Medicine Group. 2024 June 14;5(6):588-596. doi: 10.37871/jbres1931.
Experience on Utilization of Novel Modeling & Simulation Approaches in Generic Product Development
Rajkumar Boddu, Sivacharan Kollipara, Arvind Rachapally and Tausif Ahmed*
- PBPK
- PBBM
- Dissolution
- Bioequivalence
- Generic product development
Abstract
Modeling and simulation approaches such as Physiologically Based Pharmacokinetic (PBPK) and Physiologically Based Biopharmaceutics Modeling (PBBM) are gaining importance due to their impact to transform drug development paradigm. Development of such modeling approaches include integration of drug substance, drug product and physiological aspects followed by validation against clinical data. In this manuscript, we have highlighted utility of PBPK and PBBM in generic product development using case examples. An application where PBBM was utilized to obtain biowaivers and dissolution safe space was portrayed. Utility of such modeling approaches in obtaining fed bioequivalence studies was discussed with case example. Impact of gender evaluation on bioequivalence study outcome using PBPK approach was highlighted with a case example. Evaluation of safety aspects of extended release formulation with help of modeling approach was discussed with practical example. In the last case study, understanding the impact of critical bioavailability attributes with help of PBBM was indicated. Further, future direction in terms of sharing best practices across academia, industry and regulatory was highlighted. Overall, this manuscript summarizes experience on utilization of modeling and simulation approaches in generic product development with an aim to have more affordable and quality medicines to the patients.
Introduction
Of late, in silico modelling and simulation tools such as Physiological Based Pharmacokinetic (PBPK) modeling and Physiologically Based Biopharmaceutics (PBBM) modeling applications were evolved both in new drug development and generic drug development. In new drug development PBPK/PBBM modelling can be applied for evaluating API properties, selection of suitable salt or polymorphic form, selection of appropriate formulation that can aid in sufficient exposures in Phase-1 study, formulation bridging across the clinical phases of drug development and prediction of drug-drug interactions. In case of generic drug products, applications of modelling encompass biowaiver justification, scale up and post approval changes justification, dissolution specification justification, gender effect evaluation, f2 mismatch justifications, food effect evaluation, and evaluation of critical bioavailability attributes like CMA, CFV, CPP on in vivo performance [1-6].
Regulatory agencies such as the United States Food and Drug Administration (USFDA) and European Medicines Agency (EMA) have recognized the value of PBPK modeling in drug development and have provided detailed guidance’s to support its use and implementation in regulatory context. These guidelines outline the requirements for development of such models, importance of model input parameters, sensitivity analyses, strategies for verification and validation followed by specific application in the context-of-use (e.g. assessing impact of food, gender impact, establishing dissolution specifications, linking formulation attributes to in vivo performance). Specifically, the USFDA guidance on PBBM approach focusses on implementation of quality into the product development and linking drug product attributes to in vivo performance thereby embedding clinical quality into the product development. In the context of generic product development, utilization of such modeling approaches are critical because of their ability to reduce requirement of potential clinical studies. In order to obtain bioequivalence with reference product, before going ahead with pivotal clinical study, PBBM and PBPK approaches provides confidence into achieving bioequivalence. These modeling approaches also have potential to supersede traditional dissolution similarity factor (f2) thereby reducing the need of additional clinical study in case of dissolution dissimilarity. PBPK and PBBM approaches also have ability to waive critical fed bioequivalence studies thereby reducing the time required for generic product development. Additionally, such modeling approaches also can be effectively utilized to demonstrate discriminatory ability of the dissolution media thereby reducing the requirement of resources and time. Moreover, these modeling approaches enable the establishment of dissolution safe space thereby increasing probability of batch release at commercial setting. Hence, due to these plethora of advantages these modeling approaches adds critical value in generic product development thereby providing high quality, affordable medicines to the patients [7-8].
