Biology Group. 2024 July 02;5(7):683-690. doi: 10.37871/jbres1943.
Utilizing Fetal MRI and Prenatal Medical Exome Sequencing for Prenatal Diagnosis of Kinked Brainstem: A Case Series
Yunan Wang1,2, Chang Liu1,2, Ling Liu1,2, Chaoxiang Yang4, Yanlin Huang1,2, Juan Geng3, YiMing Qi1,2, Xin Zhao1,2, Ying Xiong1,2, Liyuan Fang1,2, Jing Wu1,2* and Aihua Yin1,2*
2Maternal and Children Metabolic-Genetic Key Laboratory, Guangdong Women and Children Hospital, Guangzhou, Guangdong 511442, China
3UItrasonic Diagnosis Department, Guangdong Women and Children Hospital, Guangzhou, Guangdong 511442, China
4Radiology Department, Guangdong Women and Children Hospital, Guangzhou, Guangdong 511442, China
Jing Wu, Medical Genetic Center, Guangdong Women and Children Hospital, NO.521-523, Xingnan Road, Panyu District, Guangzhou, Guangdong, 511442, P.R. China E-mail:
- Kinked brainstem
- Prenatal diagnosis
- Walker-Warburg Syndrome
- pMES
Abstract
Objective: Utilizing fetal MRI and prenatal Medical Exome Sequencing (pMES) for prenatal diagnosis in fetuses exhibiting a kinked or 'z-shaped' brainstem.
Methods: A retrospective analysis was conducted on cases of brainstem kinking identified on antenatal imaging between January 2013 and December 2022. Systematic review was performed on clinical data from these cases, encompassing maternal demographics, indications for invasive prenatal diagnosis, ultrasound findings, SNP-array results, Trio-Medical Exome Sequencing (Trio-MES) results, QF-PCR results, pregnancy outcomes and follow-ups.
Results: Two cases of fetuses presenting with a "kinked brainstem," along with other CNS abnormalities in fetal MRI were identified using Trio pMES. Prenatal phenotype features observed in our case series include "Z" shaped brainstem, hydrocephalus, hyperechogenic kidneys and generalized skin edema, cobblestone lissencephaly, cerebellar dysplasia, cerebral arachnoid cyst. Trio pMES identified a homozygous variant at c.815_816del (p.Leu272ArgfsTer117) in the FKRP gene for Patient 1. For Patient 2, a de novo heterozygous variant c.848A>G (p.H283R) was detected in the TUBA1A gene. Both variants were predicted to be likely pathogenic or pathogenic.
Conclusion: A kinked or "z-shaped" brainstem is a finding that can be more easily delineated on fetal MRI compared to fetal ultrasound, indicating an underlying dystroglycanopathy (Walker-Warburg phenotype) or tubulinopathy. Prenatal exome sequencing can aid in achieving early prenatal genetic diagnosis for cases of "kinked brainstem". Moreover, our study expands its scope by incorporating additional features such as hyperechogenic kidneys and generalized skin edema into the prenatal findings of Walker-Warburg syndrome.
Introduction
Fetal ultrasound is routinely utilized for the assessment of the posterior cranial fossa, encompassing the cerebellar hemisphere, vermis, and width of the posterior cranial fossa. However, there is a paucity of literature reporting prenatal brainstem abnormalities. Complete evaluation of the brainstem during the prenatal period can be challenging due to potential compromises in image quality caused by maternal obesity, decreased amniotic fluid levels, fetal positioning, and calvarial ossification. Fetal MRI has emerged as an effective method for evaluating suspected brain anomalies during pregnancy. Particularly in cases where fetal ultrasound alone fails to provide clear visualization of abnormalities in the fetal brainstem, fetal MRI plays a pivotal role in delineating the morphology and narrowing down differential diagnoses. It significantly enhances diagnostic accuracy compared to relying solely on fetal ultrasound findings [1]. This provides crucial information that can impact prognosis, approaches to prenatal counseling, and strategies for prenatal/perinatal management.
A kinked or "z-shaped" brainstem is an uncommon finding in fetal neuroimaging, resembling the primitive hindbrain morphology observed during early pregnancy (around 7 weeks gestational age). This phenotype may potentially cause by enzymatic dysfunction, infection, or exposure to drugs during the same developmental stage [2]. Existing literatures suggest that these brainstem features characterized by a "kinked brainstem" or a "z-shaped brainstem" may be associated with a spectrum of severe Central Nervous System (CNS) abnormalities such as Walker-Warburg Syndrome (WWS) and lissencephaly syndromes with cerebellar hypoplasia [1,3]. Most cases in the literature were clinically diagnosed postnatally, with only a limited number underwent prenatal genetic diagnosis. In this study, two cases of fetuses presenting with a "kinked brainstem," along with other CNS abnormalities in fetal MRI were identified using Trio prenatal Medical Exome Sequencing (pMES), followed by Sanger sequencing.
