Medicine Group . 2022 September 07;3(9):1007-1011. doi: 10.37871/jbres1545.
Application of Amniotic Membrane Allografts in Advanced Venous Leg Ulcer: A Case Study and Literature Review
Matthew Regulski2, John Shou1, Naomi Lambert1*, Eric Vinke1 and Tyler Barrett1
2Ocean County Foot and Ankle Center, New Jersey, USA
Abstract
Chronic Venous Insufficiency (CVI) is a lifelong, moribund and debilitating disease process with tremendous personal and financial costs. At its core, CVI involves blood pooling in the lower extremities secondary to inadequate venous blood return, resulting in venous hypertension and incompetence of the one-way valves in the lower extremity veins. As venous circulation slows, metabolic demands of the cells in the lower extremities increase, leading to stasis dermatitis, infection, cellular death, and venous ulceration. This case study aims to report the efficacy of Dehydrated Amniotic Membrane Allograft (DAMA) applications to a chronic right lateral ankle ulcer resulting from chronic venous insufficiency.
The patient in this study received DAMA applications weekly for six weeks. Upon examination at the initial application, the wound was wet and macerated due to drainage with significant hemosiderosis and lipodermatosclerosis consistent with a Venous Clinical Severity Score (VCSS) of 2. Upon inspection at the final visit, the wound was closed, with a VCSS of 0. This case study demonstrates that the application of DAMA has the potential to act as an effective barrier to cover and accelerate wound closure time. Future non-randomized and randomized controlled trials may further establish standardized protocols for DAMA application in venous ulceration, help create treatment algorithms to predict wound closure endpoints, and encourage innovation that may further accelerate healing time.
Introduction
Chronic venous insufficiency is a common and overlooked health issue that affects more than 25 million adults in the US [1]. It is estimated that up to 40% of the US population suffers from CVI [2]. CVI is the cause of 80% of lower leg ulcer cases worldwide [3]. Recent studies have approximated that the annual cost of healthcare for CVI is $3 billion [4]. These treatment costs range from $15 to $509,275 and include interventions from over-the-counter Tegaderm dressings to below-the-knee amputations. When travel expenses to doctor appointments, physical or occupational therapy appointments, and durable medical equipment evaluations are considered, the cost only rises [5]. CVI is responsible for up to 70% of all lower extremity venous ulcerations. Chronic venous ulcers, particularly in diabetic patients with CVI, warrant immediate and aggressive treatment as they precede 85% of lower extremity amputations [6].
The Venous Clinical Severity Score is used to classify venous disease of the lower extremities [7]. On a scale of zero (none) to three (severe), the VCSS affords the ability for the clinical characteristics of pain, presence of varicose veins, venous edema, skin pigmentation, presence of inflammation, induration, number of active ulcers, duration/size of active ulcers, and compliance with compressive therapy to be quantified. A multidisciplinary approach is necessary to adequately treat CVI and prevent its progression to decompensated CVI and venous ulceration. Preventative and conservative management of CVI includes the elevation of the affected leg above the heart level, daily aerobic exercise, and decompression therapy to oppose the hydrostatic forces of venous hypertension in the lower extremities [8]. Unfortunately, despite aggressive and conservative management, forestalling disease progression fails more often than not, and some ulcers never heal [9].
Patients suffering from CVI who cannot mitigate the progression of the disease may develop painful acute or chronic venous ulcerations. Ulceration from CVI ultimately develops due to the triad of venous hypertension, circulatory stasis, and tissue hypoxia triggering an inflammatory reaction and fibrosclerotic remodeling of the skin [10]. The onset of venous ulcers in CVI patients is accelerated by dry skin, dermatitis, labile edema, cellulitis, trauma, or insect bites [11]. Venous Leg Ulcers (VLUs), particularly in individuals older than 65, are often the cause of medical visits to a podiatrist, wound care specialist, primary care physician, vascular surgeon, or dermatologist [12]. VLUs often result in elevated patient discomfort due to inflammation, constant dressing changes, immobility, and increased skin sensitivity. Clinical depression, inability to work, loss of control, loss of self-identity, insomnia, and chronic pain are all hallmarks of the CVI disease progression to venous ulceration [13]. Treatment modalities for chronic venous ulceration include mitigation of swelling, mitigation of infection, and facilitating wound closure. While leg compression devices are often used to combat excessive leg swelling and CVI, the application and removal of pressure dressings, compression stockings, and decompression boots is painful and typically causes superficial scratches or abrasions, which may lead to ulceration. Any exposed venous ulcer is vulnerable to infection. Wound swabs, DNA testing, and susceptibility testing are all performed to improve sensitivity and specificity of antibiotic therapy. Should all conservative measures fail, and the wound progress, partial wound closure, reconstructive flap surgery, or even amputation may be warranted to prevent systemic spread of infection. Left unchecked, chronic ulcer infections can proliferate throughout the blood and lead to multi-system organ failure and death.
