from the dorsal surface of the body in a supine position: a preliminary study. IEEE Trans Biomed Eng. 2007 Apr;54(4):759-66. doi: 10.1109/TBME.2006.889201. PMID: 17405385.
- Lee SM, Sim KS, Kim KK, Lim YG, Park KS. Thin and flexible active electrodes with shield for capacitive electrocardiogram measurement. Med Biol Eng Comput. 2010 May;48(5):447-57. doi: 10.1007/s11517-010-0597-y. Epub 2010 Apr 2. PMID: 20361268.
- Su PC, Hsueh YH, Ke MT, Chen JJ, Lai PC. Noncontact ECG Monitoring by Capacitive Coupling of Textiles in a Chair. J Healthc Eng. 2021 Jun 16;2021:6698567. doi: 10.1155/2021/6698567. PMID: 34221299; PMCID: PMC8225447.
- Kato T, Ueno A, Kataoka S, Hoshino H, Ishiyama Y. An application of capacitive electrode for detecting electrocardiogram of neonates and infants. Conf Proc IEEE Eng Med Biol Soc. 2006;2006:916-9. doi: 10.1109/IEMBS.2006.260362. PMID: 17946008.
- Swarup S, Makaryus AN. Digital stethoscope: technology update. Med Devices (Auckl). 2018 Jan 4;11:29-36. doi: 10.2147/MDER.S135882. PMID: 29379321; PMCID: PMC5757962.
- Balogh ÁT, Kovács F. Application of phonocardiography on preterm infants with patent ductus arteriosus. Biomed Signal Process Control. 2011;6(4):337-345. doi: 10.1016/j.bspc.2011.05.009.
- Voogdt KG, Morrison AC, Wood FE, van Elburg RM, Wyllie JP. A randomised, simulated study assessing auscultation of heart rate at birth. Resuscitation. 2010 Aug;81(8):1000-3. doi: 10.1016/j.resuscitation.2010.03.021. Epub 2010 May 18. PMID: 20483522.
- Behere S, Baffa JM, Penfil S, Slamon N. Real-World Evaluation of the Eko Electronic Teleauscultation System. Pediatr Cardiol. 2019 Jan;40(1):154-160. doi: 10.1007/s00246-018-1972-y. Epub 2018 Aug 31. PMID: 30171267.
- Chong SW, Peyton PJ. A meta-analysis of the accuracy and precision of the ultrasonic cardiac output monitor (USCOM). Anaesthesia. 2012 Nov;67(11):1266-71. doi: 10.1111/j.1365-2044.2012.07311.x. Epub 2012 Aug 29. PMID: 22928650.
- Blohm ME, Obrecht D, Hartwich J, Mueller GC, Kersten JF, Weil J, Singer D. Impedance cardiography (electrical velocimetry) and transthoracic echocardiography for non-invasive cardiac output monitoring in pediatric intensive care patients: a prospective single-center observational study. Crit Care. 2014 Nov 19;18(6):603. doi: 10.1186/s13054-014-0603-0. PMID: 25407329; PMCID: PMC4261789.
- Freidl T, Baik N, Pichler G, Schwaberger B, Zingerle B, Avian A, Urlesberger B. Haemodynamic Transition after Birth: A New Tool for Non-Invasive Cardiac Output Monitoring. Neonatology. 2017;111(1):55-60. doi: 10.1159/000446468. Epub 2016 Aug 17. PMID: 27529179.
- Marchionni P, Scalise L, Ercoli I, Tomasini EP. An optical measurement method for the simultaneous assessment of respiration and heart rates in preterm infants. Rev Sci Instrum. 2013 Dec;84(12):121705. doi: 10.1063/1.4845635. PMID: 24387410.
- Ranjan AK, Gulati A. Controls of Central and Peripheral Blood Pressure and Hemorrhagic/Hypovolemic Shock. J Clin Med. 2023 Jan 31;12(3):1108. doi: 10.3390/jcm12031108. PMID: 36769755; PMCID: PMC9917827.
- Baker S, Yogavijayan T, Kandasamy Y. Towards Non-Invasive and Continuous Blood Pressure Monitoring in Neonatal Intensive Care Using Artificial Intelligence: A Narrative Review. Healthcare (Basel). 2023 Dec 6;11(24):3107. doi: 10.3390/healthcare11243107. PMID: 38131997; PMCID: PMC10743031.
- Fanaroff JM, Fanaroff AA. Blood pressure disorders in the neonate: hypotension and hypertension. Semin Fetal Neonatal Med. 2006 Jun;11(3):174-81. doi: 10.1016/j.siny.2006.01.002. Epub 2006 Mar 3. PMID: 16516569.
- Dasgupta SJ, Gill AB. Hypotension in the very low birthweight infant: the old, the new, and the uncertain. Arch Dis Child Fetal Neonatal Ed. 2003 Nov;88(6):F450-4. doi: 10.1136/fn.88.6.f450. PMID: 14602688; PMCID: PMC1763241.
- Nakamura M, Umehara N, Ishii K, Sasahara J, Kiyoshi K, Ozawa K, Tanaka K, Tanemoto T, Ichizuka K, Hasegawa J, Ishikawa H, Murakoshi T, Sago H. A poor long-term neurological prognosis is associated with abnormal cord insertion in severe growth-restricted fetuses. J Perinat Med. 2018 Nov 27;46(9):1040-1047. doi: 10.1515/jpm-2017-0240. PMID: 29267174.
- Kent AL, Kecskes Z, Shadbolt B, Falk MC. Normative blood pressure data in the early neonatal period. Pediatr Nephrol. 2007 Sep;22(9):1335-41. doi: 10.1007/s00467-007-0480-8. Epub 2007 Apr 17. PMID: 17437131.
- Giri P, Roth P. Neonatal Hypertension. Pediatr Rev. 2020 Jun;41(6):307-311. doi: 10.1542/pir.2019-0159. PMID: 32482697.
- Harer MW, Kent AL. Neonatal hypertension: an educational review. Pediatr Nephrol. 2019 Jun;34(6):1009-1018. doi: 10.1007/s00467-018-3996-1. Epub 2018 Jul 5. PMID: 29974208.
- Batton B. Neonatal Blood Pressure Standards: What Is "Normal"? Clin Perinatol. 2020 Sep;47(3):469-485. doi: 10.1016/j.clp.2020.05.008. Epub 2020 May 22. PMID: 32713445.
- Dionne JM, Bremner SA, Baygani SK, Batton B, Ergenekon E, Bhatt-Mehta V, Dempsey E, Kluckow M, Pesco Koplowitz L, Apele-Freimane D, Iwami H, Klein A, Turner M, Rabe H; International Neonatal Consortium. Method of Blood Pressure Measurement in Neonates and Infants: A Systematic Review and Analysis. J Pediatr. 2020 Jun;221:23-31.e5. doi: 10.1016/j.jpeds.2020.02.072. PMID: 32446487.
