Medicine Group. 2024 February 29;5(2):227-232. doi: 10.37871/jbres1886.
Antibacterial Effect of Indian Gooseberry (Phyllanthus emblica L.) Extract Against ESBL Producing Multi-Drug Resistant Bacteria
Vemula Sarojamma1, Eedlapalli Siddhartha2 and Ramakrishna Vadde2*
2Department of Biotechnology & Bioinformatics, Yogi Vemana University, Kadapa – 51600, Andhra Pradesh, India
- Indian gooseberry
- Amla
- Antioxidant potential
- Antimicrobial activity
- Multidrug resistant bacteria
Abstract
The most celebrating fruit in Indian traditional medicine Ayurveda is the Indian gooseberry also known as amla fruit, used the prevention of numerous diseases. No reports are available on the prevention of ESBL produced MDR bacteria has lead us to investigate amla’s bioactive compounds (total phenolics, flavonoids, tannins) and their antioxidant and antibacterial activities against MDR bacteria (E coli, P aeruginosa, S aureus, K pneumoniae, H influenza, S typhi). Higher levels of phenolics with elevated levels of antioxidant activities were noticed in amla extracts and also scavenged all types of free radicals including DPPH, ABTS, -OH, H2O2, and superoxide radicals at lowest concentrations. Disc diffusion method was used for determination of antimicrobial activities of extracts and seen temperate growth inhibition against MDR bacteria as compared to control drug, cefotaxime. The results concluded that Indian gooseberry inhibited the growth of ESBL produced MDR bacteria with high levels of phenolics and their antioxidant potential.
Introduction
β-Lactam antimicrobial agents are the routinely used in prevention of bacterial infections. The persistent exposure of bacteria to β-lactam agents induces active and continuous production and mutation of β- lactamases in bacteria, and further expand their activity even against newly developed β-lactam antibiotics. These enzymes are known as extended-spectrum β-lactamases (ESBLs) [1,2]. These enzymes in bacteria hydrolyses the β-lactam antibiotics by opening of the β-lactam ring, making antibiotics inactive. In recent years, ESBL-producing Multidrug Resistant (MDR) organisms are becoming a problematic cause of infections worldwide [3]. Recent surveys notices a significant increase in the ESBL incidence rate from all parts of the world including our previous study [1,4-6]. In spite of large number of antibiotics available in pharmaceutical market, their usage was restricted due to drug resistance among bacteria, and also toxic side effects possessed by many of prescribed antimicrobials, warranting a search for novel antimicrobial compounds that could significantly overcome these drawbacks. In recent years, traditional medicines gained special interest all around world because of safe use and also an importance resource for discovery of new antibiotics [7,8]. Numerous studies reported traditional remedies are highly effective against these MDR bacteria [9,10].
Indian gooseberry (Phyllanthus emblica Linn.) is one of the routinely used fruit in local traditional medicine Ayurveda (India), Traditional Chinese Medicine, and Tibetan medicine. The fruit has been reported to possess several pharmacological properties, such as analgesic, antioxidant, anti-inflammatory, antifungal, antibacterial, anticancer, antiviral activities and chemo protective activities [11-15]. To reduce side effects of synthetic antibiotics usage in treatment of MDR diseases, the common culinary herbs that exhibit antimicrobial activity could be a source of natural drugs. Although some authors reported that Indian gooseberry contains potent antimicrobials and antioxidants, there is no report on the antimicrobial activities especially against MDR bacteria isolated from clinical samples. Thus, the present study was designed to assess the levels of bioactive compounds, antioxidants of amla and evaluating their antimicrobial activities against multidrug resistant bacteria.
Materials and Methods
Preparation of amla extracts
Fresh amla fruits (Phyllanthus emblica L.) collected from botanical garden of Yogi Vemana University campus, Kadapa, Andhra Pradesh, India and voucher specimen with a number YVUH-1990, have been deposited at the Herbarium, Yogi Vemana University, Kadapa – 516003, A.P., India. The seed and pulp part of amla fruit were separated, air-dried at room temperature and ground into a fine powder in a blender. Amla seed or pulp powder was extracted in 80 % ethanol by vortexing for 2 minutes and incubating at 40C overnight in a shaking incubator. After incubation, the extracts were collected, filtered, and concentrated in vacuum under reduced pressure by using rota evaporator (Heidolph rotary evaporator, Germany). Concentrated extracts were obtained, aliquoted and stored at -800C until use.