Case Presentation
Production of dehydrated human amniotic membrane allografts
Following the standards established by the U.S. Food and Drug Administration (FDA) and the American Association of Tissue Banks (AATB), human amnions were obtained from consenting mothers. An independent certified laboratory tested all the donations for infectious disease per Clinical Laboratory Improvement Amendments (CLIA) of 1988, 42 CFR part 493, and FDA regulations. In addition, each birth mother was tested for Hepatitis B Core Antibody (HBcAb), Hepatitis B Surface Antigen (HBsAg), Hepatitis C Antibody (HCV), Human Immunodeficiency
Virus Antibody (HIV -1/HIV-2 Plus 0), Human T-Lymphotropic Virus Antibody (HLTV-I/11), Syphilis (RPR), HIV-1/HCV/HBV, NAT, and West Nile Virus (WNV). Each test was performed with an FDA-Approval testing kit. All test results were negative or non-reactive. The donated amnion was aseptically processed and did not involve the addition of cells, tissues, or articles other than the exceptions outlined in 21 CFR Part 1271.10(a) (3). The amnion was placed on a sterile drying tray and desiccated in a high nitrogen concentration drying chamber. The sterility of the donation was tested at an independent laboratory, Eurofins VRL Laboratories. In addition, the desiccated allografts are sterilized via gamma irradiation before distribution.
Patient History
The patient of interest is a 47-year-old male who suffered from a refractory, non-healing right lateral ankle ulceration. The patient worked as an architect and often remained seated for 10 or more hours a day. He has a family history of varicose veins from both parents. The patient developed this ulceration secondary to venous insufficiency and valvular reflux. Despite 8 months of conservative treatment, the patient's venous ulcer persisted. This prompted a referral to an interventional cardiologist. The cardiologist performed a venous reflux test and diagnosed the patient with pelvic compression syndrome, prompting surgical stent placement in the right external iliac and common femoral vein. Stent placement did not resolve the patient's venous stasis ulcer, prompting a podiatric referral for a second opinion and definitive wound closure.
Prior to the podiatric referral, the patient had failed a total of 10 months of conservative measures that included revascularization. At the time of referral, the patient was under medical treatment for Systemic Lupus Erythematosus (SLE) and was prescribed prednisone and doxycycline for his rosacea and characteristic butterfly rash. The patient had further comorbidities of labile hypertension and chronic anxiety.
Upon the initial examination, on 1 February 2022, the VLU was wet and macerated, with copious drainage, significant hemosiderosis, and lipodermatosclerosis. The wound bed was granular in appearance with significant amounts of slough and biofilm, typical of a chronically infected wound. A repeat venous flex test confirmed perforator venous incompetence, or reflux of blood, from the deep venous system back into the superficial venous system in the legs. His perforator vein incompetence was subsequently treated with sclerotherapy.
Initial wound treatment included sharp debridement to remove the biofilm and the application of Blast X with collagen and Drawtex. Triamcinolone was applied topically to the peri-wound tissues to treat the stasis dermatitis, followed by multilayer compression bandaging.
