Medicine Group. 2024 July 06;5(7):699-709. doi: 10.37871/jbres1946.
Effect of Hyperthermic Intraperitoneal Chemotherapy on Long Term Survival in Gastric Cancer with Peritoneal Metastasis Treated with Preoperative Intraperttoneal Chemotherapy plus Peritonectomy: The Phase III PM-GC-1 Trial
Yutaka Yonemura1-3*, Haruaki Ishibashi2, Takuji Fujita2, Yang Liu2, Satoshi Wakama2, Syouzou Sako2, Toshiyuki Kitai2, Yasuo Hirono2, Shouzou Sako2, Akiyoshi Mizumoto3, Sachio Fushida2, Nobuyuki Takao3, Keizou Taniguchi4 and Daisuke Fujimoto4
2Department of Regional Cancer Therapy, Peritoneal Dissemination Center, Kishiwada Tokushukai Hospital, Kishiwada City, Oosaka Prefecture, 596-8522, Japan
3Department of Regional Cancer Therapy, Peritoneal Dissemination Center, Kusatsu General Hospital, Kusatsu City, Shiga Prefecture, 525-8585, Japan
4Department of Surgery, Mizonokuchi Hospital, Teikyo University, School of medicine, Kawasaki, Kanagawa, 213-8507, Japan
5Department of Surgical Oncology, The University of Tokyo, Japan
- Peritoneal metastasis
- Gastric cancer
- HIPEC
- Peritonectomy
- Peritoneal cancer index
Abstract
The present article intends to perform a Randomized Controlled Trial (RCT) to confirm the effect of Intraoperative Hyperthermic Intraperitoneal Chemo Perfusion (IOHIPEC) after treatment by Neoadjuvant Intraperitoneal/Systemic Chemotherapy (NIPS) plus Cytoreductive Surgery (CRS) for Gastric Cancer (GC)-patients with Peritoneal Metastasis (PM).
Patients, and treatments: From January, 2013 to May, 2021, 266 GC-patients with PM were treated with NIPS. After 3 cycles of NIPS, 239 patients were selected as eligible to receive complete CRS, and were recruited onto this study (The phase III GCPM-1 Trial). The patients were randomized preoperatively into the CRS + Extensive Intraoperative Peritoneal Lavage (EIPL) and CRS +EIPL + IOHIPEC Group.
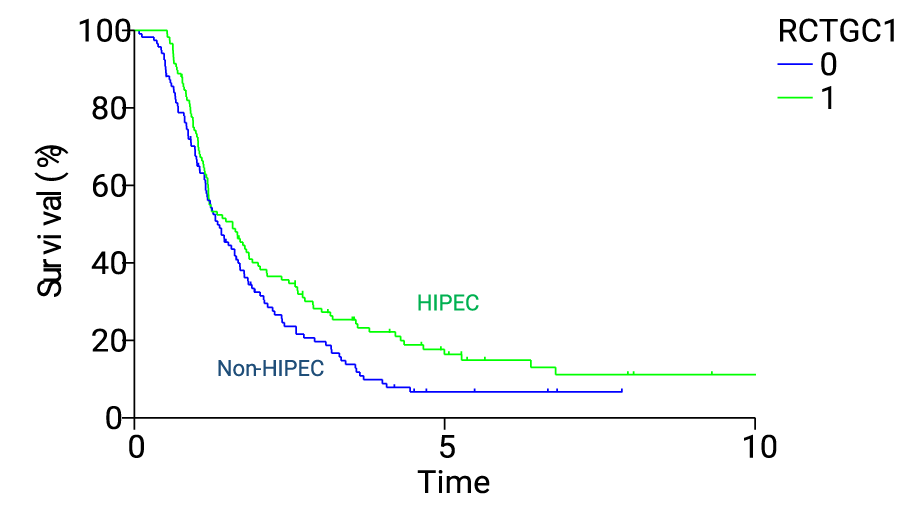
Results: All these patients were well balanced and compatible regarding major clinicopahological characteristics and surgical procedures. Median overall survival (OS) for both groups was 10.2 months (non-IOHIPEC group, 9.7 months, vs. IOHIPEC group, 10.4 months). One-3-, 5- and 10-year OS rates were 44.4%, 14.4%, 5.4% and NR in non-IOHIPEC group, and those in IOHIPEC groups were 47.5%, 22.1%, 14.7% and 10.6%, respectively (p = 0.032).
Median OS of IOHIPEC and non-IOHIPEC group after CCR-0 resection were 16.7 and 15.9 months, respectively. Five-year survival rates of IOHIPEC and non-aIOHIPEC group in CCR-0 resection were 21.7% and 8.2% (p < 0.005). However, survival after CCR-1 resection was not improved by IOHIPEC, and all patients died of disease progression within 3 years after CRS.
