Biology Group . 2023 March 16;4(3):413-425. doi: 10.37871/jbres1690.
Fungal Infection and Mycotoxins Contamination on Farm-Stored Chickpea in Major Producing Districts of Ethiopia
Samuel Alemayehu1*, Fetien Abay Abera2, Kiros Meles Ayimut3, Jagger Harvey4, Rizana Mahroof5, Bhadriraju Subramanyam6 and Jonathan Ulmer7
2Visiting Professor University of California, Davis, Department of Plant Sciences, MS/11 Shields Ave., Davis, CA 95616, USA
3Department of Dryland Crop and Horticultural Sciences, College of Dryland Agriculture and Natural Resources, Mekelle University, Mekelle, Tigray, Ethiopia
4Feed the Future Innovation Lab for the Reduction of Post-Harvest Loss; Department of Plant Pathology, Kansas State University, Manhattan, KS 66506, USA
5Department of Biological and Physical Sciences, South Carolina State University, Orangeburg, SC 29117, USA
6Department of Grain Science and Industry, Kansas State University, Manhattan, KS 66506, USA
7Feed the Future Innovation Lab for the Reduction of Post-Harvest Loss, Department of Agricultural Education, Kansas State University, Manhattan, KS 66506, USA
- Chickpea samples
- Ethiopia
- Fungal infection
- Mycotoxins
- Moisture
Abstract
Crop susceptibility to moisture content, quality of storage facilities in the farm contributes to fungal infections and mycotoxin contamination. Chickpea grain loss in many parts of the world has been due to inadequate and poor storage facilities, post-harvest activities leading to mycotoxins and fungal infections. The current research investigated the prevalence of fungal infection and mycotoxin level in farm chickpea across five major growing districts in Ethiopia. In the current study, fungal infection and mycotoxin concentrations were investigated in two Desi and Kabuli type varieties of samples containing 150 chickpea kernels collected from five districts in Ethiopia. Additionally, moisture content, relative humidity, and temperature were also investigated during sampling. Moisture levels ranged from 13.3 - 22.3% with a mean value of 16.4% across the five districts. There was no significant difference between the two varieties studied. Survey of different storage techniques used by farmers showed that polypropylene bags were most common and accounted for 54.7%, followed by gotta 45.3%. The total fungal infection in chickpea kernels across the sampled districts range 23 - 79%. Infection with Aspergillus genera was predominant, accounting for approximately 44.3% of the total (25 - 62.5%), followed by Penicillium spp. at 34.3% (10.9 - 55.3%) and Fusarium spp. 21.4% (9.6 - 42.3%) as the lowest. A cross the studied districts, chickpea germination ranged from 68.8% to 75.5%. Total aflatoxins levels ranged from 2.5 to 31.1 ppb and a mean of 17.4 ppb. Ochratoxin A concentrations ranged from 4.3 to 35.0 ppb, with a mean value of 10.6 ppb. Fumonisins (18.7%), ranged from 0.2 to 2.9 ppm and Deoxynivalenol (6.7%) ranged from 0.2 to 2.9 ppm. Chickpea samples had a high level of total aflatoxins (ppb), followed by ochratoxin A. Even though, the current co-occurrence of mycotoxins found is at low levels, it may adversely affect the health of regular consumers and the quality of Chickpea. Further investigations should be performed in different regions to help in advising and making decisions to the relevant government institutions on the appropriate measures to be undertaken.
Introduction
Chickpea (Cicer arietinum L.) is the third most widely grown and consumed pulse crop in the world after dry beans and peas [1,2]. Chickpeas play an important role in increasing soil fertility through biological nitrogen fixation, increasing soil organic matter through leaf fall and root decomposition, and facilitating the access of immobilized soil phosphorus [3,4]. They are an excellent choice for vegetarians as they are low in fat, high in fiber and complex carbohydrates, and a good source of vitamins and minerals [5-7]. Ethiopia is the leading producer, consumer, and exporter of chickpeas in Africa, mainly grown by smallholder farmers in rain-fed areas [8-14]. According to the Central Statistical Agency [15], nearly 57% of total chickpea production in Ethiopia is consumed by households, with the remainder exported. In Ethiopia, abiotic and biotic factors reduce grain production and seed quality [16-18]. Conventional storage methods such as Gota, Jute bags, and woven polypropylene bags are widely used, yet they are prone to infestations from stored grain pests and may expose chickpeas to high moisture and temperature levels, leading to fungal growth and mycotoxin contamination [5,19,20,21]. Mycotoxins from contaminated crops can cause health concerns such as allergic reactions, carcinoma implications, and, in extreme situations, death [21]. Aspergillus, Penicillium, and Fusarium genera are the three most prevalent fungi of chickpeas in the world, which can increase their free fatty acid content and cause adverse biochemical changes [21,22]. These changes may lead to reduced germination and yield of protein and carbohydrates but also produce toxic contaminants during the post-harvest storage period [23,24]. Mycotoxins are one of the most threatening contaminants in chickpeas and chickpea-based products from all over the world and their consumption can be harmful to human health [21,25-28]. The extensive use of conventional storage practices by smallholder farmers results in considerable post-harvest losses in several crops as a result of fungal growth and mycotoxin contamination [29-35]. Due to limited information on the importance of mycotoxins on stored chickpeas, postharvest protection research in Ethiopia did not receive adequate attention, and chickpeas have not been surveyed for the prevalence and prominence of mycotoxins. The changes in chickpea grain quality due to fungal infection and mycotoxin levels that occur during seed storage need to be investigated. Therefore, the present study was conducted to assess the major mycotoxins of stored chickpea and associated losses in quality (germination) in the major growing districts of Ethiopia.
Materials and Methods
Description of study areas
The study was carried out in five districts across four regional states of Ethiopia i.e. Dambia and Gondar Zuria (Amhara); Laeilaymaichew (Tigray); Ada’a (Oromia) and Meskan (South Nations Nationalities and Peoples Region) (Figure 1). These districts were purposively chosen because they represent the major chickpea growing districts in Ethiopia.
Grain sampling
Chickpea samples were collected from 150 in-farm storages across five growing districts in 2018. Following discussions with district-level crop production experts, 30 chickpeas growing households were randomly selected from each growing districts. Each household, samples taken, was considered as a sampling unit. About 1kg chickpeas stored in storage structures were taken from every third household. To reach chickpea-growing households in five districts, eight kebeles were considered: Tech Tseda and Maksegnit (Dambia), Meskel Kiristos and Gorgora (Gondar zuria), Hatsebo (Laeilaymaichew), Dankaka and Giche (Ada'a), and Gogit II (Meskan). The use of district-level data analysis allowed for a more targeted approach to identifying households growing chickpeas and farmers in these districts have years of experience and knowledge of chickpea farming, which can help increase productivity. Purposive sampling enabled us to select districts that were representative of the chickpea-growing regions, while simple random sampling ensured that the households within those districts were chosen reasonably. Samples were properly labeled with the name of the location and date of collection. During sampling, additional information on the duration of storage (how long it was stored from harvest), type of storage structure and approximate quantity of stored seed, type of pesticide treatment used, grain moisture content, and variety of the crop were recorded.
Determining moisture content and evaluating kernel mold infection and germination
Before subsampling for laboratory analysis, the chickpea moisture content and the intergranular temperature were determined using a grain moisture meter [36], according to the manufacturer’s instructions. For mycological analysis, 100 chickpea kernels per sample were randomly taken and surface sterilized by soaking in 1% sodium hypochlorite solution for 1 minute and rinsed off three times in sterile distilled water. From each sample, 25 sterilized kernels were transferred into plates containing Potato Dextrose Agar (PDA) amended with 0.1% streptomycin and the agar plates were incubated at 25oC for 7days [25]. The percentage of fungal infection was calculated by counting the kernels with visible fungal growth and dividing it by the total number of chickpea kernels placed on plate, and multiplied by 100. Fungi infecting chickpea kernels were identified to the level of the genus on the basis of their cultural and morphological characteristics, after sub-culturing into a Czapek agar and Aspergillus differentiation media plate. Aspergillus, Fusarium, and Penicillium genera isolated from chickpea kernels were confirmed at the Kansas state university food science laboratory. From each sample, 100 randomly selected chickpea kernels were taken and placed in 215 × 215 mm plastic petri dishes lined with filter papers for germination test. The plates were kept in a germination chamber fitted with fluorescent lights, with a 12 h light and 12 h dark-light cycle, at a temperature of 25°C. Sterile distilled water was added to each plate on a daily basis to maintain adequate moisture in each plate. Taking records of germination, in each sample, commenced after seven days of incubation. The germination percentage was calculated by the number of kernels germinated*100/ total kernels used.