Material and Methods
Subject
A retrospective review was conducted on cases of kinked brainstem identified on antenatal imaging between January 2013 and December 2022, resulting in the enrollment of two cases. Each case underwent a routine ultrasound scan at a primary hospital before being referred to our Medical Genetic Center for reassessment. Following ultrasound examination, fetal MRI reassessment as well as prenatal genetic testing including chromosome analysis, viral testing, and Trio-pMES were performed. Both couples were nonconsanguineous and healthy individuals without any history of exposure to teratogens, irradiation, infections or smoking during pregnancy. They did not have diabetes or hypertension during this period. This study has received approval from the Institutional Review Board/Medical Ethics Committee of Guangdong Women and Children Hospital (IRB reference number: 202301185). Written informed consent was obtained from each participating family.
Cytogenetic and molecular analyses
The metaphase chromosomes of amniotic fluid cells were analyzed using the G-banding technique, employing standard protocols [4]. The Lab-Aid 820 automation system (Zee San Biotech Company, Fujian, China) was utilized to extract genomic DNA from uncultured fetal amniotic fluids and peripheral blood samples from the parents. For SNP-array analysis, a commercial CytoScan 750K Array (Affymetrix, Santa Clara, CA) containing a total of 750,436 oligonucleotide probes (25-85mer), including both nonpolymorphic and SNP probes (550,000 nonpolymorphic probes and 200,436 SNP probes), was employed. The genomic DNA labeling and hybridization procedures were conducted following the manufacturer's instructions. Analysis of the obtained data was performed using Affymetrix Chromosome Analysis Suite software [5].
Medical exome sequencing
