Environmental Sciences . 2022 May 27;3(5):641-659. doi: 10.37871/jbres1491.
Atlantic Forest and Caatinga: Two Threatened Tropical Biomes in Brazil and Repercussions for Public Health
Maria de Fátima Freire de Melo Ximenes1-3*, Carlos Brisola Marcondes4, Marcel Miranda de Medeiros Silva2,3, Magnólia Florêncio Fernandes de Araujo1 and Cícero Oliveira5
2Laboratório de Pesquisas em Entomologia (LABENT), Universidade Federal do Rio Grande do Norte, Natal, Rio Grande do Norte, Brazil
3Programa de Pós-graduação em Desenvolvimento e Meio Ambiente, Brazil
4Departamento de Microbiologia, Imunologia e Parasitologia, Centro de Ciencias Biológicas, Universidade Federal de Santa Catarina, Campus Trindade, 88040-900 Florianópolis, SC, Brazil
5Agencia de Comunicação, Universidade Federal do Rio Grande do Norte, Natal, Rio Grande do Norte, Brazil
- Arboviruses
- Climate
- Desertification
- Environmental degradation
- Environmental health
- Leishmaniasis
Abstract
Tropical forests are of inestimable value for their ecosystem services to current and future generations, and it is increasingly urgent to implement strategies that decrease species extinction and the main biological dangers for life on Earth. The climate scenarios and projections for South America in the 21st century show climate variations for Brazil leading to severe impacts for the Amazon and Northeastern Brazil, with repercussions for water resources. This article review aims to broaden the discussion on the inextricable relationship between the environment and health, using prevalent diseases in Brazil and other tropical countries as examples and highlighting the degradation of two Brazilian biomes. It will also call attention to the scientific evidence and urgent need to rethink the effect of anthropogenic actions on the planet and act to their mitigation. A bibliographic search was conducted using keywords associated with the objective. Major sources of information were Web of Science, MEDLINE/PubMed, ScienceDirect, Scielo, World Health Organization and sites of several scientific journals and Brazilian environment and health institutions. Initially, 2178 references and 34 additional references were obtained, 404 of which were selected by titles and abstracts and 138 considered in the review. The evidence obtained could result in other studies and primarily help decision- making in the fields of scientific investigation, education, public health and governance, with an emphasis on regions suffering from desertification.
Ecological disturbances and a decline in the ecosystem may increase loss of biodiversity and change relationships between biotic and abiotic factors, as well as soil and air composition and desertification. Infectious diseases pose biological dangers to humans, primarily in tropical countries. New values, global alliances and behavioral changes may be the path to understanding that all forms of life sustain humanity on the planet.
Introduction
Tropical forests are of inestimable value for the ecosystem services they provide to current and future generations, making it increasingly urgent to implement strategies that reduce species extinction and the main biological risks for life on Earth. In this respect, knowledge of traditional people is essential. Indigenous people believe that the Earth was meant to be handed down to subsequent generations, which would justify our presence on the planet. However, the connection of these people to nature has been lost over time. Species extinction, river pollution, new diseases, epidemics, pandemics, and incessant exploitation of natural resources reveal a global crisis, and the life model we have adopted, which is based on consumption and accumulation of material goods, contributing to the current problems humanity faces.
Water cycling is an important environmental service for Brazil and neighboring countries. Every year the prevalent winds in Amazonia supply around 10 trillion m3 of water to the region in the form of water vapor from the Atlantic Ocean. In the Amazonian, forest evapotranspiration is 8.4 trillion m3/year, resulting in 15 million m3/year of rainfall, 50% more than that originating in the ocean [1]. However, the world’s largest tropical forest urgently needs protection. Despite its mega biodiversity, rich natural resources and critical role in climate change, deforestation is increasing in the area [2]. Recent data show that in 2021 deforestation was 12.6% higher than in March 2020, the year with the highest levels in 12 years [3,4].
The degradation of the Brazilian Amazon has promoted the spread of diseases, with economic and social impact. Many pathogens have prospered due to changes in land use, deforestation and poverty, with serious consequences for the health and development of the region. There was a significant growth in malaria cases after policies to develop Amazon were implemented in the 1970s. Although transmission control measures resulted in an important decline in malaria cases between 2005 and 2016, they increased by 50% between 2016 and 2018 [5].
The SARS-CoV-2 pandemic and severe acute respiratory syndrome underscore the urgent need for a change in attitudes to reverse the current global health situation. More important is learning to live with all the organisms that make up life on the planet. We cannot claim that we were unaware of the risks of new epidemics and pandemics [6-8]. Nowadays, evidence indicates that the SARS-CoV-2 virus likely originated in bats [9] in China and Japan and in pangolins in China [10,11].
In Brazil, the death of approximately 670,000 people infected with SARS-CoV-2, and an unknown number of people with health problems such as respiratory failure has caused anguish and social and economic problems, despite the solidarity of the Brazilian people. Studies confirmed a causal relationship between meteorological factors and COVID-19 [12]. In China, the correlation between temperature and relative humidity and COVID-19 was negative, that is, a 1°C increase in temperature led to a decrease of 36 to 57% in daily confirmed cases [13]. The contamination rate in some Brazilian states was initially favored by higher average temperatures and an intermediate humidity range [14], indicating the need for additional studies.
In addition to loss of biodiversity, ecological disturbances and loss of ecosystem resilience may change relationships between biotic and abiotic factors, as well as soil and air composition and desertification. Ecosystem instability alters these relationships and may cause serious problems to human health. In this respect, knowledge of biodiversity is essential in any biome. Equally important is to guarantee its conservation, which involves research and new knowledge, legal support, individual and collective responsibility and efficient management [15,16], in order to ensure the functioning of ecosystem services compatible with life for future generations.
Some regions are more vulnerable, mainly where populations live in endemic areas of the disease, whose vectors are influenced by climate and where sanitary infrastructure and water supply are precarious. Northeastern Brazil is such a region. An increase in temperature may have a greater effect on the composition and function of ecosystems. The prevalence of infectious diseases varies according to social, ecological, climatic and economic factors with characteristics of the pathogen and according to the immune response of hosts.
This article aims to broaden the discussion on the inextricable relationship between the environment and health, using a number of prevalent diseases in Brazil and other tropical countries as examples and highlighting the degradation, changes and endemism of two Brazilian biomes in Northeastern Brazil, in order to broaden the field of study in a global perspective. It will also call attention to the scientific evidence and the urgent need to rethink the effect of anthropogenic actions on the planet and act to mitigate them. Science, collaboration and respect for different forms of knowledge, new values, global alliances and behavioral changes may be the path to understanding that all forms of life sustain humanity on the planet.
Methods
Research strategy and justification
This study was carried out to obtain data published on the environment-health relationship, focusing on two important tropical biomes and the current situation of some diseases that occur in Brazil and other countries, highlighting Northeastern Brazil.
The Atlantic Forest is severely affected and the Caatinga, an extensive, exclusively Brazilian semiarid biome, with development potential, lacks analyses and decisions that result in continuous public policies for those who live there and depend on these biomes. The pandemic and its relationship with wild animals prompted the study of articles published between August 2020 and December 21, 2021.
The study was divided into five different stages:
- Definition of what would be addressed
- Search for articles using keywords
- Title and abstract selection, with no limit for year or country
- Relevant article selection and analysis
- Systematization of information
A bibliographic search used the following keywords alone or combined: climate change, Caatinga biome, Atlantic Forest biome, global health, environmental context, environmental social, vector borne diseases. Major sources of information were Web of Science, MEDLINE/Pubmed, ScienceDirect, Scielo, World Health Organization and sites of several scientific journals and Brazilian environment and health institutions; articles published between 1990 and 2021 were selected. Exceptionally, older references were included because of their relevance and support of the studies. In Rio Grande do Norte state, there are few articles on the issue, which led us to include studies carried out by our research group on vectors of Leishmaniases and arboviruses.
An article published in The New York Times on December 3, 2021 highlighted an old problem on its front page, namely desertification in Northeastern Brazil, where millions of people live. High temperatures, low rainfall and deforestation threaten subsistence plantations, resulting in water and food scarcity [17], even though the region produces and exports food and exhibits potential for solar and wind energy production. Wind energy generation in Rio Grande do Norte has been increasing, showing its potential for clean energy production.
Inclusion criteria
Studies were selected with primary and secondary data on the importance and threats to Brazilian biomes; evidence and repercussions of global warming; vector insects and climate parameters; distribution and occurrence of vector borne diseases; environmental and social context of some arboviruses, Leishmaniases, Chagas disease and effective interventions to mitigate problems related to biome degradation. Important articles may have been left out of the review, due or the difficulty in obtaining the entire text (Table 1).
| Table 1: Topics considered in the studies, divided into the categories used in the scoping review; geographic areas and number of studies included (Twenty nine studies are cited in more than one category; *studies conducted in Northeastern Brazil). | |||
| Category | Region/country | References | No. of Eligible Studies |
| Degradation of ecosystems and health; climate; Global hotspots; emerging infectious diseases; threats to biodiversity and human health | Americas: Brazil | [1,4,7,8,10,12-14,17*,18,21,24,41,42*,46,47,50-55,81,83,94*,96,103*,105,107*,112,113, 121*,122,124,132,133,136] | 37 |
| Sustainable development, neglected diseases; prevention of epidemics and pandemics; outbreak; emerging disease surveillance | Americas: Brazil and others | [8,74,79,106,114] | 5 |
| Ecological resilience; conservation; ecosystem restoration | Brazil | [15,23,25*,26,129,130,131,135*] | 8 |
| Vector insects (biology; climate; urban area; wild area; seasonal variation, vectors infection; environmental sanitation) | Brazil; Argentina |
[19*,20*,45,48,49,53*,61-63*,102*-104*,107*,108*] | 14 |
| Deforestation; soil use in the Amazon and Cerrado biomes; climate change; diseases | Brazil | [1-3,5,80,138,39] | 7 |
| One Health; Planetary Health; Eco-Health | [6,20,126] | 3 | |
| Difficulties/failures and advances in the control of infectious diseases; social context, environmental, political | Brazil | [115,122,137] | 3 |
| Traditional peoples (indigenous leadership thinking; violence against indigenous peoples; national policy) | Brazil | [124,125] | 2 |
| Social and economic context; primary care; sanitation; morbidity | Brazil | [81,84,116] | 3 |
| Atlantic Forest Biome: | |||
| Forest characteristics; degradation; conversion of natural areas into grasslands; biodiversity loss; climate change | Brazil | [18,22,30,40,123,128,132] | 7 |
| Caatinga Biome | |||
| Forest characteristics; degradation, biodiversity loss; climate change | Brazil | 16,27-34*,35*,38*,43,134*,135*] | 14 |
| Desertification | Brazil | [31*,36,37*,42*,97,119*] | 6 |
| Diseases | |||
| Dengue, Zika, Chikungunya | Americas: Brazil | [44*,49,50-53*] | 6 |
| Yellow fever | Brazil | [56-60] | 5 |
| West Nile virus | Africa, Asia, Europa, Australia, North América; South America; Brazil | [64-67] | 4 |
| Mayaro virus; wildlife; urban area | Latin América and Caribbean; Brazil | [68-73] | 6 |
| Chagas Disease: vectors; animal hosts; environmental degradation; work; migration | Brazil | [74-80,82,84-90] | 15 |
| Visceral Leishmaniasis: expansion; urban outbreak | Americas; Brazil | [19*,91-93*,95*,96*,98,99*,100,101*,109,111] | 12 |
| Others | [9,11,97,110,117,118,127] | 7 | |
Exclusion criteria
Duplicate references, comments, letters, editorials, opinions, conference abstracts, unavailable articles were excluded.
