Medicine Group . 2022 August 30;3(8):980-984. doi: 10.37871/jbres1540.
Improving Invasive Breast Cancer Care Using Machine Learning Technology
Clement G Yedjou1*, Solange S Tchounwou2, Jameka Grigsby3, Keara Johnson4 and Paul B Tchounwou4
2Department of Pathology and Laboratory Medicine. School of Medicine, Tulane University, 1430 Tulane Avenue, New Orleans, LA, 70112, United States
3Department of Biological Sciences, School of Arts and Sciences, Alcorn State University, 1000 ASU Drive, Alcorn State, MS 39096, United States
4Department of Biology, College of Science, Engineering and Technology, Jackson State University, 1400 Lynch Street, Box 18750, Jackson, MS 39217, United States
- Machine Learning
- Breast cancer
- Infiltrating ductal carcinoma
- Infiltrating lobular carcinoma
- Mucinous carcinoma
- Machine learning
- Surgery treatment
Abstract
Breast Cancer (BC) is the most common malignancy in women worldwide. In the United States, the lifetime risk of developing an invasive form of breast cancer is 12.5% among women. BC arises in the lining cells (epithelium) of the ducts or lobules in the glandular tissue of the breast. The goal of the present study was to use Machine Learning (ML) as a novel technology to assess and compare the invasive forms of BC including, infiltrating ductal carcinoma, infiltrating lobular carcinoma, and mucinous carcinoma. To achieve this goal, we used ML algorithms and collected a dataset of 334 BC patients available at https://www.kaggle.com/amandam1/breastcancerdataset and interpreted this dataset based on the form of BC, age, sex, tumor stages, surgery type, and survival rate. Among the 334 patients, 70% were diagnosed with infiltrating ductal carcinoma, 27% with infiltrating lobular carcinoma, and 3% with mucinous carcinoma. Overall, out of 334 BC patients: 64 (19.16%) were in stage I, 189 (56.59%) in stage II, and 81 (24.25%) in stage III. Sixty-six, 67, 96, and 105 patients underwent lumpectomy, simple mastectomy, modified radical mastectomy, and other types of surgery, respectively. The survival rates were 83.4% for stage I, 79.1% for stage II, and 77% for stage III. Findings from the present study demonstrated that ML provides an important tool to curate large amount of BC data, as well as a scientific means to improve BC outcomes.
Introduction
Breast Cancer (BC) is a group of diseases in which cancer cells are genetically and morphologically different from normal cells and divide in an uncontrolled manner, resulting in a lump or mass [1-3]. Most of breast cancers start in the lobules or in the ducts that connect the lobules to the nipple. Worldwide, BC is the most frequently diagnosed life-threatening cancer and the leading cause of death among women. The development of BC can begin in different areas of the breast, such as the lobules, ducts, or the tissue in between. Adenocarcinoma represents more than 95% of breast cancers [4-6]. The main two histological subtypes of BC are Invasive Lobular Carcinoma (ILC), also called Infiltrating Lobular Carcinoma (ILC), and Invasive Ductal Carcinoma (IDC), also called Infiltrating Ductal Carcinoma (IDC). IDC is the most common type of BC overall, representing approximately 80% of all breast cancers [7,8]. Other pathological subtypes of BC may still appear in patients with IDC. In comparison with IDC, ILC accounts for 10-15% of all BC [9,10], and it is characterized by small, round tumor cells growing in the stroma in a discohesive single-file pattern [11,12]. In addition, ILC is more difficult to detect by standard imaging techniques such as mammography and 18F-FDG-ET [13-15]. ILC is mostly detected in older patients and in advanced stages of BC [16,17]. Furthermore, ILC patients display relatively late recurrences and worse long-term survival compared to IDC patients [18-20]. Mucinous BC is a rare type of invasive BC that accounts for less than 2% of all breast cancers. Like other types of invasive BC, mucinous BC begins in the milk duct of the breast before spreading to the tissues around the duct [21]. Most patients with mucinous breast carcinoma present with a palpable breast mass. Mucinous BC grows slowly and may reach a large size at the moment of diagnosis. This can be explained by the fact that the mucinous content of the tumor does not feel firm or solid upon examination [22,23]. It is important to distinguish between the various subtypes of breast cancers because they have different prognoses and treatment implications. The treatment of BC is highly effective when the disease is diagnosed at an early stage. The current treatment options for BC consist of a multidisciplinary approach involving surgical oncology, chemotherapy, radiotherapy, hormone/endocrine therapy, targeted cancer drugs, and bone strengthening drugs (bisphosphonates). Such treatment modalities have been associated with a reduction of BC growth and spread, reduction in breast cancer mortality, and an increase in survival rates, thereby saving the life of BC patients [24]. Researchers are increasingly using Machine Learning (ML) approaches for modelling the progression and treatment of cancer due to its ability to detect key features from complex datasets [25,26]. The application of ML models for the prediction and prognosis of disease development has become an irrevocable part of cancer research. Recent studies in our laboratory have shown that ML is an effective scientific tool to accurately predict, and classify BC patients with benign and malignant tumors [27]. ML holds the potential to transform healthcare and hence opens a world of incredible promise. The goal of the present study was to use ML as a novel technology to assess and compare the invasive forms of BC including, infiltrating ductal carcinoma, infiltrating lobular carcinoma, and mucinous carcinoma.
