General Science . 2023 March 14;4(3):401-412. doi: 10.37871/jbres1689.
Characterization and Selection by Optical Absorption and Emission Spectrophotometry of a Series of Red Dyes Capable of Destroying Far UV Rays by Absorption
Zakaria Rached, Mohammed El-Amine Nouairi*, Ghalem Raho Bachir, Wahiba Amrani and Ali Bellil
- Red dyes (beet red
- Congo red
- Methyl red
- Neutral red
- Phenol red and carminic acid)
- Absorption and emission spectrophotometry
- Destroying far UV
- Certain red dyes have the ability to absorb light in the far ultraviolet, below 200 nm.
- Red dyes can dodge far ultraviolet rays.
- Red dyes emit above 600 nm.
Abstract
The far ultraviolet rays are harmful to human health, particularly for their mutagenic effects; it can cause skin cancer, cataracts, photochemical pollution. In the future, chemists plan to use it as weapons for the destruction of objects. Therefore, we should meet this future challenge by scientific means in order to snuff out its far-ultraviolet rays.
In this paper, we’ve intend to carry out a deep spectral study on a series of red dyes such as: beet red; congo red; methyl red; neutral red; phenol red and carminic acid, to find out which are able to damage far ultraviolet rays by absorption. The experimental results have been carried out in our laboratory; it has shown that certain red pigments have an ability to absorb light in the far ultraviolet range due to their chemical structures. Therefore, they are able to dodging far ultraviolet rays. Moreover, the spectral analyzes by the emission of the series of red dyes studied, have showed that these red dyes emit beyond 600 nm.
Introduction
Dyes are natural and synthetic substances which put in contact with a support in appropriate conditions, attach themselves to the latter in a lasting manner by imparting a certain color to it. As well as, synthetic dyes are obtained by chemical synthesis and natural dyes come from animal, mineral and vegetable sources.
There are several classes of dyes according to their chemical structures, their colorations and their areas of use. They are widely used today and play an essential role in many fields such as: dyeing, food, chemistry, biology and health.
In 1876, the German chemist Otto Witt had proposed that the coloring of molecules would be due to the existence in the molecule of groups of atoms called chromophores, having the capacity to absorb certain incident light frequencies. These groups of atoms, more or less complex, comprising numerous double bonds, they are responsible for the coloring. The most important is the nitro group (-NO2 or =NO-OH), linked to an aromatic ring, the azo group (R-N=N-R'), linked to two aromatic rings, the stilbene group (C6H5- CH=CH -C6H5), nitroso (-NO or -N-OH), carbonyl (>C=O), vinyl (-C=C-), sulphide (>C=S), triarylmethane groups, acridine, anthraquinone, indigoid etc. . Other groups of atoms can intensify or change the color due to the chromophore called auxochrome groups, such as amines (-NH2), (-NHR), (-NR2), hydroxyl (-OH) and alkoxyl (-OR), electron donor groups etc.
These groups of atoms can change the absorption frequency of a chromophore by increasing the electronic delocalization, thus modifying the energies of absorption therefore the molar extinction coefficients and the frequencies of absorption.
Natural dyes of mineral origin are mainly used in lotions and external preparations [1]. These inorganic dyes are generally characterized by their light stability and somehow by their opacity.
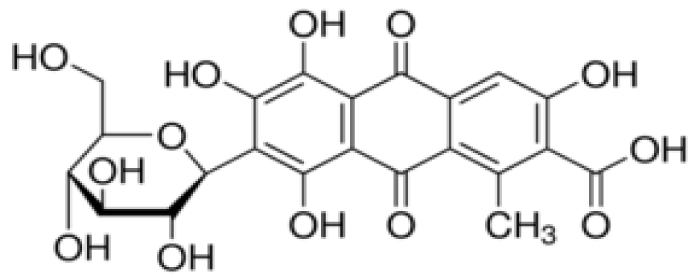
Natural dyes of animal origin are obtained from dried insects, such as carminic acid (or β-D-glucopyranosyl-7-dihydro-9,10-tetrahydroxy-3,5 acid, 6,8-methyl-1-dioxo-9,10-anthracenecarboxylic-2) (Figure 1), an anthraquinone red dye (E120: European identification code) extracted from the female cochineal (Dactylopius coccus), an insect that lives in the cacti of Peru and the Canary Islands. Carminic acid and its aluminum or calcium lake (called carmine) are relatively stable to light, heat and oxidation. The color displays changes to orange in an acid medium (pH < 5) and to bluish red at a pH of around 8 (Figure 2). It is soluble in water, ethanol and sulfuric acid and practically insoluble in organic solvents.
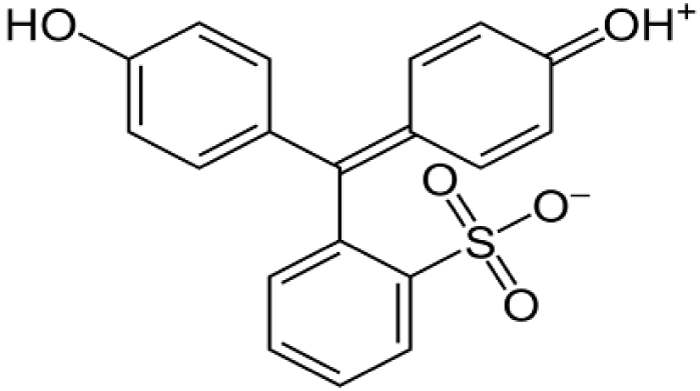
Natural dyes of vegetable origin are the most numerous and generally the most used by the food and pharmaceutical industries. Among these colorants is beet red or betanin, is the main component of the red colorant extracted from beets, which accounts for about 75-95% of all coloring matter found in beets [2-12]. It is a glucose glycoside (betanidin 5-O-glucose) and its aglycone is betanidine (Figure 3), isobetamine, neobetamine and prebetanin, responsible for the red-violet coloration [6,7]. Also, beet red exists as an internal salt, as a zwitterion in beet vacuoles. It is an anthocyanin which has a water soluble dye and gives colors ranging from purple and blue to most shades of red. The color of betanin depends on the pH [11-17], at acidic pH (pH 4-5) it is red in color and gradually turns purple-red as the pH decreases (Figure 4).
At alkaline pH (pH 11-12), betanin hydrolyzes and takes on a yellow-brown color (Figure 5). It is sparingly soluble in oil (lipophilic) and is soluble in water and ethanol (hydrophilic) [7-16].
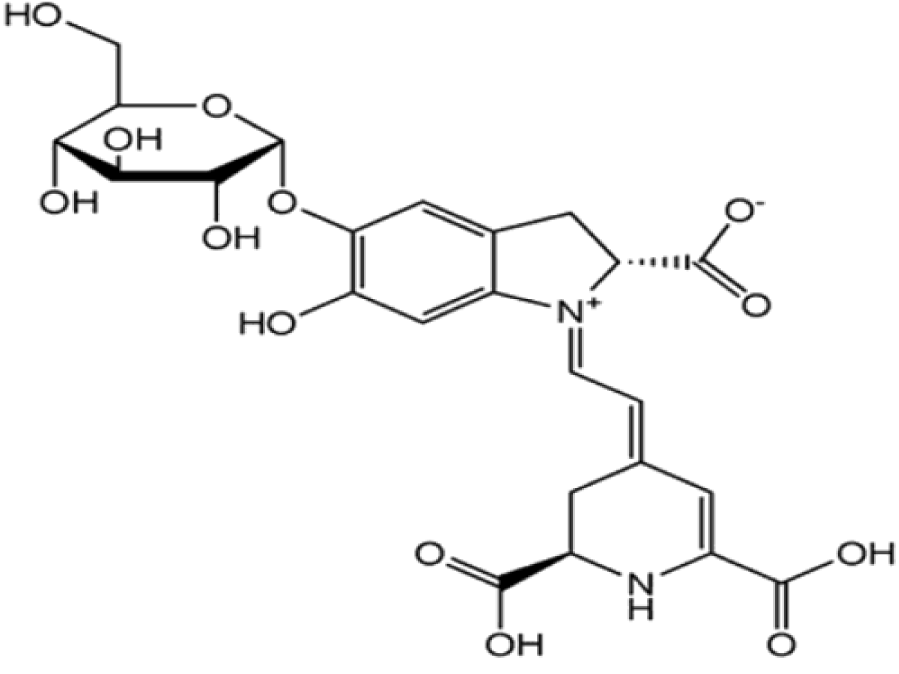
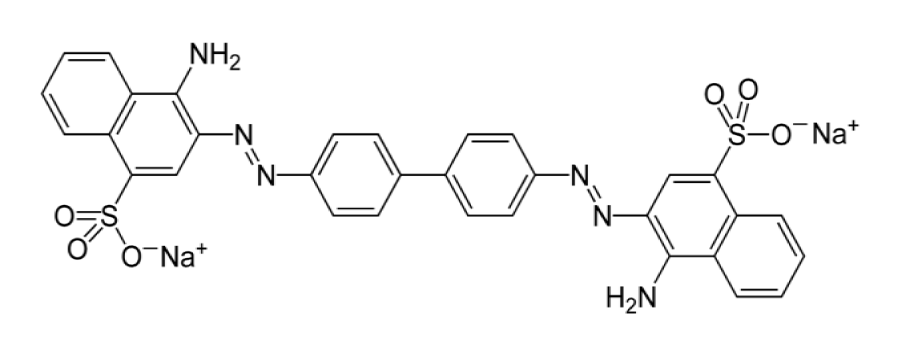
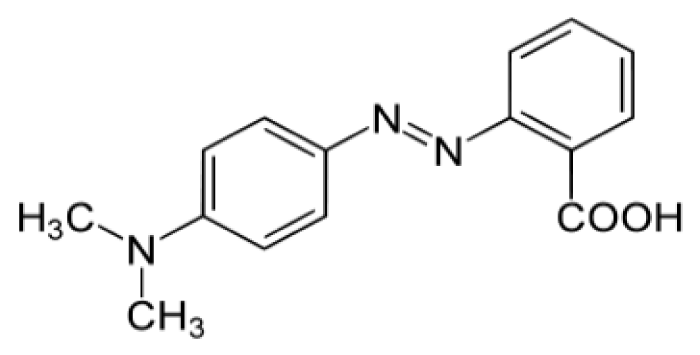
Among the synthetic dyes, we’ve found: congo red, methyl red, phenol red and neutral red. Congo red is a dye that belongs to the polyazo category because it has two azo type chromophores (Figure 6), which has obtained by the action of di-azobenzidine on a naphthalene sulphonic acid. Also, the coupling of the bis-diazo of benzidine with naphthionic acid gives congo red [18]. Its acid form is blue; its basic form is red (Figure 7) and its zone of change in the pH scale is between 3.0 and 5.2 [19]. Methyl red is an azo dye, composes mainly of 4-dimethylamino-2-phenylazo-benzoic acid (Figure 8) [20] and is a color indicator used in Hydrogen potential (pH) chemistry. It is red in color at pH < 4.4 (Figure 9), yellow above 6.2 and orange between the two. Phenol red, known as phenolsulfonephthalein (Figure 10), is a colored indicator used in chemistry in acid-base assays. Its potential acidity (pKa) is 8.0 its acid form is yellow its basic form is red (Figure 11). Its turning zone in the pH scale is between 6.6 and 8.4. Neutral red (or tolylene red or basic red V, or Basic Red 5) is a non-toxic, heterocyclic aromatic chemical compound (Figure 12), often used as a dye or color indicator by biologists. Its color changes from red to yellow when the pH goes from 6.8 (slightly acidic) to a more basic pH (8.0) (Figures 13,14).