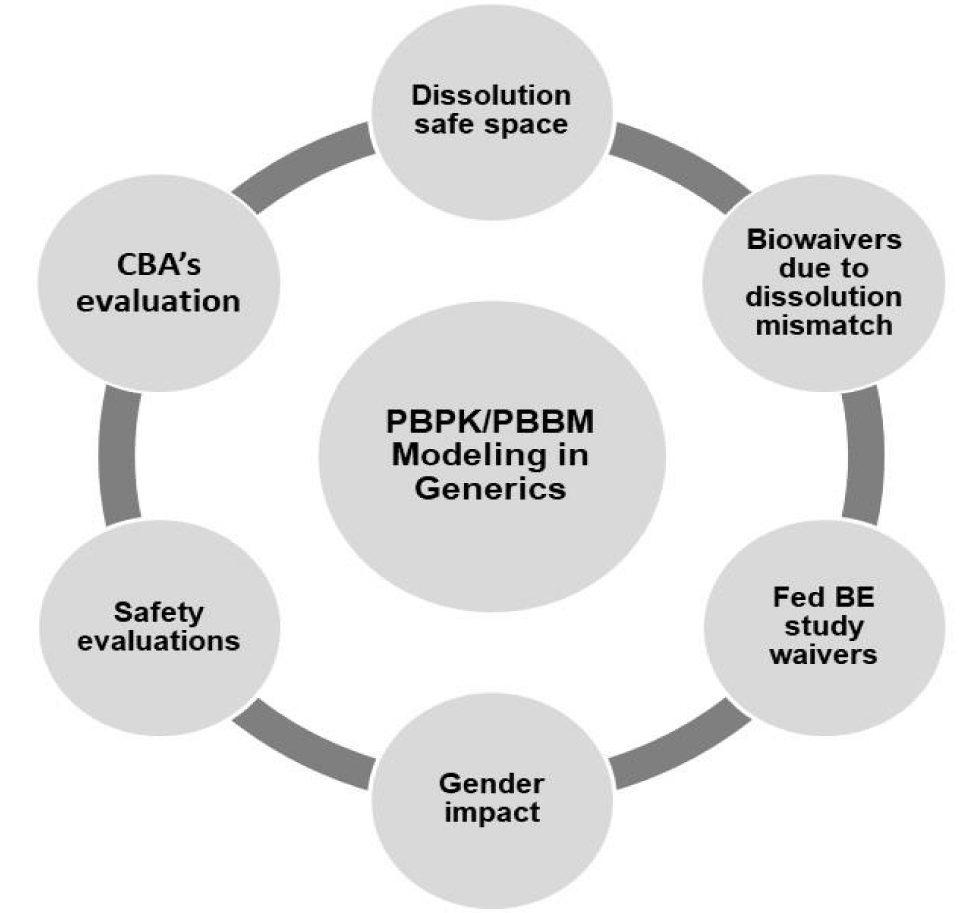
In this context, we have made an effort to summarize our understanding and experience of utilizing novel modeling and simulation approaches in generic product development. We have discussed various examples on applications of PBPK and PBBM approaches in generic product development with help of literature reported case examples [9-14]. A summary of various applications of modeling approaches is provided in table 1 and schematically described in figure 1. In the manuscript, utility of PBBM in establishing dissolution safe space, biowaivers is discussed with case example. Application of PBBM in obtaining fed bioequivalence study waiver is portrayed with a case study. Further, gender impact on bioequivalence study outcome is critically discussed with help of an example. Further, evaluation of safety aspects of extended release formulation with help of modeling approach was discussed with practical example. In the last case study, the impact and identification of Critical Bioavailability Attributes (CBA) with help of PBBM was performed. Finally, we also made an attempt to discuss futuristic aspects of modeling approaches together with latest developments in regulatory context. Overall, we believe that in this manuscript we have summarized our experience on utilization of novel modeling approaches in generic development and thus this manuscript can be of great relevance to pharmaceutical, biopharmaceutics and modeling scientists.
| Table 1: Literature examples demonstrating utility of PBBM in generic product development. | |||
| S. No | Title | Application | Approach |
| 1 | Utility of Physiologically Based Biopharmaceutics Modeling (PBBM) in Regulatory Perspective: Application to Supersede f2, Enabling Biowaivers & Creation of Dissolution Safe Space [3] | PBBM utility in safe space and biowaivers | Product DRL (50mg and 100mg) is a generic IR tablet with BCS Class-III. The reference and test formulations have salt-A & Salt-B of API but both products were bioequivalent for 100mg. However, while leveraging the generic product to different market, f2 < 50 vs RLD in pH 6.8 for both strengths, hindering a bioequivalence waiver. To address this, PBBM model was used and, justified by establishing dissolution safe space (>85% released up to 60 min) and consequently BE was proved against the reference for both strengths. |
| 2 | Biopharmaceutics Risk Assessment-Connecting Critical Bioavailability Attributes with in vitro, in vivo Properties and Physiologically Based Biopharmaceutics Modeling to Enable Generic Regulatory Submissions [4] | PBBM utility in evaluation of CBA’s | This article proposes a framework: initial CBA identification, confirmatory evaluation, and control strategy definition. Tools like bio-discriminatory dissolution methods and PBBM aid in practical assessment. A case study illustrates detailed CBA evaluation using PBBM. Overall, this manuscript guides biopharmaceutics risk assessment for generic submissions, future directions include integrating CBA evaluation with FDA's KASA initiative, creating risk assessment. |
| 3 | Power of integrating PBPK with PBBM (PBPKBM): a single model predicting food effect, gender impact, drug-drug interactions and bioequivalence in fasting & fed condition [5] | Multi-purpose PBPK-BM model | In this article, an integrated PBPK-PBBM model was used where single model able to apply for bioequivalence prediction, gender impact assessment, food effect evaluation, and predicting drug-drug interactions. This demonstrates the utility of a combined PBPK-BM model in multiple regulatory justifications and reducing review timelines. |
| 4 | Physiologically Based Pharmacokinetics Modeling in Biopharmaceutics: Case Studies for Establishing the Bioequivalence Safe Space for Innovator and Generic Drugs [6] |
PBBM in establishing dissolution safe space | This article discusses detailed case studies illustrating PBBM's application in establishing BE safe spaces. BE safe space applications to set in vitro dissolution/Particle Size Distribution (PSD) specs, widening dissolution specs to supersede f2 similarity and for Scale-Up And Post-Approval Changes (SUPAC) bio waivers were described. Further, proper PBBM work flow, and approaches are discussed, along with parameter sensitivity analyses and Virtual Bioequivalence (VBE) modeling. |
| 5 | Novel application of PBBM to justify impact of faster dissolution on safety and pharmacokinetics - A case study and utility in regulatory justifications [9] | PBBM to demonstrate safety | This manuscript explores new application PBBM to assess the impact of faster dissolution profiles on safety of a Narrow Therapeutic Index (NTI). The justification was addressed through proper PBBM modelling. Using developed and validated model, simulated plasma profiles for faster dissolution profiles generated and compared against reported safety levels and found to be safe. This innovative use of PBBM suggests potential to expedite drug approval by potentially avoiding clinical safety studies. |
| 6 | Using Mechanistic Modeling Approaches to Support Bioequivalence Assessments for Oral Products [10] | PBBM in BE assessment | This article summarizes FDA and CRCG public workshop -2022. The workshop aimed to modernize BE demonstration approaches, integrate them into generic drug development, and establish modeling best practices for regulatory submissions. |
