Medicine Group . 2022 August 26;3(8):953-973. doi: 10.37871/jbres1538.
Carob Antioxidants in Human Health: From Traditional Uses to Modern Pharmacology
Abdullatif Azab*
- Carob
- Ethnomedicine
- Antioxidants
- Polyphenols
- Carbohydrates
- Volatiles
- Human health
Abstract
Carob has been used by humans since antiquity. Its major use is food, but traditional medicines of many nations used it for treatments of various health disorders. The fruits (pods or kibbles) were the main source for nutrition and medicinal uses, but decoctions and extracts were prepared from other parts of the tree, especially leaves. Modern science has analyzed most of the chemical compositions of the different parts, and among the phytochemicals that were found, antioxidants play very important roles in Carob nutritional and medicinal activities. So, in addition to having strong antioxidant activity and due to it, these natural products, their extracts, and foods that contain them, have anticancer, neuroprotective, hepatoprotective, antiaging, skin care, antidiabetic, and others. Phenolics and carbohydrates are the strongest antioxidants, but some volatile compounds have the same activity, to some extent. However, this review will present Carob antioxidants, their major nutritional and medicinal activities, and suggest future horizons for their use in human wellbeing.
Abbreviations
ABTS: 2,2’-Azino-bis(3-ethylbenzothaiazoline-6-sulfonic acid); AChE: Acetylcholinesterase; °Bx: Brix; CUPRAC: CUpric ion Reducing Antioxidant Capacity; DPPH: 2,2-Diphenyl-1-picrylhydrazyl; FAST: Florescence of Advanced (Millard products) and Soluble Tryptophan; FRAP: Ferric Reducing Ability of Plasma; GC-MS: Gas Chromatography Mass Spectroscopy; GI: Glycemic Index; HPLC: High Performance Liquid Chromatography; LBG: Locust Bean Gum; LC-MS: Liquid Chromatography Mass Spectroscopy; LAO: Linoleic Acid Oxidation; NMR: Nuclear Magnetic Resonance; ORAC: Oxygen Radical Absorbance Capacity; OxHLIA: Oxidative Hemolysis Inhibition; PAHBAH: p-Hydroxybenzoic acid hydrazide; PPL: Porcine Pancreatic Lipase; PPMD: Phosphomolybdenum (method); ROS: Reactive Oxygen Species; SPME: Solid Phase Microextraction; TBARS: Thiobarbituric Acid Reactive Substances; TEAC: Trolox Equivalent Antioxidant Capacity; TC: Total Carbohydrate (content); TF: Total Flavonoid; TPC: Total Phenolic Content
Introduction
Carob (Ceratonia siliqua L.) is an evergreen tree belonging to the Fabaceae (Legumes) family, which consists of about 791 genera and 19325-19560 species [1]. Until 1979, Carob was considered a single species of the Ceratonia genus, when another species, C. oreothauma n. sp. was discovered in Oman and Somali Republic [2]. Evidently, this species is way less fruitful than Carob and its fruits are less nutritional. Humans utilized wild Carob trees and they domesticated this important nutritional source relatively late in Roman period [3]. Even though, peoples of the Mediterranean basin are still using wild varieties of Carob in minor scale in addition to the domesticated and way more fruitful cultivars [4]. Finally, while it takes 7-8 years for wild varieties to fruit for the first time, grafted domesticated cultivars fruit after 3-4 years [5].
Traditional Uses of Carob
Carob fruits, named pods or kibbles, have been an important nutritional source since early dawn of humanity. Even though it was very rare, archeological findings indicate that it was used by humans as early as around 43000 years ago [6]. In Greek islands, Carobs were present and used 9000 years before present [7].
In our first and comprehensive review article about Carob, we listed many traditional uses of Carob, and the interested readers can view it [8]. Evidently, food was and still the major traditional and modern use of Carob pods, but one of the earliest medicinal uses is treating mouth inflammations in Arab folk medicine [9]. But in addition to the traditional uses mentioned in [8], few other reports were either overlooked or published later. In Southern Italy, Carob pods are used as human and animal food, to treat intestine inflammations, and their decoction used as expectorant [10].
Moroccan ethnomedicine is one of the finest in the world, and as a major Carob cultivating country, Carob is extensively used [8]. In an interesting study, Bou-Idra M, et al. [11] presented different statistical aspects of Carob traditional uses in the Zerhoun region. In addition to various facts about the region and its inhabitants, they listed the uses by gender, age groups, education, and method of preparation, marital status, dose, tree parts, treatment objectives, treatment results, administration method, and sources of ethnomedicine information. In a recent study, Bachar M, et al. [12] reported that in the Bouhachem, Carob pods powder is mixed with honey and orally administered to treat diarrhea and stomach pains.
In Sulaymaniyah province (Kurdistan, Iraq), herbalists use Carob powder treat abdominal pain and diarrhea [13]. Contrary to the previous limited use, Palestinian ethnomedicine uses Carob tree parts for the wellbeing of humans and animals, as reported in ethnoveterinary research [14]. Fruits are served as animal’s food, their aqueous extracts are used to treat eye and skin diseases, and leaves extract is used for the same purpose. Similarly, an extensive use is reported by Palabaş Uzun S, et al. [15] from Kahramanmaraş region in Turkey. All parts of the tree are used, and their decoctions are used to treat urinary disorders, anemia, and sexual problems. In Marmaris region, like in all Turkey, Carob pods molasses (concentrated aqueous extract) is named Pekmez. This sticky, viscous liquid is used as food, and for treatment of prostatitis, anemia, and liver disorders [16,17]. Akbulut, et al. [18] estimated that Carob is one of the most traded plants for medicinal purposes in Turkey, and they mention its use to treat intestinal parasites, among other uses.
One of the major nutritional health concerns in Arab-Islamic world is thirst feeling during the fasting month of Ramadan, where Muslims avoid drinking and eating from dawn to sunset. So, in addition to many uses of Carob products for preparation of different foods, Carob powder aqueous extracts is served as healthy, thirst relieving for fasting people [19]. Since fasting can be a burden for different parts of human body, Emara MH, et al. [20] states that the mentioned Carob drink “can be safely consumed during Ramadan fasting by patients with liver diseases.
Finally, one of the traditional uses of Carob seeds is an equal and the origin of the mass unit “Carat” or “Karat”, used for gemstones and gold. It was firstly defined in Paris as equal to Carob seed mass, 200 mg, accepting the ancient belief that masses of Carob seeds are accurate and identical [21]. But modern studies have shown that seeds mass can differ by up to 5% even collected from the same tree, season, and ripening stage [22].
Major Medicinal and Nutritional Properties of Carob
Traditional medicines of peoples who used Carob fruits for food, noticed since ancient times the capability of this food to treat some health disorders. Modern science followed this knowledge and numerous studies of Carob medicinal activities were and are being published. In table 1 we summarized these activities in alphabetical order.
| Table 1: Major medicinal properties of carob. | |
| Property | Experimental Procedure and Results, Reference |
| Analgesic | 50 mg/kg Body weight in rats. Seed aqueous extract was most efficient [23]. |
| Anti-alopecia | Pods aqueous extract (with 5 other plants) downregulated IL-1α in HaCaT cells, suggesting nonscarring hair loss prevention activity [24]. |
| Anti-Alzheimer | Pods methanolic extracts showed glucose-mediated glycation and AChE inhibition [25]. |
| Antibacterial | Seeds aqueous and ethanolic extracts were found against two species of gram-positive and three species of gram-negative bacteria. These bacteria were isolated from diabetic feet [26]. Methanolic and aqueous bark extracts showed activity against diarrheagenic E-coli. Methanolic extract was more efficient [27]. Leaves were separately extracted with water and ethanol, and both extracts were active against three bacteria strains. Ethanolic extract was more active [28]. Ethanolic fruits extract was tested against six bacteria species. Results are ambiguous [29]. Pods aqueous extract was active against four out of six bacteria species [30]. Mature pods powder was mixed (4%) with fermented milk and the mixture was active against four bacteria species. |
| Anticancer | Pulp aqueous extract was most effective against leukemia, breast, and colon cancer cell lines [23]. Ethanolic leaves extract found more cytotoxic to PLHC-1 cells than aqueous extract [28]. Pods were extracted using HPLC with mobile phase of water-methanol gradient containing 2% acetic acid. This extract had different activities on HT29 and LT97 cell lines [31]. Methanolic leaf extracts were prepared from male and hermaphrodite trees, had stronger activities against HeLa cell lines, compared with extract of female trees [32]. Leaves were extracted with methanol-acetone-water (7:7:6 v/v/v) to obtain phenolic-rich (gallic and p-coumaric acids) extract, which had strong activity against HCT-116 and CT-26 cell lines [33]. Ripe pods were extracted with ethyl acetate, diethyl ether, ethanol, and water; and unripe pods were extracted with ethyl acetate and diethyl ether. All extracts were tested against breast cancer cells. Some were active and others were not [34]. Methanolic extract of sapwood was prepared and tested against four types of human cancer cell lines. |
| Anti-cobalt-toxicity | Seeds aqueous extract had protective effect against cobalt (Co+2) toxicity in Onion (Allium cepa L.) bulbs [35]. |
| Antidepressant | Fresh pods were extracted with 70% aqueous acetone (70% v/v), and the extract had notable effects in some tests and no effect in other depression tests in mice [36]. Seeds peel was extracted with 70% aqueous solvents (v/v, ethyl acetate, EA, AC and methanol, MT). All extracts had activity in four depression tests [37]. |
| Antidiabetic | Twenty healthy humans were supplemented with insoluble fiber in combination with glucose load. Consequently, their glycemic control worsened [38]. Immature beans, in different ripening stages, were extracted with water. Extracts were tested in rats with or without STZ-induced diabetes. In all cases, treatment proved to lower blood glucose, even in animals that with glucose load [39]. Immature pods were dried and extracted with methanol, and extract proved non-toxic for rats. It exerted in vitro (α-glucosidase and α-amylase inhibition) and in vivo in rats with STZ-nicotinamide-induced type II diabetes [40]. Rats were supplied with D-Pinitol- and fructose-enriched drink with 1.65% soluble fiber, compared with (control) group which was given a drink that contained almost same total amount of sugar, without the three previously mentioned components. Study group blood glucose status was significantly improved [41]. Diabetic rats were fed with diet that contained cocoa-carob powder and had the same energy value (15048 KJ/kg) of the control diet. Test group showed better biochemical test results, indicating possible potential of this powder in blood sugar control [42]. |
| Antidiarrhea | Residue of pods extraction contained 21.2% polyphenols and 40% tannins, showed significant activity in children with bacterial diarrhea. Vomiting duration was also shorted in study group [43]. Children with acute diarrhea that were treated with bean juice, had 46 h duration of the illness, compared with 85 in the control group [44]. |
| Anti-inflammatory | Pods aqueous extract was active against dextran sulfate-sodium-induced ulcerative colitis in rats [45]. Bark was extracted successively with hexane, dichloromethane, ethyl acetate and methanol. The extract was active against carrageenan-induces paw edema in rats [46]. Diet obesity and dextran sulfate sodium inflammation were induced in mice. Animals were treated with a combination of Carob leaves and Opuntia ficus-indica cladodes polyphenol-rich infusion, resulting clear positive effect [47]. Industrial Carob by-products were extracted with 50% aqueous methanol. Extract was active in macrophage cell line 264.7 [48]. Powder of Carob pods and Wakame (Undaria pinnatifida) was extracted with water, and extract showed in vitro activity (mature 3T3-L1 adipocytes and RAW 264.7 macrophages) [49]. |
