Biology Group . 2023 April 04;4(4):600-609. doi: 10.37871/jbres1715.
Inter-Relationship between Malaria, Probiotic Food Intake and Gut Microbiota Status among Malaria Patients
Achu Jordan Awah-Nanzdum1, Theresia Njuabe Metoh1*, Mabel Kaghou Mbifung1, Chi Tchampo Fru1, Achille Chi Djouosseu1, Nina Ghislain Yensii1, Ndi Bertrand Bongjo2, Marthe Ngo-Matip3 and Carl Moses Mbofung4
2Centre for Food Technology and Research, Department of Chemistry, Benue State University-Makurdi, Nigeria
3National Polytechnique, Douala and Spirulina Producer’s Center Nomayos, Yaounde, Cameroon
4Department of Food and Bioresource Technology, COLTECH, The University of Bamenda, Cameroon
- Artemether-lumefantrine
- Arthrospira platensis
- Gut microbiota
- >Parasite density
- >Plasmodium falciparum
Abstract
Plasmodium infection results in clinical presentations that range from symptomatic to severe malaria, resulting in about 500,000 deaths annually worldwide. Artemisinin-based Combination Therapies (ACTs) has largely been responsible for the significant reduction in malaria-related mortality in tropical and sub-tropical regions. However, this progress is seriously threatened by the reduced clinical efficacy of artemisinins, which is characterized by delayed parasitic clearance and high rate of recrudescence. In order to evaluate the synergistic effect of probiotics and antimalarial drug, in the treatment of malaria, the combination of artemether-lumefantrine and arthrospira platenis was administered to malaria patients and the gut microbiota and malaria parasite burden assessed during seven days follow-up. Of 313 subjects aged 2 to 18 years screened for malaria parasites, 75 participants were eligible to participate in this study. These participants were randomized and assigned to 3 groups to receive either the combination of Artemether-Lumefantrine and Arthrospira platensis (AL + AP), n = 25), designated as malaria positive group1 or Artemether-Lumefantrine (AL, n = 25), labeled malaria positive group 2 or to receive no antimalarial drug for malaria negative participants (n = 25, group 3). Fecal samples were collected on day 0 pre-treatment from all study participants and days 3 and 7 post-treatment from malaria positive patients for culture analysis and identification of gut microbiota communities. There was a significant change in the number of bacteria communities among treatment groups on days 3 and 7 post-treatment. Results from fecal sample culture analysis showed that E. coli was relatively abundant on day 3 (9 × 104 CFU/ml) and day 7 (9 × 104 CFU/g) as compared to day 0 (7 × 104 CFU/ml), Klebsiella was relatively abundant on day 3 (6 × 104 CFU/g) and day 7 (6 × 104 CFU/g) as compared to day 0 (5 × 104 CFU/ml), Pseudomonas was relatively abundant on day 3 (5 × 104 CFU/g) as compared to day 0 (4 × 104 CFU/ml) and day 7 (4 × 104 CFU/ml), Enterococci faecalis was relatively abundant on day 0 (5 × 104 CFU/ml) as compared to day 3 (3 × 104 CFU/ml) and day 7 (4 × 104 CFU/ml). In all treatment groups, the results showed that administration of arthemether-lumefantrine and Arthrospira to malaria positive subjects had a significant change on gut bacteria community on day 3 and day 7 post-treatment. In addition, malaria infected patients administered clinically relevant doses of artemether-lumefantrine and Arthrospira platensis had a significantly lower average parasitaemia on day 1 and day 2 as compared to malaria infected patients administered artemether-lumefantrine only. Therefore, the combination of artemether-lumefantrine and Arthrospira platensis in the treatment of malaria modifies the gut bacteria community which intend modulates malaria severity rapidly than artemether-lumefantrine alone. Collectively, these results identify the treatment of malaria with artemether-lumefantrine and Arthrospira platensis as potential treatment to decrease parasite burden.
Abbreviations
Background
Malaria is a disease transmitted by the female Anopheles mosquito carrying the Plasmodium parasite [1]. Malaria remains one of the most prevalent infectious diseases globally and in 2021, the World Health Organization (WHO) estimated that malaria still causes about 247 million cases and 619,000 deaths globally with children below 5 years and pregnant women being the most vulnerable group to the disease [2]. Despite substantial efforts to control this disease in the last few decades, the emerging resistance of Plasmodium species to currently available drugs remain a public health concern [1]. In Cameroon, malaria is a wide spread endemic disease with Plasmodium falciparum being the predominant parasite specie and Anopheles gambiae as the primary vector responsible for transmission [1,3]. Artemisinin-Based Combination Therapies (ACTs) is currently recommended by the WHO as the first line treatment for patients infected with uncomplicated Plasmodium falciparum as artemisinin can rapidly reduce the level of parasites in blood [2]. Artemether-Lumefantrine (AL) is one of the approved and most successful fixed dose ACT used in the treatment of uncomplicated P. falciparum malaria [4,5]. However, resistance to this drug was detected in a number of countries [6] and there is a concern that this resistance will spread to other countries which would pose a major health problem because no other antimalarial with an equivalent efficacy have been found as of now. In order to mitigate this effect, prevention and control strategies have focused on vector control through the use of Insecticide Treated Bed Nets (ITNs), Indoor Residual Spraying (IRS), and larval source management. These measures have been progressive in the control of malaria but have faced challenges due to resistance [7,8] to antimicrobial drugs and difficulties in the development of vaccines [9]. The nature of human immune response that protects individuals from Plasmodium falciparum is complex [10] such that, host and parasite genetic factors contribute to the heterogeneity in the immune and clinical responses to malaria [11], but environmental factors such as host microbiota could also play a role. Recently, the role of gut microbiota for the control of infectious diseases, including viral disease is being elucidated [12]. Previous studies demonstrated that anti-Alpha-Gal antibodies induced by the presence of Escherichia coli in the gut are cytotoxic to Alpha-Gal expressing Plasmodium sporozoite and thus protect mice from mosquito-transmitted Plasmodium infection [13]. However, it is currently unknown to humans if the composition of the gut microbiota modulates the risk of Plasmodium falciparum infection or the risk of developing malaria disease once Plasmodium falciparum blood stage infection is established. Arthrospira platensis is one of the most consumed microalgae as food supplement worldwide [14], because of its nutritional beneficial value and therapeutic properties in human health [15,16]. Oral supplementation of Arthrospira in human studies have been shown to potentiate the innate immune system, ameliorate hyperlipidemia, reduce the body mass, improve antioxidant status and enhance anti-inflammatory and antihypertensive effects [15,17]. Although the underlying mechanism of claimed biological functions of Arthrospira has not yet been fully understood, recent studies in health and disease has shown that Arthrospira can modulate the composition of gut microbiota that may lead to improved health status [18-20]. This study characterized the interactions between the gut microbiota, the host and Plasmodium falciparum in the outcome of malaria treatment and investigated the effect of orally co-administered Arthrospira platensis and Artemisinin-based Combination Therapy on the human gut microbiota and malaria parasite burden in malaria infected patients.