| 7 | Establishing the Bioequivalence Safe Space for Immediate-Release Oral Dosage Forms using Physiologically Based Biopharmaceutics Modeling (PBBM): Case Studies [11] | PBBM to establish safe space | The manuscript details a few case studies using PBPK modeling to establish safe spaces for BCS Class 2 and 4 drugs across different companies, for both internal decision-making and regulatory applications. Case studies detailed practical versus ideal dataset for safe space development, methodologies for integrating dissolution data, and criteria for model validation and application. |
| 8 | Development, validation and application of physiologically based biopharmaceutics model to justify the change in dissolution specifications for DRL ABC extended release tablets [12] | PBBM in justifying dissolution specifications | In this this work, author detailed the application of PBBM as to dissolution specification justification. The integrated PBBM with a mechanistic In Vitro-In Vivo Relationship (IVIVR) was developed, validated and and successfully justified changes dissolution specification. |
| 9 | Role of Physiologically Based Biopharmaceutics Modeling (PBBM) in Fed Bioequivalence Study Waivers: Regulatory Outlook, Case Studies and Future Perspectives [13] | PBBM in fed bioequivalence study waivers | The manuscript discusses PBBM's role in generic fed bioequivalence study waivers, highlighting industry practices and practical considerations. Analysis of 36 products reveals the predictability of PBBM for fed bioequivalence, correlated with BCS class, formulation, and food effect. Two case studies illustrated successful fed bioequivalence study waivers with PBBM. The article also outlines future directions for regulatory perspectives and best practices in PBBM for generic fed bioequivalence study waivers. |
| 10 | Best Practices for Integration of Dissolution Data into Physiologically Based Biopharmaceutics Models (PBBM): A Biopharmaceutics Modeling Scientist Perspective [15] | Incorporation of dissolution into PBBM | This article summarizes the understanding of data dissolution data inputs and dissolution modelling in PBPK modelling. It discusses incorporating dissolution data and challenges for fitting the data Z-factor and P-PSD model applications, weibull fitting etc for immediate, modified, and delayed-release formulations, are detailed. It also covers generating virtual dissolution profiles using weibull and DDD plus and time scaling approaches for dissolution safe space generations. Finally provided a practical guide for biopharmaceutics modeling scientists to effectively integrate dissolution data into PBPK models. |
| 11 | Modelling Based Approaches to Support Generic Drug Regulatory Submissions‑Practical Considerations and Case Studies [16] | PBBM to support generic product development | This article described Model-Informed Drug Development (MIDD) which utilizes the tools like Physiologically Based Pharmacokinetic Modelling (PBPK) to predict drug exposure during development. This article focuses on PBPK application in generic drug development, especially for bio-waiver applications. It highlights approaches and pitfalls in regulatory submissions. Case studies illustrate PBPK's role in obtaining bio-waivers, bridging theory and practical application in generic drug development. |
| 12 | Experience Learned and Perspectives on Using Model‑Integrated Evidence in the Regulatory Context for Generic Drug Products-A Meeting Report [17] | PBBM to support generic product development | The article summarizes a 2022 FDA workshop on modeling in generic drug development. It discusses using modeling for long-acting injectable, virtual bioequivalence assessments, food's impact on bioequivalence, and the success of model-informed drug development under PDUFA VI. Collaboration between regulators and industry is emphasized. |
Applications of Pbbm and Pbpk in Generic Product Development
Dissolution safe space & biowaivers
The concept of a "safe space" in drug product quality and biopharmaceutics defines boundaries, typically based on in vitro dissolution and other quality attributes, within which different variants of a drug product are expected to be bioequivalent. This approach expands dissolution safe space beyond traditional regulatory frameworks like the BCS and In-Vitro-In-Vivo Correlations (IVIVC), in vivo wherein safe space can be established using in vivo clinical studies or through modeling approaches. However, while IVIVCs are considered the gold standard, their success rates, particularly for Immediate-Release (IR) products, remain low due to various challenges. PBBM is emerging as a promising alternative, offering a mechanistic understanding of in vivo drug release and its interaction with physiology, leading to in vitro/in vivo Relationships (IVIVRs) and potentially enabling regulatory flexibility, especially for IR products. Defining a BE safe space with PBBM/PBPK allows the establishment of mechanistic IVIVR to better understand absorption mechanism and Critical Bioavailability Attributes (CBA). Defining a Bioequivalence (BE) safe space is critical for the identification of newer bioequivalent formulations or for setting of clinically relevant in vitro specifications to ensure drug product quality [3,4].