Mycotoxin analysis
A representative 300 grams of each chickpea sample were ground to a fine powder using an electrical grinder (DE-500g), then sieved using a 1 mm mesh size sieve and stored in sealed Ziplock bags at 4ºC. Sample preparation, mycotoxin extraction, and analysis were performed according to the manufacturer’s instructions (Romer Labs, Union, Missouri, USA).Total aflatoxin was determined using the AgraStrip test kits, COKAS1600W and COKAS1600WS while total fumonisins were determined using the COKAS3000A test kit, Deoxynivalenol using COKAS4000A watex lateral flow testing strips, and AgraQuant ochratoxin A using the COKAQ2000 test kits. All test kits used for the determination of the different toxin levels in the current study were USDA-GIPSA approved and purchased from Romer Labs. The Mycotoxins test kits were validated with chickpea samples of known standard levels provided by Romer Labs. All samples were run in duplicate wells and readings were obtained using an Agravision reader with a built-in calibration curve provided by the manufacturer and associated with each test kit lot. Levels of total aflatoxin, total fumonisins, and deoxynivalenol were determined using a lateral flow AgraVision reader. Ochratoxin A (OTA) levels were obtained using Stat Fax 4700 Enzyme-Linked Immuno-Sorbent Assay (ELISA) reader [37-39]. The range of detection for aflatoxin was 0 to 100 ppb with a Limit of Detection (LOD) of 3.31 ppb and a Limit of Quantitation (LOQ) of 5.0 ppb. The range of detection for fumonisin was 0 to 5000 ppb with an LOD of 300 ppb and an LOQ of 400 ppb. The range of detection for deoxynivalenol was 0 to 5000 ppb with an LOD of 210 ppb and an LOQ of 250 ppb. According to the manufacturer's description, the range of detection for ochratoxin (OTA) was 2 to 40 ppb with an LOD of 1.9 ppb and an LOQ of 2 ppb. Ochratoxin was done with agraquant (COKAQ2000) methods and readings were obtained using stat fax 4700 ELISA reader [5,40]. For readings above the maximum limit, the extracts were diluted until a measurement within the range of detection was obtained, and this concentration was recorded after applying the proper dilution factor.
Data analysis
Mycotoxin occurrence data were analyzed using descriptive statistics such as frequency and central tendency measures. Means were subject to multiple comparisons using Tukey’s HSD test at a 5% level of significance. Differences in occurrence of Aspergillus, Penicillium, and Fusarium genera and aflatoxin, fumonisin, and deoxynivalenol and ochratoxin concentrations were compared using the nonparametric one-way ANOVA using R-Version 3.5.1 (2018). Pearson’s correlation coefficients were calculated to determine the relationship between aflatoxin, fumonisin, deoxynivalenol, and ochratoxin concentrations and storage conditions. Statistical analysis was performed using Reversion 3.5.1 (2018), and all tests were performed at a probability level of p < 0.05.
Results
Storage methods, chickpea variety, and insecticide application
Among storage methods, polypropylene bags (54.7%) is the most commonly used method of chickpea storage followed by gotta (45.3%) across studied districts (N=150). More than 40% of the surveyed households in the districts of Laeilaymaichew, Gondar Zuria, and Dambia stored their chickpea in gotta storage structures (Table 1).
| Table 1: Proportion of chickpea type variety and storage methods among samples across studied districts (N = 150). | ||||
| Districts | The proportion of chickpea type (%) | Storage methods (%) | ||
| Desi | Kabuli | Gotta/Gimbi (% of all types) | polypropylene bag (% of all types) | |
| Dambia | 93.3a | 6.7d | 40.0c | 60.0b |
| Ada’a | 10.0d | 90.0a | 0.0d | 100a |
| Laeilaymaichew | 43.3c | 56.6b | 100a | 0.0d |
| Gondar Zuria | 43.3c | 56.6b | 86.7b | 13.3c |
| Meskan | 50.50b | 50.0c | 0.0d | 100a |
| P-values | 0.001 | 0.001 | 0.001 | 0.001 |
| Groups within a column sharing the same letter are not significantly different at Fisher’s 5% level of significance. | ||||
The data collected show that gotta storage is the most common method of storing chickpeas in these three districts (Table 1). Where 100% of households in Ada’a and Meskan districts and 60% in Dambia districts stored their chickpeas in polypropylene bags. While using polypropylene bags to store a chickpea is much more common in the Ada’a and Meskan districts.
Regarding chickpea type varietal distribution, 52% of samples belonged to the Desi and 48% were Kabuli type across study area. There were significant differences among samples between the two chickpea type across five districts (p < 0.001). The proportion of Desi type in Dambia, Meskan, Laeilaymaichew, Gondar Zuria, and Ada’a were 93.3%, 50%, 43.3%, and 10.0%, respectively (Table 1). The majority (93.3%) of chickpea samples from the Dambia district was of the Desi type and the rest (6.7%) were of the Kabuli type. The proportion of Desi type (43.3%) and Kabuli type (56.7%) were similar in Laeilaymaichew and Gondar Zuria districts. Kabuli type accounts for 90.0% of the total sample from Ada’a while 10.0% was Desi type. The proportion of Desi and Kabuli type in chickpea samples were 50% of each from Meskan district. None of the above discussed samples received any insecticide treatment.