- Di Biase M, Casani A, Orfeo L. Invasive arterial blood pressure in the neonatal intensive care: a valuable tool to manage very ill preterm and term neonates. Ital J Pediatr. 2015 Sep 24;41(Suppl 1):A9. doi: 10.1186/1824-7288-41-S1-A9. PMCID: PMC4594163.
- Rao A, Eskandar-Afshari F, Weiner Y, Billman E, McMillin A, Sella N, Roxlo T, Liu J, Leong W, Helfenbein E, Walendowski A, Muir A, Joseph A, Verma A, Ramamoorthy C, Honkanen A, Green G, Drake K, Govindan RB, Rhine W, Quan X. Clinical Study of Continuous Non-Invasive Blood Pressure Monitoring in Neonates. Sensors (Basel). 2023 Apr 2;23(7):3690. doi: 10.3390/s23073690. PMID: 37050750; PMCID: PMC10098632.
- Wu J, Mu D. Vascular catheter-related complications in newborns. J Paediatr Child Health. 2012 Feb;48(2):E91-5. doi: 10.1111/j.1440-1754.2010.01934.x. Epub 2010 Dec 29. PMID: 21199061.
- Yi Z, Zhang W, Yang B. Piezoelectric approaches for wearable continuous blood pressure monitoring: A review. Journal of Micromechanics and Microengineering. 2022. doi: 10.1088/1361-6439/ac87ba.
- Wang H, Wang L, Sun N, Yao Y, Hao L, Xu L, Greenwald SE. Quantitative Comparison of the Performance of Piezoresistive, Piezoelectric, Acceleration, and Optical Pulse Wave Sensors. Front Physiol. 2020 Jan 14;10:1563. doi: 10.3389/fphys.2019.01563. PMID: 32009976; PMCID: PMC6971205.
- Rwei P, Qian C, Abiri A, Zhou Y, Chou EF, Tang WC, Khine M. Soft iontronic capacitive sensor for beat-to-beat blood pressure measurements. Adv Mater Interfaces. 2022;9(18):2200294. doi: 10.1002/admi.202200294.
- Bijender, Kumar S, Soni A, Yadav R, Singh SP, Kumar A. Noninvasive Blood Pressure Monitoring via a Flexible and Wearable Piezoresistive Sensor. ACS Omega. 2024 Feb 1;9(6):6355-6365. doi: 10.1021/acsomega.3c04786. PMID: 38375497; PMCID: PMC10876045.
- Min S, Kim DH, Joe DJ, Kim BW, Jung YH, Lee JH, Lee BY, Doh I, An J, Youn YN, Joung B, Yoo CD, Ahn HS, Lee KJ. Clinical Validation of a Wearable Piezoelectric Blood-Pressure Sensor for Continuous Health Monitoring. Adv Mater. 2023 Jun;35(26):e2301627. doi: 10.1002/adma.202301627. Epub 2023 May 11. PMID: 36960816.
- Kim J, Chou EF, Le J, Wong S, Chu M, Khine M. Soft Wearable Pressure Sensors for Beat-to-Beat Blood Pressure Monitoring. Adv Healthc Mater. 2019 Jul;8(13):e1900109. doi: 10.1002/adhm.201900109. Epub 2019 Apr 29. PMID: 31033256.
- Luo N, Dai W, Li C, Zhou Z, Lu L, Poon CCY, Shen SC, Zhang YT, Zhao N. Flexible piezoresistive sensor patch enabling ultralow power cuffless blood pressure measurement. Adv Funct Mater. 2016;26(8):1178-1187. doi: 10.1002/adfm.201504560.
- Abiri A, Chou EF, Qian C, Rinehart J, Khine M. Intra-beat biomarker for accurate continuous non-invasive blood pressure monitoring. Sci Rep. 2022;12(1):16772. doi: 10.1038/s41598-022-19096-6.
- Lakhal K, Martin M, Ehrmann S, Boulain T. Noninvasive monitors of blood pressure in the critically ill: what are acceptable accuracy and precision? Eur J Anaesthesiol. 2015 May;32(5):367-8. doi: 10.1097/EJA.0000000000000229. PMID: 25844845.
- Lakhal K, Ehrmann S, Boulain T. Noninvasive BP Monitoring in the Critically Ill: Time to Abandon the Arterial Catheter? Chest. 2018 Apr;153(4):1023-1039. doi: 10.1016/j.chest.2017.10.030. Epub 2017 Nov 3. PMID: 29108815.
- Li J, Jia H, Zhou J, Huang X, Xu L, Jia S, Gao Z, Yao K, Li D, Zhang B, Liu Y, Huang Y, Hu Y, Zhao G, Xu Z, Li J, Yiu CK, Gao Y, Wu M, Jiao Y, Zhang Q, Tai X, Chan RH, Zhang Y, Ma X, Yu X. Thin, soft, wearable system for continuous wireless monitoring of artery blood pressure. Nat Commun. 2023 Aug 17;14(1):5009. doi: 10.1038/s41467-023-40763-3. PMID: 37591881; PMCID: PMC10435523.
- Elgendi M, Fletcher R, Liang Y, Howard N, Lovell NH, Abbott D, Lim K, Ward R. The use of photoplethysmography for assessing hypertension. NPJ Digit Med. 2019 Jun 26;2:60. doi: 10.1038/s41746-019-0136-7. PMID: 31388564; PMCID: PMC6594942.
- Mukkamala R, Hahn JO, Inan OT, Mestha LK, Kim CS, Töreyin H, Kyal S. Toward Ubiquitous Blood Pressure Monitoring via Pulse Transit Time: Theory and Practice. IEEE Trans Biomed Eng. 2015 Aug;62(8):1879-901. doi: 10.1109/TBME.2015.2441951. Epub 2015 Jun 5. PMID: 26057530; PMCID: PMC4515215.
- Chowdhury MH, Shuzan MNI, Chowdhury MEH, Mahbub ZB, Uddin MM, Khandakar A, Reaz MBI. Estimating Blood Pressure from the Photoplethysmogram Signal and Demographic Features Using Machine Learning Techniques. Sensors (Basel). 2020 Jun 1;20(11):3127. doi: 10.3390/s20113127. PMID: 32492902; PMCID: PMC7309072.
- Samimi H, Dajani HR. A PPG-Based Calibration-Free Cuffless Blood Pressure Estimation Method Using Cardiovascular Dynamics. Sensors (Basel). 2023 Apr 21;23(8):4145. doi: 10.3390/s23084145. PMID: 37112490; PMCID: PMC10146008.
- González S, Hsieh WT, Chen TP. A benchmark for machine-learning based non-invasive blood pressure estimation using photoplethysmogram. Sci Data. 2023 Mar 21;10(1):149. doi: 10.1038/s41597-023-02020-6. PMID: 36944668; PMCID: PMC10030661.