| Antiobesity | Powder of Carob pods and Wakame (Undaria pinnatifida) was administered to rats as a snack, showing clear positive effect [49]. Healthy adults consumed meals (2520 KJ) enriched with fibers and polyphenols, resulting decreasing the acylation of ghrelin [50]. Healthy adults consumed 40 g fiber and polyphenols-enriched Carob snack, resulting lowering GI and increasing satiety [51]. Leaves ethanolic extract showed notable PPL inhibition activity [52]. Rats were fed with normal diet and divided into two groups. Test (1) group was also supplemented with 20% Carob pulp. In another main group, rats were fed with high fat foot. This group was also divided into two groups, and test (2) group was supplemented with 20% Carob pulp. Both test groups showed weight loss [53]. Randomly recruited adults were supplemented with 1.5 g seed powder, in three meals (0.5 g each) every day, for 8 weeks. The supplement alone had no effect but with physical activity, it resulted weight loss in overweight participants [54]. Roasted pods powder was extracted with methanol, and extract suppressed the differentiation of 3T3-L1 preadipocytes into adipocytes in vitro, suggesting anti-obesity potential [55]. |
| Determined by three methods: ABTS, DPPH, and FRAP. Pods aqueous extract was most active [23]. Ethanolic leaves extract showed stronger activity (DPPH) than aqueous extract [28]. Fruits aqueous extracts had strong activity determined by four methods [30]. Methanolic leaf extracts of hermaphrodite, male and female trees, showed almost same activity (DPPH, linoleic acid method) [32]. Ripe pods were extracted with ethyl acetate, diethyl ether, ethanol, and water; and unripe pods were extracted with ethyl acetate and diethyl ether. All extracts had significant activity (DPPH) [34]. Seeds peel was extracted with 70% aqueous solvents (v/v, ethyl acetate, EA, AC and methanol, MT). All extracts had activity in two tests (DPPH, FRAP) [37]. |
|
| Antioxidant | Immature beans, in different ripening stages, were extracted with water. Extracts showed high activity in DPPH and ABTS tests [39]. Immature pods were dried and extracted with methanol, and extract had notable activity (DPPH, FRAP) [40]. Bark was extracted successively with hexane, dichloromethane, ethyl acetate and methanol. The extract was active in DPPH test [46]. Industrial Carob by-products were extracted with 50% aqueous methanol. Extract was active in four tests: ORAC, FRAP, DPPH, TEAC [48]. Leaves ethanolic extract showed strong activity (DPPH) [52]. Pods were repeatedly extracted with water, and the final combined extract was analyzed for general composition and tested by LAO method [56]. Deseeded pods were extracted with several solvents resulting that 80% aqueous acetone (v/v) had the highest activity. Compared to other powerful antioxidants, its second only to pure gallic acid [57]. Pods were extracted successively with several solvents with ranging polarities, and activity of extract was determined by DPPH method [58]. Bark was extracted successively with solvents having increasing polarity. The final extract had strong activity determined by DPPH and FRAP methods. General composition was also determined [59]. Methanolic extracts of seed germ of several cultivars had significant activity tested by DPPH and FRAP method. General composition was also determined [60]. Seeds were separated from pods and aqueous extracts of both materials were prepared. Extracts had myeloperoxidase inhibition and ROS-scavenging activities [61]. Pods were repeatedly extracted with water, and the final combined extract was analyzed for general composition and tested by DPPH and ABTS methods [62]. Pods were roasted in various condition and were subjected to in vitro digestion simulation. The resulting liquids were tested with DPPH and TEAC methods [63]. Pods were roasted in various condition and were extracted with water. Extracts were tested with DPPH method, and general composition was determined [64,65]. Leaves aqueous extract had myeloperoxidase inhibition and ROS-scavenging, and protection against rat’s intestinal fluids activities [66]. Leaves were extracted successively with solvents having increasing polarity. The final extract had strong activity determined by DPPH, FRAP and PPMD methods. General composition was also determined [67]. Leaves were harvested from three locations, and they were extracted with methanol. Extracts differed slightly in their activity (DPPH) [68]. Pods flour aqueous extract had significant activity, determined by DPPH and FRAP methods [69]. Deseeded pods at different ripening stages were dried and extracted with 70% aqueous acetone (v/v). Samples of this extract were encapsulated with methyl cellulose. Encapsulated and non-encapsulated extracts were subjected to gastric and intestinal digestion conditions (pH and enzymes). Antioxidant activity (DPPH, FRAP, ORAC), TPC and TF were higher after in encapsulated extract after digestive simulation [70]. Pods at different ripening stages were dried and extracted separately with hexane, chloroform, ethyl acetate and 70% aqueous acetone (v/v). For all extracts TPC and TF were determined. Aqueous acetone extract of ripe pods had the highest values [71]. Pods were roasted in different conditions after sugar removal. The pods powder was extracted with water and samples of it were encapsulated with β-cyclodextrin. Testing TPC and antioxidant activity (TEAC, ORAC, FRAP), showed that encapsulation protected phenolics in different oxidation systems [72]. Ripe pods were extracted separately with methanol and water. Methanolic extract had notable activity (DPPH) [73]. Pods from two cultivars were subjected to ultrasound-assisted extraction with several solvents. TPC and TF of extracts were determined, and their activity was tested in chemical (DPPH, FRAP) and four food systems. Acidic aqueous acetone (H2O:Me2CO:HCl = 80:19:1, v/v/v) had the highest contents and strongest activities [74]. Fruits in different ripening stages and/or locations, were extracted with water (or other solvents) and their general compositions (TPC, TF, TC) were determined, and the extracts activity (DPPH and other methods) was tested. Clear differences resulted in both categories [75-81]. Feeding rabbits with Carob powder alone or with cow whey, improved antioxidant biomarkers such as reduction of lipid peroxidation [82]. Pods were roasted in different conditions (mainly temperature) and their general chemical composition and antioxidant activity (DPPH/ABTS) were determined. High temperatures and long roasting times resulted clear reduction in both tests, while short term and medium temperature resulted increase [83-85]. Administration of pods water extract to male mice reduced levels of superoxide dismutase [86]. Pods powder was supplemented to aging roosters resulting reduction of superoxide dismutase levels [87]. Leaves aqueous extract had significant activity in three tests: DPPH, FRAP and Fe(II)-chelating. General composition was determined in this study [88]. |
| Leaves were extracted successively with hexane and 40% aqueous methanol (v/v). TPC was determined and antioxidant activity was tested by DPPH method. Metabolite profile was determined by GC-MS. This procedure was performed to six other aromatic plants of the Mediterranean region. Carob had the highest antioxidant capacity [89]. Commercial pods syrup was supplemented to fish (Tilapia, Oreochromis niloticus) as part of meals that contained fixed amounts of other food ingredients. Carob syrup lowered the concentrations of superoxide dismutase [90]. Ethanolic extract of ripe seeds was prepared and it’s capacity was tested with DPPH, ABTS, FRAP and CUPRAC methods [91]. Pods powder was supplemented as “whole meal” to pigs, resulting improvement in body weight and shorter diarrhea periods, compared with control group. General composition of supplement and antioxidant capacity (DPPH, ABTS) were determined [92]. Ripe pods powder was subjected to three digestion simulations. For undigested/digested materials, general composition, and antioxidant activity (DPPH, ORAC, ABTS) were determined. There was a clear effect on both properties [93,94]. Methanolic extract of sapwood was prepared and tested with DPPH and ABTS methods. General composition of the extract was determined [95]. Aqueous extract of immature pods was tested by lipid peroxidation measurement and superoxide dismutase inhibition [96]. LBG was extracted with 70% aqueous acetone, 80% aqueous methanol and 80% aqueous ethanol (all v/v). Extracts activities were tested with DPPH and FRAP methods. General composition of extracts was determined. Aqueous acetone extract had highest TPC [97]. Preprepared pods aqueous extracts were analyzed for general composition and their activity was determined by ABTS method [98]. Mature pods powder was mixed (4%) with fermented milk and the mixture had notable activity (DPPH). General composition was determined [99]. |
|
| Deseeded pods were powdered and part of it was roasted. Raw and roasted powders were mixed and extracted with water. General chemical composition was determined, and its activity was tested with DPPH method [100]. Commercially pods powder was mixed with other food ingredients and muffins were prepared, and their antioxidant capacity was tested with TEAC or DDPH and ABTS methods. Pulps were subjected to aqueous ultra-sound-assisted extraction. Extract was tested for chemical (DPPH, FRAP, ABTS) and in vivo antioxidant activity in mice. |
|
| Antiviral | Pods aqueous extract was active against herpes simplex type I virus [30]. |
| Antivenom | Pods aqueous extract had weak activity against Egyptian horned viper (Cerastes cerastes) venom [101]. |
| Cardioprotective | Preprepared pods aqueous extracts had cardioprotective activity in mice with metabolic syndrome that resulted from high fat diet [98]. Hypercholesterolemic participants received commercial, non-soluble, polyphenol-rich fiber. Comparing with control group, in test group, a blood cholesterol lowering effect was observed [102]. Commercial non-soluble, polyphenol-rich fiber was supplemented to male rabbits, resulting improvement is cardiovascular system functioning, especially expression of protective proteins in aorta [103]. |
| Corrosion inhibition | Pods methanolic extract had corrosion inhibition activity of steel (98.689% Fe, 0.38% C, and other elements) in 1 M HCl medium [104]. |
| Enzyme inhibition | Pods aqueous extract inhibited amylase, maltase, sucrase and lactase [30]. Industrial Carob by-products were extracted with 50% aqueous methanol. Extract inhibited AChE [48]. Ripe pods powder was subjected to three digestion simulations. It is reported that digested material had higher α-amylase and α-glucosidase inhibition activity [93]. Mature pods powder was mixed (4%) with fermented milk and the mixture had α-amylase and α-glucosidase inhibition activity [99]. Fermented pods powder was mixed with Konjac, and the mixture had activity tested with DDPH, FRAP, ORAC and TEAC methos. TPC was also determined [100]. |
| Fertility effects | Administration of pods water extract to male mice increased the quantity and quality of sperm biomarkers [86]. Pods powder was supplemented to aging roosters resulted improvement of sperm quality and quantity [87]. Male mice were supplemented with mixed Carob and Pumpkin aqueous extract (20% Carob seeds, 80% Pumpkin seeds, w/w), resulting in sperm improvement in several tests [105]. |