From other perspective of dissolution similarity, over the past decade BCS based biowaivers and lower strength biowaivers has been based on the dissolution data evaluation in QC and multimedia, where f2 similarity factor should be greater than 50. Different regulatory agencies have varying requirements for comparing dissolution profiles between higher and lower strengths of immediate release product. While the USFDA focuses on QC media, the EMA and NMPA require multiple pH conditions (1.2, 4.5, and 6.8). If the f2 value (a measure of similarity) is below 50, it could lead to rejection thereby leading to impediment on grant of the biowaivers of additional strengths. However, with the help of PBPK/PBBM modelling approach can supersede f2 dissimilarity. In cases where absorption is controlled by permeability (e.g., BCS III), f2 may not be relevant. By integrating dissolution and permeability models, bioequivalence can still be demonstrated, extending approval possibilities for BCS and lower strength biowaivers. This approach provides dissolution safe space wherein the dissolution dissimilarity is superseded by the equivalent in vivo performance [3,4, 15-20]. Further, when such modeling approaches are integrated together with dissolution safe space, they can help to set Clinically Relevant Dissolution Specifications (CRDS), define the boundaries of particle size specifications, can enable wider boundaries of dissolution safe space, and hence can support Scale-Up and Post Approval Changes (SUPAC) effectively [21,22].
The concept of dissolution safe space establishment and superseding f2 can be demonstrated through literature example Bhattiprolu AK, et al. [3]. In this example, the formulation was an IR tablet with BCS class III API. Pivotal fasting bioequivalence study was conducted for Market-A and while leveraging the same study to Market-B, f2 dissimilarity was observed between generic product and Market-B reference product. In order to supersede this dissimilarity, PBBM approach has been utilized where dissolution data was inputted into the model using multiple z-factor vs pH. The results indicated that there is no impact of f2 dissimilarity on bioequivalence and thus bioequivalence was established between pivotal test product and Market-B reference product using PBBM approach. Further, dissolution safe space was established wherein the release of 85% up to 60 min can be considered to be bioequivalent to reference product due to permeability driven absorption. This establishment of dissolution safe space has not only enabled biowaivers in the present case but also helped to widen the traditional dissolution criteria for BCS based biowaivers thereby resulting in the possibility of more and more candidates to be eligible for BCS based biowaivers.
Fed be study waivers
In generic formulation development, establishing bioequivalence against the reference product is pivotal for regulatory approval. Companies carefully design formulations, considering factors like patent constraints, BCS classification, formulation composition and expected variability in performance. Pilot studies are conducted to assess formulation behaviour, followed by further optimization if necessary, before pivotal studies. Depending on the product specific bioequivalence guidance, fasting and fed studies as a single dose or multiple doses are conducted to demonstrate bioequivalence. To enhance confidence in bioequivalence outcomes, in silico approaches like PBBM modeling, coupled with bio predictive dissolution media, are gaining traction. Recent discussions within the generic industry and regulatory conferences have emphasized the utility of PBPK modeling in predicting the impact of food on bioequivalence and supporting waivers of fed studies. PBPM modeling has demonstrated its usefulness in simulating food effects on drug absorption, aiding in risk assessment, and predicting bioequivalence results. Such approaches can be utilized in both at new drug development (e.g. to avoid repeat food effect studies due to formulation changes) and at generic product development (e.g. avoidance of fed BE studies). These approaches possess strong ability to integrate all the physiochemical properties, dissolution data together with physiological changes that happens after food intake and thus can predict in vivo scenario more effectively. Importantly, physiological changes resulting after intake of food with different fat and meal content can be effectively simulated thereby mimicking the in vivo scenario accurately. After the development and validation of the model, PBBM can be utilized to obtain fed bioequivalence study waiver as indicated by following example.