Conservative treatment continued for four weeks to prepare the wound for DAMA application. Once the wound had achieved granulation, AmnioText™, a dehydrated amniotic membrane allograft, was applied over the wound to serve as a protective barrier. Each allograft application process included AmnioText™, silicon non-adherent dressing, BlastX, collagen, Drawtext, and Urgo2K multilayer compression bandaging. The patient was encouraged to keep his leg elevated while working and take a 30-minute walk daily. He was prescribed vitamin C, D, multivitamins, and Juven twice daily. Once the AmnioText™ allograft applications commenced, his prednisone prescription was stopped, and his dose of doxycycline was decreased by half. Complete closure was achieved after four weeks of intensive wound preparation, followed by six weeks of AmnioText™ applications. After successful wound closure, the patient was encouraged to wear 30-40 mmHg compression stockings daily and to perform 30-60 minutes of aerobic exercise daily to facilitate venous return. Follow-up appointments consisted of regular assessments for an additional three months due to the high reoccurrence rate for venous ulceration. To date, the patient has been compliant with his post AmnioText™ allograft application regimen and has maintained successful wound closure.
Discussion
Since the first use of amnion tissue as a skin graft in 1910, the use of perinatal tissue allografts in regenerative medicine has increased dramatically [14]. Dehydrated Amniotic Membrane Allografts (DAMA) are a collagen-rich extracellular matrix produced by the maternal placenta. Amnion is a thin membrane on the inner side of the fetal placenta; it surrounds the embryo and delimits the amniotic cavity, serving as a barrier and covering to contain amniotic fluid [15]. DAMA has been successfully utilized for nearly a decade in podiatric and vascular medicine to close other chronic leg and foot ulcers found in diabetic patients and those with other comorbidities. In 2014, Stephen Barr demonstrated the benefits of using DAMA in seven patients with surgical wounds, venous leg ulcers, and diabetic foot ulcers [16]. Of the seven patients, all but one experienced at least 86% wound closure in an average of 8-14 weeks. Compared with wound closure rates of 6-12 months with conservative management, medical literature supports the fact DAMA used as a barrier or covering accelerates wound closure time in ulcers.
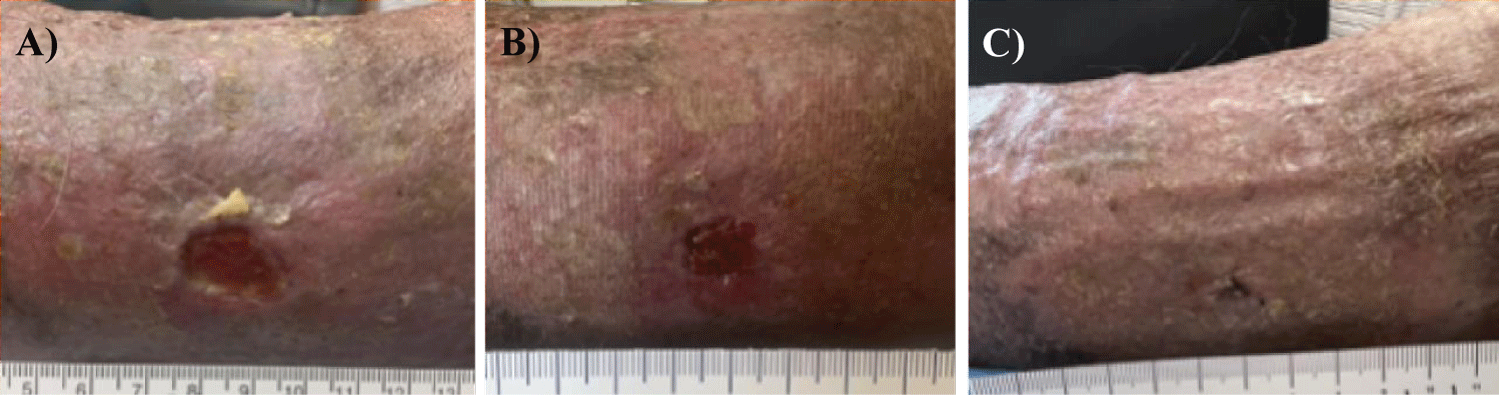
CVI has a pathophysiologic basis consistent with chronic, ambulatory venous hypertension [17]. CVI is insidious and despite revascularization, may lead to venous ulceration, as presented in this case. Despite months of conservative management, this patient suffered from a treatment-refractory wound. Similar observations were reported in this case study. Wound contracture was consistent and clinically evident after each of the six allograft applications. Each allograft was applied based on medical necessity. Weekly allograft applications were terminated upon wound closure, as shown in table 1. The healing process of the ulcer can be observed qualitatively in figure 1 and quantitatively in table 1.
