Medicine Group. 2024 June 28;5(6):660-669. doi: 10.37871/jbres1940.
Short-Chain Fatty Acids Suppress MCP-1 and Cellular Aging Induced by Lipopolysaccharides under High-Concentration Glucose
Kazuo Sonoki1* and Kosuke Muraoka2
2Division of Clinical Education Development and Research, Department of Oral Function, Kyushu Dental University, Kitakyushu, Fukuoka, Japan
- SCFAs
- lipopolysaccharide
- HUVECs
- MCP-1
- Telomere length
Abstract
Background: High glucose levels, as observed in diabetic patients, enhances the inflammatory response of Porphyromonas gingivalis-Lipopolysaccharide (Pg-LPS) in various cell types and may thus worsen atherosclerosis and other inflammatory diseases. Short-Chain Fatty Acids (SCFAs) have been suggested to defend against inflammation and atherosclerosis, but, it is unknown whether SCFAs also affect aging, which accelerates atherosclerosis. We investigated the inhibitory effects of SCFAs on an aging marker, i.e., telomere length, and an atherogenic factor, Monocyte Chemoattractant Protein-1 (MCP-1), in Human Umbilical Vein Endothelial Cells (HUVECs) exposed to Pg-LPS under high glucose.
Methods: We treated HUVECs with the three SCFAs acetic acid, propionic acid and butyric acid and then stimulated the cells with 0.1 µg/mL Pg-LPS + high (20 mM) glucose. We then measured the MCP-1 mRNA expression and telomere length by real-time RT-PCR.
Results: Acetic acid or propionic acid at ≥0.5 mM and butyric acid at ≥0.25 mM suppressed the enhanced expression of MCP-1 mRNA induced by Pg-LPS + high glucose to the level in unstimulated HUVECs. The NF-κB activation, which promotes the transcription of MCP-1 mRNA, and MCP-1 protein production enhanced by Pg-LPS + high glucose were suppressed by acetic acid. The telomere length of HUVECs exposed to Pg-LPS + high glucose was significantly decreased by 30% compared to that in unstimulated HUVECs. Acetic acid or propionic acid at 1 mM and butyric acid 0.5 mM restored the telomere length to levels comparable to those in unstimulated HUVECs.
Conclusion: SCFAs may reduce the atherogenic risk and aging risk of periodontal disease in individuals with diabetes mellitus.
Background
Periodontal disease is a chronic inflammatory condition caused by periodontal pathogens, and it is known that prolonged inflammation in the oral cavity can lead to various systemic diseases, including cardiovascular disease due to atherosclerosis [1]. Diabetes mellitus, which is due to chronic hyperglycemia, also contributes to the onset and progression of atherosclerosis [2]. When periodontal disease and diabetes overlap, there is a concern that atherosclerosis may be further accelerated. Our own previous study using Human Umbilical Vein Endothelial Cells (HUVECs) revealed that high glucose enhances the production of MCP-1, an atherosclerosis-promoting factor, induced by Pg-LPS [3]. Specifically, the administration of Pg-LPS at a concentration of 0.1 μg/mL under 5.5 mM glucose did not alter the expression of MCP-1 in HUVECs compared to HUVECs without Pg-LPS treatment. However, under 20 mM glucose, the expression of MCP-1 was significantly enhanced. This means that glucose has a synergistic effect with Pg-LPS on the induction of MCP-1 expression in HUVECs, and it indicates that in patients with both periodontal disease and diabetes mellitus, atherosclerosis may be accelerated compared to that in patients with periodontal disease alone.
Atherosclerosis is classed as a disease of aging, and thus increasing age is an independent risk factor for the development of atherosclerosis [4]. Atherosclerosis is also associated with premature biological aging, as irreversible growth arrest and apoptosis, elevated DNA damage, epigenetic modification, and telomere shortening and dysfunction have been associated with atherosclerosis [4]. There is a also a relationship between periodontal disease and aging, and it is well known that the prevalence and severity of periodontal disease are closely associated with aging [5]. Moreover, chronic inflammation has been reported to accelerate aging [6]. In patients with chronic inflammatory diseases such as periodontitis, the length of leukocyte telomeres, which is a marker of aging, has been reported to be shortened [7-10]. This suggests the potential contribution of periodontal disease to the acceleration of aging. We speculated that if this contribution exists, our experimental system in which HUVECs are stimulated with Pg-LPS under high-glucose exposure could be used to determine whether telomere shortening occurs in HUVECs in addition to enhanced MCP-1 expression. We therefore decided to investigate telomere length under these conditions.
Short-Chain Fatty Acids (SCFAs) are fatty acids with a carbon number between two and six. The anaerobic bacterial fermentation of dietary fibers in the human colon produces SCFAs such as acetic acid (C2), propionic acid (C3), and butyric acid (C4) predominantly. The SCFAs are provided to the host human and used by local enterocytes as an energy resource, or they are transported across the gut epithelium into the bloodstream where they can interact with peripheral cells and tissues as signaling molecules. Many studies have revealed that SCFAs exert a wide range of functions from immune regulation to metabolism in a variety of tissues and organs, and they thus have both direct and indirect influences on the human body. SCFAs are also considered to be defensive against inflammation and atherosclerosis11. We thus conducted the present study to clarify the effects of SCFAs on MCP-1 expression and telomere length in HUVECs.
Methods
Cell culture
HUVECs were harvested from umbilical cord veins and cultured in M199 medium supplemented with 10% fetal calf serum (FCS; Invitrogen, Carlsbad, CA), 20 µg/mL endothelial cell growth supplement (Sigma-Aldrich, St. Louis, MO), 100 µg/mL heparin (Sigma-Aldrich), and 5 μL/mL penicillin-streptomycin liquid (Sigma-Aldrich) in 10-cm gelatin-coated dishes (cat.# 4020-020, Iwaki, Tokyo). This cell culture medium contains 5.5 mM glucose. Cells were used within six passages.