Kollipara, et al. [13] demonstrated the utility of PBBM from fed bioequivalence studies perspective. Before development of PBBM, it is essential that a biopredictive media has been identified that can provide insight into in vivo performance. Various factors such as formulation, administration condition (fasting/fed), BCS class, pH vs solubility profile can be considered for development of biopredictive media. For media simulating fed condition, biorelevant media’s such as FeSSIF, or fat containing media’s such as Ensure-Plus, peanut oil, milk based media’s or aqueous media’s with surfactants such as pH 4 with sodium lauryl sulfate can be utilized. Once the predictive ability of such media’s is demonstrated with pilot studies, further data generated in these media’s can be incorporated into PBBM to understand in vivo behavior and to estimate bioequivalence using virtual bioequivalence approach. This example demonstrated two case studies wherein fed bioequivalence studies waiver has been obtained with PBBM approach. In one of the example, the reference formulation consisted of BCS class II API formulated with micronized API in tablet dosage form in order to enhance dissolution rate. The generic product consisted of solid dispersion approach to enhance solubility and dissolution rate. While pivotal fasting bioequivalence study was conducted for Market-A, when the same study was leveraged to Market-B, the agency asked to perform fed bioequivalence study. To support waiver of fed bioequivalence study, PBBM approach was utilized wherein the solubility, dissolution rate and particle size were incorporated into the model. Upon validating the model against pivotal fasting study, virtual bioequivalence approach was utilized wherein fed bioequivalence was demonstrated successfully. Together with modeling, biopharmaceutics risk assessment has been made and considering totality of evidence, agency accepted the approach and granted waiver for fed bioequivalence study. This work signifies the impact of PBBM approach in obtaining complex study waivers for fed bioequivalence and highlights the importance of identification of biopredictive media mimicking fed condition. Together with modeling approach, biopharmaceutics risk assessment can be appropriately considered in order to establish study waiver justification based on “totality of evidence”.
Gender impact on bioequivalence study
Any drug product typically is intended for administration in both genders in order to yield required pharmacological effect. Hence, in general the efficacy of safety of the drug product has to be evaluated in both populations before releasing it into the market. While this aspect of inclusion of female subjects is possible and evident in new drug development in order to establish gender impact, inclusion of female subjects in bioequivalence studies in generic development possess few challenges and thus the bioequivalence studies typically are conducted in male population only.
Because of this aspect, various regulatory bodies, USFDA, EMA and NMPA have been issuing deficiencies for Bioequivalence (BE) studies conducted due to the absence of female subjects. This exclusion of female subjects in BE representation stems from socio-cultural reasons. In the bioequivalence studies conducted for generic products, If the drug is intended to be used in both the genders i.e. male and female, then the subjects should also include both the genders in equal proportions But because of the socio cultural reasons female subjects may not be willing to volunteer in the bioequivalence study because of which regulatory agencies asks queries with respect to extrapolation of BE results in males to both genders Hence alternate approaches such as PBPK modeling can be utilized to address this deficiency.
These approaches aims to address regulatory queries on gender effect by integrating virtual female subjects into the study. The PBPK models can incorporate physiologies of various populations (e.g. male, female, diseased, pediatric, geriatric) and in this case, specifically the physiology of female subjects can be incorporated into the PBPK model to assess pharmacokinetics. The PBPK model then can be simulated to get the exposures in both female and male subjects, which then can be subsequently compared against reported values. By employing PBPK-BM, a mechanistic understanding of the differences between males and females in terms of pharmacokinetics can be gained. These models not only provide a robust framework for such evaluations but also offer valuable insights that can aid in justifying regulatory inquiries. By utilizing these approaches, potential repeat BE studies can be avoided thereby enabling faster approvals, launches and reduced development timelines.
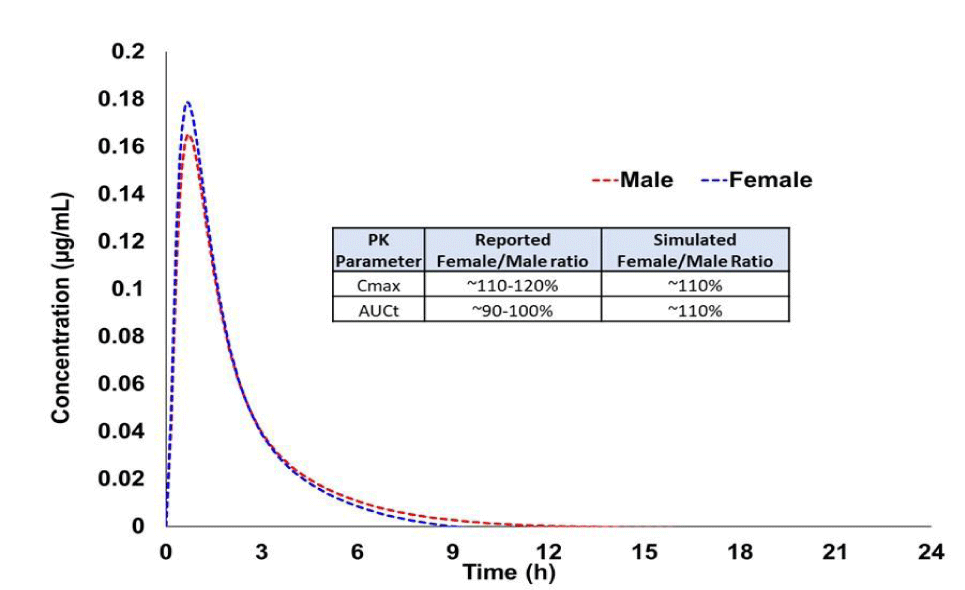
An example of addressing gender impact through modeling is portrayed in Boddu, et al. [5] In this example, PBPK model was developed through incorporation of enzymes, transporters involved and biopharmaceutical properties and physiological properties (Age, weight and BMI). The developed model was validated using the in house pivotal BE study data which was performed in only male subjects and then applied to predict the female subjects PK parameters. Subsequently, PK parameters comparison was made between male and female populations and also compared to against literature reported data (Figure 2). Simulated gender effect is matching with reported data and justification was submitted to agency. This justification of absence of gender impact on bioequivalence using PBPK modeling approach has demonstrated high impact wherein potential clinical study has been avoided. This example also highlights use of appropriate physiology in the model in order simulate specific individual in the modeling approach.