- Slapničar G, Mlakar N, Luštrek M. Blood Pressure Estimation from Photoplethysmogram Using a Spectro-Temporal Deep Neural Network. Sensors (Basel). 2019 Aug 4;19(15):3420. doi: 10.3390/s19153420. PMID: 31382703; PMCID: PMC6696196.
- Addison PS. Slope Transit Time (STT): A Pulse Transit Time Proxy requiring Only a Single Signal Fiducial Point. IEEE Trans Biomed Eng. 2016 Nov;63(11):2441-2444. doi: 10.1109/TBME.2016.2528507. Epub 2016 Feb 12. PMID: 26890527.
- Elgendi M. On the analysis of fingertip photoplethysmogram signals. Curr Cardiol Rev. 2012 Feb;8(1):14-25. doi: 10.2174/157340312801215782. PMID: 22845812; PMCID: PMC3394104.
- Liang Y, Elgendi M, Chen Z, Ward R. An optimal filter for short photoplethysmogram signals. Sci Data. 2018 May 1;5:180076. doi: 10.1038/sdata.2018.76. PMID: 29714722; PMCID: PMC5928853.
- Tang D, Goh S, Wong D, Lew E. PPG signal reconstruction using a combination of discrete wavelet transform and empirical mode decomposition. IEEE. 2016;1-4. doi: 10.1109/ICIAS.2016.7824118.
- Hassani A, Foruzan AH. Improved PPG-based estimation of the blood pressure using latent space features. Signal Image Video Process. 2019;13(6):1141-1147. doi: 10.1007/s11760-019-01460-1
- Ceran C, Taner OF, Tekin F, Tezcan S, Tekin O, Civelek B. Management of pulse oximeter probe-induced finger injuries in children: report of two consecutive cases and review of the literature. J Pediatr Surg. 2012 Nov;47(11):e27-9. doi: 10.1016/j.jpedsurg.2012.06.033. PMID: 23164026.
- Mukherjee R, Ghosh S, Gupta B, Chakravarty T. A Literature Review on Current and Proposed Technologies of Noninvasive Blood Pressure Measurement. Telemed J E Health. 2018 Mar;24(3):185-193. doi: 10.1089/tmj.2017.0068. Epub 2017 Aug 7. PMID: 28783442.
- Quan X, Liu J, Roxlo T, Siddharth S, Leong W, Muir A, Cheong SM, Rao A. Advances in Non-Invasive Blood Pressure Monitoring. Sensors (Basel). 2021 Jun 22;21(13):4273. doi: 10.3390/s21134273. PMID: 34206457; PMCID: PMC8271585.
- Le T, Ellington F, Lee TY, Vo K, Khine M, Krishnan SK, Dutt N, Cao H. Continuous non-invasive blood pressure monitoring: A methodological review on measurement techniques. IEEE. 2020. doi: 10.1109/ACCESS.2020.3040257.
- C Bramwell MJ, Hill AV. The velocity of the pulse wave in man. Archv fiir Physiol. 1922.
- Finnegan E, Davidson S, Harford M, Jorge J, Watkinson P, Young D, Tarassenko L, Villarroel M. Pulse arrival time as a surrogate of blood pressure. Sci Rep. 2021 Nov 23;11(1):22767. doi: 10.1038/s41598-021-01358-4. PMID: 34815419; PMCID: PMC8611024.
- Poon C, Zhang YT, Wong G, Poon WS. The beat-to-beat relationship between pulse transit time and systolic blood pressure. IEEE. 2008;129(3):342-343. doi: 10.1109/ITAB.2008.4570616.
- Chen W, Kobayashi T, Ichikawa S, Takeuchi Y, Togawa T. Continuous estimation of systolic blood pressure using the pulse arrival time and intermittent calibration. Med Biol Eng Comput. 2000 Sep;38(5):569-74. doi: 10.1007/BF02345755. PMID: 11094816.
- Njeru CM, Ansermino JM, Macharia WM, Dunsmuir DT. Variability of respiratory rate measurements in neonates- every minute counts. BMC Pediatr. 2022 Jan 3;22(1):16. doi: 10.1186/s12887-021-03087-z. PMID: 34980049; PMCID: PMC8722355.
- MacLean JE, Fitzgerald DA, Waters KA. Developmental changes in sleep and breathing across infancy and childhood. Paediatr Respir Rev. 2015 Sep;16(4):276-84. doi: 10.1016/j.prrv.2015.08.002. Epub 2015 Aug 14. PMID: 26364005.
- Jorge J, Villarroel M, Chaichulee S, Guazzi A, Davis S, Green G, McCormick K, Tarassenko L. Non-contact monitoring of respiration in the neonatal intensive care unit. IEEE. 2017;286-293. doi: 10.1109/FG.2017.44.
- Steinschneider A. Prolonged apnea and the sudden infant death syndrome: clinical and laboratory observations. Pediatrics. 1972 Oct;50(4):646-54. PMID: 4342142.
- Fieselmann JF, Hendryx MS, Helms CM, Wakefield DS. Respiratory rate predicts cardiopulmonary arrest for internal medicine inpatients. J Gen Intern Med. 1993 Jul;8(7):354-60. doi: 10.1007/BF02600071. PMID: 8410395.
- Schechtman VL, Lee MY, Wilson AJ, Harper RM. Dynamics of respiratory patterning in normal infants and infants who subsequently died of the sudden infant death syndrome. Pediatr Res. 1996 Oct;40(4):571-7. doi: 10.1203/00006450-199610000-00010. PMID: 8888285.
- Reuter S, Moser C, Baack M, Falls S. Respiratory distress in the newborn. Pediatrics in Review. 2014;35 (10):417-429. doi: 10.1542/pir.35-10-417
- Williamson M, Poorun R, Hartley C. Apnoea of Prematurity and Neurodevelopmental Outcomes: Current Understanding and Future Prospects for Research. Front Pediatr. 2021 Oct 25;9:755677. doi: 10.3389/fped.2021.755677. PMID: 34760852; PMCID: PMC8573333.
- Perlman JM, McMenamin JB, Volpe JJ. Fluctuating cerebral blood-flow velocity in respiratory-distress syndrome. Relation to the development of intraventricular hemorrhage. N Engl J Med. 1983 Jul 28;309(4):204-9. doi: 10.1056/NEJM198307283090402. PMID: 6866033.
- Qudsi H, Gupta M. Low-cost, thermistor based respiration monitor. In: Proceedings - 29th Southern Biomedical Engineering Conference, SBEC 2013. 2013;23-24. doi: 10.1109/SBEC.2013.20.