Cox regression analysis revealed HIPEC, lymph node metastasis (pN0,1 v.s. pN2,3, completeness of cytoreduction (CCR-0 v.s. CCR-1) were independent prognostic factors for OS (a hazard ratio of 0.63, 95% CI, 0.49 to 0.88 for use of HIPEC), ( a hazard ratio of 1.62, 95% CI, 1.21 to 2.17), and (a hazard ratio of 1.70, 95% CI 1.13 to 2.57, respectively
Grade 3,4, and 5 postoperative complications in non-HIPEC group were 17 (14.2%), 9 (7.5%) and 2 (1.7%), and those in HIPEC group were 14 (11.7%), 18 (15.1%), and 3 (2.5%), respectively. However, no significant difference in the rates of grade 3, 4, and 5 complications between non-HIPEC and HIPEC group.
The present RCT trial to confirm the effects of IOHIPEC on survival after NIPS + CRS (GCPM-1 trial).
Introduction
Peritoneal Metastasis (PM) has been considered a lethal condition, and the median survival time after surgery or systemic chemotherapy alone was reported 6 months [1]. In 1999, Peritoneal Surface Oncology Group International (PSOGI) proposed an innovative treatment named Comprehensive Treatment (COMPT) that combined Cytoreductive Surgery (CRS) using peritonectomy technique and Perioperative Chemotherapy (POC) [2-4]. Aim of COMPT is to achieve cure of PM. Cure could be achieved with a combination of complete removal of macroscopic tumors by peritonectomy technique and complete eradication of Micro Metastasis (MM) left after peritonectomy by POC [2,3]. COMPT is now performing as a standard treatment for peritoneal metastasis from colorectal cancer, mesothelioma, and pseudomyxoma peritonei [5-9]. In Gastric Cancer (GC) patients with PM, however, complete removal of macroscopic PM in combination with gastrectomy plus regional lymph adenectomy is very difficult to perform safely, because such a procedure has a high risk of developing postoperative mortality and morbidity. Additionally, incomplete cytoreduction with remaining PM after preoperative systemic chemotherapy plus gastrectomy alone could not improve survival of GC-patients with PM [10]. POC comprises Neoadjuvant Chemotherapy (NAC), intraoperative Hyperthermic Peritoneal Chemotherapy (HIPEC), and postoperative chemotherapy. Among these forms of chemotherapies, intraoperative HIPEC may have an important role in the complete elimination of MM, because the tumor burden is least just after Cytoreductive Surgery (CRS). Chemotherapeutic agents used in HIPEC can kill proportion of cancer cells at specific points in the cell cycle, but hyperthermia at temperatures over 43 Celsius introduces irreversible damage to cancer cells in a time-dependent and exponential manner without relation to the cell cycle [11]. However, number of residual cancer cells left on the preserved peritoneal surface killed by intraoperative HIPEC is limited. Accordingly, micro metastasis that will be left after CRS should be reduced by NAC as much as possible [12]. Neoadjuvant Intraperitoneal/Systemic chemotherapy (NIPS) shows more effectively eradicate intraperitoneal MM burden as compared with systemic chemotherapy does by introducing significant higher locoregional dose intensity [12]. To increase cure rate, the MM burden that may be left after CRS could be decreased below the threshold level that eradicated by intraoperative HIPEC. By a combination of NIPS and HIPEC, the MM after CRS is effectively eliminated, resulting in the increase of cure rate [12]. Up to now only two RCTs in GC-patients with PM were reported [13,14]. In the two studies, patients were treated with CRS +/- intraoperative HIPEC. The present article intends to perform a randomized trial to confirm the effect of HIPEC after treatment by NIPS plus CRS for GC-patients with PM.
Patients and Methods
Patients
From January, 2013 to May, 2021, 266 GC-patients with PM, including 124 men and 142 women, aged 23-75 years (mean 54.5 years) were treated with Neoadjuvant Intraperitoneal/Systemic chemotherapy (NIPS) [3,4]. NIPS protocol was as followings: oral administration of S1 of 60 mg/m2 from day 1 to 14, and intraperitoneal infusion of docetaxel of 30mg y/m2 and CDPP of 30mg/m2 in 500 ml of saline on day 1 and 14 from intraperitoneal port system, after 7 days of drug holiday, this cycle repeats 3 cycles. All the patients were diagnosed having PM by histologic or cytologic examination. After 3 cycles of NIPS, 239 patients were selected as eligible to receive complete CRS by computed tomography and/or positron emission tomography, and were recruited onto this study (The phase III GCPM-1 Trial). According to the method by Spiliotis J, et al. [15] the patients were randomized preoperatively into two groups with similar demographic, clinical and therapeutic features. Randomization was performed by a member (Y. L.) of the Department of Medical Trial Center who did not have any information regarding patients characteristics or medical records Before treatment all patients signed informed consent and the study protocol was approved by the Ethics Commettee of Kishiwada Tokushukai Hospital (The protocol No. H-19-2). Using online statistic tools (GraphPad Software), these patients were randomized into the CRS + Extensive Intraoperative Peritoneal Lavage (EIPL) [16] and CRS +EIPL + HIPEC Group according to a computer-generated randomized number. Routine preoperative studies included physical examination, blood test, serum biochemistry and electrolytes, liver and renal function evaluation and coagulation studies. Patient inclusion criteria were (1) age 20-75 years old, (2) performance status of ≤ 2, (3) life expectancy of >9 weeks, (4) normal peripheral blood white blood cell count ≥ 3000/mm3 and platelet count ≥100,000/mm3, (5) acceptable liver function, with bilirubin no greater than 2 upper limit of normal level, and aspartic amino transferase and alanin aminotransferase no greater than 2 upper limit of normal level, (6) accepted renal function with serum creatinine no longer than 1.5 mg/ml, and (7) cardiovascular and pulmonary and other major organ function can tolerate on major operation. Major exclusion criteria were (1) age ≤ 20 years old ≥76 years old, (2) any hematogenous metastasis, or prominent retroperitoneal metastasis during preoperative image diagnosis. (3) Co-existence of cancer in the other organs (Figure 1).