Safety evaluations utilizing pbbm
Evaluation of safety together with efficacy becomes paramount importance and thus should be studied in detail before releasing the drug product into the market. In case of new drug development, both efficacy and safety aspects are weighed equally and studied during phases of clinical trials. In case of generic product development, typically the traditional bioequivalence approaches evaluate the equivalency in the efficacy of drug product against reference product. However, despite being generic product, evaluation of safety aspects is also of paramount importance. While efficacy evaluations have been performed significantly with PBBM approach, understanding the impact of safety is an upcoming area. Safety evaluations due to faster dissolution profiles, different formulation compositions are of importance to evaluate as they can have impact on labelling aspects. Especially in the cases where faster dissolution profiles were observed during stability, it becomes pertinent to evaluate impact of such faster dissolution profiles on adverse events and safety. In such cases, PBBM approach can be utilized to evaluate impact of faster dissolution profiles on safety aspects of drug product. One of such example is presented below:
The work performed by Boddu, et al. [9] elucidates the importance of PBBM in evaluating safety aspects. The drug product has BCS class I API formulated as ER formulation using release controlling excipients. However, the product exhibited faster dissolution profiles during stability and as the API belongs to NTI category, agency asked to evaluate impact of such dissolution profiles on potential safety aspects. To address this, a PBBM model was developed using inputs of physicochemical properties, physiology and dissolution profile with help of Weibull fitting. The model was extensively validated with literature reported IVIVC and also with population simulations of pivotal fasting and fed studies. Upon inputting the faster dissolution profiles into the model, it was observed that the highest Cmax was lower than the reported safety levels and thus no impact on safety was established. This case example clearly highlights other spectrum of applications of PBBM modeling to establish safety. Utilization of modeling approaches in this case has avoided potential safety studies and helped to bring the product to the market as early as possible.
Impact of formulation variables on bioequivalence
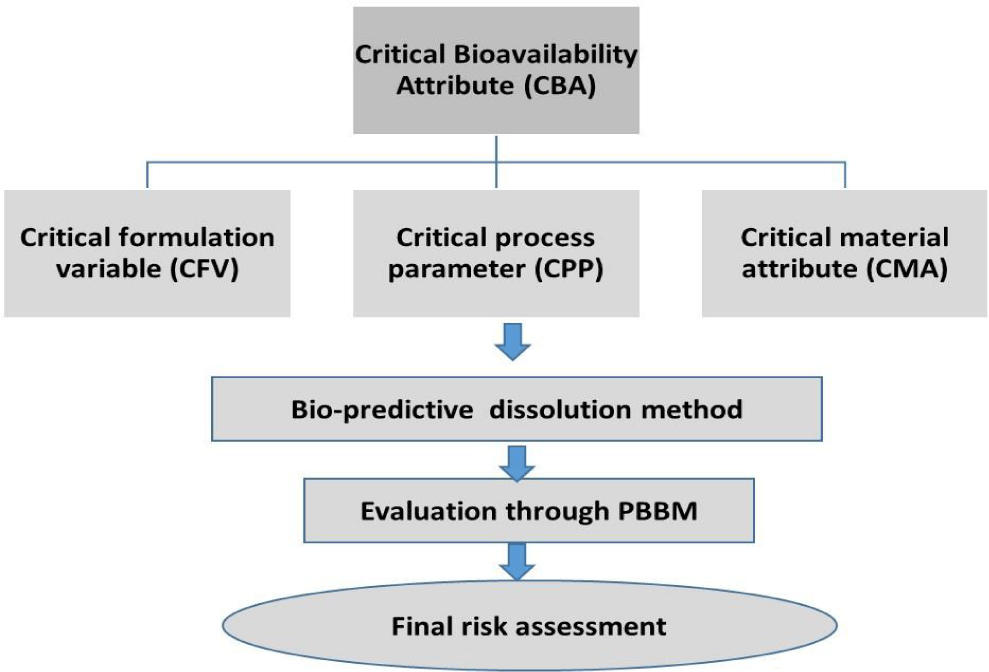
During the process of pharmaceutical development, it is important to evaluate the impact of various formulation and process variables on drug product in vivo performance. These variables are termed as Critical Bioavailability Attributes (CBA) that can have impact on in vivo behavior of the drug product. The CBA’s can be of Critical Material Attributes (CMA), Critical Process Parameters (CPP) and Critical Formulation Variables (CFV) as indicated in figure 3. Understanding the impact of these variables on in vivo performance helps to define ranges of these parameters that can result in consistent in vivo behavior. The aspects of CBA’s evaluation using PBBM approach for efficient biopharmaceutics risk assessment is demonstrated by following example.
