General Science . 2022 December 31;3(12):1567-1569. doi: 10.37871/jbres1637.
Vapor-Gas Deposition of Polymer Coatings on Metals and Mineral Carriers
Makarychev Yu B*
In modern medicine and biotechnology, metal frames and mineral carriers modified with polymer coatings are widely used. In most cases, during polymerization, hydrolytically stable bonds between the polymer and the carrier are not formed on the surface, and the modifying layer is retained on the surface due to adsorption forces or geometric factors. To ensure good adhesion of polymers with metals and mineral carriers, primers or primers on an aqueous or organic basis are used. Recently, the most widely used trialkoxysilanes of the general formula Z(CH2)nSi(OR)3 have been found, where R = CH3 or C2H5, and Z = NH2, SH, CN, Hal, CH = CH2. Aqueous solutions of trialkoxysilanes are often used to inhibit various metals from corrosion [1-3]. There are few publications of steam-gas coating on iron, copper and aluminum [4-6]. To form conversion coatings on aluminum and magnesium, sol-gel technologies based on triethoxysilanes are used [7,8]. At the same time, the formation of a strong covalent bond of the polymer with the carrier is guaranteed and the polymerization conditions in the surface layer are softened. These processes are based on hydrolysis reactions with the formation of silanols and their subsequent crosslinking by reactions.
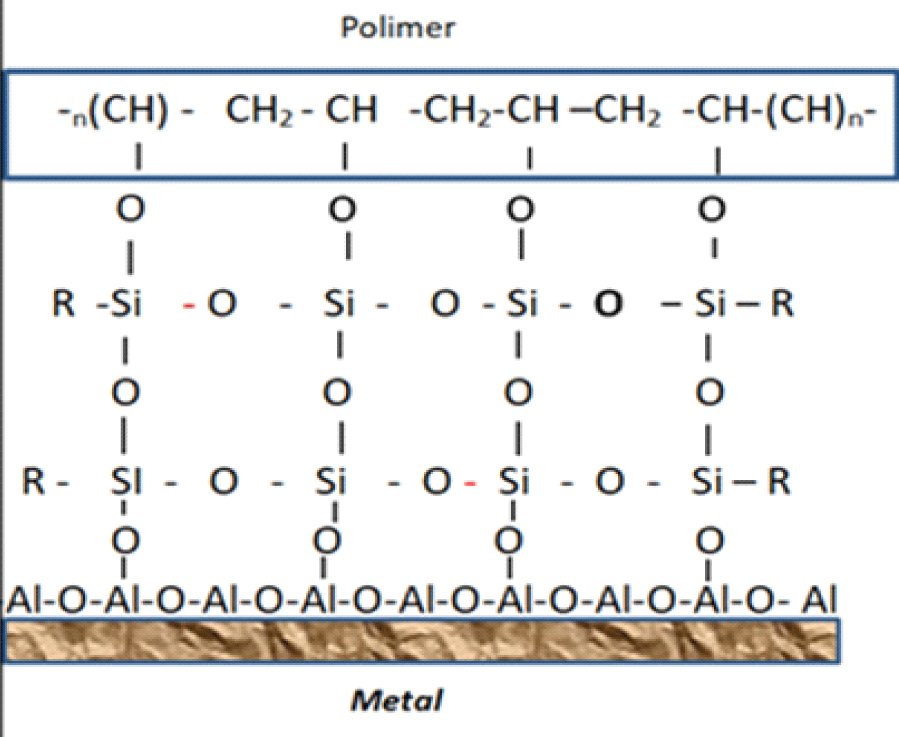
A block diagram of the interaction of primer and polymer coating molecules is shown in figure 1.
The main obstacles to the use of such materials in aqueous solutions are the difficulty of controlling the course of polymerization on the surface and the difficulty of obtaining a uniform coating. The studies carried out by us in work [4] showed that siloxane coatings on metals can be obtained by vapor-gas deposition of Vinyltrimethoxysilane (VS) with its subsequent polymerization at T = 120°C. Such coatings can be obtained on any substrates with a given thickness and chemical compositions. This is achieved by selecting copolymers with different functional groups and the composition of azeotropic mixtures that equalize the partial pressures of the components in the vapor phase. For example, the compositions given in table 1 can be used to apply a polymer coating based on VS and Ethylene Glycol (EG).Vapor-gas deposition of siloxane coatings was carried out in a sealed chamber with a volume of 3 liters, equipped with a disk heater and a set of crucibles for evaporation of azeotropic mixtures.