Measurement of the expression of MCP-1 mRNA by a real-time reverse transcriptase-polymerase chain reaction analysis: For the quantitation of MCP-1 mRNA expression induced by co-stimulation with 0.1 μg/mL Pg-LPS (cat.# tlrl-pglps, InvivoGen, San Diego, CA) and 20 mM glucose, HUVECs were grown to confluence in 35-mm gelatin-coated dishes (cat.# 4000-020, Iwaki) in 2.0 mL of the cell culture medium. The cell culture medium was replaced with 1.0 mL of fresh cell culture medium, and we added 2.0 μL of 0.05 mg/mL Pg-LPS to provide the final concentrations of 0.1 μg/mL Pg-LPS and 14.5 μL of 1 M glucose to provide the final concentration of 20 mM glucose. After 2-h incubation, total RNA was extracted using TRIzol™ reagent (Invitrogen), and a real-time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) for MCP-1 mRNA was performed with the same primers as used in our previous study [3]. The standard curve of MCP-1 mRNA was obtained by using the total RNA of the unstimulated HUVECs, and the expression of MCP-1 mRNA is given relative to that in the unstimulated HUVECs.
For the evaluation of the inhibitory effect of SCFAs on the MCP-1 mRNA expression, at 2 h before the co-stimulation with Pg-LPS + high glucose, we added acetic acid (cat.# 017-00256, Nacalai Tesque, Kyoto, Japan) or propionic acid (cat.# 402907-100ML, Sigma-Aldrich) at the final concentrations of 0.1, 0.5, and 1.0 mM, and butyric acid (cat.# B103500-100ML, Sigma-Aldrich) at the final concentrations of 0.05, 0.25, and 0.5 mM to 1.0 mL of the cell culture medium. After 2 h of the co-stimulation by Pg-LPS + high glucose with or without SCFAs, total RNA was extracted, and real-time RT-PCR analyses of MCP-1 mRNA were performed.
To investigate the involvement of Toll-Like Receptor 4 (TLR4) receptor in the enhancement of the MCP-1 mRNA expression induced by Pg-LPS + high glucose, we added either a cell-permeable inhibitor of TLR4 signaling, TAK-242 (cat.# 13871, Cayman Chemicals, Ann Arbor, MI), at 10 μM or an anti-TLR4 antibody (cat.# mabg-htlr4, InvivoGen) at 10 μg/mL to the HUVECs at 1 h before co-stimulation by Pg-LPS + high glucose.
Measurement of MCP-1 protein expression in the cell culture medium by ELISA: For the quantification of the production of MCP-1 protein induced by the co-stimulation with 0.1 μg/mL Pg-LPS and 20 mM glucose, HUVECs were grown to confluence in 24-well gelatin-coated plates (cat.# 4820-020, Iwaki) in 1.0 mL of the cell culture medium. The cell culture medium was replaced with 1.0 mL of fresh cell culture medium, and we added Pg-LPS to provide the final concentration of 0.1 μg/mL Pg-LPS and glucose to provide the final concentration of 20 mM glucose. After 12-h incubation with Pg-LPS + high glucose, supernatants were collected and the concentrations of MCP-1 protein were measured by an enzyme-linked immunosorbent assay (ELISA) using an MCP-1 ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. The concentrations of MCP-1 protein were given relative to the concentration in the unstimulated HUVECs.
To examine the inhibitory effect of SCFAs on the MCP-1 protein production, at 2 h before the co-stimulation with Pg-LPS + high glucose we added acetic acid or propionic acid at the final concentration of 1.0 mM and butyric acid at the final concentration of 0.5 mM to 1.0 mL of the cell culture medium. After 12-h co-stimulation by Pg-LPS + high glucose with or without SCFAs, the supernatants were collected and the concentrations of MCP-1 protein were measured.
Measurement of NF-κB activation by western blot analysis: For the quantitation of the activation of nuclear factor kappa- B (NF-κB), which promotes the expression of MCP-1 mRNA induced by the co-stimulation with 0.1 μg/mL Pg-LPS and 20 mM glucose, HUVECs were grown to confluence in 10-cm gelatin-coated dishes in 8.0 mL of the cell culture medium. The cell culture medium was replaced with 3.0 mL of fresh cell culture medium, and Pg-LPS and glucose were added to final concentrations of 0.1 μg/mL and 20 mM, respectively. After 1-h incubation, nuclear extracts were prepared by a modification of the method described by Schreiber, et al. [11]. The nuclear extracts were stored at −80℃ until use after the determination of protein concentrations.
To examine the inhibitory effect of SCFAs on the NF-κB activation, at 2 h before co-stimulation by Pg-LPS + high glucose, we added acetic acid to a final concentration of 1.0 mM in 3.0 mL of fresh cell culture medium. As a positive control of the inhibition on NF-κB activation, we added two antioxidants, i.e., vitamin C (L(+)-ascorbic acid; Wako Pure Chemical Industries, Osaka, Japan) at a final concentration of 100 μM beginning 1 h before the co-stimulation by Pg-LPS + high glucose, and vitamin E (DL-α-tocopherol; Nacalai Tesque) at a final concentration of 50 μM beginning 24 h before the co-stimulation by Pg-LPS + high glucose, as in our earlier study [3].
Nuclear extracts (approximately 20 μg) were applied to 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, and the separated proteins were transferred to nitrocellulose membranes (Immobilon-P Transfer Membranes, Millipore, Billerica, MA). The membranes were subjected to immunoblotting with a mouse monoclonal antibody for human phosphorylated NF-κB p65 (cat.# sc-166748, Santa Cruz Biotechnology, Santa Cruz, CA) or human Lamin B1 (cat.# sc-365214, Santa Cruz Biotechnology) as the internal standard, and with goat anti-mouse IgG, horseradish peroxidase-conjugated antibodies (cat.# 12-349, Millipore). Immunoreactive proteins were detected by an ECL Prime Western Blotting Detection Reagents (Amersham Biosciences, Buckinghamshire, UK), and the band density was analyzed with the U.S. National Institutes of Health (NIH) image analysis software ImageJ 1.42q. The density of NF-κB p65 was divided by that of Lamin B1, and the expressions of NF-κB p65 activation are given relative to that in unstimulated HUVECs.