We know that far ultraviolet rays are harmful to human health, particularly for their mutagenic effects; it can cause skin cancer, cataracts, photochemical pollution. In the future, chemists plan to use it as weapons for the destruction of objects. Therefore, we should meet this future challenge by scientific means in order to snuff out its far-ultraviolet rays.
The main objective in this work, is to carry out an experimental study by spectrophotometric methods on series of red dyes mentioned previously, to know which one is able to damage the far ultraviolet rays by absorption, i.e. there is an appearance of an absorption peak in the far ultraviolet range.
Experimental Methods and Characterization
Extraction of beet red (betanin)
Generally the primary method used to isolate red food coloring is solid-liquid extraction a process commonly used in the food industry. Being a water-soluble pigment, betanin can be extracted from red beets using one of the following solvents acidified water, water-alcohol mixture or acidified alcohol solution [21].
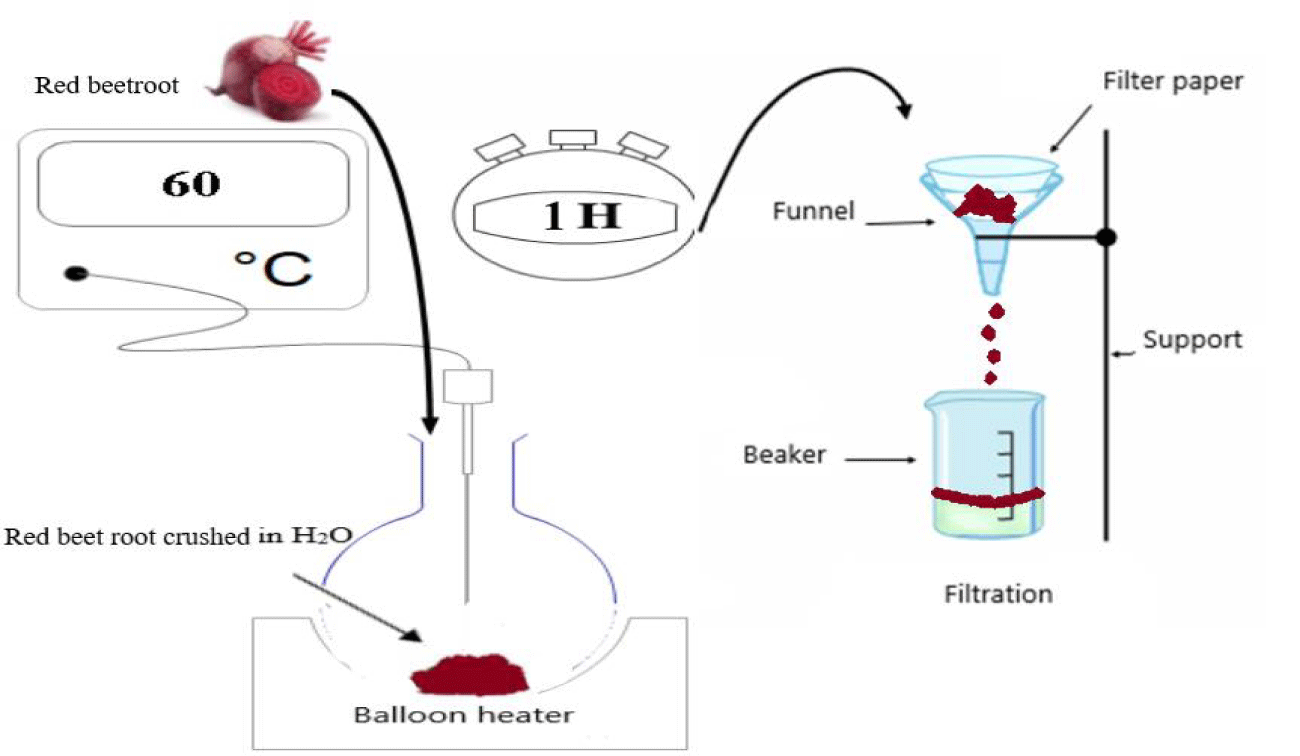
The separation method is physical, where water is used as the extraction solvent. Commercially available Algerian beetroot is used as the extraction material. This raw material is scraped and ground in a mortar until a fine purity is obtained. 40 g of grated beetroot is transferred to a flask containing 100 ml of distilled water, heated to 60°C in a heating mantle for one hour to eliminate bacteria. Then filtered to obtain a pure liquid extract, without impurities (Figure 15).
Preparation of red dye solutions
The preparation of red dye solutions depends on their physico chemical properties such as their solubilities in appropriate solvents.
Dissolution of methyl red
The solubility of methyl red in ethanol at 20°C is 2 g/l. Therefore, for the total mass dissolution of the methyl red powder in ethanol, we should dissolve 0.02 g of the methyl red in 10 ml of ethanol.
The solubility of methyl red in water at 20°C is 0.1 g/l. So, for the total mass dissolution of methyl red powder in water, we should dissolve 0.01 g of methyl red in 100 ml of water.
Dissolution of congo red
The solubility of congo red in water at 20°C is 25 g/l, and it is very soluble in alcohol. Therefore, for the total mass dissolution of the congo red powder, 0.25 g of congo red should be dissolved in 10 ml of the appropriate solvent (ethanol or distilled water).
Dissolution of carminic acid
The solubility of carminic acid in water at 25°C is 1.3 g/l. In our experiment, we have used ethanol as a solvent. So, for the dissolution of 80% by mass of carminic acid powder, we should dissolve 0.04 g of carminic acid in 50 ml of ethanol.
Dissolution of phenol red
The solubility of phenol red in water at 20°C is 0.77 g/l and in ethanol is 2.9 g/l. In our experiment, we have used ethanol as a solvent. Therefore, for the dissolution of 80% by mass of the powder of phenol red, we should dissolve 0.11 g of phenol red in 50 ml of ethanol.
Dissolution of neutral red
The solubility of neutral red in water at 25°C is 50 g/l. So, for the total dissolution by mass of the powder of neutral red in water, we should dissolve 0.5 g of neutral red in 10 ml of water.
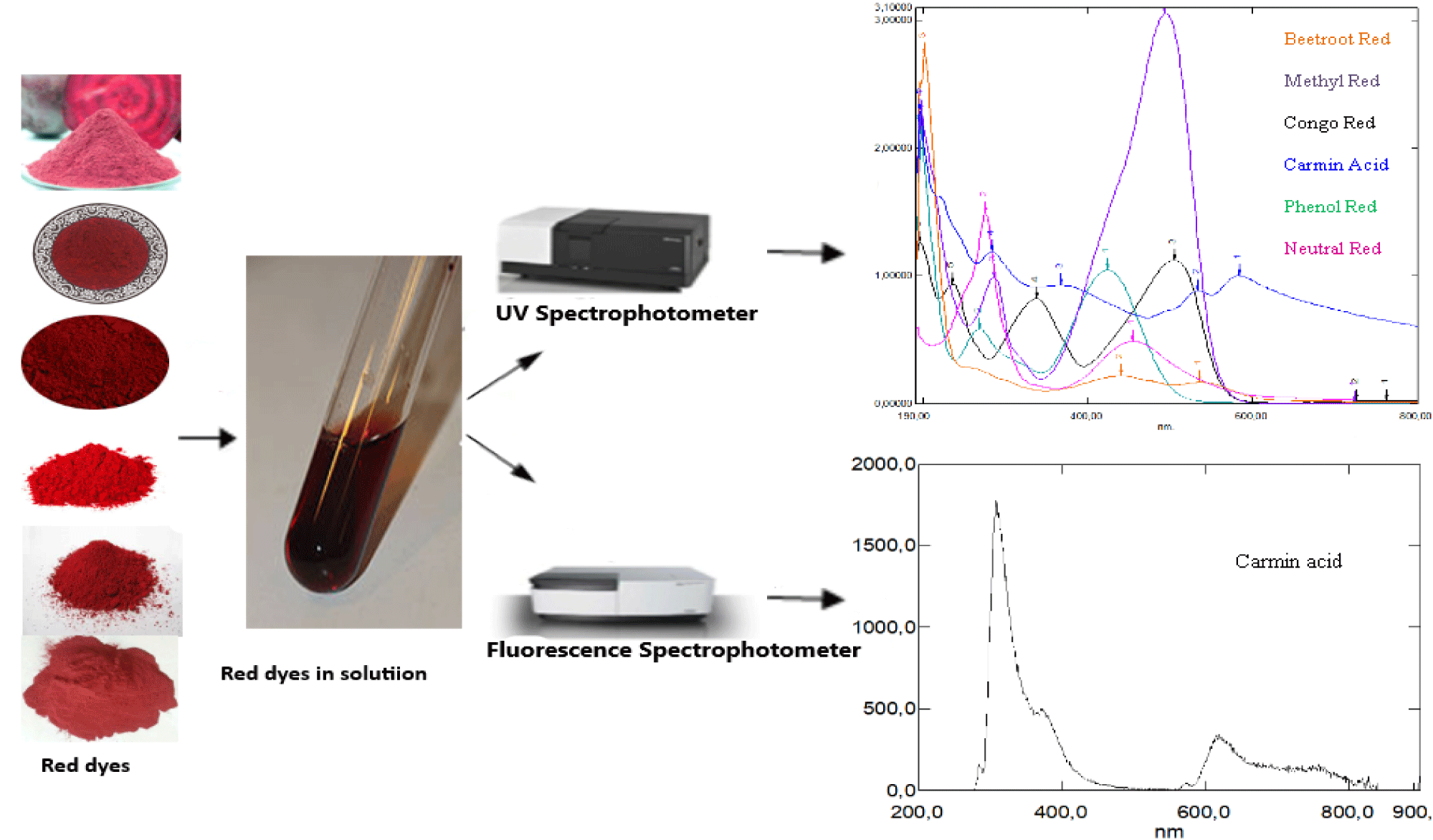
Before performing the spectrophotometric analyses, several dilutions are prepared to obtain high resolution spectra. The analysis methods used are UV-Vis absorption spectrophotometry and emission spectrophotometry using the following devices Shimadzu 3600i Plus UV-Vis-NIR Spectrophotometer and Shimadzu RF-6000 Fluorescence Spectrophotometer.