Moisture, relative humidity, and temperature inside chickpea across districts
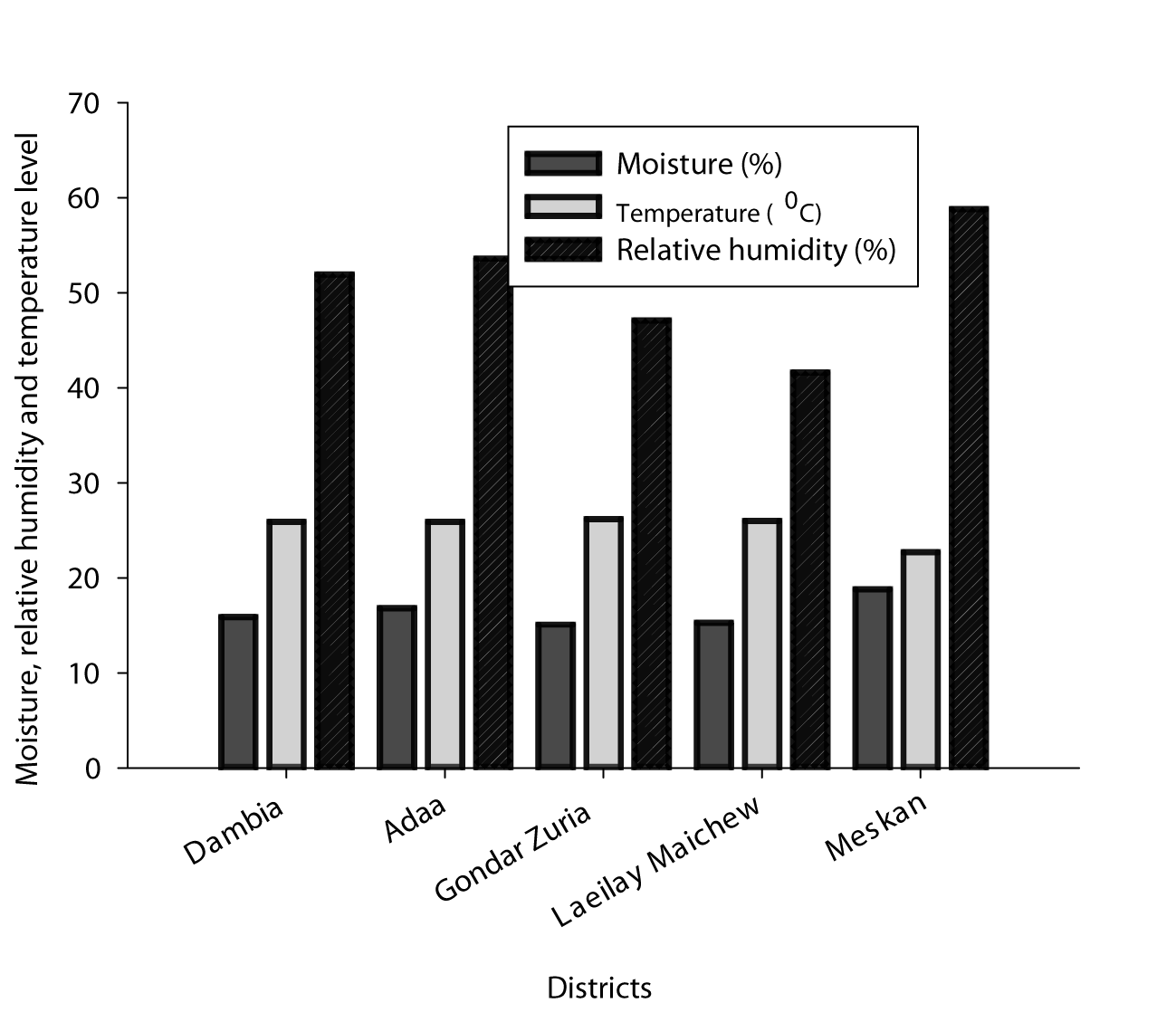
Moisture content in chickpea samples ranged from 13.3% to 22.3%, with a mean value of 16.4% (N = 150) across the districts (Figure 2). Overall, 93.3% of chickpea samples contained moisture content above the ESA limit (14% for chickpea storage in Ethiopia). Moisture content varied significantly across chickpea growing districts (p < 0.001). Chickpea samples collected from Meskan districts had the highest grain moisture content (18.8%), followed by Ada’a (16.8%). Dambia (15.9%), Laeilay Maichew (15.3%) and Gondar Zuria (15.1%), had the lowest moisture content and the difference was not statistically significant. The inter-granular temperature across the studied districts ranged from 22.70C to 26.20C. However, Ada’a, Dambia, Laeilay Maichew, and Gondar Zuria were statistically similar but lower in Meskan districts. Relative humidity inside the traditional storage structures ranged from 29.5 to 77.4%. Meskan districts had the highest relative humidity, whereas Laeilay Maichew the lowest. There was no significant difference between the two chickpea type varieties regardless of moisture, temperature, and relative humidity.
Fungal incidence of chickpea kernels
The proportion of chickpea kernels infected by Aspergillus, Penicillium, and Fusarium genera in the five districts are shown in table 2. On average, 48.7% of chickpea kernels were infected with level of infection (range: 23 – 79%). Aspergillus genera 44.3% (25 – 62.5%) followed by Penicillium 34.3% (10.9 – 55.3%) were the dominant fungal genera isolated while the proportion of chickpea infection by Fusarium genera was the lowest 21.4% (9.6-42.3%). Analysis of variance showed significant (p < 0.05) differences in the proportion of infection among the fungi within the district. The proportion of infection of Aspergillus genera was significantly higher in Ada’a (48.0 ± 8.2%), Meskan (44.6 ± 7.0%), Laeilaymaichew (44.0 ± 6%), and Gondar Zuria (46.4 ± 7.6%) and levels among these four districts were statistically similar. Lower proportion of chickpea kernels infected by Aspergillus genera was observed from Dambia (38.2 ± 6.0). The proportion of kernels infected by Penicillium genera varied significantly (p ≤ 0.001) among chickpea sampled districts. Overall, the highest proportion of Penicillium genera infected kernels was observed from Meskan (24.1 ± 6%) followed by Ada’a (23.3 ± 6%) and Laeilaymaichew (23.1 ± 8%) districts and levels among these three districts were statistically similar to one another. The proportion of kernels infected by Fusarium genera varied significantly (p ≤ 0.001) among chickpea sampled districts. The proportion of kernels infected by Fusarium genera from Dambia district (44.7 ± 7.5) was higher than other sampled districts. Chickpea samples from Meskan, Ada’a and Laeilaymaichew had statistically similar Fusarium infection.
| Table 2: The proportion of chickpea kernels infected by Aspergillus, Penicillium and Fusarium genera in five chickpea growing districts (N = 150). | ||||||
| Districts | Fungal genera (%) | |||||
| Aspergillus | Penicillium | Fusarium | ||||
| Range | Mean ± SE | Range | Mean ± SE | Range | Mean± SE | |
| Dambia | 25 - 49.3 | 38.2 ± 3.0b | 9.6 - 30.6 | 17.1 ± 1.7c | 26.8 - 55.3 | 44.7 ± 2.5a |
| Ada’a | 36.4 - 62.5 | 48.0 ± 2.7a | 11.1 - 37.0 | 23.3 ± 3.0ab | 10.9 - 43 | 28.6 ± 2.8c |
| Laeilay maichew | 34.6 - 55.7 | 44.0 ± 3.0a | 12.1 - 42.3 | 23.1 ± 2 .7ab | 23.1 - 41.3 | 32.8 ± 0.6bc |
| Gondar Zuria | 33.3 - 61.9 | 46.4 ± 2.5a | 10.3 - 30.6 | 19.5 ± 1.7bc | 21.6 - 43.4 | 34 ± 1.9b |
| Meskan | 32.1 - 57.4 | 44.6 ± 3.5a | 10.0 - 35.7 | 24.1 ± 3.0a | 18.6 - 46 | 31.2 ± 2.3c |
| F value | 9.5 | 7.3 | 24.0 | |||
| p value | <0.001 | <0.001 | <0.001 | |||
| Groups within a column sharing the same letter are not significantly different 5% level of significance. | ||||||
Kernels infection and chickpea seed germination
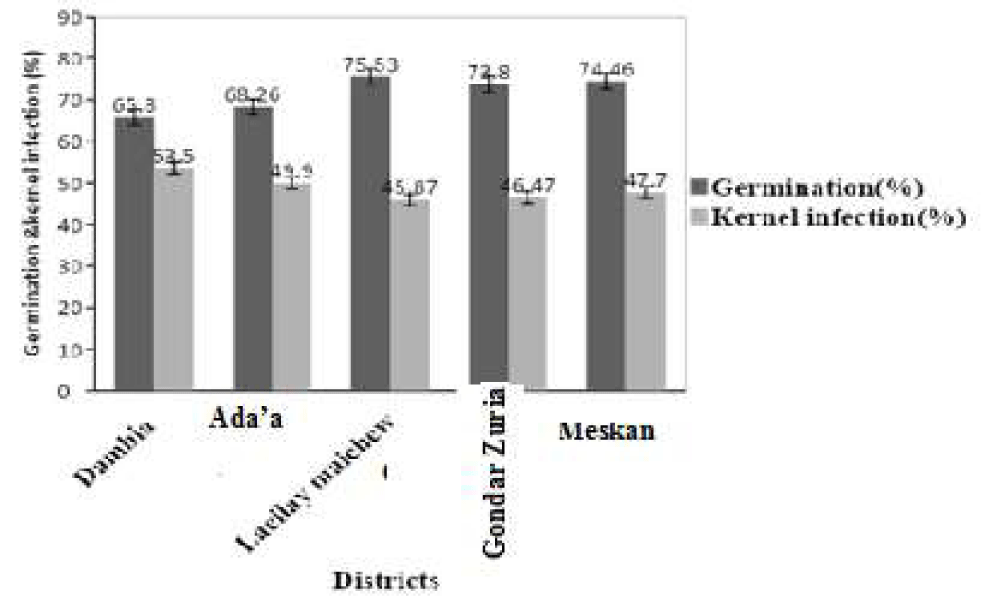
Percent seed germination and kernel infection in five chickpea growing districts are presented in in figure 3. The germination of chickpea kernels in all the growing districts ranged from 68.8% to 75.5%. The results of the experiment showed that there were significant differences (p < 0.001) in the percent chickpea germination across the five chickpea growing districts. The mean percent kernel germination in sampled districts were 65.80% (range = 50-90%), 68.7% (range = 46-8%), 75.5% (range = 53-90%), 73.8 % (range = 45-85%) and 74.7% (range = 53-85%) in Dambia, Ada’a, Laeilay maichew, Gondar Zuria and Meskan, respectively. The percentage germination rate in the chickpea samples collected from Laeilay maichew, Gondar Zuria and Meskan were higher in comparison to the samples from Dambia and Ada’a. There was no significant difference (P=0.283) of Kernel infection between chickpea growing districts where the samples were collected.
