Environmental Sciences Group. 2024 June 14;5(6):597-606. doi: 10.37871/jbres1932.
Performance of Waste Paper as a Substrate for Oyster Mushroom (Pleurotus streatus L.) Cultivation
Tsega Mussie, Sirak Berhe, Selamawit Teklemichael and Tedros Gebrezgiabhier*
- Food security
- Growth parameters
- Performance
- Significant
- Substrate
Abstract
Purpose: Cultivation of Pleurotus spp. (Oyster mushroom) is economically feasible, exploiting its great capacity to degrade cellulose, lignin and hemicelluloses present in organic wastes. Waste paper is a very common pollutant thrown as garbage with no recycling plan in the environment. In view of the growing importance of food security and environmental sanitation this study was conducted to evaluate the performance of waste paper as a substrate for cultivation of Oyster mushroom (Pleurotus ostreatus L.), supplemented with sawdust and wheat bran.
Methods: Pure culture of Pleurotus ostreatus was propagated using sterilized wheat grain as substrate for spawn preparation. The collected waste paper along with the saw dust and wheat bran was mixed as per the design [three different substrate mixtures/treatments (T-1, T-2 and T-3) were prepared], composted for a week, filled in polyethene bags and sterilized. Substrate bags were inoculated with Oyster spawn and incubated in a cultivation room with controlled conditions (light, temperature, humidity and ventilation). Data for growth parameters (number of days for primordia growth, number of fruiting bodies per bag, crop yield, biological efficiency and contamination rate) were recoded for each bag of each treatment, compared against the control group (records of evaluated parameters from the mushroom extension unit) and were analyzed statistically using two-factor Analysis of Variance (ANOVA) with replication technique.
Results: Treatment-3 (waste paper supplied with saw dust and wheat bran) showed high performance in terms of mean crop yield (150.58 ± 1.2g) and BE (43.1 ± 1.1%); while Treatment-1(waste paper alone without any supplement) scores lower BE (17.3±2.1%). Based on the Analysis of Variance (ANOVA) this study shows that all the growth parameters were found significantly different at p-value (p < 0.01) considering each growth parameter among the treatments.
Conclusion: Therefore, waste paper along with required percentage of sawdust and wheat bran can be used as alternative substrate for cultivation of this prized mushroom reducing substrate expenses, adding a new food recipe to nutritional cuisine and reducing waste paper pollution on the environment on the other hand.
Introduction
Mushrooms are macroscopic fungi with heterotrophic absorptive mode of nutrition; which involves secreting of powerful hydrolytic enzymes into their surroundings in order to break down complex organic compounds to small sized particles to enhance rate of absorption in to their body [1]. Mushrooms have a distinctive fruiting body which can be either epigeous or hypogenous in which most species are grouped under Basidiomycota and Ascomycota (like truffle and morel) [2]. Even though mushrooms can be edible or nonedible, there is no clear-out-cut delimitation between edible and poisonous fungi (eg: Amanita muscaria). Since antiquity, humans have regarded mushrooms as a source of healing and culinary delight [3]. Psychedelic mushrooms were employed for ritualistic purposes by many prehistoric and current societies around the world, demonstrating that the narcotic and hallucinogenic characteristics of certain mushrooms have been known since ancient times [4]. Increased recognition of the nutritional value of many species, combined with the realization of the income-generating potential of fungi through trade, has resulted in a growing interest in cultivation to supplement, or replace, wild harvest [5].
Mushroom cultivation is the practice of obtaining fruit bodies artificially by repeating the growing stages. As the popularity of mushrooms in the diets and awareness about their health benefits increases in many societies in the world, interest in growing gourmet mushrooms increases equivalently which paves the way of understanding the materials and conditions required for mushroom growth [6]. Oyster mushroom is the second widely cultivated mushroom worldwide following Agaricus bisporus. Even though Agaricus bisporus is a highly tasty and nutritious mushroom, it does not appear to have specific medical value [7]. Chinese were the first professional mushroom farmers, documenting the cultivation process for Shiitake on wood logs, and valuing mushrooms for both medicinal and culinary purposes [8].
Domestication, cultivation practices, and commercialization of many mushroom species are all part of an agricultural crop improvement program that focuses on low-cost substrate materials that are readily available [9]. Approximately 14,000 mushroom species have been documented, accounting for 10% of the total expected mushroom species on the human planet, with around 2,300 useful mushroom species, 20 of which are cultivated commercially. China is said to have between 1500 and 2000 edible mushroom species, with 981 of them identified. Oyster mushroom cultivation began in the early 1900s, when tree stumps and logs were used as substrate to mimic their natural growth [3].
Even though the first successful experimental cultivation of P. ostreatus was achieved in Germany in 1917, the late attempts to grow oyster mushrooms on sawdust in the 1950s in Germany marked a historic milestone for mushroom cultivation [10]. Oyster mushroom mass production began in the 1960s using a straw-based substrate, and since then, the mushroom's popularity and production have grown steadily [11]. Oyster is a crucial decomposer of dead trees in the woodlands it inhabits, as it is mostly saprobic and only occasionally parasitic. Oysters are seasonal in the wild, but they have a longer season in parts of southern Europe [8].
