Medicine Group. 2024 July 05;5(7):691-698. doi: 10.37871/jbres1944.
Diabetic Retinopathy and Vision Loss: A Public Health Concern
Jossias Genao Cruz1, Deepkumar Patel2 and Karen Allison3*
2University of San Francisco, School of Public Health, San Francisco, CA
3University of Rochester, Flaum Eye Institute, Rochester, New York
- Equity
- Blindness
- Diabetic retinopathy
- Vision loss
- Diabetes
Abstract
Diabetic Retinopathy (DR), a known complication of diabetes, is one of the top three leading causes of blindness in the U.S. Minorities are disproportionately affected and, most often, diagnosed at advanced stages. This review consolidates current epidemiological data, pathophysiological insights, clinical classifications, and treatment approaches for DR. Despite advancements in therapies like anti-VEGF drugs and laser treatments, significant disparities persist, particularly affecting racial minorities and those with lower socioeconomic status. Early detection via regular eye exams and improved healthcare access are crucial in mitigating DR's increasing global impact. Public health strategies must address these disparities and ensure inclusivity in clinical research to improve DR management and outcomes. Continued awareness of primary and secondary forms of prevention is crucial in all populations with DR, especially minorities, because progression to severe stages can be recognized, treated, and ameliorated. Although anti-vascular endothelial growth factors have advanced, further research is needed to aid understanding of DR in populations disproportionately affected by severe diseases.
Introduction
It has been assumed by the public that diabetes is a disease of the elderly. However, diabetes can be a lifelong disease that chronically affects multiple organs in the body. The number of individuals with diabetes has been steadily increasing compared to recent decades, with a current burden of 37.3 million in the United States [1,2]. Diabetes is commonly known as a disease that prevents insulin production (type 1) or the uptake of sugars (type 2). Generally, Type 2 Diabetes (T2D) accounts for up to 90-95% of diagnosed cases in this country [1-3]. Diabetic Retinopathy (DR) is a known complication, and it is crucial to diagnose early. The longer the time between the development of diabetic retinopathy and diagnosis, the higher the chances of blindness. Adults suffering for more than ten years post-diagnosis have a 12.2% chance of developing the disease and a 5.9% chance of vision loss [4]. Preventive interventions in the initial stages, such as routine dilated eye examinations, help diagnose and subsequently treat retinopathy, reducing the chances of severe complications of vision loss by up to 94%. However, it is a public health concern that the current inequalities in DR diagnosis affect racial and ethnic minorities, as well as those of lower socioeconomic status. More than half of patients diagnosed with diabetes do not obtain the assessment required by recommended guidelines, and others lack access to eye care [5]. This paper aims to provide a comprehensive review of diabetic retinopathy to create more awareness in the medical community and incentivize protective public health measures.
Epidemiology
Diabetes has global consequences. In 2010, the global burden of diabetes was estimated to be 285 million people [6]. Recent diabetic studies reveal an increasing trend [7]. According to the International Diabetes Federation, 537 million adults, or one in ten people aged 20-79 years, suffer from diabetes globally, which is estimated to increase to 463 million by 2030 [8]. The overall burden of Type 1 Diabetes (T1D) is approximately 8.75 million people. In children and younger adults under 20, it is estimated that 17% (1.52 million people) suffer from T1D, and among individuals aged 20-59, the estimate is 64%, or 5.56 million people. The United States is the country with the highest percentages [9]. Moreover, sociodemographic factors appear to influence the diabetic population negatively.
Diabetic retinopathy is a known complication of diabetes and a significant contributor to preventable blindness worldwide. An investigation by Teo ZL, et al. [10] estimated the worldwide prevalence of DR to be 22.27%, 6.17% for Vision-Threatening Diabetic Retinopathy (VTDR) and 4.07% for Clinically Significant Macular Edema (CSME). Compared to previous studies [11,12]. Trends appear to be decreasing. Even though public health and systematic quality improvement measures for diabetes appear to be factors ameliorating previous statistics, it is crucial to recognize that DR, VTDR, and CSME cases are anticipated to increase to 160.50 million, 44.82 million, and 28.61 million, respectively, by 2045 [10]. Proliferative Diabetic Retinopathy (PDR) is the most prevalent VTDR, disproportionately affecting patients with T1D and consistently affecting T2D patients. Furthermore, literature gaps in diabetic macular edema should be further studied [6,10].