The Qiagen DNA blood kit (Qiagen GmbH, Hilden, Germany) was utilized for the extraction of genomic DNA. To prepare the library and enrich the target, we followed the manufacturer's instructions using an Agilent Technologies SureSelectXT Clinical Research Exome kit (Santa Clara, CA). Subsequently, Then, Trio-MES was performed using 2 × 150 bp in the paired end mode of the NextSeq 500 platform (Illumina, San Diego, CA) to obtain an average coverage of above 110x, with 97.6% of target bases covered at least 10x. Sequence quality analysis and filtering of mapped target sequences were performed with the ‘varbank’ exome and genome analysis pipeline v.2.1 as described previously. Analysis of genetic results was based on the genomic variation database (http://dgv.tcag.ca/dgv/app/home), DECIPHER database (https://decipher.sanger.ac.uk/), and OMIM database (http://www.ncbi.nlm.nih.gov/omim). Found variants were further verified by Sanger sequencing [6].
Results
Intrauterine phenotypes
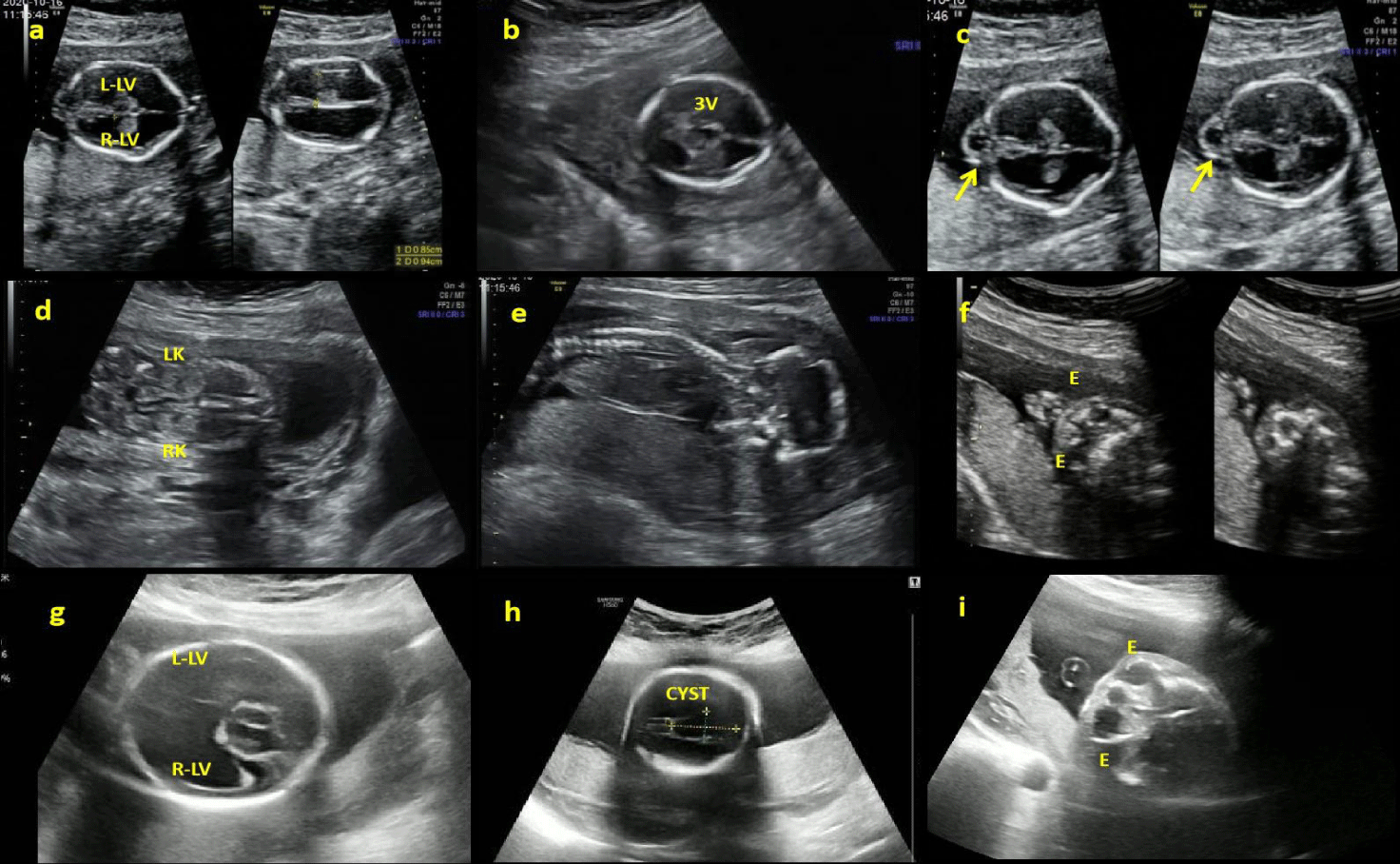
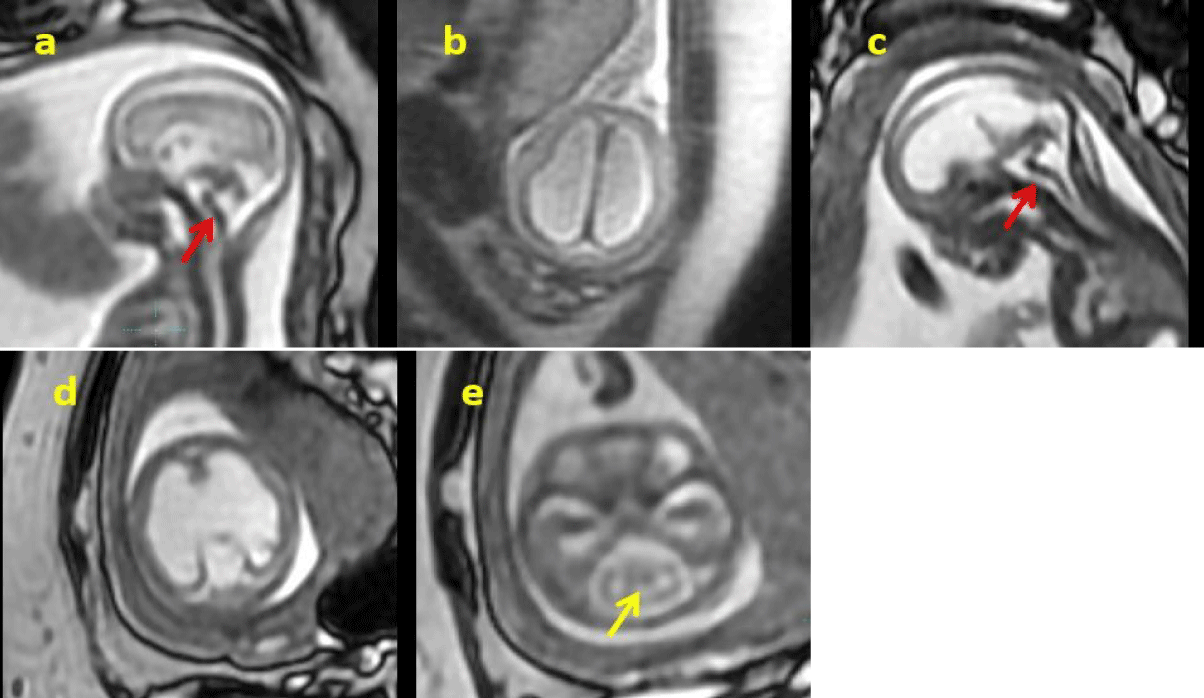
In Patient 1 (P1), ultrasonography at 16+4 weeks revealed evident bilateral ventricular dilation and enlargement of the third ventricle ( 8.5 mm left, 9.4 mm right, 2.6 mm the third ventricle), indicating hydrocephalus (Figure 1). Bilateral hyperechogenic kidneys and generalized skin edema were also noted. Fetal Central Nervous System Magnetic Resonance Imaging (CNS-MRI) was conducted at 17+2 weeks revealed a "Z" shaped brainstem along with hydrocephalus and cobblestone lissencephaly.
In Patient 2 (P2), ultrasonography at 23+3 weeks showed significant bilateral ventricular enlargement (18.2 mm) cerebellar dysplasia and cerebral arachnoid cyst. Fetal CNS-MRI conducted at 24+2 weeks confirmed hydrocephalus, cobblestone lissencephaly, absence of midline structures such as septum pellucidum and corpus callosum, cerebellar dysplasia, as well as a distinctive "Z" shaped appearance with brainstem dysplasia (Figure 2).