Methods
Study area
The study was carried out at the Regional hospital Bamenda in the North West Region of Cameroon. Bamenda is located between latitudes 5056" to 5058" North of Equator and between longitude 100 08" and 10010" East of Greenwich Meridian and is found in the Mezam division [21] occupying a total surface area of 3,125 hectares. The climate of the Northwest Region of Cameroon is characterized by long rainy seasons from March to November with rainfall averaging 1800-2500 mm per year, average relative humidity of 97% to 98% and mean annual temperature of 24°C [21,22] conducive for the breeding of anopheles mosquitoes [3].
Study design
The study design is an analytical, clinical trial and longitudinal study conducted on malaria infected patients in the Northwest Region of Cameroon from the month of May to August 2021. The objective of the study was explained to the parents or guardians of participants prior to running and confirming a positive malaria test. Following parental approved proxy-consent to allow their kids participate in the study, participants were randomized and assigned to three groups; including group 1 involving malaria infected patients administered artemether-lumefantrine and Arthrospira platensis, group 2; malaria infected patients administered artemether-lumefantrine only and group 3; malaria negative subjects (control group).
Study participants and eligibility criteria
The study population consisted of participants aged 2-18 years, who had signs and symptoms of malaria and on performing malaria parasite microscopy were confirmed to have a parasite density of 2000 trophozoites/uL. Inclusion criteria involved participants who agreed to obtain and consume the antimalarial treatment and food supplement provided and were resident in Mezam.
Sampling and participant recruitment
From the consultation unit in the hospital, patients who came to the laboratory reception with a form requesting malaria test were identified. From the parasitology unit, positive malaria cases were identified and patients traced. The process of selecting study participants was done according to the WHO protocol in 2009 [23]. With prior informed proxy-consent from parents and positive eligibility criteria, children were recruited into the study and administered the antimalarial treatment in conjunction with Arthrospira platensis.
Drug regimen for artemeter-lumefantrine
Treatment with AL was done for 3 days in line with WHO weight-based regime [23]. A fixed-dose combination of 20 mg of artemether and 120 mg lumefantrine per tablet was administered, translating to one, two or three tablets per patient depending on individual weight. A full course of AL consisted of three tablets taken two times in a day (8h apart on day 0, and 12h apart on days 1 and 2). After drug administration, subjects were observed for 20 min to make sure they did not vomit. If vomiting occurred, a repeat dose was given. Any subject who persistently vomited was withdrawn and treated with parenteral artesunate or quinine according to the guidelines for the management of severe malaria [23,24]. Besides, paracetamol was given to all subjects with a body temperature ≥ 38°C.
Arthrospira platensis supplementation
Arthrospira platensis used for this study was provided from a farm in Nomayos (Yaounde- Cameroon). Using an electronic scale balance, 500 mg of dried and powdered Arthrospira platensis was encapsulated in a 1 gram capsule. A variety of clinical dosages for Arthrospira platensis have been used in previous scientific studies ranging from 1-10 grams per day [25,26]. For this study, the dosages per body weight of Arthrospira platensis were estimated based on a standard of 8 g/70 kg daily body weight [27,28].
Determination of parasite density
Thick and thin blood films were prepared by finger-pricking and smearing approximately 5 uL of blood on microscopic slide. Blood films were stained with 10% Giemsa for 20 mins and screened microscopically under *100 oil immersion lens using a light microscope (Olympus, USA). Intensity of infection was estimated by counting the number of asexual parasites in relation to 200 white blood cells. Parasite density was calculated assuming a mean leukocyte count of 8,000 per µL blood [29].
Stool sample collection and analysis
Fresh stool samples were collected before and after treatment in sterile cups. Stool samples were stored at a temperature of 40°C prior to microbiota analysis. Then different culture media were prepared such as the MacConkay culture media, blood nutrient agar, Cystine-Lactose-Electrolyte-Deficient (C.L.E.D) agar and Eosin Methylene Blue (EMB) agar.
Culturing technique
Fresh stool samples were collected in sterile stool cups and stored at a temperature of 80°C. Colonies on the primary or subculture plates were identified by standard laboratory procedures [30]. Briefly, for each sample, 1 gram of fecal matter was homogenized in 5 ml of distilled and filtrated using a filter paper. Filtrate was centrifuged at 25000 RPM (Revolution per Minute) for 5 mins to separate insoluble particles from diluent. Serial dilution from 10-1 to 10-5 was performed in other to enumerate the different bacteria colonies. Fives tubes were labelled 10-1, 10-2, 10-3, 10-4 and 10-5 and to each transferred 9 ml of distilled water. A micropipette was used to transfer 1 ml of sample broth to tube labelled 10-1, 1 ml of from 10-1 tube to 10-2, 1 ml from 10-2 to 10-3, 1 ml from 10-3 to 10-4 and 1 ml from 10-4 to 10-5. Using a calibrated micropipette, 10 µl aliquot was transferred from the tube labelled 10-4 to the culture plate and spread by mere tilting or gentle twirling of the plate followed by incubation at a temperature of 37°C. CFU enumeration was done after 24 hours using the formula: Number of colonies on plate × reciprocal of dilution of sample = number of bacteria/ml.
Statistical analysis
All statistical calculations were performed with SPSS version 17.0. Difference in colonic bacteria community and variation among treatment groups and days of intervention were compared by one-way Analysis of Variance (ANOVA) following post-hoc method with p < 0.05 considered statistically significant.