In the United States, the economic affliction of diabetes is significant to the healthcare system [13]. Generally, ethnicity and sociodemographic elements play a significant role in diabetic retinopathy [5,6]. Multiple studies such as the National Health and Nutrition Examination Survey (NHANES), the Los Angeles Latino Eye Study (LALES), Proyecto Vision Evaluation Research (VER), and The New Jersey 725 revealed inequalities in diabetic retinopathy primarily affecting non-Hispanic Black and Hispanic individuals, compared to non-Hispanic white individuals [14].
Further, it is necessary to emphasize other complex sociodemographic factors that may contribute to the development of diabetic retinopathy. The financial and educational demographic data reveal a higher prevalence of DR in the underprivileged population. First, investigations in the United States, UK, and Spain linked this finding to multiple factors: screening, treatment monitoring, and insurance [14]. The NHANES revealed that an education below high school negatively impacted eye care visits. Second, a low-income community environment may lead to higher glycemic levels in individuals and lower levels of screening [15]. Third, access to healthcare services and cost-effectiveness were influential. Factors such as distance to a healthcare facility, rural vs. urban locations, health insurance, and lack of coverage for specific treatments and/or procedures, language barriers, cultural sensitivity, and patient education can either negatively or positively affect patient care. Further, reminders, education, cultural sensitivity, and overcoming language barriers have positively affected physician–patient relationships and improved screening. [14,16].
Pathophysiology, classification, and progression
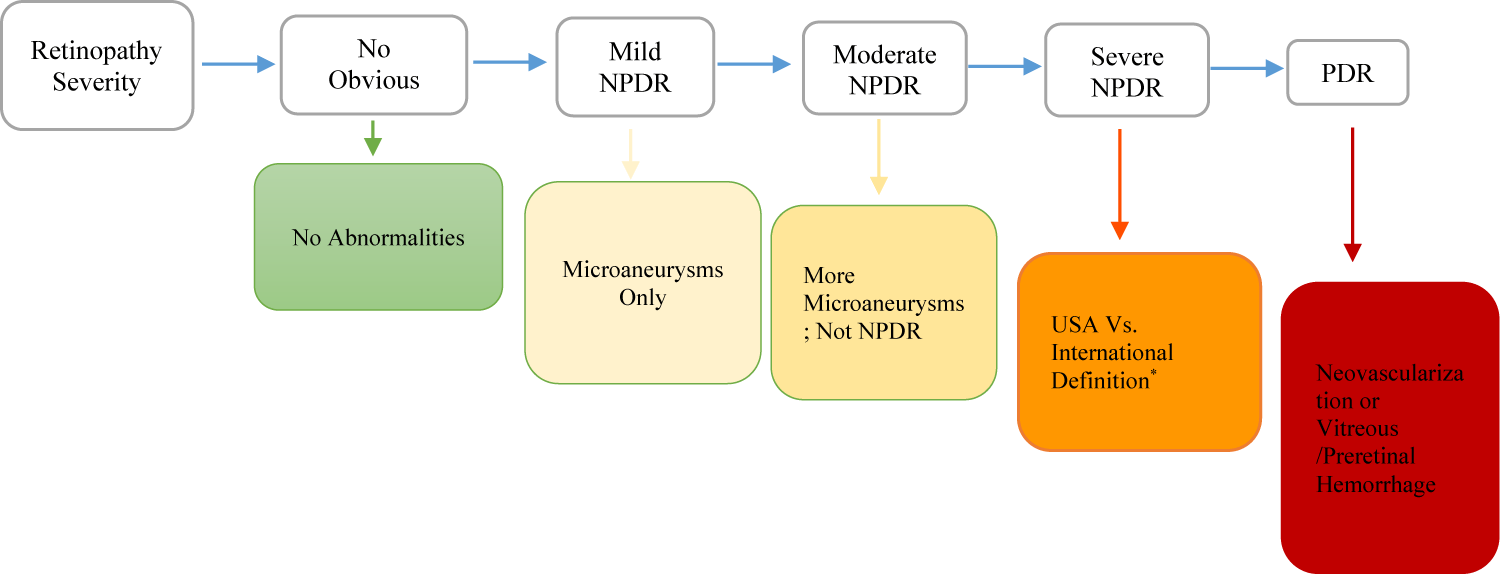
Diabetic retinopathy affects multiple areas of the eye, most detrimentally the retinal region. Without appropriate management, it progresses through an organized manner of stages (Figure 1) [15,17].