Results and Discussion
Initially, 2178 references and 34 additional references were obtained, 404 of which were selected and 138 were included. The interrelationship between the topics analyzed and presented as primary or secondary data in the studies selected hinders their separation into only one category. Twenty-nine studies appear in more than one category (Table 1).
The selected studies show the relationship between the ecosystems and human health threatened by the environmental degradation of Brazilian tropical biomes and their biological, social and economic repercussions, where the current global crisis highlights the urgency of implementing mitigation measures focused on sustainable development.
Atlantic forest biome: Biodiversity hotspots
After the Portuguese invasion in the 16th century, a large part of the Atlantic Forest vegetation was destroyed by intense exploration. Brazilwood (Paubrasilia echinata (Lam.) Gagnon, H. C. Lima & G. P. Lewis) and other woods were extracted and several species became extinct. Ancient reports describe a dense almost pristine forest, inhabited by large populations of indigenous peoples. In Northeastern Brazil, the extinction was nearly total, which aggravated the survival conditions of the population, causing food restriction and a rural exodus [18].
The biological diversity of forests includes inert agents (virus) and thousands of microscopic organisms that circulate among their natural hosts. Mining, livestock raising, development and urbanization, habitat deterioration by pollution, salinity, acidity, changes in temperature and humidity, large crops for feed, drug production, fuel and fibers and the introduction of exotic species and diseases are among the factors that directly affect loss of biodiversity and habitats [16].
Based on the biology of vector insects and seasonal temperature and rainfall curves, the predictability of the occurrence of a number of diseases such as Leishmaniases, dengue fever and chikungunya has been analyzed [19,20]. Infectious diseases pose biological danger to public health, primarily in tropical countries. Influenza, malaria, diarrhea, tuberculosis, Chagas disease, Leishmaniasis, filariasis, dengue fever, Zika, chikungunya, Mayaro fever, yellow fever, and schistosomiasis infect millions of people worldwide.
Current habitat degradation and fragmentation seem to be accelerating the emergence of diseases in humans and other animals caused by viruses, protozoa and other infectious agents, high-risk areas considered global hotspots for zoonotic diseases, including the Atlantic Forest of Brazil [21]. The decline in financial resources aimed at monitoring deforestation in conservation units suggests the destructuring of environmental policies in Brazil.
The Atlantic Forest is one of the largest rainforests in the Neotropical region [18]. It originally occupied an area of 1,110,182 Km2 in Brazil and recent data indicate that the remaining plant cover is around 28%, double previous estimates, and where approximately 70% of Brazilians live [22].
Designated by UNESCO as a World Heritage Center and designated one of the world´s biodiversity hotspots by Conservation International, the Atlantic Rainforest is one of the greatest repositories of biodiversity on the planet. Although its plant cover has declined by at least 70%, it continues to harbor more than 60% of all species on Earth, with endemism indices of up to 50%, depending on the taxonomic group observed [22,23].
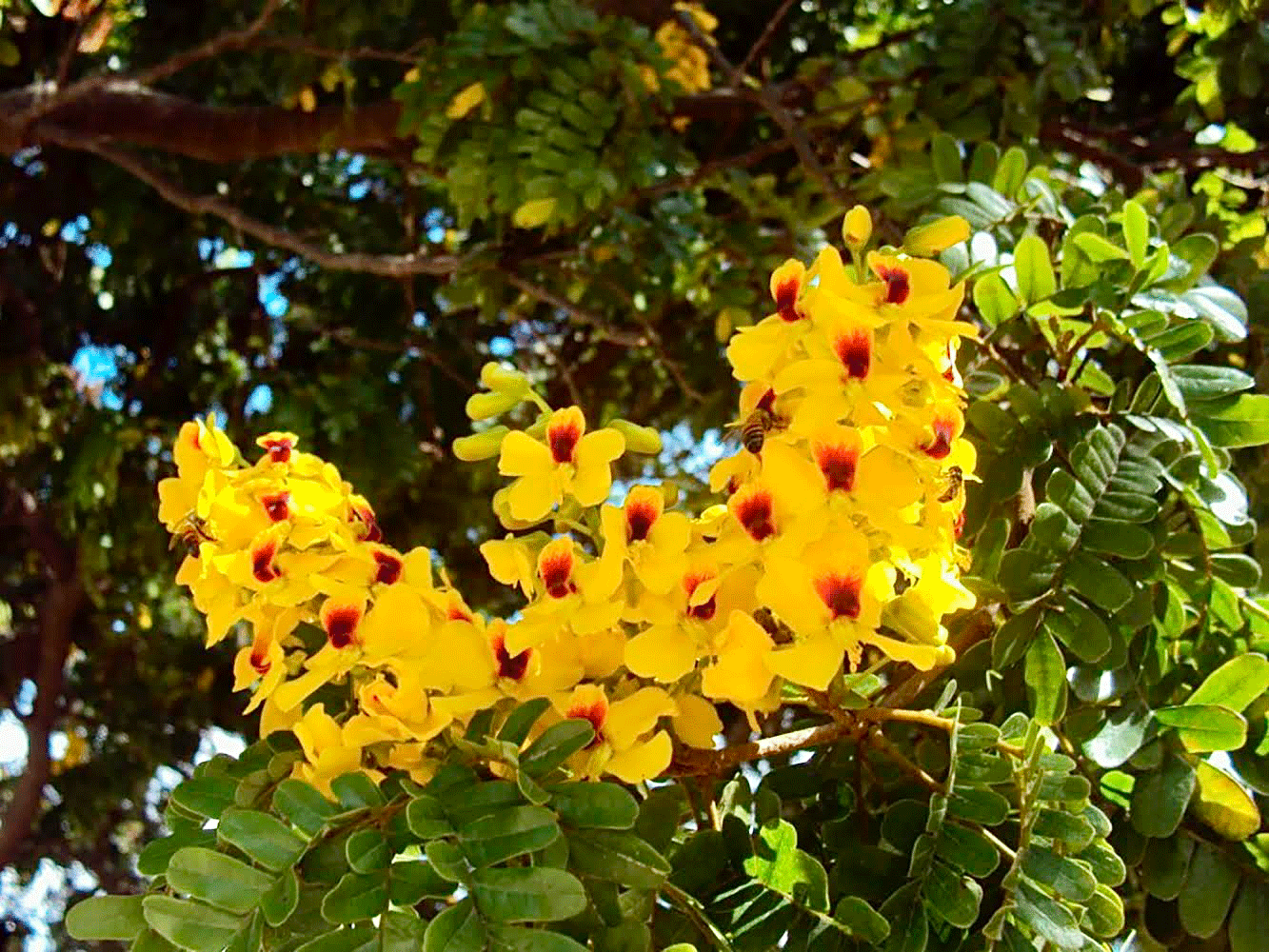
Biodiversity is one of the most suitable indicators for monitoring and assessing biodiversity protection of species and biomes. For example, pollination, an important ecosystem service, is threatened by the declining bee population (Figure 1) caused by anthropogenic disturbance and loss of natural habitat [24]. Species diversity in the Atlantic Forest biome is large, but not homogeneous. Eight main centers of endemism are recognized. The Pernambuco center of endemism, encompassing the forest region north of the mouth of the São Francisco River, the states of Alagoas, Paraíba, Pernambuco and Rio Grande do Norte, is considered one of the most threatened [25]. Restoration of these areas requires technology, investment, explicit laws, training and a large number of personnel over the long-term [26].
Rio Grande do Norte contains a small swath of Atlantic Forest covering around 3,362.89 km2 of the 52,983.90 km2 in the state. Natal, the capital of the state, harbors a number of Atlantic Forest areas as conservation units. The environmental protection zone (Dunes State Park of Natal), the second largest urban park in Brazil (Figure 2), is located near densely populated neighborhoods. Nevertheless, Natal´s Dunes Park is one of the largest Atlantic Forest conservation units in the country [18].
Caatinga biome: Desertification areas
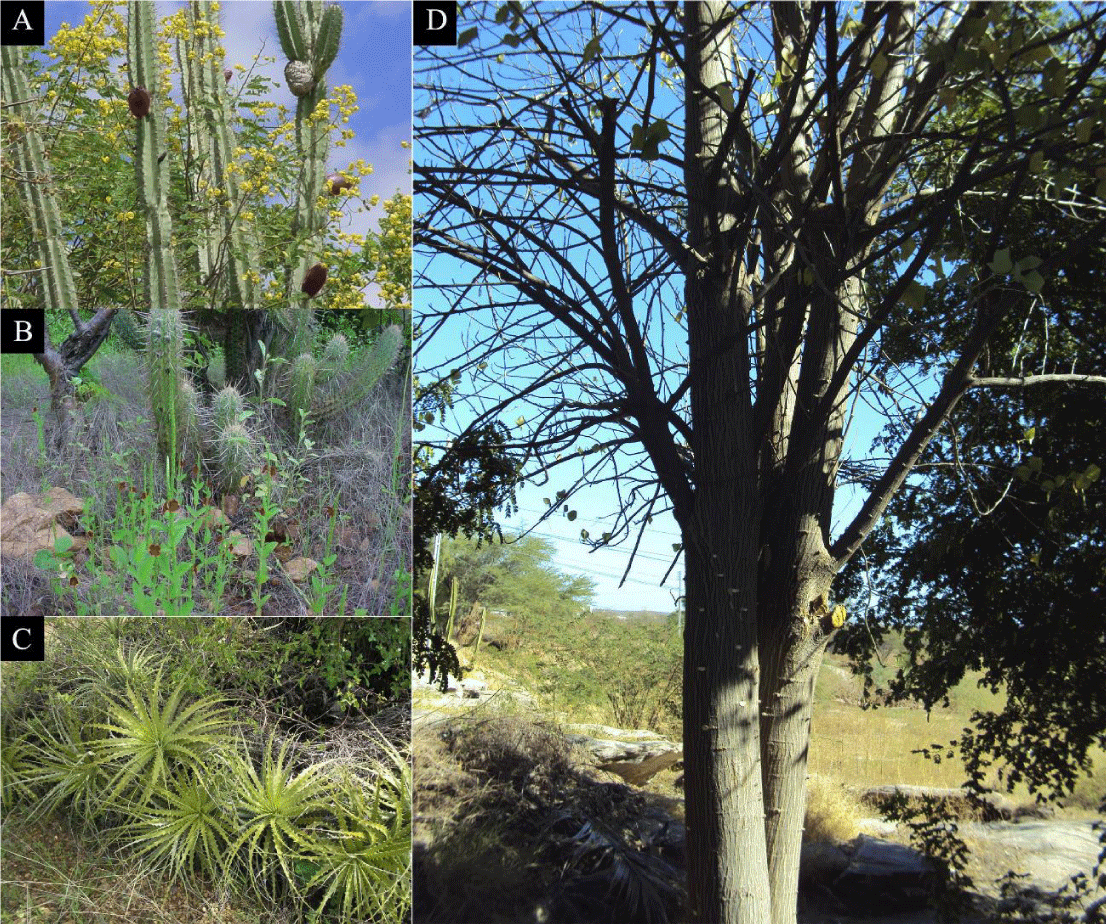
Caatinga means “white forest” in the indigenous Tupi-Guarani language, due to the color of trees after losing their leaves in the dry season. However, in the rainy season the landscape changes from whitish to different shades of green. It is one of the largest biomes in Brazil, covering 11% of the country and occupying an area of 844,453 Km2 [27], with flora and fauna typical of the semiarid and species found nowhere else on Earth. The lizards Tropidurus semitaeniatus and Phyllopezus periosus are examples of typical caatinga species whose feeding behavior includes invertebrates and are affected by the lower rainfall in drier periods [28], in addition to contributing to insect population balance.