Cultivation of edible mushrooms combines both skill and scientific technology in which agricultural wastes are recycled to produce a protein rich but cheap human food. So being an indoor activity mushroom cultivation can help reduce vulnerability to poverty and strengthens livelihoods through the generation of a fast yielding and nutritious source of food [12] and a reliable source of income creating a great complete contentment for landless poor families and villages. The present day cultivation technology of oyster mushroom is a result of various successive high technology steps which evolved throughout the world during 20th century [13].
In Eritrea currently only oyster mushroom is being cultivated with the help of Ministry of Agriculture and in other small unions using saw dust supplemented with wheat bran as a substrate. Mushroom cultivation can be a sustainable and environmentally sound socio-economic activity in Eritrea. Mushroom growers are facing many problems i.e. poor knowledge of cultivation, less availability of the best substrates, expenses for substrates and low mushroom yield per amount of substrate used. In addition to this no much work has been done on the use of different agro-industrial waste substrates to optimize the mushroom cultivation in Eritrea, therefore, this study is initiated to use readily available waste material (waste paper) which can considerably increase environmental pollution if not treated well.
Materials and Methods
Study site
The study was conducted at Mai-Nefhi College of Science (MCS) subzone Gala–Nefhi in Asmara (15.3317°N, 38.9300°E) Eritrea. All required laboratory activities of this study was conducted in the Microbiology laboratory located in MCS.
Substrate collection
Waste paper (wasted lab-report papers collected on garbage collection baskets in the lab) was collected (4 kg) from the Biology laboratory in MCS. Sawdust (5 kg) was collected from Sembel Metal and Wood Work Company in Asmara. Other nutrient supplement such as wheat bran, gypsum and lime were obtained from markets in Asmara city.
Strain selection, collection and propagation
Cultivated spawn was obtained from mushroom extension unit (Ministry of Agriculture) and was propagated by inoculating it on sterilized wheat grain in Microbiology laboratory, MCS. Oyster mushroom was selected for this study due to its highest ability to grow on wastes, resistance to different environmental conditions, high yield coupled with nutritional and culinary functions over other mushroom species and for its short life cycle.
Spawn production
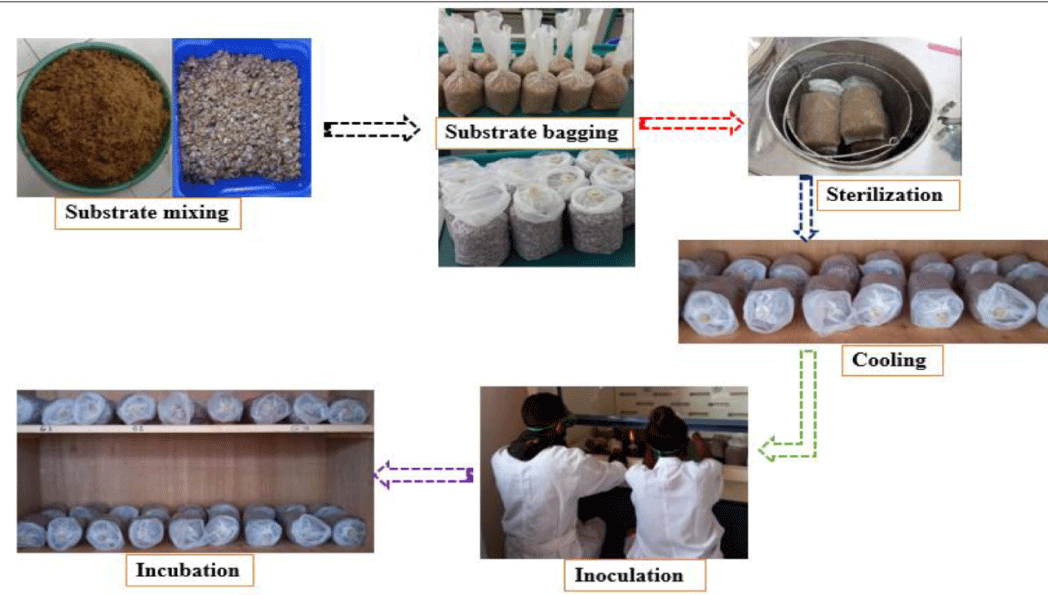
Spawn is the vigorous mycelial growth of a single fungus on a chosen substrate material (like cereals or saw dust). Wheat grain (1kg) was boiled on a dish and cooled by spreading on a floor mat. After cooling, 250 g of wheat was filled on a sterilized bottle followed by inoculation of each bottle with 7 days old culture (obtained from Mushroom extension unit) which grew on sawdust and was incubated for 20 days at 25°C until the new wheat substrate is fully colonized by the fungal mycelia (Figure 1).