CRS
All the CRS procedures were performed by the same surgeon (Y.Y) through a midline xiphoid-pubic incision. After the abdominal wall was opened, peritoneal cavity was washed with saline (EIPL) [16] and peritoneal cancer index (PCI) was determined [17]. Peritoneal cytologic status was studied from ascites or by peritoneal wash cytology when ascites was not found. Then, maximum CRS was performed, including the resection of primary tumor with acceptable margins, any involved adjacent structures, D2 lymphadenectomy, peritonectomies, where the total resection of involved peritoneal sectors with peritoneal metastases was performed [4,17,18]. The peritoneal sectors without macroscopic metastasis were preserved. After washing peritoneal cavity with 10 liter of saline (EIPL) [16], HIPEC was performed before reconstruction by an open procedure that is believed to provide optimal thermal homogeneity by a rigorous stirring with hands. The skin of the abdomen is attached to a retractor ring and four liter of warmed saline with 60 mg/body of docetaxel and 50mg/body of cisplatinum was introduced into the peritoneal cavity. The temperature probes were placed on the upper abdominal cavity and pelvis, and plastic sheet was covered on the abdominal wound. The saline was heated by the tubes connecting with stainless coil in the warmed bath set in the extracorporeal system. The abdominal temperature maintained between 43 to 43.5 °C for 40 min. When the thermal dose reached 40 min. [11], HIPEC was stopped. Then, the perfusion solution in the peritoneal cavity was removed through the suction tube, and the peritoneal cavity was again washed with 6 liter of warned saline, and drainage tubes were placed on the pelvis and bilateral space of diaphragm. The wound was closed and patients were delivered to the intensive care unit for recovery. The extent of CRS was determined by Sugarbaker’s criteria on the Completeness of Cytoreduction (CCR score) [17]. A score of CCR-0 indicates no residual disease and CCR-1 refers to the residual disease. The primary end point was Overall Survival (OS), defined as time interval from the date of CRS to death due to disease from any causes. The secondary end points were adverse events, according to Clavien PA [19].
Statistical Framework
This study was designed to have 80% power to detect an improvement in 5-year survival rate from 5% to 15% by intraoperative HIPEC. Sample size was determined 224 patients requiring to reach 110 events for final analysis, based on the use of the log-rank test with a two-sided significance of 5%, and b = 20%.
Statistical Analysis
All patients were followed and no patients were lost to follow-up. Outcome data were obtained from medical records and patients’ interview. All statistical analyses were performed using SPSS software statistical computer package version 17 (SPSS Inc., Chicago, USA). The clinical variables were analyzed by X2 tests and student T-test. Statistical significance was defined as a p-value ≤ 0.05. Survival times were estimated using Kaplan-Meier method, and survivals of each group of patients were compared with univariate analysis.
Results
Baseline data and surgical intervention
By May 2021, 27 patients were ineligible most commonly because of selection criteria not being met. A total of 239 patients were randomized into CRS + EIPL (N = 120) and CRS+EIPL+ HIPEC (N = 119) groups. These patients were well balanced and conpartible regarding major baseline clinicopahological characteristics (Table 1) and surgical procedures (Table 2). Four to 6 weeks after the last intraperitoneal administration of docetaxel and cisplatinum, CRS was done in all 239 patients. As shown in table 1, age and male/female ratio of non-HIPEC and HIPEC group were not significantly different (Table 1). Type 2,3/ Type 4 primary tumors were 36/74, and 39/80 in non-HIPEC and HIPEC group, respectively (NS). Lymph node status of these two groups was not statistically significant. Positive cytology after NIPS in non-HIPEC and HIPEC groups were 29 (24%) and 25 (21%), respectively (NS). Positive cytology before NIPS in non-HIPEC and HIPEC groups were 73 (61%) and 68 (58%) (NS). Cytology positive patients without macroscopic PM in non-HIPEC and HIPEC group were 7 (5.8%) and 7 (5.9%), respectively. Differentiated type in histology of non-HIPEC and HIPEC groups were 17 (14.2%), and 14 (11.8%), respectively (NS). Recurrent cases of non-HIPEC and HIPEC groups were 32 (26.7%) and 36 (30.3%) (NS).