Incidences and levels of mycotoxins in chickpea samples
Total aflatoxin: Table 3 showed that total aflatoxins were detected in 92% of the chickpea samples (N = 150). The overall level of total aflatoxins in detected samples ranged from 2.5 ppb to 31.1ppb with a mean value of 17.4 ppb. About 83.3% of chickpea samples exceeded the 10ppb total aflatoxin limit set by Ethiopian standard authority, whereas only 26% of chickpea samples exceeded the 20ppb, the maximum tolerable limit set in the United States. The total aflatoxins levels were significantly different among samples across surveyed districts (p < 0.001). The overall mean of total aflatoxins was higher in samples from Meskan (21.1 ± 0.8ppb), Ada’a (18.6 ± 0.8ppb), and Laeilay Maichew (16.72 ± 0.73ppb) districts, whereas, the lowest level (12.5±0.7ppb) was recorded in samples from Dambia districts. Total aflatoxins varied significantly between the chickpea type varieties (Table 4). The highest total aflatoxins were detected in the Kabuli type (18.5 ± 0.6 ppb). A lower concentration of total aflatoxins was found in the Desi type (local). The mean concentration of total aflatoxins between the two storage methods was similar. These have significant effects on food safety, health, the economy as well as international trade in the country. Considering the findings in this study, steps toward mold and aflatoxin mitigation strategies in grain storage are necessary. Aflatoxin levels had shown a positive correlation with temperature, moisture, relative humidity, and fungal infections.
| Table 3: Comparison of mycotoxin concentrations in farm-stored chickpea samples with regulatory standards (N = 150). | |||||
| Mycotoxin | No of positive samples (%) | Mycotoxin level in positive samples | (%) samples exceed tolerable limit | ||
| Range | Mean | ||||
| Total aflatoxins | 92 | 2.5 - 31.1 | 17.38 | 83.3 >10μg/kg(ESA) | 26>20μg/kg(FDA) |
| Ochratoxin A | 92.7 | 4.3 - 35.0 | 10.6 | 84>5μg/kg (EU) | |
| Deoxynivalenol | 6.7 | 0.5 - 4.9 | 2.05 | 4.7>750μg/kg(EU) | |
| Total fumonisin | 18.7 | 0.2 -2.9 | 0.82 | 2>2000μg/kg(ESA) | |
| ESA = Ethiopian Standard Authority; FDA = Food and Drug Administration; EU = European Union. Mean concentrations were calculated from positive samples. | |||||
Ochratoxin A: Ochratoxin A concentrations detected in infected chickpea ranged from 4.3 to 35.0ppb with the mean value of 10.6ppb. The infection of chickpea samples contaminated with ochratoxin A ranged from 83.3 to 100% (N = 150) across sampled districts. The highest mean ochratoxin A was detected in chickpea samples collected from Ada’a (11.9 ± 0.8ppb), while the lowest was in Dambia (8.76 ± 0.8ppb). The mean level of ochratoxin A in chickpea samples collected from Gondar Zuria, Laeilay Maichew, and Meskan districts was statically similar. The mean concentration of ochratoxin A also varied among chickpea varieties. Kabuli type chickpea variety had the highest ochratoxin A concentration (11.2 ± 0.5ppb), while the lower concentration was found in Desi type chickpea variety (9.7 ± 0.5). There was no significant difference between the mean concentrations of ochratoxin A among the two storage methods. The results showed that 150 samples, 15.3%, exhibited levels below the ochratoxin A MTL 5ppb, maximum level set by the European Union, whereas, 126 (84%) samples of the chickpea had a content exceeding the limit.
Total fumonisin and deoxynivalenol: The levels of total fumonisin contamination in chickpea samples examined in the current study are shown in tables 2,3.
Total fumonisin was detected on 18.7% samples. The mean concentration detected ranged from 0.32 ppm to 1.1ppm. Only 4.7% of chickpea samples exceeded the 2ppm maximum level set by the Ethiopian standard authority for total fumonisin. The mean concentration of total fumonisins was statistically similar across the districts, varieties and chickpea storage methods. However, overall fumonisin was not detected in samples collected from Meskan areas. Deoxynivalenol was detected in 6.7% of chickpea samples and the level of deoxynivalenol concentration ranged from 0.5ppm to 4.9 ppm. There was no significant difference in concentration of deoxynivalenol across districts, varieties, and storage methods.
The percentage of chickpea samples with mycotoxin concentrations exceeding regulatory limits are described in table 3. Chickpea samples in overall exceed the FDA 20μg/kg and ESA (10μg/kg) tolerable limits for total aflatoxins by 26% and 83.3%, respectively. Nevertheless, the average level of total aflatoxins across all chickpea samples was within FDA's acceptable range. Owing to the unavailability of Ochratoxin A and Deoxynivalenol regulation limits in Ethiopia (ESA), the current research used EU tolerable limits for comparison. Accordingly, 84% chickpea samples exceeded EU tolerable limit (5μg/kg) for Ochratoxin A and 4.7% samples exceeded tolerable limit (750μg/kg) for Deoxynivalenol.
Aflatoxin, Fumonisins, DON and Ochratoxin levels showed a positive correlation with temperature, moisture, relative humidity, and fungal infections. However, germination negatively correlated with temperature, moisture, relative humidity, and fungal infections, it decreased with the increase in biotic factors. Thus, it can be concluded that fungal infections have a strong correlation with temperature, moisture, and relative humidity, but negatively with kernel.
