Medicine Group . 2022 August 12;3(8):908-914. doi: 10.37871/jbres1531.
Hyperhaemolysis Syndrome: A Review of Cases
Muhammad Amirfarhan Zainudin and Asral Wirda Ahmad Asnawi*
- Hyperhaemolysis
- Alloantibody
- Autoantibody
- Transfusion
- Delayed
Abstract
Hyperhaemolysis is characterized by a haemolytic transfusion reaction that leads to life-threatening anaemia. It is usually suspected when there is a drop in haemoglobin level at least 24 hours after receiving allogeneic red cell transfusion. Decreasing haematocrit level, failure to respond to repeated RBC transfusions, markedly elevated LDH, absent haptoglobin, and gross haemoglobinuria are all suggestive of a haemolytic process. The aim of this review is to summarize and evaluate the cases from published literature to determine the features, management and outcome of reported cases of hyperhaemolysis syndrome.
Introduction
Hyperhaemolysis syndrome is a rare life-threatening delayed complication of blood transfusion [1]. This hyperhaemolytic reaction is characterized by the destruction of both transfused and autologous erythrocytes. This clinical entity is defined by an abrupt onset of accelerated intravascular haemolysis, evidenced by a dramatic fall within the post-transfusion haemoglobin below the pre-transfusion level, markedly elevated Lactate Dehydrogenase (LDH), indirect hyperbilirubinemia and haemoglobinuria. Of note reticulocytopenia may be additionally present, in contrast to other types of haemolytic reactions, where reticulocytosis is sometimes observed. Hyperhaemolysis most typically occurs in patients with a red blood cell disease requiring repeated red blood cell transfusion, while occasionally it has been reported in patients with thalassemia and in individuals without underlying hematologic diseases [2]. This immune haemolytic reaction is in part due to the formation of alloantibodies as a response to previous exposure to foreign red blood cell antigen. The level of alloantibodies may deteriorate with time in the circulation however, this may cause a rapid response upon re-exposure to specific red cell antigen via an amnestic immune response. In certain patients, this may also induce the concomitant formation of autoantibodies that attach to the red blood cells causing a severe form of immune haemolysis. The clinical features of hyperhaemolysis and its management have not been well defined. Recently, there has also been an increase in awareness of this serious delayed haemolytic transfusion reaction due to more haemovigilance amongst clinicians and the transfusion unit. A series of theories have been proposed to clarify the haemolytic destruction of both allogeneic and autologous erythrocytes in this haemolytic reaction, including excessive complete complement activation, phagocytosis by hyperactivated macrophages of erythrocytes covered with C3b in a setting of incomplete complement activation, and HLA antigen-alloantibody reactions. Additionally, accumulating evidence suggests a compounded role between autoimmunization and alloimmunisation, indicating a non-negligible risk of autoimmunisation and AIHA development because of alloantibody formation following an allogeneic erythrocyte transfusion. This phenomenon defines, among others, a condition termed bystander immune haemolysis. The proposed rationale for its occurrence is that alloantibody binding to transfused erythrocytes triggers conformational changes in antigenic epitopes that subsequently stimulate the unregulated production of an autoantibody and/or generates an overwhelming amount of activated complement components resulting in direct red blood cell lysis. Therefore, the general aim of this review was to investigate the different cases and reports relating to hyperhaemolytic transfusion reaction. The role of IVIG, steroids, other therapies as well as the patient outcome are also discussed.
Methodology
Search strategy
For identifying related research publications, we used PubMed, Scopus and Ovid-MEDLINE with unrestricted date. The following terms were used for Medical Subject Heading (MeSH); hyperhaemolysis and antibody. The search strategy involved a combination (“AND”) of the following three sets of keywords: 1) Hyperhaemolysis OR Hyperhemolysis OR Hyperhemolytic OR Hyperhaemolytic 2) Antibody OR Antibodies OR Isoantibody OR Alloantibody OR Isoantibody 3) Haemolysis OR Hemolysis OR Haemolytic OR Hemolytic. Synonym keywords were created from MeSH term in Cochrane library.
Inclusion criteria: All original articles and case reports with abstracts that reported the outcome of patient having hyperhaemolysis syndrome undergoing treatment were included. The study subject included all studies with discussion on hyperhaemolysis syndrome associated with various types of red cell antibodies. This analysis only included manuscripts written in English, due to limited sources.