| Food | Mature pods powder was mixed (4%) with fermented milk and the mixture had significant probiotic activity [99]. Fermented pods powder was mixed with Konjac, and the mixture had probiotic activity [100]. Seeds powder was added to some foods (Brownies, Coffee, Cocoa milk) resulting improvement of their tastes, textures, and nutritional compositions [106]. Pods powder was fermented, and the product’s nutritional parameters were compared to starting material, in attempt of testing its potential as ruminants’ food [107]. Deseeded pods were powdered and part of it was roasted. Raw and roasted powders were mixed and extracted with water, and the extract was vacuum concentrated. This viscous product was added to three types of sweets, resulting improvement of their tastes, nutritional values, and textures [108]. Commercial pods flour was mixed with other food ingredients to prepare snacks. The product was tested for taste, color, texture, nutritional values, and general acceptance by children. Results were satisfactory [109]. Commercial condensed pods water extract (Rob, 72 °Bx) or powder were added to yogurt and/or ice creams. Taste and sensory properties were improved, and nutritional content increased, especially polyphenols and D-Pinitol [110-112]. Commercially pods powder was mixed with other food ingredients and muffins were prepared. General composition, taste, texture, and color were tested. In addition, Millard products presence due to baking, was determined by FAST method [113]. Muffins were prepared containing cocoa powder or commercial Carob powder substitute, up to 15%. Taste, color, and sensory parameters were compared after baking. Several compounds were determined in the products such as sterols, tocopherols and isoflavones [114]. Replacing barley in rabbits’ food decreased the digestibility of crude protein, neutral detergent fiber and acid detergent fiber [115]. |
| Gastroprotective | Ripe pods were extracted separately with methanol and water. Aqueous extract was protective against indomethacin-induced gastric ulcer in rats [73]. Pods aqueous extract proved to have both laxative and antidiarrheal (castor oil-induced) effects, depending on pods in animal model. Mature fruit can be laxative while immature pods may cause constipation [116]. Humans with irritable bowel syndrome were treated with aqueous seed extract, resulting physical and psychological comfort [117]. Deseeded pods were added to sheep diet (12%) resulting protective activity in animals with Haemonchus contortus or Trichostrongylus colubriformis nematode infection. |
| Hepatoprotective | Aqueous extract of immature pods was active against ethanol-induced liver (oxidative stress) toxicity [96]. Mice were infected with Schistosoma mansoni resulting schistosomal liver pathology. Treating them with deseeded pods aqueous extract ameliorated liver injuries resulted from the infection [118]. Pulps were subjected to aqueous ultra-sound-assisted extraction. Extract had positive effect against paracetamol hepatotoxicity in mice [119]. Pods were extracted with water at 50°C for 72 h. The dried extract was administered to rats with dextran sulfate sodium-induced hepatotoxicity. Improvement was observed especially in oxidative stress biomarkers [120]. |
| Immune system | Commercial pods syrup was supplemented to fish (Tilapia, Oreochromis niloticus) as part of meals that contained fixed amounts of other food ingredients. Carob syrup had clear positive effect on immune biomarkers [90]. |
| Nanoparticles preparation | Fresh leaves aqueous extract reduced Ag(I) ions (AgNO3 solution) to prepare AgNP’s, and their antibacterial activity was tested [121]. Ce(NO3)3x6H2O solution reacted with leaves aqueous extract yielding CeO2NP’s.*The antioxidant (DPPH) and cytotoxic (MCF7 cells) activities of the NP’s were tested [122]. *In this reaction, Ce(III) is oxidized by nitrate ions to Ce(IV) and nitrite ions [123]. |
| Nephroprotective | Pods were extracted with water at 50°C for 72 h. The dried extract was administered to rats with dextran sulfate sodium-induced nephrotoxicity. Improvement was observed especially in oxidative stress biomarkers [120]. |
| Nervous system, brain | Leaves aqueous extract inhibited AChE and improved cognitive parameters (memory) in 6-hydroxydopamine-induced impairment in zebrafish [88]. Pods were extracted with water at 50°C for 72 h. The dried extract was administered to female rats that were subjected to different types of stress. Improvement was observed emotional and biochemical stress biomarkers [124]. Pods aqueous extract attenuated brain injury caused by waterpipe smoke in rats [125]. |
| Pulmoprotective | Pods aqueous extract attenuated lungs injury caused by waterpipe smoke in rats [126]. |
| Skin care | Aerial parts (bark, leaves and pods) were collectively or separately extracted with ethanol. The extracts were analyzed for active compounds and their antioxidant capacities were tested (DPPH). All extracts had depigmentation activity in vitro (tyrosinase and melanin inhibition) and in vivo (treating humans with pigmentation spots). Leaves extract had the highest activity. All products proved to be safe in toxicity tests [127]. Pods aqueous extract was used to produce cosmetic moisturizer formulation acting as anti-wrinkle and antiaging [128]. |
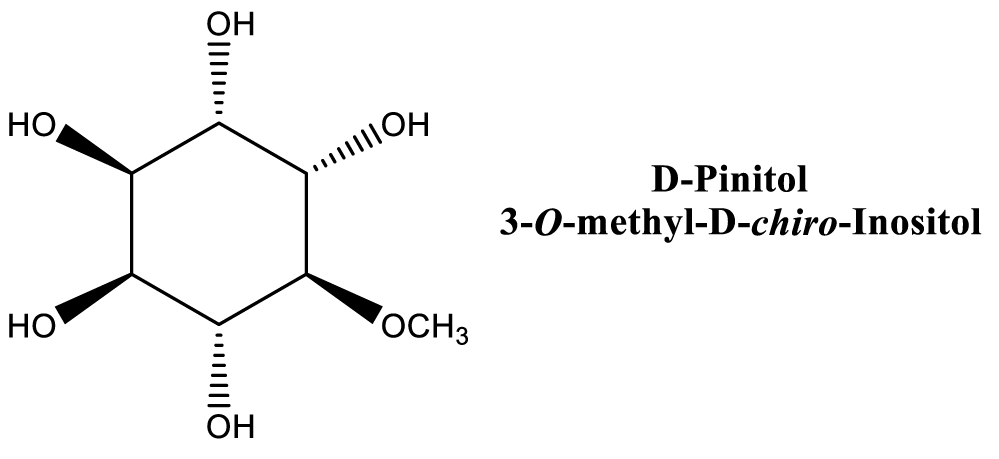
It is very important to emphasize that table 1 did not include properties of pure compounds contained in Carob tree different parts such as D-Pinitol, shown in figure 1 [129].
Chemical Composition of Carob
Numerous publications have reported chemical composition of Carob. Most of these publications reported general composition, meaning compound families, such as polyphenol, carbohydrates, amino acids etc. Another type of publications reported detailed compositions, but the natural product presented in them are previously known, even though some publications of this type have reported spectral and other characterization data (manly NMR and GC-MS). Very few publications reported novel, previously unknown compounds. Evidently, most publications focus on polyphenols since they are the major source of Carob health, medicinal and most of its other properties.
A summary of published reports of Carob chemical composition is shown in table 2. Figures mentioned in table 2 are presented after it.
| Table 2: Reported chemical composition of Carob. |
| Reported Composition, Reference |
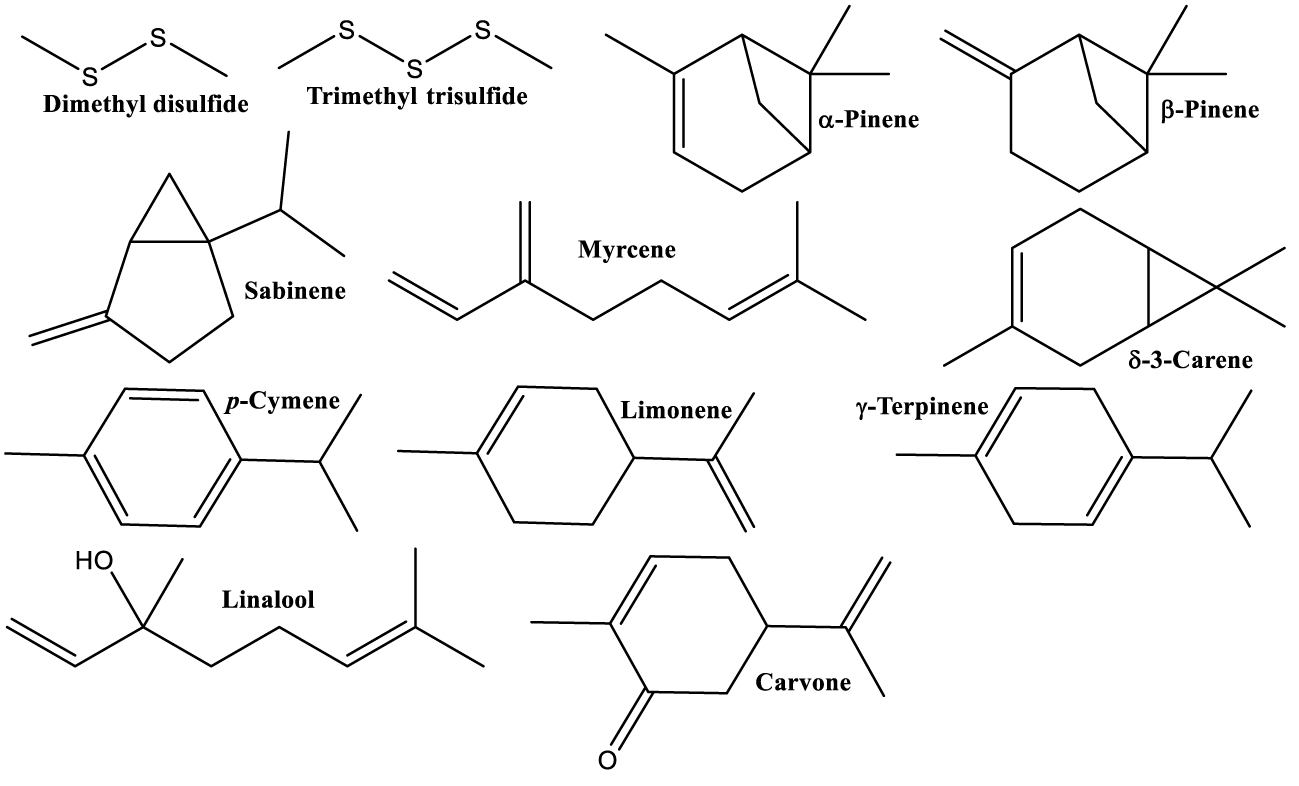
| TPC and different phenolic compounds group were determined in molasses, locally named Rob, pulp and seeds. Rob had highest content. Volatiles highest concentrations were found in seeds. The structures of major volatiles with antioxidant capacity are shown in figure 2 [23]. |
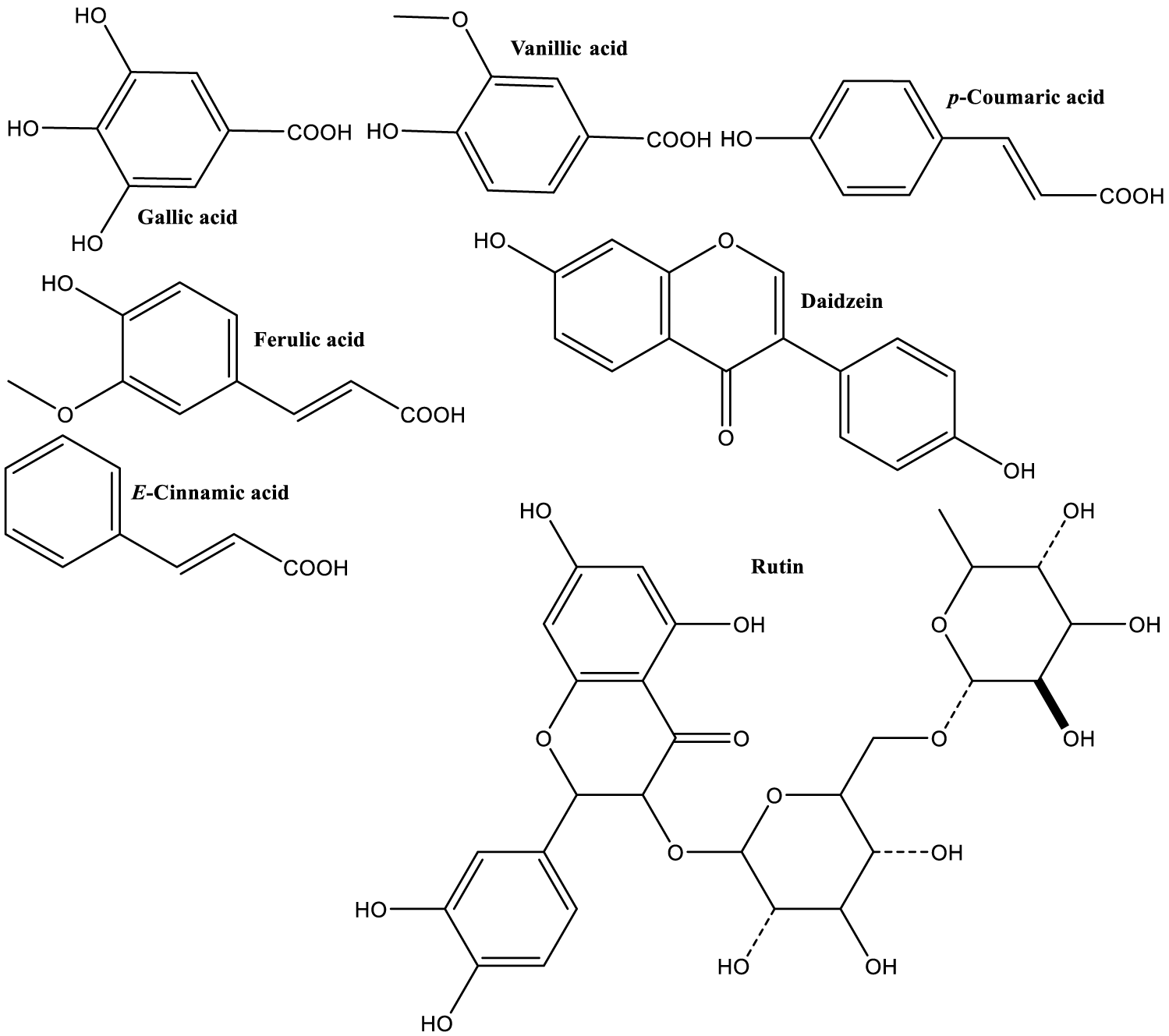
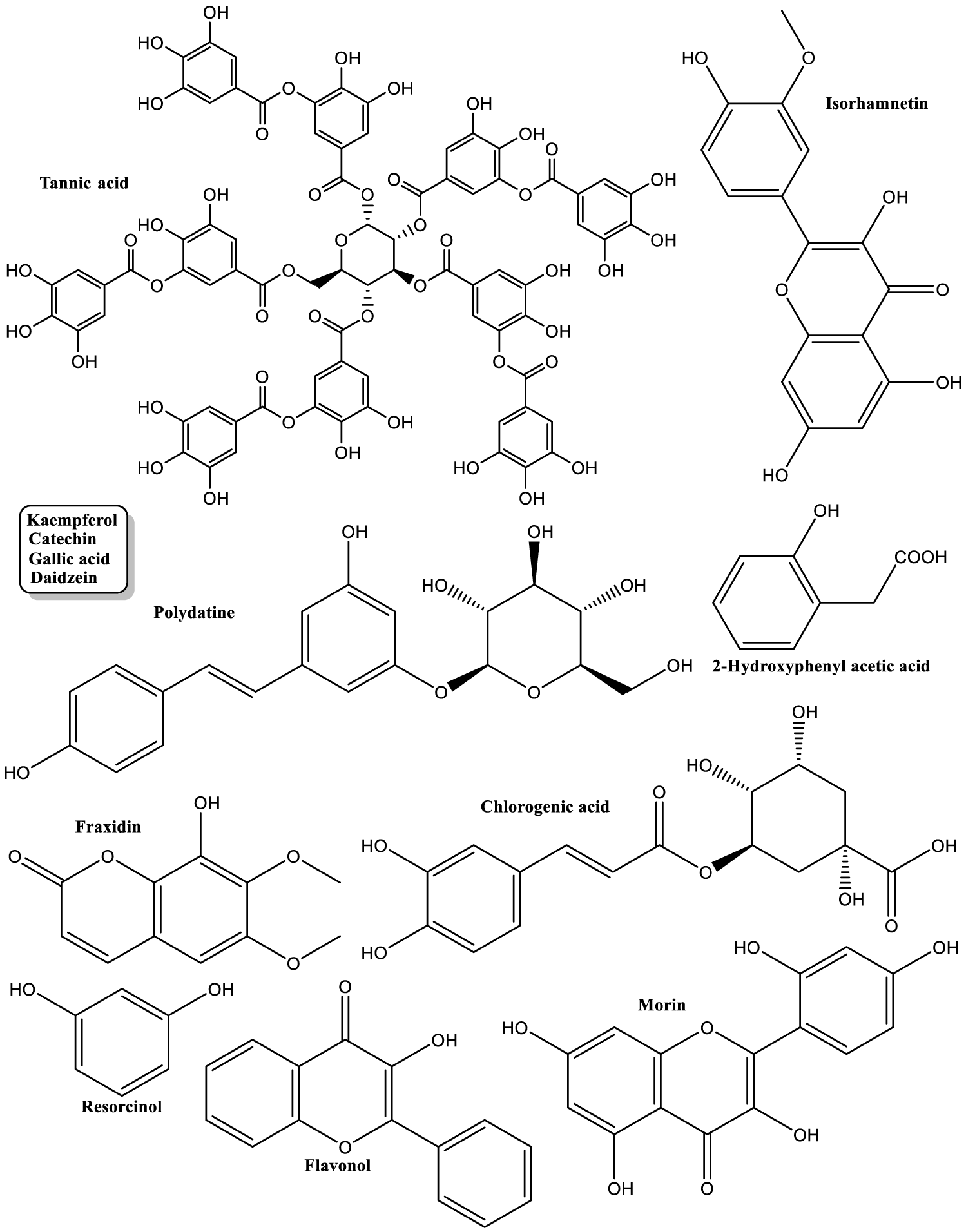
| Phenolics contained in pods methanolic extract were mainly: gallic acid, vanillic acid, p-coumaric acid, ferulic acid, rutin, daidzein and t-cinnamic acid (Figure 3) [25]. |
| General compositions of aqueous and ethanolic leaves extracts were determined: TPC and/or flavonoid and/or condensed tannin contents, and/or sugar profile [28,37]. |
| Pods aqueous extract was analyzed for general composition (TPC, total flavonoids, total amino acids, total alkaloids, and total carbohydrates contents). Major phenolics were also determined by HPLC (Figure 4) [30]. |
| Ripe pods were extracted with ethyl acetate (EA), diethyl ether (DE), ethanol (ET), and water (WA); and unripe pods were extracted with ethyl acetate and diethyl ether. EA extract had highest TPC (140.41 mg/g). EA extract of ripe pods contained seven detectable phenolics shown in figure 5 [34]. |
| Deseeded pods were extracted with several solvents resulting that 80% aqueous acetone (v/v) had the highest TPC, TF and TP/TF yields [57]. |
| Deseeded pods, seeds and syrup were analyzed separately using HPLC. This is the earliest, most comprehensive, and detailed study of pods polyphenolic composition [58]. |
| Leaves aqueous extract was analyzed with HPLC. Structures of identified compounds are shown in figure 6 [66]. |
| Leaves were harvested from three locations, and they were extracted with methanol. Their TPC and TF content were determined, indicating some little to moderate differences. But the fatty acid composition showed notable difference: C16:0 265%, C20:1 (cis 11) 500% [68]. |
| Pods flour aqueous extract was prepared and its TPC, TF and TC contents were determined. Its mono and polysaccharide contents were determined by HPLC, and its reducing sugars content was tested with PAHBAH method [69]. |
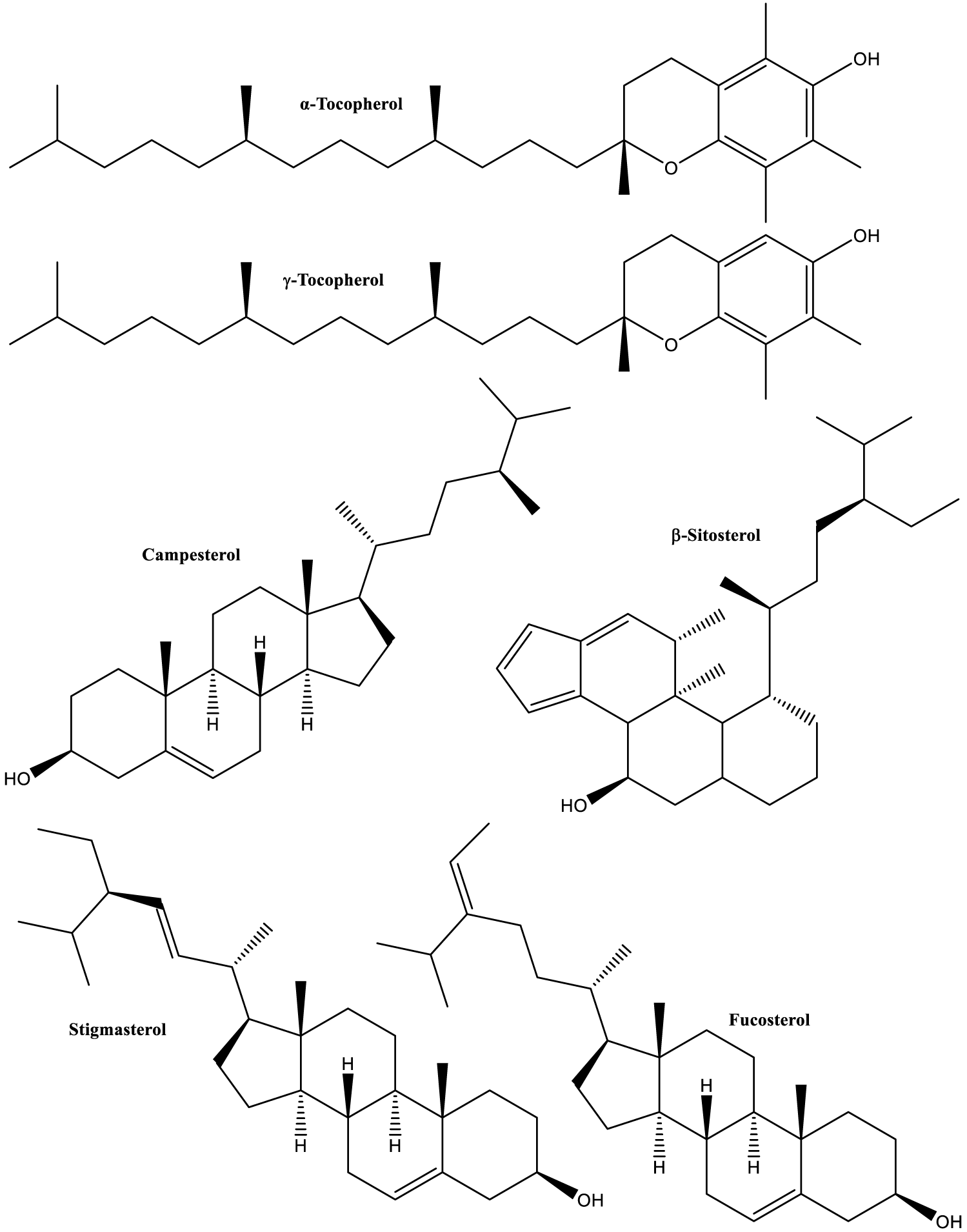
| Seeds from ripe pods were thoroughly analyzed for functional properties and chemical composition. In the second group, TPC, TF, mineral content, monosaccharide composition, protein content, amino acid composition, polysaccharide composition, sterols, fatty acids, and tocopherols. Polar fractions were extracted with ethanol, while non-polar compounds were extracted with hexane. In figure 7 the structures of major tocopherols and sterols are shown [91]. |
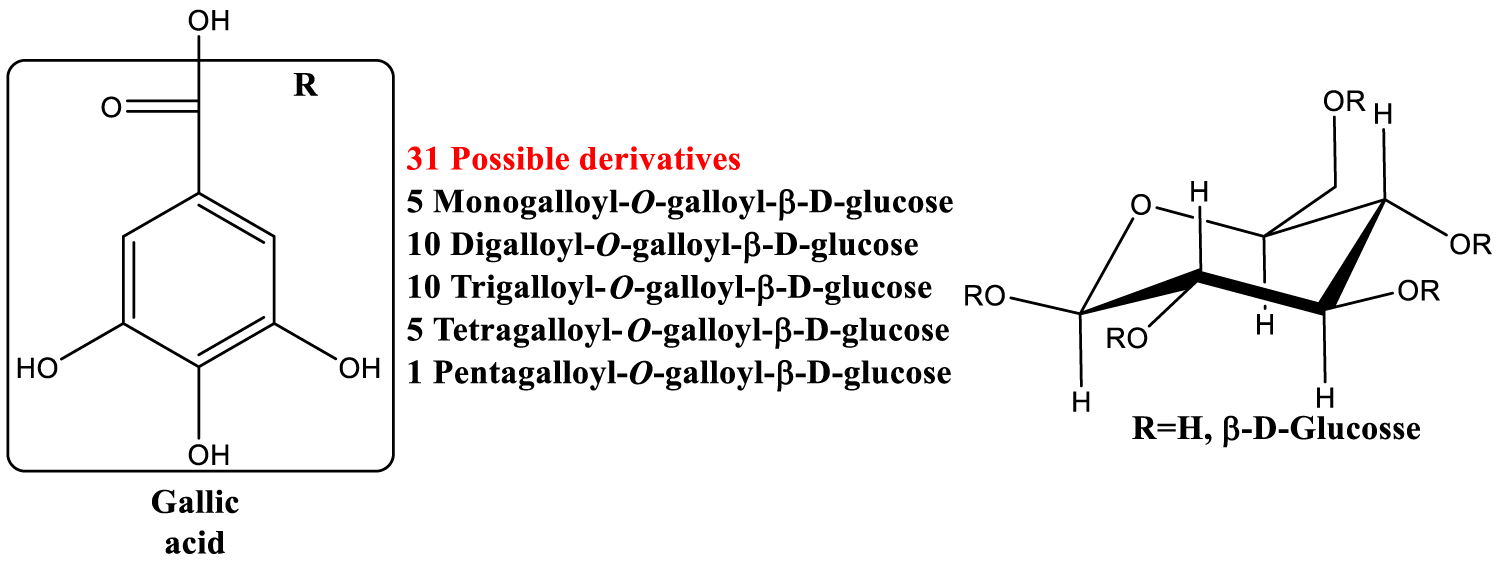
| Preprepared pods aqueous extracts were analyzed for general composition and special focus was given to galloyl hexosides and their derivatives (Figure 8) [98]. |
| The following publications reported general, partially, or completely detailed chemical compositions of some compound families or minerals, without special focus or related activities. Attention should be given to some reported compositions are related to Carob products such as flour, powder, or molasses. In addition, some publications report compositions and extraction methods [130-146]. |
| The following publications reported known chemical compositions but with special focus: seeds sugar profile [147], germ proteins and amino acids [148], seed ultrasound-assisted extraction, detailed polyphenols, and flavonoid compositions, antiglycation and antioxidant (ORAC) activities [149], seeds composition using different extraction techniques, antifungal, and antioxidant (TBARS, OxHLIA) activities [150], previously known phenolics with NMR characterization [151], very detailed composition of phenolics with GS-MS and LC-MS data [152], very detailed mineral content [153], very detailed phenolic content of Rob, with complete data of compounds pKa’s and effect of pH on extraction [154], comparison between ultrasound-assisted and classical extraction methods [155,156], relationship between D-Pinitol and glucose contents [157], negative effect of roasting on polyphenols capacity to bind glycoconjugates of bile acids [158], comparison of volatiles content of roasted and unroasted pods, determined by SPME [159]. |
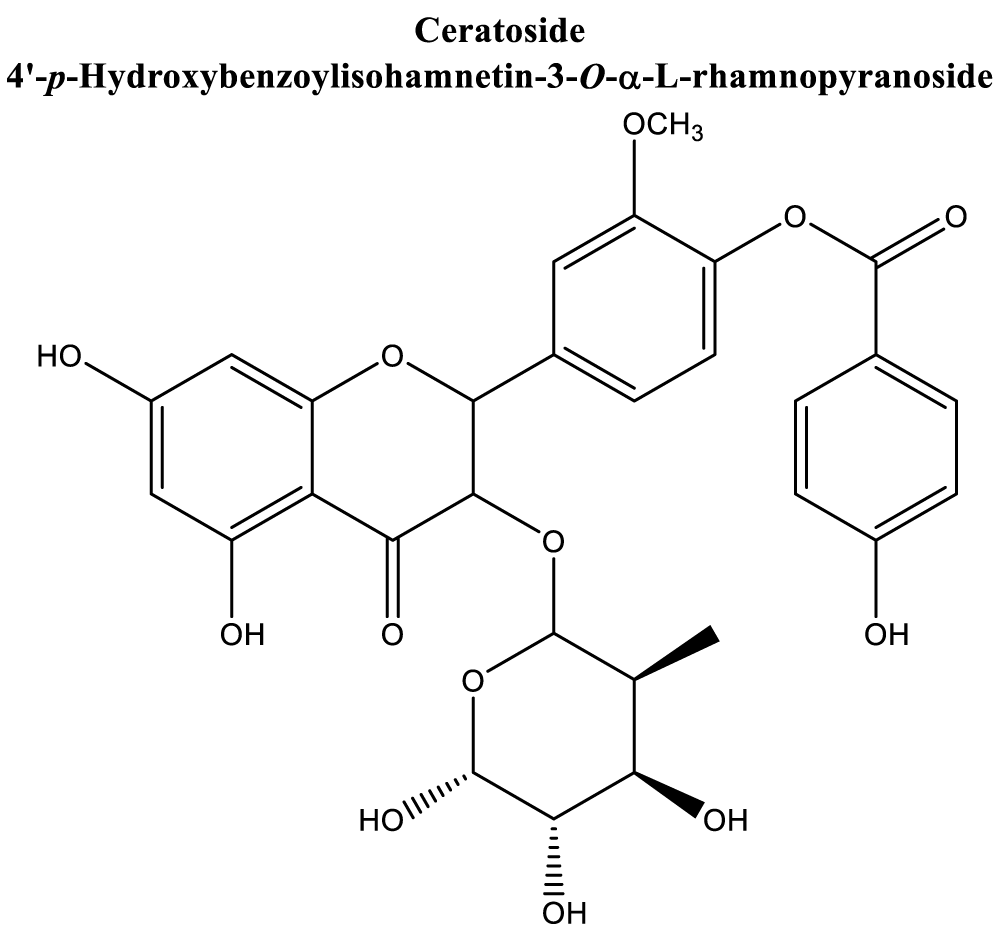
| Two novel acylated flavonol: Ceratoside. Figure 9 [160] |
Antioxidant Activity of Carob
Antioxidant activity of different parts Carob tree is the most studied and most important among all reported properties of this plant so far, as can be clearly seen in table 1. This activity is a result of the presence of some of the most powerful, natural antioxidants, polyphenols (Figures 3-6 and 8-9). But it is very important to emphasize that other natural products contribute to this notable activity: sugars, volatiles (Figure 2) and non-polar compounds (Figure 7).
This joint antioxidant activity was presented by many published studies, especially the influence of reducing sugars. A. Kashif-Shaheen, et al. [161] used DPPH method for testing the antioxidant activity of hydrolyzed Manno-oligosaccharides, where mannose was the main monosaccharide responsible for this activity. Recent studies indicated clearly that reducing sugars presence influences not only the results of antioxidant activity tested by various methods, TPC determined by Folin-Ciocalteu test [162,163]. Moreover, studies have shown that there is clear interaction between these antioxidant compound families in testing this activity [164,165].
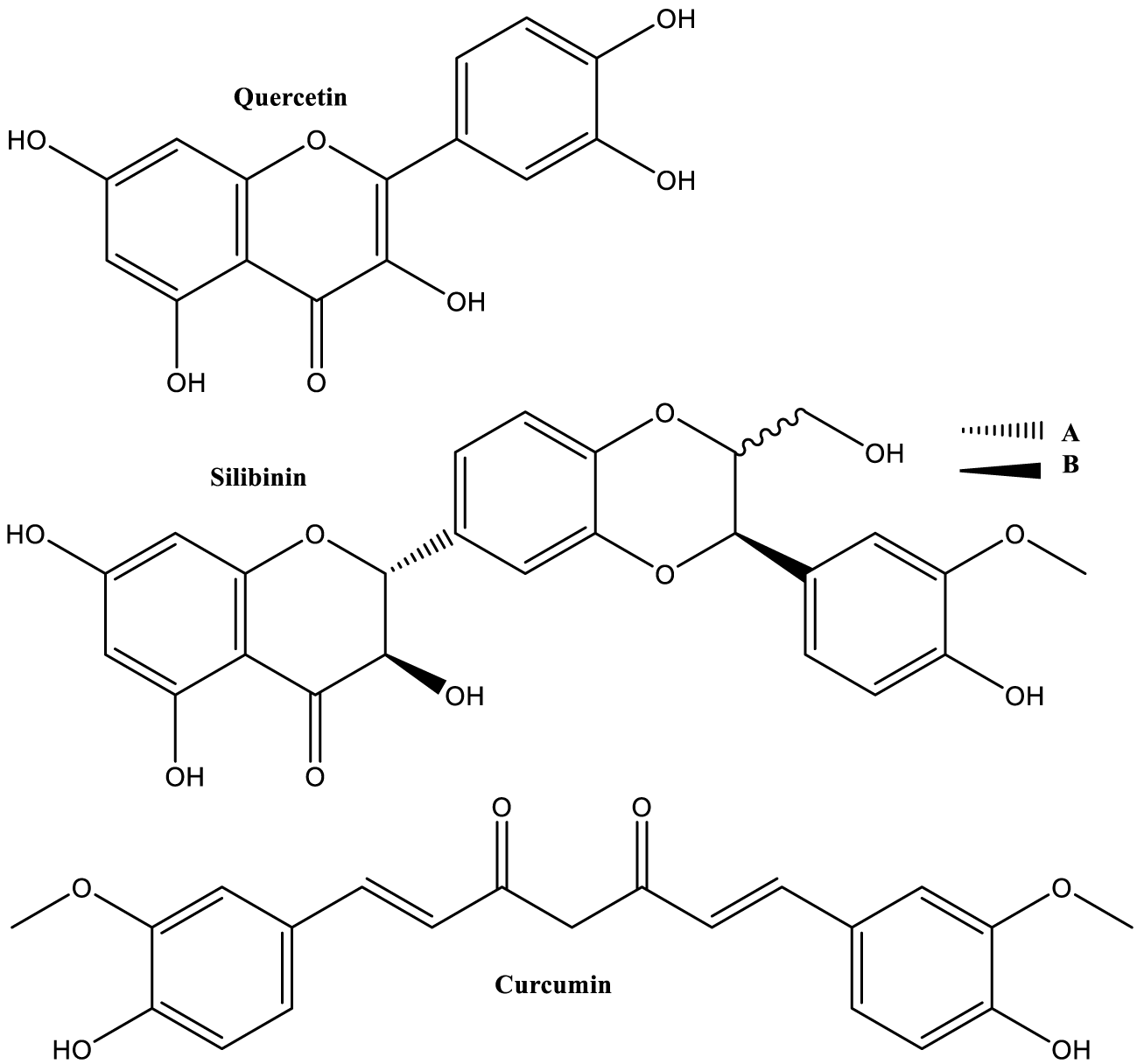
But these interactions exist also between polyphenols, and consequently, have effect on different properties of compound combinations, compared with single compound systems. For example, Baranowska M, et al. [166] showed clear mutual influence between quercetin (Figure 10) and naringenin (Figure 5) in redox-related chemical and biological properties of their mixtures.