Substrate preparation
Collected waste paper was chopped manually in to small pieces. In order to prepare, aerobic composted substrate through outdoor solid-waste fermentation process, waste paper was mixed with sawdust and wetted with 2% lime water. Moreover; portion of the chopped waste paper (without sawdust supplement) was soaked in to 2% lime water and composted alone. After 20 days each of these composted substrates were supplemented with wheat bran and gypsum according to the required proportion [14]. In addition; portion of the composted waste paper alone were kept unsupplemented with wheat bran to evaluate it as standalone substrate for mushroom cultivation. Generally substrate mixtures were designed to group under three treatments (Table 1).
| Table 1: Proportions of assessed substrate mixtures along with supplements. | ||||||
| Treatments | Waste paper (%) | Sawdust (%) | Wheat bran (%) | Lime (%) | Gypsum (%) | No. of replicates |
| T1 | 98 | 0 | 0 | 1 | 1 | 10 |
| T2 | 80 | 0 | 18 | 1 | 1 | 10 |
| T3 | 40 | 40 | 18 | 1 | 1 | 10 |
| Control treatment (Tc): 80% saw dust + 18% wheat bran + 1% gypsum + 1% lime | ||||||
Bagging and sterilization of substrates
The composted substrate mixtures were filled equally into plastic bags at the rate of 350 g of substrate per bag and sterilized for 2hrs at 1210 C, 15 IBS using an autoclave. After 4hrs the sterilized bags were removed out from the autoclave and kept for 20hrs on shelves to be cooled then ultimately the filled bags were transferred to the cultivation room for inoculation [15]. During inoculation step the inoculation box was first sterilized with fumigant smoke. Then cooled substrate bags were inoculated with the spawn (one glass bottle of spawn per 5 bags) with in the inoculation box and mixed thoroughly to facilitate rapid and uniform mycelia growth. The mouth of the bags was closed using a plastic closure ring cup and incubated in the dark incubation room at 25 +10 C with 65 -70% relative humidity for 20 days until mycelial colonization was completed [16]. Holes were made over the polythene bags for aeration parallel to the running mycelium down the substrate using sterilized tooth sticks. Incubation room and its surrounding was sterilized with formalin diluted with water to avoid contaminants at least one time per week (Figure 2).
Fruiting and harvesting
After incubation time was completed bags were opened by removing the cup and ring for fruiting. Temperature and relative humidity was maintained at 18°C - 23°C and 80 - 90% RH, respectively. During fruiting time water was sprayed in the form of fine mist using sprayer simultaneously for two weeks, to maintain moisture up to the desired level [17]. The floor of the mushroom cultivation house was sterilized with formalin to avoid contamination and then covered with sacks to be wetted with water 3times in a day and water buckets filled with water were placed in the house to maintain humidity. Mature mushroom was identified by curl margin of the cap and harvested by twisting to uproot from the base.
Fruiting bag assessment of different substrate mixtures
Mushroom growth parameters for each fruiting bag of different substrate mixtures were continuously observed and recorded in order to select the most promising substrate mixture of this study. The experiment comprised three treatments in which each treatment further involves ten replicates each.
Data collection and harvesting of mushroom
The number of primordial growth in each bag of all treatments, number of mature mushrooms per bag, harvest / yield per bag, biological efficiency and number of contaminated bags encountered in each treatment was recorded periodically during culturing. The yield of the mushrooms was determined in terms of fresh weights of the harvested mushrooms.
The biological efficiency (%) was calculated as follows:
E= (wf/wd) ×100,
where:
E-is the biological efficiency, wf-is the weight of fresh harvested fruiting body, and
wd-is the weight of dry matter of the substrate in grams [18].
Data analysis
The Complete Randomized Design (CRD) was applied in this study and the data thus obtained for first flash only were analysed statistically using two-factor Analysis of Variance (ANOVA) with replication technique to check the significant difference between treatments in each growth parameter at significance level (P < 0.01) and to predict the correlation between recorded growth parameters (Figure 3).
Results and Discussion
Spawning
A spawn is like what a seed is to plant. A medium through which the mycelium of a fruiting culture has grown and which serves as the inoculum of “seed” for the substrate in mushroom cultivation is called the “mushroom spawn. Unlike spore, spawn is already at its mycelial stage growing on its own substrate such as sorghum, barley or wheat and is inoculated into the substrates [19]. The quality and amount of spawn is one of the most decisive factors for successful mushroom cultivation. The stock culture selected should be acceptable in terms of yield, flavor, texture, fruiting time, and so on. Failure to achieve a satisfactory harvest may often be traced to unsatisfactory spawn used [20].
Spawning was done under aseptic condition and bottles filled with wheat grain were inoculated in the same day to observe the mycelial colonization time. During spawning mycelial growth was appeared in all battles after 6th day and gradually invaded the wheat grains. The growth of the mycelium is faster if it is kept in incubator under optimal temperature (19-23°C) within 14-16 days. In this study full white mycelial invasion of the substrate was observed after 20 days of incubation and was ready for inoculation on solid substrate similar to other studies [19] (Figure 4).
