Environmental Sciences Group. 2024 July 08;5(7):718-728. doi: 10.37871/jbres1947.
Environmental Impacts of Emerging Micropollutants in the Environment: Chemical Properties, Behavior, Toxicology, Health Effects and Fate: A Review
Hakime Mohammadzade1, Halime Almasi2*, Mojtaba kalantar3 and Sahand Jorfi4
2Department of Environmental Health Engineering, Shoushtar Faculty of Medical Sciences, Shoushtar, Iran
3Department of Occupational Health and Safety, Shoushtar Faculty of Medical Sciences, Shoushtar, Iran
4Environmental Technologies Research Center, Department of Environmental Health Engineering, Ahvaz Jundishapur University of Medical Sciences. Ahvaz, Iran
E-mail:
- Emerging micro pollutant
- Properties
- Behavior
- Toxicology
Abstract
In recent years, the contamination of the environment by emerging micro-pollutants has become a significant concern for various communities. Due to the recent identification of these substances as environmental pollutants, there is limited information available regarding their fate and toxicity in the environment. Consequently, the scientific community has extensively debated the spread and fate of these emerging environmental contaminants. Additionally, the continuous production of many of these substances in societies has left their potential impacts on humans and the environment unclear. At present, more information is needed about the current concentrations of emerging pollutants. This study provides an overview of the most significant micropollutants, focusing on the most significant emerging pollutants, assess their potential toxic effects on human health, and understand their behavior once they enter the environment.
Introduction
Emerging Micropollutants (EMPs) refers to rare organic compounds that are currently in use and are released into the environment. The category of EMPs compasses substances that exhibit distinct toxicity, behavior, and treatment methods compared to other pollutants [1,2]. The European Union has compiled a list of 242 chemical substances, approximately 70 percent of which belong to the categories of pharmaceuticals (antibiotics, steroids) and personal care products (antimicrobials, UV filters) while the remaining 30 percent includes industrial substances like Endocrine-Disrupting Chemicals (EDCs), Pesticides, Perfluorinated Compounds (PFCs), Disinfection by-Products in water (DBPs), additives, preservatives, detergents, surfactants, fire retardants, and softeners along with their derived products [3,4]. These compounds are usually reported in very small amounts in the body (typically in the range of micrograms to nanograms per liter). Even in low concentrations, they can harm human health, the environment or drinking water sources [5,6]. UV filters protect against the harmful effects of the sun's UV rays. Many personal care products contain these chemicals, including sunscreens, shampoos, creams, and industrial applications like food packaging, textiles, vehicle coatings, and photographic gear. Furthermore, many personal care products, including soap, lotions, toothpaste, sunscreen, perfumes, and moisturizers, contain micropollutants like triclosan [7].
Human activity has resulted in the presence of tiny biological micro-contaminants like bacteria, mycoplasma, viruses, and protozoa in water reservoirs. These small pollutants cause many water-borne diseases, contributing to high death rates worldwide. Examples of these pathogens in water include cryptosporidium, legionella, rotavirus, and hepatitis A virus [2,8].
Based on the concerns highlighted, the primary goal of this article is to provide a comprehensive review of the most significant Emerging Micropollutants (EMPs), focusing on their sources, behaviors, and potential toxic effects on human health and the environment. By synthesizing the latest research and data, this study aims to enhance our understanding of EMPs and underscore the urgent need for more detailed environmental monitoring and effective management strategies. Through this work, we hope to contribute to the broader scientific discourse and inform policy-making to better address the risks associated with these pollutants.
Endocrine disrupting compounds
Endocrine-Disrupting Compounds (EDCs) are increasingly worrying for environmental health because they interfere with natural hormones in living organisms are known as endocrine disruptors. Despite their significance, we still don't know much about how these chemicals behave or their full environmental impact, as only a few have been studied globally [9]. There are many types of EDCs, including pesticides, herbicides, fungicides, industrial chemicals (such as PCBs), heavy metals (such as cadmium), plastic-related products (such as bisphenol A and phthalates), and household products (such as alkyl phenols) (Table 1) [10,11]. In addition to biocides, pesticides, moisturizers, and detergents, they are found in many household products. EU guidelines identify some EDCs as priority pollutants because of their harmful effects on estrogen in organisms, such as 4-nonylphenols and diclofenac. Bisphenol A is still debated as an environmental and health hazard [12].
| Table 1: Endocrine Disrupting Chemicals: Sources, Environments, and Biological Mechanisms [15] . | ||
| Chemical class | Resources | Matrix |
| Organic chlorinated compounds | Banned under the Stockholm Convention, many compounds still persist globally. | Water and wastewater, biosolids, soil, sediments, tissue, urine and breast milk serum |
| Halogenated aromatic hydrocarbons (HAHs) | Banned under the Stockholm Convention, PCBs, as well as restricted compounds like PCDDs and PCDFs, still be released into the environment from sources such as incinerators and cement kilns. | Water and wastewater, biosolids, soil, sediments, tissue, urine and breast milk serum |
| Brominated flame retardants | While BDEs (Brominated Flame Retardants) are restricted under the Stockholm Convention, they still persist in the environment. As substitutes, flame retardants such as TBBPA, HBCDs, and DBDPE | Water and wastewater, biosolids, soil, sediments, tissue, urine and breast milk serum |
| Phenolic compounds | Nonylphenol is a degradation product of alkylphenol ethoxylate surfactants, commonly found in detergents, pesticide formulations, and other products. Octylphenol, on the other hand, is utilized as an industrial surfactant. "BPA, which is present in polycarbonate plastics and epoxy resins, has raised concerns regarding its potential health effects. As a result, structural analogs are being increasingly employed as alternatives to BPA | Water and wastewater, biosolids, soils and sediments, food and beverages, urine and hair serum |
| Phthalates | Lubricants are commonly used as additives in PVC plastics. It's important to note that monoester metabolites of these additives may have potential endocrine effects | Water and wastewater, soil biosolids, sediments, food and drinking tissue, urine and serum |
| Drugs, illegal drugs and hormones | Drugs discharged from municipal sewage treatment plants | Biosolids water and wastewater, soil and sediments |
| Personal care products | Discharge from sewage treatment plants. Sunscreen and insect repellent may be washed off the skin by outdoor bathers. | Water and sewage, biosolids, soil and sediments |
EDCs, similar to steroid hormones, can be identified through hydroxyl groups separated by a hydrophobic region. Their profound and permanent effects on children during critical development periods, including birth defects and behavioral changes, are a major concern [13].