Further, Non-Proliferative Diabetic Retinopathy (NPDR) modifies blood flow and vascular permeability, and causes basement membrane enlargement, damage of pericytes, acellular angiogenesis [17], and cotton-wool spots (signs of retinal neural ischemia). The progression of NPDR leads to venous abnormalities and intraretinal vascular remodeling, consequently decreasing perfusion and increasing ischemia [15]. Proliferative Diabetic Retinopathy (PDR) causes vascular structures to rupture, progressing to vitreous hemorrhage, fibrosis, scarring, and retinal detachment, leading to considerable vision loss [17].
Vascular Endothelial Growth Factors (VEGFs) are released to compensate for ischemic retinal events and damages, causing neovascularization and contributing to Diabetic Macular Edema (DME), a thickening of macular tissue. There is a direct proportionality between DR stage progression and DME [18].Central macular thickening exponentially increases the risk of vision loss [15,17,18]. DME is the most common cause of vision loss [15,17].
Diabetes has an inflammatory component involving the activation of innate immunity. Investigations revealed the involvement of macrophage-restricted Protein Tyrosine Phosphatase 1B (PTP1B), an inflammatory regulator, and monocyte occlusion of the microvasculature [19].
Importance of metabolic control
The benefits of long-term glycemic control have been proposed by the Diabetic Control and Complication Trial (DCCT), Stockholm Interventional Trial, and the UK study [20].Investigations revealed that preventive measures like blood sugar control substantially decrease the risk of DR in patients with T1D [21]. Maintaining normal hemoglobin A1c reduces the risk of DR by more than half [22,23]. Moreover, insulin treatment decreases the advancement to DR and eyesight-threatening pathologies by close to 50%, and for a patient with T2D, a hemoglobin A1c level below 6% reduced progression [14].There is disagreement in the literature over whether a lower hemoglobin A1c level may cause DR in minorities [24].
Individuals of lower sociodemographic status, African American and Hispanic ethnicities are disproportionately affected by DR compared to non-Hispanic white individuals. In particular, the LALES study demonstrated an association in Hispanic participants that for every 1% hemoglobin A1c increase (up to a value of 11%), there was a greater than 20% risk of DR emergence [14,15,24].
Hypertension increases the risk of DR and DME [6]. Studies have shown that tight blood pressure control, lower than 150/90 mmHg, in individuals with T2D decreases the risk of microvascular complication, DR, and worsening of visual acuity by greater than 30%. Hypertension is a risk factor in African American and Hispanic populations [6,15]. The current antihypertensive treatment in patients with diabetes aims to inhibit the Renin–Angiotensin–Aldosterone System (RAAS) system. Consequently, these patients benefit from anti-hypertensive treatment from the early stages of DR [25].
Studies investigating the effect of hyperlipidemia and DR revealed mixed results. Evidence suggests that statins and fibrates for treating DR and DME are inconclusive. Controlling diabetes is crucial in order to prevent the progression of diabetic retinopathy and other damage to vital organs. Currently, there are more than sixty medications available for the treatment of this disease, with ongoing research studying over one hundred additional medications in clinical trials. Many of these medications offer innovative approaches to managing diabetes, providing benefits not only to the heart, kidneys, and body weight, but also to other organs. Among the new classes of medications, the Sodium-Glucose co-Transport 2 (SGLT2) inhibitors have gained significant popularity. These inhibitors work on the kidneys to eliminate excess glucose and sodium through urine. As a result, they not only lower blood glucose levels but also offer benefits to the heart and kidneys. Another type of medication, the SGLT1/2 inhibitors, targets SGLT1 proteins in the intestine as well as SGLT1 and SGLT2 proteins in the kidneys. These medications have been shown to reduce HbA1c levels and lower the risk of heart failure and death. In addition to insulin injections, non-insulin medications like Semaglutide (Ozempic), a glucagon-like peptide 1 (GLP-1) agonist, are also available. This medication is injected once a week and mimics a gut hormone that increases insulin release in response to food, blocks glucose production in the liver, and induces a feeling of fullness. It effectively lowers HbA1c levels and promotes weight loss. While the treatment of both type 1 and type 2 diabetes is advancing rapidly, the treatment of diabetic retinopathy is progressing at a slower pace [41].