Materials and Methods
Source of dataset and information
Clinical and pathologic data were extracted from the electronic medical record on BC publicly available on Machine Learning Repository: https://www.kaggle.com/amandam1/breastcancerdataset. The dataset consists of a group of BC patients, who underwent surgical treatment to remove their tumors.
Machine learning methods
This study was based on the application of Machine Learning (ML) algorithms to analyze and interpret a dataset of 334 BC patients. ML is a branch of Artificial Intelligence (AI) that is used to classify data based on models which have been developed and for predictive analytics, in particular, breast cancer [28,29]. It provides tools by which large quantities of data can be automatically analyzed. In the case of the present study, we utilized ML algorithms and collected a scientific dataset of BC patients from Kaggle (https://www.kaggle.com/amandam1/breastcancerdataset) and interpreted this dataset based on different parameters, including patient age at diagnosis, gender, tumor stage, histology, type of surgery, and patient status (alive or dead).
Data selection and collection
We collected a dataset consisting of a group of 334 BC patients, who had surgery to remove their tumors. The dataset consists of the following variables: (1) Age (age at diagnosis); (2) Gender (male/female); (3) Tumor stage (I, II, III); (4) Histology (infiltrating ductal carcinoma, infiltrating lobular carcinoma, and mucinous carcinoma); (5) Surgery type (lumpectomy, simple mastectomy, modified radical mastectomy, other); and (6) Patient status (alive, dead, no reported [no information available whether the patient is alive or dead]).
Results
Invasive breast cancer
Among the 334 BC patients evaluated in this study, 70% of patients were diagnosed with infiltrating ductal carcinoma, also called invasive ductal carcinoma, and 27% of patients were diagnosed with infiltrating lobular carcinoma, also called invasive lobular carcinoma. Also, 3% of patients were diagnosed with mucinous carcinoma. As seen in the case of the present study, invasive ductal carcinoma is the most common type of breast cancer overall, representing over 70% of all breast cancers.
Sex and age at diagnosis
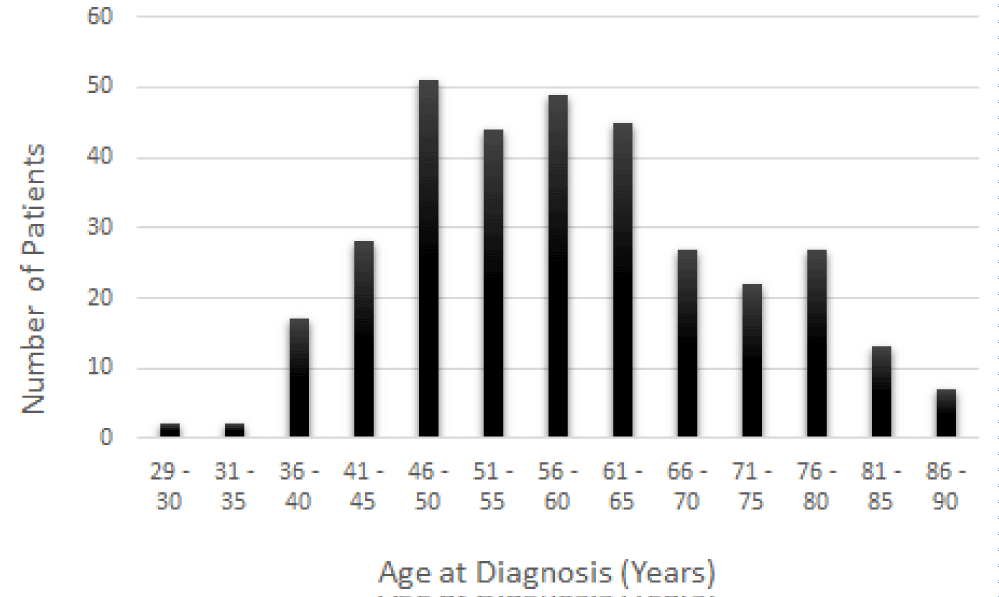
BC is most often found in women, but men also can suffer from it. Among the 334 BC patients evaluated in this study, 330 patients were women aged from 29 to 90 years old, and the remaining four patients were men aged from 44 to 84 years. BC is a rare disease in men. It has been reported that 1% of BC diagnosed in the United State is found in men [30-32]. In the present study, the patients were subsequently divided into thirteen groups of age as follows: 29 to 30 years; 31 to 35 years; 36 to 40 years; 41 to 45 years; 46 to 50 years; 51 to 55 years; 56 to 60 years; 61 to 65 years; 66 to 70 years; 71 to 75 years; 76 to 80 years; 81 to 85 years; and 86 to 90 years. As seen in figure 1, the prevalence of BC increases with age. Also, a very large proportion of BC patients were diagnosed between the age of 46 and 65 compared to the age ranges of 29-45 and 66-90. Although BC can strike at any age, younger females in general are not at high risk of developing BC.