There is growing concern because EDCs have been shown to cause harm even at very low concentrations, though we still lack comprehensive knowledge about their effects on aquatic life and human health. Disruption of the Estrogen Receptor (ER) is the most reported mechanism of endocrine disruption. EDCs tend to weakly bind to Estrogen Receptors (ER) compared to natural and synthetic steroid hormones, resulting in a much weaker response [14]. However, some of these low-potency EDCs are present in the environment at much higher concentrations than steroid hormones. For example, the industrial chemicals nonylphenol and bisphenol have been detected in surface waters at concentrations in the micrograms per liter range, while steroid hormones are present only in low concentrations. Although the concentration of most EDCs in the aquatic environment is less than the amount required to cause visible physiological effects, these small effects accumulate over time until the cumulative amount of these effects reaches an irreversible change [14].
Organic chlorinated compounds
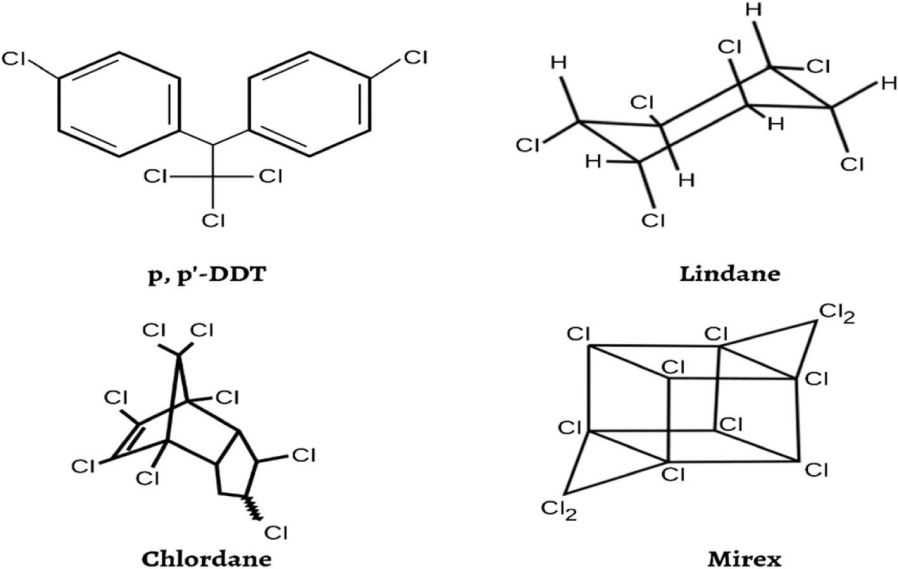
Environmental pollution from organochlorine compounds, especially pesticides, has increased due to rapid urbanization and industrial activities. Between 1950 and 1970, organochlorine insecticides were widely used in pest management (Figure 1). Despite some being phased out, certain OCPs like lindane and endosulfan are still in use. OCPs possess chlorine-substituted aliphatic or aromatic ring structures, making them slightly soluble and semi-volatile. Their exceptional stability enables them to persist in the environment for extended periods, and they are globally ubiquitous [16,17].
The tendency of a chemical to approach depends on the ethanol/water partition coefficient, which is very important for predicting the behavior of that substance in the environment. The fate and behavior of pollutants like OCPs are influenced by phase distribution and transfer processes, such as vapor pressure, water solubility, octanol-water partition coefficient, Henry's law constant, octanol-air partition coefficient, and ethanol solubilit [18]. OCPs were among the first Persistent Organic Pollutants (POPs) included in the Stockholm Protocol, including HCB, DDT, chlordane, heptachlor, dieldrin, and endrin. Substances like pentachlorobenzene and hexachlorobenzene are used as raw materials or by-products in certain industrial chemicals and are contaminants in various pesticide formulations. They have diverse structures with multiple chlorine substitutions [19].
Because of its usage and physicochemical characteristics (toxicity, resistance to degradation, lipophilicity, poor water solubility), DDT is spreading globally, which is alarming. Bird populations are negatively pacted by the stable metabolites that DDT produces, such as Dichlorodiphenyldichloroethylene (DDE) and dichlorothyelene. As a result of growing awareness of these risks, DDT use and manufacturing were restricted beginning in the 1970s [16].
Toxicology and health effects: The Stockholm Convention highlights significant health risks associated with twelve Organochlorine Pesticides (OCPs), emphasizing DDT and HCH due to their potential to cause organ failure, endocrine disruption, and malignant tumours [20]. HCH compounds exist in the form of α, β, γ and δ isomers and are the most common organic halogenated insecticides. The persistent nature, toxicity and tendency of HCHs to accumulate in organisms led to their ban. Also, the US Environmental Protection Agency has reported DDT as a potential carcinogen [21,22].