The Nuchal Translucency thickness (NT) of the P1 was 5.8mm (99.9th), and the NT of P2 were 1.1mm (< 95th) percentile for its gestational age.
Cytogenetic and molecular analyses
Both pregnant women tested negative for G-banding karyotype, chromosome microarray (SNP array), TORCH analysis (Toxoplasmosis, Other: syphilis, varicella-zoster, Rubella, Cytomegalovirus, and Herpes infections), as well as parvovirus B19 analysis.
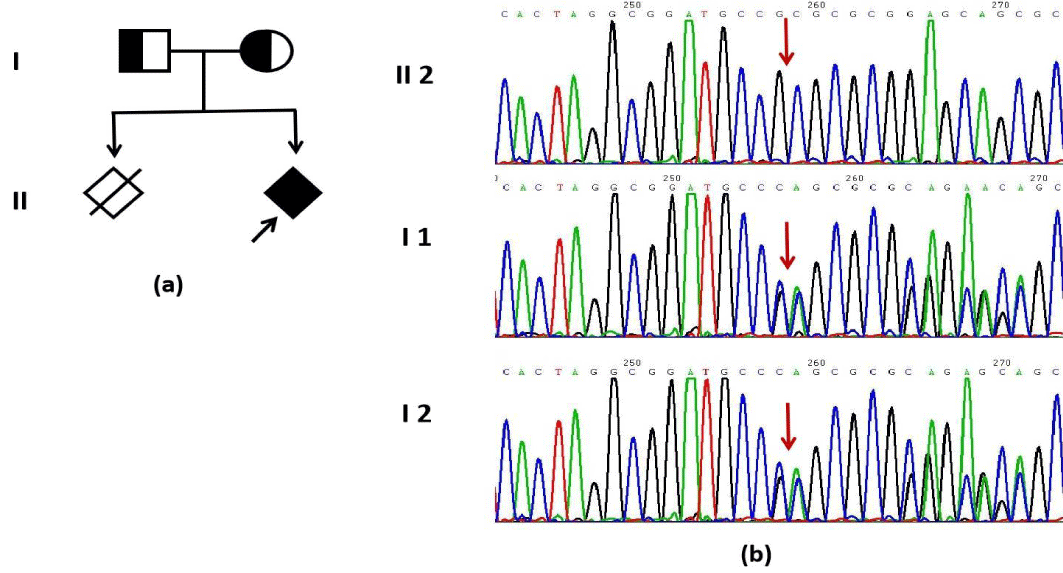
In Patient 1 (P1), Trio-pMES identified a homozygous variant (c.815_816del, p.Leu272ArgfsTer117) in the FKRP gene, inherited from both parents. The identified variant was not annotated in the gnomAD-exome database (PM2_Supporting). It is a frameshift variant predicted to induce mRNA degradation (PVS1), and the clinical phenotype of the patient aligns with the primary disease phenotype (PP4). This specific variant has no record in either Clinvar or HGMD databases. In conclusion, according to ACMG guidelines, this variant is classified as pathogenic. It is associated with muscular dystrophy-dystroglycanopathy (congenital with brain and eye anomalies), type A,5 and muscular dystrophy-dystroglycanopathy (congenital with or without impaired intellectual development), type B,5 (OMIM ID:613153/606612). Clinical manifestations include brain and ocular malformations, congenital hydrocephalus, pontine and cerebellar dysplasia, as well as profound cognitive impairment. Additional features may include microphthalmia, eye asymmetry, cataracts, muscular dystrophy, hypotonia, and feeding difficulties.
In Patient 2 (P2), novo heterozygous variant (c.848A>G, p.His283Arg) in the TUBA1A gene was identified, as shown in figure 3. The variant was not documented in the gnomAD-exome database (PM2_Supporting). Additionally, various bioinformatics software tools such as REVEL/AlphaMissense predicted that the mutation would have deleterious effects on the gene or gene product (PP3_Moderate). The clinical phenotype of the proband was consistent with the primary phenotype of the disease (PP4), and there was confirmed kinship between the fetus and a new mutation (PS2). Although this variant is classified as Likely Pathogenic (LP) in Clinvar databases, it is not recorded in HGMD databases. In conclusion, according to ACMG guidelines, this variant is classified as LP. It is associated with Lissencephaly 3 (OMIM ID: 611603), which is an autosomal dominant disorder characterized by microcephaly, abnormal gyrus formation, aberrant corpus callosum development, hypoplasia of the cerebellum and brain stem, potentially accompanied by intellectual impairment, motor retardation, epilepsy.
Follow-ups
After genetic counseling, both couples chose termination of the pregnancy. But they declined additional pathological investigations.
Discussion
The development of the posterior cranial fossa, including the brainstem, occurs early in pregnancy. Brainstem folding, a distinctive characteristic, occurs between weeks 3 and 8, while cerebellar development typically reaches completion by week 16. The presence of a kinked or "z-shaped" brainstem suggests a genetic or environmental insult around 7 weeks gestational age, interrupting brainstem and cerebellum development [2]. Thus, brainstem kinking often indicates severe CNS disorders and may be an initial manifestation in some cases [2].