Exclusion criteria: Publications without primary data, such as letters to editors, editorials, conference proceedings and narrative review were excluded. This review focuses on the various case reports and patient management in different countries and regions. Therefore, studies that did not provide information regarding their patients, immunohaematological results, management outline and patient outcome were excluded.
Screening of articles for eligibility
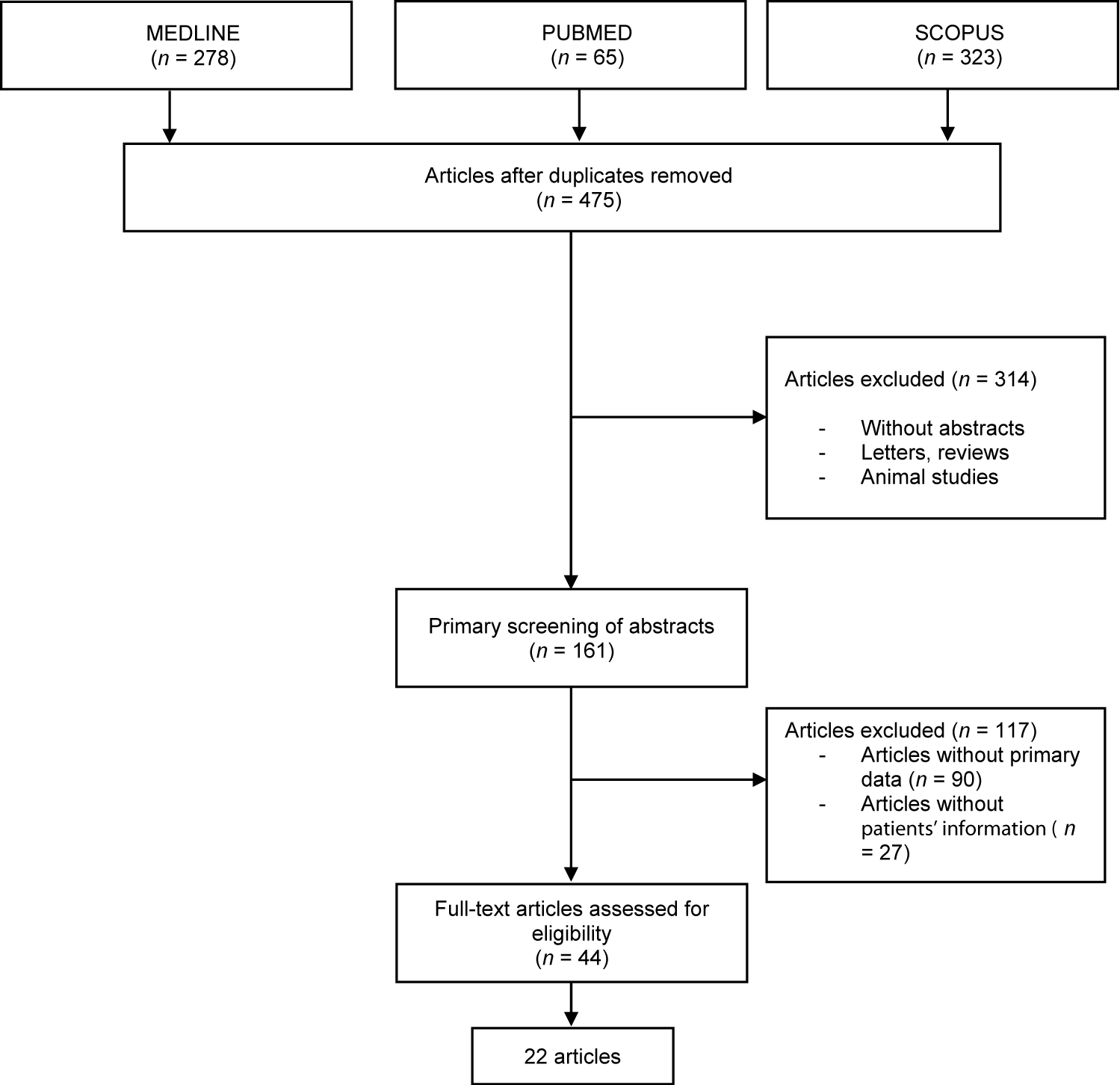
Article selection was performed in three steps. Articles with titles that did not meet the inclusion criteria was rejected in the first step. The second phase was screening the abstract of the articles. Abstracts that met the criteria of keywords were included. In the third and final step, the complete text of the articles was thoroughly read and evaluated to exclude articles which did not meet the criteria of inclusion. All systematic reviews or meta-analysis were excluded and all duplicates were removed. All authors were mentioned in the process of collection and extraction of data. Any differences in opinion among authors were resolved through discussion. For data collection standardisation, all data extraction was performed independently using a method of data collection. Records on reasons for rejection was kept (Figure 1).