Genomic DNA extraction and the measurement of the telomere length: To measure the change in telomere length by co-stimulation with 0.1 μg/mL Pg-LPS and 20 mM glucose, we seeded HUVECs onto 35-mm gelatin-coated dishes in 2.0 mL of the cell culture medium, and added Pg-LPS to a final concentration of 0.1 μg/mL and glucose to a final concentration of 20 mM. The HUVECs were cultured until they reached confluence, with a change of 2.0 mL of culture medium and Pg-LPS + high glucose administration every 2 days. The HUVECs typically reached confluence in 3 days.
We then extracted the genomic DNA from the HUVECs with the use of a Genomic DNA Purification Kit (MagExtractor, NPK-101; Toyobo, Osaka, Japan). We measured the telomere lengths in the HUVECs by real-time Polymerase Chain Reaction (PCR) using a Telomere Length qPCR Kit (Absolute Human Telomere Length Quantification qPCR Assay Kit, #8918; ScienCell Research Laboratories, Carlsbad, CA) and a LightCycler 96® PCR system (Roche, Rotkreuz, Switzerland). In brief, 1 µL of an extracted genomic DNA sample or a reference human genomic DNA sample (telomere length 726 ± 70 kb per diploid cell) was added to 9 µL of stock solution consisting of 2 µL of a telomere primer set or a Single-Copy Reference (SCR) primer set and 7 µL of nuclease-free H2O in each well of LightCycler 8-Tube Strips. Then, 10 µL of 2×GoldNStart TaqGreen qPCR master mix (dye: SYBR™ Green I) was added to reach a total reaction liquid volume of 20 µL. Several LightCycler eight-tube strips were mounted symmetrically on the block cycler unit of the LightCycler 96 instrument, and the real-time PCR was started.
The telomere length was calculated using the quantification Cycle (Cq) obtained from the LightCycler 96. The ΔCq(telomere) is the difference in the quantification cycle's number of telomeres between the target and the reference genomic DNA samples. The ΔCq(SCR) is the difference in the quantification cycle's number of SCRs between the target and the reference genomic DNA samples. The ΔΔCq is the difference between the ΔCq(telomere) and the ΔCq(SCR). The relative telomere length of the target sample to the reference sample is expressed as 2−ΔΔCq. The total telomere length of the target sample per diploid cell is expressed as the reference sample telomere length (726 ± 70 kb) × 2−ΔΔCq. The total telomere length of each group was normalized with the value of the unstimulated HUVECs set to 1.00.
For the evaluation of the inhibitory effect of SCFAs on the telomere shortening, at 2 h before the co-stimulation with Pg-LPS + high glucose, we added acetic acid or propionic acid at the final concentrations of 1.0 mM, and butyric acid at the final concentrations of 0.5 mM to 2.0 mL of the cell culture medium. In addition to the SCFAs, as a positive control, we added resveratrol 5 µM at 24 h before the co-stimulation with Pg-LPS + high glucose.
Cell proliferation, death, and cytotoxicity: The cell proliferation of HUVECs was analyzed by a BrdU (5-bromo-2'-deoxyuridine) incorporation assay using a commercially available kit (BrdU Cell Proliferation Assay Kit; cat.# X1327K1, Exalpha Biologicals, Shirley, MA). BrdU is incorporated in de-novo-synthesized DNA of actively proliferating cells. In brief, 100 µL of HUVECs suspension was plated in a 96-well gelatin-coated plate (cat.# 354689, Corning, Tewksbury, MA) at a density of 1 × 104 cells per well, and 100 µL of culture medium was added. The HUVECs were cultured in a total volume of 200 µL of cell culture medium with or without Pg-LPS + high glucose until they reached confluence. Twenty-four hours prior to the completion of cultivation, 20 µL of BrdU was added to each well for labeling the HUVECs. After labeling, the BrdU-treated cells were fixed and the DNA was denatured. The BrdU incorporated into newly synthesized cellular DNA was reacted with a peroxidase-conjugated anti-BrdU antibody, and the immune complexes were quantified by the subsequent substrate reaction. The amount of reaction product was determined by measuring the absorbance at 450 nm using a plate reader (iMark; Bio Rad Laboratories, Hercules, CA). The absorbance value (OD) was directly correlated to the amount of DNA synthesis and the number of proliferative cells.
The cell death rate was assessed using the trypan blue dye exclusion method. Briefly, HUVECs were cultured in 2.0 mL of cell culture medium with or without Pg-LPS + high glucose in 35-mm gelatin-coated dishes until they reached confluence. After removing the medium from the dish, cells were washed with 1 mL of Phosphate-Buffered Saline (PBS). Cells were detached by adding 0.5 mL of PBS containing 0.5% trypsin + 0.02% EDTA-2Na, followed by the addition of 0.5 mL of cell culture medium. To 100 µL of the obtained cell suspension, 100 µL of 0.4% trypan blue (cat.# 33201, Muto Pure Chemicals Co., Tokyo) was added, and then the mixture was mixed and allowed to stand for 5 min. Subsequently, 20 µL aliquots were placed on a Neubauer hemacytometer. Within 5 min, the numbers of viable and dead cells (stained blue) were counted under an optical microscope in 40 × microscope fields per dish.
To assess the cytotoxicity of Pg-LPS + high glucose on HUVECs, we used the Cytotoxicity LDH Assay Kit-WST (cat.# 347-91751, Dojindo, Kumamoto, Japan), which is designed to measure cell damage by quantifying the activity of Lactate Dehydrogenase (LDH) released from cells into the culture medium. In brief, HUVECs were seeded onto 35-mm gelatin-coated dishes in 2.0 mL of the cell culture medium with or without Pg-LPS + high glucose and cultured until they reached confluence. We transferred 100 µL of the supernatant from each dish to each well of a 96-well plate, and then 100 µL of Working Solution (included in the kit) was added to each well. After 30 min, 50 µL of Stop Solution (included in the kit) was added to each well. The activity of LDH, which is indicative of cell damage, was measured at 490 nm using a microplate reader (iMark; Bio Rad Laboratories) and is expressed as a ratio relative to the LDH activity of unstimulated HUVECs.