| Table 1: Clinicopathological parameters between the non-HIPEC and HIPEC groups. | ||
| Non-HIPEC group | HIPEC group | |
| Age (years) | 55.0 ± 12.7 | 54.2 ± 13.3 |
| Male/Female | 51/69 | 53/66 |
| Macroscopic type (Type 2,3/4) | 36/74 | 39/80 |
| Age (65< vs ≥ 65 | 86/34 | 82/36 |
| CCR-0/CCR-1 | 79/41 | 78/41 |
| Lymph node metastasis (pNo,1/ pN2,3 | 57/63 | 62/58 |
| Cytology (class V) after NIPS | 29 (24%) | 25 (21%) |
| Cytology (classV) before NIPS | 73 (61%) | 68 (58%) |
| P0Cy1 | 7 (5.8%) | 7 (5.9%) |
| Histologic type (differentiated/ poor) | 17/103 | 14/105 |
| Primary/ recurrent | 88/32 | 83/36 |
| PCI | 8.1 ± 8.6 | 8.1 ± 8.5 |
| PCI ≤ 12 / ≥ 13 | 86/34 | 85/34 |
| Small bowel PCI (SB-PCI) | 2.3 ± 3,1 | 2.4 ± 3.1 |
| Residual PCI | 3.1 ± 6.4 | 4.1 ± 12.6 |
| No. of resected organs | 4.9 ± 2.4 | 4.6 ± 2.5 |
| No. of resected peritoneal sectors | 6.1 ± 7.7 | 5.5 ± 8.5 |
| No. of involved peritoneal sectors | 4.9 ± 5.8 | 4.8 ± 4.5 |
| Serum CEA | 3.6 ± 4.7 | 3.2 ± 3.3 |
| Serum CA19-9 | 87.8 ± 251 | 85.1 ± 231 |
| Bleeding volume (ml) | 1400 ± 1060 | 1333 ± 1025 |
| Operation time (min) | 209 ± 70 | 255 ± 87 |
| Table 2: Operation procedures between the two groups. | ||
| Non-HIPEC group | HIPEC group | |
| Gastrectomy Total | 60 | 70 |
| Others | 24 | 17 |
| Colon resection + G1E3:G14 | ||
| Total colectomy | 22 | 22 |
| Right hemicolectomy30 | 30 | 27 |
| Left hemicolectomy | 11 | 10 |
| Rectal resection | 3 | 7 |
| Hysterectomy + BSO | 53 | 53 |
| Small bowel mesentery | 70 | 62 |
| Right diaphragmatic peritonectomy | 33 | 23 |
| Left diaphragmatic peritonectomy | 43 | 38 |
| Distal pancreas | 14 | 12 |
| Splenectomy | 60 | 70 |
| Pelvic peritonectomy | 81 | 79 |
| Table 3: Multivariate analysis of the study. | ||||
| Variables | p value | X2 | Hazard ratio | 95% CI |
| Gender: Male vs. Female | 0.979 | 0.559 | 0.917 | 0.688 - 1.224 |
| CCR-0 vs. CCR-1 | 0.01 | 6.49 | 1.703 | 1.130 - 2.567 |
| Cytology before NIPS | 0.55 | 1.479 | 0.822 | 0.600 - 1.127 |
| Cytology after NIPS | 0.104 | 2.753 | 1.342 | 0.945 - 1.969 |
| pN0,1 vs. pN2,3 | 0.001 | 10.604 | 1.622 | 1.212 - 2.170 |
| PCI ≥ 13 vs. ≤ 12 | 0.008 | 7.001 | 1.158 | 1.676 - 3.833 |
| Non-HIPEC vs. HIPEC | 0.005 | 8.037 | 0.662 | 0.498 - 0.880 |
| small bowel PCI ≥ 3 vs. ≤ 2 | 0.042 | 4.109 | 1.569 | 1.015 - 2.427 |
PCI and operation procedures
As shown in table 1, PCI of non-HIPEC and HIPEC groups were 8.1 ± 8.6 and 8.1 ± 8.5, respectively (NS). Small bowel PCI of each group were 2.3 ± 3.1 and 2.2 ± 3.1 (NS). Serum CEA and CA19-9 levels in each group were not significantly different (NS). Table 2 shows the resected organs in each group. Total gastrectomy plus D2 lymph node dissection was done in 60 (50%) and 70 (58.8%) of non-HIPEC and HIPEC group (NS). Colon resection was performed in 86 and 76 patients of non-HIPEC and HIPEC group. In non-HIPEC group, total colectomy, right hemicolectomy, left hemicolectomy, and rectal resection were done in 22, 30, 11, and 3 patients. Those procedures were performed in 