The spectroscopic measurements are carried out on all the far and visible ultraviolet paths as it’s indicated in the Figures cited below (Figures 16-28). The experiments are carried out using a quartz cuvette. Thus, six spectra are acquired, also for absorption and emission.
Experimental Results and Discussion
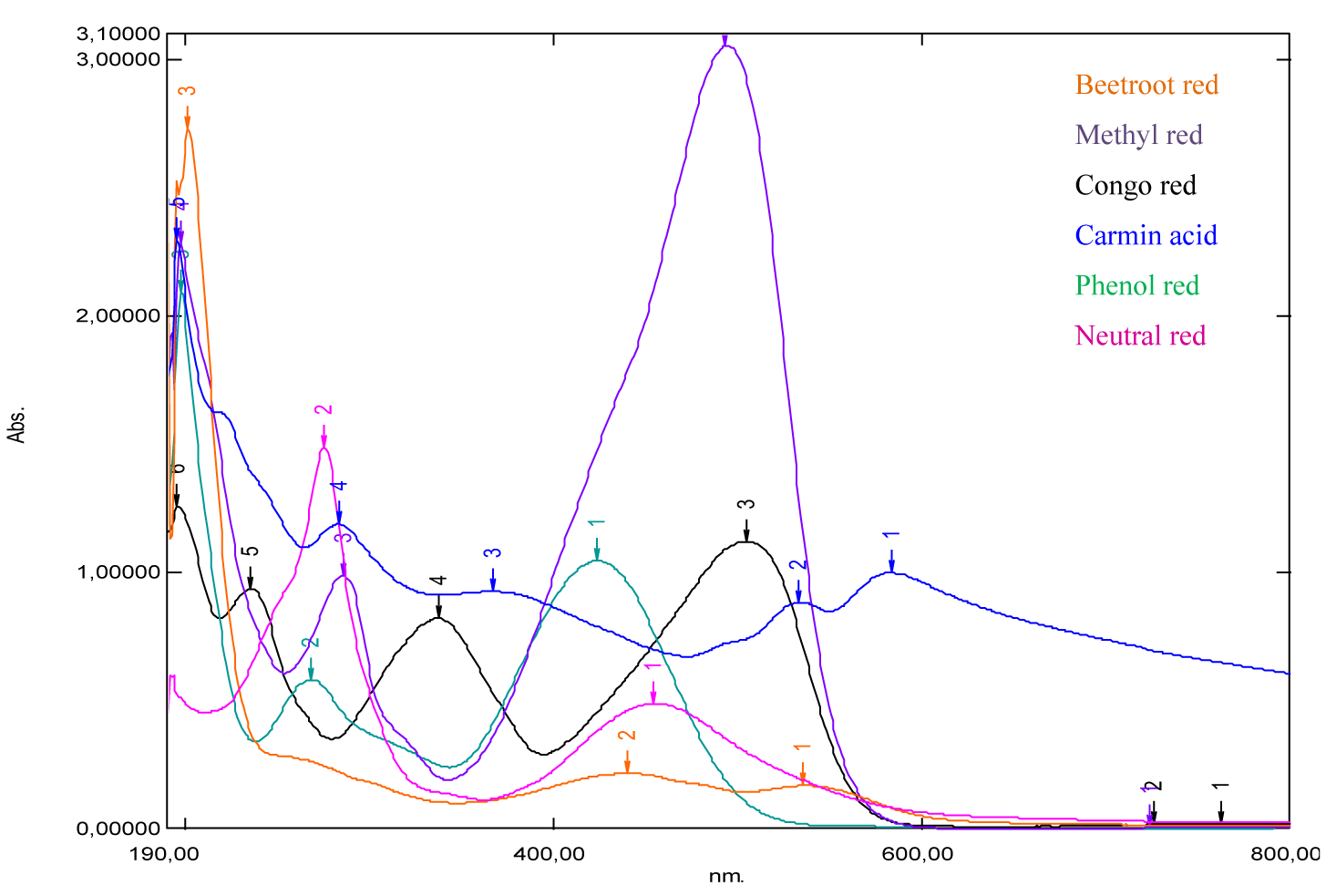
Analysis by UV-Vis absorption spectrophotometry is carried out on a series of red dyes, such as beet red, congo red, methyl red, neutral red, phenol red and carminic acid. Indeed, dilute solutions of red dyes have been prepared to produce well-resolved spectra (Figures 16-22). These Figures have been the subject of disclosure of the possible absorptions of this series of molecules of red colors. Otherwise, a spectral analysis by emission, is carried out on this series of red dyes at different excitatory wavelengths (Figures 23-28), these Figures have been the subject of disclosure of the possible emissions of this series of molecules of red colors.
The table below (Table 1) groups the values of maximum absorption wavelengths (λmax absorbed), excitatory wavelengths (λexcited) and emitted wavelengths (λemitted) of the series of dyes studied.
| Table 1: Values of wavelengths maximum absorbed (λmax), wavelengths excited (λ excited) and wavelengths emitted (λemitted) for red colorants studied. | ||||||