Determination of bioactive compounds (total phenolics, flavonoids, and tannins)
The bioactive compounds of amla were determined by using Folin-Ciocalteu method for total phenolics, Aluminium chloride method for flavonoids and Vanillin-HCl method for tannins as previously described [16,17].
Determination of antioxidant activities of amla
Antioxidant activity of ethanol extracts of amla were quantified through free radical scavenging assays as described previously [17]. The free radicals used in the study includes DPPH (2,2-diphenyl-1-picrylhydrazyl), ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid), hydrogen peroxide (H2O2), hydroxyl (-OH), and superoxide (O-2). Free radical scavenging activities evaluated in % of inhibition and expressed in IC50 values.
Determination of antibacterial activity
Bacterial strains and clinical isolates: All ESBL producing multidrug resistant bacterial isolates (Escherichia coli, Klebsiella pneumoniae, staphylococcus aureus, Salmonella typhi, Pseudomonas aeruginosa and Proteaus vulgaris) were obtained from tertiary care hospital after ESBL screening and confirmation performed by modified Kirby Bauer’s disc diffusion method as described previously [5]. Cefotaxime, ceftazidime, ceftriaxone discs were used for screening ESBLs production according to NCCLS guidelines [18].
Preparation of inoculum: Loop full of 24 h bacteria culture was taken and mixed in 5 ml of nutrient broth. Tube was incubated in a shaking incubator until culture reaches the 0.5 McFarland units which is equal to bacterial concentration 1.5x108 CFU.
Antimicrobial activity
Bacterial culture spread over the Muller-Hinton agar plate with cotton swab. Whatman No.1 filter paper discs of size (6 mm) were placed at four corners of petri dishes and dropped 20 µl amla extract over discs. The entire set up was incubated at 37 0C for 24 h, the Zone of Inhibition (ZOI) was measured and results expressed in cm. Cefotaxime (30 μg/disc) was used as the standard antibiotic.
Statistical analysis
Each experiment was carried out five times. All results depicted in figures and tables are in Mean ± Standard error. Statistical significance was determined by using one-way ANOVA post-hoc multiple comparisons from Duncan Multiple Range (DMR) Test at a significance level of p ≤ 0.05.
Results and Discussion
High-dose and prolonged antimicrobial therapy and drug resistance in bacteria decimates beneficial commensals and predisposing to pathogen invasion [19]. Natural bioactive compounds or nutrients isolated from plant sources are possessing antioxidant, antibacterial, preventative and nutritive values could be used as safe antimicrobials in the suppression of invasive pathogens [20]. In this connection, ethanolic extracts of amla are used in present study for evaluation of bioactive compound composition (total phenolics, flavonoids, tannins) and their antioxidant property with free radical scavenging activities along with antimicrobial activities against ESBL produced MDR bacteria.
Bioactive rich amla having potential antioxidant property
Indian gooseberry fresh fruits were extracted with 80% ethanol and found significant levels of bioactive compounds and represents a promising source of human health promoting agents. The results were depicted in (Table 1). Amla pulp comprises of high levels of total phenolic compounds (85 ± 6.8 mg GAE/g) as compared to seed (67 ± 4 mg GAE/g) in fresh amla fruits. Total phenolics have been proved to be responsible for the antioxidant and antimicrobial activity of amla fruit [9,13,21]. The amounts of flavonoids from 6 to 10 QE/gm and tannins from 8 to 9 mg/g, respectively were observed. These finding are in agreement with previously published data reporting higher levels of polyphenols, and flavonoids responsible antioxidant and antibacterial activity [13,22,23]. Antioxidant potential of Amla extracts were evaluated by determining the DPPH, ABTS, Hydrogen peroxide, Hydroxyl and Superoxide free radical scavenging assays and the results were depicted in (Table 1). Amla extracts exhibited DPPH and ABTS free radical scavenging properties at lowest IC50 concentrations indicating highest antioxidant potential as compared to standard antioxidants ascorbic acid (Vitamin C)/BHT. The results in table 1 indicating the higher values of DPPH, ABTS, OH and H2O2 free radical scavenging activity in amla seed with superoxide radical scavenging activities higher in amla pulp region. Based on these results amla extracts are good source of free radical scavengers. These results are in agreement with previous studies showing presence of emblicanins and rutin which favors for ascorbic acid formation [13]. It is identified that the amla extracts exhibited stronger antioxidant activity because of higher phenolics, and tannins, which could be acted as antioxidant agents. The results reveal that the amla is possessing excessive antioxidant potential in suppressing highly toxic free radicals (Table 1). Fresh amla pulp is having high superoxide radical scavenging activity compared to seed. Phenolic compounds of amla enhances overall antioxidant activities by the mechanisms of free radical inactivation and supressing hydroperoxides decomposition into free radicals. Correlations with free radical scavenging activity (antioxidant activity) with phenolic content of amla extracts exhibited a significant correlations indicating phenolics are responsible for antioxidant properties. In connection, Kumar GS, et al. [9] observed in his studies that phenolic fraction is the major antioxidant compounds in amla. The superoxide generates dangerous hydroxyl radicals as well as singlet oxygen, both of which contribute to the oxidative stress [24]. These free radical activity was effectively suppressed by amla and its bioactive compounds. These antioxidant properties of amla may contribute the pharmaceutical properties to cure various diseases.