Results
Socio-demographic data
Of a total of 220 screened, 50 were malaria positive giving a prevalence of 22.72%. Malaria patients included 29 (58%) females and 21 (42%) males aged 2 to less than 18 years (Table 1).
| Table 1: Gender and prevalence of malaria in 3 health facilities in the North-west region Cameroon. | |||
| Gender | MP Positive | MP Negative | Prevalence |
| Male | 21 | 104 | 20.19% |
| Female | 29 | 116 | 25.0% |
| Total | 50 | 220 | 22.72% |
Comparison of microbiota community between malaria positive and malaria negative patients
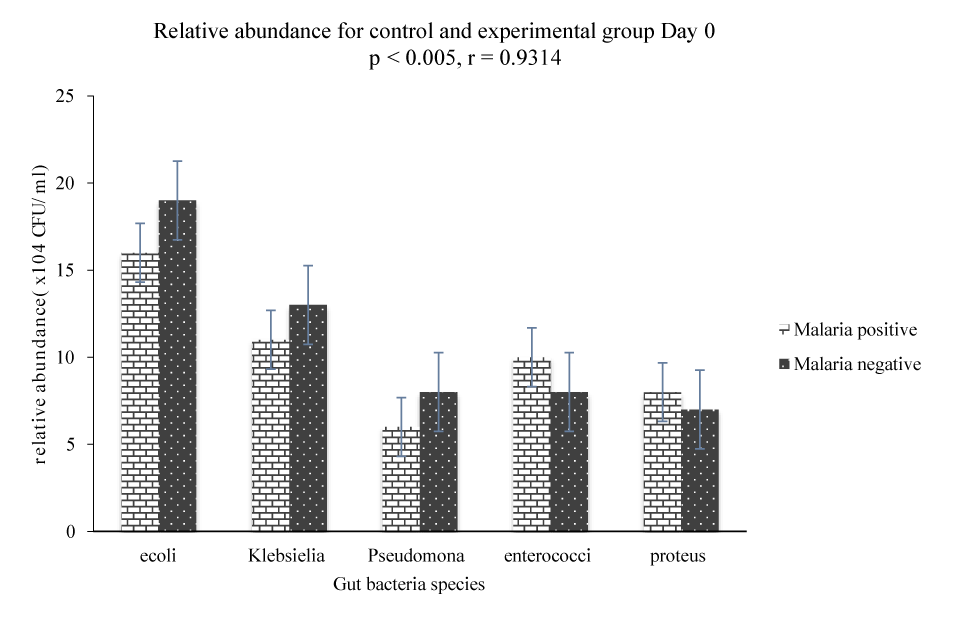
The results in figure 1 show that there is a significant difference (p < 0.005) in the gut bacteria community between malaria negative and malaria positive subjects on day 0 (before treatment). Fecal analysis of the gut bacteria community for malaria positive subjects showed that E coli had an average growth of 16 × 104 CFU/ml, Klebsiella 11 × 104 CFU/ml, Pseudomonas 6 × 104 CFU/ml, Enterococci faecalis 10 × 104 CFU/ml and Proteus 8 × 104 CFU/ml as compared to malaria negative subjects where E. coli had a growth of 19 ×104 CFU/ml, Klebsiella 13 × 104 CFU/ml, Pseudomonas 8 × 104 CFU/ml, Enterococci faecalis 8 × 104 CFU/ml and proteus 7 × 104 CFU/ml. Variability in the gut microbiota between malaria positive and malaria negative subjects showed that E. coli, Klebsiella and Pseudomonas were relatively abundant in the fecal sample of malaria negative subjects as compared to the growth E. coli, Klebsiella and Pseudomonas in the fecal sample of malaria positive subjects (Figure 1). On the other hand, the average growth of Enterococci faecalis and Proteus was relatively abundant in the fecal sample of malaria positive subjects as compared to the growth of Enterococci faecalis and Proteus in the fecal sample of malaria negative subjects.
Comparison of gut bacteria community and structure amongst malaria positive subjects administered with AL + AP and AL after treatment (day 3 and 7).
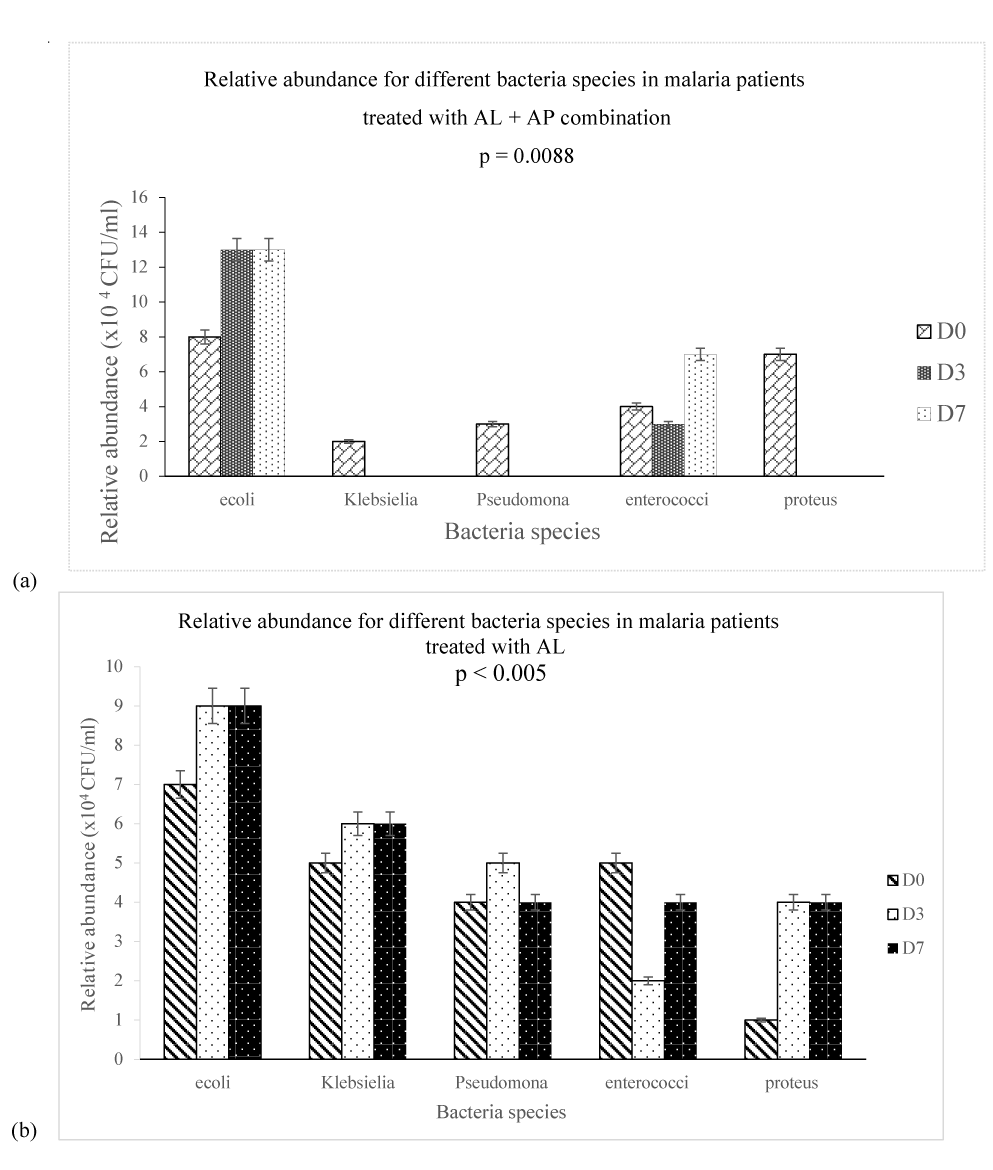
Analysis of the relative abundance of fecal bacteria species for malaria positive subjects on day 0, day 3 and day 7 showed that there was a significant difference (p = 0.008) in gut bacteria community between E. coli, Klebsiella, Pseudomonas, Enterococci faecalis and Proteus between day 0, day 3 and day 7 as shown on figure 2. Hence, fecal analysis of malaria positive patients showed an average growth of 13 × 104 CFU/ml for E. coli on day 3 and day 7 as compared to 8 × 104 CFU/ml on day 0, Klebsiella had a relative abundance of 2 × 104 CFU/ml on day 0 and no growth on day 3 and day 7, Pseudomonas had a relative abundance of 3 × 104 CFU/ml on day 0 and no growth on day 3 and day 7, Enterococci faecalis had a relative