Stages of breast cancer in relation to the age
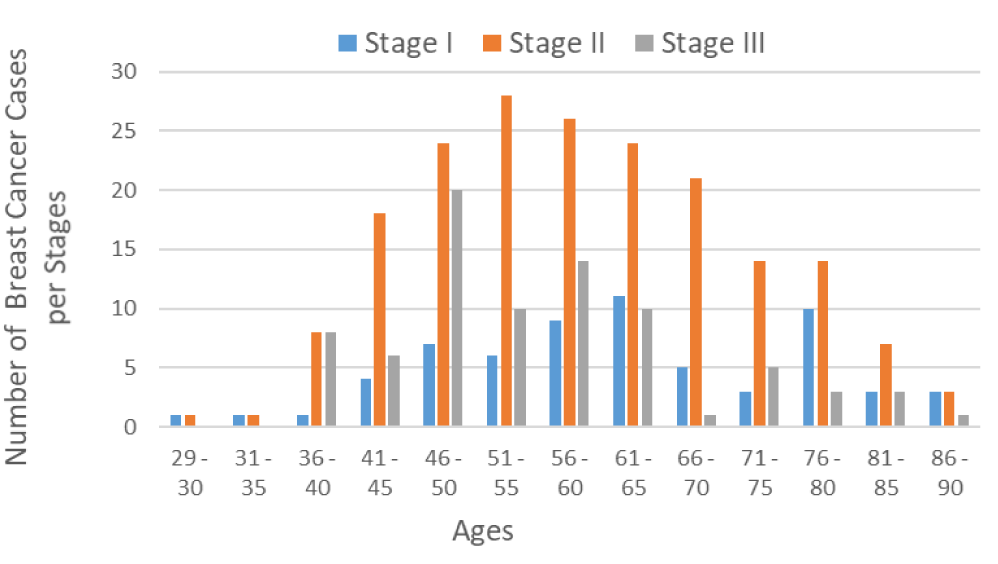
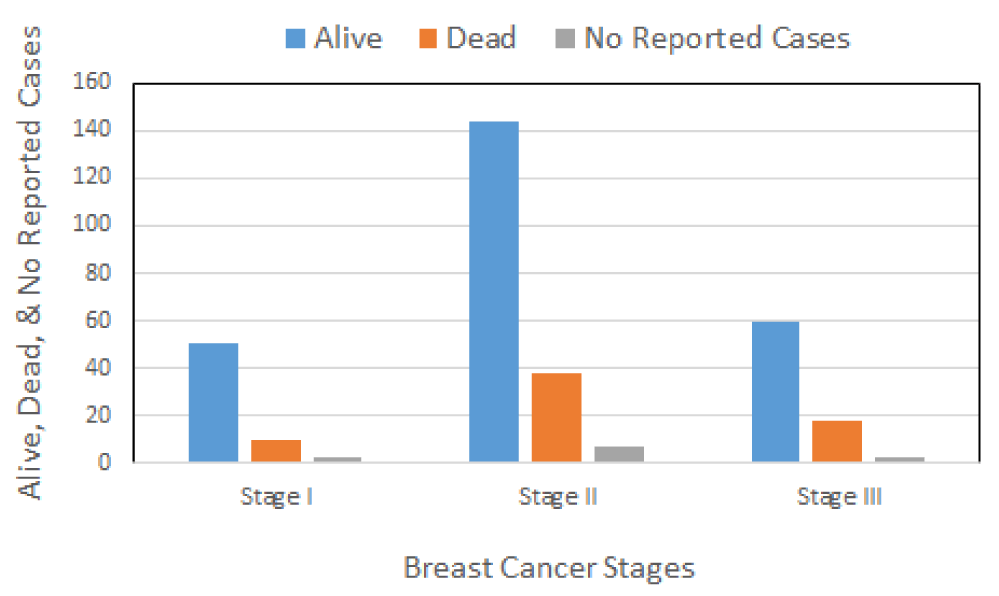
Staging describes the spread of cancer at the time of diagnosis, and is used to determine the treatment options. Here, 64 out of 334 BC patients were diagnosed in stage I, 189 patients in stage II, and 81 patients in stage III (Figure 2). As seen in figure 2, the risk of developing BC increases with age. BC is rare in females younger than 25 years. Approximately 6 to 7% of all breast cancer in the United are diagnosed in women under 40 years of age. As shown in figure 1, its prevalence gradually increases with age and reaches a plateau in women aged 46-65 years. Most BC is diagnosed in stage II (Figure 2).