Halogenated aromatic hydrocarbons
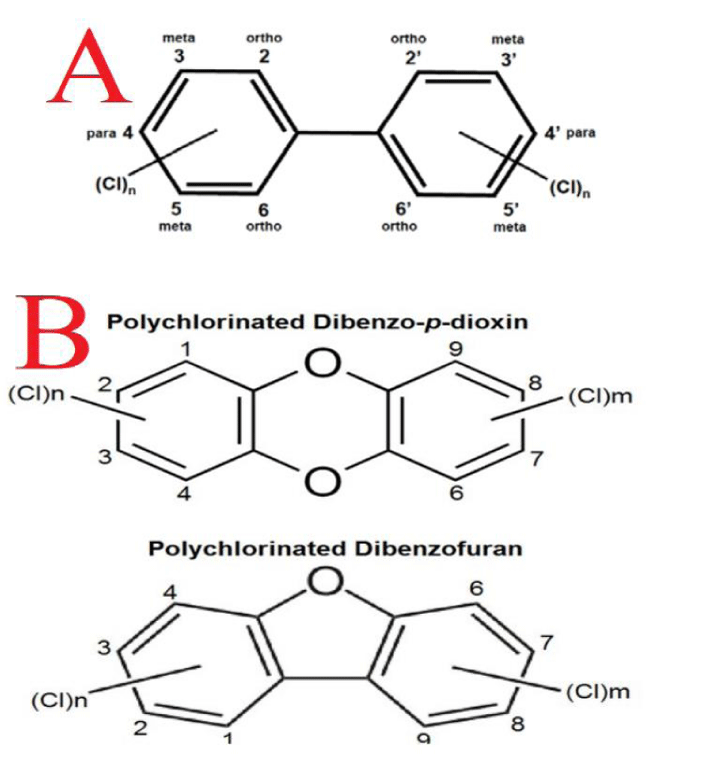
Increased attention is being given to Halogenated Aromatic Hydrocarbons (HAHs) like Polychlorinated Biphenyls (PCBs) and Polychlorinated Dibenzodioxins and Furans (PCDD/DFs). PCBs are synthetic compounds with two benzene rings and multiple chlorine atoms (Figures 2(A,B)). There are 209 possible PCB compounds, with about 100 detectable in the environment [23]. Due to the chemical stability of PCBs, it is not possible to break them through conventional methods. PCBs have a high affinity for lipids and can easily dissolve or accumulate in the fatty tissues of animals and magnify as they move up the food chain. Also, Polychlorinated Dibenzo-P-Dioxins (PCDD) and Dibenzofurans (PCDFs) have diverse structures (Figure 2B) [23].
The most toxic are those substituted at the 2, 3, 7, and 8 positions, especially 2,3,7,8-TCDD(2,3,7,8-Tetrachlorodibenzo-p-Dioxin). The main sources of these chemicals are the combustion of chlorinated organic molecules or the industrial production of organic compounds in the presence of chlorine [24]. PCBs (polychlorinated biphenyls) are formed in the synthesis of chlorobenzene, chlorinated solvents (such as chloroform), chlorinated alkanes, certain pigments, and during the thermal decomposition of chlorinated organic compounds, such as municipal waste incinerators [19].
Toxicology and health effects: PCBs and PCDD/DFs, categorized as Persistent, Bio accumulative, and Toxic (PBT) compounds, pose significant environmental and health risks due to their persistence and bioaccumulation in the food chain. Depending on their chlorination pattern, PCBs can mimic estrogen, act as anti-androgens, and even interfere with thyroid hormone and Ah receptors [25]. The number and placement of chlorine substituents around the double phenolic ring, along with its half-life in the environment or tissue, determine the structure and function of a PCB compound. As shown by Zhang W, et al. [26] four homologous PCBs have chlorine atoms in lateral positions (i.e., meta or para) and none of them are substituted in the ortho position. These are homologous PCBs 77, 81, 126, and 169. The structure and function of PCBs depend on the number and placement of chlorine atoms. PCBs with chlorine atoms in lateral positions (homologous PCBs 77, 81, 126, 169) exhibit dioxin-like toxicity by binding to the Ah receptor.
"Low-chlorine" PCBs (1-3 chlorine atoms) are weakly estrogenic, while highly chlorinated PCBs are more persistent, with half-lives of 20 years or more. Despite bans, PCBs are still found in human and wildlife tissues, and climate change may release more PCBs into the environment [19]. Research shows that PCDD/DFs and PCBs strongly bind to the Ah receptor, but other compounds in this class can still cause endocrine disorders by interfering with hormone binding to thyroid and steroid receptors [25].
Brominated flame retardants
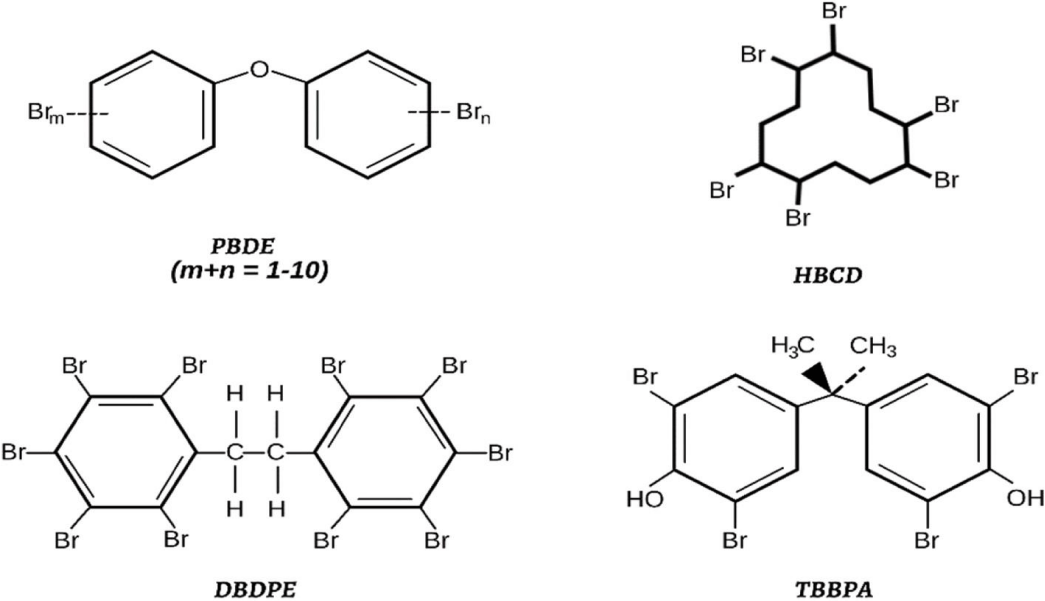
In 1999, high levels of polybrominated diphenyl ethers (PBDEs) were found in the serum of workers at an electronics assembly plant. PBDEs and other brominated flame retardants (BFRs) used in commercial materials have been detected globally in environmental and human samples [19]. The structure of some BFR classes is shown in figure 3. Recent study examined 63 new BFRs in indoor air, dust, consumer goods, and food. There is still limited data on identifying these compounds. New BFRs have also been found in living organisms and human tissues. Current data suggests that new BFRs are not necessarily safer than the established classes [27,28].