| Table 1: Bioactive compounds and their antioxidant potential of amla extracts. | ||||||||
| Plant extracts | Bioactive compounds (mg/gm of amla extract) |
Free radical scavenging properties (IC50 in µg GAE/ml) |
||||||
| Total phenolics | Flavanoids | Tannins | DPPH | ABTS | -OH | H2O2 | Superoxide | |
| Amla Seed | 85 ± 6.8a | 6.2 ± 0.43a | 7.8 ± 1.3a | 70 ± 5a | 62 ± 4a | 80 ± 5.8a | 52 ± 2a | 65 ± 7.2a |
| Amla Pulp | 67 ± 4b | 10 ± 2.4b | 9.0 ± 1.6b | 15 ± 1b | 55 ± 6b | 45 ± 3.8b | 38±4b | 98 ± 8.3b |
| Standard antioxidant | - | - | - | 83 ± 6c Vit C |
78 ± 6c Trolox |
68 ± 5.8c Mannitol |
64 ± 5c Vit C |
48 ± 3.2c BHT |
| The data represented as mean ± SE for five independent determinations and the mean value bearing different alphabets (shown in superscript) in the same column are significantly different at p < 0.05 according to DMR test. | ||||||||
Amla suppresses the growth of ESBL produced MDR bacteria
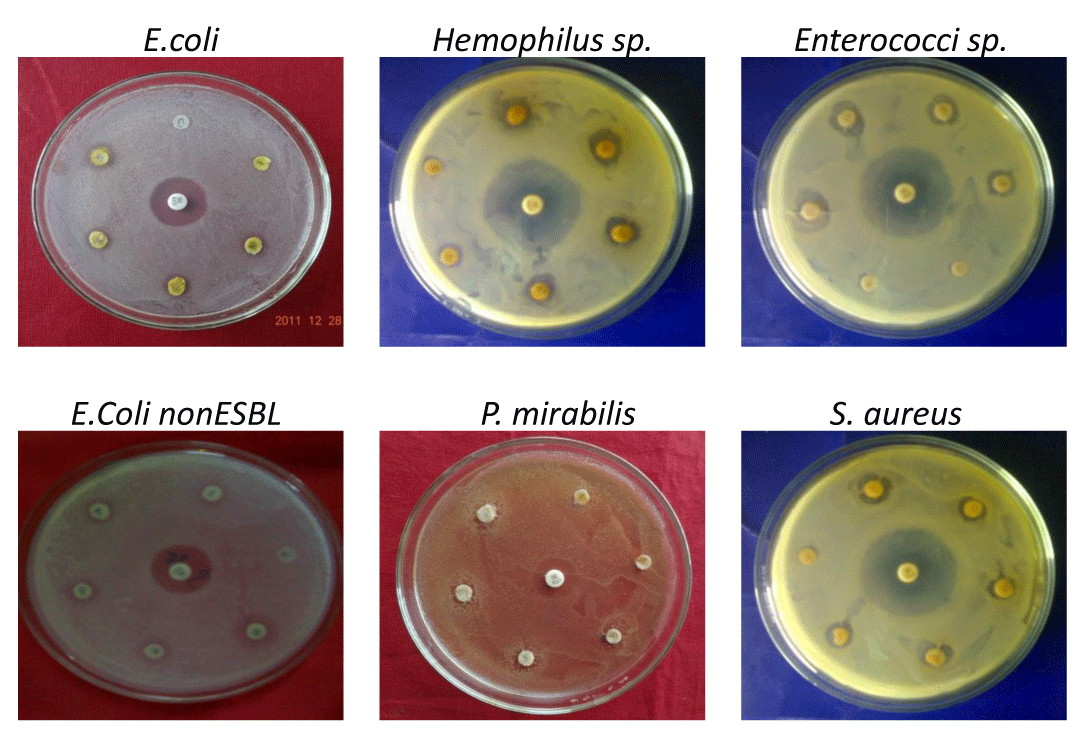
The antimicrobial activity of the amla extracts against ESBL producing multidrug resistant bacteria was examined qualitatively by the presence or absence of bacterial growth in terms of zone inhibition determined through disc diffusion method. ESBL positive strains of E. coli, Klebsiella pneumoniae, Staphylococcus aureus, Salmonella typhi, Pseudomonas aeruginosa, Hemophilus sp., Enterococci sp., and Proteaus vulgaris were used in this study. Results from the antimicrobial disc diffusion method are summarized in figure 1 and table 2, respectively. Most of the extracts showed good inhibitory activity against all ESBL producing multidrug resistant bacteria which were isolated from clinical samples of tertiary care hospital. The concentration of extract placed on the disc in terms of total phenolics at the concentration of 25 and 50 µg GAE, however, the results exhibited highest activities at 50 µg GAE/disc. Amla seed extract is having high inhibitory activity as compared to pulp extract due to higher levels of bioactive compounds and their antioxidant properties of amla seed. The zone of inhibition of bacterial growth in the presence of amla extracts is 40 – 70% of cefotaxime (positive control antibiotic) zone of inhibition indicating the amla comprising of effective bioactive compounds which inhibits the bacterial growth as similar to standard antibiotic