Surgery type in relation to the stage of breast cancer
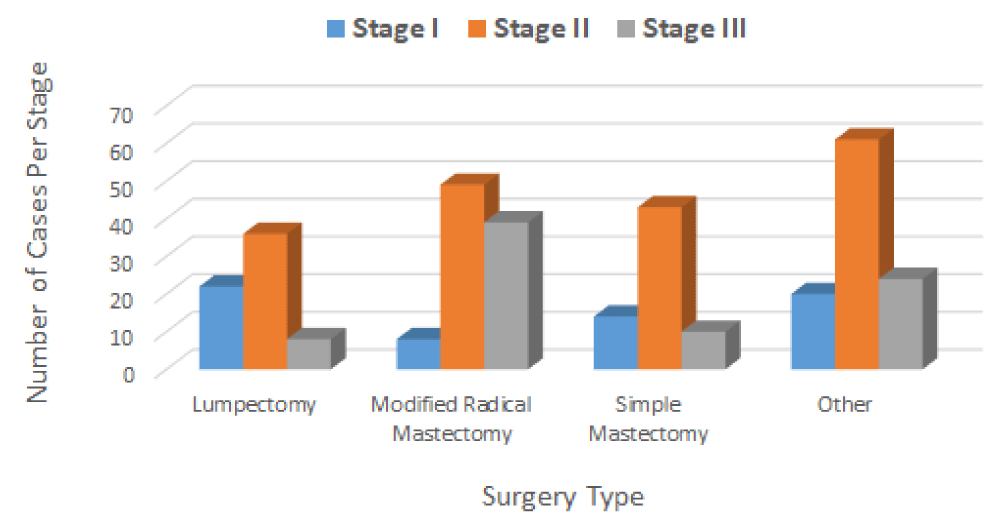
Surgery has been a mainstay of BC treatment for many years. In the case of the present study, the physicians performed different types of surgery (localized therapy), including lumpectomy, simple mastectomy, modified radical mastectomy, and other kinds of surgery to remove the breast tumor. Among the 334 BC patients who had surgery, sixty-six (66) patients underwent a lumpectomy, with a total of 22 patients in stage I, 36 in stage II, and 8 in stage III. Ninety-six 96 patients underwent a modified radical mastectomy, with a total of 8 patients in stage I, 49 in stage II, and 39 in stage III. Sixty-seven (67) patients underwent a simple mastectomy, with a total of 14 patients in stage I, 43 in stage II, and 10 in stage III. One hundred five (105) patients underwent other types of surgery with a total of 20 patients in stage I, 61 in stage II, and 24 in stage III (Figure 3). As seen in figure 3, most patients in stages II and III undergone modified radical mastectomy (a surgery technique that removes the entire breast including the breast tissue, skin, areola, and nipple) compared to simple mastectomy (a surgery technique that removes the breast including the breast tissue, nipple, areola, skin, and skin but not all the lymph nodes).