| λ(nm) | λmax1 absorbed | Amax1 absorbed | λmax2 absorbed | λmax3 absorbed | λ excited | λ emitted |
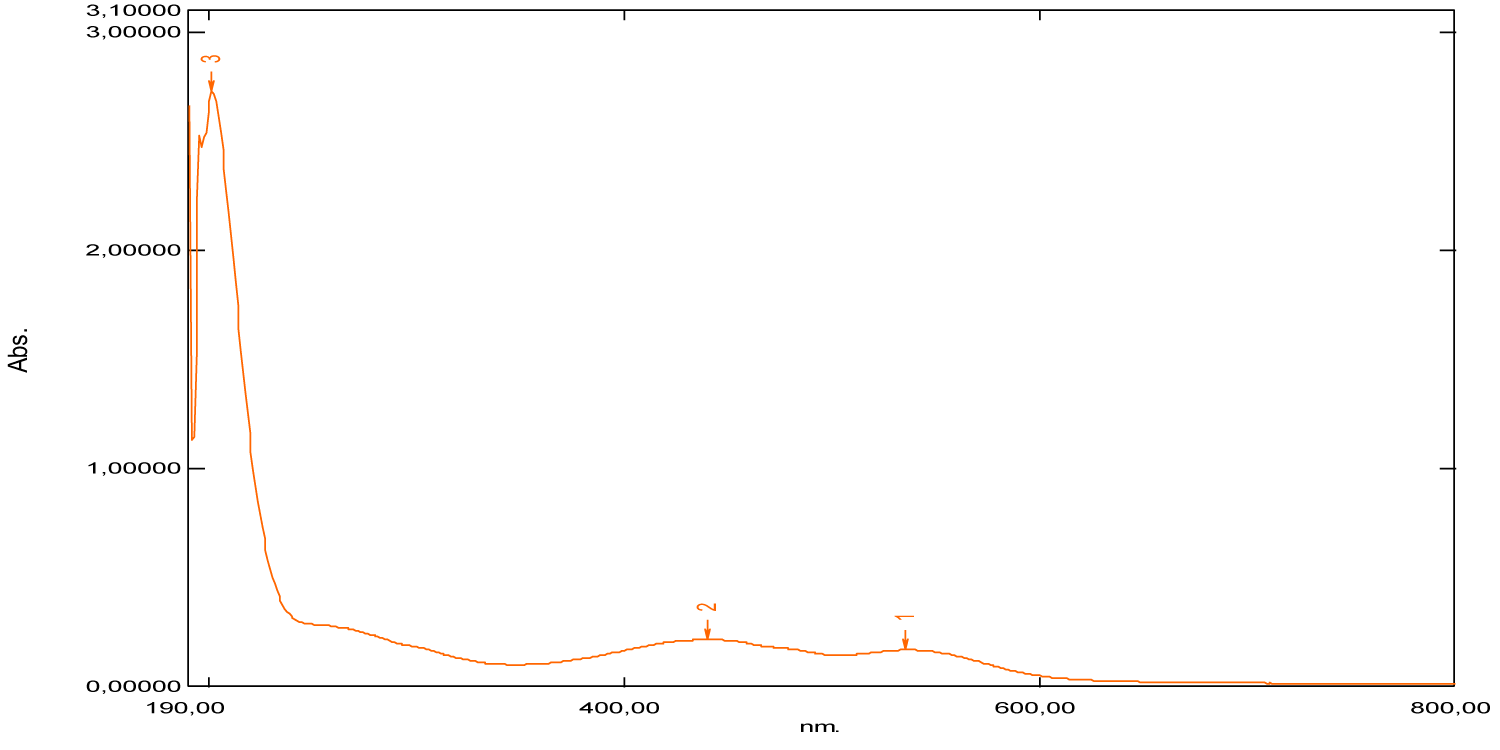
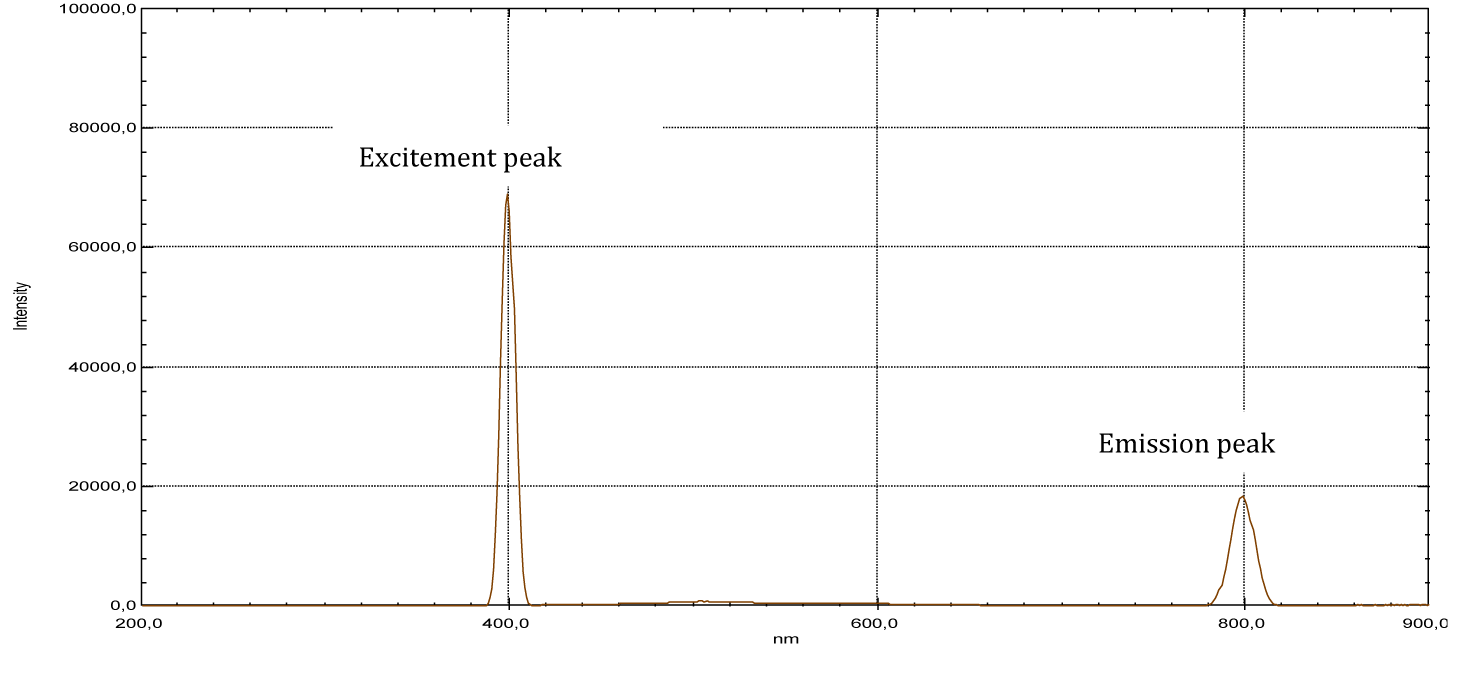
| Beet red | 201 | 2.73 | 480 | 537 | 400 | 800 |
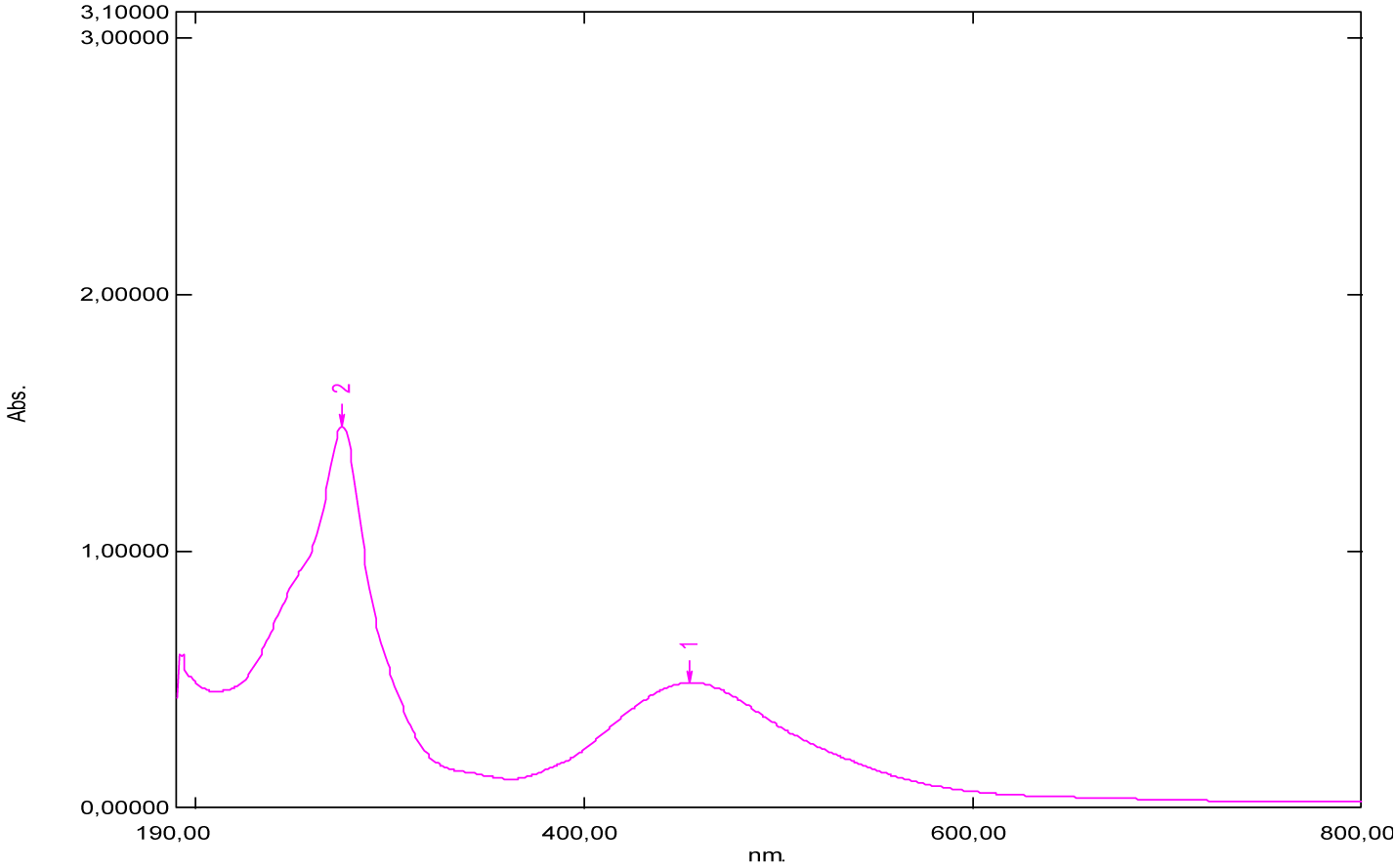
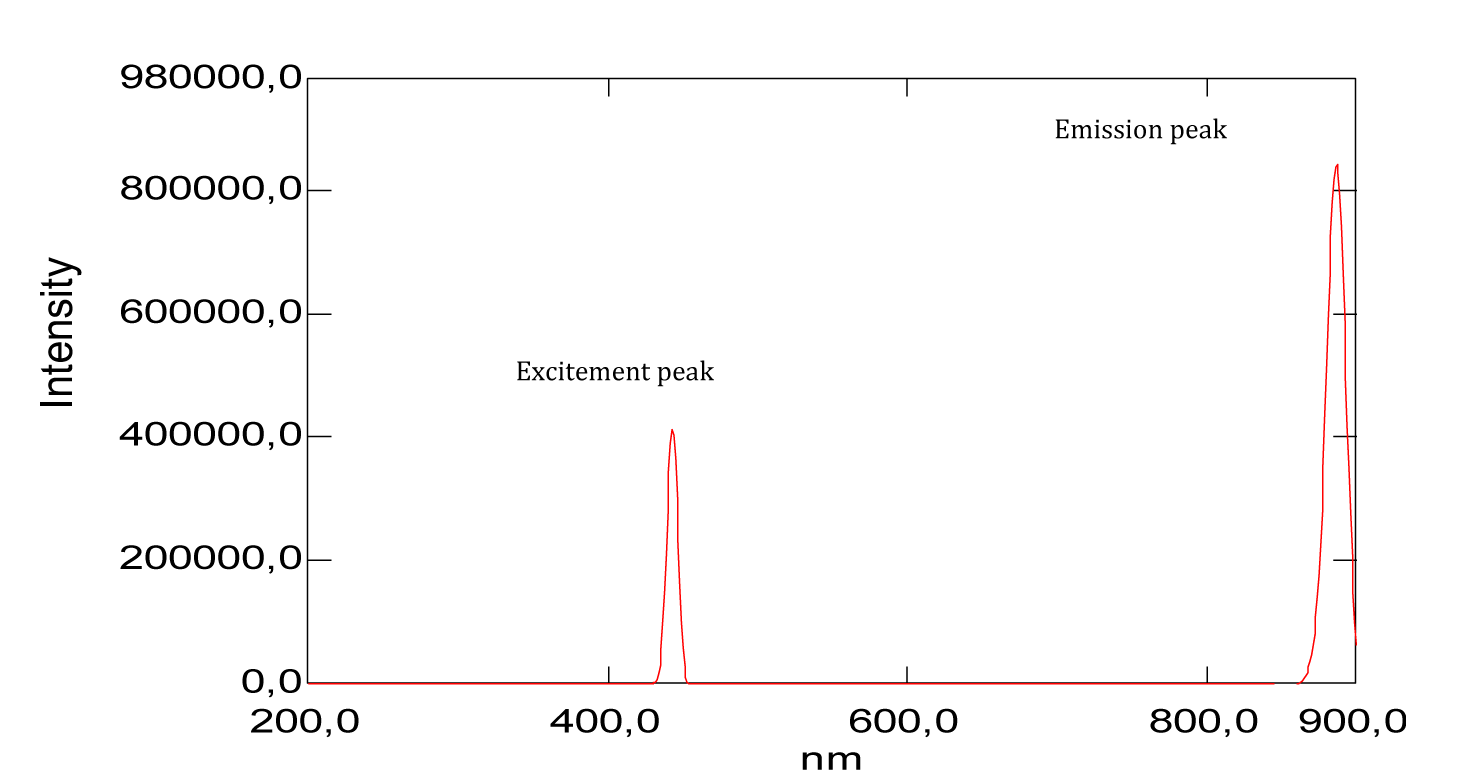
| Methyl red | 197 | 2.28 | 256.5 | 429.5 | 291 | 600 and 900 |
| Congo red | 195 | 1.25 | 200 | 340 and 500 | 339 | 690 |
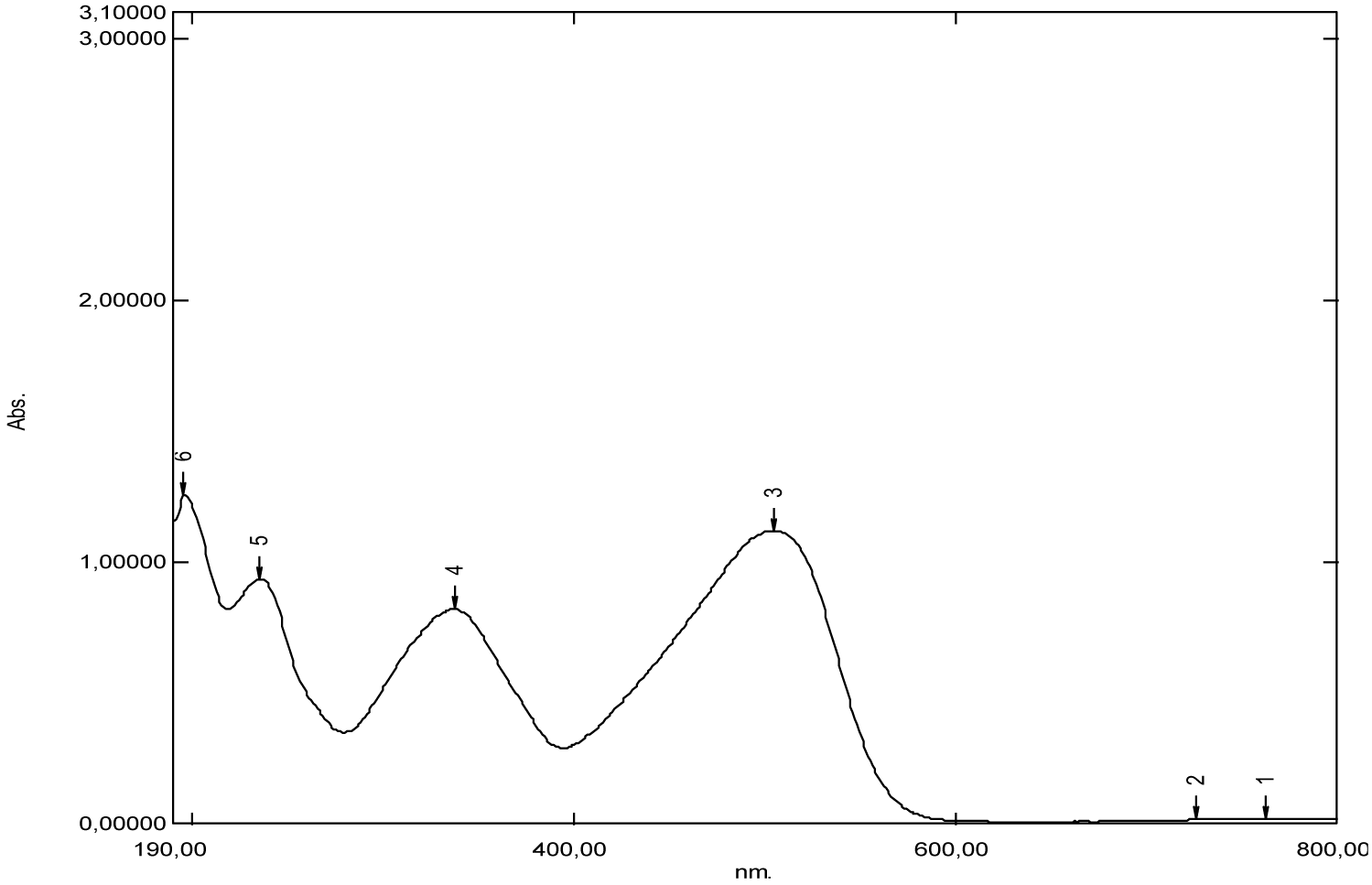
| Carmin acid | 195 | 2.29 | 250 | (500-600) | 286 | (600-900) |
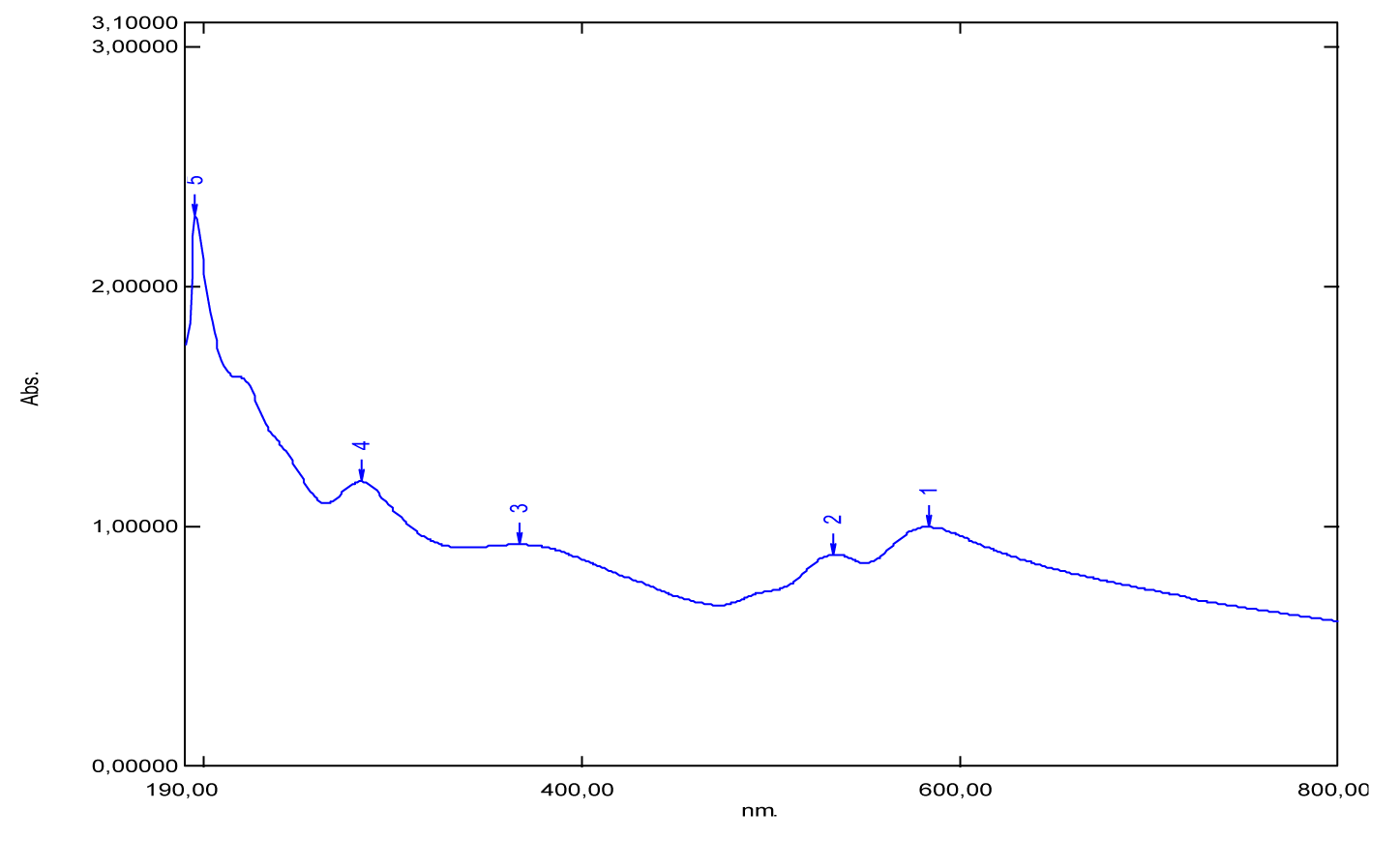
| Phenol red | 196 | 1.20 | 269 | 426 | 442 | 900 |
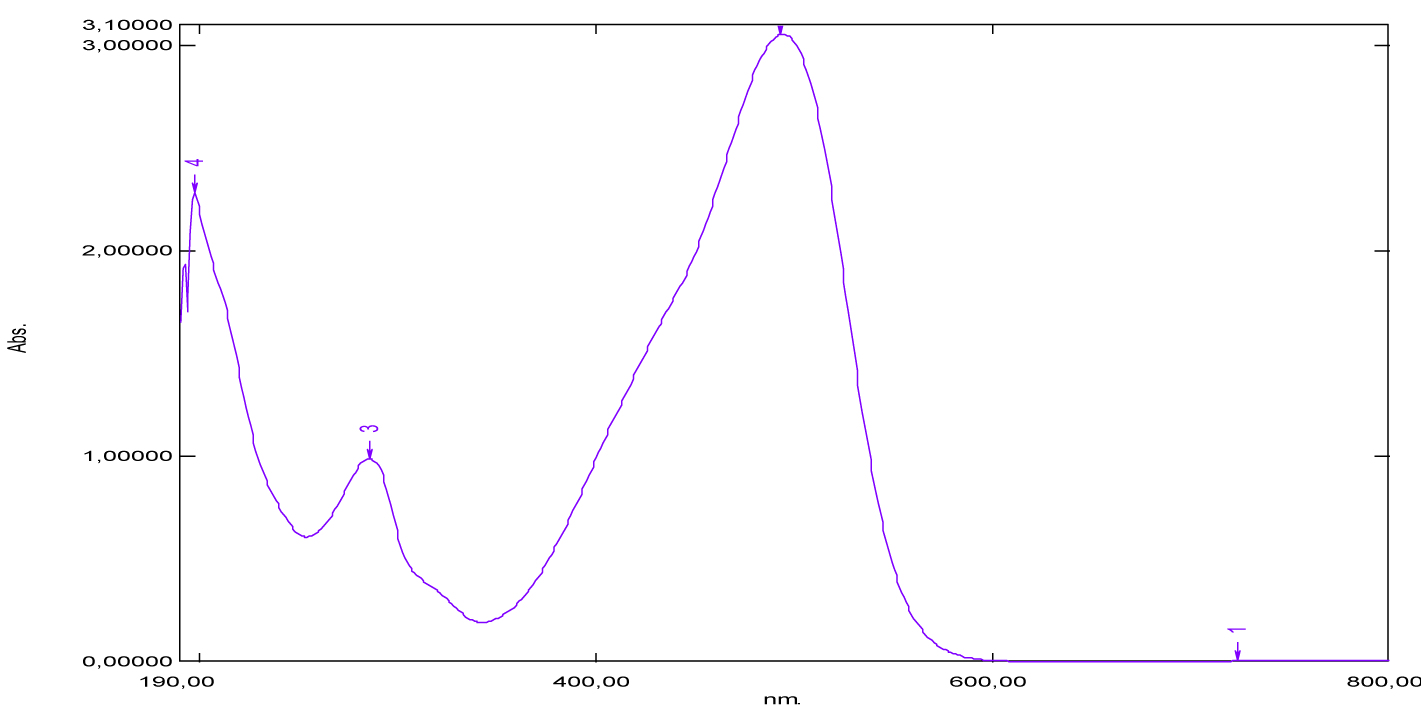
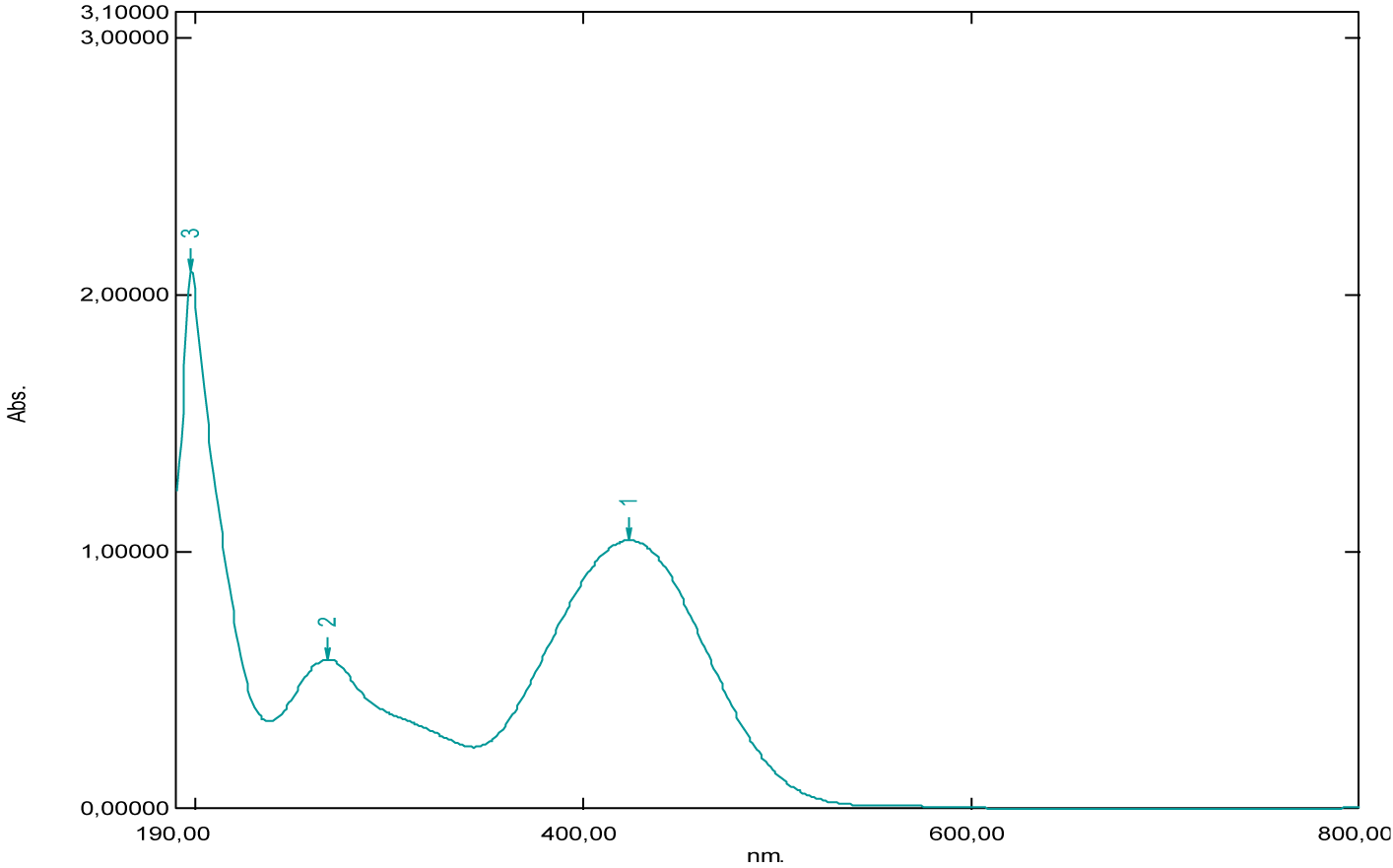
| Neutral red | 275 | 1.48 | - | 514 | 286 | (600-800) |
Generally, for organic compounds, the different types of transitions are σ →σ*, n →σ*, π →π* and n →π*. The maximum absorption wavelengths of these types of transitions are respectively: λ max (σ →σ*) < 150 nm (far UV), 150 nm < λ max (n → σ*) < 250 nm, λ max (π →π*, n →π*) > 190 nm, λ max (π →π*) > 400 nm for highly conjugated systems. The intensities of absorption bands depend on the values of the molar extinction coefficient of the solvent used. According to the