Toxicology and health effects: In the European Union, certain Brominated Flame Retardants (BFRs) are prohibited or restricted due to environmental persistence and health risks. However, BFR-treated products can still release these chemicals, contaminating air, soil, and water, and entering the food chain, particularly in animal-derived foods like fish, meat, and milk [28].
Mixtures of Polybrominated Diphenyl Ethers (PBDEs) and other BFRs such as Hexabromobiphenyl (HBB) and Hexabromocyclododecane (HBCD) are recognized as Persistent Organic Pollutants (POPs) and are targeted for elimination under the Stockholm Convention. Despite the phase-out, concerns remain about the endocrine effects of other BFRs like Tetrabromobisphenol A (TBBPA) and Decabromodiphenylethane (DBDPE). Recent researchs are exploring the transformation and environmental fate of both established and novel Brominated Flame Retardants (BFRs) [19].
Phenolic compounds
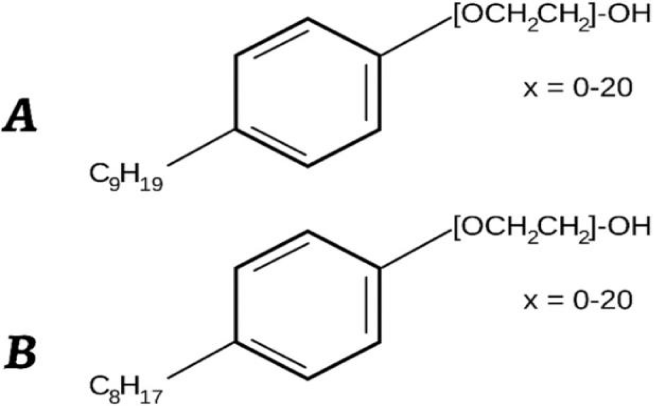
Researchers closely monitor alkylphenol compounds like Nonylphenol Ethoxylates (NPEOs) and their breakdown product, Nonylphenol (NP), which are known as xenoestrogens. Octylphenol (OP), also found in some commercial products, is a known xenoestrogen. Analyzing Alkylphenol Ethoxylates (APEs) in the environment is complex due to the various isomers of NP, OP, and NPEOs attached to a phenol ring in commercial products (Table 2) [29].
| Table 2: The main characteristics of the most abundant phenolic compounds [29]. | |||
| Bisphenol A (BPA) | Nonylphenol (NP) | Octylphenol (OP) | |
| Structure | |||
| Molecular Weight (g/mol) | 228.3 | 220.3 | 206.3 |
| Solubility (mg/L) | 300 | 6.23 | 4.8 |
| log Kow | 3.4 | 4 | 4.6 |
| pKa | 9.6 | 10.7 | 10.3 |
There are different isomers depending on which carbon of the phenolic ring the alkyl chain is attached to, resulting in 2-, 3-, and 4-alkylphenols for meta, ortho, and para positions, respectively. However, 4-nonylphenols (4-NP) and 4-octylphenols (4-OP) make up over 80% of the total production. In NPEOs, an ethoxylate chain with a terminal hydroxyl group replaces the hydroxyl group on the phenolic ring of NPs (Figure 4) [19].
Octylphenol (OP) and Nonylphenol (NP) are common alkylphenols used in various industries such as detergents, emulsifiers, dyes, pesticides, pharmaceuticals, cosmetics, and plastics, having been in use for over 60 years [30]. Table 1 provides the main physical and chemical properties of these compounds. OP and NP are hydrophobic, with solubility values of 4.8 mg/L and 6.23 mg/L, respectively, while Bisphenol A (BPA) is more soluble in water at 300 mg/L [30]. BPA is used in making polycarbonate plastics and epoxy resins, commonly found in food storage containers, water bottles, and can coatings [31].
Human exposure to BPA mainly occurs through diet, and it has been linked to endocrine-disrupting effects on mammary gland growth, brain development, and memory function [32]. The US EPA and Health Canada have set daily intake limits for BPA at 50 μg/kg and 25 μg/kg body weight, respectively. Canada has regulated BPA as a toxic substance and banned polycarbonate baby bottles containing BPA since 2010 [32].
Phenolic compounds like NP, OP, and BPA are stable, toxic, and accumulate in the environment, mainly from wastewater discharge, industrial areas, landfills, and agricultural runoff. These compounds are semi-volatile and can be transported to aquatic and terrestrial ecosystems through sedimentation. They are often found as stable intermediates of Alkylphenol Polyethoxylates (APEOs) in the environment, with low solubility and hydrophobic properties leading to accumulation in sediments and aquatic organisms [33]. LogKoc values for OP, NP, and BPA range from 4.63 to 83.5, 4.95 to 6.62, and 5.12 to 32.5, respectively. Biological concentration factors have been reported between 21 and 1300 for NP, 276 and 471 for OP, and 13.10 and 147.71 for BPA [34].
Toxicology and health effects: Phenolic compounds NP, OP, and BPA can mimic estrogen effects similar to 17β-estradiol. Soto, et al. [35], first observed NP inducing mammary tumor cell proliferation in experimental tests. Additionally, NP and OP were found to induce vitellogenin proliferation in male fish [36]. BPA poses specific risks to unborn babies, infants, and young children, as highlighted by Gusmaroli [37]. In 2015, the European Food Safety Authority reduced the acceptable daily intake of BPA from 50 to 4 micrograms per kilogram of body weight, affirming that current exposure levels do not pose a human health risk [38].
Perfluorinated compounds
Perfluorinated Compounds (PFCs) or Perfluoroalkyl Compounds (PFAS) are highly stable chemicals with fully fluorinated hydrophobic carbon chains and polar hydrophilic end groups. Since the late 1950s [39], they have been widely used in industrial and consumer products like firefighting foams, paints, non-stick cookware, textiles, and Teflon. The most commonly detected PFCs in the environment are Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS), extensively produced since 1940 [29].