Statistical Analyses
All data are presented as the mean ± Standard Error of the Mean (SEM). Matched pairs from two groups were compared by the Wilcoxon test. The comparison of multiple groups was performed by the Kruskal–Wallis test. If a significant difference was observed in the Kruskal–Wallis test (p < 0.05), a post hoc Mann-Whitney U-test with a significance level of 0.0033 (adjusted using the Bonferroni correction) was performed for pairwise comparisons between the two groups. Results were considered significant when the p-value was <0.05. All statistical analyses were conducted with the software program SPSS 17.0 (SAS, Cary, NC).
Results
Increased MCP-1 mRNA expression by co-stimulation with Pg-LPS + high glucose and the inhibitory effects of the SCFAs
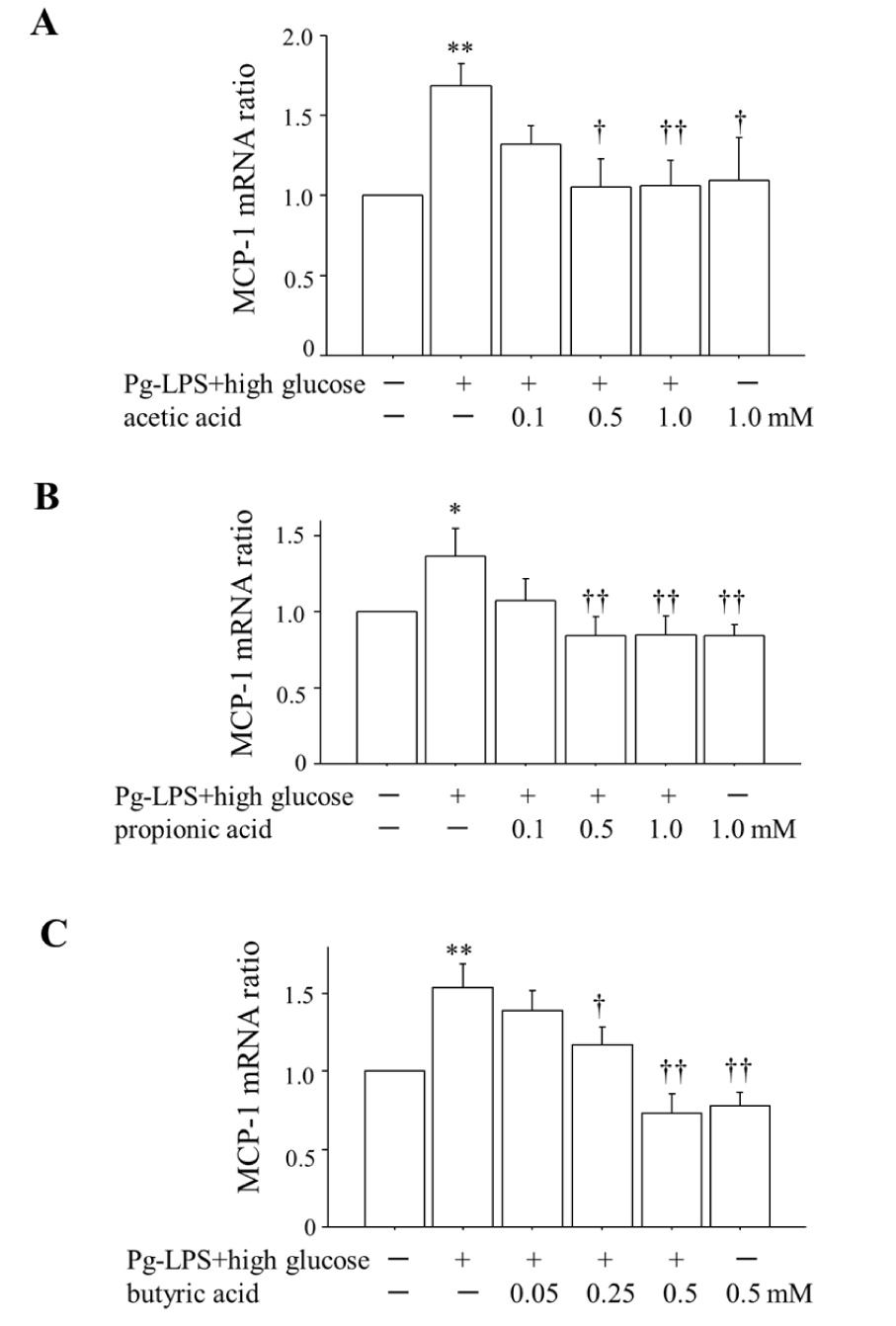
Figure 1 shows that (i) co-stimulation by 0.1 μg/mL Pg-LPS plus 20 mM glucose for 2 h significantly enhanced the expression of MCP-1 mRNA compared to that in the unstimulated HUVECs, and (ii) the SCFAs suppressed this enhancement of MCP-1 mRNA at the concentration of ≥0.5 mM in the cases of acetic acid (Figure 1A) and propionic acid (Figure 1B) and at the concentration ≥0.25 mM in the case of butyric acid (Figure 1C). There was no difference in MCP-1 mRNA expression between the unstimulated HUVECs and the unstimulated HUVECs administered 1.0 mM of acetic acid, 1.0 mM of propionic acid or 0.5 mM of butyric acid.