Survival rate in relation to the age
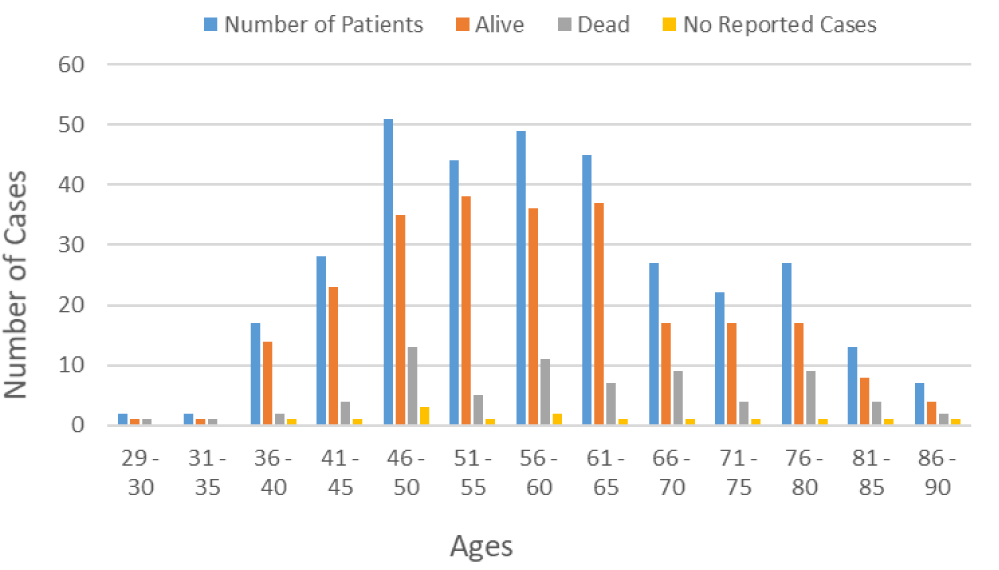
Age is a key risk factor for BC, and several scientific studies have suggested that patient age at diagnosis is associated with BC survival [33-36]. As seen in figure 4, young patients have a higher survival rate compared to older patients.
Survival rate in relation to breast cancer stages
Figure 5 presents the relative survival rates after surgery among 334 BC patients in relation to the stage. The total number of survivors detected among 334 BC patients diagnosed with invasive BC was 255 or 76.35%, including stage I, II, and III breast cancer. Sixty-six (19.76%) patients died after surgery and 13 (3.89%) of patients were not reported (there is no information available on whether those patients remained alive or dead).
Discussion
Breast Cancer (BC) is the most common cancer in women worldwide, with age considered to be an important risk factor 33. Its incidence and prevalence rates continue to increase, especially in wealthy nations. Although it has been reported that 1 in 8 women will develop metastatic BC at some time in their life, a recent study has further stratified the risk of developing BC according to the age group, showing that the risk is 1 in 15,000 women up to age 25, 1 in 1900 up to age 30, and 1 in 200 women up to age 40 [37]. Similarly, our data demonstrated that the incidence and mortality rates of BC increase with age (Figure 1). The treatment of BC has traditionally employed a tri-modality approach: surgery followed by adjuvant chemotherapy and radiation therapy. Current BC treatment may include localized therapies such as surgery, cryotherapy, radiation therapy, chemical ablation, and/or systemic therapies such as chemotherapy, hormonal therapy, immune therapy, and targeted therapy used alone or in combination. In the case of the present study, the physicians performed different types of surgery (localized therapy), including lumpectomy, simple mastectomy, modified radical mastectomy, and others of surgery to remove the breast tumor. After the surgery, 255 out of 334 patients were alive, including patients with BC stages I, II, and III. Sixty-six (66) died, and the status of the 13 remaining patients was not reported (Figure 5). According to SEER Cancer Statistics Review, the overall 5-year relative survival for BC in females is 90 percent 38. For females diagnosed with stage I BC, the five-year relative survival is approximately 100%. However, it declines to 26% for females diagnosed with stage IV BC [38]. In addition to age and stage, other risk factors that influence BC survival include hormone receptor status, HER2 status, tumor grade, treatment, health insurance, and financial resources [39,40]. Furthermore, patient and physician factors such as attitudes, beliefs, and preferences influence treatment recommendations and delivery which are more likely to contribute to the survival differences [41].
Conclusion
The application of machine learning (ML) algorithms to the clinical dataset analyzed in this study revealed that among the 334 BC patients, 330 patients were women aged from 29 to 90 years old, and the remaining 4 patients were men aged from 44 to 84 years. Seventy percent (70%) of these patients were diagnosed with infiltrating ductal carcinoma, 27% with infiltrating lobular carcinoma, and 3% with mucinous carcinoma. These patients underwent surgery to remove the tumors. Among these individuals, 66 patients (22 in stage I, 36 in stage II, and 8 in stage III) underwent a lumpectomy; 96 patients (8 in stage I, 49 in stage II, and 39 in stage III) underwent a modified radical mastectomy; 67 patients (14 in stage I, 43 in stage II, and 10 in stage III) underwent a simple mastectomy; and 105 patients (20 in stage I, 61 in stage II, and 24 in stage III) underwent other types of surgery. After the surgery, 255 patients (252 women and 3 men) who underwent treatment for BC stages I, II, and III, remained alive, and sixty-six (65 women and 1 man) patients died. The status of the remaining 13 women patients was not reported. It is evident from our study that ML can help physicians to make quicker health decisions regarding the diagnosis, detection, prediction, and better treatment of BC patients.