In a very recent study, Thayumanavan G, et al. [167] presented synergistic effect of naringenin and silibinin (Figure 10) in treatment of bisphenol A-induced neurotoxicity in Zebrafish model.
Carob major polyphenols are among most active natural antioxidants: gallic acid, catechin, chlorogenic acid, naringenin, myricetin, kaempferol and quercetin. Numerous research and review articles presented these natural products, their uses and large number of properties. Dabeek WM, et al. [168] reviewed the bioavailability and cardiovascular-related activity of kaempferol and quercetin in humans. They listed major plant sources of these compounds: for quercetin, Leeks 0.9 to Dill 79, and for kaempferol, Apples 0.14 to Spinach 55, all in mg/100 g fresh weight. Strangely enough, Carob is not listed in this publication, even though its content of each one of these compounds is higher than some plants listed there [58,83].
Quercetin is powerful antioxidant and possesses numerous health benefits and other biological activities, and consequently, it was reviewed in many publications. For examples, Anand David AV, et al. [169] published a general review about quercetin, and so did Salehi B, et al. [170]. As for activity-focused review articles, Deepika, et al. [171] recently published the benefits of quercetin in age-related diseases, while Nguyen TLA, et al. [172] presented the antibacterial activity of this natural product.
Finally, it’s important to emphasize the fact that quercetin is stronger antioxidant than curcumin [173], which is considered “an extremely potent lipid soluble antioxidant” [174].
Discussion
Humans have discovered the importance of Carob tree since antiquity, mainly as a food source, but also as medicinal plant. Since its domestication, many attempts were done to improve its fruits quantity and quality. Expectedly, the results depend on many variables, which part of them are controllable and some are not: cultivar, locality (weather, soil, water, air quality) and seasonality [175].
For production of natural products from Carob, like all plants, seasonality determines the ripeness stage of the plant part to be used, and this evident in our presentation in table 1 [75,76], and we mentioned the importance of locality [70]. Numerous publications have presented these crucial variables of ripeness stage and location on the chemical compositions of the plant, and consequently their medicinal activities, and we will cite here only two recent examples: pumpkin [176] and onion [177].
Viewing figures 3-5, reveals another very important variable that effects the outcome of the extraction of natural products from Carob, as in all other plants: extraction solvent(s) [25,30,34]. The resulting extracts contain many joint phenolics but differ in others according to the polarity of the extracting solvent: methanol, water, ethyl acetate, respectively. For phenolics, it was shown that aqueous acetone (70-80%, v/v) is one of the most efficient extracting solvents, and low concentration acidity improves the extraction yields [74]. This result is consistent with others that were presented in many publications cited in the review article [178]. However, in recent years there is increasing use of modern extraction techniques such as ultrasound-assisted extraction [155,156], with super-critical-CO2 [179], which is also consistent with using these techniques for extraction of other plants [180].
The role of Carob products as laxative vs. antidiarrheal is questioned sometimes. It has been presented in several publications that Carob extracts have antidiarrheal activity [44], and this was explained by their amino acid content. These results are in accordance with finding of other plant studies [181]. However, the results presented by Aissa A, et al. [115], are not clear when compared with other literature findings. This group reported that partial substitution of barley in rabbits food resulted digestibility adverse effect. Contrary to that, Karabulut A, et al. [182] found that feeding sheep with Carob pods improved digestibility. In later reports, it was found that including Carob pulps up to 35% of Lambs food, had no adverse effect on the animals [183]. But Carob’s positive effect on digestibility was also recorded in human infants: LBG was included in infants (1-6 months old) diet (0.45 g/100 g diet formula) resulted notable decrease in gastroesophageal reflux [184].
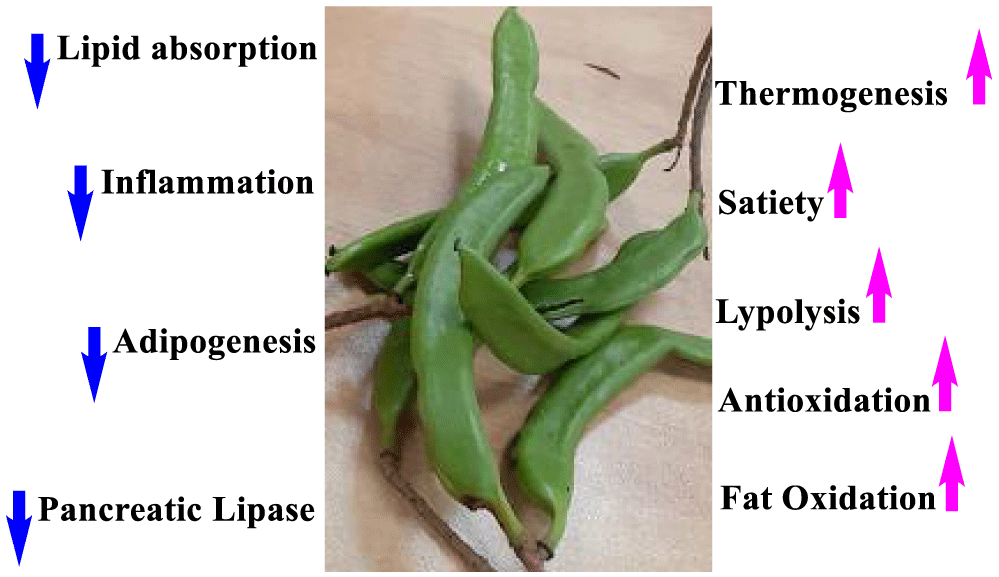
One of the nutritional-medicinal properties of Carob that is gaining increasing attention, is antiobesity activity [49-55]. This activity is well known for fruits of the Fabaceae plant family, that Carob is member of it. For example, it is well known among people of the Middle East in general and particularly Egyptians, that eating Broad bean (or Faba bean, Vicia faba L.) enhances satiety feeling, and modern research have approved this property [185]. Jamous RM [52] presented antiobesity and antioxidant of some medicinal plants that have this property according to traditional Palestinian medicine. They give Carob special focus and summarize the mechanisms that Carob products help attaining this activity and its related beneficial effects, and summarize these properties in a useful figure, that we present here (Figure 11) with some modifications.
Many research groups have studied the effect of roasting on Carob products [83-85]. These studies investigated the effects of roasting in various conditions, on the taste, texture, color, sensory properties, as well as the nutritional and medicinal activities of the roasted products. The overall findings are in good accordance with roasting other plant materials such as coffee beans [186]. Generally, weak roasting has almost no effect, mild roasting can enhance the products activities, while high temperature and/or long-time roasting have damaging effect on the content and activities of natural products, especially antioxidant activity.
In recent decades, there is a rapid increase in human infertility, and this trend is slightly higher among males than females [187]. One of the major reasons for this health disorder is oxidative stress for both genders [188,189]. Based on this very important fact, there is an increasing interest in Carob as spermatogenesis and male fertility enhancer [87,88]. The main reason for this is the quality and quantity of Carob polyphenols, which is in accordance with finding of other studies [190]. To conclude this part, it is important to mention that sperm-enhancing activity of Carob polyphenols have no relation to male Carob trees odor, which is close to human semen. The compounds responsible for this smell are volatiles that the trees attract insects with them [191].
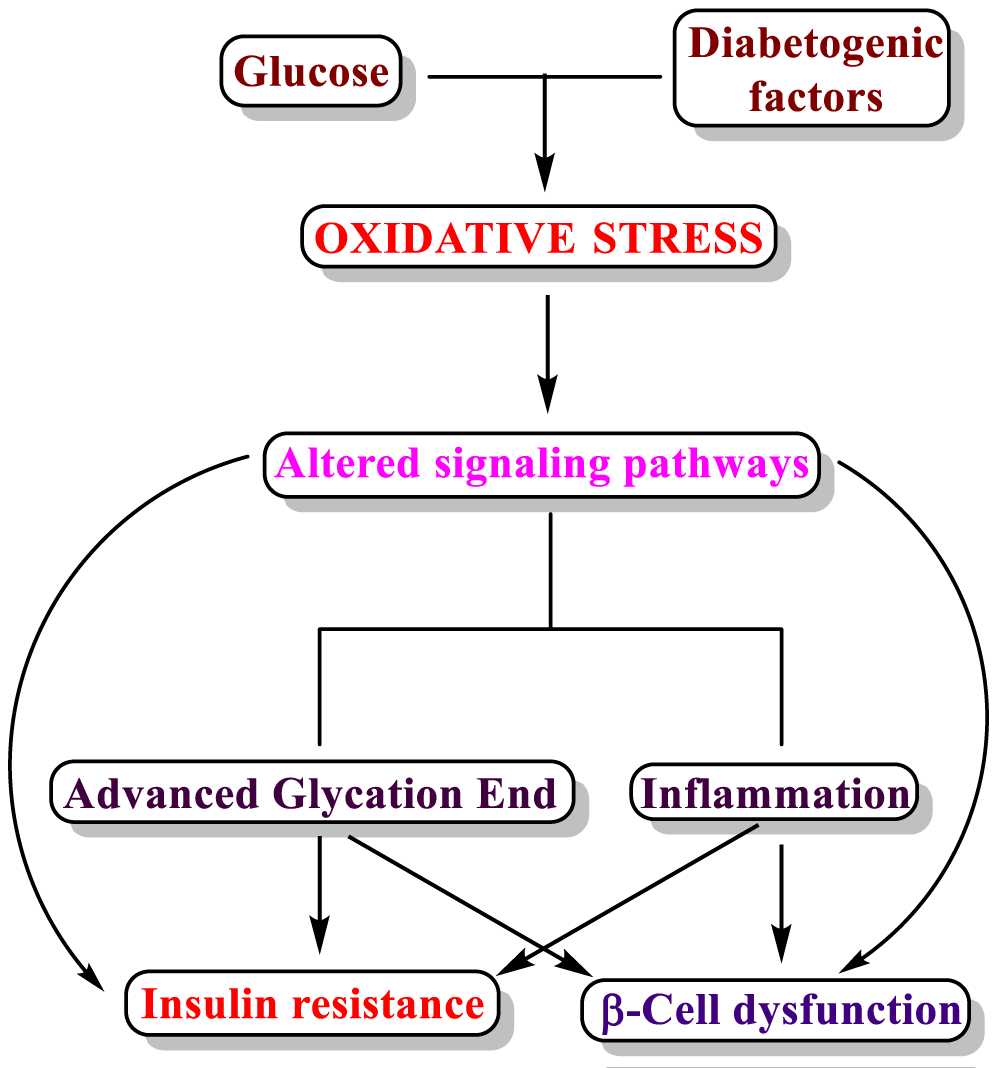
Diabetes mellitus is one of the major health concerns all over the world, and it is “a leading cause of mortality and reduced life expectancy” [192]. According to this publication, in 2017 there were 451 million diabetics in the world, and this number is expected to rise to 693 million by 2045. The global financial burden caused by this disease was USD 760 billion in 2019 and expected to be USD 845 billion by 2045 [193]. Oxidative stress is one of the leading causes of diabetes mellitus, since it alters some enzymatic systems, lipid peroxidation, glutathione metabolism and vitamin C levels [194]. The interrelation between diabetes mellitus and oxidative stress can be simplified as can be seen in figure 12 that was edited from the publication of Chiuchiu V [195].
Antidiabetic activity of Carob was extensively studied [38-42], especially in the context of insulin-resistance and D-Pinitol activity, which is the major insulin regulator in Carob [129]. But Carob contains other natural products that have insulin regulation, as well as other health promoting activities: phytosterols (Figure 7) [91]. This result is consistent with other finding, especially of clinical trials in humans [196].
Gallic acid is one of the most powerful natural antioxidants, and it has been very extensively studied, especially for its healthy properties [197]. Recent studies showed that also the galloyl glucosides (Figure 8) have cardioprotective effect [98], in addition to other notable medicinal activities [198], such as antiviral (Hepatitis B virus) of 1,2,4,6-tetra-O-galloyl-β-D-glucose.
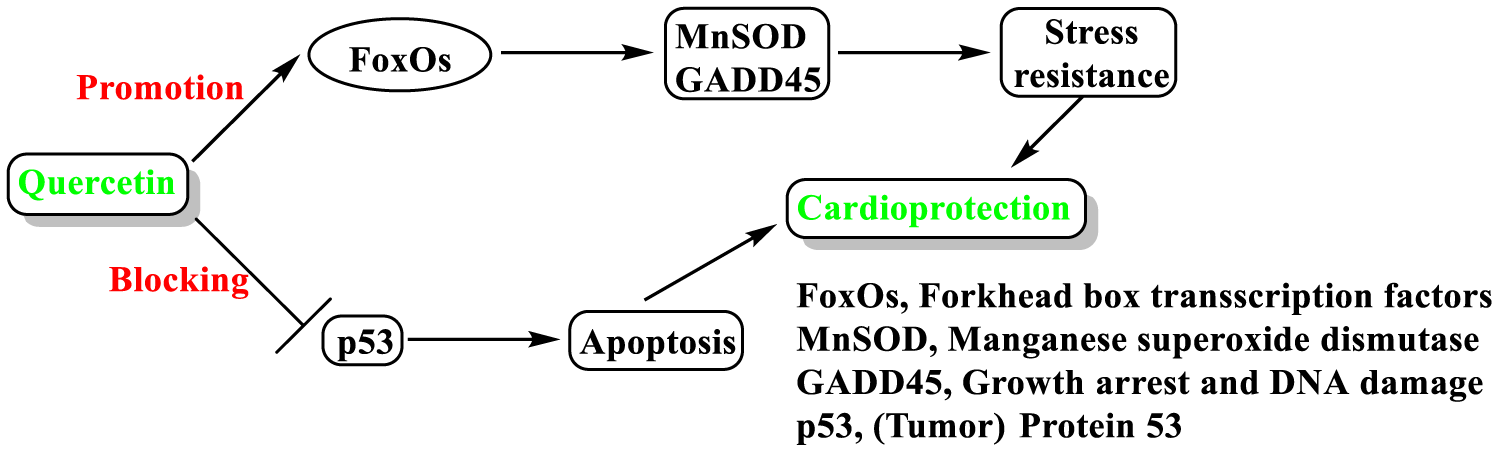
Other polyphenols of Carob have significant contribution to the cardioprotective activity: quercetin and epicatechin [58,74]. These findings are consistent with literature results [199-201]. According to these studies, quercetin cardioprotective mechanism of action involves inhibition of inflammatory-oxidative stress, through modulation of Sirtuin1 (SIRT1) activity. This activity is schematically shown in figure 13.
Interestingly, the tole of Carob’s myricetin was not published so far in context of cardioprotective activity, even though this is a very well-known property of this natural product [202], like many other polyphenols.
Traditional societies used Carob for medicinal purposes, but its major use was for nutrition [203,204]. Differences of use are slight, regardless of socioeconomic status or geographical location. Modern science is following traditional wisdom and many pharmacological discoveries about Carob were published, but the quest for healthier foods remains a top priority [110-114]. In this context, the search for gluten-free pastries found real support in the form of Carob flour that is replacing conventional flours, but also being studied to improve it, for example, by addition of hydroxypropyl methyl cellulose [205].
Pods molasses is main food prepared from Carob. This is the reason that methods of preparation are modified and improved continuously. And since this is practically an extraction process, it is studied and published ceaselessly [206]. And in the quest of healthy foods, humans used traditionally to mix Carob molasses with other basic foods such as milk, yogurt and bread, modern food technologies are exploring broader range of possibilities [207].
Based on growing Carob farming, food production and medicinal research, safety concerns were raised, and several studies were published. The Cosmetic Ingredient Review Expert Panel examined several Carob products used for cosmetics and concluded that they are safe [208]. Cariñanos P, et al. [209] concluded that use of Carob products “do not present any problem in regard to direct or crossed allergenicity, and its fruits, in addition to being edible, present other properties of interest”. Finally, Cariñanos P, et al. [210] reached the same conclusion in a follow-up study two years later.
Research of Carob and its products is flourishing, and this trend is expected to last in the coming few years or even increase. One of the best indications for this situation is the significant number of studies that were published very recently. These focus on the potential of Carob as very healthy nutritional resource, its medicinal activities, and the attempts to discover new natural products that it contains.
Frühbauerová M, et al. [211] tested the antioxidant properties of Carob powder that was prepared with a special technique of cryogenic and vibratory grinding. Their findings indicate that this powder had high antioxidant capacity, and its phenolic components (such as ferulic acid) had high bioaccessibility. In a closely related study, Vilas-Boas AM, et al. [212] studied the bioaccessibility of the ingredients of Carob flour, by testing their stability during gastrointestinal digestion simulation. Their results clearly show that particles size have key importance for this bioaccessibility. Medina-Vera D, et al. [213] a leading Spanish research group of Carob and its natural products, proposed the possible potential of D-Pinitol for tauopathies treatment, and they presented a possible mechanism of action.