Ahmed, et al. [4] proposed biopharmaceutics risk assessment strategy and CBA’s evaluation by extending principles of ICH Q9, Quality risk assessment. In this publication, identification of initial CBA’s (iCBA) based on formulation, API, historical properties was proposed and determination of final CBA using biopredictive dissolution coupled with PBBM was portrayed using an example of extended release formulation. The iCBA’s identified for drug product were % fluid uptake, compression force, polymer 1, 2 amounts and ratio of polymer 1 to polymer 2. The CBA evaluation using PBBM indicated that compression force and polymer levels had significant impact on dissolution rate and thus on in vivo performance. Based on this evaluation, final CBA risk assessment was performed and the appropriate ranges of above mentioned parameters were determined and incorporated into batch manufacturing record to ensure consistent performance. This exercise helped to integrate manufacturing quality with clinical quality in order to come up with formulation that can yield reproducible clinical performance. This approach can also establish ranges of various formulation, process variables that can yield in consistent drug product performance. The ranges of these formulation and process variables can be correlated with that of ranges in batch manufacturing record so that these variables appropriately can cover both clinical and manufacturing quality. This approach embeds significant quality into the drug product thereby providing confidence of its consistent performance in humans.
Conclusions and Future Perspectives
In conclusion, PBBM modeling offers a comprehensive approach to address the complexities of generic drug development, including the food effect, gender variation, and dissolution safe space specifications, biowaivers, safety evaluations and formulation variables impact on in vivo performance. By integrating PBPK modeling into their workflows, generic manufacturers can enhance the efficiency, accuracy, and regulatory compliance of their drug development efforts, ultimately benefiting patients worldwide with safe, effective, and affordable medications. Significant increase in the publications over the years in the area of modeling and simulations indicate the plethora of applications. Such approaches can be deployed during the early stages of development in order to rationalize formulation design so that the formulation yield consistent in vivo performance. Further, such understanding can be summarized and shared in regulatory, academic conferences in order to encourage pharmaceutical and biopharmaceutics scientists to use such approaches in the drug development program. Recent developments in this area such as Model Master File (MMF) helps to share the modeling practices across the sponsor companies in order to enable more generic submissions and approvals with an aim to have more affordable and quality medicines to the patients.
Acknowledgement
The authors would like to thank Dr. Reddy’s Laboratories Ltd., for providing an opportunity to publish this manuscript.
References
- Yuvaneshwari K, Kollipara S, Ahmed T, Chachad S. Applications of PBPK/PBBM modeling in generic product development: An industry perspective. J Drug Del Sci Technol. 2022;69:103152. doi: 10.1016/j.jddst.2022.103152.
- Aishwarya R, Murthy A, Ahmed T, Chachad S. A Novel Approach to Justify Dissolution Differences in an Extended Release Drug Product using Physiologically Based Biopharmaceutics Modeling and Simulation. J Pharm Sci. 2022 Jun;111(6):1820-1832. doi: 10.1016/j.xphs.2022.02.007. Epub 2022 Feb 23. PMID: 35217007.
- Bhattiprolu AK, Kollipara S, Ahmed T, Boddu R, Chachad S. Utility of Physiologically Based Biopharmaceutics Modeling (PBBM) in Regulatory Perspective: Application to Supersede f2, Enabling Biowaivers & Creation of Dissolution Safe Space. J Pharm Sci. 2022 Dec;111(12):3397-3410. doi: 10.1016/j.xphs.2022.09.003. Epub 2022 Sep 9. PMID: 36096285.
- Ahmed T, Kollipara S, Boddu R, Bhattiprolu AK. Biopharmaceutics Risk Assessment-Connecting Critical Bioavailability Attributes with In Vitro, In Vivo Properties and Physiologically Based Biopharmaceutics Modeling to Enable Generic Regulatory Submissions. AAPS J. 2023 Jul 27;25(5):77. doi: 10.1208/s12248-023-00837-y. PMID: 37498474.
- Boddu R, Kollipara S, Vijaywargi G, Ahmed T. Power of integrating PBPK with PBBM (PBPK-BM): a single model predicting food effect, gender impact, drug-drug interactions and bioequivalence in fasting & fed conditions. Xenobiotica. 2023 Apr;53(4):260-278. doi: 10.1080/00498254.2023.2238048. Epub 2023 Jul 25. PMID: 37471259.