22, 27, 10, and 7 patients in HIPEC group. Hysterectomy and Bilateral Salpingo-Oophorectomy (BSO) were performed in 53 (76.8%) of 69, and 53 (80.3%) of 66 female patients in non-HIPEC and HIPEC group (NS). Small bowel and mesenteric resection were in 70 (58.3%) and 62 (52.1%) in each group (NS). Right and left diaphragmatic peritonectomy were performed in 33 (27.5%) and 43 (35.8%) in non-HIPEC group. Those were done in 23 (19.3%) and 38 (31.9%) in HIPEC group. Distal pancreatectomy was performed in 14 (11.7% and 12 (10.1%) in non-HIPEC and HIPEC group. Pelvic peritonectomy was done in 81 (67.5%) and 79 (65.4%) in non-HIPEC and HIPEC group, respectively (NS). As shown in table 1, No. of resected organs in non-HIPEC and HIPEC groups were 4.9 ± 2.4 and 4.6 ± 2.5, respectively (NS). No. of resected peritoneal sectors of non-HIPEC and HIPEC groups were 6.1 ± 7.7 and 5.5 ± 8.5 (NS). No. of involved peritoneal sectors in non-HIPEC and HIPEC groups were 4.9 ± 5.8 and 4.8 ± 4.5, respectively (NS). Bleeding volume of non-HIPEC and HIPEC groups were 1400 ± 1060, and 1333 v 1025 ml (NS). Operation time of HIPEC group (255 ± 87 min) was significantly longer than non-HIPEC group (209 ±70 min) (p < 0.005). The median observation time for the primary end point was 7.7 years (range, 2.1-16 years). Median OS for both groups was 10.2 months (non-HIPEC group, 9.7 months, vs. HIPEC group, 10.4 months). One-3-, 5 and 10 year OS rates were 44.4%, 14.4%, 5.4% and NR in non-HIPEC group, and those in HIPEC groups were 47.5%, 22.1%, 14.7% and 10.6%, respectively (p = 0.032). Median OS of HIPEC and non-HIPEC group after CCR-0 resection were 16.7 and 15.9 months, respectively. Five year survival rates of HIPEC and non-HIPEC group in CCR-0 resection were 21.7% and 8.2% (p < 0.005). However, survival after CCR-1 resection was not improved by HIPEC, and all patients died of disease progression within 3 years after CRS. Survival of GC-PM-patients with PCI≤6 was significantly improved by HIPEC (p = 0.014), as compared with that of patients with PCI ≤ 5 or PCI ≥ 7. Median survival months of HIPEC and non-HIPEC group were 18.3 and 14.8 months, and 5-year survival rate of each group were 23.0% and 8.6%, respectively. Cox regression analysis revealed HIPEC, lymph node metastasis (pN0,1 vs. pN2,3, completeness of cytoreduction (CCR-0 vs. CCR-1 were independent prognostic factors for OS (a hazard ratio of 0.63, 95% CI, 0.49 to 0.88 for use of HIPEC), (a hazard ratio of 1,62, 95% CI, 1,21 to 2,17), and (a hazard ratio of 1.70, 95% CI 1.13 to 2.57, respectively. Four of 120 (3.3%) and 12 of 119 (10.1%) patients in non-HIPEC and HIPEC group survived without recurrence longer than 5 years after CRS (p = 0.036, X2 = 4.36). Grade 3,4, and 5 postoperative complications in non-HIPEC group were 17 (14.2%), 9 (7.5%) and 2 (1.7%), and those in HIPEC group were 14 (11.7%), 18 (15.1%), and 3 (2.5%), respectively. However, no significant difference in the rates of grade 3, 4, and 5 complications between non-HIPEC and HIPEC group.