Annual rainfall ranges from 150 to 1300 mm, with an average of 700 mm. Temperatures are high and vary only slightly, with annual averages between 25 and 30°C, and little difference between the coldest and hottest months [29]. The area exhibits high demographic pressure and is the most densely populated semiarid region in the world, with a very low Human Development Index (HDI) and socioeconomic inequalities that have persisted throughout its history (Figure 3) [30,31].
Three distinct vegetation patterns occur along its topographic profile: arboreal Caatinga; bushy Caatinga predominant in the Seridó region and the open herbaceous Caatinga in the lowest region, characterized by scattered grasses and bushes, a typical ecosystem in the semiarid. Its conservation units, including the Seridó Ecological Station, harbor numerous animal species, some of which constitute new records and/or centers of endemism for the region [32,33].
Floristic diversity is high for a biome with severe water shortages. Phenology confirms the nearly total leaf loss on most bush and tree species in some months of the year, from the wettest Caatinga in the scrubland region of Pernambuco to the driest in the Seridó region of Rio Grande do Norte (Figure 4) [34,35].
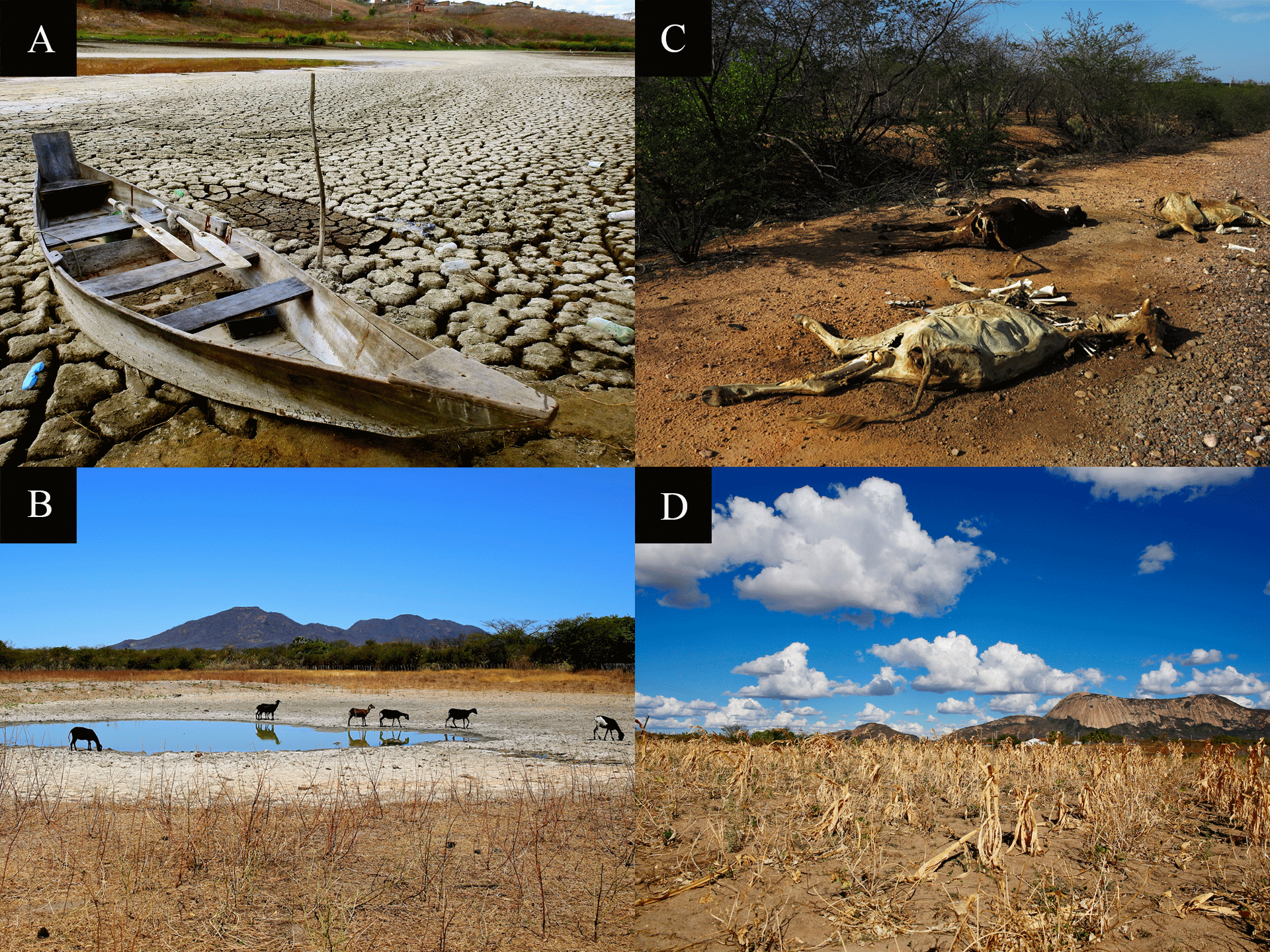
More than 1 billion people living in Africa, Asia and Latin America are affected by desertification. In Brazil, desertification threatens this biome, where the major drivers are the historically severe deforestation and livestock expansion [36]. In general, desertification triggers changes in richness and diversity as well as the floristic composition of the Caatinga [37]. Despite the environmental policies and creation of protection units, a large portion of the Caatinga continues to be subjected to the disordered use of natural resources and formation of desertification areas (Figure 5) [38].
According to Lapola, et al. [39], in 2012, only 54% of primary native Caatinga vegetation remained. There are 131 endangered species in the Caatinga, agribusiness being the main pressure vector, followed by hunting and trapping, mining, urban expansion, disordered tourism and energy production. Trapping for consumption has affected species such as the yellow-legged tinamou (Crypturellus noctivagus zabele), white-lipped peccary (Tayassu pecari), three-banded armadillo (Tolypeutes tricinctus) and rock cavy (Kerodon rupestris) [40].
The interannual variability of rainfall, soil degradation and desertification could make Northeastern Brazil one of the most vulnerable regions in the world in the next century [41,42]. In addition to the restrictive climate conditions and the reduced protected area, the impact of human activity on the region is intense. Insect-borne diseases are influenced by anthropic actions, population development, social and economic changes and demographic growth.
Arboviruses
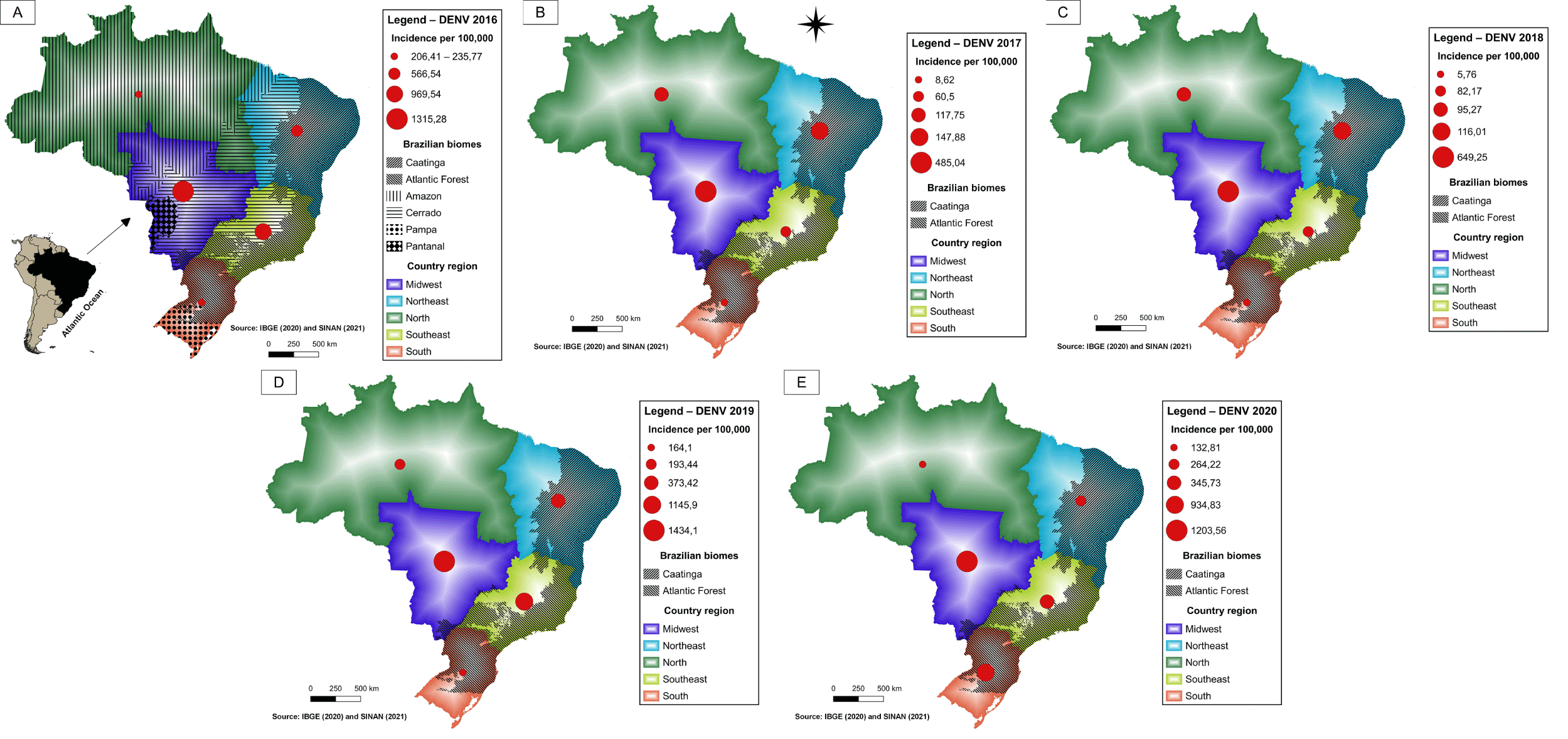
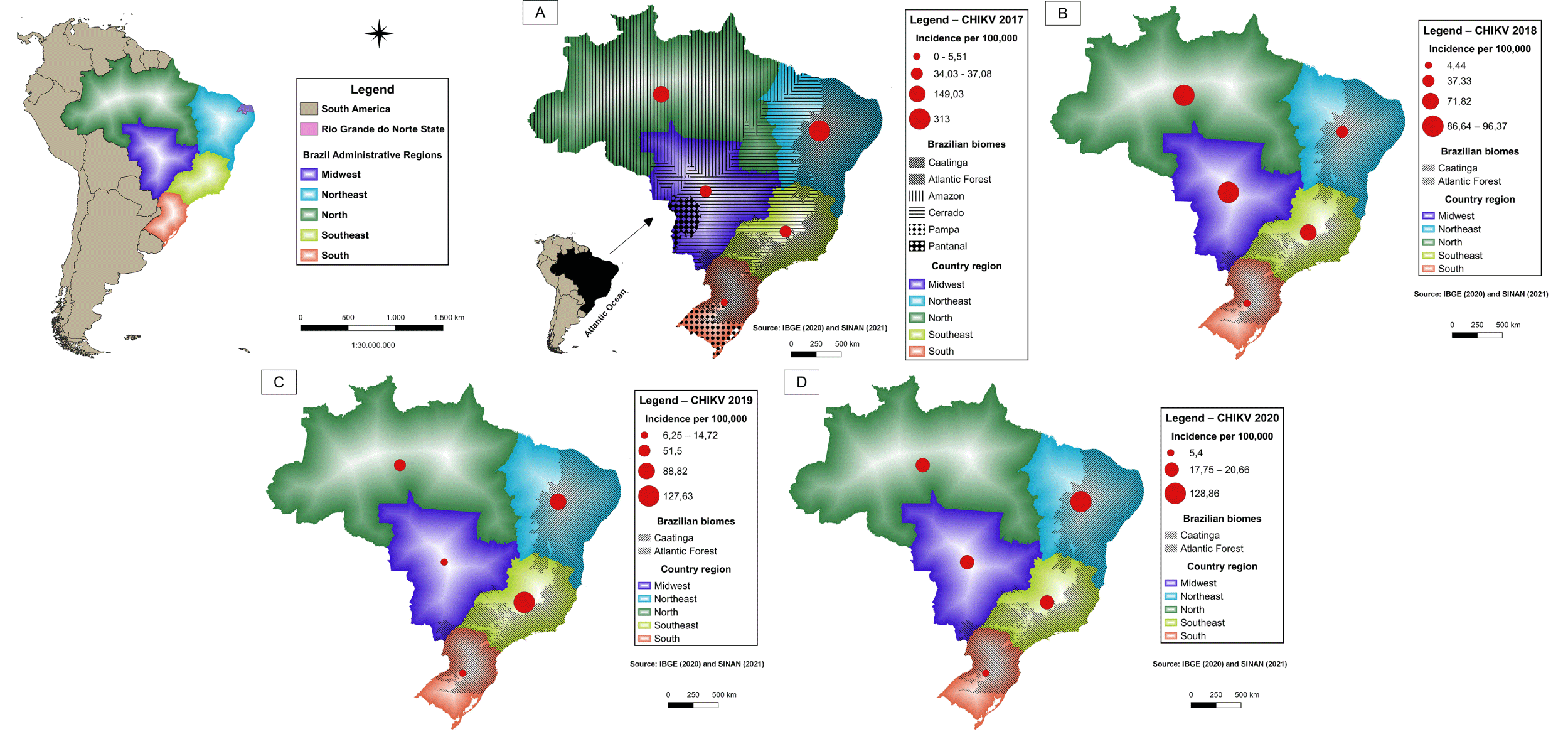
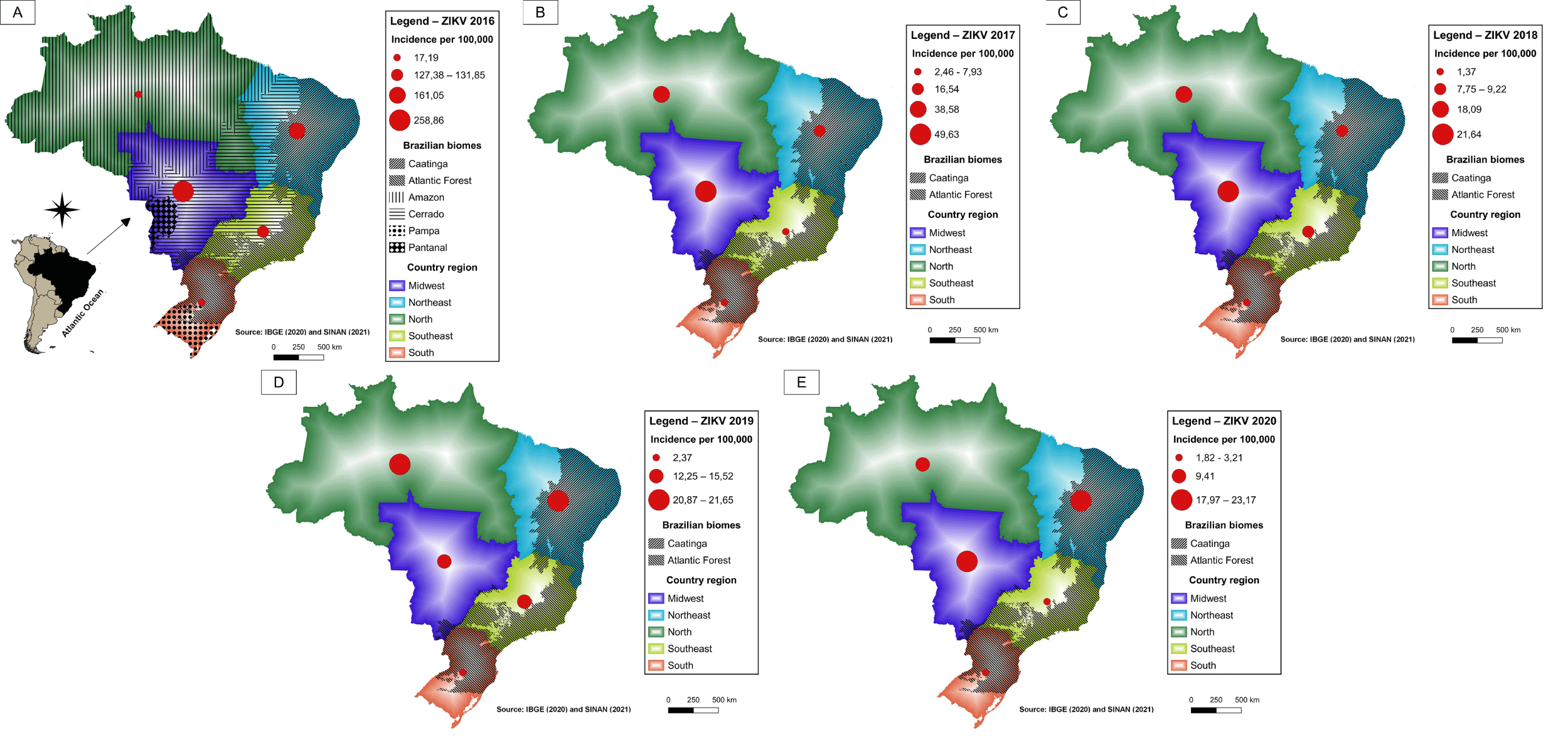
In recent years, Brazil has faced arbovirus epidemics. Dengue fever, the most prevalent, had an incidence of 392 cases per 100,000 inhabitants in 2020 (Figure 6), while, in the same period, chikungunya (Figure 7) and Zika (Figure 8) had incidences of 22.4 and 2.8 cases per 100,000, respectively [43]. In Brazil, up to the 18 week of 2022, the number of Dengue cases increased by 113.7% when compared to the same period in 2021. The incidence rate no Rio Grande do Norte was 323,93 cases per 100,000 inhabitants [44]. Aedes aegypti is a vector of three arbovirus in different regions of the world. Aedes albopictus, originally found in forest environments, is also a competent vector of arboviruses found in densely urbanized areas of Southeastern Brazil and other sites, revealing the importance of entomological surveillance and monitoring of this species [45].
The maps show the Brazilian geographic regions and the extent of the biomes. The regions containing Atlantic Forest and Caatinga areas exhibited higher levels of dengue fever and Chikungunya (Figure 6,7) during the periods analyzed. In 2019 and 2020, the highest incidence of Zika virus occurred in the northeastern states of Bahia and Rio Grande do Norte (Figure 8).
The dissemination of these diseases in addition to yellow fever, Mayaro fever and other arboviruses, whose viral transmission occurs via the bite of an insect vector, seems to be more influenced by environmental and climate change is associated with human actions that result in environmental imbalances, insect vector development, social and economic changes, demographic growth, changes in urban infrastructure and aspects inherent to vertebrate hosts, including humans, in the development of infection.