- Baker K, Alfvén T, Mucunguzi A, Wharton-Smith A, Dantzer E, Habte T, Matata L, Nanyumba D, Okwir M, Posada M, Sebsibe A, Nicholson J, Marasciulo M, Izadnegahdar R, Petzold M, Källander K. Performance of Four Respiratory Rate Counters to Support Community Health Workers to Detect the Symptoms of Pneumonia in Children in Low Resource Settings: A Prospective, Multicentre, Hospital-Based, Single-Blinded, Comparative Trial. EClinicalMedicine. 2019 Jun 10;12:20-30. doi: 10.1016/j.eclinm.2019.05.013. PMID: 31388660; PMCID: PMC6677646.
- Schmalisch G. Current methodological and technical limitations of time and volumetric capnography in newborns. Biomed Eng Online. 2016 Aug 30;15(1):104. doi: 10.1186/s12938-016-0228-4. PMID: 27576441; PMCID: PMC5004292.
- Lee H, Rusin CG, Lake DE, Clark MT, Guin L, Smoot TJ, Paget-Brown AO, Vergales BD, Kattwinkel J, Moorman JR, Delos JB. A new algorithm for detecting central apnea in neonates. Physiol Meas. 2012 Jan;33(1):1-17. doi: 10.1088/0967-3334/33/1/1. Epub 2011 Dec 7. PMID: 22156193; PMCID: PMC5366243.
- Koyama S, Sato Y, Maru H, Shiokawa S, Martinka M. Wearable FBG sensor system for respiratory strain measurement during daily activities. IEEE Sens J. 2024;24(13):20595-20605. doi: 10.1109/JSEN.2024.3396784.
- Olsson E, Ugnell H, Oberg PA, Sedin G. Photoplethysmography for simultaneous recording of heart and respiratory rates in newborn infants. Acta Paediatr. 2000 Jul;89(7):853-61. doi: 10.1080/080352500750043774. PMID: 10943970.
- Foo JY, Wilson SJ. Estimation of breathing interval from the photoplethysmographic signals in children. Physiol Meas. 2005 Dec;26(6):1049-58. doi: 10.1088/0967-3334/26/6/014. Epub 2005 Oct 31. PMID: 16311452.
- Johansson A, Oberg PA, Sedin G. Monitoring of heart and respiratory rates in newborn infants using a new photoplethysmographic technique. J Clin Monit Comput. 1999 Dec;15(7-8):461-7. doi: 10.1023/a:1009912831366. PMID: 12578044.
- Nilsson L, Johansson A, Kalman S. Monitoring of respiratory rate in postoperative care using a new photoplethysmographic technique. J Clin Monit Comput. 2000;16(4):309-15. doi: 10.1023/a:1011424732717. PMID: 12578078.
- Pimentel MAF, Johnson AEW, Charlton PH, Birrenkott D, Watkinson PJ, Tarassenko L, Clifton DA. Toward a Robust Estimation of Respiratory Rate From Pulse Oximeters. IEEE Trans Biomed Eng. 2017 Aug;64(8):1914-1923. doi: 10.1109/TBME.2016.2613124. Epub 2016 Nov 18. PMID: 27875128; PMCID: PMC6051482.
- Addison PS, Watson JN, Mestek ML, Ochs JP, Uribe AA, Bergese SD. Pulse oximetry-derived respiratory rate in general care floor patients. J Clin Monit Comput. 2015 Feb;29(1):113-20. doi: 10.1007/s10877-014-9575-5. Epub 2014 May 6. PMID: 24796734; PMCID: PMC4309914.
- Zhou Y, Zheng Y, Wang C, Yuan J. Extraction of respiratory activity from photoplethysmographic signals based on an independent component analysis technique: Preliminary report. Instrum Sci Technol. 2006;34(5):537-45. doi: 10.1080/10739140600809678.
- Wertheim D, Olden C, Savage E, Seddon P. Extracting respiratory data from pulse oximeter plethysmogram traces in newborn infants. Arch Dis Child Fetal Neonatal Ed. 2009 Jul;94(4):F301-3. doi: 10.1136/adc.2008.145342. Epub 2008 Nov 17. PMID: 19015221.
- Shelley KH, Awad AA, Stout RG, Silverman DG. The use of joint time frequency analysis to quantify the effect of ventilation on the pulse oximeter waveform. J Clin Monit Comput. 2006 Apr;20(2):81-7. doi: 10.1007/s10877-006-9010-7. Epub 2006 Jun 1. PMID: 16779621.
- Johnston WS, Mendelson Y. Extracting breathing rate information from a wearable reflectance pulse oximeter sensor. Conf Proc IEEE Eng Med Biol Soc. 2004;2004:5388-91. doi: 10.1109/IEMBS.2004.1404504. PMID: 17271561.
- Karlen W, Raman S, Ansermino JM, Dumont GA. Multiparameter respiratory rate estimation from the photoplethysmogram. IEEE Trans Biomed Eng. 2013;60(7):1946-53. doi: 10.1109/TBME.2013.2246160.
- Nilsson L, Johansson A, Kalman S. Respiration can be monitored by photoplethysmography with high sensitivity and specificity regardless of anaesthesia and ventilatory mode. Acta Anaesthesiol Scand. 2005 Sep;49(8):1157-62. doi: 10.1111/j.1399-6576.2005.00721.x. PMID: 16095458.
- Cysarz D, Zerm R, Bettermann H, Frühwirth M, Moser M, Kröz M. Comparison of respiratory rates derived from heart rate variability, ECG amplitude, and nasal/oral airflow. Ann Biomed Eng. 2008 Dec;36(12):2085-94. doi: 10.1007/s10439-008-9580-2. Epub 2008 Oct 15. PMID: 18855140.
- Mishra S, Norton JJS, Lee Y, Lee DS, Agee N, Chen Y, Chun Y, Yeo WH. Soft, conformal bioelectronics for a wireless human-wheelchair interface. Biosens Bioelectron. 2017 May 15;91:796-803. doi: 10.1016/j.bios.2017.01.044. Epub 2017 Jan 25. PMID: 28152485; PMCID: PMC5323068.
- Sun Y, Wang W, Long X, Meftah M, Tan T, Shan C, Ronald MA, de With PHN. Respiration monitoring for premature neonates in NICU. Applied Sciences (Switzerland). 2019 Dec 1;9(23). doi: doi.org/10.3390/app9235246.
- Kim YS, Mahmood M, Kwon S, Maher K, Kang JW, Yeo WH. Wireless, Skin-Like Membrane Electronics With Multifunctional Ergonomic Sensors for Enhanced Pediatric Care. IEEE Trans Biomed Eng. 2020 Aug;67(8):2159-2165. doi: 10.1109/TBME.2019.2956048. Epub 2019 Nov 26. PMID: 31794383.
- Al-Khalidi FQ, Saatchi R, Burke D, Elphick H, Tan S. Respiration rate monitoring methods: a review. Pediatr Pulmonol. 2011 Jun;46(6):523-9. doi: 10.1002/ppul.21416. Epub 2011 Jan 31. PMID: 21560260.