Mushroom growth
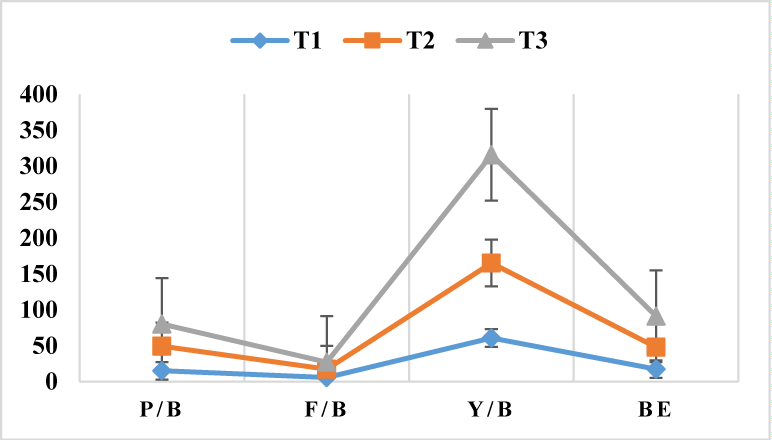
During incubation the mycelium in the inoculated spawn starts to spread down from the neck of the bag down colonizing the whole substrate. Holes were created at the boundary of a growing mycelium using sterilized tooth sticks to allow aeration which is necessary for facilitating mycelial running/colonization through the substrate. After full substrate colonization the bags were opened and primordia starts to grow after 3 days. Primordia are tiny pinheads or young fruit bodies which later develops into mature fruit body. The formation of fruiting bodies of P. ostreatus was observed within the range of 5-8 days after primordial initation. Comparing the three treatments; T3 and T2 shows good growth of P. ostreatus mycelium as evidenced by the complete and heavy colonization of substrates forming a compact white mass of mycelium within 18-20 days of inoculation. Cultivation of oyster mushroom on the three treatments has manifested variable levels of biological efficiency. These variations are mainly related to the substrate’s carbon content and proportion of supplement added to it like previous studies [21]. The performances of the three treatments were also evident by their elevated fruiting body values (yield) on T3 followed by T2 (Table 2).
| Table 2: Comparative results of recorded growth parameters in evaluated treatments. | |||||
| Treatment | Average no. of Primordia / bag | Mean no. of mature mushrooms / bag | Mean Yield/bag (g) | Mean of Biological efficiency (%) | No. of bags showing contamination |
| T1 | 15 ± 0.0 | 5.5 ± 0.5 | 60.6 ± 0.9 | 17.3 ± 2.1 | 2 |
| T2 | 34.5 ± 0.5 | 11 ± 0.0 | 104.5 ± 1.01 | 30.5 ± 1.8 | 2 |
| T3 | 30.5 ± 0.5 | 9.5 ± 0.5 | 150.58 ± 1.2 | 43.1 ± 1.1 | 1 |
| Tc | 52 ± 00 | 17 ± 00 | 200 ± 0.39 | 49 ± 0.1 | 1 |
| ± Standard deviation, Tc = control treatment | |||||
The yield and quality of mushroom produced is determined by three factors: the type of mushroom strain, the environmental conditions in which the mushroom is grown and the physiological and nutritional requirements of different strains [22]. Waste paper is evaluated as carbon source in this study showed good mushroom yield at 40% level indicating that the yield of mushroom can further be increased by supplementing the substrate with suitable nitrogen source which is comparable with previous findings [23].
Substrates and mushroom cultivation
The organic materials, on which mushrooms derive their nutrition, are referred to as substrates. The substrate must be rich in essential nutrients and be free of toxic substances that inhibit growth of the mycelium [24]. Oyster mushroom decomposes variety of substrates using extensive enzymatic toolkits. Commercial production of P. ostreatus mushrooms is largely determined by the availability and utilization of cheap by products / waste materials, which can be agricultural or industrial wastes that are ideal and most promising substrates for cultivation [17]. The substrates used in this study can be considered practical and economically feasible because these are readily available everywhere at no or low cost in large quantities. Utilization of these wastes for the production of P. ostreatus mushrooms could be more economical, ecological and can provide nutritional food option and similarly reduce food scarcity [21]. According to previous findings waste paper is rich in cellulose, hemicellulose and lignin content [25].
Non-green revolution (mushroom cultivation) convert huge lignocellulosic biomass wastes into human food and notable nutraceuticals. Mushroom requires carbon, nitrogen and inorganic compounds as its nutritional sources for growth however, required amount of each nutritional source differs according to mushroom species [21]. Mushroom is the only saprophyte which is able to dissolve and transform cellulose into food for mankind. Oyster mushroom requires high carbon and lower nitrogen input, thus demanding a high C/N ratio [26].
Growth conditions
Mushroom cultivation requires an appropriate environment both for vegetative and reproductive growth. So success or failure of mushroom cultivation depends on the control of growing conditions and evading contamination. These environmental factors (named as “triggers” or “environmental shocks”) include temperature, humidity, light and ventilation in which optimal levels of these factors at vegetative stage and reproductive stage are different [27]. According to previous studies it was found that electrical shock treatment and high sound intensity can enhance the growth and yield of oyster mushroom [28]. Oyster mushroom has maximum number of commercially cultivated species (varying in shape, color, texture and aroma), longer shelf life, highest productivity, high tolerance for variations in environmental shocks, higher growth rate, short cropping days and resistance to diseases and pests [26].