Comorbidities
Diabetic kidney disease is the leading cause of chronic kidney disease in the world [14]. Some of the clinical manifestations of diabetic nephropathy are associated with hypertension, proteinuria, and abnormal Glomerular Filtration Rate (GFR). Nephropathy is reported to be higher in patients with T1D [26]. Studies have shown a relationship between DR, nephropathy, and microvascular complications. An elevated microalbuminuria ratio is also associated with DR [6,14]. Chronic levels of normoglycemia may decrease the advancement of late diabetes complications [27]. A study by Saini D, et al. [26] identified a relationship between the severity of DR and diabetic nephropathy and neuropathy, which may be used as chronic kidney disease markers.
Management and Treatment
The management of diabetic retinopathy is multifactorial. Individuals with diabetes benefit from uninterrupted lifestyle modifications (diet, exercise, and disease education) and medical management (primary and secondary interventions of comorbid conditions, i.e., glycemic control, blood pressure, renal diseases, neurological diseases, and lipids). Specifically, tight glycemic control, approximately a 10% decrease in hemoglobin A1c, decreases the risk of DR by 39%, and its effects are long-lasting [28,29]. Ophthalmologic evaluation, treatment, and recommendations must be implemented early in patient care. Patient eye care visualization can be performed with a simple light source, and a specific lens can guide the decision of DR. Non-invasive coherence tomography can help confirm the diagnosis. Overall, good synchronization of patient care among practitioners improves along with fundus photography optical treatment, compliance, and surveillance [29].
Early and advanced stages of DR can present asymptomatically. Primary and secondary intervention helps decrease disease progression. Medical treatment of diabetic retinopathy depends on disease stages and damaged areas. The current treatment utilizes intravitreal (in-eye) drugs known as Vascular Endothelial Growth Factor Inhibitors (Anti-VEGF). FDA-approved drugs such as faricimab-svoa (Vabysmo), ranibizumab (Lucentis), and aflibercept (Eylea). At stages of sight-threatening retinopathy, aflibercept performed better at improving vision [30]. A fourth drug, bevacizumab (Avastin), can be used off-label for the treatment of diabetic macular edema [31]. In patients with DR who developed macular edema, aflibercept, bevacizumab, or ranibizumab helped to improve vision in Center-Involved Diabetic Macular Edema (CI-DME) [30]. Steroid benefits and/or their combination with Anti-VEGF are uncertain and regarded as second-line drugs [30]. Topical anesthesia is utilized to administer drugs, and side effects range from mild discomfort to increased intraocular pressure and infection. These injections will need to be repeated. In some cases, the medication is used with panretino photocoagulation. Photocoagulation is a targeted laser treatment; it stops or slows down vascular leakage in the eye with laser ablation. Panretinal photocoagulation, known as scattered laser treatment, helps shrink abnormal blood vessels. During the procedure, the areas of the retina away from the macula are ablated. The burns cause the abnormal new blood vessels to shrink and scar. The ETDRS study and a Cochrane systematic review demonstrated that photocoagulation slows progression to sight-threatening vision loss [29]. These procedures are usually carried out over multiple sessions at a doctor's office or eye clinic. Patients may experience, even at twenty-four hours post-intervention, blurry vision, and some peripheral or night vision loss. Vitrectomy is another procedure that invades the middle of the eye (vitreous) and help removes blood and scar tissue that is affecting the retina. This procedure is performed under anesthetic at a hospital or surgical center according to the American Academy of Ophthalmology (Table 1). Unfortunately, patients of lower sociodemographic status (Hispanic, Black, and Medicaid-insured) typically present with advanced stages and do not receive similar improvement compared to non-Hispanic white patients. Consequently, anti-VEGF treatment offers a lower probability of improvement. Additionally, our current system of clinical trials does not have a fair or good representation of minorities even though the disease burden is skewed towards this population [29,31].