abundance of 4 × 104CFU/ml on day 0 and no growth on day 3 and day 7 and Proteus 7 × 104 CFU/ml on day 0 and no growth on day 3 and day 7. Time dependently, there was a significant change in gut bacteria community between day 0, day 3 and day 7 with arthemether-lumefantrine and Arthrospira exerting an antibacterial effect on Klebsiella, Pseudomonas, Enterococci faecalis and proteus on day 3 and day 7 (Figure 2a). There was a significant difference (p < 0.005) in the relative abundance of gut microbiota between day 0, day 3 and day 7 figure 2b for malaria positive subjects administered arthemether-lumefantrine. The fecal sample culture analysis showed that E. coli was relative abundant on day 3 (9 × 104 CFU/ml) and day 7 (9 × 104 CFU/g) as compared to day 0 (7 × 104 CFU/ml), Klebsiella was relatively abundant on day 3 (6 × 104 CFU/g) and day 7 (6 × 104 CFU/g) as compared to day 0 (5 × 104 CFU/ml), Pseudomonas was relative abundant on day 3 (5 × 104 CFU/g) as compared to day 0 (4 × 104 CFU/ml) and day 7 (4 × 104 CFU/ml), Enterococci faecalis was relatively abundant on day 0 (5 × 104 CFU/ml) as compared to day 3 (3 × 104 CFU/ml) and day 7 (4 × 104 CFU/ml). In all treatment groups, the results on figure 2a showed that administration of arthemether-lumefantrine and Arthrospira to malaria positive subject had a significant change on gut bacteria community on day 3 and day 7 while figure 2b show that administration of arthemether-lumefantrine had a significant effect on the relative abundance of bacteria species in the gut.
Correlation between gut bacteria community and parasitemia
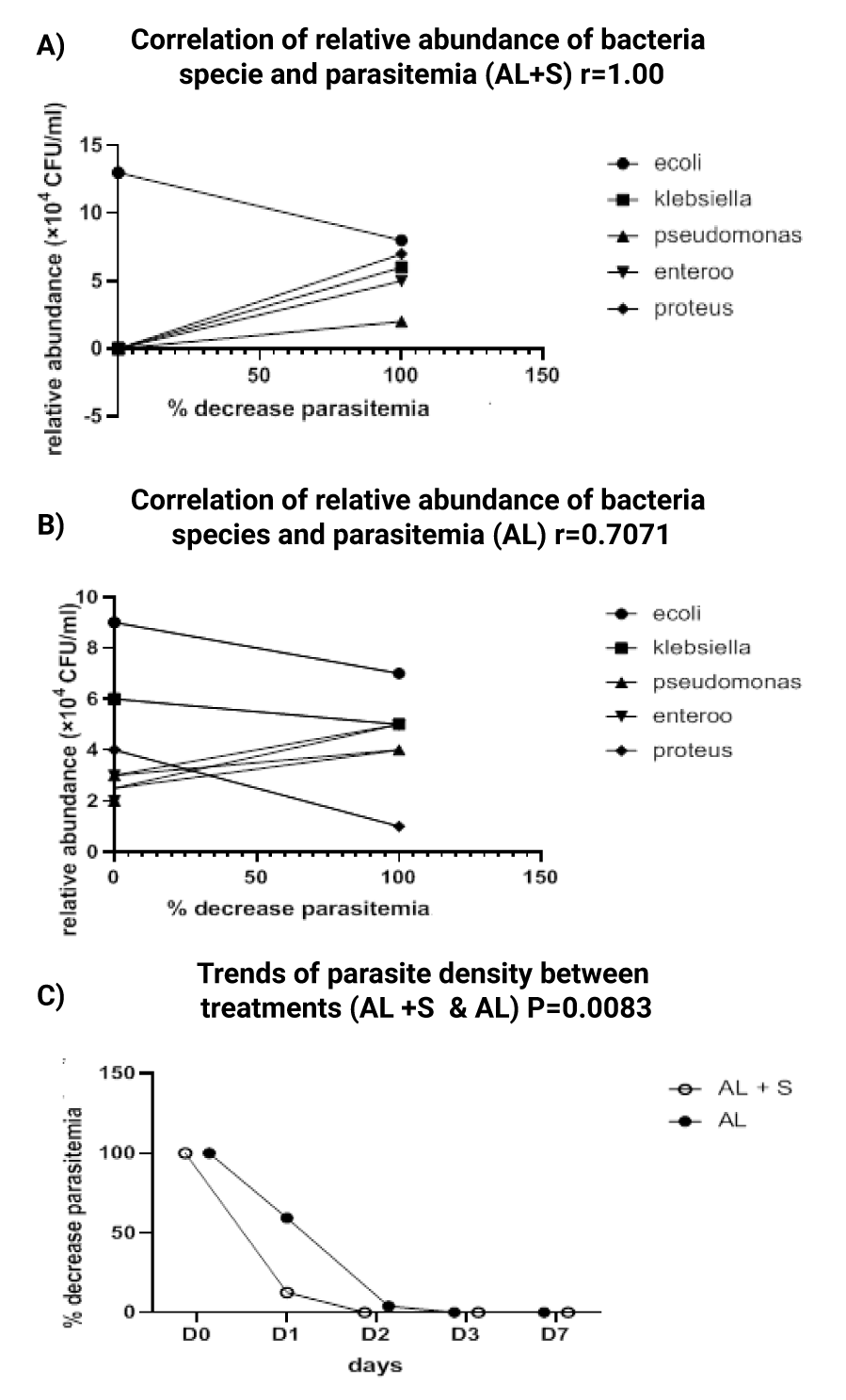
The correlation between gut bacteria community and parasitemia on day 0, 1, 2, 3 and 7 in malaria positive subjects administered AL + AP is presented on figure 3a. This observation shows that, there is a strong positive correlation (r = 1.00) between the gut bacteria community and parasitemia for malaria positive subjects administered AL + AP at all treatment days (day 0, 1, 2, 3, and 7). Each malaria positive subject in this group was assumed 100% parasitemia on day 0. Day 1 (during treatment) had an average of 12.56% parasitemia accounting for 87.44% parasite clearance and day 2 and 3 had 0% parasitemia accounting for 100% parasite clearance figure 3c with a significant relative abundance of E. coli at 100% parasite clearance whereas there was weak positive correlation (r = 0.7071) between gut bacterial community and parasitemia for malaria positive subjects administered AL (Figure 3b). Day 1 had an average parasitemia of 59.37% accounting for 40.639% clearance in parasitemia, day 2 had average parasitemia of 3.95% accounting for 96.05% parasite clearance and day 3 100% parasite clearance (Figure 3b).