Brainstem abnormalities are not clearly defined on fetal ultrasound, and in such cases, fetal MRI is crucial for revealing brainstem structure and narrowing potential diagnoses [1,7]. The Z-shaped appearance of the brainstem was previously reported by Stroustrup Smith, et al. [2] who utilized fetal MRI scans between 19 and 34 weeks of gestation in a unique series comprising seven cases. They identified a correlation between the "Z"-shaped appearance of the brainstem and severe neurodysgenesis occurrence. Subsequently, this kinked brainstem was recognized as a characteristic manifestation of WWS, which can be detected as early as 14 weeks [8], particularly when accompanied by agyria and hydrocephalus. Literature has also documented associations between Z-shaped brainstems with L1CAM mutation or tubulinopathy [9,10]. In our case series, both cases exhibited hydrocephalus and cobblestone lissencephaly, with one diagnosed with WWS and the other with tubulinopathy. Thus, when detecting a "Z"-shaped brainstem with agyria and hydrocephalus, WWS, L1CAM mutation, or tubulinopathy should be highly suspected, with implications for prenatal counseling and prognosis.
The most likely diagnosis in cases with a kinked brainstem is WWS, a lethal autosomal recessive disorder characterized by hydrocephalus, agyria, retinal dysplasia with or without encephalocele. It has been reported that patients with WWS typically do not survive beyond infancy [11]. Therefore, early detection and diagnosis of WWS are crucial for the prevention of birth defects. However, prenatal detection of WWS remains challenging due to some diagnostic criteria, such as muscular hypotonia and elevated creatine kinase levels, cannot be reliably identified prenatally. Additionally, lissencephaly, cerebellar malformations, and retinal abnormalities observed on antenatal imaging are often nonspecific and insensitive indicators, found in less than one-quarter of reported cases [11]. In our case series, we present a prenatal diagnosis of WWS in the second trimester using prenatal ultrasonography, MRI scans, and pMES. The fetus exhibited hydrocephalus, agyria, brainstem dysplasia, and a distinctive "Z"-shaped appearance on prenatal MRI scans, along with bilateral hyperechogenic kidneys and generalized skin edema.
Hyperechogenic kidneys often accompany additional abnormalities in the renal tract and extra-renal structures, as well as chromosomal and genetic disorders like Autosomal Recessive Polycystic Kidney Disease (ARPKD), Autosomal Dominant Polycystic Kidney Disease (ADPKD), and Beckwith-Wiedemann syndrome. These factors influence the overall outcome more significantly than the hyperechogenicity of the kidneys [12]. In our case series, case 1 presented with bilateral hyperechogenic kidneys. Previous studie have reported urinary malformations characterized by large cystic kidneys associated with POMT2-related WWS [13]. Gasser B, et al. [14] reported prenatal diagnosis of WWS in three siblings, where subsequent pregnancies revealed fetal hydrocephalus during ultrasound examination. Termination occurred at 20 weeks for the third female fetus, which exhibited postmortem findings including dilated ventricles, thin cortex, type II lissencephaly with microscopic evidence of disorganized architecture, retinal dysplasia, cystic changes, and stenosis at the pyeloureteral junction. Fetal generalized skin edema, often an initial manifestation of hydrops fetalis, is valuable for detecting genetic abnormalities such as aneuploidy or Noonan syndrome, and structural malformations, especially in early pregnancy stages [15]. Notably, previous literature on WWS symptoms has not mentioned skin edema, thus it broadening our comprehension of the clinical spectrum to encompass hyperechogenic kidneys and generalized skin edema.
Approximately one-third of WWS cases involve mutations in genes like FKRP, FKTN, LARGE, POMT1, and POMT2. However, the genetic cause for most WWS patients remains unknown [16]. Exome sequencing has become essential for uncovering molecular-level fetal malformations by identifying Single Nucleotide Variations (SNVs) and insertions/deletions (indels) in gene coding regions. Recent advancements in exome sequencing have increased studies investigating pathogenic variations associated with WWS. In our current case series, trio medical exome sequencing followed by Sanger sequencing revealed variants in the FKRP gene in fetuses with WWS.
FKRP variations are associated with a spectrum of muscular dystrophies, including Limb-Girdle Muscular Dystrophy 2I (LGMD2I), congenital muscular dystrophies type 1C (MDC1C), CMD with mild structural brain involvement, Muscle-Eye-Brain disease (MEB), and Walker-Warburg Syndrome (WWS) [17]. Case 1 carried a homozygous nonsense mutation at c.815_816del (p.Leu272ArgfsTer117) in the FKRP gene, inherited from both parents. This variant, classified as likely pathogenic, is de novo. The homozygous nonsense mutation in the FKRP gene can explain the anatomopathological features of the fetus, including WWS features, with hydrocephalus, cobblestone lissencephaly, brainstem anomalies, bilateral hyperechogenic kidneys, and generalized skin edema.