PFOS became a listed Persistent Organic Pollutant (POP) under Annex B of the Stockholm Convention, while the US EPA aimed to phase out PFOA through its 2010/2015 Stewardship Plan. Manufacturing now focuses on shorter-chain (8C chain) PFCA and PFSA compounds, which are more soluble and less likely to bioaccumulate [40]. PFOA, representative of PFCA, has high water solubility (3400 mg/L) and behaves as an organic acid (pKa 2.5), primarily existing as free anions in aqueous environments, where they can form micelles under certain conditions [41].
PFOS and PFSAs have lower solubility compared to carboxylic acids like PFOA. For example, the solubility of PFOS is about 370 mg/L, and its estimated pKa is 3.3 [42]. PFOS anions can strongly bind with cations in solution, enhancing the desalination effect in water with higher dissolved solids. In seawater, PFOS solubility is much lower, measuring less than 12.4 mg/L [41]. Generally, the solubility of both PFCA and PFSA compounds decreases as molecular weight increases due to longer hydrophobic carbon chains [37]. Evaluating the octanol-water partition coefficient (Kow) for PFCs, which is important for understanding their potential to accumulate in living organisms [43].
However, logKow is not suitable for predicting their bioaccumulation properties because PFCs typically bind to proteins rather than lipids. PFCs enter the environment through industrial and consumer products, with secondary releases from melting ice, precipitation, or sediment resuspension impacting their environmental fate [44]. In biological organisms, PFCs' bioconcentration and bioaccumulation depend largely on their carbon chain length. Perfluoro Sulfonic Acids (PFSAs) are more likely to bioaccumulate compared to Perfluoro Carboxylic Acids (PFCAs), with PFOS being the most commonly detected compound [45,46]. Longer-chain PFCs exhibit higher bioconcentration potential, whereas PFHxA and PFHpA show lower Bioconcentration Factors (BCF). PFCs undergo biomagnification in the food chain, evidenced by Biomagnification Factors (BMF>1) in predator-prey PFC concentration comparisons [47].
Unlike many organic pollutants, PFCs preferentially accumulate in proteins rather than lipids, leading to their highest concentrations in blood, liver, and kidneys. Blood samples typically contain lower PFOA concentrations than PFOS, but muscle tissue remains a concern due to human exposure risks from contaminated fish and meat consumption [48].
The behavior of PFCs after release into the environment largely depends on their perfluorocarbon chain length and functional groups, which impart their hydrophobic properties. PFSAs, due to their structure, strongly adsorb onto sediments compared to PFCAs [48]. On average, PFSAs exhibit stronger adsorption onto sediments than PFCAs. Many studies have indicated that a key environmental parameter influencing pollutant transport and adsorption in soil or sediment is the organic carbon content, which dictates the equilibrium between the hydrophobic forces of PFCs and the organic matter water repellency in soil, and the repulsive forces between anionic PFCs and negatively charged carboxylic groups present in soil organic matter. A common parameter used to estimate PFC adsorption is the solid-water distribution coefficient (Kd) specific to PFCs, correlated with normalized organic carbon content (Koc) [49]. Generally, longer-chain PFCs (> C8) exhibit stronger adsorption to organic matter with a linear relationship between logKoc and perfluoroalkyl chain length. Various laboratory studies have shown that the soil-water distribution coefficient (Kd) increases linearly with the addition of organic matter [49].
Fate
The fate, distribution, and reactions of a chemical substance in the environment depend on several factors: 1-Sources and Release Characteristics: Including production volume, type of use, and release patterns, influencing its overall environmental presence [50]. 2- Ecosystem Properties: Such as temperature, pH, salinity, suspended load, sedimentation rate, nutrient cycles, and redox conditions, affecting the substance's behavior and distribution [50]. 3- Transformation Processes: Including photolysis, hydrolysis, redox reactions, and biological degradation, which can alter the chemical structure and behavior of the substance in the environment. 4- Substance Properties: Such as its molecular structure, solubility, volatility, partition coefficients, and potential for bioaccumulation and biodegradation, which impact its environmental fate and effects [50].
When organic chemicals, including micropollutants, enter the environment, they undergo physical, chemical, and biological processes that can either maintain their chemical structure or transform them into different substances products products [50]. These processes are broadly categorized into two groups:
- Maintaining Chemical Structure: Includes transfer and mixing processes within water bodies or sediments, precipitation, and absorption by organisms.
- Chemical, Photochemical, and Biological Changes: Involves transformations driven by chemical reactions, sunlight, and microbial activity. Adsorption onto sediments and suspended particles is a critical non-transformative process that shields micropollutants from degradation processes like biodegradation and photodegradation, thereby reducing their bioavailability products [50].
The extent of adsorption in water environments depends on the chemical structure of the pollutant and the presence of minerals and organic substances in sediments or suspended materials. Chemicals with charged functional groups tend to adsorb onto oppositely charged surfaces like mineral surfaces through electrostatic interactions [50]. Neutral chemicals preferentially adsorb onto sediments and suspended particles with neutral hydrophobic surfaces, and this adsorption is enhanced in systems with higher organic carbon content. Triclosan, with a pKa of 1.8, exists predominantly in its anionic form (40-50%) in seawater with a pH around 8, influencing its adsorption behavior [51,52].
The capacity of organic carbon to absorb micropollutants is assessed through the partition coefficient normalized to organic carbon, indicating their affinity for sediments and suspended particles [50]. Another measure, the n-octanol/water distribution coefficient (KOW), highlights those compounds with high molecular weight and KOW values less than 5 are readily absorbed by sediments. Persistent organic pollutants typically have KOW values greater than 5 [52]. Micropollutants accumulate in aquatic organisms primarily through direct partitioning among sediment, water, and organisms, and also through consumption of contaminated food and internal transfers within organisms, accumulating in tissues such as lipids, proteins, and polysaccharides [51,52].
The fate of Organic Micropollutants (OMPs) in aquatic environments hinges on their physicochemical properties and the efficiency of wastewater treatment. Each OMP possesses distinct characteristics—polarity, adsorption tendencies, solubility, pKa, ability to dissolve in water, chemical structure (including reactive groups), and volatility—all influencing their distribution in the environment and susceptibility to microbial degradation or alteration [53,54]. Polar OMPs like Pharmaceuticals and Personal Care Products (PPCPs), soluble in water, are often preferentially removed by microbial processes from wastewater. Conversely, semi-polar and lipophilic compounds tend to adsorb onto carbon-based surfaces such as sludge, soil, or plant roots [53,54].