As shown on figures 3a,b E. coli had a dominant relative abundance of 13 × 104 CFU/g while other bacteria species including Klebsiella, Pseudomonas, Proteus, Enterococci feacalis were completely excluded at 100% parasite clearance in malaria positive subjects administered AL + AP as compared to malaria positive subjects administered AL where there was no significant change in the gut bacteria community in the course of the study. Furthermore, a parasite clearance of 100% was obtained on D2 in malaria positive subjects administered AL + AP as compared to malaria positive subjects administered AL whose maximum parasite clearance was obtained on D3 (figure 3c).
Relationship between malaria severity and gut microbiota composition and structure
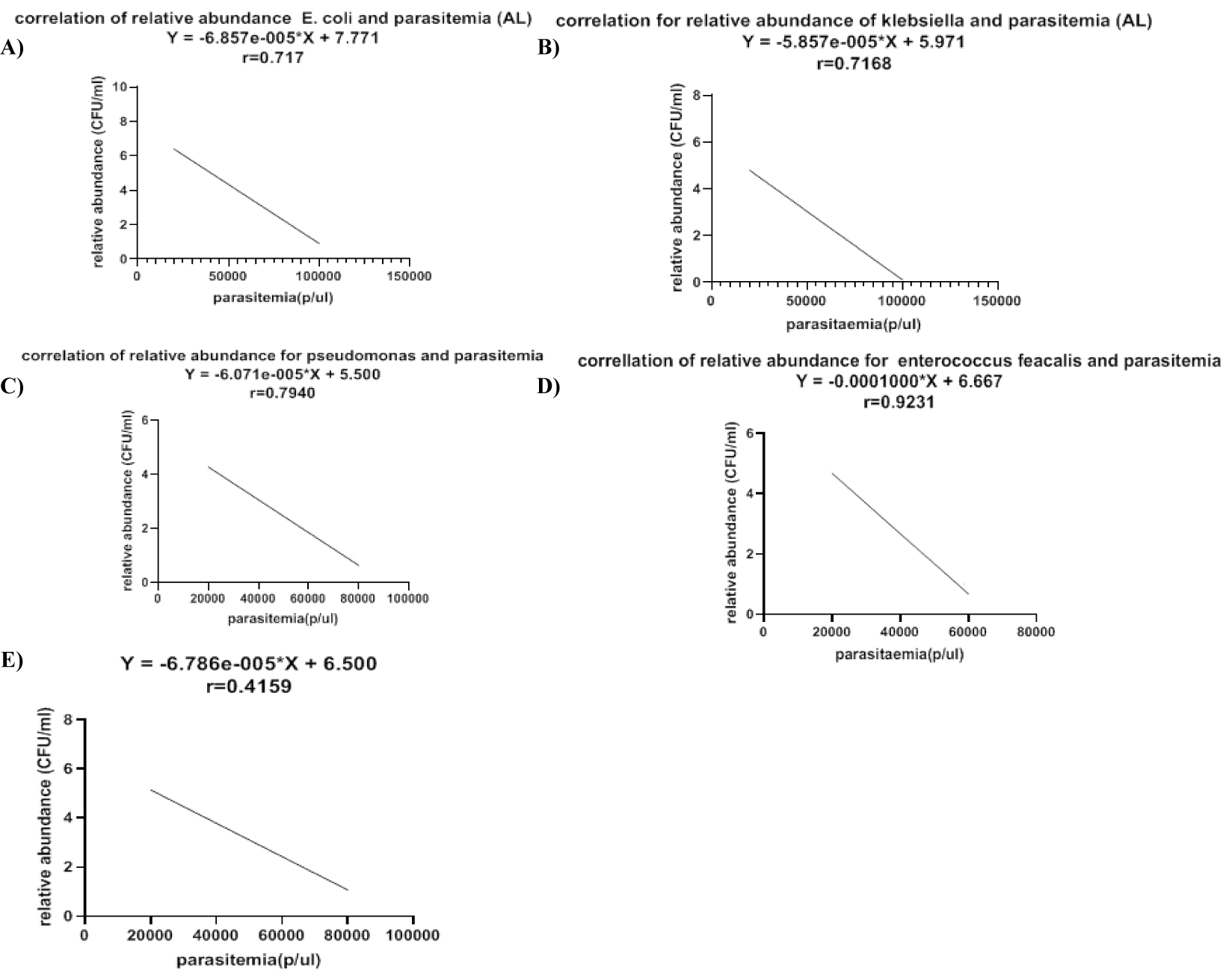
The results on figure 4 represent the relationship between relative abundance of bacterial communities and malaria parasite density. For all bacteria species there is a negative correlation between relative abundance of E. coli (r = 0.717), Klebsiella (r = 0.7168), Pseudomonas (r = 0.7940), Enterococci feacalis (r = 0.9231) and proteus (r = 0.4159) and malaria parasite range (figure 4). As malaria parasite density reduces there is a significant increase in relative abundance of E coli figure 4a, Klebsiella figure 4b, Pseudomonas figure 4c, Enterococci faecalis figure 4d, proteus figure 4e in the gut microbiota.