Climate change may alter the geographic distribution of insects and prolong or shorten the survival time of the infectious agent in the vector insect, increasing the frequency of bites and thereby raising the risk of infection. People are becoming more vulnerable to calamities and temperature variability associated with waterborne diseases and viral fevers caused by Aedes aegypti and other species [46,47]. The emergence of ancient or new diseases varies according to the transmission mode of the pathogen. Diseases that emerge because of changes in land use, water shortages, habitat fragmentation and loss of biodiversity are generally transmitted by insect vectors [48].
Dengue, zika and chikungunya
Despite a number of social and economic advantages in urban areas, such as easier access to medical treatment and schools, human occupation, the precarious infrastructure in the peripheral areas of cities, and mainly the aspects related to trash and sanitation favor the development of mosquitoes and may cause outbreaks of dengue fever and the emergence of new diseases caused by viruses, as occurred with Zika and chikungunya in Brazil. Cases of Dengue [44] and hospitalizations in Northeastern Brazil are rising, certainly related to the difficulties and disruption of preventive measures that have occurred during the pandemic.
At the onset of the Zika epidemic in African and Asian countries, Marcondes and Ximenes [49] underscored the risk of the virus spreading in Brazil due to the high infestation by Aedes spp. in the country. Cases of Zika (Figure 8) occurred initially in the Northeast and resulted in a serious public health problem, caused mainly by microcephaly in newborns. Zika outbreaks were associated with urbanization and deforestation, likely favoring the habitat for the mosquito vector [50]. Urban and wild cycles are maintained by different mosquito species with occasional spillover by bridge vectors that feed on animals and humans [51]. Human activity in areas where the sylvatic cycle is spreading can lead to infection in humans, which may give rise to the onset of an urban cycle by anthropophilic vectors such as Ae. aegypti [50]. Models that predict Zika virus transmission and the relationship with temperature show that in the worst-case scenario, over 1.3 billion more people could face transmission temperatures suitable for ZIKV by 2050, with North American and European populations most at risk. Mitigating gas emissions could reduce ZIKV expansion, potentially protecting 200 million people who live in these areas [52].
In a conservation unit of the Atlantic Forest in an urban area of Natal, Rio Grande do Norte, we recorded two new sylvatic mosquito species infected by the chikungunya virus and an association with the epidemic outbreak of the disease in residents living in neighboring areas. The data reveal the possibility of the virus’ spreading by new mosquito species [53]. The Dunas State Park of Natal conservation unit is located among densely populated neighborhoods, which contributes to the advance of mosquito species. Environmental degradation has an initial impact on the population closest to the degraded area. Later, populations further away may also be affected [54,55], whether because the disease assumes epidemic proportions or because the pathogen is spread by insect vectors.
In the Caatinga, mosquitoes use reproduction strategies whereby they lay their eggs in tree trunk holes, rocks, fruit skins, and other sites that can store small or large amounts of water (Figure 9). In urban environments, solid wastes, primarily plastics, are used as breeding sites by mosquitoes, and consequently disseminate arboviruses in large or small cities.
Yellow fever
Yellow fever outbreaks in the Congo and Brazil in 2016 and 2017 showed that the virus spread from forest to urban areas. The Congo outbreak was associated with high population mobility and low vaccination coverage [56]. In Brazil, the outbreak was linked to deforestation that altered the habitat and the geographic distribution of primates and vectors [57,58]. Due to habitat fragmentation by deforestations in endemic areas or not, the entomological surveillance of the mosquitoes Ae. albopictus, Haemagogus sp. and Sabethes sp. is essential to mitigate the risk of transmission, despite the existence of a vaccine. Aedes scapularis, a widely distributed mosquito species, was suspected as vector in the first Brazilian study of sylvatic yellow fever, in Espírito Santo state [59] and was infected by yellow fever virus in São Paulo state [60].
Haemagogus mosquitoes are widely distributed throughout the Americas, with 28 known species, some of which transmit yellow fever virus, Mayaro, and other arbovirus [61]. Data from Rio Grande do Norte state show that the abundance and diversity of Aedes mosquitoes, transmitters of the dengue, Zika and chikungunya viruses and their Haemagogus counterparts, transmitters of the yellow fever virus, vary according to the temperature and rainfall in the two biomes: the Atlantic Forest [62] and Caatinga [63].