Tubulinopathies represent another less common genetic etiology associated with cases featuring a kinked brainstem. Previous literature has identified two distinct prenatal imaging patterns linked to tubulinopathies [18]. The more severe presentation is characterized by voluminous germinal matrices, indicating trapped precursor cells, leading to significant parenchymal reduction and a smooth cortical surface, often referred to as "microlissencephaly," along with severe brainstem dysgenesis. In our case, the thin and kinked brainstem was better visualized using MRI rather than ultrasound. This pattern was also observed in Case 2, diagnosed prenatally with tubulinopathy, exhibiting hydrocephalus, cobblestone lissencephaly, absence of midline structures (including septum pellucidum and corpus callosum), cerebellar dysplasia, and a distinctive "Z"-shaped appearance of the brainstem with brainstem dysplasia.
Tubulin proteins play a critical role in cortical development, influencing neuronal proliferation, migration, differentiation, and cortical lamination. Mutations in tubulin genes often affect patients with complex malformations of the cortex, commissures, posterior fossa, and varying degrees of ventriculomegaly. Tubulins are structural subunits that form microtubules, essential for cell structure, intracellular transport, and cell division [19]. Dysfunctional tubulins and microtubule-associated proteins, known as tubulinopathies, can lead to a wide range of complex brain malformations. The alpha and beta tubulins are the most common isoforms in humans responsible for assembling microtubules. Since their first description in 2007, at least eight genes encoding α- (TUBA1A, TUBA8), β- (TUBB2A, TUBB2B, TUBB3, TUBB4A, TUBB), and γ-tubulins (TUBG1) have been clinically reported. Mutations in these tubulin genes, highly expressed during central nervous system development, result in cortical malformations [20].
The TUBA1A gene (OMIM #605529) encodes tubulin α-1A, a crucial protein involved in the function and stability of microtubules [21]. Mutations in this gene can lead to various malformations, primarily due to impaired neuronal migration and/or proliferation. Cerebral cortex development relies on a sequential process involving neurogenesis, cell migration, and terminal differentiation, facilitated by the dynamic nature of the neuronal cytoskeleton. Mutations in the tubulin gene can disrupt cytoskeletal dynamics, affecting aggregation, stability, and association with microtubule protein isolates. Malformations associated with TUBA1A mutations include lissencephaly, microlissencephaly, cerebellar hypoplasia, agenesis of the corpus callosum, pachygyria, and polymicrogyria [22,23]. Additionally, TUBA1A is expressed in both the fetal brain [24] and retina [25], contributing to ophthalmologic abnormalities such as microphthalmia, congenital cataracts, and microcephaly. Fetus 2 described in our case carried a de novo heterozygous missense mutation at c.848A>G (p.H283R) in the TUBA1A gene. Although this variant was not previously reported, it was classified as likely pathogenic, consistent with an autosomal dominant inheritance pattern associated with TUBA1A mutations. The heterozygous missense mutation in the TUBA1A gene could explain the anatomopathological features observed in the fetus, including hydrocephalus, absence of midline structures, and brainstem anomalies. However, no ocular abnormalities were evident in this case.
Conclusion
In this case series, two fetuses presenting with a "kinked brainstem" and other CNS abnormalities on fetal MRI were identified using trio prenatal Medical Exome Sequencing (pMES) followed by Sanger sequencing. The "kinked" or "z-shaped" appearance of the brainstem is more easily delineated on fetal MRI compared to ultrasound, indicating an underlying dystroglycanopathy (Walker-Warburg phenotype) or tubulinopathy. Prenatal exome sequencing facilitates early genetic diagnosis for cases of "kinked brainstem." Furthermore, our study expands the prenatal findings of WWS by incorporating additional features such as hyperechogenic kidneys and generalized skin edema.
Declarations
Ethics approval and consent to participate
This study has been approved by the Institutional Review Board/ Medical Ethics Committee of Guangdong Women and Children Hospital (IRB reference number: 202301185). Written informed consent was obtained from each participating family.
Consent for publication
Written informed consent for publication of their clinical details and clinical images was obtained from the patient.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due individual privacy but are available from the corresponding author (Aihua Yin, E-mail: yinaihua0131@163.com & Jing Wu, E-mail: wujing_548@163.com) on reasonable request.
Competing interests
The author(s) have no conflicts of interest relevant to this article.
Funding
This study was supported by the Guangzhou Basic and Applied Basic Research Funding Project (Grant No. 202102020847), the Medical Science and Technology Research Project of Guangdong Province (Grant No. A2023500) and the Guangzhou Science and Technology Planning Project (Grant No. 202103000047). The funding body did not play a role in the design of the study, collection, analysis, interpretation of data, or in writing the manuscript.
Authors Contributions
YW, CL, JW and AY conceived and designed the study. YW, CL, CY, YH, JG, XZ, YX, JW and AY carried out the research. LL performs laboratory experiments. YH, CY, JG and JW analyzed and interpreted data. YW, CL and YX drafted the manuscript, and AY revised it. All authors read and approved the final manuscript.