Discussion
In the treatment of colorectal cancer with PM, Nagata, et al. [20] reported that 2 of 7 colorectal cancer-patients with PM who underwent complete resection without perioperative chemotherapy were still alive without recurrence five years after CRS. PCIs of the patients were less tan 2 (20). In the treatment of PM from gastric cancer, cure could not be achieved by surgery alone [12]. In the treatment of PM from cancers with highly malignant behavior like gastric cancer, most patients treated with surgery alone will die due to the growth of residual MM on the unremoved peritoneum left after complete resection of macroscopic metastasis [21]. Even in complete responders to systemic chemotherapy, multi-drug resistant cancer cells contaminate in the PM always regrow, and consequently systemic chemotherapy will fail. Additionally, chemotherapy cannot be continued due to the development of severe side effects after several cycles. These factors are considered to account for treatment failure with chemotherapy. A key for cure of COPMT is the complete elimination of MM by intraoperative HIPEC (IOHIPEC), because the MM burden is least just after marosocopic complete resection of PM. When the residual MM burden is less than the threshold level that can be completely eliminated by IOHIPEC, even patients with MM will be cured in combination with macroscopic complete resection and IOHIPEC. On the contrary, COMPT will be failed if the residual MM burden is bigger than the threshold level. In this sense, NAC has a big role in reducing MM burden below the threshold level. Intraperitoneal chemotherapy can generate significantly higher loco-regional dose intensity in the peritoneal cavity than systemic chemotherapy. Additionally, Neoadjuvant Intraperitoneal/Systemic chemotherapy (NIPS) has higher power for the reduction of MM burden than systemic or intraperitoneal chemotherapy due to the bidirectional attack to MM not only from peritoneal surface but also from submesothelial arterial capillary [3]. Accordingly, NIPS is belived to be more effective for PM from gastric cancer, ovarian cancer and pseudomyxoma peritonei, than systemic chemotherapy [22,23]. Accordingly, the trial was designed to confirm the effects of IOHIPEC on long-term survival after NIPS and cytoreductive surgery. After 3 cycles of NIPS, candidates for CRS are selected by laparoscopy, Computed Tomography (CT), Magnetic Resonance Imaging (MRI), and/or positron emission tomography (PET). As the results, 239 patients were selected as the candidates for randomization. If the small bowel and its mesentery are diffusely involved, complete cytoreduction cannot be performed. Additionally, patients with PCI values that exceed the threshold levels are excluded from consideration for CRS. To validate the direct effects of HIPEC on PM from GC, Laparoscopic HIPEC (LHIPEC) was performed, and PCI levels and cytologic status were studied one month after one cycle of LHIPEC [24]. The PCI before LHIPEC was 13.49± 11.00 (n = 55) and after LHIPEC significantly reduced to 11.64± 10.65 (p= 0.0381) [24]. Twenty-six patients showed positive cytology before LHIPEC, but positive cytology became negative in 15 (58%) patients after LHIPEC [24]. These results indicate that LHIPEC effectively reduces PCI and the burden of MM and PFCCs. Regarding the effects of IOHIPEC on survival after CRS, Spiliotis J, et al. [15] and van Driel WJ, et al. [25] reported that IOHIPEC using CDDP resulted in longer recurrence-free survival and overall survival than surgery alone in stage III ovarian cancer patients. In PM from colorectal cancer, however, Quenet F, et al. [26] described that IOHIPEC using oxaliplatin plus intravenous 5FU and folic acid had no beneficial effects on survival as compared with surgery alone. The effect of IOHIPEC on long term survival in GC-PM patients was studied in three randomized controlled trials. In 2011, Yang XJ, et al. [13] reported an RCT involving 68 GC-PM-patients, randomly allocated to CRS alone v.s. CRS+IOHIPEC. Patients treated with CRS + IOHIPEC (MST: 11.0 months) survived significantly longer than those treated with CRS alone (MST: 6.5 months). Recently, Rau B, et al. [14] studied the effects of HIPEC on CRS in GC -patients with synchronous PM in a phase III randomized trial (GASTRIPEC-1 trial). Fifty-two GC-PM-patients were randomly assigned to 53 patients to CRS alone, and 52 to CRS + IOHIPEC. IOHIPEC comprised mitomycin C 15 mg/m2 and CDDP 75 mg/m2 in 5 L of saline perfused for 60 minutes at 42 ℃. The progression free survival was 3.5 months in CRS alone group and 7.1 month in the CRS + HIPEC group. (p = 0.047). The present RCT trial to confirm the effects of IOHIPEC on survival after NIPS + CRS (GCPM-1 trial). IOHIPEC was performed by the open method, with 60mg/body of docetaxel and 50mg/body of CDDP were added in 4 L of saline intraperitoneally at temperature of 43 to 43.5℃. HIPEC was performed until the thermal doses reach 40 min. by using the equation by Sapareto and Dewey [11]. In the other studies [13-15,25,26], thermal dose was not described. Accordingly, it is doubtful that all patients were treated with the same thermal dose. By calculating thermal doses, every patient can be treated with the same thermal doses. In the present study, there was no significant difference in the clinico-pathological prognostic factors between the two groups. Median survival times with CRS alone and with CRS + IOHIPEC were 15.6 and 17.6 months, and the overall survival curve for CRS + IOHIPEC was significantly better with CRS alone (X2 = 6.78, p = 0.011). Regarding the complications after COMPT, There was no significant difference in grade 3 or 4 adverse events in ovarian cancer and gastric cancer trials [13-15,25]. In the present study, the incidence of grade 3, 4, and 5 complications was found in 28 (23.4%) in the CRS alone group, and 35 (29.3%) with CRS + IOHIPEC. There was no difference in grade >=3 complications between the two groups. However, Quenet F [26] reported that grade 3 or worse adverse events at 60 days in IOHIPEC group were more common than surgery alone group. These studies of GC-PM strongly suggest that IOHIPEC improve postoperative survival, and MM left after CRS in some segments of patients treated with NIPS could be eliminated using IOHIPEC. Additionally, NIPS can reduce MM burden that will be left in the peritoneum even after peritonectomy. Because all our patients had been treated with NIPS, we could not assess the values of NIPS on survival. However, in the RCTs conducted by Rau B, et al. [14] and Yang XJ, et al. [13], NIPS was not performed, but the survival of this study was superior to that of the previous trials. These results may indicate NIPS may be important role in reducing MM before CRS and IOHIPEC. Further studies must be performed to confirm the effects of NIPS for peritoneal metastasis from gastric cancer.