Nine species of Haemagogus have been reported in Brazil, two of which are found in Rio Grande do Norte: Hg. leucocelaenus and Hg. spegazzinii. These species have been found naturally infected by the yellow fever virus in other regions and countries [61,63]. Yellow fever epizootics and suspected human cases have occurred in some Brazilian states between April 2021 and April 2022. Transmission of the virus has been confirmed in the states of Pará, Minas Gerais, Santa Catarina and Rio Grande do Sul. Four people exposed in forest areas died. This demonstrates the active circulation of the virus and the increased risk of transmission to human populations [44].
We identified five plant species typical of the Caatinga as development sites. Haemagogus spegazzinii is apparently a generalist in terms of the plant species it colonizes and is frequently dominant. In the semiarid region, where trees are small, these mosquitoes tend to lay their eggs in breeding sites with a small opening, located in shady areas up to 3 m from the ground, in order to reduce evaporation. The results show that natural receptacles, such as tree hollows, cut bamboo, coconut husks, are the main breeding sites of Haemagogus species. It is important to note that the dengue II virus was sequenced in Hg. spegazzinii mosquitoes in a rural area Rio Grande do Norte, where caatinga vegetation occurs [43].
West nile virus
In the last three decades, the West Nile Virus (WNV) has become a public health problem in Europe and the Americas. The virus belongs to the family Flaviviridae, genus Flavivirus. WNV was originally isolated from a human in 1937 in Uganda. It was later detected in the Middle East, France, South Asia and Australia. In 1999, the virus was introduced into the USA, followed by Canada and Argentina [64]. West Nile virus epidemics have increased globally as a result of droughts. In periods with low rainfall, the rate of infection of the WNV mosquito vector increases [65]. In Brazil, the virus was isolated for the first time in 2019 in a horse in Espírito Santo state, and was also reported in Minas Gerais, São Paulo and Piauí states, the last in Northeastern Brazil [66,67]. It affects the animal’s brain and is mostly transmitted by Culex mosquitoes; some birds are natural reservoirs and equines and humans are occasional hosts.
Infections in humans are mostly subclinical, but fatal meningoencephalitis cases can occur. The transmission model proposed for Brazil, based on serological evidence of infection by the virus, involves the mosquitoes Culex quinquefasciatus; Ae. albopictus; nine bird species, including migratory birds (Calidris alba and Arenaria interpres); the house sparrow (Passer domesticus); equids, reptiles and humans, although questions remain about the epidemiological importance of the findings [66,67].
Mayaro virus
The Mayaro virus of the family Togaviridae was isolated for the first time in a rural worker in Trinidad and Tobago in 1954 [68]. Since then, outbreaks and isolated cases have been notified in regions of South and Central America. The virus causes an acute febrile syndrome similar to dengue, with myalgia, eye pain, chills, arthralgia, rash, and cough less frequently reported.
The Pantanal region of Brazil is rich in biodiversity. The subtropical climate of the Cerrado biome favors the introduction and maintenance of vectors and arboviruses. Equids of the region are used as indicators of the local circulation of the Mayaro virus. Information on these cases between 1954 and 2019 shows that Brazil has the highest number of recorded MAYV cases in Latin America and the Caribbean, followed by Peru and Venezuela [69,70]. Analyses reveal the need for greater surveillance of the Mayaro virus in its hosts [71-73], considering the presence of insect vectors of the virus.
In forest environments, the enzootic cycle occurs in primates, rodents, marsupials, birds, sloths and mosquitoes from species of Haemagogus, Sabethes, Culex, Psorophora, Coquillettidia and Aedes. These animals are common in the Atlantic Forest. In rural areas, the virus is transmitted by Haemagogus spp. and Aedes spp., and human cases of Mayaro fever have mostly occurred due to accidental spillover from the sylvatic cycle. Transmission by Ae. aegypti, Ae. scapularis and Ae. albopictus mosquitoes increases the risk of urbanizing the disease.
Chagas disease
It is believed that the disease rarely affected humans before European colonization and deforestation. However, the new production relationships, forms of land occupation and precarious housing, such as mud and straw huts inhabited by poor families from rural areas, contributed to the formation of ecotopes for wild triatomine bugs, followed by the domestication and transmission of T. cruzi to humans and their domestic animals.
Chagas disease extends from the Southern United States to Argentina and Chile with important occurrence in Brazil and originally associated with Brazilian biomes. In 1908, the flagellated protozoan T. cruzi was found in the digestive system of the hematophagous insect known as barbeiro in Brazil and vinchuca in Argentina (Hemiptera: Reduviidae: Triatominae).
In Latin America, Chagas disease is an example of the clear relationship between environmental degradation and human health. There are distinct epidemiological cycles and circulation of the protozoan Trypanosoma cruzi in different triatomine bugs in different ecotopes, depending on the mammal species and triatomine adapted to peridomestic areas and strains of T. cruzi that circulate among animals [74-78]. For many families, migration from rural to urban environments has provided better access to education and health services. On the other hand, it favors the dispersion of vectors and reservoirs to non-endemic areas, including other countries, and the risk of the disease spreading over time [75-79].
Expansion of T. cruzi has been associated with environmental impacts. The protozoan was isolated in bats captured near a hydroelectric dam in the Brazilian Amazon [80]. The discovery suggests the potential risk of zoonotic spillover to the fast-growing cities in the vicinity of hydroelectric dams. Building dams requires a large workforce, which not only increases the impact of humans on the environment, but also the opportunity for disease spillover [81-84], putting human populations at risk of contracting zoonotic diseases in new areas.
Trypanosoma cruzi can infect wild and domestic mammals of dozens of species in different biomes of Brazil, in different ecotopes that constitute natural transmission foci. Sylvatic, peridomestic and domestic transmission cycles ensure the circulation of the protozoan and the intersection of these cycles results in a risk of infection for humans. Predation and agonistic encounters between dogs and wild mammals have been reported as a source of spillover and spillback for parasitic infection in rural areas [7,85].
Domestic animals such as dogs and cats, synanthropic cavies and rats act as reservoirs in domestic environments while opossums and armadillos are sources of infection for insects. Rodents are major sylvatic reservoir hosts [75]. According to Xavier, et al. [84], canine infection was shown to be an efficient indicator of a reduction in wild mammalian fauna richness and acts as a signal for the presence of small wild mammals with high parasitemia. The lower richness of small mammal species is discussed as a risk factor for the reemergence of Chagas disease.
The rate of T. cruzi infection in the five Brazilian biomes showed that 17% of mammals were seropositive and 8% of all animals had positive hemocultures indicative of parasitemia and consequently potential infectiousness. Opossums, mainly Philander spp. and Didelphis spp., the coati Nasua nasua, the capuchin monkey Sapajus libidinosus and the golden lion tamarin Leontopithecus rosalia demonstrated higher rates of positive hemocultures. Bats (Chiroptera) stood out for hosting the greatest diversity of species and genotypes of Trypanosoma spp. [78,85,86].
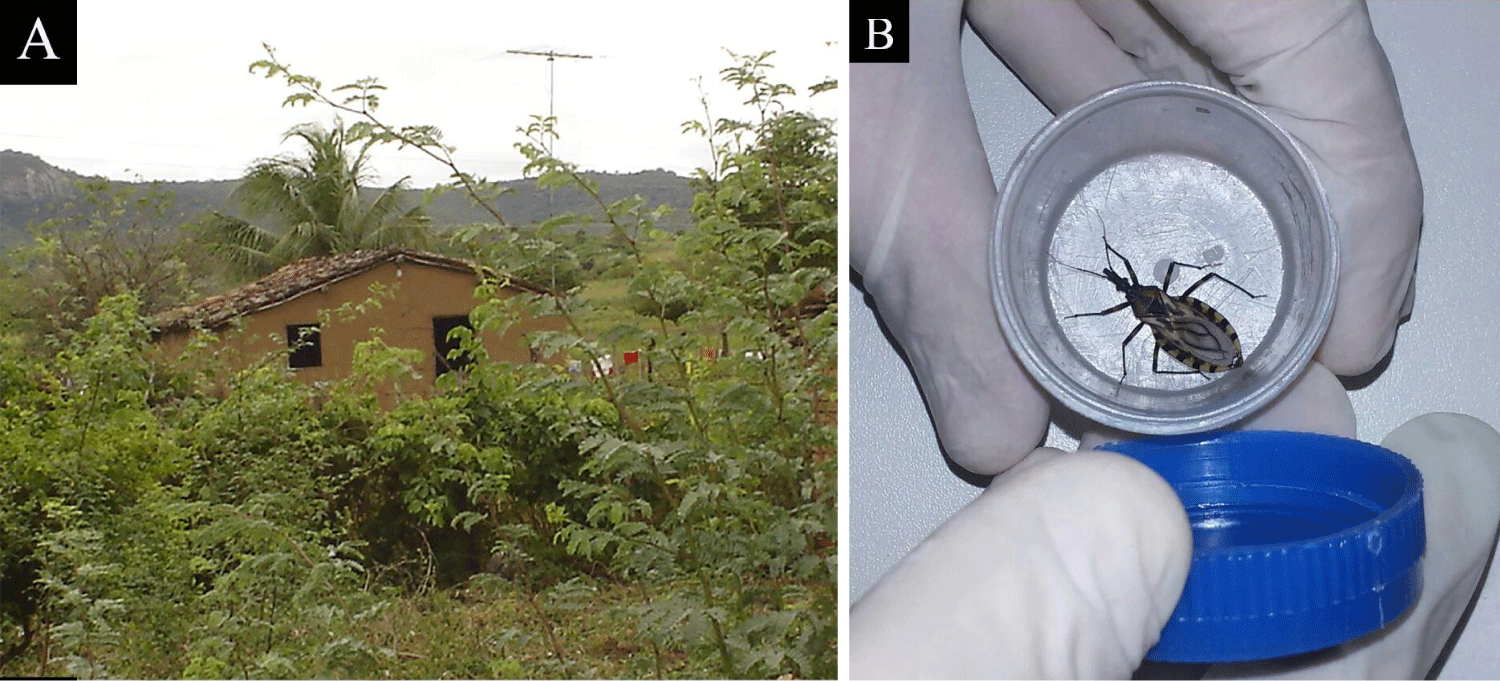
Rattus rattus was found in the Caatinga with high rates of positive blood cultures (21%); Cerdocyon thous (fox) was also co-infected by T. cruzi and Leishmania spp., demonstrating the importance of carnivores in maintaining these parasites in sylvatic cycles [85]. Triatoma brasiliensis and T. pseudomaculata are the main vectors of T. cruzi in the Northeast [86]. Triatoma brasiliensis is widely distributed in the Caatinga in habitats near farms (Figure 10), occupying henhouses, pigpens, corrals, perches and piles of bricks, tiles and wood [87,88]. In Rio Grande do Norte, among the triatomines captured in the intradomestic area, the T. cruzi infection rate was higher in Rhodnius nasutus, followed by T. braziliensis, T. pseudomaculata, and Panstrongylus lutzi colonization in the peridomestic and intradomestic environment [89].
There was a significant decline in the Southeast, South and Midwest, but relevant growth in the Northeast and North of Brazil. Despite the progress, the risk of vector transmission of Chagas disease persists due to the existence of triatomine bugs with high colonization potential. The persistence of residual foci of Triatoma infestans in some municipalities in the states of Bahia and Rio Grande do Sul [90] could lead to the spreading of the disease.