Increased MCP-1 protein concentration by co-stimulation with Pg-LPS + high glucose and the inhibitory effects of the SCFAs
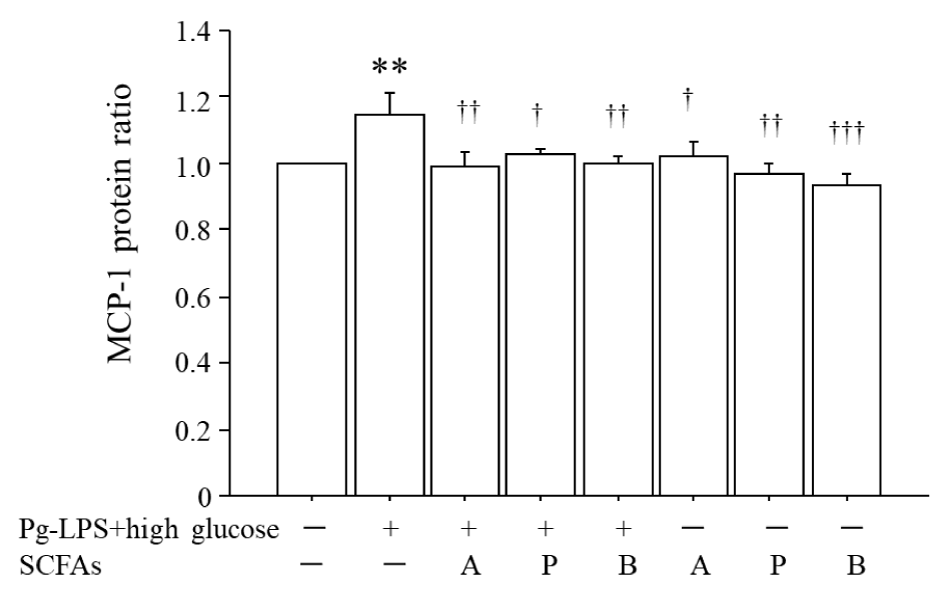
Figure 2 shows that (i) co-stimulation by 0.1 μg/mL Pg-LPS plus 20 mM glucose for 12 h increased the ratio of MCP-1 protein by 1.15-fold compared to that in the unstimulated HUVECs, and (ii) the application of 1.0 mM acetic acid (A) or propionic acid (P) and 0.5 mM butyric acid (B) beginning 2 h before co-stimulation by Pg-LPS + high glucose significantly suppressed the MCP-1 protein ratio to the level of the unstimulated HUVECs. There were no significant differences in the production of MCP-1 protein between the unstimulated HUVECs and unstimulated HUVECs administered 1.0 mM acetic acid, 1.0 mM propionic acid, or 0.5 mM butyric acid.
Increase in NF-κB activation by co-stimulation with Pg-LPS + high glucose and the inhibitory effects of the SCFAs
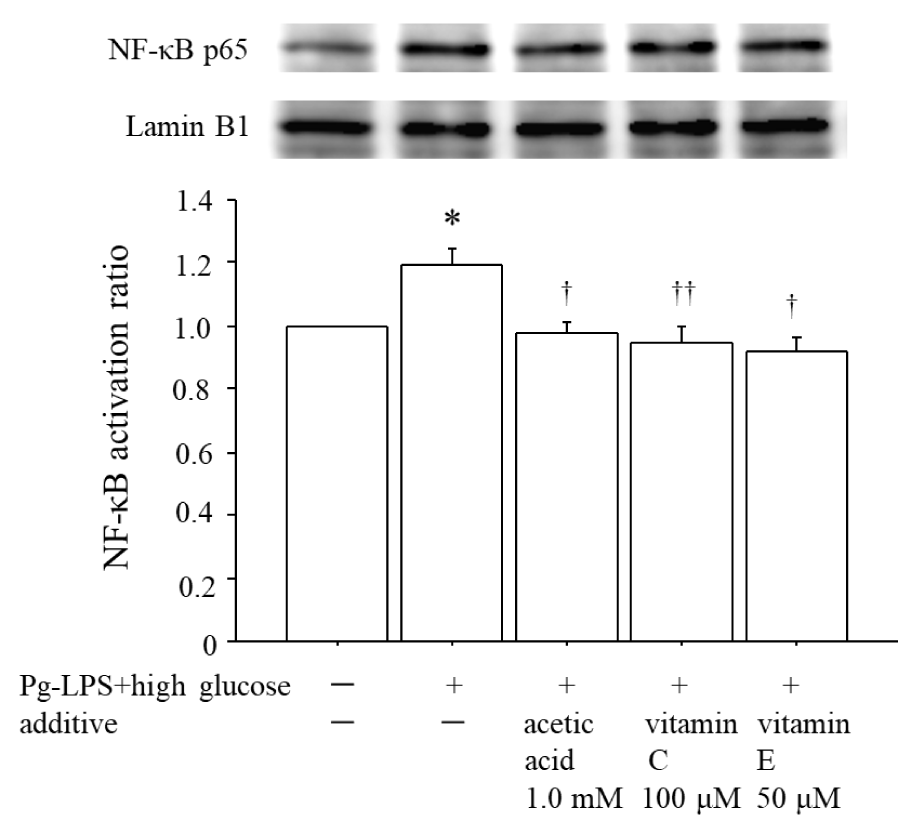
Figure 3 illustrates that co-stimulation by 0.1 μg/mL Pg-LPS plus 20 mM glucose for 1 h enhanced the NF-κB activation by 1.19-fold compared to that in the unstimulated HUVECs, whereas 1.0 mM acetic acid significantly suppressed this enhancement of NF-κB activation to the level in unstimulated HUVECs. In addition, as we expected, both 100 μM vitamin C and 50 μM vitamin E significantly suppressed the enhancement of NF-κB activation that was induced by co-stimulation with Pg-LPS + high glucose.