Formation of Heterocyclic Aromatic Amines (HAAs) during meat processing, is a major concern due to the potential risks that HAA have [214]. This risk can be reduced by cooking some plants with meat or adding plant extracts [215]. Turkish researchers Erdoğan B, et al. [216] have shown that adding a combination of Carob pods aqueous extract and propolis, significantly reduced the formation of HAAs during the cooking of meatballs using various methods. Another Turkish group of Ozdemir Y, et al. [217] made a smart, healthy, and sustainability-promoting use of Carob: they added pulp that was left after production of molasses from pods to the production mixture of ice cream cones. This resulted in enrichment of food qualities of these cones, as well as consumers higher satisfaction. And the high advantages of the molasses that was presented in numerous publications, were highlighted in a new study of Toufeili I, et al. [218] from Lebanon. The compared the nutritional qualities of Carob syrup with those of Date and Apple and found that Carob syrup had higher health benefits. To improve the qualities of Carob molasses even more, the Italian research group of Clodoveo ML, et al. [219] developed a new ultrasound-assisted extraction method. This method proved to be quantitatively and qualitatively efficient since it made the use of extreme methods such as extensive heating, unnecessary.
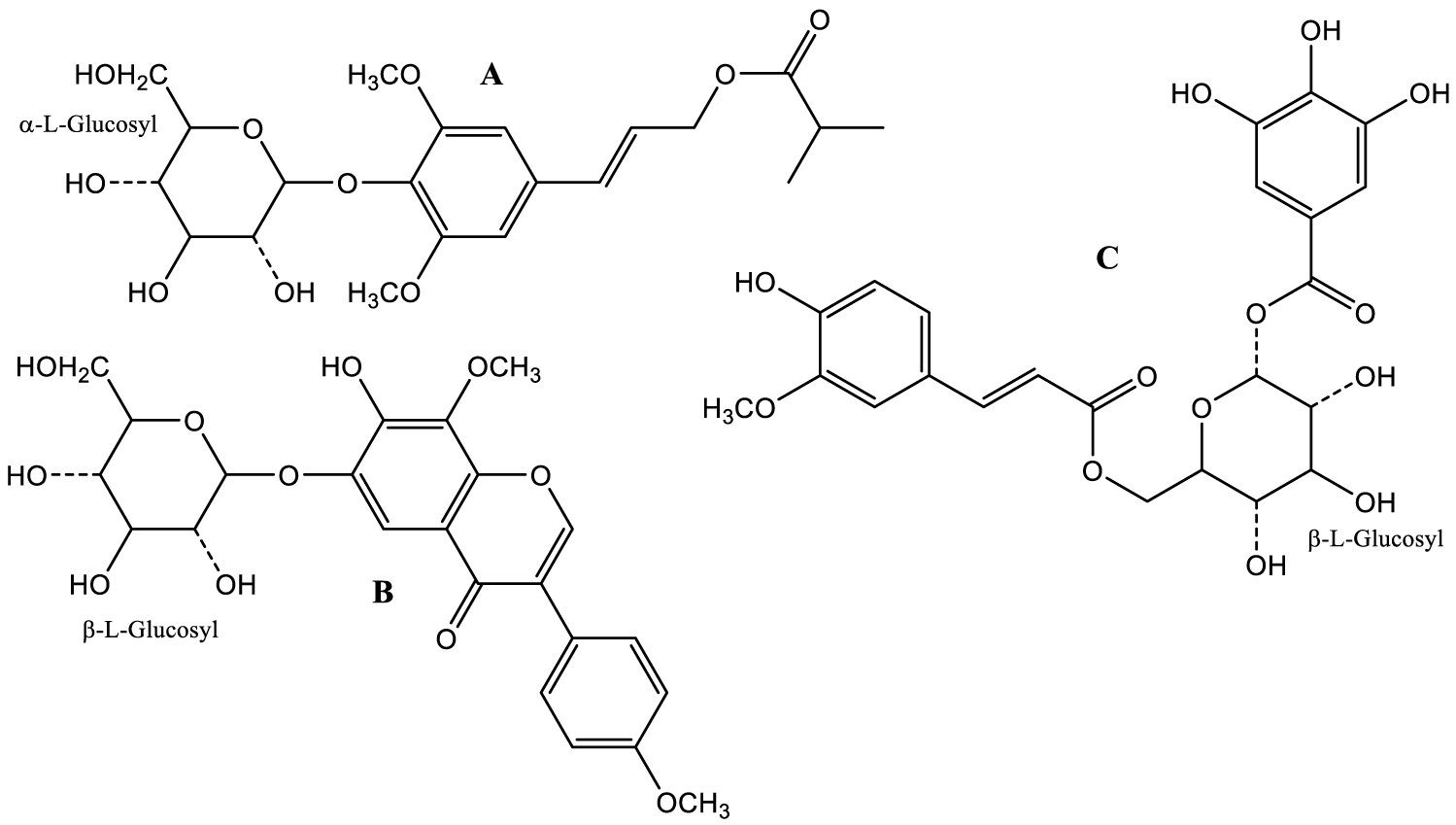
Finally, the search for novel natural products in Carob is continuing, and Z-T. Peng and her colleagues from China recently reported the isolation and characterization of a new isoflavone (A) and two phenylpropanoids (B,C) from Carob pods, shown in figure 14 [220].
Conclusions and Future Vision
- Carob is a plentiful source of important antioxidants as well as other very healthy natural products.
- Since interactions between natural products have effects on their properties, it should be carefully considered if these compounds are to be consumed separately or in combinations such as extracts, in foods, supplementations or drugs.
- Cardioprotective activity of Carob should be studied in more depth, especially in connection with myricetin.
- Carob products can be extensively used in oxidative stress-related health disorders. Extended research is needed.
- Carob and its products are safe. There is a potential for new healthy foods and cosmetics, based on Carob alone or in combinations with other materials, especially synergism with other natural products of other plants.
Conflicts of Interest
The author declares that there is no conflict of interest regarding the publication of this article.
Funding
This work received no funding.
References
- Jiang KW, Pan B, Tian B. Recent taxonomic changes for Fabaceae (Leguminosae) genera in China. Biodiv Sci. 2019;27:689-697. doi: 10.17520/biods.2019032.
- Hillcoat D, Lewis GP, Verdcourt B. A new species of Ceratonia (Leguminosae-Caesalpinioideae) from Arabia and the Somali Republic. Kew Bull. 1980;35:261-271. doi: 10.2307/4114570.
- Zohary D. Domestication of the carob (Ceratonia siliqua L.), Israel. J Plant Sci. 2002;50:141-145. doi: 10.1560/BW6B-4M9P-U2UA-C6NN.
- Gharnit N, Ennabili A. Categories of carob tree (Ceratonia siliqua L.) from Morocco. Int J Fruit Sci. 2016;16:259-274. doi: 10.1080/15538362.2015.1102674.
- Gubbuk H, Gunes E, Ayala-Silva T, Ercisli, S. Rapid vegetative propagation method for carob. Not Bot Hort Agrobot Cluj. 2011;39:251-254. doi: 10.15835/nbha3916074.
- Liphschitz N. Ceratonia siliqua in Israel: An ancient element or a newcomer? Isr J Bot. 1987;36:191-197. doi: 10.1080/0021213X.1987.10677083.
- Bottemal S, Sarpaki A. Environmental change in Crete: A 9000-year record of Holocene vegetation history and the effect of the Santorini eruption. Holocene. 2003;13:733-749. doi: 10.1 191/0959683603hl659rp.
- Azab A. Carob (Ceratonia siliqua): Health, medicine and chemistry. Eur Chem Bull. 2017;6:456-469. doi: 10.17628/ecb.2017.6.456-469.
- Khalifa AB. Herbs: Nature's Pharmacy. 1st ed. Arab Cultural Center: Casablanca, Morocco: 2004. p.286-288.
- Guarino C, De Simone L, Santoro S. Ethnobotanical study of the Sannio area, Campania, Southern Italy. Ethnobot Res App. 2008;6:255-317. doi: 10.17348/era.6.0.255-317.
- Bou-Idra M, Rhafouri R, Zekkori B, Bahri H, El Omari M, Bentayeb A. Floristic and ethnobotanical studies of three plants, Cactus, Carob and Caper used in the Zerhoun region (Morocco). Int J Recent Adv Multidiscip Res. 2015;2:1027-1034.
- Bachar M, ElYacoubi H, Zidane L, Rochdi A. Ethnomedicinal and traditional phytotherapeutic plants used in Bouhachem Natural Regional Park (Rif of Morocco): Case of Bni-Leit and Al-Oued districts. J Pharm Pharmacogn Res. 2021; 9:284-312.
- Ahmed HM. Ethnopharmacobotanical study on the medicinal plants used by herbalists in Sulaymaniyah Province, Kurdistan, Iraq. J Ethnobiol Ethnomed. 2016 Jan 28;12:8. doi: 10.1186/s13002-016-0081-3. PMID: 26821541; PMCID: PMC4730727.
- Ali-Shtayeh MS, Jamous RM, Jamous RM. Traditional Arabic Palestinian ethnoveterinary practices in animal health care: A field survey in the West Bank (Palestine). J Ethnopharmacol. 2016 Apr 22;182:35-49. doi: 10.1016/j.jep.2016.02.005. Epub 2016 Feb 8. PMID: 26869545.
- Palabaş Uzun S, Koca C. Ethnobotanical survey of medicinal plants traded in herbal markets of Kahramanmaraş. Plant Divers. 2020 Dec 29;42(6):443-454. doi: 10.1016/j.pld.2020.12.003. PMID: 33733012; PMCID: PMC7936109.
- Gürdal B, Kültür S. An ethnobotanical study of medicinal plants in Marmaris (Muğla, Turkey). J Ethnopharmacol. 2013 Mar 7;146(1):113-26. doi: 10.1016/j.jep.2012.12.012. Epub 2012 Dec 20. PMID: 23261486.
- Erbay MS, Anil S, Melikoğlu G. Plants used in traditional treatment against anemia in Turkey. Marmara Pharm J. 2016;20:164-171. doi: 10.12991/mpj.20162044391.
- Akbulut S, Bayramoglu MM. the trade and use of some medical and aromatic herbs in Turkey. Stud Ethno-Med 2013;7:67-77. doi: 10.1080/09735070.2013.11886446.
- Yousif AK, Alghzawi HM. Processing and characterization of carob powder. Food Chem. 2000;69:283-287. doi: 10.1016/S0308-8146(99)00265-4.
- Emara MH, Soliman HH, Elnadry M, Mohamed Said E, Abd-Elsalam S, Elbatae HE, Zaher TI, Ezzeldin S Bazeed S, Abdel-Razik A, Youssef Mohamed S, Elfert A; “Egyptian Ramadan Fasting, Liver Diseases Interest Group”. Ramadan fasting and liver diseases: A review with practice advices and recommendations. Liver Int. 2021 Mar;41(3):436-448. doi: 10.1111/liv.14775. Epub 2021 Jan 9. PMID: 33369880.
- Zhengzhang T. On the origin of the carat as the unit of weight for gemstones. Chin J Geochem. 1991;10:288-293. doi: 10.1007/BF02843332.
- Turnbull LA, Santamaria L, Martorell T, Rallo J, Hector A. Seed size variability: from carob to carats. Biol Lett. 2006 Sep 22;2(3):397-400. doi: 10.1098/rsbl.2006.0476. PMID: 17148413; PMCID: PMC1686184.
- Ben Ayache S, Behija Saafi E, Emhemmed F, Flamini G, Achour L, Muller CD. Biological Activities of Aqueous Extracts from Carob Plant (Ceratonia siliqua L.) by Antioxidant, Analgesic and Proapoptotic Properties Evaluation. Molecules. 2020 Jul 8;25(14):3120. doi: 10.3390/molecules25143120. PMID: 32650498; PMCID: PMC7397290.
- Pekmezci E, Dundar C, Turkoglu M. Proprietary Herbal Extract Downregulates the Gene Expression of IL-1α in HaCaT Cells: Possible Implications Against Nonscarring Alopecia. Med Arch. 2018 Apr;72(2):136-140. doi: 10.5455/medarh.2018.72.136-140. PMID: 30302033; PMCID: PMC6126931.
- Abidar S, Yildiz O, Degirmenci A, Amakran A, El Maadoudi M, Nhiri M. Glucose-mediated protein glycation: Contribution of methanolic extract of Ceratonia siliqua L. in protection and in vitro potential inhibition of acetylcholinesterase. J Food Biochem. 2019 Nov;43(11):e13009. doi: 10.1111/jfbc.13009. Epub 2019 Aug 8. PMID: 31393019.
- Al-Timimi LAN, Al-Tameemi NAN. Role of carob seed in characterization of MDR bacteria in diabetic foot ulcer. Biochem Cell Arch. 2020;20:927-934. doi: 10.35124/bca.2020.20.1.927.
- Abdulkareem RS, Al-Hayali WRY, Ibrahim II. Antimicrobial activity of Ceratonia silique L. extract against diarrheagenic E-coli. Sys Rev Pharm. 2020;11:2139-2141.
- Ben Othmen K, Elfalleh W, García Beltrán JM, Esteban MÁ, Haddad M. An in vitro study of the effect of carob (Ceratonia siliqua L.) leaf extracts on gilthead seabream (Sparus aurata L.) leucocyte activities. Antioxidant, cytotoxic and bactericidal properties. Fish Shellfish Immunol. 2020 Apr;99:35-43. doi: 10.1016/j.fsi.2020.02.005. Epub 2020 Feb 4. PMID: 32032761.
- Adjal F, Guitone A, Almi S. Antibacterial properties of Ceratonia siliqua ethanolic extract and Artemisia herba alba essential oil. Acad Lett. 2021;1943. doi: 10.20935/AL1943.
- Darwish WS, Khadr AES, Kamel MAEN, Abd Eldaim MA, El Sayed IET, Abdel-Bary HM, Ullah S, Ghareeb DA. Phytochemical Characterization and Evaluation of Biological Activities of Egyptian Carob Pods (Ceratonia siliqua L.) Aqueous Extract: In Vitro Study. Plants (Basel). 2021 Nov 29;10(12):2626. doi: 10.3390/plants10122626. PMID: 34961100; PMCID: PMC8706755.
- Klenow S, Glei M, Haber B, Owen R, Pool-Zobel BL. Carob fibre compounds modulate parameters of cell growth differently in human HT29 colon adenocarcinoma cells than in LT97 colon adenoma cells. Food Chem Toxicol. 2008 Apr;46(4):1389-97. doi: 10.1016/j.fct.2007.09.003. Epub 2007 Sep 11. PMID: 17950517.
- Custódio L, Fernandes E, Escapa AL, López-Avilés S, Fajardo A, Aligué R, Alberício F, Romano A. Antioxidant activity and in vitro inhibition of tumor cell growth by leaf extracts from the carob tree (Ceratonia siliqua). Pharm Biol. 2009;47:721-728. doi: 10.1080/13880200902936891.
- Ghanemi FZ, Belarbi M, Fluckiger A, Nani A, Dumont A, De Rosny C, Aboura I, Sayed Khan A, Murtaza B, Benammar C, Boucif Farid L, Patoli D, Delmas D, Rébé C, Apétoh L, Akhtar Khan N, Ghringhelli F, Rialland M, Hichami A. Carob leaf polyphenols trigger intrinsic apoptotic pathway and induce cell cycle arrest in colon cancer cells. J Funct Foods. 2017;33: 112-121. doi: 10.1016/j.jff.2017.03.032.
- Gregoriou G, Neophytou CM, Vasincu A, Gregoriou Y, Hadjipakkou H, Pinakoulaki E, Christodoulou MC, Ioannou GD, Stavrou IJ, Christou A, Kapnissi-Christodoulou CP, Aigner S, Stuppner H, Kakas A, Constantinou AI. Anti-Cancer Activity and Phenolic Content of Extracts Derived from Cypriot Carob (Ceratonia siliqua L.) Pods Using Different Solvents. Molecules. 2021 Aug 19;26(16):5017. doi: 10.3390/molecules26165017. PMID: 34443605; PMCID: PMC8401790.
- Macar O, Kalefetoğlu Macar T, Çavuşoğlu K, Yalçın E. Determination of protective effect of carob (Ceratonia siliqua L.) extract against cobalt(II) nitrate-induced toxicity. Environ Sci Pollut Res Int. 2020 Nov;27(32):40253-40261. doi: 10.1007/s11356-020-10009-6. Epub 2020 Jul 13. PMID: 32661972.
- Agrawal A, Mohan M, Kasture S, Foddis C, Frau MA, Loi MC, Maxia A. Antidepressant activity of Ceratonia siliqua L. fruit extract, a source of polyphenols. Nat Prod Res. 2011 Feb;25(4):450-6. doi: 10.1080/14786419.2010.527447. PMID: 21328139.
- Lakkab I, El Hajaji H, Lachkar N, Lefter R, Ciobica A, El Bali B, Lachkar M. Ceratonia siliqua L. seed peels: Phytochemical profile, antioxidant activity, and effect on mood disorders. J Funct Foods. 2019;54:457-465. doi: 10.1016/j.jff.2019.01.041.
- Gruendel S, Otto B, Garcia AL, Wagner K, Mueller C, Weickert MO, Heldwein W, Koebnick C. Carob pulp preparation rich in insoluble dietary fibre and polyphenols increases plasma glucose and serum insulin responses in combination with a glucose load in humans. Br J Nutr. 2007 Jul;98(1):101-5. doi: 10.1017/S0007114507701642. Epub 2007 Mar 21. PMID: 17381962.
- Rtibi K, Selmi S, Grami D, Saidani K, Sebai H, Amri M, Eto B, Marzouki L. Ceratonia siliqua L. (immature carob bean) inhibits intestinal glucose absorption, improves glucose tolerance and protects against alloxan-induced diabetes in rat. J Sci Food Agric. 2017 Jun;97(8):2664-2670. doi: 10.1002/jsfa.8091. Epub 2016 Nov 11. PMID: 27739095.
- Qasem MA, Noordin MI, Arya A, Alsalahi A, Jayash SN. Evaluation of the glycemic effect of Ceratonia siliqua pods (Carob) on a streptozotocin-nicotinamide induced diabetic rat model. PeerJ. 2018 May 23;6:e4788. doi: 10.7717/peerj.4788. PMID: 29844959; PMCID: PMC5970558.
- Lambert C, Cubedo J, Padró T, Vilahur G, López-Bernal S, Rocha M, Hernández-Mijares A, Badimon L. Effects of a Carob-Pod-Derived Sweetener on Glucose Metabolism. Nutrients. 2018 Feb 27;10(3):271. doi: 10.3390/nu10030271. PMID: 29495516; PMCID: PMC5872689.
- García-Díez E, López-Oliva ME, Caro-Vadillo A, Pérez-Vizcaíno F, Pérez-Jiménez J, Ramos S, Martín MÁ. Supplementation with a Cocoa-Carob Blend, Alone or in Combination with Metformin, Attenuates Diabetic Cardiomyopathy, Cardiac Oxidative Stress and Inflammation in Zucker Diabetic Rats. Antioxidants (Basel). 2022 Feb 21;11(2):432. doi: 10.3390/antiox11020432. PMID: 35204314; PMCID: PMC8869324.