These findings also show the need to analyze the ecology of these species, the risk of infection and the importance of instructing local populations on how to preserve forest areas. The T. cruzi transmission cycle in the wild can be observed in the two biomes studied here: the Atlantic Forest and Caatinga. According to Jansen, et al. [85], in the Atlantic Forest, 44.4% of the animals examined consisted of two species of marmosets (Primates: Callitrichidae), 37.6% the marsupial Didelphis spp., 10.7% Philander spp. and 0.35% rodents with T. cruzi multiplication in culture media. Among the wild mammals sampled, Nasua nasua (coati) proved to be a key species, where 67% were positive with one or more species of parasite and 44% co-infected.
Visceral Leishmaniasis
Recurring epidemics of Visceral Leishmaniasis (VL) affect communities in East Africa, with high rates of morbidity and mortality. It is a chronic and lethal disease. Of the total number of VL cases in 2020, 97% were recorded in Brazil [91].
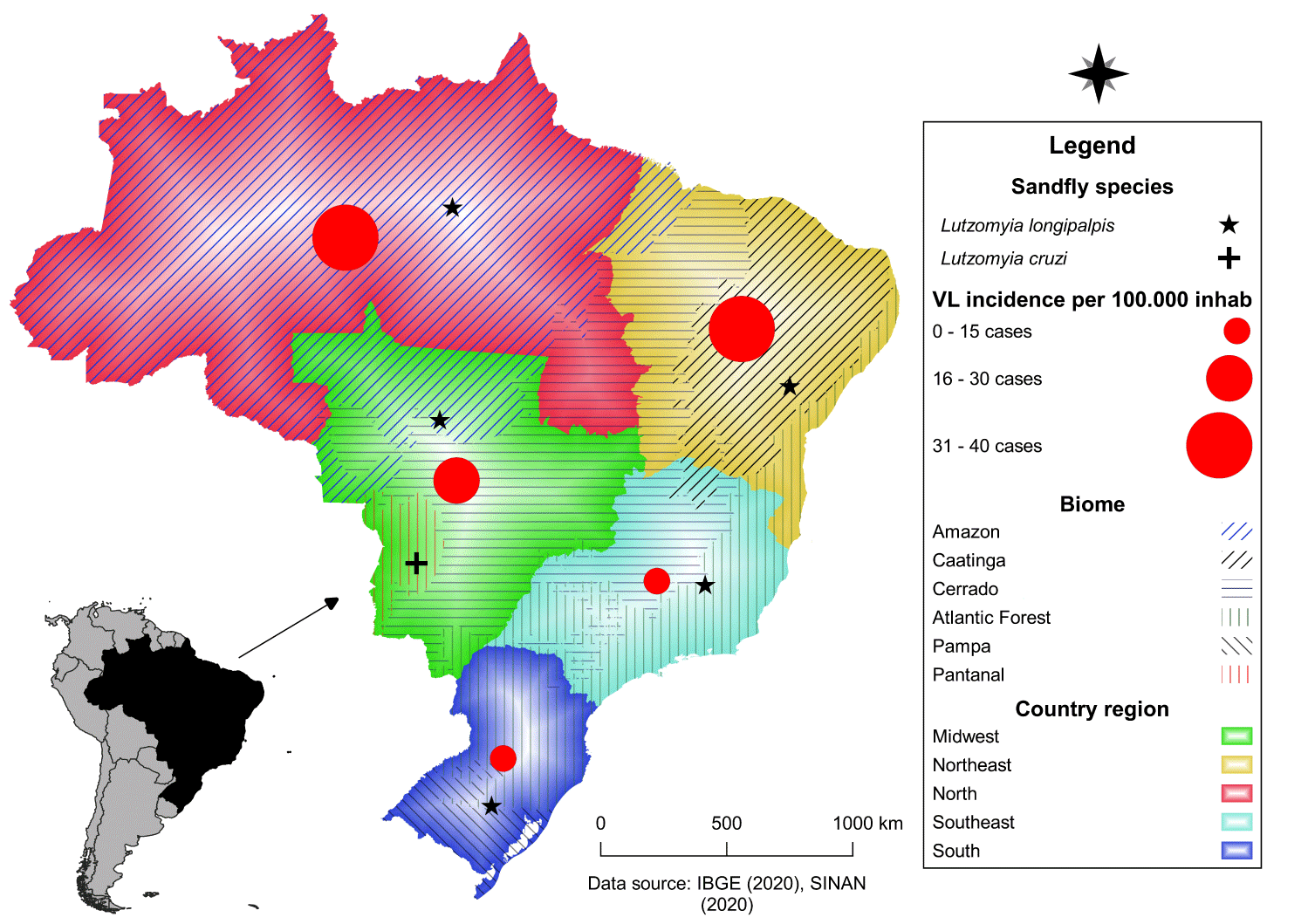
In Brazil, Venezuela, Colombia, and other areas of Latin America, VL typically occurs in rural areas, but large urban outbreaks have been reported in Northeastern Brazil [92]. The urban expansion of VL in Brazil occurred with an urban outbreak in Teresina, Piauí state, between 1981 and 1985, with the disease initially limited to the rural area, later spreading to other areas of the city [93-95]. The map (Figure 11) shows the incidence of visceral Leishmaniasis in Brazil in recent years, with the Northeast recording the largest number of infected persons. The sandfly Lutzomyia longipalpis, vector of Leishmania infantum, is found in all the regions of the country and Lutzomyia cruzi only in Mato Grosso do Sul state. The incidence of visceral Leishmaniasis in Brazil in recent years is highest in Mato Grosso do Sul state.
In Brazil, VL occurs in all five Brazilian regions, the Northeast being the main area of occurrence with 44.5% of cases, affecting mainly adult men and children aged 0 to 5 years [96,97]. From 2017 to 2019, the Northeast recorded 19,094 cases of Leishmaniasis in humans, 6,000 visceral and 13,094 tegumentary (cutaneous + mucocutaneous) [97]. However, knowledge of the ecological relationships as risk factors for epidemic episodes of VL in the Northeast is insufficient to elucidate these episodes [98]. In Rio Grande do Norte, the disease has shown a periurban epidemiological pattern, with a decline in cases among children younger than 10 years old and a rise in adults [99,100], which has persisted over the years. In Ceará, another state in the same region, children younger than five years old are more vulnerable, with an incidence of 12.67 cases per 100,000 inhabitants in 2017 [97,101-104].
The disease is present in all the regions of the country, as subsequently observed in the Northeast, affecting people of all ages. There is a strong correlation between the incidence of VL and socially vulnerable populations exposed to insect vectors and living with host animals, thereby classifying cutaneous and visceral Leishmaniases in the group of 20 Neglected Tropical Diseases (NTDs). NTDs affect more than 1 billion people worldwide, causing death and compromising the lives of the people affected, limiting their productivity and contributing to maintaining the cycles of poverty and disease [105,106].
The vectors of Leishmania parasites in the Americas are species and subspecies of Phlebotomine sand flies. The biology of each species is unique and directly affects the incidence, epidemiology and control of VL and cutaneous Leishmaniasis. The expansion of VL in the rural, periurban and urban areas of Brazil is directly associated with adaptation of the insect vector Lutzomyia longipalpis to human-modified environments [19,107-108]. In Rio Grande do Norte, Lu. longipalpis is found in all eight geographic zones [102,107]. The insect vectors of L. infantum have been found in the conservation units of the Atlantic Forest and Caatinga as well as in the peridomestic environment of dwellings located near forests [19,20,108]. Among the mammals used as the blood meal source of Lu. longipalpis are domestic [108,109] and wild animals, including the fox (C. thous) and the six-banded armadillo Euphractus sexcinctus, typical of the Caatinga [108,110].
Visceral Leishmaniasis is associated with malnutrition, immunological debility, precarious living conditions, climate change, deforestation, disordered urbanization, the construction of dams, roads and hydroelectric power plants, and population migrations [102,106,111-113]. Thus, it is necessary to improve the living conditions of people who reside in areas with concentrations of poor populations and resilient agricultural systems in order to combat malnutrition.
Leishmaniases show a clear association with forest degradation and growth in urban areas, especially American visceral Leishmaniasis. The evidence obtained could result in other studies and primarily help decisions in the fields of scientific investigation, education, public health and governance, with an emphasis on regions suffering from desertification.
Mitigating environmental impacts will be a significant challenge for countries in the coming decades. Processes that change the environment, causing fragmentation and changing forest area, alter the composition of sand fly fauna in Brazil and Argentina, with a reduction, absence or adaptation of Leishmania, vertebrate and invertebrate hosts to a new environments [19,111,112].
Food and water-related diseases
Access to clean drinking water is one of the most serious problems worldwide, and several countries have improved this situation, but this is not the case for basic sanitation. In 2025, half of the world’s population will still be living in areas with water shortages [114], perpetuating the same health problems.
In Brazil, an association between sewage, water pollution, morbidity and mortality caused by diseases related to inadequate sanitation systems is found in some parts of the country. In the North and Northeast, the hospitalization rate for waterborne diseases is more than double the national average. A significant portion of hospitalization expenses incurred by the National Health System (SUS in Portuguese) could be avoided with preventive measures, which is also observed in other regions and countries [115].
Diarrhea in children and older adults remains a problem, although there has been a significant decline in morbidity and mortality in Brazil [116]. Water management needs to improve to guarantee the supply and quality of the water consumed. The construction of water tanks by the Cistern Program is a good example of social technology and cooperation between civil society and the federal government. However, the program lost more than 90% of its funding after 2016, which compromised access to clean drinking water, increasing insecurity and the risk of conflicts in water-dependent rural communities [117,118]. At the time, collecting rainwater and maintaining these artisanal reservoirs allowed greater water access for family farmers from several northeastern states and boosted safe food production, health and even access to education for their children, who no longer needed to seek water sources outside their homes. The policy received an award from the World Future Council (WFC), in cooperation with the United Nations Convention to Combat Desertification (UNCCD).
Health, environmental degradation and impacts on life on earth
Climate projections and trends demonstrate that in the last forty or fifty years of the 20th century the global temperature increase was higher than at any time in the last six hundred years, and that this warming trend is accelerating [41,119]. The greenhouse effect does not occur uniformly on the planet. Projections of climate scenarios by the Intergovernmental Panel on Climate Change (IPCC) [41] for South America in the 21st century show that climate variations in Brazil will have serious impacts in Amazonia and the Northeast, with ramifications for water resources a significant threat for the semiarid region of the latter, whose per capita water supply is already insufficient [119]. Accurate predictions of the effect of global warming on arbovirus transmission are essential [120].
Brazilian cities harbor 86% of the country’s population, and are more affected by a temperature increase than most cities worldwide. According to Lapola, et al. [121], analysis of the vulnerability of urban dwellers in six metropolitan regions of Brazil (Manaus, Natal, São Paulo, Vitória, Curitiba and Porto Alegre) reveals high and very high risk in the underdeveloped areas of Manaus and Natal, capitals of the states of Amazonas and Rio Grande do Norte, respectively. Restoring and increasing protection in the green areas of these cities may be a short-term strategy to minimize the problem.
The degradation of natural capital has an impact on the sharing of spatial, temporal and trophic dimensions [122]. Among the main threats to Brazilian species and biomes are habitat destruction, deforestation, burning, wood exploitation, conversion of fields into pastureland, dam construction, invasive species, hunting, and endangered animal and plant trafficking [123]. According to Davi Kopenawa [124], an indigenous Yanomami leader, regarding mining and actions that impact forests and human health:
If we let prospectors dig everywhere, like pigs in the forest, the rivers and forests will become cesspools filled with motor oil and trash. They also wash the gold dust mixing it with what they call quicksilver. All these dirty and dangerous things make the waters sick and the fish soft and rotten. Whoever eats them runs the risk of dying emaciated from dysentery, with painful abdominal cramps and dizzy spells.