Acknowledgment
This work was funded by the National Institutes of Health (NIH), Grant # 1U54MD015929-01 at Jackson State University, Jackson, MS, United States, and Faculty Research Award Program (FRAP) at Florida Agricultural and Mechanical University, Tallahassee, FL, United States.
Author contributions
Conceptualization, C.G.Y., S.S.T., and R.A.A.; Methodology, C.G.Y., J.G., and S.S.T; formal analysis, S.S.T., B.M., C.G.Y and R.E.; investigation, C.G.Y, S.S.T., K.J., and J.G.; supervision, K.S., and P.B.T.; writing-original draft preparation, all authors; writing-reviewing and editing, all authors; All authors have read and agreed to the published version of the manuscript.
Conflicts of intevrest
The authors declare no conflict of interest.
Data availability statement
The breast cancer dataset that support the findings in this paper was made publicly available in Kaggle.com (https://www.kaggle.com/amandam1/breastcancerdataset).
References
- Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021 Apr 5. doi: 10.1002/ijc.33588. Epub ahead of print. PMID: 33818764.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68(6):394-424. doi: 10.3322/caac.21492. Epub 2018 Sep 12. Erratum in: CA Cancer J Clin. 2020 Jul;70(4):313. PMID: 30207593.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019 Jan;69(1):7-34. doi: 10.3322/caac.21551. Epub 2019 Jan 8. PMID: 30620402.
- DeSantis CE, et al. Breast cancer statistics 2019 CA. Cancer J Clin. 2019;69.
- Hoda SA, Hoda RS. Robbins and Cotran Pathologic Basis of Disease. Am J Clin Pathol. 2020;154.
- Kumar V, Abbas A, Aster J, Turner J. Robbins Cotran Pathologic Basis of Disease Tenth Edition. Elsevier. 2021.
- Badowska-Kozakiewicz AM, Liszcz A, Sobol M, Patera J. Retrospective evaluation of histopathological examinations in invasive ductal breast cancer of no special type: an analysis of 691 patients. Arch Med Sci. 2017 Oct;13(6):1408-1415. doi: 10.5114/aoms.2015.53964. Epub 2016 Mar 4. PMID: 29181072; PMCID: PMC5701672.
- Chand P, Garg A, Singla V, Rani N. Evaluation of Immunohistochemical Profile of Breast Cancer for Prognostics and Therapeutic Use. Niger J Surg. 2018 Jul-Dec;24(2):100-106. doi: 10.4103/njs.NJS_2_18. PMID: 30283220; PMCID: PMC6158994.
- Tsagkaraki IM, Kourouniotis CD, Gomatou GL, Syrigos NK, Kotteas EA. Orbital metastases of invasive lobular breast carcinoma. Breast Dis. 2019;38(3-4):85-91. doi: 10.3233/BD-190398. PMID: 31640079.
- Suchman K, Zimmerman BS, Ru M, Cascetta KP, Tiersten A. Distribution of oncotype recurrence scores in invasive lobular carcinomas. J Clin Oncol. 2020;38.
- Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004;6(3):R149-56. doi: 10.1186/bcr767. Epub 2004 Feb 17. PMID: 15084238; PMCID: PMC400666.
- Cao AY, Huang L, Wu J, Lu JS, Liu GY, Shen ZZ, Shao ZM, Di GH. Tumor characteristics and the clinical outcome of invasive lobular carcinoma compared to infiltrating ductal carcinoma in a Chinese population. World J Surg Oncol. 2012 Jul 17;10:152. doi: 10.1186/1477-7819-10-152. PMID: 22805492; PMCID: PMC3502188.
- Han B, Gu Z, Liu Z, Ling H. Clinical Characteristics and Survival Outcomes of Infiltrating Lobular Carcinoma: A Retrospective Study of 365 Cases in China. Cancer Manag Res. 2022 Feb 16;14:647-658. doi: 10.2147/CMAR.S346319. PMID: 35210861; PMCID: PMC8858761.
- Johnson K, Sarma D, Hwang ES. Lobular breast cancer series: imaging. Breast Cancer Res. 2015 Jul 11;17(1):94. doi: 10.1186/s13058-015-0605-0. PMID: 26163296; PMCID: PMC4499185.
- Shakoor MT, Ayub S, Mohindra R, Ayub Z, Ahad A. Unique presentations of invasive lobular breast cancer: a case series. Int J Biomed Sci. 2014 Dec;10(4):287-93. PMID: 25598762; PMCID: PMC4289705.