- Greneker EF. Radar sensing of heartbeat and respiration at a distance with applications of the technology. IEEE. 1997. doi: 10.1049/cp:19971650.
- Uenoyama M, Matsui T, Yamada K, Suzuki S, Takase B, Suzuki S, Ishihara M, Kawakami M. Non-contact respiratory monitoring system using a ceiling-attached microwave antenna. Med Biol Eng Comput. 2006 Sep;44(9):835-40. doi: 10.1007/s11517-006-0091-8. Epub 2006 Aug 29. PMID: 16941101.
- Gleichauf J, Herrmann S, Hennemann L, Krauss H, Nitschke J, Renner P, Niebler C, Koelpin A. Automated Non-Contact Respiratory Rate Monitoring of Neonates Based on Synchronous Evaluation of a 3D Time-of-Flight Camera and a Microwave Interferometric Radar Sensor. Sensors (Basel). 2021 Apr 23;21(9):2959. doi: 10.3390/s21092959. PMID: 33922563; PMCID: PMC8122919.
- Beltrão G, Stutz R, Hornberger F, Martins WA, Tatarinov D, Alaee-Kerahroodi M, Lindner U, Stock L, Kaiser E, Goedicke-Fritz S, Schroeder U, R BSM, Zemlin M. Contactless radar-based breathing monitoring of premature infants in the neonatal intensive care unit. Sci Rep. 2022 Mar 25;12(1):5150. doi: 10.1038/s41598-022-08836-3. PMID: 35338172; PMCID: PMC8956695.
- Addison PS, Jacquel D, Foo DMH, Antunes A, Borg UR. Video-Based Physiologic Monitoring During an Acute Hypoxic Challenge: Heart Rate, Respiratory Rate, and Oxygen Saturation. Anesth Analg. 2017 Sep;125(3):860-873. doi: 10.1213/ANE.0000000000001989. PMID: 28333706.
- McDuff DJ, Estepp JR, Piasecki AM, Blackford EB. A survey of remote optical photoplethysmographic imaging methods. Annu Int Conf IEEE Eng Med Biol Soc. 2015;2015:6398-404. doi: 10.1109/EMBC.2015.7319857. PMID: 26737757.
- Tan KS, Saatchi R, Elphick H, Burke D. Real-time vision based respiration monitoring system. IEEE. 2010;770-4. doi: 10.1109/CSNDSP16145.2010.5580316.
- Aoki T, Takemura S, Mimura F, Nakajima T. Development of non-restrictive Sensing System for sleeping person using fiber grating vision sensor. IEEE. 2001;155-60. doi: 10.1109/MHS.2001.965238.
- Rossol SL, Yang JK, Toney-Noland C, Bergin J, Basavaraju C, Kumar P, Lee HC. Non-Contact Video-Based Neonatal Respiratory Monitoring. Children (Basel). 2020 Oct 6;7(10):171. doi: 10.3390/children7100171. PMID: 33036226; PMCID: PMC7600716.
- Lorato I, Stuijk S, Meftah M, Kommers D, Andriessen P, van Pul C, de Haan G. Multi-camera infrared thermography for infant respiration monitoring. Biomed Opt Express. 2020 Aug 3;11(9):4848-4861. doi: 10.1364/BOE.397188. PMID: 33014585; PMCID: PMC7510882.
- Maurya L, Zwiggelaar R, Chawla D, Mahapatra P. Non-contact respiratory rate monitoring using thermal and visible imaging: a pilot study on neonates. J Clin Monit Comput. 2023 Jun;37(3):815-828. doi: 10.1007/s10877-022-00945-8. Epub 2022 Dec 4. PMID: 36463541; PMCID: PMC10175339.
- Pereira CB, Yu X, Goos T, Reiss I, Orlikowsky T, Heimann K, Venema B, Blazek V, Leonhardt S, Teichmann D. Noncontact Monitoring of Respiratory Rate in Newborn Infants Using Thermal Imaging. IEEE Trans Biomed Eng. 2019 Apr;66(4):1105-1114. doi: 10.1109/TBME.2018.2866878. Epub 2018 Aug 23. PMID: 30139045.
- Costanzo I, Sen D, Rhein L, Guler U. Respiratory Monitoring: Current State of the Art and Future Roads. IEEE Rev Biomed Eng. 2022;15:103-121. doi: 10.1109/RBME.2020.3036330. Epub 2022 Jan 21. PMID: 33156794.
- Liao SH, Chen WJ, Lu MSC. A CMOS MEMS capacitive flow sensor for respiratory monitoring. IEEE Sens J. 2013;13(5):1401-2. doi: 10.1109/JSEN.2013.2245320.
- Moradina S, Abdolvand R. MEMS-based passive wireless respiration profile sensor. IEEE. 2016;1-3. doi: 10.1109/ICSENS.2016.7808422.
- Mahbub I, Oh T, Shamsir S, Islam S, Pullano SA, Fiorillo AS. Design of a pyroelectric charge amplifier and a piezoelectric energy harvester for a novel non-invasive wearable and self-powered respiratory monitoring system. IEEE. 2017;105-8. doi: 10.1109/R10-HTC.2017.8288917.
- Vicente BA, Sebastião R, Sencadas V. Wearable devices for respiratory monitoring. Advanced functional materials. John Wiley and Sons Inc. 2024. doi: 10.1002/adfm.202404348.
- Vaidya R, Visintainer P, Singh R. Tidal volume measurements in the delivery room in preterm infants requiring positive pressure ventilation via endotracheal tube-feasibility study. J Perinatol. 2021 Aug;41(8):1930-1935. doi: 10.1038/s41372-021-01113-7. Epub 2021 Jun 10. PMID: 34112962; PMCID: PMC8191447.
- Poulton DA, Schmölzer GM, Morley CJ, Davis PG. Assessment of chest rise during mask ventilation of preterm infants in the delivery room. Resuscitation. 2011 Feb;82(2):175-9. doi: 10.1016/j.resuscitation.2010.10.012. Epub 2010 Nov 12. PMID: 21074926.
- Soliman MM, Ganti VG, Inan OT. Towards Wearable Estimation of Tidal Volume via Electrocardiogram and Seismocardiogram Signals. IEEE Sens J. 2022 Sep 15;22(18):18093-18103. doi: 10.1109/jsen.2022.3196601. Epub 2022 Aug 10. PMID: 37091042; PMCID: PMC10120872.
- Laufer B, Hoeflinger F, Docherty PD, Jalal NA, Krueger-Ziolek S, Rupitsch SJ, Reindl L, Moeller K. Characterisation and Quantification of Upper Body Surface Motions for Tidal Volume Determination in Lung-Healthy Individuals. Sensors (Basel). 2023 Jan 22;23(3):1278. doi: 10.3390/s23031278. PMID: 36772318; PMCID: PMC9920533.