Krenak [125], another indigenous leader in Brazil adds the following:
Because of our divorce from the integration and interactions with our mother Earth, she is leaving us as orphans, not only the so-called Indians or indigenous peoples, but everyone.
Natural systems are being rapidly degraded to an unprecedented degree in human history. Theoretical analysis of One Health, Eco Health and Planetary Health reveals differences and similarities, strategies that can be implemented as policies between nations [120,126].
A model for assessing biodiversity loss in Northeastern Brazil estimates that the decrease in the bird, mammal and scaly reptile population in the study area is at an “extreme” level [127]. Reptiles, from their origin at the end of the Carboniferous, have favored the coevolution of pathogens and vectors, such as Ae. albopictus and Phlebotomine sand flies [127]. This helps understand the evolutionary process that resulted in blood feeding on mammals and the emergence of new zoonotic diseases originating from pressures on the wild animal hosts.
Attempts to explain the extraordinary diversity of the tropics frequently require interactions between climate variability in space, time and topography [128]. Knowing and monitoring the species in these areas is essential for restoration, protection, preservation and sustainable use projects, wherever possible. Despite the advances and technologies available for monitoring forests [129], the measures adopted have been insufficient to protect them.
These critical areas occupy less than 25% of the earth’s surface, hot spots where more than 1 billion people live [23] in urban and rural areas. In these critical points, many species, including humans, share a common vulnerability and struggle for survival. These ecosystems are responsible for water production, regulation and supply; climate regulation and equilibrium, in addition to providing scenic landscapes and preserving a historical and cultural heritage for millions of traditional Brazilian peoples [130,131].
With a view to conserving the different biomes in the country, the Brazilian government created conservation units, particularly after the year 2000. However, despite the creation of more than 650 conservation units, most are small, but demarcating indigenous lands may promote native vegetation conservation more efficiently. Thus, indigenous peoples may be key elements of conservation strategies [131]. Over time, the use of land previously destined to forest conservation for grasslands or agricultural fields has had a significant impact on forest cover associated with watersheds, with a negative effect on river sources and consequently water availability and flow. This left only around 20% of watersheds in the biome with natural plant cover [132,133].
Despite their slow recovery, regenerating forests are important in mitigating climate and conserving biodiversity, since they potentially sequester carbon and harbor a series of species. Achieving restoration and landscape conservation objectives through passive restoration is a challenge that requires investment in management planning in areas with low resilience, high biodiversity and carbon conservation [134,135].
On the other hand, Demange [131] underscores that ecological resilience can also be induced by guidelines, institutions and decision-making processes that promote, among other objectives, continuous learning about ecosystem functioning, biodiversity conservation and maintaining ecological functions everywhere. However, degradation increases faster than the improvements obtained by environmental protection, because enforcement of legislation seems to be inadequate. Careful oversight is mandatory. There is also an urgent need to change production and consumption patterns and man’s relationship with nature by implementing new ecological and ethical objectives for environmental preservation.
By 2050, the agricultural areas of several municipalities will likely be reduced by rising temperatures throughout the semiarid of Northeastern Brazil [119]. With climate change, extreme rainfall will likely be more recurrent and droughts will be more prolonged and frequent, compromising natural and socioeconomic systems [41], which could affect food safety in the region. Although the Caatinga Biosphere Reserve, part of the Man and Biosphere (MaB) program created by UNESCO in 1971, has warned about the areas susceptible to desertification in Brazil, social vulnerability in the region has increased.
Biome protection policies are described in scientific articles, the rhetoric of political leaders and the history of those living in the Caatinga biome. The government program to construct a million cisterns in Brazil, a public policy to provide access to water in the Northeast, was enacted to mitigate the constant droughts in the area [117] and was strengthened by the Brazilian government with its Zero Hunger (Fome Zero) program in 2003. The processes and decisions were based on local knowledge and practices in order to value the “sertanejo” (rural) culture, its knowledge and centuries-long interaction with drought, to subsequently establish public policies to conserve what remains. The creation of new conservation units with alternative uses, sustainable exploitation, monitoring and environmental education is essential and will improve the quality of life of the region’s inhabitants.
In addition to the challenge of supplying food to everyone, rapid urbanization increases diseases, causes epidemiological changes and overloads health systems. In urban tropical areas, there is a clear association between arboviruses, water shortage and nonexistent or poor basic sanitation. The number of conservation units and number of trees per capita, among others, are important indicators of the socioenvironmental vulnerability of Brazilian cities. Although some infectious diseases such as tuberculosis and hanseniasis have declined in Brazil, others have emerged and reemerged, remaining a challenge to overcome [118,136,137].
Global goals
In a recent article, Fearnside [138] reported that the only positive effect of the COVID-19 pandemic was making the public aware of the risks of emerging diseases. Indeed, the disease resuscitated an old concern about the relationship between environmental degradation and infectious diseases, also demonstrated in a study of mosquitoes and arboviruses in a conservation unit of the Atlantic Forest, in an urban area of Natal in Northeastern Brazil [53].
The WHO Conference 2021 report on climate change shows opportunities to correct the directions and strengthen the measures to face the health crisis related to environmental and climate changes. It is up to countries to find solutions to comply with the Paris Agreement [106] and adopt measures to reconstruct the economy and protect communities post-pandemic, in addition to preventing epidemics of old and new viruses.
The twenty diseases considered Neglected Tropical Diseases (NTDs) affect more than 1 billion poor and marginalized people worldwide, primarily in tropical and subtropical regions. With a view to ending the negligence and achieving sustainable development, at the 73rd World Health Assembly, the World Health Organization established a road map for neglected tropical diseases for 2021-2030, proposing that national health plans consider neglected diseases. Governments should establish their agendas and national and regional goals in order to prevent and control endemic NTDs in their countries [106].
The new road map for the next decade reveals the strategies, targets and goals for NTDs, including a 90% decline in people that need intervention for some type of NTD, based on 2010 data; eliminating at least one NTD in 100 countries; eradicating two NTDs; and reducing by 75% Disability Adjusted Life Years (DALY) caused by NTDs, such as invalidity and premature death [106].
Transversal goals to achieve in 2030 include a reduction to 0% in dengue fever lethality; decline in visceral Leishmaniasis lethality to less than 1%; treatment of 100% of notified cases of cutaneous Leishmaniasis; increase in the number of countries with a decrease in Chagas disease by vectorial, food, transfusion and congenital transmission from ten countries in 2025 to 15 in 2030; elimination as a public health problem of soil-transmitted helminthiasis and schistosomiasis and other diseases. All of these diseases are associated with living conditions in rural and urban areas, the presence of biological vectors (mosquitos, sandflies, triatomines, snails); poor basic sanitation; degradation of green areas and local climate. Thus, it remains a daunting challenge, albeit possible, to achieve the Sustainable Development Goals (SDGs) agreed upon by 190 countries in Agenda 2030 or other agendas being considered.
Conclusion
Despite the complexity of the relationships between development, environment and health, evidence reveals that the planet and human civilization cannot wait. Global plans and strategies to avoid deforestation, which include guidelines, laws and enforcement, agreements and advances in global partnerships need to be established and adhered to. Much has to be done to sustain a world population of 9 to 10 billion people in the next few decades. Sensitization and awareness of the problems, investments in education and scientific research are essential to achieve sustainable, resilient and healthy cities, with life at the center of discussions.
Fragmented habitats result in an imbalance in the relationships between organisms and seem to be accelerating the emergence of diseases in humans and other animals, caused by viruses, protozoa and other agents, revealing global hotspots for zoonotic diseases, including the forests of Brazil. People are living with the risk directly linked to the degradation of green areas, droughts and climate change and the lack of access to clean drinking water, giving rise to problems, such as the emergence of infectious agents, disease and death. The parasite-host relationship shows advantageous adaptations for parasites, but is enough known to control them?
The Caatinga biome, a seasonal dry tropical forest, with distinct characteristics of the Atlantic Forest, exhibits similar problems that are aggravated by drought, high temperatures, problems with land use and the ongoing desertification. The region is densely inhabited and contains municipalities with very low human development indices, despite the potential development of the region. The intensity of human activities and the speed with which they occur indicate a difficulty in overcoming public health problems and favor the emergence of others, considering the biological, social, environmental aspects and the forecast of warming caused by climate change. As such, this biome requires significant attention and mitigation strategies for these problems. The most vulnerable populations in Brazil and other countries cannot continue living in poverty and permanent risk of contracting infectious diseases, some of which can be easily prevented through improved living conditions and food safety. Urgent measures are needed to guarantee sustainable life.
Despite the reduced morbidity and mortality of some diseases in Brazil, such as Chagas disease, over the last fifty years, there is much to be done, primarily in relation to diseases involving insect vectors, such as visceral Leishmaniasis and dengue fever. In spite of the complexity, certain factors that cause the proliferation of their vectors may be mitigated by public policies, basic sanitation, access to clean water and educational measures. Without the tropical forests, Brazil and the world will be unable to slow down the climate extremes that cause environmental catastrophes, diseases and deaths.
Preventing the spread of diseases, new epidemics and new pandemics requires absolute restructuring of how mankind relates to ecosystems, and the problem requires diversified knowledge, regional and global policies and concrete actions. Theoretical-conceptual analysis of the One Health, Eco Health or Planetary Health initiatives, which shows differences and similarities, may be important paths for the scientific community to assess, such as recognizing the wisdom of traditional peoples, respecting the social and cultural differences of each ethnicity. These groups occupy and use their lands and natural resources from a sustainable perspective, via the knowledge, innovation and practices generated and transmitted by tradition.
The global health crisis reflects the disconnection between mankind and nature, and traditional peoples are essential to understanding what has been lost and what can be done. Indigenous peoples, Quilombola communities, babassu coconut breakers, mangaba fruit pickers, artisanal fishermen, shellfish pickers, beach dwellers, countryside dwellers, raftsmen, Caatinga dwellers, among others who live in the Brazilian biomes, occupy 25% of Brazilian territory and certainly have much to teach us. It is essential to contain deforestation and the advance of climate change and restore forests.
The evidence obtained in the studies of different biomes, highlighting the Atlantic Forest and Caatinga biomes, and anticipating damage to health can be used in disease prevention strategies and to mitigate public policies. Engaging people and organizations in the pursuit of comprehensive health, taking part in all areas of knowledge and the active participation of society rekindles hope that it is possible to halt the incessant and growing exploitation of natural resources, despite the uncertainties and the following questions that need to be answered: What more must be learned? Will change come from more technology or more humanization? What are the core values of humanity? What do we want to leave our children, grandchildren and future generations, who do not exist yet and whom we will never know, but will be here on Earth? Do we need to restructure university curricula? How do we involve more teachers, students and communities? How do we sensitize government authorities, politicians and legislators? Despite the complexity of the issue, we need to keep the hope alive that change is possible.
Acknowledgment
To the Federal University of Rio Grande do Norte, National Council for Scientific and Technological Development (CNPQ), Coordination for the Improvement of Higher Education Personnel (CAPES), Chico Mendes Institute for Biodiversity Conservation (ICMBIO), Ecological Station of the Seridó (ESEC-SERIDÓ); Nísia Floresta National Forest, Agricultural Research Corporation of Rio Grande do Norte (EMPARN), and Department of Health of Rio Grande do Norte (SESAP) for promoting studies, research and guiding students with a view to understanding the relationships between nature and human health. To all the undergraduate and graduate students, professors and laboratory technicians for the knowledge accumulated and their invaluable collaboration. Some of our studies cited in this review were funded by CAPES/MEC; CNPq/MCTI Decit/SCTIE/MoH (440638/2016-0).
Competing Interest
The authors declare they no conflict of interest.