Results
Based on the articles included a total of 22 articles that met the inclusion criteria were reviewed. The number of studies originating from each continent was as follows: ten from America, eleven from Europe and one from Asia. All were individual case reports except for one which reported a series of nine patients. The total number of individual cases was thirty.
Patient demographic
The youngest reported case was 13 months old while the oldest was 67 years of age. Of these cases eleven were known to have sickle cell disease, twelve had severe forms thalassaemia while seven did not have any history of haemoglobinopathy. Since haemaglobinopathy is largely an ethnic-based disease, although a large number of reports did not mention the patient’s ethnicity, we found that a majority were of African-decent. The reason for admission or the presenting complaints varied, however all cases had a recent history of allogeneic red cell transfusion. The commonality of all cases was the drop in haemoglobin level at least 24 hours after the transfusion was completed with a median drop of 1.9 g/dl (range: 0.3 - 4.0) (Table 1).
| Table 1: Summary of case reports and patient characteristics. | ||||||||
| Ref no. | Country | Patient (age, sex, ethnicity) | Underlying disease | Presenting complaint | Red cell antibodies detected | Haemoglobin level (g/dl) | ||
| Alloantibody | Autoantibody | Pre-transfusion | Post-transfusion | |||||
| [3] | UK | 36 years Female Chinese |
β-thalassemia at 31 week of gestation |
Routine blood test | Anti-Jka Anti-P1 |
Autoanti-IgG | 7.7 | 7.4 |
| [4] | USA | 18 years Female Caucasian |
No known underlying disease | Thigh pain, dark urine, haematuria within 72 hours receiving blood transfusion | None detected | None detected | 11.2 | 8.8 |
| [5] | Malaysia | 32 years Female Nigerian |
Sickle cell disease Primigravida in first trimester |
Shortness of breath, lower abdominal pain and vaginal bleeding 2 days after receiving blood transfusion |
Anti-Fya Anti-Jkb Anti-E |
None detected | 10.0 | 6.0 |
| [6] | USA | 35 years Female African- American |
Sickle cell disease | Severe fatigue, worsening jaundice, and total body pain 2 weeks post-transfusion, LDH 5000 IU/L | anti-C, anti-E, anti-K, anti-S, anti-Fya, anti-Jka, and anti-Sda |
anti-IH | 7.4 | 3.8 |
| [7] | Spain | 13 months Male Guinea |
Sickle cell disease | Fever and vomiting | None detected | None detected | 7.0 | 6.0 |
| [8] | USA | 30 years Female Caucasian |
No known underlying disease | Vaso-occlusive crisis | Anti-Jsa Anti-Fya Anti C |
Autoanti-IgG | 7.9 | 6.2 |
| [9] | USA | 55 years Male Caucasian |
No known underlying disease | Multiple fracture after motor accident Severe dyspnea and fatigue after transfusion |
anti-Jka, | Autoanti-IgG and complement |
Normal, not mentioned | 5.4 |
| [10] | Ireland | 3 years Male Ethnicity not mentioned |
Sickle cell disease | Transfusion started at 3 years 6 months, but stopped at 3 years 10 months as hyperhaemolysis suspected | Not detected | Autoantibodies were suspected | 7.4 | 5.7 |
| [11] | France | 30 years Female African |
Sickle cell disease admitted at 23 weeks of gestation |
Multifocal vasoocclusive crisis | anti-E, anti-C, anti-S, anti-Jkb, anti-Fya anti-Fy3, Anti-M |
Anti-IH | 9.5 | 5.5 |
| [12] | UK | 36 years Female Ethnicity not mentioned |
Sickle cell disease | Symptomatic aneamia | anti-e anti-Fya anti-C anti-K |
Not detected | 5.5 | 5.1 |
| [13] | USA | 28 years Female Ethnicity not mentioned |
Sickle cell disease | Vasoocclusive crisis | anti-C anti-Jkb anti-e anti-K |
Autoanti-IgG | 5.8 | 3.6 |
| [14] | UK | 30 years Male Ethnicity not mentioned |
Sickle cell disease | Vasoocclusive crisis | Anti-Leb Anti-M |