Co-occurrence of mycotoxins in chickpea samples
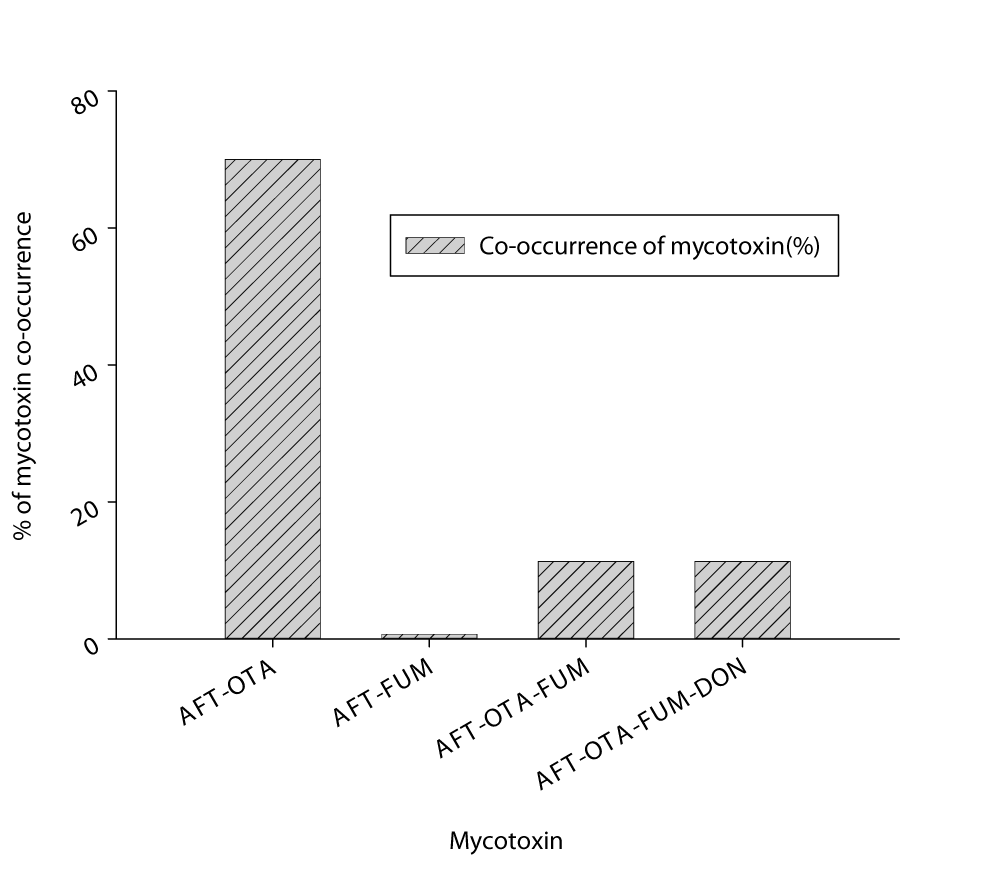
Figure 4 shows the co-occurrence of mycotoxins observed in chickpea samples. Total Aflatoxin (AFT) and Ochratoxin (OTA) co-occurred in 70% of chickpea samples, whereas Total Aflatoxin (AFT) and Total Fumonisin (FUM) co-occurred within only 0.7% of chickpea samples. In 11.3% of the samples, Total Aflatoxin (AFT), Ochratoxin (OTA), and Fumonisin (FUM) were detected together. AFT-OTA-FUM-DON, four mycotoxins, co-occurred in 11.3% of the samples. Our results emphasize the need of monitoring and reducing mycotoxin contamination in chickpeas to maintain food safety and prevent health risks.
Discussion
Farmers in the studied districts produced a considerable amount of chickpea for food, seed and trade. Traditional methods were used to store chickpea on-farm yard. The lack of fungal resistant varieties, inadequate storage facilities, and a prolonged storage period contributed to the major postharvest losses of chickpea in Ethiopia [6,19,41]. Except for total aflatoxin and ochratoxin A, there was a significant difference in physical environments, chickpea kernel germination, fungal incidence, and mycotoxin levels among chickpea type varieties (Table 4). This suggests that different chickpea varieties may have varying levels of resistance to fungal infections and mycotoxin contamination, which could have implications for food safety and crop management strategies. Thus, to minimize these mycotoxin contaminations and make chickpea production more sustainable in Ethiopia, there is a need for developing new fungal resistant varieties of chickpea that are adapted to local agroecological zones as well as enhancing modern storage infrastructure. The current findings indicated that large population of small-scale farmers use conventional chickpea storage strategies, which is consistent with earlier studies conducted in the region [30,42,43]. This implies that farmers are limited to modern storage techniques as alternatives to traditional storage strategies, which contribute to molds and mycotoxins in stored products in Ethiopia [44,45]. This limitation has caused stored products to be of inferior quality, as evidenced by the presence of molds and mycotoxins in the current study. This is a major concern to the Ethiopian farmers since fungi and mycotoxins affect stored products and thus, hinder trade. The current study found that Aspergillus and Penicillium genera, as well as their toxins, are predominant in chickpea kernels across all sampled districts in Ethiopia. In particular, the highest frequency of toxin-producing Aspergillus and Penicillium species was observed in chickpea kernels from all districts. This is consistent with previous studies [22], which found that Aspergillus and Penicillium species are abundant in chickpea storage. The research highlighted that this increase in storage fungi was most likely due to the presence of organic material in the grain, which provided an abundant source of food for their growth. Chickpea samples from Ada’a, Laeilaymaichew, Gondar Zuria, and Meskan districts had high levels of Aspergillus, Penicillium, total aflatoxins, and ochratoxin A. The research further indicated that high temperatures and relative humidity in grain storage conditions favored the growth of fungi, which contributed to an increase in total aflatoxins and ochratoxin A in the grains. Due to their larger surface area and higher nutritional composition, Kabuli type chickpeas may be more susceptible to fungal infection and mycotoxin contamination. The findings of this research have important implications for grain storage practices in Ethiopia, as high temperatures and humidity can facilitate the growth of fungi, leading to increased levels of mycotoxins in stored grains. In some of the sampled districts, the presence of a high proportion of local Desi chickpea and a low proportion of Kabuli chickpea revealed that farmers had limited access to improved seed distributions [2,46-48]. This indicates that the majority of farmers relied on conventional agricultural practices, which, despite their durability and drought resistance, had limited yield potential as compared to those who utilized improved seed varieties. A lack of quality control mechanisms, storage technologies, and awareness regarding storage molds and mycotoxins magnifies poor seed quality in small-scale farmer systems [49,50]. Farmers have limited control over the quality of their produce and do not have access to the most up-to-date agronomic practices due to a lack of access to quality seed. Overall, moisture levels in 93.3% of chickpea samples from farmers' storage systems exceeded the 14% limit recommended for short-term storage [1]. The high grain moisture levels and relative humidity in some study districts may create an ideal environment for fungi to grow and mycotoxin production in chickpea. As a consequence, farmers may face large economic losses as a result of low seed quality, as well as potential health risks from eating contaminated crops. According to studies conducted by Bhandari, et al. [51], Jayaraman, et al. [52] and Likhayo, et al. [43], higher temperatures and increased moisture content create an ideal environment for mold infection. These results showed that Aspergillus, Penicillium, and Fusarium spp. were in the chickpea kernels from the main producing areas. The study also found that Aspergillus and Penicillium species were more likely to be identified in kernels from colder and drier locations, whilst Fusarium species were more likely to be detected in warmer and more humid configurations. This is consistent with previous studies from India [21,25], Pakistan [53] and Turkey [27], which found similar fungal genera in chickpea grown in these countries. Chickpea infections with these pathogens also contaminate with their respective mycotoxins [27]. Mycotoxins are toxic chemicals that may harm people and animals. The germination rate of chickpea kernels obtained from all growing districts ranged from 68.8% to 75.5%, which was lower than the recommended range of 85-90%. This suggests that a fungal pathogen infection harmed the germination potential of these chickpeas [51,54,55]. The results of this study indicate that better crop management strategies are urgently needed to lower the prevalence of chickpea infections by fungal pathogens and consequent mycotoxin contamination. Likewise, it is evident that mycotoxin contamination may considerably reduce crop yield, resulting in economic losses in agriculture. Lack of proper storage facilities, crop susceptibility, and moisture level are all factors that lead to fungal infections and mycotoxin contamination, resulting in post-harvest chickpea grain loss [21,44,56,57]. Such losses are a major issue for smallholder farmers in developing countries, since chickpeas provide an important source of dietary nutrients as well as a key contributor of income. Yet, the mean total aflatoxin concentration (range from 2.5 to 31.1μg/kg) in the current study's chickpea samples was lower than that found in previous Indian reports [25], which found 5.5-205μg/kg in stored chickpea seeds. Nevertheless, 83.3% of chickpea samples exceeded the Ethiopian Standard Authority's acceptable limit 10μg/kg). This indicates that a significant proportion of the chickpea samples assessed have an unusually high level of aflatoxin, which poses major health risks. Similarly, in terms of frequency and concentration, the chickpea samples in the current study revealed higher levels of ochratoxin A than those from Iran [39]. This is a concern since ochratoxin A has been linked to cancer and to toxicity of the liver, kidneys, and cardiovascular system. These findings indicate that, although there are still some issues with aflatoxin and ochratoxin A contamination in chickpeas, these levels are generally above acceptable ranges. Climatic changes, poor storage strategies, old grain residue that might contaminate fresh grains, and the growing environment could be a cause [58-60]. Consequently, it is important that the necessary steps be taken to reduce the contaminants' levels. Several studies [61,62] have shown that mycotoxins can get into food while it is being stored. Mycotoxins, which are fungal metabolites, can contaminate crops while they are being stored. High levels of mycotoxins and the presence of four toxins may cause both quantity and quality losses in chickpeas, increasing hunger, malnutrition, reduced household income and health problems. These implications need urgent attention in creating awareness about their effects and adopting preventive strategies to limit mycotoxin contamination in food. Our findings revealed a significant variation in fungal incidence and mycotoxin contamination in chickpea samples from the studied districts. According to Pinotti, et al. [63], crop susceptibility, grain morphological variations, and moisture content variations between chickpea varieties might all contribute to the observed difference in mycotoxin prevalence rate. The present investigation found a link between fungal infections and mycotoxin contamination and high moisture content, which is consistent with previous findings [54,63]. This was also confirmed by the existence of positive and significant associations between fungal infections and all physical environments. Even at low concentrations, the co-occurrence of mycotoxins in this research may make them more toxic and pose serious health risks to individuals who eat chickpeas on a daily basis. Previous studies [60,62] demonstrated that exposure to these toxins over an extended period of time might have long-term effects. Mycotoxins are becoming more common in Ethiopia for a variety of reasons, including climatic conditions, poor storage practices, lack of resistant varieties, modern storage facilities, and regulatory restrictions. Since chickpeas are such a popular household nutritional and economic source in Ethiopia, strategies to improve their quality and limit mycotoxin levels are crucial. Despite efforts to prevent fungal contamination, many countries throughout the globe have established regulatory guidelines for mycotoxin [60,64]. These mycotoxin regulation rules are critical for food safety and security, as well as consumer health. We compared current mycotoxin levels in chickpea with ESA, FDA, and EU tolerable limits (Tables 5,6).