- Loeb H, Vandenplas Y, Würsch P, Guesry P. Tannin-rich carob pod for the treatment of acute-onset diarrhea. J Pediatr Gastroenterol Nutr. 1989 May;8(4):480-5. doi: 10.1097/00005176-198905000-00010. PMID: 2723939.
- Akşit S, Cağlayan S, Cukan R, Yaprak I. Carob bean juice: a powerful adjunct to oral rehydration solution treatment in diarrhoea. Paediatr Perinat Epidemiol. 1998 Apr;12(2):176-81. doi: 10.1046/j.1365-3016.1998.00093.x. PMID: 9620567.
- Rtibi K, Jabri MA, Selmi S, Sebai H, Marie JC, Amri M, Marzouki L, El-Benna J. Preventive effect of carob (Ceratonia siliqua L.) in dextran sulfate sodium-induced ulcerative colitis in rat. RSC Adv. 2016;6:19992-20000. doi: 10.1039/c5ra21388f.
- Lachkar N, l-Sobarry M, El Hajaji H, Lamkinsi T, Lachkar M, Cherrah Y, Alaoui K. Anti-inflammatory and antioxidant effect of Ceratonia siliqua L. methanol barks extract. J Chem Pharm Res. 2016;8:202-210.
- Aboura I, Nani A, Belarbi M, Murtaza B, Fluckiger A, Dumont A, Benammar C, Tounsi MS, Ghiringhelli F, Rialland M, Khan NA, Hichami A. Protective effects of polyphenol-rich infusions from carob (Ceratonia siliqua) leaves and cladodes of Opuntia ficus-indica against inflammation associated with diet-induced obesity and DSS-induced colitis in Swiss mice. Biomed Pharmacother. 2017 Dec;96:1022-1035. doi: 10.1016/j.biopha.2017.11.125. Epub 2017 Dec 6. PMID: 29221725.
- Rico D, Martín-Diana AB, Martínez-Villaluenga C, Aguirre L, Silván JM, Dueñas M, De Luis DA, Lasa A. In vitro approach for evaluation of carob by-products as source bioactive ingredients with potential to attenuate metabolic syndrome (MetS). Heliyon. 2019 Jan 30;5(1):e01175. doi: 10.1016/j.heliyon.2019.e01175. PMID: 30775572; PMCID: PMC6357213.
- Martínez-Villaluenga C, Peñas E, Rico D, Martin-Diana AB, Portillo MP, Macarulla MT, de Luis DA, Miranda J. Potential Usefulness of a Wakame/Carob Functional Snack for the Treatment of Several Aspects of Metabolic Syndrome: From In Vitro to In Vivo Studies. Mar Drugs. 2018 Dec 17;16(12):512. doi: 10.3390/md16120512. PMID: 30562926; PMCID: PMC6315385.
- Gruendel S, Garcia AL, Otto B, Mueller C, Steiniger J, Weickert MO, Speth M, Katz N, Koebnick C. Carob pulp preparation rich in insoluble dietary fiber and polyphenols enhances lipid oxidation and lowers postprandial acylated ghrelin in humans. J Nutr. 2006 Jun;136(6):1533-8. doi: 10.1093/jn/136.6.1533. PMID: 16702317.
- Papakonstantinou E, Orfanakos N, Farajian P, Kapetanakou AE, Makariti IP, Grivokostopoulos N, Ha MA, Skandamis PN. Short-term effects of a low glycemic index carob-containing snack on energy intake, satiety, and glycemic response in normal-weight, healthy adults: Results from two randomized trials. Nutrition. 2017 Oct;42:12-19. doi: 10.1016/j.nut.2017.05.011. Epub 2017 May 31. PMID: 28870473.
- Jamous RM, Abu-Zaitoun SY, Akkawi RJ, Ali-Shtayeh MS. Antiobesity and Antioxidant Potentials of Selected Palestinian Medicinal Plants. Evid Based Complement Alternat Med. 2018 Jun 13;2018:8426752. doi: 10.1155/2018/8426752. PMID: 30026782; PMCID: PMC6031216.
- Sour S, Fridi C, Taif A. Beneficial Effects of carob pulp (Ceratonia siliqua) on lipids profile and oxidant/antioxidant status in obese rats. Agrobiologia. 2019;9:1200-1206.
- Jaffari H, Abedi B, Fatolahi H. The effect of 8 weeks of carob supplementation and resistance training on lipid profile and irisin in obese men. Int J Sport Exerc Health Res. 2020;4:91-95. doi: 10.31254/sportmed.4212.
- Fujita K, Norikura T, Matsui-Yuasa I, Kumazawa S, Honda S, Sonoda T, Kojima-Yuasa A. Carob pod polyphenols suppress the differentiation of adipocytes through posttranscriptional regulation of C/EBPβ. PLoS One. 2021 Mar 8;16(3):e0248073. doi: 10.1371/journal.pone.0248073. PMID: 33684156; PMCID: PMC7939365.
- Kumazawa S, Taniguchi M, Suzuki Y, Shimura M, Kwon MS, Nakayama T. Antioxidant activity of polyphenols in carob pods. J Agric Food Chem. 2002 Jan 16;50(2):373-7. doi: 10.1021/jf010938r. PMID: 11782210.
- Makris DP, Kefalas P. Carob pods (Ceratonia siliqua L.) as a source of polyphenolic antioxidants. Food Technol Biotechnol. 2004;42:105-108.
- Papagiannopoulos M, Wollseifen HR, Mellenthin A, Haber B, Galensa R. Identification and quantification of polyphenols in carob fruits (Ceratonia siliqua L.) and derived products by HPLC-UV-ESI/MSn. J Agric Food Chem. 2004 Jun 16;52(12):3784-91. doi: 10.1021/jf030660y. PMID: 15186098.
- El Hajaji H, Lachkar N, Alaoui K, Cherrah Y, Farah A, Ennabili A, El Bali B, Lachkar M. Antioxidant activity, phytochemical screening, and total phenolic content of extracts from three genders of carob tree barks growing in Morocco. Arab J Chem. 2011;4:321-324. doi: 10.1016/j.arabjc.2010.06.053.
- Custódio L, Escapa AL, Fernandes E, Fajardo A, Aligué R, Alberício F, Neng N, Nogueira JM, Romano A. Phytochemical profile, antioxidant and cytotoxic activities of the carob tree (Ceratonia siliqua L.) germ flour extracts. Plant Foods Hum Nutr. 2011 Mar;66(1):78-84. doi: 10.1007/s11130-011-0214-8. PMID: 21399924.
- Rtibi K, Jabri MA, Selmi S, Souli A, Sebai H, El-Benna J, Amri M, Marzouki L. Carob pods (Ceratonia siliqua L.) inhibit human neutrophils myeloperoxidase and in vitro ROS-scavenging activity. RSC Adv. 2015;5:84207-84215. doi: 10.1039/C5RA14719K.
- Roseiro LB, Tavares CS, Roseiro JC, Rauter AP. Antioxidants from aqueous decoction of carob pods biomass (Ceretonia siliqua L.): Optimisation using response surface methodology and phenolic profile by capillary electrophoresis. Ind Crops Prod. 2013:44:119-126. doi: 10.1016/j.indcrop.2012.11.006.
- Vitali Čepo D, Mornar A, Nigović B, Kremer D, Radanović D, Dragojević IV. Optimization of roasting conditions as an useful approach for increasing antioxidant activity of carob powder.LWT-Food Sci Technol. 2014:58:578-586.doi: 10.1016/j.lwt.2014.04.004.
- Boublenza I, Lazouni HA, Ghaffari L, Ruiz K, Fabiano-Tixier AS, Chemat F. Influence of roasting on sensory, antioxidant, aromas, and physicochemical properties of carob pod powder (Ceratonia siliqua L.). J Food Qual. 2017. doi: 10.1155/2017/4193672.
- Grigoriou AM, Pinakoulaki E. Linking the Dynamic Changes in the In Vitro Antioxidant Activity of Carob Kibbles upon Roasting to the Chemical and Structural Changes Revealed by FTIR Spectroscopy. Antioxidants (Basel). 2021 Dec 20;10(12):2025. doi: 10.3390/antiox10122025. PMID: 34943128; PMCID: PMC8698282.
- Rtibi K, Jabri MA, Selmi S, Sebai H, Amri M, El-Benna J, Marzouki L. Ceratonia siliqua leaves exert a strong ROS-scavenging effect in human neutrophils, inhibit myeloperoxydase in vitro and protect against intestinal fluid and electrolytes secretion in rats. RSC Adv. 2016;6:65483-65493. doi:10.1039/c6ra11297h.
- El Hajaji H, Lachkar N, Alaoui K, Cherrah Y, Farah A, Ennabili A, El Bali B, Lachkar M. Antioxidant properties and total phenolic content of three varieties of carob tree leaves from Morocco. Rec Nat Prod. 2010;4:193-204.
- Dallali S, Aloui F, Selmi H, Sebei H. Comparison of the chemical composition and the antioxidant activity of the leaves of Carob tree (Ceratonia siliqua L.) collected in three sites of Djebel Zaghouan (Tunisia). J New Sci Agric Biotechnol. 2018;21;3429-3438.
- Petkova N, Petrova I, Ivanov I, Mihov R, Hadjikinova R, Ognyanov M, Nikolova V. Nutritional and antioxidant potential of carob (Ceratonia siliqua) flour and evaluation of functional properties of its polysaccharide fraction. J Pharm Sci Res. 2017;9:2189-2195.
- Ydjedd S, Bouriche S, López-Nicolás R, Sánchez-Moya T, Frontela-Saseta C, Ros-Berruezo G, Rezgui F, Louaileche H, Kati DE. Effect of in Vitro Gastrointestinal Digestion on Encapsulated and Nonencapsulated Phenolic Compounds of Carob (Ceratonia siliqua L.) Pulp Extracts and Their Antioxidant Capacity. J Agric Food Chem. 2017 Feb 1;65(4):827-835. doi: 10.1021/acs.jafc.6b05103. Epub 2017 Jan 17. PMID: 28094929.
- Ydjedd S, Chaalal M, Richard G, Kati DE, López-Nicolás R, Fauconnier ML, Louaileche H. Assessment of antioxidant potential of phenolic compounds fractions of Algerian Ceratonia siliqua L. pods during ripening stages. Int Food Res J. 2017;24:2041-2049.
- Čepo DV, Jug M, Rajković, MG, Jablan J. Formulation of a nutraceutical derived from carob: β-cyclodextrin encapsulation of antioxidants from carob pod. J Food Nutr Res. 2017;56:48-60.
- Belhocine M, Sakmeche C, Azzouz F. Antioxidant activity and gastro-protective effets of carob podsa queous extracts on indomethacin-induced gastric ulcer in wistar rats. Int J Biosci. 2018;12:48-69. doi: 10.12692/ijb/12.6.48-69.
- Goulas V, Georgiou E. Utilization of Carob Fruit as Sources of Phenolic Compounds with Antioxidant Potential: Extraction Optimization and Application in Food Models. Foods. 2019 Dec 24;9(1):20. doi: 10.3390/foods9010020. PMID: 31878230; PMCID: PMC7022565.
- Ben Othmen K, Elfalleh W, Lachiheb B, Haddad M. Evolution of phytochemical and antioxidant activity of Tunisian carob (Ceratonia siliqua L.) pods during maturation. EuroBiotech J. 2019;3:135-142. doi: 10.2478/ebtj-2019-0016.
- Kyriacou MC, Antoniou C, Rouphael Y, Graziani G, Kyratzis A. Mapping the Primary and Secondary Metabolomes of Carob (Ceratonia siliqua L.) Fruit and Its Postharvest Antioxidant Potential at Critical Stages of Ripening. Antioxidants (Basel). 2021 Jan 5;10(1):57. doi: 10.3390/antiox10010057. PMID: 33466561; PMCID: PMC7824902.
- Vekiari AS, Ouzounidou G, Gork G, Ozturk M, Asfi M. Compositional changes of major chemical compounds in Greek carob pods during development. Bull Chem Soc Ethiop. 2012;26:343-351. doi: 10.4314/bcse.v26i3.3.
- Benchikh Y, Paris C, Louaileche H, Charbonnel C, Ghoul M, Chebil L. Comparative characterization of green and ripe carob (Ceratonia siliqua L.): physicochemical attributes and phenolic profile. J Food Sci Technol. 2016;1:85-91.
- El Batal H, Hasib A, Dehbi F, Zaki N, Ouatmane A, Boulli A. Assessment of nutritional composition of Carob pulp (Ceratonia siliqua L.) collected from various locations in Morocco. J Mater Environ Sci. 2016;7:3278-3285.
- Simsek S, Ozcan MM, Al Juhaimi F, ElBabiker E, Ghafoor K. Amino acid and sugar contents of wild and cultivated carob (Ceratonia siliqua) pods collected in different harvest periods. Chem. Nat Compd. 2017;53:1008-1009. doi: 10.1007/s10600-017-2187-9.
- Ben Othmen K, Garcia‑Beltran JM, Elfalleh W, Haddad M, Esteban MA. Phytochemical compounds and biological properties of carob pods (Ceratonia siliqua L.) extracts at different ripening stages. Waste Biomass Valorization. 2021;12:3. doi: 10.1007/s12649-021-01352-x.
- Rtibi K, Marzouki K, Salhi A, Sebai H. Dietary supplementation of carob and whey modulates gut morphology, hemato-biochemical indices, and antioxidant biomarkers in rabbits. J Med Food. 2021 Oct;24(10):1124-1133. doi: 10.1089/jmf.2020.0185. Epub 2021 Mar 18. PMID: 33739870.
- Jambi HA. Effect of roasting process on polyphenols content of Carob powder. Life Sci J. 2015;12:1-5.
- Şahin H, Topuz A, Pischetsrieder M, Özdemir F. Effect of roasting process on phenolic, antioxidant and browning properties of carob powder. Eur Food Res Technol. 2009;230:155-161. doi: 10.1007/s00217-009-1152-7.
- Boublenza I, Lazouni HA, Ghaffari L, Ruiz K, Fabiano-Tixier AS, Chemat F. Influence of roasting on sensory, antioxidant, aromas, and physicochemical properties of carob pod powder (Ceratonia siliqua L.). J Food Qual. 2017. doi: 10.1155/2017/4193672.
- Sadat SS, Mohammadi S, Sazegar G, Fazel A, Ebrahimzadeh A, Mobarhan MG, Beheshti F, Attari SS, Tavallaei S. Effects of carob fruit extract on spermatogenesis, antioxidant status, and apoptosis in adult male mice. Pharm Sci. 2019;25:184-189. doi: 10.15171/PS.2019.28.
- Nemati Z, Dehgani P, Besharati M, Amirdahri S. Dietary carob fruit (Ceratonia siliqua L.) supplementation improves spermatogenesis, semen quality and embryonic death via antioxidant effect in aging broiler breeder roosters. Anim Reprod Sci. 2022 Apr;239:106967. doi: 10.1016/j.anireprosci.2022.106967. Epub 2022 Mar 12. PMID: 35299115.
- Abidar S, Boiangiu RS, Dumitru G, Todirascu-Ciornea E, Amakran A, Cioanca O, Hritcu L, Nhiri M. The Aqueous Extract from Ceratonia siliqua Leaves Protects Against 6-hydroxydopamine in Zebrafish: Understanding the underlying mechanism. Antioxidants (Basel). 2020 Apr 8;9(4):304. doi: 10.3390/antiox9040304. Erratum in: Antioxidants (Basel). 2020 Jun 09;9(6): PMID: 32276477; PMCID: PMC7222174.
- de Falco B, Grauso L, Fiore A, Bonanomi G, Lanzotti V. Metabolomics and chemometrics of seven aromatic plants: Carob, eucalyptus, laurel, mint, myrtle, rosemary and strawberry tree. Phytochem Anal. 2022 Jul;33(5):696-709. doi: 10.1002/pca.3121. Epub 2022 Mar 30. PMID: 35354224.
- Yilmaz E. Effect of dietary carob (Ceratonia siliqua) syrup on blood parameters, gene expression responses and ammonia resistance in tilapia (Oreochromis niloticus). Aquac Res. 2020;51:1903-1912. doi: 10.1111/are.14540.
- Fidan H, Stankov S, Petkova N, Petkova Z, Iliev A, Stoyanova M, Ivanova T, Zhelyazkov N, Ibrahim S, Stoyanova A, Ercisli S. Evaluation of chemical composition, antioxidant potential and functional properties of carob (Ceratonia siliqua L.) seeds. J Food Sci Technol. 2020 Jul;57(7):2404-2413. doi: 10.1007/s13197-020-04274-z. Epub 2020 Jan 31. PMID: 32549590; PMCID: PMC7270304.
- Spoljaric D, Marencic D, Benkovic M, Spoljaric B, Cvitanovic AB, Mrsic G, Vlahovic K, Popovic M, Srecec S, Stolic I. Effect of dietary carob wholemeal on blood parameters in weaned pigs. Vet Arh. 2019;89:351-366. doi: 10.24099/vet.arhiv.0314.
- Chait YA, Gunenc A, Bendali F, Hosseinian F. Simulated gastrointestinal digestion and in vitro colonic fermentation of carob polyphenols: Bioaccessibility and bioactivity. Lwt-Food Sci Technol. 2020;117:108623.doi:10.1016/j.lwt.2019.108623.
- Goulas V, Hadjisolomou A. Dynamic changes in targeted phenolic compounds and antioxidant potency of carob fruit (Ceratonia siliqua L.) products during in vitro digestion, LWT-Food Sci Technol. 2018;101:269-275. doi: 10.1016/j.lwt.2018.11.003.
- Custódio L, Escapa AL, Patarra J, Aligué R, Alberício F, Neng NR, Florencio-Norueira JM, Romano A. Sapwood of carob tree (Ceratonia siliqua L.) as a potential source of bioactive compounds. Rec Nat Prod. 2013;7:225-229.
- Souli A, Sebai H, Chehimi L, Rtibi K, Tounsi H, Boubaker S, Sakly M, El-Benna J, Amri M. Hepatoprotective effect of carob against acute ethanol-induced oxidative stress in rat. Toxicol Ind Health. 2015 Sep;31(9):802-10. doi: 10.1177/0748233713475506. Epub 2013 Jan 30. PMID: 23363576.
- Mekhoukhe A, Kicher H, Ladjouzi A, Medouni-Haroune L, Brahmi F, Medouni-Adrar S, Madani K. Antioxidant activity of carob seeds and chemical composition of their bean gum by- products. J Complement Integr Med. 2018 Aug 15;16(1). doi: 10.1515/jcim-2017-0158. PMID: 30110252.