| Table 1: Management and recommendations for patients diagnosed with diabetes [29]. | |||
| Severity of Retinopathy | Macular Edema | Follow-Ups (Months) | Notes |
| Normal or Minimal NPDR | No | 12 | Lifestyle Modification and medical management-optimal glycemic control Close monitoring Avoid rapid glycemic reduction in patients with high Hgb A1c [28] |
| Mild NPDR | No NCI-DME CI-DME | 12 3-6 1* | The data suggest benefits of early treatment [37] |
| Moderate NPDR | No NCI-DME CI-DME | 6-12*** 3-6 1* | Pharmacotherapy or (Anti VEGF) helps delay NPDR progressing to PDR [37] Overall and annual risk of PDR 26% and 8%, respectively [3] |
| Severe NPDR | No NCI-DME CI-DME | 3-4 2-4 1* | Pharmacotherapy helps delay NPDR progressing to PDR [37] Overall and annual risk of PDR 50% and 15 %, respectively [3] |
| Non-High-Risk PDR | No NCI-DME CI-DME | 3-4 2-4 1* | PRP and anti-VEGF sometimes used in combination |
| High-Risk PDR | No NCI-DME CI-DME | 2-4 2-4 1* | |
| Anti-VEGF = Anti-Vascular Endothelial Growth Factor; CI-DME = Center-Involved Diabetic Macular Edema; NCI-DME = Non-Center-Involved Diabetic Macular Edema; PDR = Proliferative Diabetic Retinopathy; NPDR = Non-Proliferative Diabetic Retinopathy. *Adjunct treatment with anti-VEGF and/or (+/- Laser) increased visual improvement. Patients re-examined as early as one month. *** Shorter intervals between re-examination/follow-up. | |||
The literature shows that non-compliance with treatment tends to have worse patient outcomes. Factors that decrease compliance with treatment in minorities are medical underinsured, lack of medical insurance, unaffordable co-pays, access to care, lower sociodemographics, lower socioeconomics and even lower eyesight [14,32].There are multiple methods for glycemic detection, monitoring, and control; the most common are invasive, where tissue interference occurs, and according to healthcare guidelines, glucose monitoring should occur multiple times per day [33]. For patients struggling with diabetes, noninvasive methods of glycemic monitoring may offer an advantageous solution, especially for lower sociodemographic populations. While these technologies are still being perfected, most patients may benefit from these technologies as an alternative or second choice to current methods. Hence, their investigation in clinical trials in a diverse population is necessary [33].
Treatment Approach Consideration
There is a general tendency for type 1 diabetes to develop into DR. Tight daily glucose measures in type 1 diabetics reduce the development of DR by more than 75% and decrease the progression of retinopathy by more than half [15]. Glycemic control of hemoglobin A1c of 6-7% has been shown to reduce the progression of DR [34]. Early stages of DR-treated laser surgery reduce the incidence of vision loss by half [35]. The incidence of development of eye-threatening disease or blindness in untreated eyes compared to those who received treatment was more than 50% [15]. Overall, the literature reveals multiple modalities of treatment at different stages benefiting patients with DR.
Public Health Awareness
Worldwide, diabetic retinopathy can cause blindness in a working-age population if untreated. Approximately one in three people diagnosed with diabetes develop DR, and management can be overwhelming. Social Determinants of Health (SDoH) are factors that are not medically related but weigh on patients' health. There are tangible sociodemographic factors (economic, educational, healthcare access, environmental, and community) that are deleterious to DR and patient outcomes [32]. Certain communities have been associated with decreased risk of type 2 diabetes. Food deserts and nutritional deserts are correlated with increasing BMI. Consequently, the management of chronic health diseases in lower socioeconomic areas is challenging due to its multifactorial causes [14,32].
Czapp P and Kovach K argued that poor neighborhoods or poverty, in general, can decrease access to healthy nutrition, safe access to education, infrastructure, jobs, clean air, water, and utilities, and hence cause increasing stress and concomitant chronic diseases. Low socioeconomic level of patients with T1D has been associated with the development of DR. Moreover, having public-funded health insurance has been associated with obtaining fewer medical appointments and a lower compliance rate with the completion of medical visits.
Ethnic minorities often lack health insurance and access to eye doctors can be financially and conveniently challenging in rural areas. As a result, preventive health measures services are lacking. In fact, studies have shown that the quality of care for some minorities with diabetes is substandard compared to individuals of higher socioeconomic status [36-39]. An investigation by Li J, et al. [40] graphically demonstrates racial/ethnic disparities in DR complications and ophthalmologic examination. Hence, African American and Hispanic patients are more likely to develop advanced retinopathy.