| Table 4: Table Incidence and levels of mycotoxins in chickpea samples from each district of Ethiopia. | ||||||||
| Mycotoxin incidences and level in positive samples | ||||||||
| Total aflatoxins(ppb) | Ochratoxin A(ppb) | Total Fumonisins(ppm) | DON(ppm) | |||||
| District | Incidence (%) | Mean ± SE | Incidence (%) | Mean ± SE | Incidence (%) | Mean ± SE | Incidence (%) | Mean ± SE |
| Dambia | 96.7 | 12.5 ± 0.8c | 100 | 8.7 ± 0.8b | 30.0 | 0.9 ± 0.2 | 23.3 | 2.1 ± 0.6 |
| Ada’a | 86.7 | 17.5 ± 0.8b | 93.3 | 11.9 ± 0.8a | 20.0 | 1.1 ± 0.3 | 0.0 | 0.0 ± 0.0 |
| Laielayamychw | 100 | 16.7 ± 0.8b | 83.3 | 10.7 ± 0.8ab | 3.3 | 0.3 ± 0.0 | 3.3 | 0.6 ± 0.0 |
| Gondar Zuria | 100 | 18.7 ± 0.8ab | 96.7 | 10.0 ± 0.8ab | 40.0 | 0.6 ± 0.25 | 6.7 | 2.0 ± 1.4 |
| Meskan | 90.0 | 21.1 ± 0.8a | 93.3 | 11.2 ± 0.8ab | 0.0 | 0.0 ± 0.0 | 0.0 | 0.0 ± 0.0 |
| Groups within a column sharing the same letter are not significantly different at 5% level of significance. | ||||||||
| Table 5: Storage conditions and quality of chickpea across varieties and storage methods. | ||||||||||
| Varieties | No samples | Moisture (%) | Temperature (°C) | Humidity (%) | Germination (%) | Incidence | Totalaflatoxins (ppb) | Ochratoxin A(ppb) | Fumonisins(ppm) | Deoxynivalenol (ppm) |
| Desi | 72 | 16.6 ± 0.2 | 25.3 ± 0.3 | 50.5 ± 0.9 | 70.8 ± 1.0 | 49.4 ± 1.8 | 15.9 ± 0.6b | 9.7 ± 0.5b | 0.8 ± 0.2 | 1.8 ± 0.6 |
| Kabuli | 78 | 16.2 ± 0.2 | 25.4 ± 0.3 | 50.7 ± 0.9 | 72.3 ± 1.3 | 48.0 ± 1.7 | 18.5 ± 0.6a | 11.2 ± 0.5a | 10.0 ± 0.2 | 2.1 ± 0.9 |
| F1,148 | 1.17 | 0.103 | 0.018 | 0.659 | 0.346 | 9.98 | 4.22 | 0.452 | 0.078 | |
| p-value | 0.28 | 0.749 | 0.895 | 0.418 | 0.557 | 0.001 | 0.04 | 0.507 | 0.789 | |
| Method | ||||||||||
| Gota | 69 | 15.3 ± 0.2b | 26.1 ± 0.3a | 45.5 ± 0.8b | 72.4 ± 1.4 | 47.4 ± 1.8 | 16.5 ± 0.6 | 9.96 ± 0.5 | 0.78 ± 0.1 | 2.5 ± 0.6 |
| Ppbags | 71 | 17.3 ± 0.2a | 24.8 ± 0.3b | 54.8 ± 0.8a | 70.9 ± 1.2 | 49.7 ± 1.7 | 17.8 ± 0.5 | 10.9 ± 0.5 | 0.95 ± 0.1 | 1.1 ± 0.7 |
| F1,148 | 61.76 | 9.45 | 67.05 | 0.61 | 0.824 | 2.45 | 1.64 | 0.419 | 2.367 | |
| p-value | 0.001 | 0.0024 | 0.001 | 0.436 | 0.366 | 0.12 | 0.202 | 0.523 | 0.167 | |
| Table 6: Pearson correlation between fungal infection, physical environment, and mycotoxin concentration. | ||||||||
| Variables | Temperature | Moisture | Relative humidity | AFT (ppb) |
FUM (ppm) |
DON (ppm) |
OTA (ppb) |
Kernel Germination % |
| AFT | 0.4*** | 0.5*** | 0.5*** | |||||
| FUM | 0.4*** | 0.2NS | 0.3** | 0.4*** | ||||
| DON | 0.3** | 0.2NS | 0.2NS | 0.3** | 0.6*** | |||
| OTA | 0.4*** | 0.3** | 0.3** | 0.3** | 0.3** | 0.2NS | ||
| Germination | -0.6*** | -0.4*** | -0.5*** | -0.3** | -0.5*** | -0.4*** | -0.2NS | |
| Infections | 0.7*** | 0.5*** | 0.6*** | 0.4*** | 0.4*** | 0.3** | 0.3** | -0.8*** |
| AFT = Total Aflatoxins; DON = Deoxynivalenol; FUM = Total Fumonisin; OTA = Ochratoxin A; Ppb = Parts Per Billion; Ppm = Parts Per Million; *, **, *** Correlations Are Significant At 5% And 1% Levels Of Significance. NS = Correlations Are Not Significant At 5% Level of Significance. | ||||||||
Conclusions and Recommendations
The results of the survey led to the following conclusions: Due to a lack of access to adequate and modern storage facilities, small-scale farmers in Ethiopia are facing the challenge of chickpea post-harvest losses. Fungal infection and mycotoxin contamination were found in chickpea samples collected from various districts, with total aflatoxin and ochratoxin A being the most frequently detected toxins. total aflatoxin and ochratoxin A levels varied significantly across locations and chickpea type varieties, with some districts exceeding recommended human consumption guidelines. However, the majority of chickpea samples had lower levels of deoxynivalenol and total fumonisin than the EU and ESA, respectively. The coexistence of mycotoxins, even at low levels, may result in increased toxicity and serious health consequences for regular chickpea consumers due to their potential synergistic effects on humans. Chickpea kernel germination rates were lower than the recommended range. Due to the long-term effects of mycotoxins on consumer health, prioritize appropriate on-farm management practices, the establishment of regular monitoring programs, and routine testing of chickpea grains for mycotoxins, which are critical in Ethiopia. Furthermore, these findings can serve as a foundation for future research and regulatory bodies to reduce the risk of mycotoxin exposure in humans and animals, as well as to set regulatory limits for remaining toxins. Collaboration among government agencies, researchers, and industry stakeholders is also essential for creating effective mycotoxin management strategies in chickpea production. It is important to involve and educate farmers on the importance of mycotoxin management and provide them with the necessary resources to ensure the safety and quality of their chickpea. This may require additional resources and support, particularly for smallholder farmers who may face financial constraints.