Even though majority of mushroom mycelia grow well at a temperature range of 20-300 C and forms primordia at 10-200 C; oyster mushrooms fruits with good quality between 10-23°C and 80-85 RH respectively coupled with sufficient ventilation[29]. Over 80% of the fruit body is water so substrate moisture content should be 60-75%. During fruiting, different relative humidity levels, ranging from 80-95%, sufficient light and ventilation are required. So the temperature and relative humidity of the cultivation room for this study was kept at 20-22°C and 85-90 RH [30]. Deviation from the optimal conditions can cause change in color and morphology of a fruit body and reduces the yield of cultivated mushroom species [31]. In favor of this, during fruiting time temporary increase in temperature above the optimal level in the sunny days had affected the color and development of mushroom caps i.e. deep brown curled caps while during lower temperature in the night time which reached 17°C the fruited mushrooms became dark in color similar to previous discussions [32].
Moreover; during mycelial growth, CO2 concentrations in the bags could rise up to 40%. Mycelial growth of P. ostreatus tends to stimulate in CO2 concentrations up to 28%. So the ambient CO2 concentration in the growing room should be controlled by ventilation, especially during fruit body formation and development excessive CO2 can inhibit pilus differentiation and enhance premature aging of mushrooms [33]. In this study mushrooms were produced with long stipes carrying tiny caps in the first fruiting week in some of the bags, while later after sufficient ventilation was allowed in the growing room the remained bags flushes fruit bodies with short stipes having broad caps. This may be attributed to the fact that ventilation should be sufficient during fruiting stage as discussed in previous studies [34]. So mushroom growers must consider CO2 concentration in the substrate bags during mycelial colonization and the ambient CO2 concentration during fruit body development.
Fruiting bag assessment of different substrate mixtures
Mycelium growth rate for each type of substrate was measured every week after the mycelium colony reached the neckline of the bags. Bags containing 98% waste paper showed poor mycelial growth that eventually stopped after spreading to certain extent, leading to failure of colonisation of the whole substrate and those containing mixture of 40% wastes paper +40% saw dust exhibited faster mean colonization days (18 days) compared to T2 (19days). Mycelium running rate in substrate bags was found to be differed due to different levels of supplements used and compaction degree of a substrate mixture.
Substrate mixtures containing 40% of waste paper obtained higher yield following to the standard treatment than waste paper alone (98%) which displays poor mycelial growth which is similar to previous studies [35]. 100 % waste paper substrate do not support vast mycelial growth through the whole substrate bag. This suggests that substrate mixtures used had a significant effect on number of days taken for successful colonisation of fruiting bags by oyster mushroom mycelium and on mean weight of mushrooms harvested. This finding shows that the nutritional feebleness of waste paper as a standalone substrate can be reduced by supplementing it with a substrate rich in nitrogen content as carbon to nitrogen ratio (C/N) determines the decomposition rate of a waste material by the fungal mycelia [36] (Figure 5).
Treatment and substrate evaluation
Considering the mean weight of mushrooms produced; T1 (60.6 ± 0.9g) obtained the lowest while T2 (104.5 ± 1.01g) and T3 (150.58 ± 1.2g) scored higher harvest. Highest mean number of mushrooms per bag was observed in T2 (11 ± 0.0) and T3 (9.5 ± 0.5) while T1 (5.5 ± 0.5) produced the lowest number of mushroom. Mean of biological efficiency was found the highest in T3 (43.1 ± 1.1%) and T2 (30.5 ± 1.8%) compared to T1 (17.3 ± 2.1). This shows T3 scores an appreciable result in relation to the control treatment records (Table 2). In addition these results tends to corporate with previous study done by Tesfay T, et al. [35] where waste paper was evaluated as substrate mixed with different proportions of corn stalk and wheat bran for oyster mushroom cultivation.