| Table 1: Measurements of the Venous Ulcer at each visit. | |
| Date of Examination | Venous Leg Ulcer Measurement |
| o2/1/2022 | 2.50 cm x 1.80 cm |
| o2/8/2022 | 2.45 cm x 1.75 cm |
| o2/22/2022 | 2.50 cm x 1.90 cm* |
| o3/1/2022 | 2.00 cm x 1.50 cm |
| ◊3/8/2022 | 1.50 cm x 1.00 cm |
| ◊3/14/2022 | 1.50 cm x 1.00 cm |
| ◊3/22/2022 | 1.00 cm x 1.00 cm |
| ◊3/29/2022 | 0.75 cm x 0.75 cm |
| ◊4/5/2022 | 0.65 cm x 0.75 cm |
| ◊4/12/2022 | 0.50 cm x 0.25 cm |
| ∆4/19/2022 | 0.25 cm x 0.15 cm |
| ∆4/26/2022 | closed |
| oWound Preparation *Post Debridement ◊DAMA applications Δ Wound Healed | |
The use of human amniotic membrane allografts as an effective method in VLU treatment coincides with a 2017 multicenter randomized controlled trial comparing the efficacy of human amnion/chorion membrane allografts simultaneously with compression therapy, and standard wound care with compression therapy alone [18]. This trial followed 109 patients receiving allografts over 16 weeks and used a Kaplan-Meier analysis to compare healing time without allografts. At week 16, the mean VLU area was reduced by 72% for allograft-treated wounds compared to 39% with standard of care. In a 2013 prospective study, 40 patients with non-healing venous ulcers were observed for one to three years following an Amniotic Membrane (AM) transfer [19]. The inclusion criteria for this study included; patients between 40-50 years of age, non-healing venous leg ulcers 3 cm in size or greater, ulcer duration greater than 3 months despite surgery or split skin graft. By the 90-day mark, 80% of patients had experienced a greater than 50% reduction in baseline ulcer area and re-epithelization. Not a single patient required further surgery for their ulcers in the 1-3 years of follow-up.
While amniotic membrane allografts have been shown in repeat clinical studies and case presentations to accelerate the time to wound closure in venous leg ulcers, they are also comparable in cost to the standard of care [20]. Not only is the standard of care for VLUs shown to be less effective in wound closure rate, but it is also higher in cost when considering additional quality of life metrics. When accounting for the expenses involved in treating VLU's, patient travel to and from a wound care clinic, wound care supplies not covered by insurance, lost wages from time off work, and caregiver fees for home care nurses or aids are all out-of-pocket expenditures for patients. Extending wound closure rates by weeks or even months may result in medical bankruptcy for patients and their families. Medicare currently recognizes the medical necessity of DAMA allografts and provides some reimbursement for their application when used as an intervention for DFUs and venous stasis ulcers [21].
Consequently, many patients seeking care in “site of service 11” locations can rely on Medicare to assist with the costs associated with VLU treatment [22]. This assistance is critical in underserved communities where resources are limited, medical supply costs are high, availability is low, and transportation is challenging. As the population in underserved communities is generally in more frail health and suffers from multiple comorbidities, rapid treatment of venous ulcers could exponentially improve their quality of life and long-term vitality.
Results
Upon initial examination on February 1, 2022, the VLU measured 2.00 cm x 1.80 cm (Figure 1A). The wound was open and showed clear signs of lipodermatosclerosis. The edges and outline of the ulcer were poorly defined. At the final examination on April 26th, the VLU (Figures 1B,C) was closed. Despite wound closure evident at the final visit, a slight indention was present in the skin topography with minimal traces of skin irritation. The patient's wound contracted consistently and clinically significantly throughout six DAMA applications, as seen in table 1. Noting that the patient’s VLU had been open for ten months despite revascularization prior to the first application of DAMA, aggressive VLU management with DAMA allografting was instrumental in accelerating the time to VLU closure. Among the most important outcomes from this case study, and confirmed by other studies, the use of DAMA may decrease the risk of infection, reduce the pain associated with an open wound, and accelerate the time to wound closure.