Not detected | 8.5 | 6.9 |
| [15] | Greece | 30 years Female Greek |
Thalassemia | Scheduled for RBC transfusion | Anti-C Anti-P1 |
Not detected | 7.0 | 5.0 |
| [16] | Spain | 49 years Male Caucasian |
Caroli disease | Septic shock | Anti-E | Not detected | 7.7 | 6.5 |
| [17] | UK | 32 years Female Nigerian |
No known underlying illness | Admitted for exchange transfusion prior to total hip replacement | Anti-E Anti-C Anti-Jsb |
Autoanti-IgG | 7.2 | 5.4 |
| [18] | UK | 65 years Female Ethnicity not mentioned |
HbH disease | Breathlessness | Anti-E Anti-Jkb |
Not performed | 7.7 | 5.1 |
| [19] | UK | 9 patients | Sickle cell anaemia (6) Hb Sickle/C (2) HbH dsiease (1) |
Fever (9), sickle pain (8) and dark-coloured urine (4) | Anti-C Anti-Fya Anti-M Anti-S Anti-E Anti-Cw |
Autoanti-e | 8.1 | 5.6 |
| [20] | USA | 67 years Female African- American |
Anaemia of chronic disease | Symptomatic anemia | Anti-K Anti-Fya Anti-Jka |
Autoanti-IgG | 8.0 | 7.5 |
| [21] | USA | 19 years Male African- American |
Sickle cell disease | Back, arm and leg pain | Not detected | Not detected | 8.1 | 6.2 |
| [22] | USA | 33 years Male Ethnicity not mentioned |
Sickle cell disease | Nausea, vomiting and blood in urine | Anti-C Anti-E Anti-K Anti-Jkb |
Autoanti-IgG | 5.5 | 4.9 |
| [23] | Brazil | 18 years Female Ethnicity not mentioned |
Sickle cell disease | Severe anemia and signs of haemolysis | Not detected | Not detected | 7.2 | 4.0 |
| [24] | USA | 58 years Female Ethnicity not mentioned |
HIV, hepatitis C and chronic obstructive airway disease | Dyspnea and cough | Anti-S Anti-Fyb Anti-E Anti-C |
Autoanti-IgG | 10.0 | 6.0 |
Investigation
There were several alloantibody specificities found from immunohaematological investigation. The alloantibodies and autoantibodies detected are summarised in table 2. Most cases reported presence of more than one clinically significant alloantibody. Alloantibodies included mainly IgG-type antibodies; anti-Jka, anti-Jkb, anti-Fy3, anti-Fya, anti-Fyb, anti-E, anti-C, anti-K, anti-S, and a small proportion of IgM-types; anti-M and anti-P1. The commonest type found was anti-Jkb, anti-C and anti-E. Warm auto-IgG antibodies were documented in a majority of cases.
| Table 2: Summary of alloantibodies and autoantibodies detected in reported cases of hyperhaemolysis. | |||||
| Alloantibodies | Autoantibodies | ||||
| Blood group system | Number of cases | Ref no. | Type | Number of cases | Ref no. |
| RH (ISBT No.004) | 13 | [5,6,8,11-13,15-19,22,24] | Auto-IgG antibodies | 10 | [3,8,9,10,13,17,19,20,22,24] |
| Kidd (ISBT No.009) | 9 | [3,5,6,9,11,13,18,20,22] | |||
| Duffy (ISBT No.008) | 8 | [5,6,8,11,12,19,20,24] | |||
| Kell (ISBT No.006) | 7 | [6,8,12,13,17,20,22] | |||
| MNS (ISBT No.002) | 6 | [6,11,14,19,24] | |||
| P1PK (ISBT No.003) | 2 | [3,15] | |||
| Lewis (ISBT No.007) | 1 | [14] | |||
| Others: Sid (901 series) |
1 | [6] | Others: Anti-IH |
2 | [6,11] |
| Not detected | 5 | [4,7,10,21,23] | Not detected | 9 | [4,5,7,12,14-16,21,23] |
| Not mentioned | 1 | [3] | |||
| ISBT: International Society of Blood Transfusion; 901 series; High-incidence antigens | |||||
Management of hyperhaemolysis
In most cases, further red blood cell transfusion was avoided. However, in four cases exchange transfusion was opted to alleviate symptoms and reduce the effects of haemolysis. Other forms of treatment included instituting steroid therapy, intravenous immunoglobulin and in isolated cases immunotherapy such as Rituximab, an anti-CD20 monoclonal antibody; Eculizumab, a complement C5 inhibitor and Tocilizumab, an Interleukin (IL)-6 receptor inhibitor. Iron supplementation was also opted in some reported cases (Table 3).