Involvement of the TLR4 receptor in the enhancement of MCP-1 mRNA expression
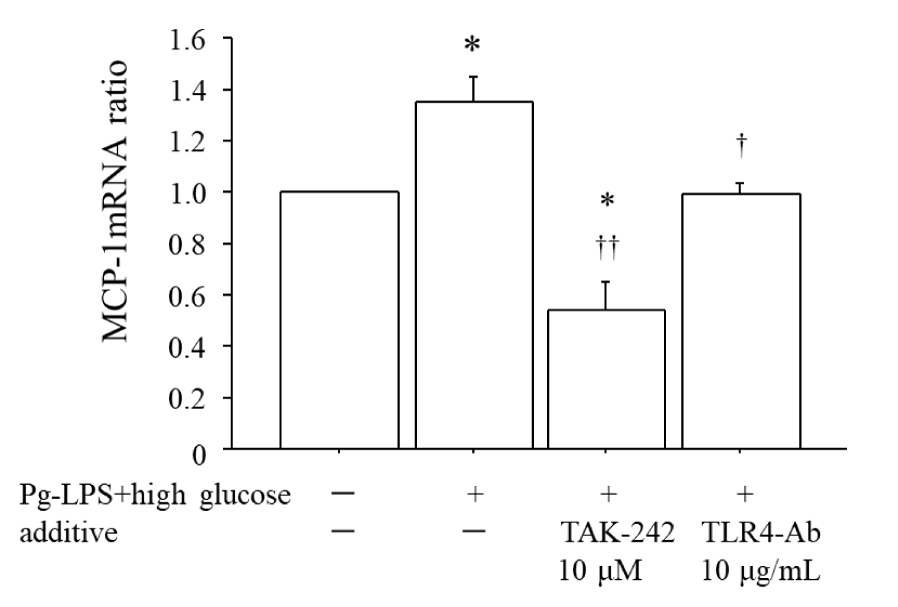
Figure 4 shows that (i) co-stimulation by 0.1 μg/mL Pg-LPS plus 20 mM glucose for 2 h significantly enhanced the expression of MCP-1 mRNA by 1.35-fold compared to the unstimulated HUVECs, and (ii) both the cell-permeable inhibitor of TLR4 signaling, i.e., TAK-242, at 10 μM and the anti-TLR4 antibody at 10 μg/mL significantly suppressed this enhancement of MCP-1 mRNA. Notably, TAK-242 suppressed the expression of MCP-1 mRNA to a level below that in the unstimulated HUVECs.
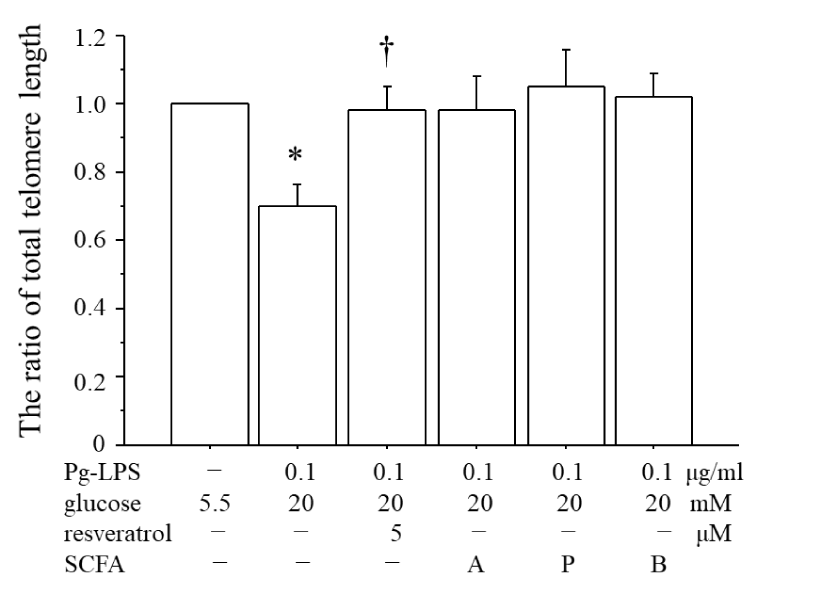
The telomere shortening of the HUVECs stimulated by Pg-LPS + high glucose and the inhibitory effects of the SCFAs
Figure 5 illustrates the total telomere length of HUVECs after approximately 72 h of cultivation following the initiation of stimulation with Pg-LPS + high glucose until cell confluence was achieved. The comparison was made with the total telomere length of unstimulated HUVECs during the same period until cellular confluence was reached (the ratio of total telomere length). The total telomere length was significantly decreased by 30% with the Pg-LPS + high glucose stimulation. In contrast, the administration of resveratrol at 5 µM did not lead to a reduction in the total telomere length. When the SCFAs (1.0 mM acetic acid (A) or propionic acid (P) and 0.5 mM butyric acid (B)) were administered 2 h before co-stimulation by Pg-LPS + high glucose, there was no significant difference compared to the total telomere length observed with Pg-LPS + high glucose stimulation. However, there was also no significant difference compared to the total telomere length of the unstimulated HUVECs, suggesting that the SCFAs suppressed the shortening of the total telomeres induced by the Pg-LPS + high glucose stimulation.
Cell proliferation, death, and cytotoxicity by Pg-LPS + high glucose
The length of telomeres gradually shortens with each cell division. Therefore, if the co-stimulation of Pg-LPS + high glucose accelerated the cell division of HUVECs, the telomere length would decrease. We thus investigated the effects of Pg-LPS + high glucose administration on cell proliferation, death, and cytotoxicity. However, in this study, no changes in cellular morphology were observed throughout the entire research.
The cell proliferation of HUVECs, analyzed by the BrdU incorporation assay, was significantly decreased in the HUVECs stimulated with Pg-LPS + high glucose compared to the unstimulated HUVECs (OD 0.224 ± 0.026 for the unstimulated HUVECs, OD 0.193 ± 0.024 for Pg-LPS + high glucose, n = 4, p = 0.021).
The cell death rate of HUVECs, which we assessed using the trypan blue dye exclusion method, showed no significant difference between unstimulated HUVECs and HUVECs stimulated with Pg-LPS + high glucose (cell death rate of unstimulated HUVECs: 5.85 ± 2.60%; cell death rate of Pg-LPS + high glucose-stimulated HUVECs: 5.01 ± 2.22%, n = 7, p = 0.705).
The cytotoxicity of HUVECs was evaluated by the activity of LDH in the culture medium. The LDH activity of the HUVECs stimulated with Pg-LPS + high glucose was increased by 2.80 ± 0.53 times compared to that in the unstimulated HUVECs, the LDH activity of which was normalized to 1.00 (n = 7, p = 0.012) [12].