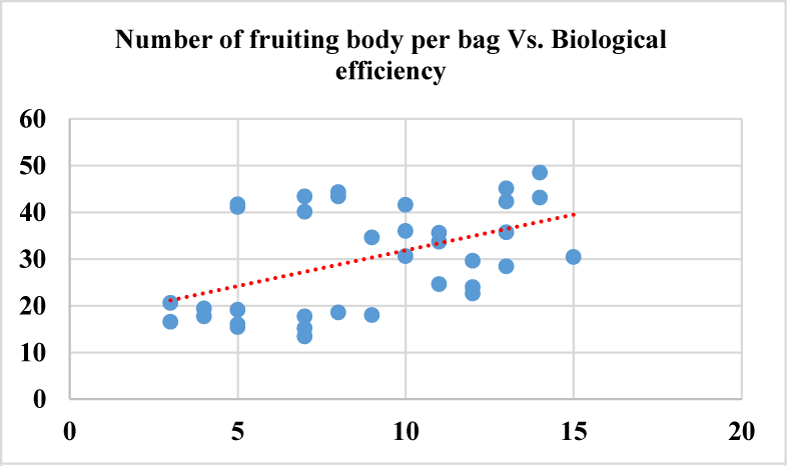
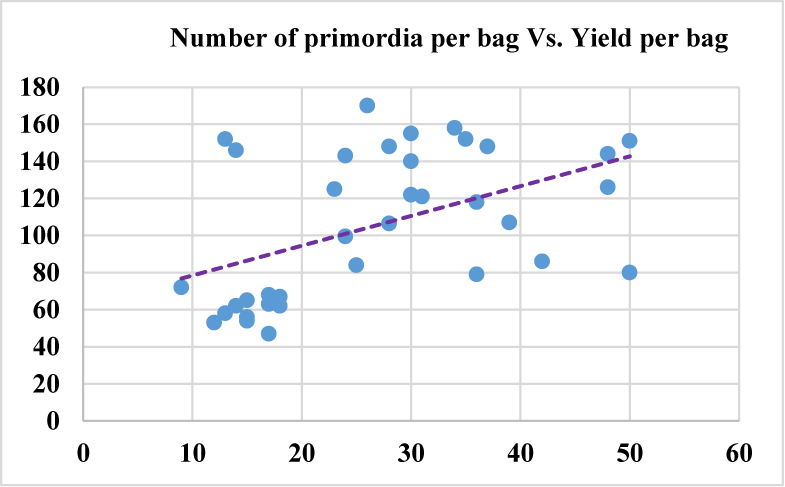
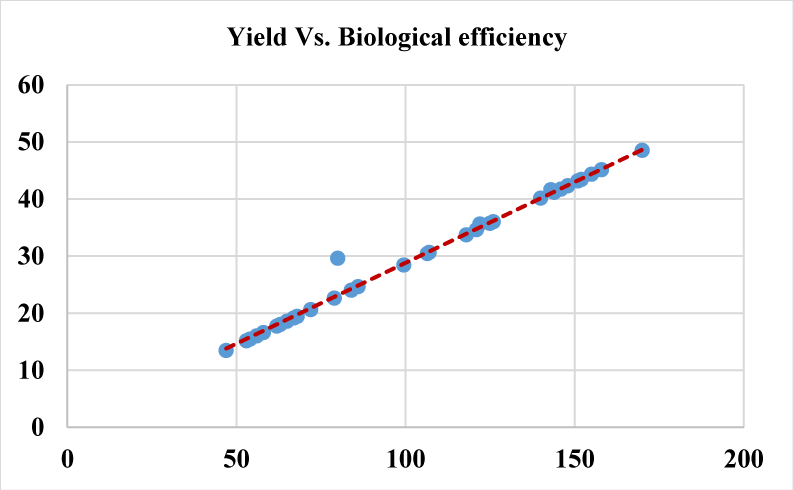
Based on the Analysis Of Variance (ANOVA) this study shows that all the evaluated growth parameters were found significantly different at p-value (p < 0.01) considering each growth parameter among the treatments. Number of primordia per bag displayed mild positive correlation with number of mature fruiting bodies per bag (p = 0.55), yield per bag (p = 0.48) (Figure 7) and biological efficiency (0.52). While Yield per bag shows strong positive correlation (p = 0.99) with biological efficiency, where dots are closely touching the trend line in figure 8 indicating the correlation strength. The higher the yield per bag the higher biological efficiency will be attained upon dividing by the dry mass of the substrate used per bag. Number of fruiting body per bag showed mild correlation with yield per bag (p = 0.46) and biological efficiency (p = 0.48) (Figure 6) because the higher the number of the fruiting bodies in each bag the smaller will be the size of each fruiting body thereby posing little effect on yield per bag and indirectly to biological efficiency.
Conclusion
The result obtained from the trials set in this study, reveals that the opportunities exist for the use of waste paper in oyster mushroom cultivation in Eritrea. Results obtained in the experiment showed that if waste paper is mixed with some other substrates it can produce good yield. As it shows in treatment 3 (the substrate mixture containing 40% Saw dust + 40% waste paper +18% wheat bran supplemented with 1% lime and 1% gypsum) yielding about 150.58 ± 1.2g mean harvest of fresh oyster mushroom approaching to the yield of the control treatment (Tc). Additional benefit of using waste paper achieved positive outcomes since it will lower the cost of substrate bag preparation using sawdust. In addition to this if the local populace will involve in mushroom cultivation, definitely it will help in ensuring food scarcity and enhance the environmental sanitation since the cultivation of oyster mushroom involves recycling of waste materials. Moreover, mushroom can serve as source of income to families.
Acknowledgment
Authors express their deepest appreciation for Mr. Teklemariam Yohannes (mushroom expert in the extension unit of Ministry of Agriculture) for providing pure spawn culture and his technical advice during the study.
References
- Alday JG, Martínez de Aragón J, de-Miguel S, Bonet JA. Mushroom biomass and diversity are driven by different spatio-temporal scales along Mediterranean elevation gradients. Sci Rep. 2017;7:45824. doi: 10.1038/srep45824.
- Krishnapriya PJ, Geetha D, Priya RU. The first report of Tricholoma giganteum from Kerala, its molecular characterization and production studies. Mushroom Research. 2017;26(2):137-141.
- Valverde ME, Hernández-Pérez T, Paredes-López O. Edible mushrooms: improving human health and promoting quality life. Int J Microbiol. 2015;2015:376387. doi: 10.1155/2015/376387. Epub 2015 Jan 20. PMID: 25685150; PMCID: PMC4320875.