| Table 3: Management of cases and patient outcome. | |||
| Ref no. | Management | Outcome | |
| Exchange transfusion | Other therapy | ||
| [3] | Not given | None given | Patient recovered |
| [4] | Not given | IVIG, Steroids | Patient recovered |
| [5] | Red cell exchange with compatible packed cell and reconstituted plasma | None given | Patient died |
| [6] | Not given | Eculizumab | Patient recovered |
| [7] | Not given | IVIG, Methylprednisolone | Patient recovered |
| [8] | Not given | Erythropoietin | Patient recovered |
| [9] | Not given | Iron supplementation | Patient recovered |
| [10] | Not given | Erythropoietin | Patient recovered |
| [11] | Exchange transfusion | Erythropoietin | Patient died |
| [12] | Not given | Methylprednisolone, IVIG Tocilizumab |
Patient recovered |
| [13] | Not given | Eculizumab, Iron supplementation, Hemopure | Patient recovered |
| [14] | Not given | Rituximab, Methylprednisolone | Patient recovered |
| [15] | Not given | Methylprednisolone, IVIG | Patient recovered |
| [16] | Not given | Rituximab, IVIG | Patient recovered |
| [17] | Not given | IVIG Corticosteroid |
Patient recovered |
| [18] | Not given | IVIG, Methylprednisolone, Iron supplementation, Folic acid supplementation | Patient recovered |
| [19] | Exchange transfusion | IVIG, Methylprednisolone | All patients recovered |
| [20] | Not given | Steroids, IVIG, Recombinant erythropoietin | Patient recovered |
| [21] | Exchange transfusion | Methylprednisolone, Subcutaneous darbopoeitin | Patient recovered |
| [22] | Not given | Prednisone | Patient recovered |
| [23] | Not given | IVIG methylprednisolone, Folic acid, Erythropoietin | Patient recovered |
| [24] | Not given | Prednisone, Erythropoietin | Patient recovered |
| IVIG: Intravenous Immunoglobulin | |||
Patient outcome
Most patient survived the hyperhaemolysis episode after they showed good response to their treatment where the haemoglobin level started to normalise again. However, deaths have been reported.
Discussion
Hyperhaemolysis Syndrome (HHS) is considered a potentially catastrophic illness that occurs when the body produces an immune haemolytic reaction [24]. It is described as acute intravascular lysis triggered by the formation of specific alloantibodies that destroy the foreign red cells. As such, it is a complication that would be more susceptible to patients who have acquired these alloantibodies in previous exposure to foreign red cells especially in patients who are transfusion dependent. However, there is growing evidence that hyperhaemolysis is not restricted only to patients with haemoglobinapathies [16].
Hyperhaemolysis is a subtype of Delayed Haemolytic Transfusion Reaction (DHTR) [18]. It is commonly present in patients with underlying sickle cell disease, but it can also be seen in patients with other underlying haemoglobinapathies. Hyperhaemolysis can be differentiated with the other subtype of DHTR by some features, although it is not specific and obvious. The most common and primary feature in hyperhaemolysis syndrome would be a reduction in the RBC level below the level before transfusion occurs. Other features would be the presence of serologic findings of RBC alloantibodies during lab investigation. It is well-known that sickle cell RBCs are more susceptible to lysis. If laboratory findings suggest the absence of alloantibodies, it was suspected that the development of platelet antibodies resulting from the increase in the number of red blood cells during transfusion could lead to the lytic reaction [18]. Besides that, some proportion of it was noted to be due to antibodies to human leukocyte antigen. On top of that, macrophage activation during hyperhaemolysis is enhanced especially for patients with underlying SCD. In this proposed mechanism activated macrophages displayed a unique feature in which they will bind to a specific red blood cells surface glycoprotein via the macrophage adhesion molecules. For example, if sickled red blood cells express a specific surface glycoprotein that enables the macrophage to bind to them, then haemolysis will occur. This response can be triggered even after the antigen has been cleared from the circulation. HHS, which is triggered by the alloantibody response, also shares some common features with the syndrome of Haemophagocytic Lymphohistiocytosis (HLH) when it also causes uncontrolled haemolysis reaction. This condition could only be triggered by the inappropriate activation of the macrophage, which is also a characteristic seen in HLH. This inappropriate activation of macrophage might explain the underlying clinical practise of corticosteroid to treat hyperhaemolysis syndrome. Another proposed mechanism was a defect in the complement regulation pathway resulting to an immune-mediated haemolysis. This will lead the red blood cell, particularly the sickle cells to be more vulnerable to lysis by the immune system. The subsequent antibodies form against non–RBC-transfused antigens, results in the formation of complexes that interact with the red blood cells. Finally, the mechanism behind the reduction in the RBC level was transfusion-induced suppression of erythropoiesis, the process of making new RBC. This was because an inappropriate reduction in reticulocyte count was a common presentation. However, the usual response of this syndrome to steroid could account for the suppression of erythropoiesis process alone but is unlikely to be the reason behind the entire process.