Discussion
As they age, cells acquire the Senescence-Associated Secretory Phenotype (SASP), which is characterized by secretion of inflammatory cytokines such as IL-6, TNF-α, and MCP-1. As aging progresses, the accumulation of senescent cells in the body leads to chronic inflammation in surrounding tissues due to the secretion of SASP factors from these senescent cells [13]. SASP is thus considered one of the important mechanisms in periodontal disease, increasing both the prevalence and severity of this condition with aging [14]. Our present findings, specifically the shortening of telomeres induced by Pg-LPS + high glucose, suggest that similar to the expression of MCP-1, in individuals with both diabetes and periodontal disease, cell aging may be accelerated compared to that in individuals with periodontal disease alone. This acceleration in cell aging, coupled with the enhancement of SASP, implies not only an increased susceptibility to the worsening of periodontal disease locally but is also anticipated to contribute to the systemic promotion of atherosclerosis.
As shown in figure 3, it became evident that NF-κB activation was involved in the enhancement of MCP-1 expression induced by the co-stimulation with Pg-LPS + high glucose. In addition, when we used a cell-permeable inhibitor of TLR4 signaling (TAK-242) and an anti-TLR4 antibody before co-stimulation with Pg-LPS + high glucose, the MCP-1 mRNA expression was suppressed in both cases (figure 4). These results indicate that the co-stimulation with Pg-LPS + high glucose enhanced the MCP-1 expression through the receptor of TLR4 with the activation of NF-κB. According to previous reports, the downstream effects of SCFAs by which SCFAs suppress the transduction of signals such as NF-κB and mitogen-activated protein kinase (MAPK) are ascribed mainly to two pathways: (1) the activation of SCFA receptors, including free fatty acid receptor type 2 (FFA2) and 3 (FFA3) and GPR109A, and (2) the inhibition of histone deacetylases (HDACs) [15]. It is thus necessary to gather more information about the receptors and HDACs that mediate the effects of SCFAs in the development of inflammatory disease, including atherosclerosis. Furthermore, while this study demonstrates the suppression of MCP-1 mRNA expression and NF-κB activation, the precise molecular mechanisms by which SCFAs exert these effects are not fully elucidated. Additional experiments focusing on signaling pathways and gene expression profiles are expected to strengthen the understanding of these mechanisms.
The estimated total concentrations of SCFAs in healthy adult human peripheral blood are 79 μM with the molar ratio of 91% acetate, 5% propionate, and 4% butyrate [16], and in our present experiments we used considerably higher concentrations of the SCFAs (≥500 μM acetic acid and propionic acid, ≥250 μM butyric acid) to suppress the enhancement of MCP-1 expression induced by Pg-LPS + high glucose. It was recently reported that a 30-day high-fiber dietary intervention administered to 29 patients with the chronic inflammatory disease rheumatoid arthritis resulted in decreased serum concentrations of the proarthritic cytokines MCP-1, IL-18, and IL-33 along with increased SCFAs (max. concentrations = 500 μM acetic acid, 28 μM propionic acid, and 13 μM butyric acid) [17]. Epidemiological evidence indicates that an increased consumption of dietary fibers improves cardiovascular function and reduces systemic inflammation and atherosclerosis as well as the risk of immune disorders such as gastrointestinal disease. A clarification of the relationship between dietary fiber uptake and circulating SCFAs may contribute to the prevention and treatment of atherosclerosis.
In our present investigation of cell proliferation using BrdU, we observed that the HUVECs stimulated by Pg-LPS + high glucose exhibited a significant decrease in proliferative capacity compared to unstimulated HUVECs. The LDH activity, which is indicative of cellular damage, was also elevated. We thus suspect that the telomere shortening that occurred with the stimulation by Pg-LPS + high glucose in this study was not a result of the promotion of cell division. The telomere shortening observed in this experiment, similar to the enhancement of MCP-1 expression, was likely attributable to oxidative stress induced by Pg-LPS + high glucose. We also speculate that the anti-inflammatory effects of the SCFAs, possibly through the activation of SCFA receptors or the inhibition of HDACs, may have suppressed telomere shortening.
In this study, several limitations and drawbacks need to be addressed to enhance the robustness and generalizability of the findings. First, using only HUVECs as an in vitro model might limit the applicability of the findings to in vivo conditions. Endothelial cells from other vascular beds might respond differently to SCFA treatment and including other types of endothelial cells, such as those from arterial or venous origins, could provide a more comprehensive understanding of SCFA effects on the vasculature. Furthermore, in vivo studies are necessary to confirm the translational potential of our results. For example, considering the variability in responses among different human populations, such as age, sex, or genetic background, including a diverse range of cell lines or conducting studies on primary cells from various donors could help address this issue.
Secondly, the concentrations of SCFAs used in the study may not accurately reflect physiological levels present in humans, particularly in diabetic patients. Further research is needed to determine the effective concentrations of SCFAs in vivo and the long-term effects of SCFA treatment on inflammation and telomere length by chronic exposure studies. Furthermore, the potential side effects or cytotoxicity of SCFAs at the concentrations used were not addressed. It is important to assess whether these concentrations are safe for cells and if they could have adverse effects in vivo.
Thirdly, the concentrations of SCFAs used in this study may not accurately reflect physiological levels present in humans, particularly in diabetic patients. Further research is needed to determine the effective concentrations of SCFAs in vivo and to investigate the long-term effects of SCFA treatment on inflammation and telomere length through chronic exposure studies. Additionally, the potential side effects or cytotoxicity of the SCFA concentrations used have not been examined. It is necessary to assess whether these concentrations are safe for cells and whether they could potentially cause adverse effects in vivo.
Finally, as future experimental tasks, including control groups with normal glucose levels and Pg-LPS treatment would enable more detailed comparative analysis, allowing for the isolation of specific effects under high glucose conditions. MCP-1 is a significant marker for arteriosclerosis, but measuring other inflammatory cytokines and markers could provide a broader understanding of the inflammatory response modulation by SCFAs.