- Chu M, Nguyen T, Pandey V, Zhou Y, Pham HN, Bar-Yoseph R, Radom-Aizik S, Jain R, Cooper DM, Khine M. Respiration rate and volume measurements using wearable strain sensors. NPJ Digit Med. 2019 Feb 13;2:8. doi: 10.1038/s41746-019-0083-3. PMID: 31304358; PMCID: PMC6550208.
- Kim JS, Truong T, Kim J. Development of Embroidery-Type Sensor Capable of Detecting Respiration Using the Capacitive Method. Polymers (Basel). 2023 Jan 18;15(3):503. doi: 10.3390/polym15030503. PMID: 36771802; PMCID: PMC9920054.
- Panahi A, Hassanzadeh A, Moulavi A. Design of a low cost, double triangle, piezoelectric sensor for respiratory monitoring applications. Sens Biosensing Res. 2020:30. doi: 10.1016/j.sbsr.2020.100378.
- Wang S, Tai H, Liu B, Duan Z, Yuan Z, Pan H, Yuanjie S, Guangzhong X, Xiaosong D, Yadong J. A facile respiration-driven triboelectric nanogenerator for multifunctional respiratory monitoring. Nano Energy. 2019;58:312-21. doi: 10.1016/j.nanoen.2019.01.042.
- Sweet DG, Carnielli VP, Greisen G, Hallman M, Klebermass-Schrehof K, Ozek E, Te Pas A, Plavka R, Roehr CC, Saugstad OD, Simeoni U, Speer CP, Vento M, Visser GHA, Halliday HL. European Consensus Guidelines on the Management of Respiratory Distress Syndrome: 2022 Update. Neonatology. 2023;120(1):3-23. doi: 10.1159/000528914. Epub 2023 Feb 15. PMID: 36863329; PMCID: PMC10064400.
- Reyes-Corral M, Sola-Idígora N, de la Puerta R, Montaner J, Ybot-González P. Nutraceuticals in the Prevention of Neonatal Hypoxia-Ischemia: A Comprehensive Review of their Neuroprotective Properties, Mechanisms of Action and Future Directions. Int J Mol Sci. 2021 Mar 3;22(5):2524. doi: 10.3390/ijms22052524. PMID: 33802413; PMCID: PMC7959318.
- Cafaro RP. Hypoxia: Its Causes and Symptoms. J Am Dent Soc Anesthesiol. 1960 Apr;7(4):4-8. PMID: 19598857; PMCID: PMC2067517.
- Mohamed T, Abdul-Hafez A, Gewolb IH, Uhal BD. Oxygen injury in neonates: which is worse? hyperoxia, hypoxia, or alternating hyperoxia/hypoxia. J Lung Pulm Respir Res. 2020;7(1):4-13. Epub 2020 Jan 29. PMID: 34337150; PMCID: PMC8320601.
- Perrone S, Bracciali C, Di Virgilio N, Buonocore G. Oxygen Use in Neonatal Care: A Two-edged Sword. Front Pediatr. 2017 Jan 9;4:143. doi: 10.3389/fped.2016.00143. PMID: 28119904; PMCID: PMC5220090.
- Morgan C, Newell SJ, Ducker DA, Hodgkinson J, White DK, Morley CJ, Church JM. Continuous neonatal blood gas monitoring using a multiparameter intra-arterial sensor. Arch Dis Child Fetal Neonatal Ed. 1999 Mar;80(2):F93-8. doi: 10.1136/fn.80.2.f93. PMID: 10325783; PMCID: PMC1720901.
- Jubran A. Pulse oximetry. Crit Care. 2015 Jul 16;19(1):272. doi: 10.1186/s13054-015-0984-8. PMID: 26179876; PMCID: PMC4504215.
- O'Brien F, Walker IA. Fluid homeostasis in the neonate. Paediatr Anaesth. 2014 Jan;24(1):49-59. doi: 10.1111/pan.12326. Epub 2013 Dec 4. PMID: 24299660.
- Phattraprayoon N, Sardesai S, Durand M, Ramanathan R. Accuracy of pulse oximeter readings from probe placement on newborn wrist and ankle. J Perinatol. 2012 Apr;32(4):276-80. doi: 10.1038/jp.2011.90. Epub 2011 Jul 7. PMID: 21738120.
- Punj J, Jaryal A, Mahalingam S, Mukundan C, Pandey R, Darlong V, Chandralekha. Toe gangrene in an infant subsequent to application of adult-type pulse oximeter probe for 10 min. J Anesth. 2010 Aug;24(4):630-2. doi: 10.1007/s00540-010-0930-5. PMID: 20390308.
- Dilli D, Soylu H, Tekin N. Neonatal hemodynamics and management of hypotension in newborns. Turk Pediatri Ars. 2018 Dec 25;53(Suppl 1):S65-S75. doi: 10.5152/TurkPediatriArs.2018.01801. PMID: 31236020; PMCID: PMC6568285.
- Verstraete EH, Blot K, Mahieu L, Vogelaers D, Blot S. Prediction models for neonatal health care-associated sepsis: a meta-analysis. Pediatrics. 2015 Apr;135(4):e1002-14. doi: 10.1542/peds.2014-3226. Epub 2015 Mar 9. PMID: 25755236.
- Verstraete EH, Blot K, Mahieu L, Vogelaers D, Blot S. Prediction models for neonatal health care-associated sepsis: a meta-analysis. Pediatrics. 2015 Apr;135(4):e1002-14. doi: 10.1542/peds.2014-3226. Epub 2015 Mar 9. PMID: 25755236.
- Schallom L, Sona C, McSweeney M, Mazuski J. Comparison of forehead and digit oximetry in surgical/trauma patients at risk for decreased peripheral perfusion. Heart Lung. 2007 May-Jun;36(3):188-94. doi: 10.1016/j.hrtlng.2006.07.007. PMID: 17509425.
- Berkenbosch JW, Tobias JD. Comparison of a new forehead reflectance pulse oximeter sensor with a conventional digit sensor in pediatric patients. Respir Care. 2006 Jul;51(7):726-31. PMID: 16800905..
- Stockwell SJ, Kwok TC, Morgan SP, Sharkey D, Hayes-Gill BR. Forehead monitoring of heart rate in neonatal intensive care. Front Physiol. 2023 Apr 4;14:1127419. doi: 10.3389/fphys.2023.1127419. PMID: 37082236; PMCID: PMC10110846.
- Dassel AC, Graaff R, Meijer A, Zijlstra WG, Aarnoudse JG. Reflectance pulse oximetry at the forehead of newborns: the influence of varying pressure on the probe. J Clin Monit. 1996 Nov;12(6):421-8. doi: 10.1007/BF02199702. PMID: 8982906.