The diagnostic criteria for HHS in SCD patients should consist of three criteria [3]. Firstly, there should be an unexplained reduction in haemoglobin level after blood transfusion to a level lower than the pre-transfusion level. Secondly, haemoglobinuria associated with an increase in LDL level occurring or worsening after blood transfusion event. Lastly, the symptoms started to occur within 21 days post blood transfusion. Decreasing haematocrit level, failure to respond to repeated RBC transfusions, markedly elevated LDH, absent haptoglobin, and gross hemoglobinuria all suggestive of a currently occurring haemolytic process. In addition, it is crucial to differentiate this condition from other diseases that could cause severe haemolytic anaemia. Among them are Glucose-6-Phosphatase Deficiency Dehydrogenase (G6PD) deficiency, Drug-Induced Immune Haemolytic Anaemia (DIIHA), paroxysmal cold haemoglobinuria (PCH), paroxysmal nocturnal haemoglobinuria (PNH) and Immune Haemolytic Transfusion Reaction (IHTR).
Most of the time, patients with hyperhaemolysis are treated with IVIG and methylprednisolone for three consecutive days [4,7,12,15,18,19,23]. Strong adhesion of RBC to the macrophage has led to the role of IVIG and steroid as a choice of treatment. By administering them in this situation, the sickle cell and reticulocyte adhesion to the macrophage can be prevented. Another supplementation can also be given on case-to-case basis. For example, in case of reduction vitamin B12 level, folic acid can be prescribed [18]. Additional blood transfusion should be avoided at all costs and if indicated, should be commenced and covered by steroids and IVIG. The role of exchange transfusion was believed to be beneficial as it removes the antibody-coated RBC and circulating bilirubin. Hence, replacing them with RBCs compatible with the patient’s serum and providing albumin with new bilirubin binding sites [5,11,19,21]. The role of specific immunotherapy has also been described in some cases. Rituximab, a specific CD20 inhibitor acts on the body’s immune system by depleting B cells [14,16]. It reduces the haemolytic reaction by decreasing the production of alloantibodies. Hence, it will prevent antibody-mediated red blood cell destruction [8]. Although hyperhemolysis is not well studied, it has been suggested that its pathogenesis is mostly triggered by an immunological response. The risk of recurrence for hyperhaemolysis syndrome was not well understood as there were only a few cases report about it. Despite that, it is essential that challenging the patient in the future with further blood transfusion should be avoided. Unless it is unavoidable in certain clinical condition, it should proceed with extreme care. Eculizumab, a long-acting monoclonal antibody, had a role in treatment of hyperhaemolysis syndrome as it inhibits the complement activation [7]. This was done by inhibiting the cleavage of C5 into C5a and C5b and then further inhibiting the deployment of the terminal complement system, which includes the formation of Membrane Attack Complex (MAC) [25]. In PNH patients, eculizumab profoundly inhibits haemolysis. On the other hand, the success in treating HHS with tocilizumab, an interleukin-6 inhibiting monoclonal antibody, also provides evidence that supports the role for macrophage activation in the destruction of RBCs in antibody-negative HHS [12].
Conclusion
A high index of suspicion is key to successfully managing HHS. It is crucial to identify patients at risk of this complication for early immunohaematological workup that will allow for the prompt arrangement for genotyped red blood cells and other arrangements to increase the safety of blood transfusion that include withholding further blood transfusion, commencement of immunosuppressive therapy and supplementation such as erythropoietin. Further research tis necessary to determine individuals who are more likely to be susceptible to this fatal complication.