Discussion
This study is the first clinical investigation of the potential interplay between Plasmodium falciparum and gut microbiota in the outcome of malaria treatment following the administration of Artemisinin-Based Combination Therapy (ACT) together with Arthrospira platensis, a probiotic of interest as an adjunct treatment to malaria. The results obtained from the comparison between the gut microbiota community in malaria positive and malaria negative subjects showed a significant difference in the gut microbiota structure and function at baseline observed in the decrease of the relative abundance of some bacteria community. These findings are consistent with previous discoveries indicating that plasmodium causes intestinal malaria dysbiosis characterized by the reduction of some bacteria communities such as Firmicutes [7], and gastrointestinal pathophysiology [31]. Parasitic infections in humans have shown to influence variety of behaviors in their host including effects on the gut microbiota [32] rendering the gut microbiota unstable. Disturbances in the gut microbiota structure has been associated to the development and severity of diseases [33]. Again, results obtained shows that there is negative correlation between bacteria communities and increase malaria parasite burden. Most bacteria communities had a low relative abundance in malaria positive subjects with high parasite count in line with previous studies in which it was discovered that gut microbiota is susceptible to plasmodium-induced inflammatory related changes causing dysbiosis of the gut microbiota community [7]. Therefore, intestinal microbiota dysbiosis is also related to high parasite burden [7,34]. Overall, in this study, arthemether-lumefantrine showed antibacterial effect on gut microbiota but the effect was more pronounced when administered in combination with Arthrospira platensis. Likewise, in this study, there is a significant difference in the gut bacteria community between day 0, day 3 and day 7 in fecal sample of malaria positive subjects administered arthemether-lumefantrine and a proportionally higher relative abundance of E. coli, than Klebsiella, Pseudomonas, proteus and Enterococci feacalis on D7 post treatment as compared to day 3 and day 0. These findings are in accordance with previous studies in which artemisinin and its derivatives have shown to be differentially effective at killing specific pathogens, various fungi and bacteria including Helicobacter pylori, a human pathobionth and methicillin-resistant staphylococcus aureus [35,36].
Furthermore, this study showed that administration of the combination of arthemether-lumefantrine and Arthrospira platensis to malaria positive subjects had a significant effect on the gut microbiota community and a reduced malaria parasite burden approximately 5 folds more than the effect observed with malaria positive subjects administered arthemether-lumefantrine alone. This potential capacity of promoting the growth of commensal gut microbiota [37-39] and reducing malaria parasite burden [40-42], is due to Arthrospira platensis administered as oral non-medicinal nutraceutical supplementation derived from food sources for malaria prevention and amelioration of malaria symptoms. The antimalarial effect of Arthrospira platensis observed in this study is consistent with previous findings in which several Spirulina formulas exerted antimalarial activities against Plasmodium falciparum 3D7, in vitro [43]. Administration of arthemether-lumefantrine and Arthrospira to malaria patients proportionately maintained the relative abundance of E. coli while having antibacterial effect on Klebsiella, Pseudomonas, Proteus, Enterococci feacalis. This finding is in line with studies suggesting the effect of E. coli and of the gut microbiota composition in reducing malaria severity and providing immunity to the life threatening disease [44-46].
Conclusion
This study aimed at evaluating the effect of clinically relevant doses of artemether-lumefantrine and Arthrospira platensis on the gut microbiota and malaria parasite burden when administered to malaria positive children. From this study it can be deduced that intake of artemether-lumefantrine and Arthrospira significantly modifies the gut microbiota community by enhancing the survival of E. coli and on the other hand having an antibacterial effect on Klebsiella, Pseudomonas, Enterococci faecalis and Proteus. Artemether-lumefantrine plus Arthrospira reduced malaria parasite burden at an increased rate than artemether-lumefantrine administered separately to malaria positive subjects. Thus, it can be concluded that artemether-lumefantrine and Arthrospira combination is effective in the treatment of malaria and this effect could be associated with the presence of E. coli in the gut microbiota. The use of probiotics Arthrospira platensis for functional food development as a dietary supplement in combination with antimalarial drugs would be a promising strategy for more investigation in order to find ways to prevent malaria incidence, reduce the severity of malaria and to curtail the emergence and spread of antimalarial drug resistance
Ethical Considerations
Ethical approval was obtained from the Regional Delegation of public health Bamenda, Northwest Region Cameroon through N/Ref: 110/ATT/NWR/RDPH/BRICAD of 25th May 2021. Informed proxy-consent to participate in the study was obtained from the participant’s parent using an appended prior informed proxy-consent form.
Competing interest disclaimer
Authors have declared that no competing interests exist. The products used for this research are commonly and predominantly use products in our area of research and country. There is absolutely no conflict of interest between the authors and producers of the products because we do not intend to use these products as an avenue for any litigation but for the advancement of knowledge. Also, the research was not funded by the producing company rather it was funded by personal efforts of the authors.
References
- World malaria report 2022. World Health Organization. 2022.
- World malaria report 2020: 20 years of global progress and challenges. World Health Organization. 2020.
- Antonio-Nkondjio C, Ndo C, Njiokou F, Bigoga JD, Awono-Ambene P, Etang J, Ekobo AS, Wondji CS. Review of malaria situation in Cameroon: technical viewpoint on challenges and prospects for disease elimination. Parasit Vectors. 2019 Oct 26;12(1):501. doi: 10.1186/s13071-019-3753-8. PMID: 31655608; PMCID: PMC6815446.
- Metoh TN, Somo-Moyou R, Fon PG, Tambo E, Jun-Hu C, Zhou XN. Efficacy and safety assessment of three artemisinin-based combination therapy (acts) in the treatment of P. falciparum malaria in Cameroon. J Infect Dis Epidemiol. 2021;7:242. doi: 10.23937/2474-3658/1510242.
- Guidelines for the treatment of malaria. 3rd edition. Geneva: World Health Organization; 2015.
- Bloland Peter B & World Health Organization. Drug resistance in malaria/Peter B. Bloland. Anti-Infective Drug Resistance Surveillance and Containment Team. 2021.
- Taniguchi T, Miyauchi E, Nakamura S, Hirai M, Suzue K, Imai T, Nomura T, Handa T, Okada H, Shimokawa C, Onishi R, Olia A, Hirata J, Tomita H, Ohno H, Horii T, Hisaeda H. Plasmodium berghei ANKA causes intestinal malaria associated with dysbiosis. Sci Rep. 2015 Oct 27;5:15699. doi: 10.1038/srep15699. Erratum in: Sci Rep. 2016;6:17248. PMID: 26503461; PMCID: PMC4621605.
- Kokwaro G. Ongoing challenges in the management of malaria. Malar J. 2009 Oct 12;8 Suppl 1(Suppl 1):S2. doi: 10.1186/1475-2875-8-S1-S2. PMID: 19818169; PMCID: PMC2760237.
- Frimpong A, Kusi KA, Ofori MF, Ndifon W. Novel Strategies for Malaria Vaccine Design. Front Immunol. 2018 Nov 29;9:2769. doi: 10.3389/fimmu.2018.02769. PMID: 30555463; PMCID: PMC6281765.