- Li CI, Uribe DJ, Daling JR. Clinical characteristics of different histologic types of breast cancer. Br J Cancer. 2005 Oct 31;93(9):1046-52. doi: 10.1038/sj.bjc.6602787. PMID: 16175185; PMCID: PMC2361680.
- Zhao S, Ma D, Xiao Y, Jiang YZ, Shao ZM. Clinicopathologic features and prognoses of different histologic types of triple-negative breast cancer: A large population-based analysis. Eur J Surg Oncol. 2018 Apr;44(4):420-428. doi: 10.1016/j.ejso.2017.11.027. Epub 2018 Jan 9. PMID: 29429597.
- Ide Y, Horii R, Osako T, Ogura K, Yoshida R, Iwase T, Akiyama F. Clinicopathological significance of invasive micropapillary carcinoma component in invasive breast carcinoma. Pathol Int. 2011 Dec;61(12):731-6. doi: 10.1111/j.1440-1827.2011.02735.x. Epub 2011 Oct 31. PMID: 22126380.
- Fisher ER, Dignam J, Tan-Chiu E, Costantino J, Fisher B, Paik S, Wolmark N. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17: intraductal carcinoma. Cancer. 1999 Aug 1;86(3):429-38. doi: 10.1002/(sici)1097-0142(19990801)86:3<429::aid-cncr11>3.0.co;2-y. PMID: 10430251.
- Gokce H, Durak MG, Akin MM, Canda T, Balci P, Ellidokuz H, Demirkan B, Gorken IB, Sevinc AI, Kocdor MA, Saydam S, Harmancioglu O. Invasive micropapillary carcinoma of the breast: a clinicopathologic study of 103 cases of an unusual and highly aggressive variant of breast carcinoma. Breast J. 2013 Jul-Aug;19(4):374-81. doi: 10.1111/tbj.12128. Epub 2013 May 29. PMID: 23714006.
- Kaoku S, Konishi E, Fujimoto Y, Tohno E, Shiina T, Kondo K, Yamazaki S, Kajihara M, Shinkura N, Yanagisawa A. Sonographic and pathologic image analysis of pure mucinous carcinoma of the breast. Ultrasound Med Biol. 2013 Jul;39(7):1158-67. doi: 10.1016/j.ultrasmedbio.2013.02.014. Epub 2013 May 15. PMID: 23683410.
- Xu X, Bi R, Shui R, Yu B, Cheng Y, Tu X, Yang W. Micropapillary pattern in pure mucinous carcinoma of the breast - does it matter or not? Histopathology. 2019 Jan;74(2):248-255. doi: 10.1111/his.13722. Epub 2018 Oct 23. PMID: 30066338.
- Zhang H, Qiu L, Peng Y. The sonographic findings of micropapillary pattern in pure mucinous carcinoma of the breast. World J Surg Oncol. 2018 Jul 24;16(1):151. doi: 10.1186/s12957-018-1449-8. PMID: 30041628; PMCID: PMC6058370.
- Kesson EM, Allardice GM, George WD, Burns HJ, Morrison DS. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13 722 women. BMJ. 2012 Apr 26;344:e2718. doi: 10.1136/bmj.e2718. PMID: 22539013; PMCID: PMC3339875.
- Sharma G, Prabha C. Applications of Machine Learning in Cancer Prediction and Prognosis. in Cancer Prediction for Industrial IoT 4.0: A Machine Learning Perspective. 2021. doi:10.1201/9781003185604-8
- Deepti, Ray SA. Survey on Application of Machine Learning Algorithms in Cancer Prediction and Prognosis. In Advances in Intelligent Systems and Computing. 2021;1174.
- Yedjou CG, Tchounwou SS, Aló RA, Elhag R, Mochona B, Latinwo L. Application of Machine Learning Algorithms in Breast Cancer Diagnosis and Classification. Int J Sci Acad Res. 2021 Jan;2(1):3081-3086. Epub 2021 Oct 30. PMID: 34825131; PMCID: PMC8612371.
- Mosquera-Lopez C, Agaian S, Velez-Hoyos A, Thompson I. Computer-Aided Prostate Cancer Diagnosis From Digitized Histopathology: A Review on Texture-Based Systems. IEEE Rev Biomed Eng. 2015;8:98-113. doi: 10.1109/RBME.2014.2340401. Epub 2014 Jul 17. PMID: 25055385.
- Nir G, Karimi D, Goldenberg SL, Fazli L, Skinnider BF, Tavassoli P, Turbin D, Villamil CF, Wang G, Thompson DJS, Black PC, Salcudean SE. Comparison of Artificial Intelligence Techniques to Evaluate Performance of a Classifier for Automatic Grading of Prostate Cancer From Digitized Histopathologic Images. JAMA Netw Open. 2019 Mar 1;2(3):e190442. doi: 10.1001/jamanetworkopen.2019.0442. PMID: 30848813; PMCID: PMC6484626.