- Khatua S, Dutta AK, Chandra S, Paloi S, Das K, Acharya K. Introducing a novel mushroom from mycophagy community with emphasis on biomedical potency. PLoS One. 2017 May 26;12(5):e0178050. doi: 10.1371/journal.pone.0178050. PMID: 28552988; PMCID: PMC5446119.
- Bhambri A, Srivastava M, Mahale VG, Mahale S, Karn SK. Mushrooms as Potential Sources of Active Metabolites and Medicines. Front Microbiol. 2022 Apr 26;13:837266. doi: 10.3389/fmicb.2022.837266. PMID: 35558110; PMCID: PMC9090473.
- Suwannarach N, Kumla J, Zhao Y, Kakumyan P. Impact of Cultivation Substrate and Microbial Community on Improving Mushroom Productivity: A Review. Biology (Basel). 2022 Apr 8;11(4):569. doi: 10.3390/biology11040569. PMID: 35453768; PMCID: PMC9027886.
- Nongkynrih B, Firake Baiswar GT, Behere S, Chandra SV,Ngachan DP. Pest complex of cultivated oyster mushroom in Northeast India: Feeding losses and role of micro-climate in pest multiplication. Indian Journal of Hill Farming. 2017;30:259-267.
- Chen L, Gong Y, Cai Y, Liu W, Zhou Y, Xiao Y, Xu Z, Liu Y, Lei X, Wang G, Guo M, Ma X, Bian Y. Genome Sequence of the Edible Cultivated Mushroom Lentinula edodes (Shiitake) Reveals Insights into Lignocellulose Degradation. PLoS One. 2016 Aug 8;11(8):e0160336. doi: 10.1371/journal.pone.0160336. PMID: 27500531; PMCID: PMC4976891.
- Haukongo KN, Horn L. Effects of different substrates as medium for mushrooms cultivation. Academia Journal of Food Research. 2021;9(2):032-037.
- Ladli. A review on oyster mushroom Pleurotus ostreatus cultivation. Int J Curr Microbiol App Sci. 2020;11:1653-1665.
- Banik S, Nandi R. Effect of supplementation of rice straw with biogas residual slurry manure on the yield, protein and mineral contents of oyster mushroom. Ind Crops Prod. 2004;20:31-319. doi: 10.1016/j.indcrop.2003.11.003.
- Gupta N, Mehta M, Singh K. Benefits, challenges and opportunities in mushroom production: A review. The Pharma Innovation Journal. 2022;360-364.
- Kumar P, Bharty S, Singh RK, Kumar K, Rani N. Impact of oyster mushroom (Pleurotus ostreatus) training on socio-economic and knowledge of tribal woman of Hazaribag, Jharkhand, India. Int J Curr Microbiol App Sci. 2018;7:1106-11112018.
- Baysal E, Peker H, Yalinkiliç MK, Temiz A. Cultivation of oyster mushroom on waste paper with some added supplementary materials. Bioresour Technol. 2003 Aug;89(1):95-7. doi: 10.1016/s0960-8524(03)00028-2. PMID: 12676506.
- Itelima J. Cultivation of mushroom Pleurotus ostreatus using corn cobs and saw dust as the major substrate. Global Journal of Agricultural Sciences. 2012;11(1). Doi: 10.4314/gjass.v11i1.9.
- Grimm D, Wösten HAB. Mushroom cultivation in the circular economy. Appl Microbiol Biotechnol. 2018 Sep;102(18):7795-7803. doi: 10.1007/s00253-018-9226-8. Epub 2018 Jul 19. PMID: 30027491; PMCID: PMC6132538.
- Aditya, Neeraj, Jarial RS, Jarial K, Bhatia JN. Comprehensive review on oyster mushroom species (Agaricomycetes): Morphology, nutrition, cultivation and future aspects. Heliyon. 2024 Feb 19;10(5):e26539. doi: 10.1016/j.heliyon.2024.e26539. PMID: 38434383; PMCID: PMC10907667.
- Silva RM da, Carmo Co do, Oliveira TAS de, Figueirêdo VR de, Duarte EAA, Soares ACF. Biological efficiency and nutritional value of Pleurotus ostreatus cultivated in agroindustrial wastes of palm oil fruits and cocoa almonds. Arq Inst Biol. 2020;87(19). doi: 10.1590/1808-1657000852018.
- Kortei NK, Odamtten GT, Obodai M, Wiafe-Kwagyan M, Narh-Mensah DL. Correlations of cap diameter (pileus width), stipe length and biological efficiency of Pleurotus ostreatus (Ex.Fr.) Kummer cultivated on gamma-irradiated and steam-sterilized composted sawdust as an index of quality for pricing. Agric Food Secur. 2018;7(1). doi: 10.1186/s40066-018-0185-1.
- Boadu KB, Nsiah-Asante R, Antwi RT, Obirikorang KA, Anokye R, Ansong M. Influence of the chemical content of sawdust on the levels of important macronutrients and ash composition in Pearl oyster mushroom (Pleurotus ostreatus). PLoS One. 2023 Jun 29;18(6):e0287532. doi: 10.1371/journal.pone.0287532. PMID: 37384658; PMCID: PMC10309632..