drug. Recently, similar results were reported in amla with efficient antioxidant properties with enhanced antibacterial activity due to highest phenolic content [25-28]. These results reveal that the amla extract is having moderate antibacterial activity and can be used in therapeutics to cure bacterial infectious diseases. Further it is observed that the naturally antibiotics containing extracts like amla are as potential as chemical synthesized standard antibiotic drugs in inhibiting bacterial growth and main advantage is not developing any type of drug resistance with safe use.
| Table 2: Antibacterial activity against ESBL producing multidrug resistant bacteria. | |||||
| Bacteria | Diameter of Zone of inhibition in cm | Control Cefotaxime (30 μg/disc) |
|||
| Amla seed | Amla pulp | ||||
| 25 µg | 50 µg | 25 µg | 50 µg | ||
| E. coli (Non ESBL) | 0.55 | 0.95a | 0.5 | 0.78b | 1.5c |
| E. coli | 0.5 | 0.8a | 0.5 | 0.65b | 1.2c |
| Klebsiella pneumoniae | 0.5 | 0.81a | 0.5 | 0.78a | 2.0b |
| P aeruginosa | 0.5 | 0.79a | 0.5 | 0.65b | 1.5c |
| Staphylococcus aureus | 0.5 | 0.88a | 0.5 | 0.61b | 1.2c |
| Haemophilous sp. | 0.5 | 0.79a | 0.5 | 0.74a | 2.0c |
| Proteus mirabilis | 0.5 | 1.04a | 0.5 | 0.84b | 1.8c |
| Enterococci sp. | 0.5 | 0.7a | 0.5 | 0.72a | 1.8b |
| Salmonella typhi | 0.5 | 0.95a | 0.5 | 0.65b | 1.5c |
| The data represented as mean of five independent determinations and the mean value bearing different alphabets (shown in superscript) at 50 µg GAE of amla seed and amla pulp in the same row are significantly different at p < 0.05 according to DMR test. | |||||
Conclusion
The results conclude that amla fruits indeed comprises of high levels of phenolics displaying antioxidant potential at lowest concentrations which will further exhibited good antibacterial activity against all ESBL producing multidrug resistant bacteria. This study indicate that the Indian gooseberry is having beneficial biochemical characteristics and can be used in treatment of various diseases. The study proposes amla can be used in therapeutics to cure bacterial infectious diseases as a good source of natural antimicrobial compound.
Conflicts of Interest
Authors declare that no conflicts of interest.
References
- Gupta V. An update on newer beta-lactamases. Indian J Med Res. 2007 Nov;126(5):417-27. PMID: 18160745.