Conclusion
The increasing contamination of the environment by Emerging Micropollutants (EMPs) such as endocrine-disrupting chemicals, organic chlorinated compounds, halogenated aromatic hydrocarbons, brominated flame retardants, phenolic compounds, and perfluorinated compounds is a significant environmental and public health concern. These substances, often present in minute quantities, can have profound and lasting impacts on human health and ecosystems due to their persistence, bioaccumulation, and potential to disrupt hormonal systems. Despite regulatory efforts and restrictions on many of these compounds, their continued presence in the environment highlights the need for improved monitoring and management strategies. The toxicological effects of these pollutants, particularly on vulnerable populations like children, and their complex behavior in the environment underscore the urgent necessity for comprehensive research and effective policy interventions. This review emphasizes the critical importance of advancing our understanding of EMPs to mitigate their risks and protect both environmental and human health.
Acknowledgment
The Research Department has provided at the Shoushtar Faculty of Medical Sciences.
Ethical Approval
IR.SHOUSHTAR.REC.1403.
Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author Contributions
S J, HM, MHE and MK conceived and designed the study. HA and SJ performed the literature search and wrote the manuscript. All authors participated in the data acquisition, analysis and interpretation. All authors critically reviewed, refined and approved the manuscript.
Funding
This research was financially supported by the Research Department at Shoushtar Faculty of Medical Sciences.
rticle Highlights
- Significant challenges facing the environment and human health is control of risks associated with emerging pollutants.
- Harmful effects of endocrine disruptors on wildlife have been extensively studied, less is known about their adverse effects on human health.
- Summary of chemical, fate and health effects of emerging pollutants in different environments.
References
- Rizzo L, Malato S, Antakyali D, G. Beretsou V, B. Đolić M, Gernjak W, Heath E, Ivancev-Tumbas I, Karaolia P, R. Lado Ribeiro A, Mascolo G, S. McArdell C, Schaar H, M.T. Silva A, Fatta-Kassinos D. Consolidated vs. new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Science of the Total Environment. 2019;655:986-1008. doi: 10.1016/j.scitotenv.2018.11.265.
- Rede D, Teixeira I, Delerue-Matos C, Fernandes VC. Assessing emerging and priority micropollutants in sewage sludge: environmental insights and analytical approaches. Environ Sci Pollut Res Int. 2024 Jan;31(2):3152-3168. doi: 10.1007/s11356-023-30963-1. Epub 2023 Dec 12. PMID: 38085484; PMCID: PMC10791843.
- Arslan M, Ullah I, Müller JA, Shahid N. Organic micropollutants in the environment: Ecotoxicity potential and methods for remediation. Enhancing cleanup of environmental pollutants. 2017; 65-99. doi: 10.1007/978-3-319-55426-6.
- Das S, Ray NM, Wan J, Khan A, Chakraborty T, Ray MB. Micropollutants in wastewater: Fate and removal processes. Physico-chemical wastewater treatment and resource recovery. 2017;3:75-117. doi: 10.5772/65644.
- Rogowska J, Cieszynska-Semenowicz M, Ratajczyk W, Wolska L. Micropollutants in treated wastewater. Ambio. 2020 Feb;49(2):487-503. doi: 10.1007/s13280-019-01219-5. Epub 2019 Jul 10. PMID: 31292910; PMCID: PMC6965340.
- Brausch JM, Rand GM. A review of personal care products in the aquatic environment: environmental concentrations and toxicity. Chemosphere. 2011 Mar;82(11):1518-32. doi: 10.1016/j.chemosphere.2010.11.018. Epub 2010 Dec 23. PMID: 21185057.
- Rajasekar M, Mary J, Sivakumar M, Selvam M. Recent developments in sunscreens based on chromophore compounds and nanoparticles. RSC advances. 2024;14(4):2529-2563. doi: 10.1039/D3RA08178H.
- Gavrilescu M, Demnerová K, Aamand J, Agathos S, Fava F. Emerging pollutants in the environment: present and future challenges in biomonitoring, ecological risks and bioremediation. N Biotechnol. 2015 Jan 25;32(1):147-56. doi: 10.1016/j.nbt.2014.01.001. Epub 2014 Jan 21. PMID: 24462777.
- Encarnação T, Pais AA, Campos MG, Burrows HD. Endocrine disrupting chemicals: Impact on human health, wildlife and the environment. Sci Prog. 2019 Mar;102(1):3-42. doi: 10.1177/0036850419826802. Epub 2019 Jan 1. PMID: 31829784; PMCID: PMC10424550.
- Adegoke EO, Rahman MS, Park YJ, Kim YJ, Pang MG. Endocrine-Disrupting Chemicals and Infectious Diseases: From Endocrine Disruption to Immunosuppression. Int J Mol Sci. 2021 Apr 11;22(8):3939. doi: 10.3390/ijms22083939. PMID: 33920428; PMCID: PMC8069594.
- Stiefel C, Stintzing F. Endocrine-active and endocrine-disrupting compounds in food-occurrence, formation and relevance. NFS Journal. 2023;31:57-92. doi: 10.1016/j.nfs.2023.03.004.
- Carranza Diaz O. Behavior of selected organic micropollutants in horizontal subsurface-flow constructed wetlands operating at high organic load. 2015.
- Newbold RR, Padilla-Banks E, Jefferson WN. Environmental estrogens and obesity. Mol Cell Endocrinol. 2009 May 25;304(1-2):84-9. doi: 10.1016/j.mce.2009.02.024. Epub 2009 Mar 9. PMID: 19433252; PMCID: PMC2682588.
- Chavoshani A, Hashemi M, Amin MM, Ameta SC. Chapter 2 - Pharmaceuticals as emerging micropollutants in aquatic environments, in Micropollutants and Challenges. A. Chavoshani A, Hashemi M, Amin MM, Ameta SC, editors. Elsevier; 2020. p.35-90.
- State of the science of endocrine disrupting chemicals 2012: Summary for decision-makers. WHO; 2012.