Conclusion
The utilization of amniotic membrane allografts accelerates wound closure times in chronic VLUs and is consistent with the current medical literature. In the presented case study, the use of amniotic membrane allografts closed a 10-month-old wound, refractory to conservative measures and revascularization, in 12 weeks. The patient achieved full closure after six AmnioText allograft applications. Future research efforts centered on the preventative use of amniotic membrane allografts early in the treatment of patients with recalcitrant venous leg ulcers present a novel opportunity to reduce long-term morbidity, improve quality of life, and reduce national healthcare costs.
Acknowledgment
The authors would like to thank Ocean County Foot and Ankle Surgical Associates for their collaboration in data collection. The authors would also like to thank Justine Davis, Catherine Becker, and Jessica LaRocco for their collaboration in reviewing the manuscript.
Funding
This research received no external funding. Regenative Labs is responsible for all APC charges.
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the Institute of Regenerative and Cellular Medicine (protocol code IRCM-2022-311 and approved on 12 January 2022).
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Data availability
Data can be found in Appendix A.
Conflicts of interest
John Shou, Naomi Lambert, Eric Vinke, and Tyler Barrett are associated with Regenative Labs. Regenative Labs was involved in the design of the study, data analysis, and writing. Dr. Matthew Regulski performed the treatment and data collection at Ocean County Foot and Ankle Surgical Associates. Regenative Labs influenced the decision to publish.
References
- Kim Y, Png CYM, Sumpio BJ, DeCarlo CS, Dua A. Defining the human and health care costs of chronic venous insufficiency. Semin Vasc Surg. 2021 Mar;34(1):59-64. doi: 10.1053/j.semvascsurg.2021.02.007. Epub 2021 Feb 3. PMID: 33757637.
- O'Banion LAA, Siada S, Cutler B, Kochubey M, Collins T, Ali A, Tenet M, Dirks R, Kiguchi MM. Thrombotic complications after radiofrequency and cyanoacrylate endovenous ablation: Outcomes of a multicenter real-world experience. J Vasc Surg Venous Lymphat Disord. 2022 Jul 16:S2213-333X(22)00272-4. doi: 10.1016/j.jvsv.2022.05.009. Epub ahead of print. PMID: 35843596.
- Agale SV. Chronic leg ulcers: Epidemiology, aetiopathogenesis, and management. Ulcers. 2013;9. doi: 10.1155/2013/413604.
- Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation. 2014;130(4):333-346. doi: 10.1161/CIRCULATIONAHA.113.006898.
- Chung KC, Saddawi-Konefka D, Haase SC, Kaul G. A cost-utility analysis of amputation versus salvage for Gustilo type IIIB and IIIC open tibial fractures. Plast Reconstr Surg. 2009 Dec;124(6):1965-1973. doi: 10.1097/PRS.0b013e3181bcf156. PMID: 19952652; PMCID: PMC2788746.
- Berti-Hearn L, Elliott B. Chronic venous insufficiency: A review for nurses. Nursing. 2019 Dec;49(12):24-30. doi: 10.1097/01.NURSE.0000604688.03299.aa. PMID: 31658230.
- Vasquez MA, Rabe E, McLafferty RB, Shortell CK, Marston WA, Gillespie D, Meissner MH, Rutherford RB; American Venous Forum Ad Hoc Outcomes Working Group. Revision of the venous clinical severity score: venous outcomes consensus statement: special communication of the American Venous Forum Ad Hoc Outcomes Working Group. J Vasc Surg. 2010 Nov;52(5):1387-96. doi: 10.1016/j.jvs.2010.06.161. Epub 2010 Sep 27. PMID: 20875713.
- McNichol L, Ratliff C, Yates S. Wound, ostomy and continence nurses society core curriculum: Wound management. Lippincott Williams & Wilkins; 2021.