obtained results, mentioned in figures 16-22 and table 1, we note that: For the absorption spectrum of beet red, significant absorption is recorded between 480 nm and 540 nm (Figure 16). Thus two peaks are observed respectively at wavelengths 480 nm and 537 nm (Table 1), which correspond to the groups of atoms called chromophores present in its chemical structure, these are the functional groups [–C=N+–], [–C=C–], [–C=O–] and [–C=C–C=O–], which are responsible for the red color of the dye. Indeed, these values confirm the data of the literature [21-23]. Considering the values of the absorbances, we notice that the strong absorption is at 201 nm. This suggests that the chemical structure has an important role in specifying the absorption field, for example the presence of auxochrome groups in its chemical structure, such as the five hydroxyl groups [-OH] and the two acid groups [-COOH].
In the literature, it is given that the maximum absorption wavelength of the hydroxyl group [-OH] is 180 nm, it corresponds to the transition (n→σ*), while the maximum wavelength of absorption of the acid group [-COOH] is 205 nm, it corresponds to the transition (n→π*). This value is close to the one we’ve found (201 nm).
According to the absorption spectrum of methyl red, two absorption maxima are observed at 256.5 nm, 429.5 nm (Figure 17). Considering the values of the absorbances, we notice that the strong absorption is at 429.5 nm (Table 1), which correspond to the groupings of atoms called chromophores present in its chemical structure, these are the functional groups azo [–N= N–], [–C=C–], which are responsible for the red color of the dye. The visible wavelength of green color is absorbed by methyl red and its complement, which is transmitted red, which explains the red color appearance of the dye. In the literature, it’s given that the maximum absorption wavelength of the azo group [–N=N–] is 340 nm, it corresponds to the transition (n→π*). We recall that the absorption at 256.5 nm of the ultraviolet expresses the degree of darkness more or less clarified, because if this is not the case, the dye would be very light red. Also, we‘ve seen an absorption peak appears at 197 nm. This suggests that the chemical structure has an important role in specifying the absorption domain, for example the presence of auxochrome groups in its chemical structure, such as the tertiary amine group [-N(CH3)2] and the group acid [-COOH]. Similarly, the maximum absorption wavelength of the amine group is 190 nm, it corresponds to the transition (n→σ*) and this value is close to that found (197 nm).