- Shen L, Wania F. Compilation, evaluation, and selection of physical− chemical property data for organochlorine pesticides. Journal of Chemical & Engineering Data. 2005;50(3):742-768. doi: 10.1021/je049693f.
- Włodarczyk-Makuła M. Selected organic micropollutants in the aquatic environment. Desalination and Water Treatment. 2024;317:100061. doi: 10.1016/j.dwt.2024.100061.
- Khawar MI, Mahmood A, Nabi D. Exploring the role of octanol-water partition coefficient and Henry's law constant in predicting the lipid-water partition coefficients of organic chemicals. Sci Rep. 2022 Sep 2;12(1):14936. doi: 10.1038/s41598-022-19452-6. PMID: 36056200; PMCID: PMC9440013.
- Metcalfe CD, Bayen S, Desrosiers M, Muñoz G, Sauvé S, Yargeau V. An introduction to the sources, fate, occurrence and effects of endocrine disrupting chemicals released into the environment. Environ Res. 2022 May 1;207:112658. doi: 10.1016/j.envres.2021.112658. Epub 2022 Jan 4. PMID: 34990614.
- Mungai TM, Wang J. Occurrence and Toxicological Risk Evaluation of Organochlorine Pesticides from Suburban Soils of Kenya. Int J Environ Res Public Health. 2019 Aug 15;16(16):2937. doi: 10.3390/ijerph16162937. PMID: 31443302; PMCID: PMC6719993.
- Nakata H, Kawazoe M, Arizono K, Abe S, Kitano T, Shimada H, Li W, Ding X. Organochlorine pesticides and polychlorinated biphenyl residues in foodstuffs and human tissues from china: status of contamination, historical trend, and human dietary exposure. Arch Environ Contam Toxicol. 2002 Nov;43(4):473-80. doi: 10.1007/s00244-002-1254-8. PMID: 12399919.
- Saadati N, Abdullah MP, Zakaria Z, Rezayi M, Hosseinizare N. Distribution and fate of HCH isomers and DDT metabolites in a tropical environment-case study Cameron Highlands-Malaysia. Chem Cent J. 2012 Nov 7;6(1):130. doi: 10.1186/1752-153X-6-130. PMID: 23130650; PMCID: PMC3531265.
- Ma ECCC. Guide for physicochemical and toxicological characterization of sediments. Minist`ere du D´eveloppement durable, de l’Environnement et de la Lutte contre les changements climatiques and Environment and Climate Change Canada. 2016;59.
- Reddy AVB, Moniruzzaman M, Aminabhavi TM. Polychlorinated biphenyls (PCBs) in the environment: Recent updates on sampling, pretreatment, cleanup technologies and their analysis. Chemical Engineering Journal. 2019;358:1186-1207. doi: 10.1016/j.cej.2018.09.205.
- Gore AC, Krishnan K, Reilly MP. Endocrine-disrupting chemicals: Effects on neuroendocrine systems and the neurobiology of social behavior. Horm Behav. 2019 May;111:7-22. doi: 10.1016/j.yhbeh.2018.11.006. Epub 2018 Dec 4. PMID: 30476496; PMCID: PMC6527472.
- Zhang W, Sargis RM, Volden PA, Carmean CM, Sun XJ, Brady MJ. PCB 126 and other dioxin-like PCBs specifically suppress hepatic PEPCK expression via the aryl hydrocarbon receptor. PLoS One. 2012;7(5):e37103. doi: 10.1371/journal.pone.0037103. Epub 2012 May 16. PMID: 22615911; PMCID: PMC3353882.
- Ålander J. Concentrations of brominated flame retardants (HBB, PBEB, BTBPE, DBDPE, PBDEs and HBCD) in blood serum from first-time mothers in Uppsala 1996-2017. Livsmedelsverket. 2019.
- Zuiderveen EAR, Slootweg JC, de Boer J. Novel brominated flame retardants - A review of their occurrence in indoor air, dust, consumer goods and food. Chemosphere. 2020 Sep;255:126816. doi: 10.1016/j.chemosphere.2020.126816. Epub 2020 Apr 22. PMID: 32417508.
- Pignotti E. Contaminants of emerging concern: Occurrence and distribution in aquatic environments. 2018.
- Zhu Z, Zuo Y, Bisphenol A and other alkylphenols in the environment-occurrence, fate, health effects and analytical techniques. Adv Environ Res. 2013;2(3):179-202. doi: 10.12989/aer.2013.2.3.179.
- Bang DY, Kyung M, Kim MJ, Jung BY, Cho MC, Choi SM, Kim YW, Lim SK, Lim DS, Won AJ, Kwack SJ, Lee YK, Kim HS, Lee BM. Human risk assessment of endocrine‐disrupting chemicals derived from plastic food containers. Comprehensive Reviews in Food Science and Food Safety. 2012;11(5):453-470. doi: 10.1111/j.1541-4337.2012.00197.x.
- Beausoleil C, Emond C, Cravedi JP, Antignac JP, Applanat M, Appenzeller BR, Beaudouin R, Belzunces LP, Canivenc-Lavier MC, Chevalier N, Chevrier C, Elefant E, Eustache F, Habert R, Kolf-Clauw M, Le Magueresse-Battistoni B, Mhaouty-Kodja S, Minier C, Multigner L, Schroeder H, Thonneau P, Viguié C, Pouzaud F, Ormsby JN, Rousselle C, Verines-Jouin L, Pasquier E, Michel C. Regulatory identification of BPA as an endocrine disruptor: Context and methodology. Mol Cell Endocrinol. 2018 Nov 5;475:4-9. doi: 10.1016/j.mce.2018.02.001. Epub 2018 Feb 6. PMID: 29426018.
- Careghini A, Mastorgio AF, Saponaro S, Sezenna E. Bisphenol A, nonylphenols, benzophenones, and benzotriazoles in soils, groundwater, surface water, sediments, and food: a review. Environ Sci Pollut Res Int. 2015 Apr;22(8):5711-41. doi: 10.1007/s11356-014-3974-5. Epub 2014 Dec 30. PMID: 25548011; PMCID: PMC4381092.