Funding
This work was supported by American people through the United States Agency for International Development (USAID) under the Feed the Future initiative (www.feedthefuture.gov) with grant number AID-OAA-L-14-00002. The contents are the responsibility of the Innovation Lab for the Reduction of Post-harvest Loss (www.k-state.edu/phl) and do not necessarily reflect the views of USAID or the United States Government. Informed Consent Statement: “Not applicable.” for studies not involving humans.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jspr.2019.101526
Ddeclaration of competing interest
Authors declare that the above information about my private interests is correct to the best of my knowledge, and I am aware of my responsibilities to take reasonable steps to avoid any real or apparent conflict of interest in connection with my public service. All authors contributed to the article's conception and design, as well as its analysis and interpretation of data; drafting the article or critically revising it for important intellectual content; and final approval. This manuscript has not been submitted to, or is currently being reviewed by, any other journal or publishing venue. The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- Van Gastel AJG, Bishaw Z, Niane AA, Gregg BR, Gan Y. Chickpea seed production. Chickpea Breed Manag. 2007;417-444. doi: 10.1079/9781845932138.020.
- Boere A, Rutgers T, Willems D, Kidane D, Dolfen W. Business opportunities report: Oilseeds and pulses. Ethiopian Netherlands Bus event. 5-6 November 2015.
- Joshi PK, Rao PP, Gowda CLL, Jones RB, Silim SN, Saxena KB, Kumar J. The world chickpea and pigeon pea economies: Facts, trends, and outlook. 2001.
- Gowda C, Gowra C. Global scenario of chickpea research-present status and future thrusts. Pulses New Perspect. 2004;22.
- Alemayehu S, Abay F, Ayimut KM, Assefa D, Chala A, Mahroof R, Harvey J, Subramanyam B. Evaluating different hermetic storage technologies to arrest mold growth, prevent mycotoxin accumulation and preserve germination quality of stored chickpea in Ethiopia. J Stored Prod Res. 2020;85:101526. doi: 10.1016/j.jspr.2019.101526.
- Haile A. On-farm storage studies on sorghum and chickpea in Eritrea. African J Biotechnol. 2006;5:1537-1544.
- Thudi M, Roorkiwal M, Kudapa H, Chaturvedi SK, Singh NP, Varshney RK. An overview of chickpea research: From discovery to delivery. Pulse India. 2017;11:22-25.
- Francom MG, Counselor A. Ethiopia’s oilseed sector set to expand. GAIN Report. 2018;19:15.
- Getachew T. Pulse crops production opportunities, challenges and its value chain in Ethiopia: A Review Article. Journal of Environment and Earth Science. 2019;9:20-29. doi: 10.7176/JEES/9-1-03.
- Kassie M, Shiferaw B, Asfaw S, Abate T, Muricho G, Ferede S, Eshete M, Assefa K. Current situation and future outlooks of the chickpea sub‐sector in Ethiopia. Icrisat. 2009.
- Tadesse M. Survey of chickpea (Cicer arietinum L.) Ascochyta blight (Ascochyta rabiei) disease status in production regions of Ethiopia. Plant. 2017;5:23.
- Jones R, Audi P, Shiferaw B, Gwata E. Production and marketing of Kabuli chickpea seeds in Ethiopia: Experiences from Ada District. 2006;1-48.
- Korbu L, Damte T, Fikre A. Harnessing chickpea value chain for nutrition security and commercialization of smallho l der agriculture in Africa. Ethiopia. 2016.
- Muehlbauer FJ, Sarker A. The Chickpea Genome. Springer; 2017. p.5-13.
- CSA. Report on area and production for major crops (private peasant holdings, Meher season). Stat Bull No 584. 2017.
- Singh N, Dash S, Khan YJ. Survival of chickpea, sesame, Niger, castor and safflower seeds stored at low and ultra-low moisture contents for 16-18 years. Seed Sci Technol. 2016;44:542-555.
- Patel JV, Antala DK, Satasiya RM. Effect different packaging materials on quality of chickpea grain during storage. Int J Pure Appl Biosci. 2018;6:437-446. doi: 10.18782/2320-6754.
- Ferede S, Fikre A, Ahmed S. Assessing the competitiveness of smallholder’s chickpea production in the central highlands of Ethiopia. 2018;6(2):51-65.
- Befikadu D. Factors affecting quality of grain stored in Ethiopian traditional storage structures and opportunities for improvement. Int J Sci J Basic Appl Res. 2014;18:235-257.
- Rao N, Silim SN, Simtowe F, Gaur PM, Gowda CLL, Monyo ES, Fikre A. Assefa K, Kileo R, Thagana WM, Macharia N. Enhancing chickpea productivity and production in Eastern and Southern Africa. Agricultural Plant Science. 2009;177-190.
- Ramirez ML, Cendoya E, Nichea MJ, Zachetti VGL, Chulze SN. Impact of toxigenic fungi and mycotoxins in chickpea: A review. Curr Opin Food Sci. 2018;23:32-37. doi: 10.1016/j.cofs.2018.05.003.
- Shamsi S, Khatun A. Prevalence of fungi in different varieties of chickpea (Cicer arietinum L.) seeds in storage. J Bangladesh Acad Sci. 2016;40:37-44. doi: 10.3329/jbas.v40i1.28323.
- Adekunle AJ. Estimating post-harvest loss: A challenge of developing nations. 2018.
- Esther M, Sharon M, Abirami CVK, Alagusundaram K. Grain storage management in India. J Postharvest Technol. 2014;02:012-024.
- Ahmad SK, Singh PL. Mycofloral changes and aflatoxin contamination in stored chickpea seeds. Food Addit Contam. 1991 Nov-Dec;8(6):723-30. doi: 10.1080/02652039109374030. PMID: 1812019.
- Wu F. Mycotoxin reduction in Bt corn: potential economic, health, and regulatory impacts. Transgenic Res. 2006 Jun;15(3):277-89. doi: 10.1007/s11248-005-5237-1. PMID: 16779644.
- Gürses M. Mycoflora and aflatoxin content of hazelnuts, walnuts, peanuts, almonds and roasted chickpeas (LEBLEBI) sold in Turkey. Int J Food Prop. 2006;9:395-399. doi: 10.1080/10942910600596597.
- Mushtaq S, Akram A, Hanif NQ, Qureshi R, Akram Z, Akhund S, Nayyar BG. Natural incidence of aflatoxins, mycological profile and molecular characterization of aflatoxigenic strains in chickpea flour. Pakistan J Bot. 2015;47:1153-1160.
- Kirui MC, Alakonya AE, Talam KK, Tohru G, Bii CC. Total aflatoxin, fumonisin and deoxynivalenol contamination of busaa in Bomet county, Kenya. African Journal of Biotechnology. 2014;13:2675-2678. doi: 10.5897/AJB2014.13754.
- Wolde M. Effects of aflatoxin contamination of grains in Ethiopia. Int J Agric Sci. 2017;7:1298-1308.
- Wild CP, Gong YY. Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis. 2010 Jan;31(1):71-82. doi: 10.1093/carcin/bgp264. Epub 2009 Oct 29. PMID: 19875698; PMCID: PMC2802673.