Acknowledgment
We are grateful to the family members who consented to participate in this study. We are grateful to Li Cui for assistance with the data analysis and manuscript preparation.
References
- Nagaraj UD, Kline-Fath BM. Clinical Applications of Fetal MRI in the Brain. Diagnostics (Basel). 2022 Mar 21;12(3):764. doi: 10.3390/diagnostics12030764. PMID: 35328317; PMCID: PMC8947742.
- Stroustrup Smith A, Levine D, Barnes PD, Robertson RL. Magnetic resonance imaging of the kinked fetal brain stem: a sign of severe dysgenesis. J Ultrasound Med. 2005 Dec;24(12):1697-709. doi: 10.7863/jum.2005.24.12.1697. PMID: 16301726; PMCID: PMC1698953.
- Lacalm A, Nadaud B, Massoud M, Putoux A, Gaucherand P, Guibaud L. Prenatal diagnosis of cobblestone lissencephaly associated with Walker-Warburg syndrome based on a specific sonographic pattern. Ultrasound Obstet Gynecol. 2016 Jan;47(1):117-22. doi: 10.1002/uog.15735. PMID: 26315758.
- Stengel-Rutkowski S, Wirtz A, Hahn B, Hofmeister A, Murken JD. Routine G-banding in prenatal diagnosis of chromosomal disorders. Hum Genet. 1976 Feb 29;31(2):231-4. doi: 10.1007/BF00296151. PMID: 55378.
- Xiang J, Ding Y, Song X, Mao J, Liu M, Liu Y, Huang C, Zhang Q, Wang T. Clinical Utility of SNP Array Analysis in Prenatal Diagnosis: A Cohort Study of 5000 Pregnancies. Front Genet. 2020 Nov 6;11:571219. doi: 10.3389/fgene.2020.571219. PMID: 33240322; PMCID: PMC7677511.
- Vetro A, Pisano T, Chiaro S, Procopio E, Guerra A, Parrini E, Mei D, Virdò S, Mangone G, Azzari C, Guerrini R. Early infantile epileptic-dyskinetic encephalopathy due to biallelic PIGP mutations. Neurol Genet. 2020 Jan 2;6(1):e387. doi: 10.1212/NXG.0000000000000387. PMID: 32042915; PMCID: PMC6984131.
- Low AS, Lee SL, Tan AS, Chan DK, Chan LL. Difficulties with prenatal diagnosis of the Walker-Warburg syndrome. Acta Radiol. 2005 Oct;46(6):645-51. doi: 10.1080/02841850510021409. PMID: 16334849.
- Lacalm A, Nadaud B, Massoud M, Putoux A, Gaucherand P, Guibaud L. Prenatal diagnosis of cobblestone lissencephaly associated with Walker-Warburg syndrome based on a specific sonographic pattern. Ultrasound Obstet Gynecol. 2016 Jan;47(1):117-22. doi: 10.1002/uog.15735. PMID: 26315758.
- Guimaraes CVA, Dahmoush HM. Imaging phenotype correlation with molecular and molecular pathway defects in malformations of cortical development. Pediatr Radiol. 2020 Dec;50(13):1974-1987. doi: 10.1007/s00247-020-04674-5. Epub 2020 Nov 30. PMID: 33252763.
- Powis Z, Chamberlin AC, Alamillo CL, Ceulemans S, Bird LM, Tang S. Postmortem Diagnostic Exome Sequencing Identifies a De Novo TUBB3 Alteration in a Newborn With Prenatally Diagnosed Hydrocephalus and Suspected Walker-Warburg Syndrome. Pediatr Dev Pathol. 2018 May-Jun;21(3):319-323. doi: 10.1177/1093526617698611. Epub 2017 Mar 23. PMID: 29187032.
- Cormand B, Pihko H, Bayés M, Valanne L, Santavuori P, Talim B, Gershoni-Baruch R, Ahmad A, van Bokhoven H, Brunner HG, Voit T, Topaloglu H, Dobyns WB, Lehesjoki AE. Clinical and genetic distinction between Walker-Warburg syndrome and muscle-eye-brain disease. Neurology. 2001 Apr 24;56(8):1059-69. doi: 10.1212/wnl.56.8.1059. PMID: 11320179.
- Yulia A, Napolitano R, Aiman A, Desai D, Johal N, Whitten M, Ushakov F, Pandya PP, Winyard PJD. Perinatal and infant outcome of fetuses with prenatally diagnosed hyperechogenic kidneys. Ultrasound Obstet Gynecol. 2021 Jun;57(6):953-958. doi: 10.1002/uog.22121. PMID: 32530118.
- Nabhan MM, ElKhateeb N, Braun DA, Eun S, Saleem SN, YungGee H, Hildebrandt F, Soliman NA. Cystic kidneys in fetal Walker-Warburg syndrome with POMT2 mutation: Intrafamilial phenotypic variability in four siblings and review of literature. Am J Med Genet A. 2017 Oct;173(10):2697-2702. doi: 10.1002/ajmg.a.38393. Epub 2017 Aug 17. PMID: 28815891; PMCID: PMC6205885.