- Husna A, Rahman MM, Badruzzaman ATM, Sikder MH, Islam MR, Rahman MT, Alam J, Ashour HM. Extended-Spectrum β-Lactamases (ESBL): Challenges and Opportunities. Biomedicines. 2023 Oct 30;11(11):2937. doi: 10.3390/biomedicines11112937. PMID: 38001938; PMCID: PMC10669213.
- Boyle DP, Zembower TR. Epidemiology and Management of Emerging Drug-Resistant Gram-Negative Bacteria: Extended-Spectrum β-Lactamases and Beyond. Urol Clin North Am. 2015 Nov;42(4):493-505. doi: 10.1016/j.ucl.2015.05.005. Epub 2015 Jul 15. PMID: 26475946.
- Colodner R. Extended-spectrum beta-lactamases: a challenge for clinical microbiologists and infection control specialists. Am J Infect Control. 2005 Mar;33(2):104-7. doi: 10.1016/j.ajic.2004.07.010. PMID: 15761410.
- Sarojamma V, Ramakrishna V. Prevalence of ESBL-Producing Klebsiella pneumoniae Isolates in Tertiary Care Hospital. ISRN Microbiol. 2011 Dec 1;2011:318348. doi: 10.5402/2011/318348. PMID: 23724303; PMCID: PMC3658478.
- McDonald KL, Garland S, Carson CA, Gibbens K, Parmley EJ, Finley R, MacKinnon MC. Measures used to assess the burden of ESBL-producing Escherichia coli infections in humans: a scoping review. JAC Antimicrob Resist. 2021 Feb 14;3(1):dlaa104. doi: 10.1093/jacamr/dlaa104. PMID: 34223063; PMCID: PMC8210151.
- Gorlenko CL, Kiselev HY, Budanova EV, Zamyatnin AA Jr, Ikryannikova LN. Plant Secondary Metabolites in the Battle of Drugs and Drug-Resistant Bacteria: New Heroes or Worse Clones of Antibiotics? Antibiotics (Basel). 2020 Apr 10;9(4):170. doi: 10.3390/antibiotics9040170. PMID: 32290036; PMCID: PMC7235868.
- Antimicrobial resistance. WHO. 2023.
- Kumar GS, Harish N, Shylaja M. Dharmesh P, Salimath V. Free and bound phenolic antioxidants in amla (Emblica officinalis) and turmeric (Curcuma longa). Journal of Food Composition and Analysis. 2006;19(5): 446-452. doi: 10.1016/j.jfca.2005.12.015.
- Ekamgue B, Mbaveng AT, Kuete V. Anti-staphylococcal and antibiotic-potentiating activities of botanicals from nine Cameroonian food plants towards multidrug-resistant phenotypes. Investigational Medicinal Chemistry and Pharmacology. 2023;6(1):75. doi: 10.31183/imcp.2023.00075.
- Khatoon S, Rai V, Rawat AK, Mehrotra S. Comparative pharmacognostic studies of three Phyllanthus species. J Ethnopharmacol. 2006 Mar 8;104(1-2):79-86. doi: 10.1016/j.jep.2005.08.048. Epub 2005 Oct 19. PMID: 16236476.
- Ramakrishna V, Gopi S, Setty OH. Indian Gooseberry (Phyllanthus emblica L.)- Phytochemistry, Pharmacology, and Therapeutics. In: Medicinal plants phytochemistry pharmacology and therapeutics. Vol. 2. Ed Vijay Gupta. Daya Publishers. New Delhi. 2011;p.19-40.
- Ramakrishna Vadde, Sridhar Radhakrishnan, Kurundu HEK, Lavanya Reddivari, Jairam KP Vanamala. Indian gooseberry (Emblica officinalis Gaertn.) suppresses cell proliferation and induces apoptosis in human colon cancer stem cells independent of p53 status via suppression of c-Myc and cyclin D1. Journal of Functional Foods. 2016;25:267-278.
- Singh GP, S Jain A, B Sridhara Setty P, Bv S, S Patil S, P A, Ts G, Suresh KP, Dugganaboyana GK, Murugesan K, Gnanasekaran A, Shivamallu C, Kollur SP, Srinivasa C, Hl R, Rudrapathy P, M Basalingappa K. Antimicrobial, antioxidant and anti-inflammatory activities of seeds from emblica officinalis (Gaertn.). Bioinformation. 2022;18(8):683-691.