- Manandhar A, Milindi P, Shah A. An overview of the post-harvest grain storage practices of smallholder farmers in developing countries. Agriculture. 2018;8:57. doi: 10.3390/agriculture8040057.
- Hell K, Cardwell KF, Setamou M, Poehling H. The influence of storage practices on aflatoxin contamination in maize in four agroecological zones of Benin, west Africa. J Stored Prod Res. 2000 Oct 15;36(4):365-382. doi: 10.1016/s0022-474x(99)00056-9. PMID: 10880814.
- Ayalew A, Fehrmann H, Lepschy J, Beck R, Abate D. Natural occurrence of mycotoxins in staple cereals from Ethiopia. Mycopathologia. 2006 Jul;162(1):57-63. doi: 10.1007/s11046-006-0027-8. PMID: 16830193.
- Fuffa H, Urga K. Survey of aflatoxin contamination in Ethiopia. Ethiop J Heal Sci. 2001;11:1-25.
- Danso JK, Osekre EA, Opit GP, Manu N, Armstrong P, Arthur FH, Campbell JF, Mbata G, McNeill SG. Post-harvest insect infestation and mycotoxin levels in maize markets in the Middle Belt of Ghana. J Stored Prod Res. 2018;77:9-15. doi: 10.1016/j.jspr.2018.02.004.
- Worku AF, Merkuz A, Kalsa KK, Tenagashaw MW, Habtu NG. Occurrence of mycotoxins in farm-stored wheat in Ethiopia. African J Food, Agric Nutr Dev. 2019;19:14829-14847. doi: 10.18697/ajfand.87.18565.
- Salem NM, Ahmad R. Mycotoxins in food from Jordan: Preliminary survey. Food Control. 2010;21:1099-1103. doi: 10.1016/j.foodcont.2010.01.002.
- Rahimi K, Sani AM, Azizi EG. Effect of thermal treatment on ochratoxin content of chickpea. Nutr Food Sci. 2013;43:285-290.
- Zheng Z, Hanneken J, Houchins D, King RS, Lee P, Richard JL. Validation of an ELISA test kit for the detection of ochratoxin A in several food commodities by comparison with HPLC. Mycopathologia. 2005 Feb;159(2):265-72. doi: 10.1007/s11046-004-8663-3. PMID: 15770453.
- Getaneh DG, Tefera T, Fikre L, Seid A, Minale K, Abebe K, Abinet D. Farmers’ knowledge and practices on chickpea production and disease management in major chickpea growing areas of Ethiopia. Arch Phytopathol Plant Prot. 2021;1-22. doi: 10.1080/03235408.2021.1994269.
- Bachewe F, Minten B, Taffesse AS, Pauw K, Cameron A, Endaylalu TG. Farmers’ grain storage and losses in Ethiopia. Journal of Agricultural & Food Industrial Organization. 2019. doi: 10.1515/jafio-2019-0059.
- Likhayo P, Bruce AY, Tefera T, Mueke J. Maize grain stored in hermetic bags: Effect of moisture and pest infestation on grain quality. J Food Qual. 2018;2018:1-9. doi: 10.1155/2018/2515698.
- Alemayehu S, Abay F, Ayimut KM, Assefa D, Chala A, Mahroof R, et al. Evaluating different hermetic storage technologies to arrest mold growth, prevent mycotoxin accumulation and preserve germination quality of stored chickpea in Ethiopia. J Stored Prod Res. 2020;85.
- Worku AF, Kalsa KK, Abera M, Nigus HG. Effects of storage strategies on physicochemical properties of stored wheat in Ethiopia. AIMS Agric Food. 2019;4:578-591.
- Kabata A, Henry C, Moges D, Kebebu A, Whiting S, Regassa N, Tyler R. Determinants and constraints of pulse production and consumption among farming households of Ethiopia. J Food Res. 2016;6:41. doi: 10.5539/jfr.v6n1p41.
- Kebede E. Grain legumes production and productivity in Ethiopian smallholder agricultural system, contribution to livelihoods and the way forward. Cogent Food Agric. 2020;6. doi: 10.1080/23311932.2020.1722353.
- Zewdie A, Bedasa T. Evaluation of improved chickpea varieties for resistance to Fusarium wilt (Fusarium oxysporum) under field condition in sick plot. African Journal of Agricultural Research. 2018;13:2930-2935. doi: 10.5897/AJAR2018.13655.
- Ojiewo CO. Chickpea production, technology adoption and market linkages in Ethiopia chick pea production in Ethiopia by zone. Livingstone-Zambia. 2016.
- Sisay DT, Verhees FJHM, van Trijp HCM. Seed producer cooperatives in the Ethiopian seed sector and their role in seed supply improvement: A review. J Crop Improv. 2017;31:323-355. doi: 10.1080/15427528.2017.1303800.
- Bhandari G, Ghimire TB, Kaduwal S, Shrestha J, Acharya R. Effects of storage structures and moisture contents on seed quality attributes of quality protein maize. J Maize Res Dev. 2018;3:77-85. doi: 10.3126/jmrd.v3i1.18924.
- Jayaraman P, Kalyanasundaram I. Changes in moisture content, mycoflora and aflatoxin content of rice bran during storage. Mycopathologia. 1994 May;126(2):115-20. doi: 10.1007/BF01146203. PMID: 8065431.
- Mushtaq S, Akram A, Hanif NQ, Qureshi R, Akram Z, Akhund S, Nayyar BG. Natural incidence of aflatoxins, mycological profile and molecular characterization of aflatoxigenic strains in chickpea flour. Pakistan J Bot. 2015;47:1153-1160.
- Mabrouk Y, Belhadj O. Integrated pest management in chickpea. New Perspect Plant Prot. 2012;103. doi: 10.1080/23311932.2019.1615718.
- Tekle S, Skinnes H, Bjørnstad Å. The germination problem of oat seed lots affected by Fusarium head blight. Eur J Plant Pathol. 2013;135:147-158.
- Ashiq S. Natural Occurrence of Mycotoxins in Food and Feed: Pakistan Perspective. Compr Rev Food Sci Food Saf. 2015 Mar;14(2):159-175. doi: 10.1111/1541-4337.12122. Epub 2014 Dec 12. PMID: 33401806.
- Beheshti HR, Asadi M. Ochratoxin A in several grains in Iran. Food Addit Contam Part B Surveill. 2013;6(3):200-2. doi: 10.1080/19393210.2013.788075. Epub 2013 May 24. PMID: 24779905.
- Lutfullah G, Hussain A. Studies on contamination level of aflatoxins in some cereals and beans of Pakistan. Food Control. 2012;23:32-36. doi: 10.1016/j.foodcont.2011.06.004.
- Udomkun P, Wiredu AN, Nagle M, Bandyopadhyay R, Müller J, Vanlauwe B. Mycotoxins in Sub-Saharan Africa: Present situation, socio-economic impact, awareness, and outlook. Food Control. 2017;72:110-122. doi: 10.1016/j.foodcont.2016.07.039.
- Zain ME. Impact of mycotoxins on humans and animals. J Saudi Chem Soc. 2011;15:129-144. doi: 10.1016/j.jscs.2010.06.006.
- Bowman J, Leslie J, Wu F. How mycotoxins impact agriculture, nutrition and development. USAID Bur Food Secur. 2012.
- Ramesh V, Bhat RV, Vasanthi S. Mycotoxin food safety risk in developing countries. Food Saf Food Secur Food Trade. 2003;1-2.
- Pinotti L, Ottoboni M, Giromini C, Dell'Orto V, Cheli F. Mycotoxin Contamination in the EU Feed Supply Chain: A Focus on Cereal Byproducts. Toxins (Basel). 2016 Feb 15;8(2):45. doi: 10.3390/toxins8020045. PMID: 26891326; PMCID: PMC4773798.
- Bui-Klimke TR. Investigating the impacts of mycotoxin regulations on human health and trade. Diss Abstr Int Sect B Sci Eng. 2014;75.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.