- Shishido SN, Faulkner EB, Beck A, Nguyen TA. The effect of antineoplastic drugs in a male spontaneous mammary tumor model. PLoS One. 2013 Jun 3;8(6):e64866. doi: 10.1371/journal.pone.0064866. PMID: 23755153; PMCID: PMC3670867.
- Ellington TD, Henley SJ, Wilson RJ, Miller JW. Breast Cancer Survival Among Males by Race, Ethnicity, Age, Geographic Region, and Stage - United States, 2007-2016. MMWR Morb Mortal Wkly Rep. 2020 Oct 16;69(41):1481-1484. doi: 10.15585/mmwr.mm6941a2. PMID: 33056954; PMCID: PMC7561088.
- Flaherty DC, Bawa R, Burton C, Goldfarb M. Breast Cancer in Male Adolescents and Young Adults. Ann Surg Oncol. 2017 Jan;24(1):84-90. doi: 10.1245/s10434-016-5586-4. Epub 2016 Sep 20. PMID: 27650826.
- McPherson K, Steel CM, Dixon JM. ABC of breast diseases. Breast cancer-epidemiology, risk factors, and genetics. BMJ. 2000 Sep 9;321(7261):624-8. doi: 10.1136/bmj.321.7261.624. PMID: 10977847; PMCID: PMC1118507.
- Frank S, Carton M, Dubot C, Campone M, Pistilli B, Dalenc F, Mailliez A, Levy C, D'Hondt V, Debled M, Vermeulin T, Coudert B, Perrin C, Gonçalves A, Uwer L, Ferrero JM, Eymard JC, Petit T, Mouret-Reynier MA, Patsouris A, Guesmia T, Bachelot T, Robain M, Cottu P. Impact of age at diagnosis of metastatic breast cancer on overall survival in the real-life ESME metastatic breast cancer cohort. Breast. 2020 Aug;52:50-57. doi: 10.1016/j.breast.2020.04.009. Epub 2020 Apr 23. PMID: 32380440; PMCID: PMC7375638.
- Lofterød T, Frydenberg H, Flote V, Eggen AE, McTiernan A, Mortensen ES, Akslen LA, Reitan JB, Wilsgaard T, Thune I. Exploring the effects of lifestyle on breast cancer risk, age at diagnosis, and survival: the EBBA-Life study. Breast Cancer Res Treat. 2020 Jul;182(1):215-227. doi: 10.1007/s10549-020-05679-2. Epub 2020 May 20. PMID: 32436147; PMCID: PMC7275030.
- Brandt J, Garne JP, Tengrup I, Manjer J. Age at diagnosis in relation to survival following breast cancer: a cohort study. World J Surg Oncol. 2015 Feb 7;13:33. doi: 10.1186/s12957-014-0429-x. PMID: 25889186; PMCID: PMC4344734.
- Aebi S, Castiglione M. The enigma of young age. Ann Oncol. 2006 Oct;17(10):1475-7. doi: 10.1093/annonc/mdl330. PMID: 17005630.
- Noone AM, et al. (eds). Cancer Statistics Review, 1975-2015 - SEER Statistics. SEER Cancer Statistics Review. 2017.
- Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, Mandelblatt JS, Yakovlev AY, Habbema JD, Feuer EJ; Cancer Intervention and Surveillance Modeling Network (CISNET) Collaborators. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005 Oct 27;353(17):1784-92. doi: 10.1056/NEJMoa050518. PMID: 16251534.
- Munoz D, Near AM, van Ravesteyn NT, Lee SJ, Schechter CB, Alagoz O, Berry DA, Burnside ES, Chang Y, Chisholm G, de Koning HJ, Ali Ergun M, Heijnsdijk EA, Huang H, Stout NK, Sprague BL, Trentham-Dietz A, Mandelblatt JS, Plevritis SK. Effects of screening and systemic adjuvant therapy on ER-specific US breast cancer mortality. J Natl Cancer Inst. 2014 Sep 24;106(11):dju289. doi: 10.1093/jnci/dju289. PMID: 25255803; PMCID: PMC4271026.
- Luo T, Spolverato G, Johnston F, Haider AH, Pawlik TM. Factors that determine cancer treatment choice among minority groups. J Oncol Pract. 2015 May;11(3):259-61. doi: 10.1200/JOP.2015.003640. Epub 2015 Mar 3. PMID: 25736381.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.