- Diao P, Chen Q, Wang R, Sun D, Cai Z, Wu H, Duan S. Phenolic endocrine-disrupting compounds in the Pearl River Estuary: Occurrence, bioaccumulation and risk assessment. Sci Total Environ. 2017 Apr 15;584-585:1100-1107. doi: 10.1016/j.scitotenv.2017.01.169. Epub 2017 Feb 6. PMID: 28185731.
- Soto AM, Justicia H, Wray JW, Sonnenschein C. p-Nonyl-phenol: an estrogenic xenobiotic released from "modified" polystyrene. Environ Health Perspect. 1991 May;92:167-73. doi: 10.1289/ehp.9192167. PMID: 1935846; PMCID: PMC1519400.
- Squadrone S, Ciccotelli V, Favaro L, Scanzio T, Prearo M, Abete MC. Fish consumption as a source of human exposure to perfluorinated alkyl substances in Italy: analysis of two edible fish from Lake Maggiore. Chemosphere. 2014 Nov;114:181-6. doi: 10.1016/j.chemosphere.2014.04.085. Epub 2014 May 17. PMID: 25113200.
- Gusmaroli L. Analysis, occurrence, fate and behaviour of emerging micropollutants in wastewater and the receiving environment. Universitat de Girona. 2020.
- Bakker J, Bakker J, Hakkert BC, Hessel EVS, Luit RJ, Piersma AH, Sijm DTHM, Rietveld AG, van Broekhuizen FA, van Loveren H, Verhoeven JK. Bisphenol A: part 2. Recommendations for risk management. 2016.
- de Solla SR, De Silva AO, Letcher RJ. Highly elevated levels of perfluorooctane sulfonate and other perfluorinated acids found in biota and surface water downstream of an international airport, Hamilton, Ontario, Canada. Environ Int. 2012 Feb;39(1):19-26. doi: 10.1016/j.envint.2011.09.011. Epub 2011 Oct 29. PMID: 22208739.
- Onghena M, Moliner-Martinez Y, Picó Y, Campíns-Falcó P, Barceló D. Analysis of 18 perfluorinated compounds in river waters: comparison of high performance liquid chromatography-tandem mass spectrometry, ultra-high-performance liquid chromatography-tandem mass spectrometry and capillary liquid chromatography-mass spectrometry. J Chromatogr A. 2012 Jun 29;1244:88-97. doi: 10.1016/j.chroma.2012.04.056. Epub 2012 May 8. PMID: 22633866.
- Llorca M, Farré M, Tavano MS, Alonso B, Koremblit G, Barceló D. Fate of a broad spectrum of perfluorinated compounds in soils and biota from Tierra del Fuego and Antarctica. Environ Pollut. 2012 Apr;163:158-66. doi: 10.1016/j.envpol.2011.10.027. Epub 2012 Jan 12. PMID: 22325444..
- European Food Safety Authority (EFSA). Perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and their salts Scientific Opinion of the Panel on Contaminants in the Food chain. EFSA J. 2008 Jul 21;6(7):653. doi: 10.2903/j.efsa.2008.653. PMID: 37213838; PMCID: PMC10193653.
- Giesy JP, Naile JE, Khim JS, Jones PD, Newsted JL. Aquatic toxicology of perfluorinated chemicals. Rev Environ Contam Toxicol. 2010;202:1-52. doi: 10.1007/978-1-4419-1157-5_1. PMID: 19898760.
- Ahrens L. Polyfluoroalkyl compounds in the aquatic environment: a review of their occurrence and fate. Journal of Environmental Monitoring. 2011;13(1):20-31. doi: 10.1039/c0em00373e.
- Ferrey M, Wilson JT, Adair C, Su C, Fine D, Liu X, Washington JW. Behavior and fate of PFOA and PFOS in sandy aquifer sediment. Groundwater Monitoring & Remediation. 2012;32(4):63-71. doi: 10.1111/j.1745-6592.2012.01395.x.
- Conder JM, Hoke RA, De Wolf W, Russell MH, Buck RC. Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds. Environ Sci Technol. 2008 Feb 15;42(4):995-1003. doi: 10.1021/es070895g. PMID: 18351063.
- Olsen GW, Mair DC, Lange CC, Harrington LM, Church TR, Goldberg CL, Herron RM, Hanna H, Nobiletti JB, Rios JA, Reagen WK, Ley CA. Per- and polyfluoroalkyl substances (PFAS) in American Red Cross adult blood donors, 2000-2015. Environ Res. 2017 Aug;157:87-95. doi: 10.1016/j.envres.2017.05.013. Epub 2017 May 18. PMID: 28528142.
- Higgins CP, Luthy RG. Sorption of perfluorinated surfactants on sediments. Environ Sci Technol. 2006 Dec 1;40(23):7251-6. doi: 10.1021/es061000n. PMID: 17180974.
- Pancras T. Environmental fate and effects of poly and Perfluoroalkyl Substances (PFAS). 2016.
- Schwarzenbach RP, Gschwend PM, Imboden DM. Environmental organic chemistry. 2016: John Wiley & Sons.
- Boas M, Feldt-Rasmussen U, Main KM. Thyroid effects of endocrine disrupting chemicals. Mol Cell Endocrinol. 2012 May 22;355(2):240-8. doi: 10.1016/j.mce.2011.09.005. Epub 2011 Sep 10. PMID: 21939731.
- Pal A, Gin KY, Lin AY, Reinhard M. Impacts of emerging organic contaminants on freshwater resources: review of recent occurrences, sources, fate and effects. Sci Total Environ. 2010 Nov 15;408(24):6062-9. doi: 10.1016/j.scitotenv.2010.09.026. Epub 2010 Oct 8. PMID: 20934204..
- Luckenbach T, Epel D. Nitromusk and polycyclic musk compounds as long-term inhibitors of cellular xenobiotic defense systems mediated by multidrug transporters. Environ Health Perspect. 2005 Jan;113(1):17-24. doi: 10.1289/ehp.7301. PMID: 15626642; PMCID: PMC1253704.
- Halden RU. On the need and speed of regulating triclosan and triclocarban in the United States. Environ Sci Technol. 2014 Apr 1;48(7):3603-11. doi: 10.1021/es500495p. Epub 2014 Mar 14. PMID: 24588513; PMCID: PMC3974611.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.