- Gasser B, Lindner V, Dreyfus M, Feidt X, Leissner P, Treisser A, Stoll C. Prenatal diagnosis of Walker-Warburg syndrome in three sibs. Am J Med Genet. 1998 Mar 5;76(2):107-10. doi: 10.1002/(sici)1096-8628(19980305)76:2<107::aid-ajmg1>3.3.co;2-b. PMID: 9511971.
- Jauniaux E, Hertzkovitz R, Hall JM. First-trimester prenatal diagnosis of a thoracic cystic lesion associated with fetal skin edema. Ultrasound Obstet Gynecol. 2000 Jan;15(1):74-7. doi: 10.1046/j.1469-0705.2000.00020.x. PMID: 10776018.
- Vajsar J, Schachter H. Walker-Warburg syndrome. Orphanet J Rare Dis. 2006;1:29.
- Beltran-Valero de Bernabé D, Voit T, Longman C, Steinbrecher A, Straub V, Yuva Y, Herrmann R, Sperner J, Korenke C, Diesen C, Dobyns WB, Brunner HG, van Bokhoven H, Brockington M, Muntoni F. Mutations in the FKRP gene can cause muscle-eye-brain disease and Walker-Warburg syndrome. J Med Genet. 2004 May;41(5):e61. doi: 10.1136/jmg.2003.013870. PMID: 15121789; PMCID: PMC1735772.
- Cabet S, Karl K, Garel C, Delius M, Hartung J, Lesca G, Chaoui R, Guibaud L. Two different prenatal imaging cerebral patterns of tubulinopathy. Ultrasound Obstet Gynecol. 2021 Mar;57(3):493-497. doi: 10.1002/uog.22010. PMID: 32149430.
- Brar BK, Thompson MG, Vora NL, Gilmore K, Blakemore K, Miller KA, Giordano J, Dufke A, Wong B, Stover S, Lianoglou B, Van den Veyver I, Dempsey E, Rosner M, Chong K, Chitayat D, Sparks TN, Norton ME, Wapner R, Baranano K, Jelin AC; Fetal Sequencing Consortium. Prenatal phenotyping of fetal tubulinopathies: A multicenter retrospective case series. Prenat Diagn. 2022 Dec;42(13):1686-1693. doi: 10.1002/pd.6269. Epub 2022 Nov 28. PMID: 36403095; PMCID: PMC9805891.
- Jaglin XH, Chelly J. Tubulin-related cortical dysgeneses: microtubule dysfunction underlying neuronal migration defects. Trends Genet. 2009 Dec;25(12):555-66. doi: 10.1016/j.tig.2009.10.003. Epub 2009 Oct 26. PMID: 19864038.
- Kumar RA, Pilz DT, Babatz TD, Cushion TD, Harvey K, Topf M, Yates L, Robb S, Uyanik G, Mancini GM, Rees MI, Harvey RJ, Dobyns WB. TUBA1A mutations cause wide spectrum lissencephaly (smooth brain) and suggest that multiple neuronal migration pathways converge on alpha tubulins. Hum Mol Genet. 2010 Jul 15;19(14):2817-27. doi: 10.1093/hmg/ddq182. Epub 2010 May 12. PMID: 20466733; PMCID: PMC2893812.
- Tian G, Jaglin XH, Keays DA, Francis F, Chelly J, Cowan NJ. Disease-associated mutations in TUBA1A result in a spectrum of defects in the tubulin folding and heterodimer assembly pathway. Hum Mol Genet. 2010 Sep 15;19(18):3599-613. doi: 10.1093/hmg/ddq276. Epub 2010 Jul 5. PMID: 20603323; PMCID: PMC2928131.
- Poirier K, Saillour Y, Fourniol F, Francis F, Souville I, Valence S, Desguerre I, Marie Lepage J, Boddaert N, Jacquemont ML, Beldjord C, Chelly J, Bahi-Buisson N. Expanding the spectrum of TUBA1A-related cortical dysgenesis to Polymicrogyria. Eur J Hum Genet. 2013;21(4):381-385.
- Poirier K, Keays DA, Francis F, Saillour Y, Bahi N, Manouvrier S, Fallet-Bianco C, Pasquier L, Toutain A, Tuy FP, Bienvenu T, Joriot S, Odent S, Ville D, Desguerre I, Goldenberg A, Moutard ML, Fryns JP, van Esch H, Harvey RJ, Siebold C, Flint J, Beldjord C, Chelly J. Large spectrum of lissencephaly and pachygyria phenotypes resulting from de novo missense mutations in tubulin alpha 1A (TUBA1A). Hum Mutat. 2007 Nov;28(11):1055-64. doi: 10.1002/humu.20572. PMID: 17584854.
- Crabtree DV, Ojima I, Geng X, Adler AJ. Tubulins in the primate retina: evidence that xanthophylls may be endogenous ligands for the paclitaxel-binding site. Bioorg Med Chem. 2001 Aug;9(8):1967-76. doi: 10.1016/s0968-0896(01)00103-1. PMID: 11504633.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.