- ElHeneidy H, Omran E, Halwagy A, Al-Inany H, Al-Ansary M, Gad A. Amniotic membrane can be a valid source for wound healing. Int J Womens Health. 2016 Jun 27;8:225-31. doi: 10.2147/IJWH.S96636. PMID: 27390533; PMCID: PMC4930235.
- Ortega MA, Fraile-Martínez O, García-Montero C, Álvarez-Mon MA, Chaowen C, Ruiz-Grande F, Pekarek L, Monserrat J, Asúnsolo A, García-Honduvilla N, Álvarez-Mon M, Bujan J. Understanding Chronic Venous Disease: A Critical Overview of Its Pathophysiology and Medical Management. J Clin Med. 2021 Jul 22;10(15):3239. doi: 10.3390/jcm10153239. PMID: 34362022; PMCID: PMC8348673.
- Shai A, Halevy S. Direct triggers for ulceration in patients with venous insufficiency. Int J Dermatol. 2005 Dec;44(12):1006-9. doi: 10.1111/j.1365-4632.2005.02317.x. PMID: 16409265.
- Frykberg RG, Banks J. Challenges in the Treatment of Chronic Wounds. Adv Wound Care (New Rochelle). 2015 Sep 1;4(9):560-582. doi: 10.1089/wound.2015.0635. PMID: 26339534; PMCID: PMC4528992.
- Herber OR, Schnepp W, Rieger MA. A systematic review on the impact of leg ulceration on patients' quality of life. Health Qual Life Outcomes. 2007 Jul 25;5:44. doi: 10.1186/1477-7525-5-44. PMID: 17651490; PMCID: PMC1947954.
- Moore MC, Van De Walle A, Chang J, Juran C, McFetridge PS. Human Perinatal-Derived Biomaterials. Adv Healthc Mater. 2017 Sep;6(18):10.1002/adhm.201700345. doi: 10.1002/adhm.201700345. Epub 2017 Aug 7. PMID: 28783879; PMCID: PMC5733692.
- Mamede AC, Carvalho MJ, Abrantes AM, Laranjo M, Maia CJ, Botelho MF. Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res. 2012 Aug;349(2):447-58. doi: 10.1007/s00441-012-1424-6. Epub 2012 May 18. PMID: 22592624.
- Barr SM. Dehydrated Amniotic Membrane Allograft for Treatment of Chronic Leg Ulcers in Patients With Multiple Comorbidities: A Case Series. J Am Coll Clin Wound Spec. 2016 Feb 4;6(3):38-45. doi: 10.1016/j.jccw.2016.01.002. PMID: 27104144; PMCID: PMC4828514.
- Huether SE, McCance KL. Understanding pathophysiology. 2020.
- Bianchi C, Cazzell S, Vayser D, Reyzelman AM, Dosluoglu H, Tovmassian G; EpiFix VLU Study Group. A multicentre randomised controlled trial evaluating the efficacy of dehydrated human amnion/chorion membrane (EpiFix® ) allograft for the treatment of venous leg ulcers. Int Wound J. 2018 Feb;15(1):114-122. doi: 10.1111/iwj.12843. Epub 2017 Oct 11. PMID: 29024419; PMCID: PMC7949978.
- Francis J, Shajimon CR, Bhat AK, Kanakambaran B. Use of Amnion Transfer in Resistant Nonhealing Venous Leg Ulcers. Indian J Surg. 2015 Dec;77(Suppl 2):457-62. doi: 10.1007/s12262-013-0875-7. Epub 2013 Jan 31. PMID: 26730045; PMCID: PMC4692879.
- Ma H, O'Donnell TF Jr, Rosen NA, Iafrati MD. The real cost of treating venous ulcers in a contemporary vascular practice. J Vasc Surg Venous Lymphat Disord. 2014 Oct;2(4):355-61. doi: 10.1016/j.jvsv.2014.04.006. Epub 2014 Jun 24. PMID: 26993537.
- Becker C, Regulski M, Martin S, Barrett T. Application of dehydrated amniotic membrane allografts in advanced diabetic foot ulceration: Case Report and Review of Literature. Reports. 2022;5(3):28. doi: 10.3390/reports5030028.s.
- Place of service code set. CMS. (n.d.). 2022.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.