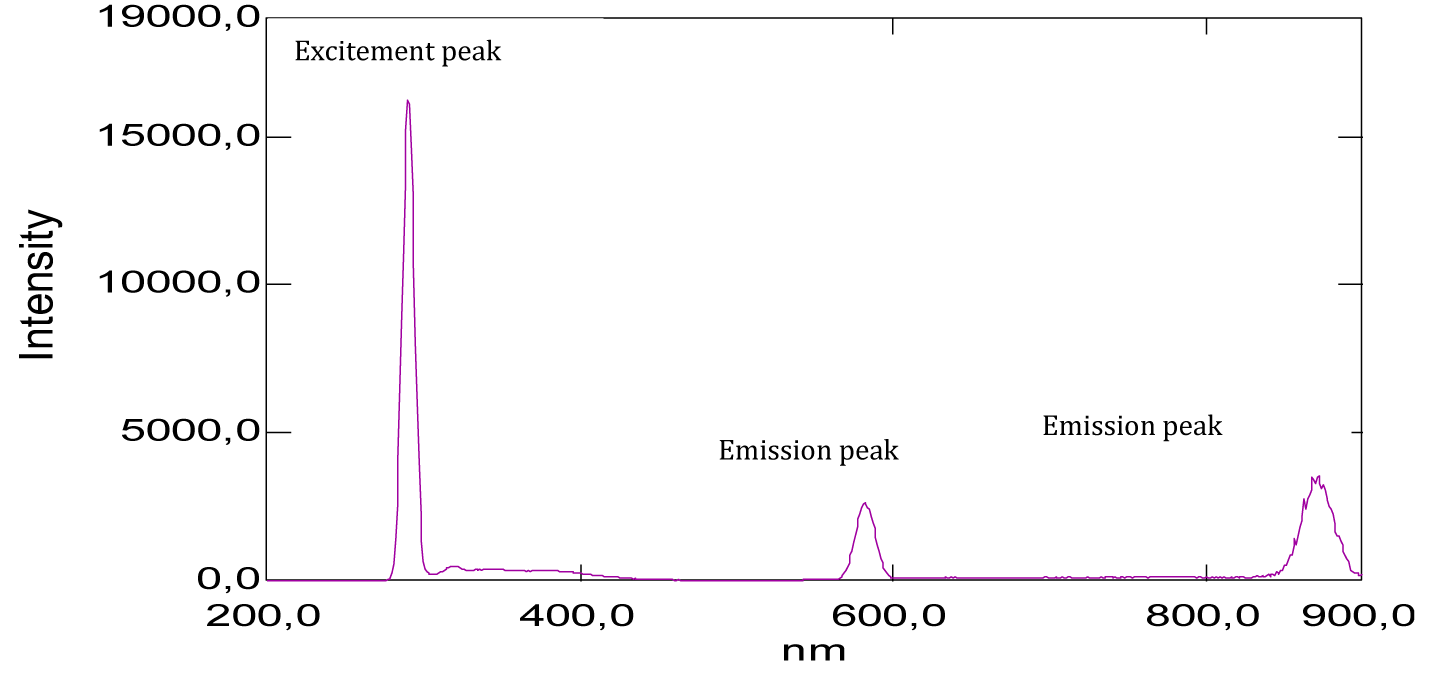
From the shape of the absorption spectrum of congo red, we can have two important remarks. The first is that of significant absorption at very low wavelengths, at 195 nm (Figure 18, Table 1). This suggests that the chemical structure has an important role in specifying the absorption domain, for example the presence of two auxochrome groups in its chemical structure, which is the primary amine [-NH2]. Likewise, in the literature is given that the maximum absorption wavelength of the amine group is 190 nm, it corresponds to the transition (n→σ*). This value is close to that found (195 nm). The second remark, is to the optical absorptions, about 340 nm and 500 nm ( Figure 18) and (Table 1), which correspond to the groupings of atoms called chromophores present in its chemical structure, these are the two functional groups azo [ –N=N–], [–C=C–] and which are responsible for the red color of the dye. In the same way, it is given that the maximum absorption wavelength of the azo group [–N=N–] is 340 nm, it corresponds to the transition (n→π*). This value is the one we’ve found (340 nm). In addition, a small light attenuation is observed on the spectrum. This may be suggesting that the extinction coefficient has a low value.
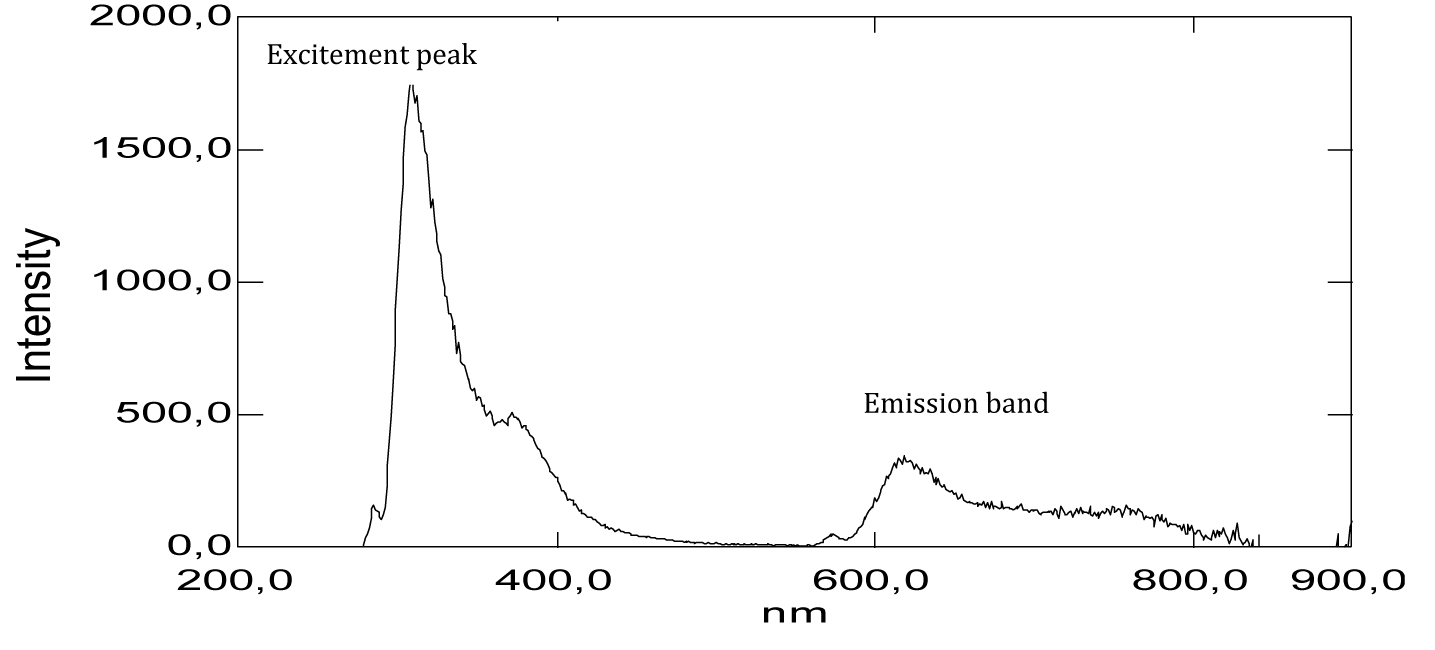
According to the absorption spectrum of carminic acid, we can have two important remarks. The first is that of significant absorption at very low wavelengths at 195 nm. This suggests that the chemical structure has an important role in the specification of the absorption domain, for example the presence of nine auxochrome groups in its chemical structure and which are the hydroxyls [-OH]. In the literature, it’s given that the maximum absorption wavelength of the hydroxyl group [-OH] is 180 nm, it corresponds to the transition (n→σ*) and this value is close to that which we have found (195 nm). The second remark is that broad absorption at around 250 nm (Figure 19) and (Table 1), two weak absorptions between 500 nm and 600 nm (Figure 19, Table 1), which correspond to the groups of atoms called chromophores present in its chemical structure are the functional groups [C=O] and [–C=C–], which are responsible for the red color of the dye. Only the optical signal is attenuated by this dilution and therefore, we notice that this substance has no optical absorption. Apparently, carminic acid does not have significant absorption peaks in the near UV and visible range.
For phenol red, is considered liquid in its physical state under normal conditions of pressure and temperature. There are three absorption bands which appear respectively at 196 nm, 269 nm and 426 nm (Figure 20) and (Table 1). Apparently, far UV light is absorbed by this molecule at 196 nm. This explains the presence of auxochromes in its chemical structure such as hydroxyls. Also, it’s given that the maximum absorption wavelength of the hydroxyl group [-OH] is 180 nm, it corresponds to the transition (n→σ*) and this value is close to that found (196 nm).