- Hoa HT, Wang CL, Wang CH. The Effects of Different Substrates on the Growth, Yield, and Nutritional Composition of Two Oyster Mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology. 2015 Dec;43(4):423-34. doi: 10.5941/MYCO.2015.43.4.423. Epub 2015 Dec 31. PMID: 26839502; PMCID: PMC4731647.
- Mamiro DP, Royse DJ. The influence of spawn type and strain on yield, size and mushroom solids content of Agaricus bisporus produced on non-composted and spent mushroom compost. Bioresour Technol. 2008 May;99(8):3205-12. doi: 10.1016/j.biortech.2007.05.073. Epub 2007 Aug 29. PMID: 17761414.
- Muswati C, Simango K, Tapfumaneyi L, Mutetwa M, Ngezimana W. The effects of different substrate combinations on growth and yield of oyster mushroom (Pleurotus ostreatus). International Journal of Agronomy. 2021; 43(4):423. doi: 10.5941/MYCO.2015.43.4.423.
- Shamugam S, Kertesz MA. Bacterial interactions with the mycelium of the cultivated edible mushrooms Agaricus bisporus and Pleurotus ostreatus. J Appl Microbiol. 2023 Jan 23;134(1):lxac018. doi: 10.1093/jambio/lxac018. PMID: 36626759.
- Zhou W, Gong Z, Zhang L, Liu Y, Yan J, Zhao M. Feasibility of lipid production from waste paper by the oleaginous yeast cryptococcus curvatus. Bioresources. 2017;12:5249-5263. doi: 10.15376/biores.12.3.5249-5263.
- Zakil FA, Sueb MSM, Isha R. Growth and yield performance of Pleurotus ostreatus on various agro-industrial wastes in Malaysia. AIP Conference Proceedings. American Institute of Physics Inc. 2019. doi: 10.1063/1.5125559.
- Sher H, Al-Yemeni M, Bahkali AHA, Sher H. Effect of environmental factors on the yield of selected mushroom species growing in two different agro ecological zones of Pakistan. Saudi J Biol Sci. 2010 Oct;17(4):321-326. doi: 10.1016/j.sjbs.2010.06.004. Epub 2010 Jun 18. PMID: 30323710; PMCID: PMC6181181.
- Takaki K, Yoshida K, Saito T, Kusaka T, Yamaguchi R, Takahashi K, Sakamoto Y. Effect of Electrical Stimulation on Fruit Body Formation in Cultivating Mushrooms. Microorganisms. 2014 Feb 12;2(1):58-72. doi: 10.3390/microorganisms2010058. PMID: 27694776; PMCID: PMC5029503.
- Hoa HT, Wang CL. The Effects of Temperature and Nutritional Conditions on Mycelium Growth of Two Oyster Mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology. 2015 Mar;43(1):14-23. doi: 10.5941/MYCO.2015.43.1.14. Epub 2015 Mar 31. PMID: 25892910; PMCID: PMC4397375.
- Saputera A, Sofyan A, Saputra RA, Sari N. Effect of watering frequency on the growth and yield of oyster mushrooms (Pleurotus ostreatus). Agrosainstek: Jurnal Ilmu dan Teknologi Pertanian. 2020;4:155-160. doi: 10.33019/agrosainstek.v4i2.91.
- Preethy S, Anbuselvi. Variables influencing the growth of golden oyster mushrooms. Nat Volatiles & Essent Oils. 2021;8(4):3576-3586.
- Rukhiran M, Sutanthavibul C, Boonsong S, Netinant P. IoT-based mushroom cultivation system with solar renewable energy integration: Assessing the sustainable impact of the yield and quality. Sustainability. 2023;15(18):13968. Doi: 10.3390/su151813968.
- Lin R, Zhang L, Yang X, Li Q, Zhang C, Guo L, Yu H, Yu H. Responses of the Mushroom Pleurotus ostreatus under Different CO2 Concentration by Comparative Proteomic Analyses. J Fungi (Basel). 2022 Jun 21;8(7):652. doi: 10.3390/jof8070652. PMID: 35887408; PMCID: PMC9321156.
- Lee HU, Ahn MJ, Lee SW, Lee CH. Effects of various ventilation systems on the carbon dioxide concentration and fruiting body formation of king oyster mushroom (Pleurotus eryngii) grown in culture bottles. J Life Sci. 2007;17:82-90. doi: 10.5352/JLS.2007.17.1.082.
- Tesfay T, Godifey T, Mesfin R, Kalayu G. Evaluation of waste paper for cultivation of oyster mushroom (Pleurotus ostreatus) with some added supplementary materials. AMB Express. 2020 Jan 18;10(1):15. doi: 10.1186/s13568-020-0945-8. PMID: 31955267; PMCID: PMC6969873.
- Paul A, Joseph P. Nutrient analysis of paper waste degenerated through the process of vermicomposting in an Institutional setup. Ecology, Environment and Conservation 2017;23:323-329.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.