Conclusion
Among GC-PM patients, the addition of HIPEC after cytoreductive surgery resulted in longer survival than surgery alone and did not result in higher rate of side effects.
References
- Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL, Faure JL, Porcheron J, Peix JL, François Y, Vignal J, Gilly FN. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000 Jan 15;88(2):358-63. doi: 10.1002/(sici)1097-0142(20000115)88:2<358::aid-cncr16>3.0.co;2-o. PMID: 10640968.
- Sugarbaker PH. Building on a consensus. J Surg Oncol. 2008;98(4):215-216.
- Yonemura Y, Canbay E, Endou Y, Ishibashi H, Mizumoto A, Miura M, Li Y, Liu Y, Takeshita K, Ichinose M, Takao N, Hirano M, Sako S, Tsukiyama G. Peritoneal cancer treatment. Expert Opin Pharmacother. 2014 Apr;15(5):623-36. doi: 10.1517/14656566.2014.879571. PMID: 24617975.
- Yonemura Y, Kawamura T, Bandou E, Takahashi S, Sawa T, Matsuki N. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg. 2005 Mar;92(3):370-5. doi: 10.1002/bjs.4695. PMID: 15739249.
- Harmon RL, Sugarbaker PH. Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer. Int Semin Surg Oncol. 2005 Feb 8;2(1):3. doi: 10.1186/1477-7800-2-3. PMID: 15701175; PMCID: PMC549516.
- Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Johung KL, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Gregory KM, Gurski LA. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021 Mar 2;19(3):329-359. doi: 10.6004/jnccn.2021.0012. PMID: 33724754.
- Colorectal Cancer. NCCN Guidelines Version 2, 2021.
- Kusamura S, Kepenekian V, Villeneuve L, Lurvink RJ, Govaerts K, De Hingh IHJT, Moran BJ, Van der Speeten K, Deraco M, Glehen O; PSOGI. Peritoneal mesothelioma: PSOGI/EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol. 2021 Jan;47(1):36-59. doi: 10.1016/j.ejso.2020.02.011. Epub 2020 Mar 12. PMID: 32209311.
- Kusamura S, Barretta F, Yonemura Y, Sugarbaker PH, Moran BJ, Levine EA, Goere D, Baratti D, Nizri E, Morris DL, Glehen O, Sardi A, Barrios P, Quénet F, Villeneuve L, Gómez-Portilla A, de Hingh I, Ceelen W, Pelz JOW, Piso P, González-Moreno S, Van Der Speeten K, Deraco M; Peritoneal Surface Oncology Group International (PSOGI) and the French National Registry of Rare Peritoneal Surface Malignancies (RENAPE). The Role of Hyperthermic Intraperitoneal Chemotherapy in Pseudomyxoma Peritonei After Cytoreductive Surgery. JAMA Surg. 2021 Mar 1;156(3):e206363. doi: 10.1001/jamasurg.2020.6363. Epub 2021 Mar 10. PMID: 33502455; PMCID: PMC7841579.
- Terashima M, Fujitani K, Yang HK, Mizusawa J, Tsujinaka T, Nakamura K, Katayama H, Lee HJ, Lee JH, An JY, Takagane A, Park YK, Choi SH, Song KY, Ito S, Park DJ, Jin SH, Boku N, Yoshikawa T, Sasako M; REGATTA study investigators. Role of reduction gastrectomy in patients with gastric cancer with a single non-curable factor: Supplementary analysis of REGATTA trial. Ann Gastroenterol Surg. 2023 Apr 16;7(5):741-749. doi: 10.1002/ags3.12674. PMID: 37663970; PMCID: PMC10472355.
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984 Jun;10(6):787-800. doi: 10.1016/0360-3016(84)90379-1. PMID: 6547421.
- Yonemura Y, Ishibashi H, Mizumoto A. A comprehensive treatment to achieve cure for peritoneal metastasis. Taiwanese J Gastroenterol Hepatol. 2023;1(1):1-6,
- Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, Cheng FL, Zhou YF, Xiong B, Yonemura Y, Li Y. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011 Jun;18(6):1575-81. doi: 10.1245/s10434-011-1631-5. Epub 2011 Mar 23. PMID: 21431408; PMCID: PMC3087875.
- Rau B, Lang H, Koenigsrainer A, Gockel I, Rau HG, Seeliger H, Lerchenmueller C, Reim D, Wahba R, Angele M, Heeg S, Keck T, Weimann A, Topp S, Piso P, Brandl A, Schuele S, Jo P, Pratschke J, Wegel S, Rehders A, Moosmann N, Gaedcke J, Heinemann V, Trips E, Loeffler M, Schlag PM, Thuss-Patience P. Effect of Hyperthermic Intraperitoneal Chemotherapy on Cytoreductive Surgery in Gastric Cancer With Synchronous Peritoneal Metastases: The Phase III GASTRIPEC-I Trial. J Clin Oncol. 2024 Jan 10;42(2):146-156. doi: 10.1200/JCO.22.02867. Epub 2023 Oct 31. PMID: 37906724; PMCID: PMC10824373.