- de la Fuente-Fernández M, González-Hedström D, Amor S, Tejera-Muñoz A, Fernández N, Monge L, Almodóvar P, Andrés-Delgado L, Santamaría L, Prodanov M, Inarejos-García AM, García-Villalón AL, Granado M. Supplementation with a carob (Ceratonia siliqua L.) fruit extract attenuates the cardiometabolic alterations associated with metabolic syndrome in mice. Antioxidants (Basel). 2020 Apr 21;9(4):339. doi: 10.3390/antiox9040339. PMID: 32326269; PMCID: PMC7222348.
- Chait YA, Gunenc AG, Bendali FB, Hosseinian F. Functional fermented carob milk: Probiotic variability and polyphenolic profile. J Food Bioact. 2021;14:114-125. doi: 10.31665/JFB.2021.14273.
- Ibrahim RM, Abdel-Salam FF, Farahat E. Utilization of carob (Ceratonia siliqua L.) extract as functional ingredient in some confectionery products. Food Nutr Sci. 2020:11:757-772. doi: 10.4236/fns.2020.118054.
- Wahby AF, Mahdy ESM, EL-mezayen HA Salama WH, Ebrahim NM, Abdel-Aty AM, Fahmy AS. Role of hyaluronidase inhibitors in the neutralization of toxicity of Egyptian horned viper Cerastes cerastes venom. J Genet Eng Biotechnol. 2012;10:213-219. doi: 10.1016/j.jgeb.2012.10.001.
- Ruiz-Roso B, Quintela JC, de la Fuente E, Haya J, Pérez-Olleros L. Insoluble carob fiber rich in polyphenols lowers totaland ldl cholesterol in hypercholesterolemic subjects. Plant Foods Hum Nutr. 2010;65:50-56. doi: 10.1007/s11130-009-0153-9.
- Valero-Muñoz M, Martín-Fernández B, Ballesteros S, Lahera V, de las Heras N. Carob pod insoluble fiber exerts anti-atherosclerotic effects in rabbits through sirtuin-1 and peroxisome proliferator-activated receptor-γ coactivator-1α. J Nutr. 2014;144:1378-1384. doi: 10.3945/jn.114.196113.
- Ghazi I, Zefzoufi M, Siniti M, Fdil R, Elattari H. Corrosion inhibition of carob pod pulp (Ceratonia siliqua L.) on carbon steel surface C38 in hydrochloric acid. J Bio Tribocorros. 2022;8:31. doi: 10.1007/s40735-022-00630-y.
- ElAkhdar N, Bawab R, Borjac J. Effect of Ceratonia siliqua and Cucurbita pepo seeds extracts on spermatogenesis in male mice. BAU J Health Wellbeing. 2020;3.
- Higazy M, ELDiffrawy E, Zeitoun M, Shaltout O, El-Yazeed A. Nutrients of carob and seed powders and its application in some food products. J Ad Agric Res. 2018;23:130-147.
- Richane A, Ismail HB, Darej C, Attia K, Moujahed N. Potential of Tunisian carob pulp as feed for ruminants: chemical composition and in vitro assessment. Trop Anim Health Prod. 2022;54:58. doi: 10.1007/s11250-022-03071-4.
- Gomaa A, Willis S, Verghese M, Boateng J. Probiotic fermentation of konjac and carob pods ceratonia siliqua and observation of related antioxidant activity. Am J Food Technol. 2021;16:18-30. doi: 10.3923/ajft.2021.18.30.
- Aydin S, Özdemir Y. Development and characterization of carob flour based functional spread for increasing use as nutritious snack for children. J Food Qual. 2017. doi: 10.1155/2017/5028150.
- Kulcan AA, Zoua Assoumou UT, Aygün M, Kuzu S, Yildiz D, Kaya N, Hacıoğlu A, Karhan M. Impact of carob extract supplementation on chemical and sensory properties of yogurt and ice cream. GIDA J Food. 2021;46:980-991. doi: 10.15237/gida.GD21043.
- El-Kholy A. Impact of carob pods powder on the physical and sensory properties of ice cream. Ismailia J Dairy Sci Technol. 2015;2:7-11.
- Guler-Akin MB, Goncu B, Akin MS. Some properties of probiotic yoghurt ice cream supplemented with carob extract and whey powder. Adv Microbiol. 2016;6:1010-1020. doi: 10.4236/aim.2016.614095.
- Červenka L, Frühbauerová M, Velichová H. Functional properties of muffin as affected by substituing wheat flour with carob powder. Potr Slovak J Food Sci. 2019;13:212-217. doi: 10.5219/1033.
- Pawłowska K, Kuligowski M, Jasińska-Kuligowska I, Kidoń M, Siger A, Rudzińska M, Nowak J. Effect of replacing cocoa powder by carob powder in the muffins on sensory and physicochemical properties. Plant Foods Hum Nutr. 2018;73:196-202. doi: 10.1007/s11130-018-0675-0.
- Aissa A, Chakroun I, Rejeb R, Ayed MH. Effect of partial dietary substitution of Carob (Ceratonia siliqua L.) to barley grains on diet digestibility in growing rabbits. J New Sci. 2021;79:4580-4585.
- Rtibi K, Selmi S, Jabri M-A, Mamadou G, Limas-Nzouzi N, Sebai H, El-Benna J, Marzouki L, Eto B, Amri M. Effects of aqueous extracts from Ceratonia siliqua L. pods on small intestinal motility in rats and jejunal permeability in mice. RSC Adv. 2016;6:44345-44353. doi: 10.1039/c6ra03457h.
- Alkhatib AJ. Therapeutic potential of aqueous extract of carob in treating irritable bowel syndrome. Curr Tr Gatsr & Hepatol. 2019;2:170-171. doi: 10.32474/CTGH.2019.02.000141.
- Al-Olayan EM, El-Khadragy MF, Alajmi RA, Othman MS, Bauomy AA, Ibrahim SR, Abdel Moneim AE. Ceratonia siliqua pod extract ameliorates Schistosoma mansoni-induced liver fibrosis and oxidative stress. BMC Complement Alternat Med. 2016;16:434. doi: 10.1186/s12906-016-1389-1.
- Martić N, Zahorec J, Stilinović N, Andrejić-Višnjić B, Pavlić B, Kladar N, Šoronja-Simović D, Šereš Z, Vujčić M, Horvat O, Rašković A. Hepatoprotective effect of carob pulp flour (Ceratonia siliqua L.) extract obtained by optimized microwave-assisted extraction. Pharmaceutics. 2022;14:657. doi: 10.3390/pharmaceutics14030657.
- Rtibi K, Selmi S, Jabri MA, El-Benna J, Amri M, Marzouki L, Sebai H. Protective Effect of Ceratonia siliqua L. Against a Dextran Sulfate Sodium-Induced Alterations in Liver and Kidney in Rat. J Med Food. 2016;19:882-829. doi: 10.1089/jmf.2016.0020.
- Awwad AM, Salem NM, Abdeen AO. Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. Int J Ind Chem. 2013;4(1):1-6. doi: 10.1186/2228-5547-4-29.
- Javadi F, Yazdi MET, Baghani M, Es-haghi A. Biosynthesis, characterization of cerium oxide nanoparticles using Ceratonia siliqua and evaluation of antioxidant and cytotoxicity activities. Mater Res Express. 2019;6:065408. doi: 10.1088/2053-1591/ab08ff.
- Tamizhdurai P, Sakthinathan S, Chen SM, Shanthi K, Sivasanker S, Sangeetha P. Environmentally friendly synthesis of CeO2 nanoparticles for the catalytic oxidation of benzyl alcohol to benzaldehyde and selective detection of nitrite. Sci Rep. 2017;7:46372. doi: 10.1038/srep46372.
- Ammari M, Othman H, Rtibi K, Sakly M, Abdelmelek H. The Effects of Carob (Ceratonia siliqua L.) on emotional behavior impairment and metabolic disorders induced by estrogen deficiency in rats. J Med Food. 2020;23:961-966. doi: 10.1089/jmf.2019.0187.
- Abdel-Rahman M, Bauomy AA, Salem FEH, Khalifa MA. Carob extract attenuates brain and lung injury in rats exposed to waterpipe smoke, Egypt. J Basic Appl Sci. 2018;5:31-40. doi: 10.1016/j.ejbas.2018.01.004.
- Saratsi K, Hoste H, Voutzourakis N, Tzanidakis N, Stefanakis A, Thamsborg SM, Mueller-Harvey I, Hadjigeorgiou I, Sotiraki S. Feeding of carob (Ceratonia siliqua) to sheep infected with gastrointestinal nematodes reduces faecal egg counts and worm fecundity. Vet Parasitol. 2020;284:109200. doi: 10.1016/j.vetpar.2020.109200.
- Lall N, Kishore N, Momtaz S, Hussein A, Naidoo S, Nqephe M, Crampton B. Extract from Ceratonia siliqua exhibits depigmentation properties. Phytother Res. 2015;29:1729-1736. doi: 10.1002/ptr.5420.
- Flagler M, Osborne R, Mullins L, d'Alessandro B, Tamura M, Ehrman M, Dowdy A, Ellis K. In vivo efficacy of a cosmetic skin care product containing carob extract. J Am Acad Dermatol. 2018;79:165. doi: 10.1016/j.jaad.2018.05.675.
- Azab A. D-Pinitol-active natural product from carob with notable insulin regulation. Nutrients. 2022;14:1453. doi: 10.3390/nu14071453.
- Nachtomi E, Alumot E. Tannins and polyphenols in carob pods (Ceratonia siliqua). J Sci Food Agric. 1963;14:464-468. doi: 10.1002/jsfa.2740140703.
- Joslyn MA, Nishira H, Ito S. Leucoanthocyanins and related phenolic compounds of carob pods (Ceratonia siliqua). J Sci Food Agric. 1968;19:543-550. doi: 10.1002/jsfa.2740190912.
- Vaya J, Mahmood S. Flavonoid content in leaf extracts of the fig (Ficus carica L.), carob (Ceratonia siliqua L.) and pistachio (Pistacia lentiscus L.). BioFactors. 2006;28:169-175. doi: 10.1002/biof.5520280303.
- Ayaz FA, Torun H, Ayaz S, Correia PJ, Alaiz M, Sanz C, Grúz J, Strnad M. Determination of chemical composition of Anatolian carob pod (Ceratonia siliqua L.): sugars, amino and organic acids, minerals and phenolic compounds. J Food Qual. 2007;30:1040-1055. doi: 10.1111/j.1745-4557.2007.00176.x.
- Ozcan MM, Arslan D, Gökçalik H. Some compositional properties and mineral contents of carob (Ceratonia siliqua) fruit, flour and syrup. Int J Food Sci Nutr. 2007;58:652-658. doi: 10.1080/09637480701395549.
- Ayaz FA, Torun H, Glew RH, Bak ZD, Chuang LT, Presley JM, Andrews R. Nutrient content of carob pod (Ceratonia siliqua L.) flour prepared commercially and domestically. Plant Foods Hum Nutr. 2009;64:286-292. doi: 10.1007/s11130-009-0130-3.
- Youssef MKE, El-Manfaloty MM, Ali HM. Assessment of proximate chemical composition, nutritional status, fatty acid composition and phenolic compounds of Carob (Ceratonia siliqua L.). Food Public Health. 2013;3:304-308. doi: 10.5923/j.fph.20130306.06.
- Owis AI, El‑Naggar EMB. Identiication and quantication of the major constituents in Egyptian carob extract by liquid chromatography–electrospray ionization-tandem mass spectrometry. Phcog Mag. 2016;12:S1-S6. doi: 10.4103/0973‑1296.176108.
- Papaefstathiou E, Agapiou A, Giannopoulos S, Kokkinofta R. Nutritional characterization of carobs and traditional carob products. Food Sci Nutr. 2018;6:2151-2161. doi: 10.1002/fsn3.776.
- Biner B, Gubbuk H, Karhan M, Aksu M, Pekmezci M. Sugar profiles of the pods of cultivated and wild types of carob bean (Ceratonia siliqua L.) in Turkey. Food Chem. 2007;100:1453-1455. doi: 10.1016/j.foodchem.2005.11.037.
- Custódio L, Fernandes E, Romano A. Quantification of polyphenols in carob tree (Ceratonia siliqua L.) fruits and leaves in Portuguese cultivars. Acta Hortic. 2009;841:503-506. doi: 10.17660/ActaHortic.2009.841.69.
- El Bouzdoudi B, El Ansari ZN, Mangalagiu I, Mantu D, Badoc A, Lamarti A. Determination of polyphenols content in carob pulp from wild and domesticated Moroccan trees. Am J Plant Sci. 2016;7:1937-1951. doi: 10.4236/ajps.2016.714177.
- El Bouzdoudi B, Ammouri N, Joly N, Martin P, Saïdi R, Nejjar El Ansari Z, Bouras M, Badoc A, Lamarti A. Total polyphenols and gallic acid contents in domesticated aarob (Ceratonia siliqua L.) pods and leaves. Int J Pure App Biosci. 2017;5:22-30. doi: 10.18782/2320-7051.5344.
- Almanasrah M, Roseiro LB, Bogel-Lukasik R, Carvalheiro F, Brazinha C, Crespo J, Kallioinen M, Mänttäri M, Duarte LC. Selective recovery of phenolic compounds and carbohydrates from carob kibbles using water-based extraction. Ind Crops Prod. 2015;70:443-450. doi: 10.1016/j.indcrop.2015.02.051.
- Antoniou C, Kyratzis A, Rouphael Y, Stylianou S, Kyriacou MC. Heat-and ultrasound-assisted aqueous extraction of soluble carbohydrates and phenolics from carob kibbles of variable size and source material. Foods. 2020;9:1364. doi: 10.3390/foods9101364.
- Fidan H, Petkova N, Sapoundzhieva T, Abanoz EI. Carbohydrate content in Bulgarian and Turkish carob pods and their products. CBU Int Conf Proc. 2016;4:796-802. doi: 10.12955/cbup.v4.855.
- Nguyen T, Aparicio M, Saleh MA. Lipid profiling of the carob fruit (Ceratonia siliqua L.) ising GC/LC/QTOF accurate mass spectrometry. Int J Anal Tech. 2017;3:1-6. doi: 10.15226/2577-7831/3/1/00108.
- Rizzo V, Tomaselli F, Gentile A, La Malfa S, Maccarone E. Rheological properties and sugar composition of locust bean gum from different carob varieties (Ceratonia siliqua L.). J Agric Food Chem. 2004;52:7925-7930. doi: 10.1021/jf0494332.
- Bengoechea C, Romero A, Villanueva A, Moreno G, Alaiz M, Millán F, Guerrero A, Puppo MC. Composition and structure of carob (Ceratonia siliqua L.) germ proteins. Food Chem. 2008;107:675-683. doi: 10.1016/j.foodchem.2007.08.069.
- Santonocito D, Granata G, Geraci C, Panico A, Siciliano EA, Raciti G, Puglia C. Carob seeds: Food waste or source of bioactive compounds? Pharmaceutics. 2020;12:1090. doi: 10.3390/pharmaceutics12111090.
- Ben Ayache S, Reis FS, Dias MI, Pereira C, Glamočlija J, Soković M, Saafi EB, Ferreira ICFR, Barros L, Achour L. Chemical characterization of carob seeds (Ceratonia siliqua L.) and use of different extraction techniques to promote its bioactivity. Food Chem. 2021;351:129263. doi: 10.1016/j.foodchem.2021.129263.
- ldahshan OA. Isolation and structure elucidation of phenolic compounds of carob leaves grown in Egypt. Curr Res J Biol Sci. 2011;3:52-55.
- Owen RW, Haubner R, Hull WE, Erben G, Spiegelhalder B, Bartsch H, Haber B. Isolation and structure elucidation of the major individual polyphenols in carob fibre. Food Chem Toxicol. 2003 Dec;41(12):1727-38. doi: 10.1016/s0278-6915(03)00200-x. PMID: 14563398.
- El Bouzdoudi B, Saïdi R, Khalid E, El Mzibri M, Nejjar AZ, El Kbiach ML, Lamarti A. Mineral composition of mature carob (Ceratonia siliqua L.) Pod: A Study. Int J Food Sci Nutr Eng. 2017;7:91-103. doi: 10.5923/j.food.20170704.04.
- Şanlı S, Güneşer O, Kılıçarslan S, Şanlı N. Screening of eighteen polyphenolic compounds in different carob pekmez by green capillary electrophoresis method. SN Appl Sci. 2020;2:1-13. doi: 10.1007/s42452-020-2387-y.
- Christou A, Stavrou IJ, Kapnissi-Christodoulou CP. Continuous and pulsed ultrasound-assisted extraction of carob's antioxidants: Processing parameters optimization and identification of polyphenolic composition. Ultrason Sonochem. 2021 Aug;76:105630. doi: 10.1016/j.ultsonch.2021.105630. Epub 2021 Jun 12. PMID: 34146974; PMCID: PMC8220390.
- Clodoveo ML, Crupi P, Muraglia M, Corbo F. Ultrasound Assisted Extraction of Polyphenols from Ripe Carob Pods (Ceratonia siliqua L.): Combined Designs for Screening and Optimizing the Processing Parameters. Foods. 2022 Jan 21;11(3):284. doi: 10.3390/foods11030284. PMID: 35159436; PMCID: PMC8833885.
- Turhan I. Relationship between sugar profile and D-pinitol content of pods of wild and cultivated types of carob bean (Ceratonia siliqua L.) Int J Food Prop. 2014;17:363-370. doi: 10.1080/10942912.2011.631255.
- van Rijs P , Fogliano V . Roasting carob flour decreases the capacity to bind glycoconjugates of bile acids. Food Funct. 2020 Jul 1;11(7):5924-5932. doi: 10.1039/d0fo01158d. Epub 2020 Jul 8. PMID: 32638775.
- Farag MA, El-Kersh DM. Volatiles profiling in Ceratonia siliqua (Carob bean) from Egypt and in response to roasting as analyzed via solid-phase microextraction coupled to chemometrics. J Adv Res. 2017 Jul;8(4):379-385. doi: 10.1016/j.jare.2017.05.002. Epub 2017 May 10. PMID: 28560053; PMCID: PMC5435580.
- Gohar A, Gedara SR, Baraka HN. New acylated flavonol glycoside from Ceratonia siliqua L. seeds. J Med Plants Res. 2009;3(5):424-428.
- Kashif Shaeen A, Park SY, Choi M, Kim SY, Yoo AY, Park JK. Antioxidant activity of manno-oligosaccharides derived from the hydrolysis of polymannan by extracellular carbohydrase of Bacillus N3. J Mar Biosci Biotechnol. 2018;10:9-17. doi: 10.15433/ksmb.2018.10.1.009.
- Muñoz-Bernal ÓA, Torres-Aguirre GA, Núñez-Gastélum JA, Rosa LA, Rodrigo-García J, Ayala-Zavala JF, Álvarez-Parrilla E. New approach to the interaction between Folin-Ciocalteu reactive and sugars during the quantification of total phenols. TIP Rev Espec Cienc Quím-Biol. 2017;20:23-28. doi: 10.1016/j.recqb.2017.04.003.