- Crompton PD, Moebius J, Portugal S, Waisberg M, Hart G, Garver LS, Miller LH, Barillas-Mury C, Pierce SK. Malaria immunity in man and mosquito: insights into unsolved mysteries of a deadly infectious disease. Annu Rev Immunol. 2014;32:157-87. doi: 10.1146/annurev-immunol-032713-120220. PMID: 24655294; PMCID: PMC4075043.
- Turner L, Lavstsen T, Berger SS, Wang CW, Petersen JE, Avril M, Brazier AJ, Freeth J, Jespersen JS, Nielsen MA, Magistrado P, Lusingu J, Smith JD, Higgins MK, Theander TG. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature. 2013 Jun 27;498(7455):502-5. doi: 10.1038/nature12216. Epub 2013 Jun 5. PMID: 23739325; PMCID: PMC3870021.
- Kamada N, Chen GY, Inohara N, Núñez G. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol. 2013 Jul;14(7):685-90. doi: 10.1038/ni.2608. PMID: 23778796; PMCID: PMC4083503.
- Yilmaz B, Portugal S, Tran TM, Gozzelino R, Ramos S, Gomes J, Regalado A, Cowan PJ, d'Apice AJ, Chong AS, Doumbo OK, Traore B, Crompton PD, Silveira H, Soares MP. Gut microbiota elicits a protective immune response against malaria transmission. Cell. 2014 Dec 4;159(6):1277-89. doi: 10.1016/j.cell.2014.10.053. PMID: 25480293; PMCID: PMC4261137.
- de Jesus Raposo MF, de Morais AM, de Morais RM. Emergent Sources of Prebiotics: Seaweeds and Microalgae. Mar Drugs. 2016 Jan 28;14(2):27. doi: 10.3390/md14020027. PMID: 26828501; PMCID: PMC4771980.
- Khan Z, Bhadouria P, Bisen PS. Nutritional and therapeutic potential of Spirulina. Curr Pharm Biotechnol. 2005 Oct;6(5):373-9. doi: 10.2174/138920105774370607. PMID: 16248810.
- Ngo-Matip ME, Pieme CA, Azabji-Kenfack M, Moukette BM, Korosky E, Stefanini P, Ngogang JY, Mbofung CM. Impact of daily supplementation of Spirulina platensis on the immune system of naïve HIV-1 patients in Cameroon: a 12-months single blind, randomized, multicenter trial. Nutr J. 2015 Jul 21;14:70. doi: 10.1186/s12937-015-0058-4. PMID: 26195001; PMCID: PMC4508814.
- Metoh TN, Siberedi RKA, Pieme CA. Evaluating the effects of spirulina (Arthrospira platensis) on artemether/lumefantrine (coartem)-induced oxidative stress in wistar rats. International Journal of Tropical Medicine. 2022;17:1-9. doi: 10.36478/ijtmed.2022.1.9.
- Yusuf MS, Hassan MA, Abdel-Daim MM, Nabtiti AS, Ahmed AM, Moawed SA, El-Sayed AK, Cui H. Value added by Spirulina platensis in two different diets on growth performance, gut microbiota, and meat quality of Japanese quails. Vet World. 2016 Nov;9(11):1287-1293. doi: 10.14202/vetworld.2016.1287-1293. Epub 2016 Nov 23. PMID: 27956783; PMCID: PMC5146312.
- Neyrinck AM, Taminiau B, Walgrave H, Daube G, Cani PD, Bindels LB, Delzenne NM. Spirulina Protects against Hepatic Inflammation in Aging: An Effect Related to the Modulation of the Gut Microbiota? Nutrients. 2017 Jun 20;9(6):633. doi: 10.3390/nu9060633. PMID: 28632181; PMCID: PMC5490612.
- Rasmussen HE, Martínez I, Lee JY, Walter J. Alteration of the gastrointestinal microbiota of mice by edible blue-green algae. J Appl Microbiol. 2009 Oct;107(4):1108-18. doi: 10.1111/j.1365-2672.2009.04288.x. Epub 2009 Mar 30. PMID: 19486425.
- Neba AS. Modern geography of the republic of Cameroon. 2nd ed. Camden: NJ Neba Pub; 1987.
- AchoChi. Human interference and environmental instability: Addressing the environmental consequences of rapid urban growth in Bamenda, Cameroon. Environment and Urbanization. 1998. doi: 10.1177/095624789801000206.
- World health statistics 2009. World Health Organization. 2022:149.
- Kishoyian G, Njagi ENM, Orinda GO, Kimani FT, Thiongo K, Matoke-Muhia D. Efficacy of artemisinin-lumefantrine for treatment of uncomplicated malaria after more than a decade of its use in Kenya. Epidemiol Infect. 2021 Jan 5;149:e27. doi: 10.1017/S0950268820003167. PMID: 33397548; PMCID: PMC8057502.
- ElFar OA, Billa N, Lim HR, Chew KW, Cheah WY, Munawaroh HSH, Balakrishnan D, Show PL. Advances in delivery methods of Arthrospira platensis (spirulina) for enhanced therapeutic outcomes. Bioengineered. 2022 Jun;13(6):14681-14718. doi: 10.1080/21655979.2022.2100863. PMID: 35946342; PMCID: PMC9373759.
- Leal-Esteban LC, Renata Nogueira C, Veauvy M, Mascarenhas B, Mhatre M, Menon S, Graz B, von der Weid D. Spirulina supplementation: A double-blind, randomized, comparative study in young anemic Indian women. Clinical Epidemiology and Global Health. 2021;12:100884. doi: 10.1016/j.cegh.2021.100884.
- Azabji-Kenfack M, Dikosso SE, Loni EG, Onana EA, Sobngwi E, Gbaguidi E, Kana AL, Nguefack-Tsague G, Von der Weid D, Njoya O, Ngogang J. Potential of Spirulina Platensis as a Nutritional Supplement in Malnourished HIV-Infected Adults in Sub-Saharan Africa: A Randomised, Single-Blind Study. Nutr Metab Insights. 2011 May 2;4:29-37. doi: 10.4137/NMI.S5862. PMID: 23946659; PMCID: PMC3738485.
- Park HJ, Lee YJ, Ryu HK, Kim MH, Chung HW, Kim WY. A randomized double-blind, placebo-controlled study to establish the effects of spirulina in elderly Koreans. Ann Nutr Metab. 2008;52(4):322-8. doi: 10.1159/000151486. Epub 2008 Aug 19. PMID: 18714150.
- Laman M, Moore BR, Benjamin J, Padapu N, Tarongka N, Siba P, Betuela I, Mueller I, Robinson LJ, Davis TM. Comparison of an assumed versus measured leucocyte count in parasite density calculations in Papua New Guinean children with uncomplicated malaria. Malar J. 2014 Apr 16;13:145. doi: 10.1186/1475-2875-13-145. PMID: 24739250; PMCID: PMC3991873.