- Yashika Gandhi, Jyotika Grewal, Vipin Jain, Hemant Rawat, Sujeet K. Mishra, Vijay Kumar, Ravi Kumar, Santosh Kumar Shakya, Preeti Sharma, Daljeet Singh Dhanjal, Shyam Baboo Prasad, Vaibhav Charde, J.C. Arya, Ch.Venkata Narasimhaji, Arjun Singh, Ravindra Singh, Naryanam Srikanth, Rabinarayan Acharya. Emblica officinalis: A promising herb confining versatile applications. South African Journal of Botany. 2023;159:519-531, doi: 10.1016/j.sajb.2023.06.041.
- Siddhartha E, Sarojamma V, Ramakrishna V. Bioactive compound rich Indian spices suppresses the growth of â-lactamase produced multidrug resistant bacteria. JKIMSU. 2017;6(1):P 10-24.
- Suresh Mallepalli, Ramakrishna Vadde. Bioactive compounds and their antioxidant activities of Ficus religiosa extract. Advances in Bioresearch. 2021;12(1):10-18. doi: 10.15515/abr.0976-4585.12.1.1018.
- Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved Standard M7-A5 and informational Supplement M100-S10. Wayne, PA: National Committee for Clinical Laboratory Standards; 2000.
- Ramirez J, Guarner F, Bustos Fernandez L, Maruy A, Sdepanian VL, Cohen H. Antibiotics as Major Disruptors of Gut Microbiota. Front Cell Infect Microbiol. 2020 Nov 24;10:572912. doi: 10.3389/fcimb.2020.572912. PMID: 33330122; PMCID: PMC7732679.
- Vaou N, Stavropoulou E, Voidarou C, Tsigalou C, Bezirtzoglou E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms. 2021 Sep 27;9(10):2041. doi: 10.3390/microorganisms9102041. PMID: 34683362; PMCID: PMC8541629.
- Anila L, Vijayalakshmi NR. Flavonoids from Emblica officinalis and Mangifera indica-effectiveness for dyslipidemia. J Ethnopharmacol. 2002 Jan;79(1):81-7. doi: 10.1016/s0378-8741(01)00361-0. PMID: 11744299.
- Mayachiew Pornpimon, Sakamon Devahastin. Antimicrobial and antioxidant activities of Indian gooseberry and galangal extracts, LWT - Food Science and Technology. 2008;41(7):1153-1159. doi: 10.1016/j.lwt.2007.07.019.
- Efenberger-Szmechtyk M, Nowak A, Czyzowska A. Plant extracts rich in polyphenols: antibacterial agents and natural preservatives for meat and meat products. Crit Rev Food Sci Nutr. 2021;61(1):149-178. doi: 10.1080/10408398.2020.1722060. Epub 2020 Feb 11. PMID: 32043360.
- Pallavi Sharma, Ambuj Bhushan Jha, Rama Shanker Dubey, Mohammad Pessarakli. Reactive oxygen species, oxidative damage, and antoxidative defense mechanism in plants under stressful conditions. Journal of Botany. 2012;26. doi: 10.1155/2012/217037.
- Sawhney R, Hazarika P, Sawhney A. Medicinal Plants as Antibacterial Agents: A Special Reference to Emblica Officinalis, Ocimum Sanctum, Aloe Vera and Aegle Marmelos. Adv. Pharm. J, 2021; 6(5), 121-130. doi: 10.31024/apj.2021.6.5.1.
- Saini R, Kumar V, Patel CN, Sourirajan A, Dev K. Synergistic antibacterial activity of Phyllanthus emblica fruits and its phytocompounds with ampicillin: a computational and experimental study. Naunyn Schmiedebergs Arch Pharmacol. 2024 Feb;397(2):857-871. doi: 10.1007/s00210-023-02624-0. Epub 2023 Jul 31. PMID: 37522914.
- Sharma P, Juhi, Halwai V, Rout S, Singh R. Antibacterial Activity of Selected Fruit Juices against Multidrug-Resistant Bacterial Pathogens Involved in Urinary Tract and Sexually Transmitted Infections among Tribal Women in Madhya Pradesh, India. J Pharmacopuncture. 2023 Sep 30;26(3):265-275. doi: 10.3831/KPI.2023.26.3.265. PMID: 37799616; PMCID: PMC10547819.
- Saeed S, Tariq P. Antibacterial activities of Emblica officinalis and Coriandrum sativum against Gram negative urinary pathogens. Pak J Pharm Sci. 2007 Jan;20(1):32-5. PMID: 17337425.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.