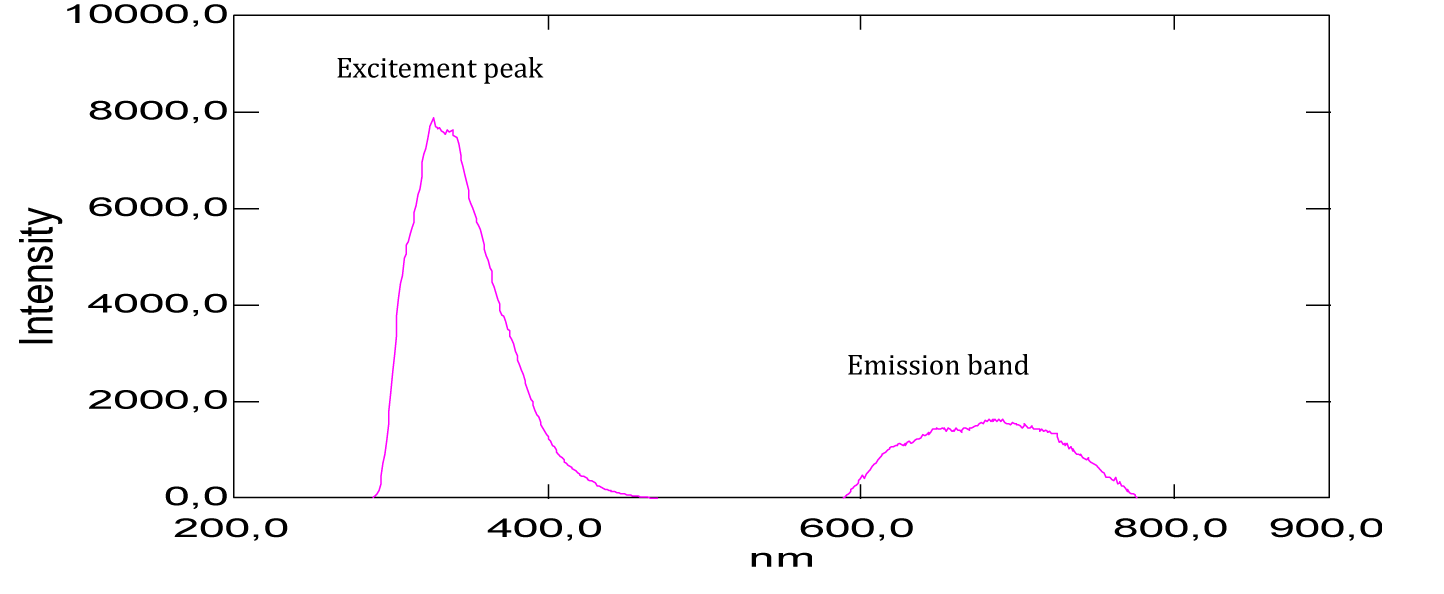
From the shape of the absorption spectrum of neutral red, we’ve noticed two absorption maxima, observed at 275 nm and 514 nm (Figure 21, Table 1). The absorption band which appears at 514 nm corresponds to the chromophore groups present in its chemical structure, these are the functional groups [–C=N–] and [–C=C–], which are responsible for the red color of the dye. Considering the absorbance values, we notice that the strong absorption is at 275 nm, which corresponds to the auxochromes present in this molecule, such as the primary and tertiary amine groups [-NH2]. Similarly, it’s given that the maximum absorption wavelength of the amine group is 190 nm, it corresponds to the transition (n→σ*) and this value is not very far from that found (275 nm). Also, we recall that the absorption at 275 nm of the ultraviolet expresses the degree of darkness more or less lightened, because the dye would be very light red if this is not the case.
According to the previous work, we can classify these dyes studied in descending order by their values of low absorption wavelengths in far ultraviolet light found, as well as the nature of the chromophore and auxochrome groups present in their chemical structures. We distinguish carminic acid, congo red, phenol red, methyl red and beet red. These pigments have a corresponding light absorbing capacity at short lengths.
From the shape of the emission spectra obtained from the series of dyes studied, we’ve observed an emission at 800 nm for beet red (Figure 23), a strong emission at 900 nm for phenol red (Figure 25), a strong emission which appears at about 690 nm for congo red (Figure 24), two emissions appear respectively at 600 nm and 900 nm for methyl red (Figure 26). There is also a spectral form of emission at higher wavelengths respectively for carminic acid (Figure 28), congo red (Figure 24) and neutral red (Figure 27). This spectral shape indicates the presence of perturbing fluorescence phenomena, such as the excimer effect. Indeed, excimers formed by collision of the fluorophore [24].
Conclusion
The optical analyzes which have carried out in our laboratory have shown that certain red dyes have the property of absorbing light in the far ultraviolet range, in the region of 200 nm. These pigments have a capacity to absorb the corresponding light at low wavelengths thanks to the presence of chromophore and auxochrome groups in their chemical structures and their natures. And therefore, they are able to dodging far ultraviolet rays. The spectral analyzes by fluorescence of the series of red pigments studied, have showed that these red dyes emit from 600 nm and strong emissions at 900 nm.
References
- Ramesh M, Muthuraman A. Flavoring and coloring agents: Health risks and potential problems. In: Natural and Artificial Flavoring Agents and Food Dyes. Elsevier; 2018. p.1-28. doi: 10.1016/B978-0-12-811518-3.00001-6.
- Omidi F, Behbahani M, Sadeghi AH, Sedighi A, Shahtaheri SJ. Application of molecular imprinted polymer nanoparticles as a selective solid phase extraction for preconcentration and trace determination of 2,4- dichlorophenoxyacetic acid in the human urine and different water samples. J Environ Health Sci Eng. 2014;12(1):1-10. doi: 10.1186/s40201-014-0137-z.
- Baião DDS, Silva DVTD, Aguila EMD, Paschoalin VMF. Nutritional bioactive and physicochemical characteristics of different beetroot formulations. Food Additives. 2017;1-24. doi: 10.5772/intechopen.69301.
- Vieira Teixeira da Silva D, Dos Santos Baião D, de Oliveira Silva F, Alves G, Perrone D, Mere Del Aguila E, M Flosi Paschoalin V. Betanin, a Natural Food Additive: Stability, Bioavailability, Antioxidant and Preservative Ability Assessments. Molecules. 2019 Jan 28;24(3):458. doi: 10.3390/molecules24030458. PMID: 30696032; PMCID: PMC6384587.
- Hanssen Maurice. E comme Additif, produits chimiques au menu. Edition Flammarion. 1988;156.
- Azeredo HMC. Betalains: Properties, sources, applications and stability: A review. Int J Food Sci Technol. 2009;44(12):2365-2376. doi: 10.1111/j.1365-2621.2007.01668.x.
- Tumbas Šaponjac V, Čanadanović-Brunet J, Ćetković G, Jakišić M, Djilas S, Vulić J, Stajčić S. Encapsulation of Beetroot Pomace Extract: RSM Optimization, Storage and Gastrointestinal Stability. Molecules. 2016 Apr 30;21(5):584. doi: 10.3390/molecules21050584. PMID: 27144556; PMCID: PMC6273385.
- Gandía-Herrero F, Escribano J, García-Carmona F. Characterization of the monophenolase activity of tyrosinase on betaxanthins: the tyramine-betaxanthin/dopamine-betaxanthin pair. Planta. 2005 Oct;222(2):307-18. doi: 10.1007/s00425-005-1526-4. Epub 2005 Jun 21. PMID: 15968512.
- Madhusudhan MC, Raghavarao KSMS. Aqueous two-phase extraction for the recovery of beet pigments and enzymes. In: Red Beet Biotechnology: Food and Pharmaceutical Applications. Springer; 2012. p.393-408. doi: 10.1007/978-1-4614-3458-0_15.
- Strack D, Vogt T, Schliemann W. Recent advances in betalain research. Phytochemistry. 2003 Feb;62(3):247-69. doi: 10.1016/s0031-9422(02)00564-2. PMID: 12620337.
- Nowacki L, Vigneron P, Rotellini L, Cazzola H, Merlier F, Prost E, Ralanairina R, Gadonna JP, Rossi C, Vayssade M. Betanin-Enriched Red Beetroot (Beta vulgaris L.) Extract Induces Apoptosis and Autophagic Cell Death in MCF-7 Cells. Phytother Res. 2015 Dec;29(12):1964-73. doi: 10.1002/ptr.5491. Epub 2015 Oct 14. PMID: 26463240.
- Delgado-Vargas F, Jiménez AR, Paredes-López O. Natural pigments: carotenoids, anthocyanins, and betalains--characteristics, biosynthesis, processing, and stability. Crit Rev Food Sci Nutr. 2000 May;40(3):173-289. doi: 10.1080/10408690091189257. PMID: 10850526.
- Cai Y, Sun M, Corke H. Antioxidant activity of betalains from plants of the amaranthaceae. J Agric Food Chem. 2003 Apr 9;51(8):2288-94. doi: 10.1021/jf030045u. PMID: 12670172.
- Schwartz SJ, von Elbe JH, Pariza MW, Goldsworthy T, Pitot HC. Inability of red beet betalain pigments to initiate or promote hepatocarcinogenesis. Food Chem Toxicol. 1983 Oct;21(5):531-5. doi: 10.1016/0278-6915(83)90136-9. PMID: 6140212.
- Kanner J, Harel S, Granit R. Betalains--a new class of dietary cationized antioxidants. J Agric Food Chem. 2001 Nov;49(11):5178-85. doi: 10.1021/jf010456f. PMID: 11714300.
- Butera D, Tesoriere L, Di Gaudio F, Bongiorno A, Allegra M, Pintaudi AM, Kohen R, Livrea MA. Antioxidant activities of sicilian prickly pear (Opuntia ficus indica) fruit extracts and reducing properties of its betalains: betanin and indicaxanthin. J Agric Food Chem. 2002 Nov 6;50(23):6895-901. doi: 10.1021/jf025696p. PMID: 12405794.
- Pedreno MA, Escribano J. Correlation between antiradical activity and stability of betanin from Beta vulgaris l roots under different temperature, pH and light conditions. J Sci Food Agric. 2001;81:627-631. doi: 10.1002/jsfa.851.
- Nikfar S, Jaberidoost M. Dyes and Colorants A2- wexler philip. In Encyclopedia of Toxicology, 3rd ed. Academic Press: Oxford; 2014. p.252-261.
- Fisher Bioblock Scientific. Produits chimiques; 2006-2007. p.403.
- Jacques Baron. Matières colorantes organiques.
- Popa A, Moldovan B, David L. ‘‘Betanin from Red Beet (Beta vulgaris L.). Extraction conditions and evaluation of the thermal stability, Babe-Bolyai”. University, Faculty of Chemistry and Chemical Engineering. Arany Janos Str. 400028, Cluj-Napoca, Romania.
- Neagu C, Barbu V. Principal component analysis of the factors involved in the extraction of beetroot betalains. J Agroaliment Proc Technol. 2014;20(4):311-318.
- Nouairi ME, Freha M, Bellil A. Study by absorption and emission spectrophotometry of the efficiency of the binary mixture (Ethanol-Water) on the extraction of betanin from red beetroot. Spectrochim Acta A Mol Biomol Spectrosc. 2021 Nov 5;260:119939. doi: 10.1016/j.saa.2021.119939. Epub 2021 May 11. PMID: 34015743.
- Albani JR. Absorption et Fluorescence principes et applications; 2001 éditions Tec-doc • J.R. Lakowicz; Principes of fluorescence spectroscopy. 3rd ed. Springer; 2006 • B. Valeur; Invitation à la fluorescence moléculaire; 2004 édition De BoecK.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.