- Sanders ER. Aseptic laboratory techniques: plating methods. J Vis Exp. 2012 May 11;(63):e3064. doi: 10.3791/3064. PMID: 22617405; PMCID: PMC4846335.
- Shimada M, Hirose Y, Shimizu K, Yamamoto DS, Hayakawa EH, Matsuoka H. Upper gastrointestinal pathophysiology due to mouse malaria Plasmodium berghei ANKA infection. Trop Med Health. 2019 Mar 4;47:18. doi: 10.1186/s41182-019-0146-9. PMID: 30872946; PMCID: PMC6399856.
- Toro-Londono MA, Bedoya-Urrego K, Garcia-Montoya GM, Galvan-Diaz AL, Alzate JF. Intestinal parasitic infection alters bacterial gut microbiota in children. PeerJ. 2019 Jan 7;7:e6200. doi: 10.7717/peerj.6200. PMID: 30643702; PMCID: PMC6327884.
- Jaeggi T, Kortman GA, Moretti D, Chassard C, Holding P, Dostal A, Boekhorst J, Timmerman HM, Swinkels DW, Tjalsma H, Njenga J, Mwangi A, Kvalsvig J, Lacroix C, Zimmermann MB. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut. 2015 May;64(5):731-42. doi: 10.1136/gutjnl-2014-307720. Epub 2014 Aug 20. PMID: 25143342.
- Jason PM, Kristen LL, Mariana XB, Michael DG, Eric MV, Franziska F, Brian PB, Gregory TW, Mohamed MA, Rashaun P, Caitlin T, Brian MMA, Shirley L, Renée MT. Inflammation-associated alterations to the intestinal microbiota reduce colonization resistance against non-typhoidal salmonella during concurrent malaria parasite infection. Sci Rep. 2015;5: 14603. doi: 10.1038/srep14603.
- Goswami S, Bhakuni RS, Chinniah A, Pal A, Kar SK, Das PK. Anti-Helicobacter pylori potential of artemisinin and its derivatives. Antimicrob Agents Chemother. 2012 Sep;56(9):4594-607. doi: 10.1128/AAC.00407-12. Epub 2012 Jun 11. PMID: 22687518; PMCID: PMC3421870.
- Sisto F, Carradori S, D'Alessandro S, Santo N, Lattuada N, Haynes RK, Taramelli D, Grande R. In Vitro Activity of the Arylaminoartemisinin GC012 against Helicobacter pylori and Its Effects on Biofilm. Pathogens. 2022 Jun 29;11(7):740. doi: 10.3390/pathogens11070740. PMID: 35889986; PMCID: PMC9324866.
- Bamgbose T, Anvikar AR, Alberdi P, Abdullahi IO, Inabo HI, Bello M, Cabezas-Cruz A, de la Fuente J. Functional Food for the Stimulation of the Immune System Against Malaria. Probiotics Antimicrob Proteins. 2021 Oct;13(5):1254-1266. doi: 10.1007/s12602-021-09780-w. Epub 2021 Apr 1. PMID: 33791994; PMCID: PMC8012070.
- Dahiya D, Nigam PS. The Gut Microbiota Influenced by the Intake of Probiotics and Functional Foods with Prebiotics Can Sustain Wellness and Alleviate Certain Ailments like Gut-Inflammation and Colon-Cancer. Microorganisms. 2022 Mar 20;10(3):665. doi: 10.3390/microorganisms10030665. PMID: 35336240; PMCID: PMC8954736.
- Vamanu E. Polyphenolic Nutraceuticals to Combat Oxidative Stress Through Microbiota Modulation. Front Pharmacol. 2019 May 3;10:492. doi: 10.3389/fphar.2019.00492. PMID: 31130865; PMCID: PMC6509743.
- Fan ZG, Li X, Fu HY, Zhou LM, Gong FL, Fang M. Gut Microbiota Reconstruction Following Host Infection with Blood-stage Plasmodium berghei ANKA Strain in a Murine Model. Curr Med Sci. 2019 Dec;39(6):883-889. doi: 10.1007/s11596-019-2119-y. Epub 2019 Dec 16. PMID: 31845218.
- Villarino NF, LeCleir GR, Denny JE, Dearth SP, Harding CL, Sloan SS, Gribble JL, Campagna SR, Wilhelm SW, Schmidt NW. Composition of the gut microbiota modulates the severity of malaria. Proc Natl Acad Sci U S A. 2016 Feb 23;113(8):2235-40. doi: 10.1073/pnas.1504887113. Epub 2016 Feb 8. PMID: 26858424; PMCID: PMC4776451.
- Morffy Smith CD, Gong M, Andrew AK, Russ BN, Ge Y, Zadeh M, Cooper CA, Mohamadzadeh M, Moore JM. Composition of the gut microbiota transcends genetic determinants of malaria infection severity and influences pregnancy outcome. EBioMedicine. 2019 Jun;44:639-655. doi: 10.1016/j.ebiom.2019.05.052. Epub 2019 May 31. PMID: 31160271; PMCID: PMC6606560.
- Wulandari DA, Sidhartha E, Setyaningsih I, Marbun JM, Syafruddin D, Asih PBS. Evaluation of antiplasmodial properties of a cyanobacterium, Spirulina platensis and its mechanism of action. Nat Prod Res. 2018 Sep;32(17):2067-2070. doi: 10.1080/14786419.2017.1360880. Epub 2017 Aug 2. PMID: 28768428.
- Yooseph S, Kirkness EF, Tran TM, Harkins DM, Jones MB, Torralba MG, O'Connell E, Nutman TB, Doumbo S, Doumbo OK, Traore B, Crompton PD, Nelson KE. Stool microbiota composition is associated with the prospective risk of Plasmodium falciparum infection. BMC Genomics. 2015 Aug 22;16(1):631. doi: 10.1186/s12864-015-1819-3. PMID: 26296559; PMCID: PMC4546150.
- Bordon Y. Microbiome: Gut bacteria cross malaria. Nat Rev Microbiol. 2015 Feb;13(2):65. doi: 10.1038/nrmicro3419. Epub 2014 Dec 22. PMID: 25534807.
- Spisni E, Turroni S, Alvisi P, Spigarelli R, Azzinnari D, Ayala D, Imbesi V, Valerii MC. Nutraceuticals in the Modulation of the Intestinal Microbiota: Current Status and Future Directions. Front Pharmacol. 2022 Mar 18;13:841782. doi: 10.3389/fphar.2022.841782. PMID: 35370685; PMCID: PMC8971809.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.