References
- Menakuru SR, Priscu A, Dhillon V, Salih A. Acute Hyperhemolysis Syndrome in a Patient with Known Sickle Cell Anemia Refractory to Steroids and IVIG Treated with Tocilizumab and Erythropoietin: A Case Report and Review of Literature. Hematology Reports. 2022;14(3):235-39
- Grainger JD, Makar Y, McManus A, Wynn R. Refractory hyperhaemolysis in a patient with beta-thalassaemia major. Transfus Med. 2001 Feb;11(1):55-7. doi: 10.1046/j.1365-3148.2001.00278.x. PMID: 11328573.
- Wu Y, Ji Y, Dai B, Guo F, Wu Y, He Z, Mo C, Wu S, Hu Y. A case of hyperhaemolysis syndrome in a pregnant Chinese woman with β-thalassemia during perinatal transfusion. Transfus Med. 2021 Feb;31(1):24-29. doi: 10.1111/tme.12748. Epub 2020 Dec 16. PMID: 33331032.
- Aragona E, Kelly M, Md MPH. Hyperhemolysis in Sickle Cell Disease. J Pediatr Hematol Oncol. 2014;36(1):e54-e56. doi: 10.1097/MPH.0b013e31828e529f.
- Asnawi AW, Sathar J, Mohamed R, Deraman R, Kumaran S, Hamid SS, Zakaria MZ. Fatal Delayed Haemolytic Transfusion Reaction and Hyperhaemolysis Syndrome in a Pregnant Woman with Sickle Cell Anaemia. Indian J Hematol Blood Transfus. 2016 Jun;32(Suppl 1):251-3. doi: 10.1007/s12288-014-0495-9. Epub 2015 Jan 1. PMID: 27408406; PMCID: PMC4925481.
- Boonyasampant M, Weitz IC, Kay B, Boonchalermvichian C, Liebman HA, Shulman IA. Life-threatening delayed hyperhemolytic transfusion reaction in a patient with sickle cell disease: effective treatment with eculizumab followed by rituximab. Transfusion. 2015 Oct;55(10):2398-403. doi: 10.1111/trf.13144. Epub 2015 May 18. PMID: 25989361.
- Chinchilla Langeber S, Osuna Marco MP, Benedit M, Cervera Bravo Á. When a transfusion in an emergency service is not really urgent: hyperhaemolysis syndrome in a child with sickle cell disease. BMJ Case Rep. 2018 Mar 27;2018:bcr2017223209. doi: 10.1136/bcr-2017-223209. PMID: 29588281; PMCID: PMC5878350.
- Dean CL, Maier CL, Roback JD, Stowell SR. Multiple hemolytic transfusion reactions misinterpreted as severe vaso-occlusive crisis in a patient with sickle cell disease. Transfusion. 2019 Feb;59(2):448-453. doi: 10.1111/trf.15010. Epub 2018 Nov 9. PMID: 30412270.
- Eberly LA, Osman D, Collins NP. Hyperhemolysis Syndrome without Underlying Hematologic Disease. Case Rep Hematol. 2015;2015:180526. doi: 10.1155/2015/180526. Epub 2015 Feb 17. PMID: 25785210; PMCID: PMC4346688.
- Hawke A, Conroy H, Geoghegan R, McMahon C. Erythropoietin as a treatment modality in hyperhaemolysis complicating sickle cell anaemia: GP44. Arch Dis Child. 2019;104:A47 doi: 10.1136/archdischild-2019-epa.110
- Ibanez C, Habibi A, Mekontso-Dessap A, Chadebech P, Chami B, Bierling P, Galactéros F, Rieux C, Nataf J, Bartolucci P, Peyrard T, Pirenne F. Anti-HI can cause a severe delayed hemolytic transfusion reaction with hyperhemolysis in sickle cell disease patients. Transfusion. 2016 Jul;56(7):1828-33. doi: 10.1111/trf.13611. Epub 2016 May 3. PMID: 27145018.
- Lee LE, Beeler BW, Graham BC, Cap AP, Win N, Chen F. Posttransfusion hyperhemolysis is arrested by targeting macrophage activation with novel use of Tocilizumab. Transfusion. 2020 Jan;60(1):30-35. doi: 10.1111/trf.15562. Epub 2019 Oct 23. PMID: 31642065.