- Spiliotis J, Halkia E, Lianos E, Kalantzi N, Grivas A, Efstathiou E, Giassas S. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol. 2015 May;22(5):1570-5. doi: 10.1245/s10434-014-4157-9. Epub 2014 Nov 13. PMID: 25391263.
- Kuramoto M, Shimada S, Ikeshima S, Matsuo A, Yagi Y, Matsuda M, Yonemura Y, Baba H. Extensive intraoperative peritoneal lavage as a standard prophylactic strategy for peritoneal recurrence in patients with gastric carcinoma. Ann Surg. 2009 Aug;250(2):242-6. doi: 10.1097/SLA.0b013e3181b0c80e. PMID: 19638909.
- Sugarbaker PH. Cytoreductive surgery and peri-operative intraperitoneal chemotherapy as a curative approach to pseudomyxoma peritonei syndrome. Eur J Surg Oncol. 2001 Apr;27(3):239-43. doi: 10.1053/ejso.2000.1038. PMID: 11373099.
- Brandl A, Yonemura Y, Glehen O, Sugarbaker P, Rau B. Long term survival in patients with peritoneal metastasised gastric cancer treated with cytoreductive surgery and HIPEC: A multi-institutional cohort from PSOGI. Eur J Surg Oncol. 2021 Jan;47(1):172-180. doi: 10.1016/j.ejso.2020.10.006. Epub 2020 Oct 14. PMID: 33071173.
- Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009 Aug;250(2):187-96. doi: 10.1097/SLA.0b013e3181b13ca2. PMID: 19638912.
- Nagata H, Ishihara S, Hata K, Murono K, Kaneko M, Yasuda K, Otani K, Nishikawa T, Tanaka T, Kiyomatsu T, Kawai K, Nozawa H, Watanabe T. Survival and Prognostic Factors for Metachronous Peritoneal Metastasis in Patients with Colon Cancer. Ann Surg Oncol. 2017 May;24(5):1269-1280. doi: 10.1245/s10434-016-5732-z. Epub 2016 Dec 19. PMID: 27995451.
- Hong SH, Shin YR, Roh SY, Jeon EK, Song KY, Park CH, Jeon HM, Hong YS. Treatment outcomes of systemic chemotherapy for peritoneal carcinomatosis arising from gastric cancer with no measurable disease: retrospective analysis from a single center. Gastric Cancer. 2013 Jul;16(3):290-300. doi: 10.1007/s10120-012-0182-1. Epub 2012 Aug 17. PMID: 22898806.
- Prabhu A, Mishra D, Brandl A, Yonemura Y. Gastric Cancer With Peritoneal Metastasis-A Comprehensive Review of Current Intraperitoneal Treatment Modalities. Front Oncol. 2022 May 26;12:864647. doi: 10.3389/fonc.2022.864647. PMID: 35719946; PMCID: PMC9204320.
- Prabhu A, Brandl A, Wakama S, Sako S, Ishibashi H, Mizumoto A, Takao N, Noguchi K, Motoi S, Ichinose M, Liu Y, Yonemura Y. Neoadjuvant Intraperitoneal Chemotherapy in Patients with Pseudomyxoma Peritonei-A Novel Treatment Approach. Cancers (Basel). 2020 Aug 7;12(8):2212. doi: 10.3390/cancers12082212. PMID: 32784670; PMCID: PMC7465601.
- Yonemura Y, Ishibashi H, Hirano M, Mizumoto A, Takeshita K, Noguchi K, Takao N, Ichinose M, Liu Y, Li Y. Effects of Neoadjuvant Laparoscopic Hyperthermic Intraperitoneal Chemotherapy and Neoadjuvant Intraperitoneal/Systemic Chemotherapy on Peritoneal Metastases from Gastric Cancer. Ann Surg Oncol. 2017 Feb;24(2):478-485. doi: 10.1245/s10434-016-5487-6. Epub 2016 Aug 9. PMID: 27506661.
- van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM, de Hingh IHJT, van der Velden J, Arts HJ, Massuger LFAG, Aalbers AGJ, Verwaal VJ, Kieffer JM, Van de Vijver KK, van Tinteren H, Aaronson NK, Sonke GS. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med. 2018 Jan 18;378(3):230-240. doi: 10.1056/NEJMoa1708618. PMID: 29342393.
- Quénet F, Elias D, Roca L, Goéré D, Ghouti L, Pocard M, Facy O, Arvieux C, Lorimier G, Pezet D, Marchal F, Loi V, Meeus P, Juzyna B, de Forges H, Paineau J, Glehen O; UNICANCER-GI Group and BIG Renape Group. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021 Feb;22(2):256-266. doi: 10.1016/S1470-2045(20)30599-4. Epub 2021 Jan 18. PMID: 33476595.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.