| Table 1: Compositions of Azeotropic mixtures for applying Siloxane coatings. | |||
| Composition of mixture | Boiling point,°C | Partial pressure, аt | The amount of the mixture in the vaporizers, ml |
| 1. EG+ 40% Phenylmethane | 110.8 | 0.26 | 10 |
| 2. VS +20 % butanol | 108.5 | 0.24 | 5 |
| 3. H2O + 70% EG | 112.3 | 0.35 | 5 |
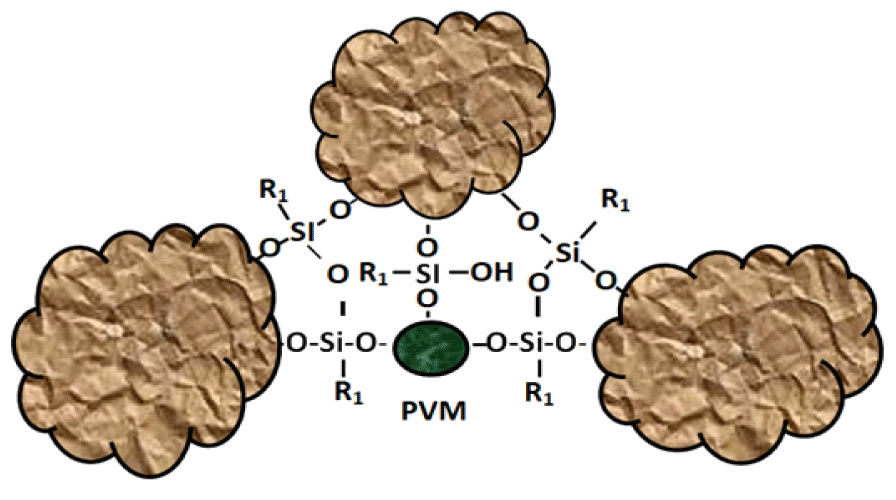
With this composition of the vapor-gas mixture, a siloxane polymer film with a thickness of 240 nm is formed on the surface of aluminum oxide at T = 120°C for 60 minutes. On mineral carriers, the scheme of interaction of siloxane coatings with the surface of the pore walls and polymer filler is shown in figure 2.
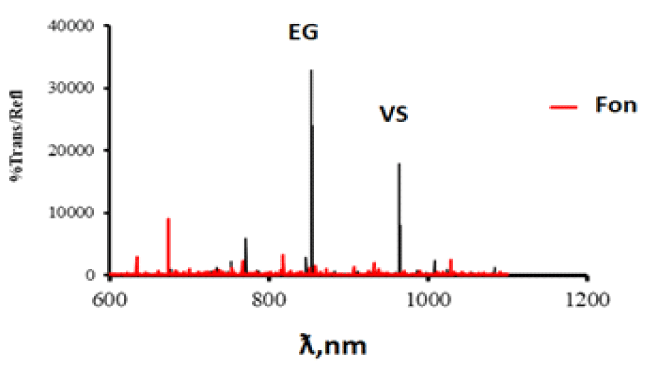
With this composition of the vapor-gas mixture, a siloxane polymer film with a thickness of 240 nm is formed on the surface of aluminum oxide at T = 120°C for 60 minutes (Figure 3).
Conclusion
The technology of steam-gas deposition of siloxane coatings of a given thickness and chemical composition on metals and porous coatings has been developed. The main application of coatings is protection from the effects of an aggressive environment, as well as providing high adhesive strength to metal and mineral substrates.
- Natalia Gladkikh, Yuriy Makarychev, Marina Maleeva, Maxim Petrunin, Ludmila Maksaeva, Alevtina Rybkina, Andrei Marshakov, Yurii Kuznetsov. Synthesis of thin organic layers containing silane coupling agents and azole on the surface of mild steel. Synergism of inhibitors for corrosion protection of underground pipelines. Progress in Organic Coatings. 2019;132:481-489
- Semiletov AM, Chirkunov AA, Kuznetsov Yu I, Andreeva NP. Passivation steel with aqueous solutions of trialkoxysilanes. J Prot Met Phys Chem Surf. 2015;51:1154-1159. doi: 10.1134/S2070205115070059
- Aramaki K. Protection of iron corrosion by ultrathin two-dimensionalpolymer films of an alkanethiolmonolayer modified with alkylethoxysilanes. J Corros Sci. 1999;41:1715-1730. doi: 10.1016/S0010-938X(99)00008-6
- Makarychev Yu B, Luchkin A Yu, Grafov O Yu, Andreev NN. Vapor-phase deposition of polymer siloxane coatings on the surface of copper and low-carbon steel, International Journal of Corrosion and Scale Inhibition, publishing house Non-profit Partnership of corrosionists "Vakor" (Moscow). 2021;11:980-1000.
- Petrunin MA, Maksaeva LB, Yurasova TA, Terekhova EV, Maleeva MA, Kotenev VA, Kablov EN, Yu Tsivadze A. Formation of organosilicon self-organizing nanolayerson an iron surface from vapor phase and their effecton corrosion behavior of metal. J Prot Met Phys Chem Surf. 2015;1010-1017. doi: 10.1134/S2070205115060179.
- Christie Ying Kei Lung, Jukka Pekka Matinlinna. Aspects of silane coupling agents and surface conditioning in dentistry: An overview Dental materials. 2012;28:467-477.
- Makarychev YB, Gladkikh NA, Redkina GV, Grafov OY, Aliev AD, Kuznetsov YI. Formation of Composite Coatings on Galvanized Steel from Organosilane Solutions Using Electrophoresis and Sol-Gel Technology. Materials (Basel). 2022 Mar 25;15(7):2418. doi: 10.3390/ma15072418. PMID: 35407753; PMCID: PMC8999879.
- Fabiola Brusciotti, Darya V Snihirova, Huibin Xue, Fatima Montemor M, Svet LM. Hybrid epoxy–silane coatings for improved corrosion protection of Mg alloy, Fabiola Brusciotti, Corrosion Science, 6792013)88-90/, Hybrid epoxy–silane coatings for improved corrosion protection of Mg alloy, Corrosion Science 6792013)88-90/.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.