- Berkenbosch JW, Tobias JD. Comparison of a new forehead reflectance pulse oximeter sensor with a conventional digit sensor in pediatric patients. Respir Care. 2006 Jul;51(7):726-31. PMID: 16800905.
- Grubb MR, Carpenter J, Crowe JA, Teoh J, Marlow N, Ward C, Mann C, Sharkey D, Hayes-Gill BR. Forehead reflectance photoplethysmography to monitor heart rate: preliminary results from neonatal patients. Physiol Meas. 2014 May;35(5):881-93. doi: 10.1088/0967-3334/35/5/881. Epub 2014 Apr 17. PMID: 24742972.
- Henry C, Shipley L, Ward C, Mirahmadi S, Liu C, Morgan S, Crowe J, Carpenter J, Hayes-Gill B, Sharkey D. Accurate neonatal heart rate monitoring using a new wireless, cap mounted device. Acta Paediatr. 2021 Jan;110(1):72-78. doi: 10.1111/apa.15303. Epub 2020 May 18. PMID: 32281685.
- Proenca M, Grossenbacher O, Dasen S, Moser V, Ostojic D, Lemkaddem A, Ferrario D, Lemay M, Wolf M, Fauchere JC, Karen T. Performance Assessment of a Dedicated Reflectance Pulse Oximeter in a Neonatal Intensive Care Unit. Annu Int Conf IEEE Eng Med Biol Soc. 2018 Jul;2018:1502-1505. doi: 10.1109/EMBC.2018.8512504. PMID: 30440677.
- van Weteringen W, Goos TG, van Essen T, Ellenberger C, Hayoz J, de Jonge RCJ, Reiss IKM, Schumacher PM. Novel transcutaneous sensor combining optical tcPO2 and electrochemical tcPCO2 monitoring with reflectance pulse oximetry. Med Biol Eng Comput. 2020 Feb;58(2):239-247. doi: 10.1007/s11517-019-02067-x. Epub 2019 Nov 18. PMID: 31741291; PMCID: PMC6994448.
- Thermal protection of the newborn: A practical guide. 1997.
- Lee EP, Yu MK, Lee SC, Gao FX, Wu HP. Predictive power of a single body temperature at different cutoff values for neonates in the nursery transferring to special care nursery. Medicine (Baltimore). 2018 Oct;97(42):e12619. doi: 10.1097/MD.0000000000012619. PMID: 30334946; PMCID: PMC6211842.
- Lunze K, Bloom DE, Jamison DT, Hamer DH. The global burden of neonatal hypothermia: systematic review of a major challenge for newborn survival. BMC Med. 2013 Jan 31;11:24. doi: 10.1186/1741-7015-11-24. PMID: 23369256; PMCID: PMC3606398.
- Nayeri F, Nili F. Hypothermia at birth and its associated complications in newborns: A follow up study. Iranian J Publ Health. 2006;35.
- Lubkowska A, Szymański S, Chudecka M. Surface Body Temperature of Full-Term Healthy Newborns Immediately after Birth-Pilot Study. Int J Environ Res Public Health. 2019 Apr 12;16(8):1312. doi: 10.3390/ijerph16081312. PMID: 31013692; PMCID: PMC6518189.
- Smith J. Methods and devices of temperature measurement in the neonate: A narrative review and practice recommendations. Newborn and Infant Nursing Reviews. 2014;14(2):64-71. doi: 10.1053/j.nainr.2014.03.001.
- Ji Y, Han D, Han L, Xie S, Pan S. The Accuracy of a Wireless Axillary Thermometer for Core Temperature Monitoring in Pediatric Patients Having Noncardiac Surgery: An Observational Study. J Perianesth Nurs. 2021 Dec;36(6):685-689. doi: 10.1016/j.jopan.2021.02.008. Epub 2021 Aug 9. PMID: 34384688.
- Hymczak H, Gołąb A, Mendrala K, Plicner D, Darocha T, Podsiadło P, Hudziak D, Gocoł R, Kosiński S. Core Temperature Measurement-Principles of Correct Measurement, Problems, and Complications. Int J Environ Res Public Health. 2021 Oct 10;18(20):10606. doi: 10.3390/ijerph182010606. PMID: 34682351; PMCID: PMC8535559.
- Callanan D. Detecting fever in young infants: reliability of perceived, pacifier, and temporal artery temperatures in infants younger than 3 months of age. Pediatr Emerg Care. 2003 Aug;19(4):240-3. doi: 10.1097/01.pec.0000086231.54586.15. PMID: 12972820.
- Press S, Quinn BJ. The pacifier thermometer. Comparison of supralingual with rectal temperatures in infants and young children. Arch Pediatr Adolesc Med. 1997 Jun;151(6):551-4. doi: 10.1001/archpedi.1997.02170430017003. PMID: 9193236.
- Mah AJ, Ghazi Zadeh L, Khoshnam Tehrani M, Askari S, Gandjbakhche AH, Shadgan B. Studying the Accuracy and Function of Different Thermometry Techniques for Measuring Body Temperature. Biology (Basel). 2021 Dec 15;10(12):1327. doi: 10.3390/biology10121327. PMID: 34943242; PMCID: PMC8698704.
- Lei D, Tan K, Malhotra A. Temperature Monitoring Devices in Neonates. Front Pediatr. 2021 Aug 24;9:732810. doi: 10.3389/fped.2021.732810. PMID: 34504819; PMCID: PMC8421638.
- Choi J, Bandodkar AJ, Reeder JT, Ray TR, Turnquist A, Kim SB, Nyberg N, Hourlier-Fargette A, Model JB, Aranyosi AJ, Xu S, Ghaffari R, Rogers JA. Soft, Skin-Integrated Multifunctional Microfluidic Systems for Accurate Colorimetric Analysis of Sweat Biomarkers and Temperature. ACS Sens. 2019 Feb 22;4(2):379-388. doi: 10.1021/acssensors.8b01218. Epub 2019 Feb 1. PMID: 30707572.
- Jeon HS, Kim JH, Jun MBG, Jeong YH. Fabrication of Thermochromic Membrane and Its Characteristics for Fever Detection. Materials (Basel). 2021 Jun 22;14(13):3460. doi: 10.3390/ma14133460. PMID: 34206427; PMCID: PMC8269493.
- Kumar A, Hsieh PY, Shaikh MO, Kumar RKR, Chuang CH. Flexible Temperature Sensor Utilizing MWCNT Doped PEG-PU Copolymer Nanocomposites. Micromachines (Basel). 2022 Jan 27;13(2):197. doi: 10.3390/mi13020197. PMID: 35208321; PMCID: PMC8875379.
- Dankoco MD, Tesfay GY, Benevent E, Bendahan M. Temperature sensor realized by inkjet printing process on flexible substrate. Materials Science and Engineering: B. 2016;205:1-5. doi: 10.1016/j.mseb.2015.11.003.