Patients continue to benefit from comprehensive screening measures like good history and physical exams, fasting blood sugar levels, hemoglobin A1c, and dilated eye exams by an ophthalmologist [14]. Moreover, tele-retinal screening, telemedicine, and artificial intelligence systems positively contribute to patient care, which appear to be cost-effective measures for the entire healthcare system.14 Furthermore, clinical trials should aim to represent minorities in their study proportionally since the burden of DR disproportionately affects this population. Our current institutions under-represent Blacks in clinical trials by factors greater than three-fold in public and private research, conversely over-represent white populations in research (Table 2) [32].
| Table 2: Clinical roles of health care providers addressing social determinants of health in diabetic retinopathy. | ||||
| Economic Stability | Education Access and Quality | Health Care Access and Quality | Neighborhood and Built Environment | Social and Community Context |
| Insurance Coverage | Health Literacy | Healthcare Coverage | Medically Underserved Areas | Healthcare Access |
| Employment Status | Lower Levels of Education < High School Increases Risk of DR | Healthcare Providers and Continuity of Care | Urban vs. Rural Impoverished Urban Areas |
Prevention Programs and Education |
| Socioeconomic Status | Preventive Education | Private Insurers as Protective Factor | Food Desert | Social Relationship-Connectedness |
| Public Assistance | ||||
Conclusion
Worldwide, the prevalence of diabetes has increased more than expected. It is a public healthcare concern that the current system has widened the gap when caring for the underserved with diabetes complications, specifically DR. Early recognition of DR will allow treatment in the early stages, delaying its complications. It will continue to benefit our society and consequently reduce eyesight-threatening vision loss. We must take advantage of technological advances in screening and treating patients for the entire population. Further, ethnic minorities continue to be disproportionately represented in clinical trials. Therefore, it is fundamental that organizations like the FDA, NEI, and NIH, along with other stakeholders, incorporate diverse populations in trials. These measures will continue to broaden our understanding of DR and better serve an underserved population.
References
- National and State Diabetes Trends. CDC.
- Diabetes Quick Facts. Basics. Diabetes. CDC.
- IDF Diabetes Atlas | Tenth Edition. Copyright © IDF Diabetes Atlas 2023. All Rights Reserved.
- Cha AE, Villarroel MA, Vahratian A. Eye Disorders and Vision Loss Among U.S. Adults Aged 45 and Over With Diagnosed Diabetes, 2016-2017. NCHS Data Brief. 2019 Jul;(344):1-8. PMID: 31442198.
- Fairless E, Nwanyanwu K. Barriers to and Facilitators of Diabetic Retinopathy Screening Utilization in a High-Risk Population. J Racial Ethn Health Disparities. 2019 Dec;6(6):1244-1249. doi: 10.1007/s40615-019-00627-3. Epub 2019 Aug 28. PMID: 31463812; PMCID: PMC6880869.
- Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond). 2015 Sep 30;2:17. doi: 10.1186/s40662-015-0026-2. PMID: 26605370; PMCID: PMC4657234.
- Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019 Nov;157:107843. doi: 10.1016/j.diabres.2019.107843. Epub 2019 Sep 10. PMID: 31518657.
- IDF Diabetes Atlas. Tenth Edition. Copyright © IDF Diabetes Atlas 2023. All Rights Reserved.
- Graham DO, Wang F, Gregory GA, Maniam J. Type 1 diabetes estimates in children and adults. International Diabetes Federation.
- Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N, Bikbov MM, Wang YX, Tang Y, Lu Y, Wong IY, Ting DSW, Tan GSW, Jonas JB, Sabanayagam C, Wong TY, Cheng CY. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology. 2021 Nov;128(11):1580-1591. doi: 10.1016/j.ophtha.2021.04.027. Epub 2021 May 1. PMID: 33940045.
- Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond). 2015 Sep 30;2:17. doi: 10.1186/s40662-015-0026-2. PMID: 26605370; PMCID: PMC4657234.
- Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O'Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY; Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012 Mar;35(3):556-64. doi: 10.2337/dc11-1909. Epub 2012 Feb 1. PMID: 22301125; PMCID: PMC3322721.
- Health and economic benefits of diabetes interventions. Power of Prevention.