In conclusion, Pg-LPS + high glucose enhanced the expression of MCP-1, a factor promoting atherosclerosis, and also shortened the telomere length in HUVECs. These results suggest that in patients with periodontitis accompanied by hyperglycemia, atherosclerosis may be further accelerated by cellular aging in the blood vessels. In contrast, SCFAs are anticipated to prevent atherosclerosis in patients with these conditions by suppressing both the enhanced expression of MCP-1 and cellular aging.
Acknowledgments
We gratefully acknowledge the exceptional assistance of Waka Yokota, Ayase Tanaka, Rio Yamasaki, Rina Ogata, Nozomi Sato, and Kaede Takachi.
Funding
This work was funded by Kyushu Dental University, Kitakyushu, Fukuoka, Japan.
Competing interest
The authors declare that they have no competing interests.
References
- Periodontal inflammation and systemic diseases: An overview Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, Orekhov AN: The diabetes mellitus-atherosclerosis connection: The role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci 21:1835. 2020. doi: 10.3390/ijms21051835.
- Sonoki K, Muraoka K, Hikiji H. Enhancement of porphyromonas gingivalis-lipopolysaccharide induced MCP-1 expression by high glucose in human endothelial cells. J Denl Oral Biol. 2020. doi: 10.47496/nl.JDOB.2020.01.01.
- Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res. 2012 Jul 6;111(2):245-59. doi: 10.1161/CIRCRESAHA.111.261388. PMID: 22773427.
- Ebersole JL, Graves CL, Gonzalez OA, Dawson D 3rd, Morford LA, Huja PE, Hartsfield JK Jr, Huja SS, Pandruvada S, Wallet SM. Aging, inflammation, immunity and periodontal disease. Periodontol 2000. 2016 Oct;72(1):54-75. doi: 10.1111/prd.12135. PMID: 27501491.
- Jurk D, Wilson C, Passos JF, Oakley F, Correia-Melo C, Greaves L, Saretzki G, Fox C, Lawless C, Anderson R, Hewitt G, Pender SL, Fullard N, Nelson G, Mann J, van de Sluis B, Mann DA, von Zglinicki T. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat Commun. 2014 Jun 24;2:4172. doi: 10.1038/ncomms5172. PMID: 24960204; PMCID: PMC4090717.
- Steffens JP, Masi S, D'Aiuto F, Spolidorio LC. Telomere length and its relationship with chronic diseases - new perspectives for periodontal research. Arch Oral Biol. 2013 Feb;58(2):111-7. doi: 10.1016/j.archoralbio.2012.09.009. Epub 2012 Nov 30. PMID: 23201158.
- Sanders AE, Divaris K, Naorungroj S, Heiss G, Risques RA. Telomere length attrition and chronic periodontitis: an ARIC Study nested case-control study. J Clin Periodontol. 2015 Jan;42(1):12-20. doi: 10.1111/jcpe.12337. Epub 2014 Dec 26. PMID: 25418689; PMCID: PMC4444215.
- Masi S, Salpea KD, Li K, Parkar M, Nibali L, Donos N, Patel K, Taddei S, Deanfield JE, D'Aiuto F, Humphries SE. Oxidative stress, chronic inflammation, and telomere length in patients with periodontitis. Free Radic Biol Med. 2011 Mar 15;50(6):730-5. doi: 10.1016/j.freeradbiomed.2010.12.031. Epub 2010 Dec 30. PMID: 21195167.
- Song W, Yang J, Niu Z. Association of periodontitis with leukocyte telomere length in US adults: A cross-sectional analysis of NHANES 1999 to 2002. J Periodontol. 2021 Jun;92(6):833-843. doi: 10.1002/JPER.20-0269. Epub 2020 Oct 27. PMID: 32996594.
- Ohira H, Tsutsui W, Fujioka Y. Are Short Chain Fatty Acids in Gut Microbiota Defensive Players for Inflammation and Atherosclerosis? J Atheroscler Thromb. 2017 Jul 1;24(7):660-672. doi: 10.5551/jat.RV17006. Epub 2017 May 27. PMID: 28552897; PMCID: PMC5517538.
- Schreiber E, Matthias P, Müller MM, Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989 Aug 11;17(15):6419. doi: 10.1093/nar/17.15.6419. PMID: 2771659; PMCID: PMC318318.
- Nelson G, Kucheryavenko O, Wordsworth J, von Zglinicki T. The senescent bystander effect is caused by ROS-activated NF-κB signalling. Mech Ageing Dev. 2018 Mar;170:30-36. doi: 10.1016/j.mad.2017.08.005. Epub 2017 Aug 25. PMID: 28837845; PMCID: PMC5861994.
- Chen S, Zhou D, Liu O, Chen H, Wang Y, Zhou Y. Cellular Senescence and Periodontitis: Mechanisms and Therapeutics. Biology (Basel). 2022 Sep 29;11(10):1419. doi: 10.3390/biology11101419. PMID: 36290323; PMCID: PMC9598109.
- Li M, van Esch BCAM, Wagenaar GTM, Garssen J, Folkerts G, Henricks PAJ. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur J Pharmacol. 2018 Jul 15;831:52-59. doi: 10.1016/j.ejphar.2018.05.003. Epub 2018 May 9. PMID: 29750914.
- Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987 Oct;28(10):1221-7. doi: 10.1136/gut.28.10.1221. PMID: 3678950; PMCID: PMC1433442.
- Dürholz K, Hofmann J, Iljazovic A, Häger J, Lucas S, Sarter K, Strowig T, Bang H, Rech J, Schett G, Zaiss MM. Dietary Short-Term Fiber Interventions in Arthritis Patients Increase Systemic SCFA Levels and Regulate Inflammation. Nutrients. 2020 Oct 20;12(10):3207. doi: 10.3390/nu12103207. PMID: 33092271; PMCID: PMC7589100.
- Anderson JW, Baird P, Davis RH Jr, Ferreri S, Knudtson M, Koraym A, Waters V, Williams CL. Health benefits of dietary fiber. Nutr Rev. 2009 Apr;67(4):188-205. doi: 10.1111/j.1753-4887.2009.00189.x. PMID: 19335713.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.