- Chen Y, Lu B, Chen Y, Feng X. Breathable and Stretchable Temperature Sensors Inspired by Skin. Sci Rep. 2015 Jun 22;5:11505. doi: 10.1038/srep11505. PMID: 26095941; PMCID: PMC4476093.
- Moser Y, Gijs MAM. Miniaturized flexible temperature sensor. Journal of Microelectromechanical Systems. 2007;16(6):1349-54. doi: 10.1109/JMEMS.2007.908437.
- Husain MD, Kennon R. Preliminary investigations into the development of textile based temperature sensor for healthcare applications. Fibers. 2013;1(1):2-10. doi: 10.3390/fib1010002.
- Oh S, Yoo JY, Maeng WY, Yoo S, Yang T, Slattery SM, Pessano S, Chang E, Jeong H, Kim J, Ahn HY, Kim Y, Kim J, Xu S, Weese-Mayer DE, Rogers JA. Simple, miniaturized biosensors for wireless mapping of thermoregulatory responses. Biosens Bioelectron. 2023 Oct 1;237:115545. doi: 10.1016/j.bios.2023.115545. Epub 2023 Jul 26. PMID: 37517336.
- Zeiner A, Klewer J, Sterz F, Haugk M, Krizanac D, Testori C, Losert H, Ayati S, Holzer M. Non-invasive continuous cerebral temperature monitoring in patients treated with mild therapeutic hypothermia: an observational pilot study. Resuscitation. 2010 Jul;81(7):861-6. doi: 10.1016/j.resuscitation.2010.03.018. Epub 2010 Apr 15. PMID: 20398992.
- Teunissen LP, Klewer J, de Haan A, de Koning JJ, Daanen HA. Non-invasive continuous core temperature measurement by zero heat flux. Physiol Meas. 2011 May;32(5):559-70. doi: 10.1088/0967-3334/32/5/005. Epub 2011 Mar 28. PMID: 21444968.
- Atallah L, Bongers E, Lamichhane B, Bambang-Oetomo S. Unobtrusive Monitoring of Neonatal Brain Temperature Using a Zero-Heat-Flux Sensor Matrix. IEEE J Biomed Health Inform. 2016 Jan;20(1):100-7. doi: 10.1109/JBHI.2014.2385103. Epub 2014 Dec 22. PMID: 25546867.
- Premarket assessment of pediatric medical devices guidance for industry and food and drug administration staff. This document supersedes “Guidance for industry and FDA staff: Premarket assessment of pediatric medical devices” dated Preface Public Comment.
- Hwang TJ, Kesselheim AS, Bourgeois FT. Postmarketing trials and pediatric device approvals. Pediatrics. 2014 May;133(5):e1197-202. doi: 10.1542/peds.2013-3348. Epub 2014 Apr 14. PMID: 24733871; PMCID: PMC4531281.
- SECTION ON CARDIOLOGY AND CARDIAC SURGERY; SECTION ON ORTHOPAEDICS. Off-Label Use of Medical Devices in Children. Pediatrics. 2017 Jan;139(1):e20163439. doi: 10.1542/peds.2016-3439. PMID: 28025239.
- Espinoza J, Shah P, Nagendra G, Bar-Cohen Y, Richmond F. Pediatric Medical Device Development and Regulation: Current State, Barriers, and Opportunities. Pediatrics. 2022 May 1;149(5):e2021053390. doi: 10.1542/peds.2021-053390. PMID: 35425971.
- Almond CS, Chen EA, Berman MR, Less JR, Baldwin JT, Linde-Feucht SR, Hoke TR, Pearson GD, Jenkins K, Duncan BW, Zuckerman BD. High-risk medical devices, children and the FDA: regulatory challenges facing pediatric mechanical circulatory support devices. ASAIO J. 2007 Jan-Feb;53(1):4-7. doi: 10.1097/01.mat.0000247958.84788.3a. PMID: 17237642.
- Bui AL, Dieleman JL, Hamavid H, Birger M, Chapin A, Duber HC, Horst C, Reynolds A, Squires E, Chung PJ, Murray CJ. Spending on Children's Personal Health Care in the United States, 1996-2013. JAMA Pediatr. 2017 Feb 1;171(2):181-189. doi: 10.1001/jamapediatrics.2016.4086. PMID: 28027344; PMCID: PMC5546095.
- Catenaccio E, Rochlin JM, Simon HK. Differences in Lifetime Earning Potential Between Pediatric and Adult Physicians. Pediatrics. 2021 Aug;148(2):e2021051194. doi: 10.1542/peds.2021-051194. PMID: 34330865.
- Gitterman DP, Langford WS, Hay WW Jr. The Fragile State of the National Institutes of Health Pediatric Research Portfolio, 1992-2015: Doing More With Less? JAMA Pediatr. 2018 Mar 1;172(3):287-293. doi: 10.1001/jamapediatrics.2017.4931. PMID: 29340575.
- Gottlieb ER, Ziegler J, Morley K, Rush B, Celi LA. Assessment of Racial and Ethnic Differences in Oxygen Supplementation Among Patients in the Intensive Care Unit. JAMA Intern Med. 2022 Aug 1;182(8):849-858. doi: 10.1001/jamainternmed.2022.2587. PMID: 35816344; PMCID: PMC9274443.
- Zonios G, Bykowski J, Kollias N. Skin melanin, hemoglobin, and light scattering properties can be quantitatively assessed in vivo using diffuse reflectance spectroscopy. J Invest Dermatol. 2001 Dec;117(6):1452-7. doi: 10.1046/j.0022-202x.2001.01577.x. PMID: 11886508.
- Qian C, Ye F, Li J, Tseng P, Khine M. Wireless and battery-free sensor for interstitial fluid pressure monitoring. Sensors. 2024;24(14):4429. doi: 10.3390/s24144429.
- Barreto J, Perez G, Kaddour AS, Georgakopoulos S V. A Study of wearable wireless power transfer systems on the human body. IEEE Open Journal of Antennas and Propagation. 2021;2:86-94. doi: 10.1109/OJAP.2020.3043579.
- Chen LY, Tee BC, Chortos AL, Schwartz G, Tse V, Lipomi DJ, Wong HS, McConnell MV, Bao Z. Continuous wireless pressure monitoring and mapping with ultra-small passive sensors for health monitoring and critical care. Nat Commun. 2014 Oct 6;5:5028. doi: 10.1038/ncomms6028. PMID: 25284074.
- Ulrich LC, Joseph FD, Lewis DY, Koenig RL. FDA's pediatric device consortia: national program fosters pediatric medical device development. Pediatrics. 2013 May;131(5):981-5. doi: 10.1542/peds.2012-1534. Epub 2013 Apr 8. PMID: 23569100.
- FDA News Release FDA awards ve grants to advance the development of pediatric medical devices. 2018.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.