- Wu D, Sanghavi M, Kollipara S, Ahmed T, Saini AK, Heimbach T. Physiologically Based Pharmacokinetics Modeling in Biopharmaceutics: Case Studies for Establishing the Bioequivalence Safe Space for Innovator and Generic Drugs. Pharm Res. 2023 Feb;40(2):337-357. doi: 10.1007/s11095-022-03319-6. Epub 2022 Jul 15. PMID: 35840856.
- Physiologically based pharmacokinetic analyses- Format and content guidance for industry. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). 2018.
- EMA Guideline on the reporting of physiologically based pharmacokinetic (PBPK) modelling and simulation. 2018.
- Boddu R, Kollipara S, Bhattiprolu AK, Ahmed T. Novel application of PBBM to justify impact of faster dissolution on safety and pharmacokinetics - a case study and utility in regulatory justifications. Xenobiotica. 2023 Oct-Nov;53(10-11):587-602. doi: 10.1080/00498254.2023.2289160. Epub 2023 Dec 19. PMID: 38062540.
- Wu F, Mousa Y, Jereb R. et al. Using Mechanistic Modeling Approaches to Support Bioequivalence Assessments for Oral Products. AAPS J. 2024;26:19. https://doi.org/10.1208/s12248-024-00886-x.
- Heimbach T, Kesisoglou F, Novakovic J, Tistaert C, Mueller-Zsigmondy M, Kollipara S, Ahmed T, Mitra A, Suarez-Sharp S. Establishing the Bioequivalence Safe Space for Immediate-Release Oral Dosage Forms using Physiologically Based Biopharmaceutics Modeling (PBBM): Case Studies. J Pharm Sci. 2021 Dec;110(12):3896-3906. doi: 10.1016/j.xphs.2021.09.017. Epub 2021 Sep 20. PMID: 34551349.
- Jaiswal S, Ahmed T, Kollipara S, Bhargava M, Chachad S. Development, validation and application of physiologically based biopharmaceutics model to justify the change in dissolution specifications for DRL ABC extended release tablets. Drug Dev Ind Pharm. 2021 May;47(5):778-789. doi: 10.1080/03639045.2021.1934870. Epub 2021 Jun 8. PMID: 34082622.
- Kollipara S, Martins FS, Sanghavi M, Santos GML, Saini A, Ahmed T. Role of Physiologically Based Biopharmaceutics Modeling (PBBM) in Fed Bioequivalence Study Waivers: Regulatory Outlook, Case Studies and Future Perspectives. J Pharm Sci. 2024 Feb;113(2):345-358. doi: 10.1016/j.xphs.2023.11.030. Epub 2023 Dec 1. PMID: 38043684.
- Kollipara S, Ahmed T, Bhattiprolu AK, Chachad S. In vitro and In silico biopharmaceutic regulatory guidelines for generic bioequivalence for oral products: Comparison among various regulatory agencies. Biopharmaceutics & Drug Disposition. 2021;42. doi: 10.1002/bdd.2292.
- Kollipara S, Bhattiprolu AK, Boddu R, Ahmed T, Chachad S. Best Practices for Integration of Dissolution Data into Physiologically Based Biopharmaceutics Models (PBBM): A Biopharmaceutics Modeling Scientist Perspective. AAPS PharmSciTech. 2023 Feb 9;24(2):59. doi: 10.1208/s12249-023-02521-y. PMID: 36759492.
- Karnati P, Murthy A, Gundeti M, Ahmed T. Modelling Based Approaches to Support Generic Drug Regulatory Submissions-Practical Considerations and Case Studies. AAPS J. 2023 Jun 23;25(4):63. doi: 10.1208/s12248-023-00831-4. PMID: 37353655.
- Tsakalozou E, Fang L, Bi Y, van den Heuvel M, Ahmed T, Tsang YC, Lionberger R, Rostami-Hodjegan A, Zhao L. Experience Learned and Perspectives on Using Model-Integrated Evidence in the Regulatory Context for Generic Drug Products-a Meeting Report. AAPS J. 2024 Jan 10;26(1):14. doi: 10.1208/s12248-023-00884-5. PMID: 38200397.
- Kollipara S, Boddu R, Ahmed T, Chachad S. Simplified Model-Dependent and Model-Independent Approaches for Dissolution Profile Comparison for Oral Products: Regulatory Perspective for Generic Product Development. AAPS PharmSciTech. 2022 Jan 13;23(1):53. doi: 10.1208/s12249-021-02203-7. PMID: 35028797.
- USFDA guidelines on dissolution testing of immediate release solid oral dosage forms. 1997.
- EMA guidelines on guideline on the Investigation of Bioequivalence. 2010
- USFDA guidelines on extended release oral dosage forms: Development, evaluation, and application of in vitro/in vivo correlations. 1997.
- EMA guidelines on the pharmacokinetic and clinical evaluation of modified release dosage forms. 2014.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.