- Zayapor MN, Abdullah A, Mustapha WAW. Influence of sugar concentration and sugar type on the polyphenol content and antioxidant activity in spiced syrup preparation. Ital J Food Sci. 2021;33:96-105. doi: 10.15586/ijfs.v33i1.1874.
- Kopjar M, Lončarić A, Mikulinjak M, Šrajbek Ž, Šrajbek M, Pichler A. Evaluation of Antioxidant Interactions of Combined Model Systems of Phenolics in the Presence of Sugars. Nat Prod Commun. 2016 Oct;11(10):1445-1448. PMID: 30549596.
- Lončarić A, Pichler A, Rašić N, Vukoja I, Leventić A, Kopjar M. Influence of phenol and sugar interactions on antioxidant activity of pomegranate juice. Acta Aliment. 2018;47:203-209. doi: 10.1556/066.2018.47.2.9.
- Baranowska M, Koziara Z, Suliborska K, Chrzanowski W, Wormstone M, Namieśnik J, Bartoszek A. Interactions between polyphenolic antioxidants quercetin and naringenin dictate the distinctive redox-related chemical and biological behaviour of their mixtures. Sci Rep. 2021 Jun 10;11(1):12282. doi: 10.1038/s41598-021-89314-0. PMID: 34112813; PMCID: PMC8192515.
- Thayumanavan G, Jeyabalan S, Fuloria S, Sekar M, Ravi M, Selvaraj LK, Bala L, Chidambaram K, Gan SH, Rani NNIM, Begum MY, Subramaniyan V, Sathasivam KV, Meenakshi DU, Fuloria NK. Silibinin and Naringenin against Bisphenol A-Induced Neurotoxicity in Zebrafish Model-Potential Flavonoid Molecules for New Drug Design, Development, and Therapy for Neurological Disorders. Molecules. 2022 Apr 15;27(8):2572. doi: 10.3390/molecules27082572. PMID: 35458770; PMCID: PMC9025613.
- Dabeek WM, Marra MV. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients. 2019 Sep 25;11(10):2288. doi: 10.3390/nu11102288. PMID: 31557798; PMCID: PMC6835347.
- Anand David AV, Arulmoli R, Parasuraman S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn Rev. 2016 Jul-Dec;10(20):84-89. doi: 10.4103/0973-7847.194044. PMID: 28082789; PMCID: PMC5214562.
- Salehi B, Machin L, Monzote L, Sharifi-Rad J, Ezzat SM, Salem MA, Merghany RM, El Mahdy NM, Kılıç CS, Sytar O, Sharifi-Rad M, Sharopov F, Martins N, Martorell M, Cho WC. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega. 2020 May 14;5(20):11849-11872. doi: 10.1021/acsomega.0c01818. PMID: 32478277; PMCID: PMC7254783.
- Deepika, Maurya PK. Health Benefits of Quercetin in Age-Related Diseases. Molecules. 2022 Apr 13;27(8):2498. doi: 10.3390/molecules27082498. PMID: 35458696; PMCID: PMC9032170.
- Nguyen TLA, Bhattacharya D. Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle. Molecules. 2022 Apr 12;27(8):2494. doi: 10.3390/molecules27082494. PMID: 35458691; PMCID: PMC9029217.
- hang M, Swarts SG, Yin L, Liu C, Tian Y, Cao Y, Swarts M, Yang S, Zhang SB, Zhang K, Ju S, Olek DJ, Schwartz L, Keng PC, Howell R, Zhang L. Okunieff P. Antioxidant Properties of Quercetin. Chapter 38 in “Antioxidant properties of quercetin” Oxygen transport to tissue XXXII. Springer Boston MA; 2011. p.283-289. doi: 10.1007/978-1-4419-7756-4_38.
- Sökmen M, Akram Khan M. The antioxidant activity of some curcuminoids and chalcones. Inflammopharmacology. 2016 Jun;24(2-3):81-6. doi: 10.1007/s10787-016-0264-5. Epub 2016 May 17. PMID: 27188988; PMCID: PMC4883448.
- Kyratzis AC, Antoniou C, Papayiannis LC, Graziani G, Rouphael Y, Kyriacou MC. Pod Morphology, Primary and Secondary Metabolite Profiles in Non-grafted and Grafted Carob Germplasm Are Configured by Agro-Environmental Zone, Genotype, and Growing Season. Front Plant Sci. 2021 Jan 13;11:612376. doi: 10.3389/fpls.2020.612376. PMID: 33519870; PMCID: PMC7838365.
- Mokhtar M, Bouamar S, Di Lorenzo A, Temporini C, Daglia M, Riazi A. The Influence of Ripeness on the Phenolic Content, Antioxidant and Antimicrobial Activities of Pumpkins (Cucurbita moschata Duchesne). Molecules. 2021 Jun 13;26(12):3623. doi: 10.3390/molecules26123623. PMID: 34199320; PMCID: PMC8231950.
- Bibi N, Shah MH, Khan N, Al-Hashimi A, Elshikh MS, Iqbal A, Ahmad S, Abbasi AM. Variations in Total Phenolic, Total Flavonoid Contents, and Free Radicals' Scavenging Potential of Onion Varieties Planted under Diverse Environmental Conditions. Plants (Basel). 2022 Mar 31;11(7):950. doi: 10.3390/plants11070950. PMID: 35406930; PMCID: PMC9002954.
- Brglez Mojzer E, Knez Hrnčič M, Škerget M, Knez Ž, Bren U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules. 2016 Jul 11;21(7):901. doi: 10.3390/molecules21070901. PMID: 27409600; PMCID: PMC6273793.
- Bernardo-Gil MG, Roque R, Roseiro LB, Duarte LC, Gírio F, Esteves P. Supercritical extraction of carob kibbles (Ceratonia siliqua L.). J Supercrit Fluids. 2011;59:36-42. doi: 10.1016/j.supflu.2011.08.007.
- Pollini L, Blasi F, Ianni F, Grispoldi L, Moretti S, Di Veroli A, Cossignani L, Cenci-Goga BT. Ultrasound-Assisted Extraction and Characterization of Polyphenols from Apple Pomace, Functional Ingredients for Beef Burger Fortification. Molecules. 2022 Mar 16;27(6):1933. doi: 10.3390/molecules27061933. PMID: 35335297; PMCID: PMC8956034.
- Kimizuka T, Seki N, Yamaguchi G, Akiyama M, Higashi S, Hase K, Kim YG. Amino Acid-Based Diet Prevents Lethal Infectious Diarrhea by Maintaining Body Water Balance in a Murine Citrobacter rodentium Infection Model. Nutrients. 2021 May 31;13(6):1896. doi: 10.3390/nu13061896. PMID: 34072947; PMCID: PMC8227537.
- Karabulut A, Canbola O, Kamalak A. Evaluation of carob Ceratonia siliqua pods as a feed for sheep. Livest Res Rural Dev. 2006;18:104.
- obindram MNE, Bognanno M, Luciano G, Lanza M, Biondi L. Carob pulp inclusion in lamb diets: effect on intake, performance, feeding behaviour and blood metabolites. Anim Prod Sci. 2016;56:850-858. doi: 10.1071/AN14733.
- Georgieva M, Manios Y, Rasheva N, Pancheva R, Dimitrova E, Schaafsma A. Effects of carob-bean gum thickened formulas on infants' reflux and tolerance indices. World J Clin Pediatr. 2016 Feb 8;5(1):118-27. doi: 10.5409/wjcp.v5.i1.118. PMID: 26862511; PMCID: PMC4737686.
- Greffeuille V, Marsset-Baglieri A, Molinari N, Cassan D, Sutra T, Avignon A, Micard V. Enrichment of pasta with faba bean does not impact glycemic or insulin response but can enhance satiety feeling and digestive comfort when dried at very high temperature. Food Funct. 2015 Sep;6(9):2996-3005. doi: 10.1039/c5fo00382b. PMID: 26190153.
- Jung S, Gu S, Lee SH, Jeong Y. Effect of roasting degree on the antioxidant properties of espresso and drip coffee extracted from Coffea arabica cv. Java Appl Sci. 2021;11:7025. doi:10.3390/app11157025.
- Aitken RJ. The changing tide of human fertility. Hum Reprod. 2022 Apr 1;37(4):629-638. doi: 10.1093/humrep/deac011. PMID: 35079808; PMCID: PMC8977063.
- Wojsiat J, Korczyński J, Borowiecka M, Żbikowska HM. The role of oxidative stress in female infertility and in vitro fertilization. Postepy Hig Med Dosw (Online). 2017 May 9;71(0):359-366. doi: 10.5604/01.3001.0010.3820. PMID: 28513460.
- Bisht S, Faiq M, Tolahunase M, Dada R. Oxidative stress and male infertility. Nat Rev Urol. 2017;14:470-485. doi: 10.1038/nrurol.2017.69.
- Ferramosca A, Lorenzetti S, Di Giacomo M, Lunetti P, Murrieri F, Capobianco L, Dolce V, Coppola L, Zara V. Modulation of Human Sperm Mitochondrial Respiration Efficiency by Plant Polyphenols. Antioxidants (Basel). 2021 Feb 2;10(2):217. doi: 10.3390/antiox10020217. PMID: 33540578; PMCID: PMC7912874.
- Hadi MY, Hameed IH, Ibraheam IA. Ceratonia siliqua: characterization pharmaceutical products and analysis of bioactive compounds: A review. Res J Pharm Technol. 2017;10:3585-3589. doi: 10.5958/0974-360X.2017.00649.7.
- Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, Song X, Ren Y, Shan PF. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. 2020 Sep 8;10(1):14790. doi: 10.1038/s41598-020-71908-9. PMID: 32901098; PMCID: PMC7478957.
- Williams R, Karuranga S, Malanda B, Saeedi P, Basit A, Besançon S, Bommer C, Esteghamati A, Ogurtsova K, Zhang P, Colagiuri S. Global and regional estimates and projections of diabetes-related health expenditure: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2020 Apr;162:108072. doi: 10.1016/j.diabres.2020.108072. Epub 2020 Feb 13. PMID: 32061820.
- Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm J. 2016 Sep;24(5):547-553. doi: 10.1016/j.jsps.2015.03.013. Epub 2015 Mar 21. PMID: 27752226; PMCID: PMC5059829.
- Chiurchiù V, Maccarrone M. Chronic inflammatory disorders and their redox control: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2011 Nov 1;15(9):2605-41. doi: 10.1089/ars.2010.3547. Epub 2011 Jun 6. PMID: 21391902.
- Li Q, Xing B. A Phytosterol-Enriched Spread Improves Lipid Profile and Insulin Resistance of Women with Gestational Diabetes Mellitus: A Randomized, Placebo-Controlled Double-Blind Clinical Trial. Diabetes Technol Ther. 2016 Aug;18(8):499-504. doi: 10.1089/dia.2016.0103. Epub 2016 May 11. PMID: 27512827.
- Gao J, Hu J, Hu D, Yang X. A Role of gallic acid in oxidative damage diseases: A comprehensive review. Nat Prod Commun. 2019;14:1-9. doi: 10.1177/1934578X19874174.
- Xiang YF, Ju HQ, Li S, Zhang YJ, Yang CR, Wang YF. Effects of 1,2,4,6-tetra-O-galloyl-β-D-glucose from P. emblica on HBsAg and HBeAg secretion in HepG2.2.15 cell culture. Virol Sin. 2010 Oct;25(5):375-80. doi: 10.1007/s12250-010-3144-y. Epub 2010 Oct 8. PMID: 20960184; PMCID: PMC7090425.
- Chun JH, Henckel MM, Knaub LA, Hull SE, Pott GB, Walker LA, Reusch JE, Keller AC. (-)-Epicatechin Improves Vasoreactivity and Mitochondrial Respiration in Thermoneutral-Housed Wistar Rat Vasculature. Nutrients. 2022 Mar 5;14(5):1097. doi: 10.3390/nu14051097. PMID: 35268072; PMCID: PMC8912787.
- Fusi J, Bianchi S, Daniele S, Pellegrini S, Martini C, Galetta F, Giovannini L, Franzoni F. An in vitro comparative study of the antioxidant activity and SIRT1 modulation of natural compounds. Biomed Pharmacother. 2018 May;101:805-819. doi: 10.1016/j.biopha.2018.03.006. Epub 2018 Mar 22. PMID: 29525677.
- Zhang F, Feng J, Zhang J, Kang X, Qian D. Quercetin modulates AMPK/SIRT1/NF-κB signaling to inhibit inflammatory/oxidative stress responses in diabetic high fat diet-induced atherosclerosis in the rat carotid artery. Exp Ther Med. 2020 Dec;20(6):280. doi: 10.3892/etm.2020.9410. Epub 2020 Oct 27. PMID: 33200005; PMCID: PMC7664594.
- Wang L, Wu H, Yang F, Dong W. The Protective Effects of Myricetin against Cardiovascular Disease. J Nutr Sci Vitaminol (Tokyo). 2019;65(6):470-476. doi: 10.3177/jnsv.65.470. PMID: 31902859.
- Akbulut S, Bayramoglu MM. Reflections of socio-economic and demographic structure of urban and rural on the use of medicinal and aromatic plants: The sample of trabzon province, stud. Ethno-Med. 2014;8:(1)89-100. doi: 10.1080/09735070.2014.11886477.
- Akbulut S. Differences in the traditional use of wild plants between rural and urban areas: The sample of adana, stud. Ethno-Med. 2015;9:141-150. doi: 10.1080/09735070.2015.11905430.
- Smith BM, Bean SR, Herald TJ, Aramouni FM. Effect of HPMC on the quality of wheat-free bread made from carob germ flour-starch mixtures. J Food Sci. 2012 Jun;77(6):C684-9. doi: 10.1111/j.1750-3841.2012.02739.x. PMID: 22671523.
- Dimassi O, Khalife R, Akiki R, Rached M. Effect of different soaking media on the efficiency of carob molasses production. Int J Environ Agric Biotechnol. 2019:4:829-834. doi: 10.22161/ijeab/4.3.33.
- Tounsi L, Kchaou H, Chaker F, Bredai S, Kechaou N. Effect of adding carob molasses on physical and nutritional quality parameters of sesame paste. J Food Sci Technol. 2019 Mar;56(3):1502-1509. doi: 10.1007/s13197-019-03640-w. Epub 2019 Feb 19. PMID: 30956330; PMCID: PMC6423243.
- Johnson W Jr, Heldreth B, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler DC, Marks JG Jr, Shank RC, Slaga TJ, Snyder PW, Andersen FA. Safety Assessment of Galactomannans as Used in Cosmetics. Int J Toxicol. 2015 Jul-Aug;34(1 Suppl):35S-65S. doi: 10.1177/1091581815586798. PMID: 26227890.
- Cariñanos P, Delgado-Capel M, Maradiaga-Marín MF, Benítez G. Considerations on the allergy-risks related to the consumption of fruits from urban trees in Mediterranean cities. Urban For Urban Green. 2019;45:126303. doi: 10.1016/j.ufug.2019.03.007.
- Cariñanos P, Marinangeli F. An updated proposal of the Potential Allergenicity of 150 ornamental Trees and shrubs in Mediterranean Cities. Urban For Urban Green. 2021;63:127218. doi: 10.1016/j.ufug.2021.127218.
- Frühbauerová M, Červenka L, Hájek T, Pouzar M, Palarčík J. Bioaccessibility of phenolics from carob (Ceratonia siliqua L.) pod powder prepared by cryogenic and vibratory grinding. Food Chem. 2022 May 30;377:131968. doi: 10.1016/j.foodchem.2021.131968. Epub 2021 Dec 29. PMID: 34995960.
- Vilas-Boas AM, Brassesco ME, Quintino AC, Vieira MC, Brandão TRS, Silva CLM, Azevedo M, Pintado M. Particle Size Effect of Integral Carob Flour on Bioaccessibility of Bioactive Compounds during Simulated Gastrointestinal Digestion. Foods. 2022 Apr 27;11(9):1272. doi: 10.3390/foods11091272. PMID: 35563995; PMCID: PMC9101685.
- Medina-Vera D, Navarro JA, Rivera P, Rosell-Valle C, Gutiérrez-Adán A, Sanjuan C, López-Gambero AJ, Tovar R, Suárez J, Pavón FJ, Baixeras E, Decara J, Rodríguez de Fonseca F. d-Pinitol promotes tau dephosphorylation through a cyclin-dependent kinase 5 regulation mechanism: A new potential approach for tauopathies? Br J Pharmacol. 2022 Jun 27. doi: 10.1111/bph.15907. Epub ahead of print. PMID: 35760415.
- Zheng W, Lee SA. Well-done meat intake, heterocyclic amine exposure, and cancer risk. Nutr Cancer. 2009;61(4):437-46. doi: 10.1080/01635580802710741. PMID: 19838915; PMCID: PMC2769029.
- Nadeem HR, Akhtar S, Ismail T, Sestili P, Lorenzo JM, Ranjha MMAN, Jooste L, Hano C, Aadil RM. Heterocyclic Aromatic Amines in Meat: Formation, Isolation, Risk Assessment, and Inhibitory Effect of Plant Extracts. Foods. 2021 Jun 24;10(7):1466. doi: 10.3390/foods10071466. PMID: 34202792; PMCID: PMC8307633.
- Erdogan B, Ozdestan‐Ocak O. Inhibitory effects of carob and propolis extracts on the formation of heterocyclic aromatic amines in beef meatballs cooked with different methods. J Food Process Preserv. 2022;e16623. doi: 10.1111/jfpp.16623.
- Ozdemir Y, Ozbek C, Ilhan S. Ice cream cone enriched with carob molasses pulp. J Food Meas Charact. 2022;1-10. doi: 10.1007/s11694-022-01489-w.
- Toufeili I, Itani M, Zeidan M, Al Yamani O, Kharroubi S. Nutritional and Functional Potential of Carob Syrup Versus Date and Maple Syrups. Food Technol Biotechnol. 2022 Jun;60(2):266-278. doi: 10.17113/ftb.60.02.22.7419. PMID: 35910270; PMCID: PMC9295627.
- Clodoveo ML, Crupi P, Muraglia M, Corbo F. Processing of carob kernels to syrup by ultrasound-assisted extraction. Processes. 2022;10:983. doi: 10.3390/pr10050983.
- Peng ZT, Xia YJ, Yashiro T, Gao X, Dong TT, Tsim KW, Wang HY. Novel phenylpropanoids and isoflavone glycoside are isolated and identified from the carob pods (Ceratonia siliqua L.). Nat Prod Res. 2022 May 14:1-7. doi: 10.1080/14786419.2022.2076230. Epub ahead of print. PMID: 35574610.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.