- Unnikrishnan A, Pelletier JPR, Bari S, Zumberg M, Shahmohamadi A, Spiess BD, Michael MJ, Harris N, Harrell D, Mandernach MW. Anti-N and anti-Doa immunoglobulin G alloantibody-mediated delayed hemolytic transfusion reaction with hyperhemolysis in sickle cell disease treated with eculizumab and HBOC-201: case report and review of the literature. Transfusion. 2019 Jun;59(6):1907-1910. doi: 10.1111/trf.15198. Epub 2019 Feb 15. PMID: 30768787.
- Bachmeyer C, Maury J, Parrot A, Bachir D, Stankovic K, Girot R, Lionnet F. Rituximab as an effective treatment of hyperhemolysis syndrome in sickle cell anemia. Am J Hematol. 2010 Jan;85(1):91-2. doi: 10.1002/ajh.21578. PMID: 20029943.
- Bezirgiannidou Z, Christoforidou A, Kontekaki E, Anastasiadis AG, Papamichos SI, Menexidou H, Margaritis D, Martinis G, Mantadakis E. Hyperhemolytic Syndrome Complicating a Delayed Hemolytic Transfusion Reaction due to anti-P1 Alloimmunization, in a Pregnant Woman with HbO-Arab/β-Thalassemia. Mediterr J Hematol Infect Dis. 2016 Oct 18;8(1):e2016053. doi: 10.4084/MJHID.2016.053. PMID: 27872733; PMCID: PMC5111518.
- Cid J, Fernández J, Palomo M, Blasco M, Bailó N, Diaz-Ricart M, Lozano M. Hyperhemolytic Transfusion Reaction in Non-Hemoglobinopathy Patients and Terminal Complement Pathway Activation: Case Series and Review of the Literature. Transfus Med Rev. 2020 Jul;34(3):172-177. doi: 10.1016/j.tmrv.2020.06.002. Epub 2020 Jun 27. PMID: 32703665.
- Cullis JO, Win N, Dudley JM, Kaye T. Post-transfusion hyperhaemolysis in a patient with sickle cell disease: use of steroids and intravenous immunoglobulin to prevent further red cell destruction. Vox Sang. 1995;69(4):355-7. doi: 10.1111/j.1423-0410.1995.tb00373.x. PMID: 8751307.
- Danaee A, Howard J, Robertson B, Robinson S. Hyperhaemolysis in a patient with HbH disease. Transfus Med. 2014 Aug;24(4):244-5. doi: 10.1111/tme.12131. Epub 2014 Jun 23. PMID: 24957792.
- Danaee A, Inusa B, Howard J, Robinson S. Hyperhemolysis in Patients With Hemoglobinopathies: A Single-Center Experience and Review of the Literature. Transfus Med Rev. 2015 Oct;29(4):220-30. doi: 10.1016/j.tmrv.2015.06.001. Epub 2015 Jun 19. PMID: 26209603.
- Darabi K, Dzik S. Hyperhemolysis syndrome in anemia of chronic disease. Transfusion. 2005 Dec;45(12):1930-3. doi: 10.1111/j.1537-2995.2005.00608.x. PMID: 16371046.
- Epstein SS, Hadley TJ. Successful management of the potentially fatal hyperhaemolysis syndrome of sickle cell anaemia with a regimen including bortezomib and Hemopure. J Clin Pharm Ther. 2019 Oct;44(5):815-818. doi: 10.1111/jcpt.12998. Epub 2019 Jun 25. PMID: 31237703.
- Ghildyal A, Alattia L, Ong M. Hyperhemolysis in a Case of Sickle Cell Anemia: 361. Am J Clin Pathol. 2018;149:S157 doi: 10.1093/ajcp/aqx131.360
- Gouveia ME, Soares NB, Santoro MS, de Azevedo FCM. Hyperhemolysis syndrome in a patient with sickle cell anemia: Case report. Revista Brasileira de Hematologia e Hemoterapia. 2015;37(4):266-68 doi: 10.1016/j.bjhh.2015.03.005
- Gupta S, Fenves A, Nance S, Sykes D, Dzik W. Hyperhemolysis syndrome in a patient without a hemoglobinopathy, unresponsive to treatment with eculizumab. Transfusion. 2015;55(3):623-28. doi: 10.1111/trf.12876
- Dubois EA, Cohen AF. Eculizumab. British journal of clinical pharmacology. 2009;68(3):318.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.