- Patel D, Ananthakrishnan A, Lin T, Channa R, Liu TYA, Wolf RM. Social Determinants of Health and Impact on Screening, Prevalence, and Management of Diabetic Retinopathy in Adults: A Narrative Review. J Clin Med. 2022 Nov 30;11(23):7120. doi: 10.3390/jcm11237120. PMID: 36498694; PMCID: PMC9739502.
- Barsegian A, Kotlyar B, Lee J, Salifu MO, McFarlane SI. Diabetic Retinopathy: Focus on Minority Populations. Int J Clin Endocrinol Metab. 2017;3(1):034-45. doi: 10.17352/ijcem.000027. Epub 2017 Nov 11. PMID: 29756128; PMCID: PMC5945200.
- Zhang X, Cotch MF, Ryskulova A, Primo SA, Nair P, Chou CF, Geiss LS, Barker LE, Elliott AF, Crews JE, Saaddine JB. Vision health disparities in the United States by race/ethnicity, education, and economic status: findings from two nationally representative surveys. Am J Ophthalmol. 2012 Dec;154(6 Suppl):S53-62.e1. doi: 10.1016/j.ajo.2011.08.045. PMID: 23158224; PMCID: PMC4169111.
- Antonetti DA, Silva PS, Stitt AW. Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat Rev Endocrinol. 2021 Apr;17(4):195-206. doi: 10.1038/s41574-020-00451-4. Epub 2021 Jan 19. PMID: 33469209; PMCID: PMC9053333.
- Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII the twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008 Nov;115(11):1859-68. doi: 10.1016/j.ophtha.2008.08.023. PMID: 19068374; PMCID: PMC2761813.
- Forrester JV, Kuffova L, Delibegovic M. The Role of Inflammation in Diabetic Retinopathy. Front Immunol. 2020 Nov 6;11:583687. doi: 10.3389/fimmu.2020.583687. PMID: 33240272; PMCID: PMC7677305.
- Rodriguez-Fontal M, Kerrison JB, Alfaro DV, Jablon EP. Metabolic control and diabetic retinopathy. Curr Diabetes Rev. 2009 Feb;5(1):3-7. doi: 10.2174/157339909787314176. PMID: 19199891.
- Frank RN. Diabetic retinopathy and systemic factors. Middle East Afr J Ophthalmol. 2015 Apr-Jun;22(2):151-6. doi: 10.4103/0974-9233.154388. PMID: 25949071; PMCID: PMC4411610.
- Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993 Sep 30;329(14):977-86. doi: 10.1056/NEJM199309303291401. PMID: 8366922.
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998 Sep 12;352(9131):837-53. Erratum in: Lancet 1999 Aug 14;354(9178):602. PMID: 9742976.
- Penman A, Hancock H, Papavasileiou E, James M, Idowu O, Riche DM, Fernandez M, Brauner S, Smith SO, Hoadley S, Richardson C, Vazquez V, Chi C, Andreoli C, Husain D, Chen CJ, Sobrin L. Risk Factors for Proliferative Diabetic Retinopathy in African Americans with Type 2 Diabetes. Ophthalmic Epidemiol. 2016;23(2):88-93. doi: 10.3109/09286586.2015.1119287. Epub 2016 Mar 7. PMID: 26950197; PMCID: PMC4851860.
- Silva PS, Cavallerano JD, Sun JK, Aiello LM, Aiello LP. Effect of systemic medications on onset and progression of diabetic retinopathy. Nat Rev Endocrinol. 2010 Sep;6(9):494-508. doi: 10.1038/nrendo.2010.122. Epub 2010 Jul 27. PMID: 20664533.
- Saini DC, Kochar A, Poonia R. Clinical correlation of diabetic retinopathy with nephropathy and neuropathy. Indian J Ophthalmol. 2021 Nov;69(11):3364-3368. doi: 10.4103/ijo.IJO_1237_21. PMID: 34708806; PMCID: PMC8725070.
- Dahl-Jørgensen K, Brinchmann-Hansen O, Hanssen KF, Ganes T, Kierulf P, Smeland E, Sandvik L, Aagenaes O. Effect of near normoglycaemia for two years on progression of early diabetic retinopathy, nephropathy, and neuropathy: the Oslo study. Br Med J (Clin Res Ed). 1986 Nov 8;293(6556):1195-9. doi: 10.1136/bmj.293.6556.1195. PMID: 3096429; PMCID: PMC1341978.
- Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007 Aug 22;298(8):902-16. doi: 10.1001/jama.298.8.902. PMID: 17712074.
- Flaxel CJ, Adelman RA, Bailey ST, Fawzi A, Lim JI, Vemulakonda GA, Ying GS. Diabetic Retinopathy Preferred Practice Pattern®. Ophthalmology. 2020 Jan;127(1):P66-P145. doi: 10.1016/j.ophtha.2019.09.025. Epub 2019 Sep 25. Erratum in: Ophthalmology. 2020 Sep;127(9):1279. doi: 10.1016/j.ophtha.2020.06.047. PMID: 31757498.
- Diabetic Retinopathy Clinical Research Network; Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, Antoszyk AN, Arnold-Bush B, Baker CW, Bressler NM, Browning DJ, Elman MJ, Ferris FL, Friedman SM, Melia M, Pieramici DJ, Sun JK, Beck RW. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015 Mar 26;372(13):1193-203. doi: 10.1056/NEJMoa1414264. Epub 2015 Feb 18. PMID: 25692915; PMCID: PMC4422053.
- Grisanti S, Ziemssen F. Bevacizumab: off-label use in ophthalmology. Indian J Ophthalmol. 2007 Nov-Dec;55(6):417-20. doi: 10.4103/0301-4738.36474. PMID: 17951896; PMCID: PMC2635984.
- Sanjiv N, Osathanugrah P, Harrell M, Siegel NH, Ness S, Chen X, Cabral H, Subramanian ML. Race and ethnic representation among clinical trials for diabetic retinopathy and diabetic macular edema within the United States: A review. J Natl Med Assoc. 2022 Apr;114(2):123-140. doi: 10.1016/j.jnma.2021.12.016. Epub 2022 Jan 22. PMID: 35078668.
- Houle J, Lauzier-Jobin F, Beaulieu MD, Meunier S, Coulombe S, Côté J, Lespérance F, Chiasson JL, Bherer L, Lambert J. Socioeconomic status and glycemic control in adult patients with type 2 diabetes: a mediation analysis. BMJ Open Diabetes Res Care. 2016 May 11;4(1):e000184. doi: 10.1136/bmjdrc-2015-000184. PMID: 27239316; PMCID: PMC4873951.
- Nathan DM; DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9-16. doi: 10.2337/dc13-2112. PMID: 24356592; PMCID: PMC3867999.
- Calderon GD, Juarez OH, Hernandez GE, Punzo SM, De la Cruz ZD. Oxidative stress and diabetic retinopathy: development and treatment. Eye (Lond). 2017 Aug;31(8):1122-1130. doi: 10.1038/eye.2017.64. Epub 2017 Apr 28. PMID: 28452994; PMCID: PMC5558229.
- Wykoff CC. Management of Diabetes-Related Retinopathy. 2019 May. In: Prevention and Management of Diabetes-Related Eye Disease. Arlington (VA): American Diabetes Association; 2019 May. PMID: 34251786.
- Spanakis EK, Golden SH. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep. 2013 Dec;13(6):814-23. doi: 10.1007/s11892-013-0421-9. PMID: 24037313; PMCID: PMC3830901.
- Czapp P, Kovach. Poverty and health- The family medicine perspective (position paper). American Academy of Family Physicians. 2015.
- Golden SH, Brown A, Cauley JA, Chin MH, Gary-Webb TL, Kim C, Sosa JA, Sumner AE, Anton B. Health disparities in endocrine disorders: biological, clinical, and nonclinical factors--an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2012 Sep;97(9):E1579-639. doi: 10.1210/jc.2012-2043. Epub 2012 Jun 22. PMID: 22730516; PMCID: PMC3431576.
- Li J, Harrison WJ, Cho SK. Disparity in diabetic eye complications among racial/ethnic minorities: An analysis of the 2009-2018 United States Medical Expenditure Panel Survey. Diabetes Epidemiology and Management. 2022;6:100070. doi: 10.1016/j.deman.2022.100070.
- Scheen AJ. Dual GIP/GLP-1 receptor agonists: New advances for treating type-2 diabetes. Ann Endocrinol (Paris). 2023 Apr;84(2):316-321. doi: 10.1016/j.ando.2022.12.423. Epub 2023 Jan 10. PMID: 36639119.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.