References
- Nobre AD. The future climate of Amazonia, scientific assessment report. 2014. https://tinyurl.com/5fn3daa2
- Instituto Nacional de Pesquisas Espaciais (INPE). Taxas de desmatamento, Amazônia legal. 2020. https://tinyurl.com/p78uuysh
- Fonseca A, Justino M, Cardoso D, Ribeiro J, Salomão R, Souza Junior C, Veríssimo A. Boletim do desmatamento da Amazônia Legal SAD. 2019. https://tinyurl.com/ec4p36nr
- Watanabre P. Desmatamento da Amazônia em março é o maior dos últimos seis anos. Amazonas atual. 2021. https://tinyurl.com/3z5kpnej
- Castro MC, Baeza A, Codeço CT, Cucunubá ZM, Dal'Asta AP, De Leo GA, Dobson AP, Carrasco-Escobar G, Lana RM, Lowe R, Monteiro AMV, Pascual M, Santos-Vega M. Development, environmental degradation, and disease spread in the Brazilian Amazon. PLoS Biol. 2019 Nov 15;17(11):e3000526. doi: 10.1371/journal.pbio.3000526. PMID: 31730640; PMCID: PMC6881077.
- Cunningham AA, Daszak P, Wood JLN. One Health, emerging infectious diseases and wildlife: two decades of progress? Philos Trans R Soc Lond B Biol Sci. 2017 Jul 19;372(1725):20160167. doi: 10.1098/rstb.2016.0167. PMID: 28584175; PMCID: PMC5468692.
- Daszak P, Cunningham AA, Hyatt AD. Emerging infectious diseases of wildlife--threats to biodiversity and human health. Science. 2000 Jan 21;287(5452):443-9. doi: 10.1126/science.287.5452.443. PMID: 10642539.
- Intergovernmental Platform on Biodiversity and Ecosystem Services. Workshop report on biodiversity and pandemics. Bonn, Germany: IPBES secretariat. 2020.
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. Addendum: A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 Dec;588(7836):E6. doi: 10.1038/s41586-020-2951-z. PMID: 33199918.
- Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T. Bats: important reservoir hosts of emerging viruses. Clin Microbiol Rev. 2006 Jul;19(3):531-45. doi: 10.1128/CMR.00017-06. PMID: 16847084; PMCID: PMC1539106.
- Wacharapluesadee S, Tan CW, Maneeorn P, Duengkae P, Zhu F, Joyjinda Y, Kaewpom T, Chia WN, Ampoot W, Lim BL, Worachotsueptrakun K, Chen VC, Sirichan N, Ruchisrisarod C, Rodpan A, Noradechanon K, Phaichana T, Jantarat N, Thongnumchaima B, Tu C, Crameri G, Stokes MM, Hemachudha T, Wang LF. Evidence for SARS-CoV-2 related coronaviruses circulating in bats and pangolins in Southeast Asia. Nat Commun. 2021 Feb 9;12(1):972. doi: 10.1038/s41467-021-21240-1. PMID: 33563978; PMCID: PMC7873279.
- Sarkodie SA, Owusu PA. Impact of meteorological factors on COVID-19 pandemic: Evidence from top 20 countries with confirmed cases. Environ Res. 2020 Dec;191:110101. doi: 10.1016/j.envres.2020.110101. Epub 2020 Aug 22. PMID: 32835681; PMCID: PMC7442571.
- Qi H, Xiao S, Shi R, Ward MP, Chen Y, Tu W, Su Q, Wang W, Wang X, Zhang Z. COVID-19 transmission in Mainland China is associated with temperature and humidity: A time-series analysis. Sci Total Environ. 2020 Aug 1;728:138778. doi: 10.1016/j.scitotenv.2020.138778. Epub 2020 Apr 19. PMID: 32335405; PMCID: PMC7167225.
- Auler AC, Cássaro FAM, da Silva VO, Pires LF. Evidence that high temperatures and intermediate relative humidity might favor the spread of COVID-19 in tropical climate: A case study for the most affected Brazilian cities. Sci Total Environ. 2020 Aug 10;729:139090. doi: 10.1016/j.scitotenv.2020.139090. Epub 2020 Apr 28. PMID: 32388137; PMCID: PMC7194794.
- Batabyal AA. On some aspects of ecological resilience and the conservation of species. J Environ Manage. 1998 Apr;52(4):373-378. doi: 10.1006/jema.1998.0183.
- Pyšek P, Hulme PE, Simberloff D, Bacher S, Blackburn TM, Carlton JT, Dawson W, Essl F, Foxcroft LC, Genovesi P, Jeschke JM, Kühn I, Liebhold AM, Mandrak NE, Meyerson LA, Pauchard A, Pergl J, Roy HE, Seebens H, van Kleunen M, Vilà M, Wingfield MJ, Richardson DM. Scientists' warning on invasive alien species. Biol Rev Camb Philos Soc. 2020 Dec;95(6):1511-1534. doi: 10.1111/brv.12627. Epub 2020 Jun 25. PMID: 32588508; PMCID: PMC7687187.
- Nicas J. A slow-motion climate disaster: the spread of barren land. Section A, The New York Times; 2021. p.1. https://tinyurl.com/2dcrewyw
- Fundação SOS Mata Atlântica. Relatório anual. 2019. https://tinyurl.com/5fj9ut5a
- Pinheiro MPG, Silva-Inacio CL, Silva MMM, Araújo PSF, Ximenes MFFM. Potential vectors of Leishmania spp. in an Atlantic Forest conservation unit in northeastern Brazil under anthropic pressure. Parasit Vectors. 2021 Jan 11;14(1):38. doi: 10.1186/s13071-020-04523-2. PMID: 33430944; PMCID: PMC7798338.
- Silva MMM, Inácio CLS, Pinheiro MPG, Ximenes MFFM. Phlebotomines (Diptera: Psychodidae) and mosquitoes (Diptera: Culicidae) surrounding an environmental protection zone in the metropolitan region of Natal: use of light-emitting diode (LED) bulbs in entomological surveillance. Neotrop Entomol. 2020 Oct;49(5):768-779. doi: 10.1007/s13744-020-00802-w. Epub 2020 Aug 14. PMID: 32797397.
- Allen T, Murray KA, Zambrana-Torrelio C, Morse SS, Rondinini C, Di Marco M, Breit N, Olival KJ, Daszak P. Global hotspots and correlates of emerging zoonotic diseases. Nat Commun. 2017 Oct 24;8(1):1124. doi: 10.1038/s41467-017-00923-8. PMID: 29066781; PMCID: PMC5654761.
- Lira PK, Portela RC, Tambosi LR. Land-cover changes and an uncertain future: will the brazilian atlantic forest lose the chance to become a hopespot? In: Marques MCM, Grelle CEV, editors. The Atlantic Forest. 1st ed. Cham: Springer International Publishing; 2021. p.233-251. doi: 10.1007/978-3-030-55322-7_11.
- Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GA, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000 Feb 24;403(6772):853-8. doi: 10.1038/35002501. PMID: 10706275.
- Winfree R, Aguilar R, Vázquez DP, LeBuhn G, Aizen MA. A meta-analysis of bees' responses to anthropogenic disturbance. Ecology. 2009 Aug;90(8):2068-76. doi: 10.1890/08-1245.1. PMID: 19739369.
- Tabarelli M, Aguiar AV, Girão LC, Peres CA, Lopes AV. Effects of pioneer tree species hyperabundance on forest fragments in northeastern Brazil. Conserv Biol. 2010 Dec;24(6):1654-63. doi: 10.1111/j.1523-1739.2010.01529.x. PMID: 20497203.
- Melo FP, Pinto SR, Brancalion PH, Castro PS, Rodrigues RR, Aronson J et al. Priority setting for scaling-up tropical forest restoration projects: early lessons from the atlantic forest restoration pact. Environ Sci Policy. 2013 Nov;33:395-404. doi: 10.1016/j.envsci.2013.07.013.
- Instituto Brasileiro de Florestas. Bioma Mata Atlântica. 2020. https://tinyurl.com/bde4v7hh
- Ribeiro LB, Freire EM. Trophic ecology and foraging behavior of Tropidurus hispidus and Tropidurus semitaeniatus (Squamata, Tropiduridae) in a caatinga area of northeastern Brazil. Iheringia Ser Zool. 2011 Dec 13;101(3):225-232. doi: 10.1590/S0073-47212011000200010.
- Barbosa HA, Kumar TV, Paredes F, Elliott S, Ayuga JG. Assessment of caatinga response to drought using meteosat-SEVIRI normalized difference vegetation index (2008-2016). ISPRS J Photogramm Remote Sens. 2019 Feb;148:235-52. doi: 10.1016/j.isprsjprs.2018.12.014.
- Ab'Saber AN. Os domínios da natureza no Brasil: potencialidades paisagísticas. 3rd ed. São Paulo, SP, Brazil: Ateliê Editorial; 2003. p.160.
- Ministério do Meio Ambiente. Caatinga. 2015. https://tinyurl.com/bddt422m
- Andrade MJ, Sales RF, Freire EM. Ecology and diversity of a lizard community in the semiarid region of Brazil. Biota Neotrop. 2013 Jul-Sep;13(3):199-209. doi: 10.1590/S1676-06032013000300023.
- Costa WJ. Six new species of seasonal killifishes of the genus Cynolebias from the Săo Francisco river basin, brazilian caatinga, with notes on C. porosus (Cyprinodontiformes: Rivulidae). Ichthyol Explor Freshw. 2014 Aug;25(1):79-96.
- Queiroz LP, Cardoso D, Fernandes MF, Moro MF. Diversity and evolution of flowering plants of the caatinga domain. In: Silva JMC, Leal IR, Tabarelli M, editors. Caatinga: The Largest Tropical Dry Forest Region in South America. 1st ed. Cham: Springer; 2017. p.23-63.
- Amorim IL, Sampaio EV, Araújo EL. Fenologia de espécies lenhosas da caatinga do Seridó, RN. R Árvore. 2009 Jul 20;33(3):491-499. doi: 10.1590/S0100-67622009000300011.
- United Nations Educational, Scientific and Cultural Organization (UNESCO). Desertification; The UNESCO Courier, Paris, France. 2006. https://tinyurl.com/2s4zp8sx
- Souza BI, Menezes R, Artigas RC. Efeitos da desertificação na composição de espécies do bioma caatinga, Paraíba/Brasil. Invest Geog. 2015;88:45-59. doi: 10.14350/rig.44092.
- Torres RR, Lapola DM, Gamarra NL. Future climate change in the caatinga. In: Silva JMC, Leal IR, Tabarelli M, editors. Caatinga: The Largest Tropical Dry Forest Region in South America. 1st ed. Cham: Springer; 2017. p.383-410.
- Lapola DM, Martinelli LA, Peres CA, Ometto JPHB, Ferreira ME, Nobre CA, Aguiar APD, Bustamante MMC, Cardoso MF, Costa MH, Joly CA, Leite CC, Moutinho P, Sampaio G, Strassburg BBN, Vieira ICG. Pervasive transition of the Brazilian land-use system. Nat Clim Chang. 2014;4(1):27-35. doi: 10.1038/nclimate2056.
- Instituto Chico Mendes de Conservação da Biodiversidade (ICMBIO). Livro Vermelho da Fauna Brasileira Ameaçada de Extinção. 1st ed. Brasília: Ministério do Meio Ambiente (MMA); 2018. p.492.
- Intergovernmental Panel on Climate Change (IPCC). Climate change 2021: the physical science basis. Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change. 2021. https://tinyurl.com/4zwa4p9r
- Marengo JA, Bernasconi M. Regional differences in aridity/drought conditions over Northeast Brazil: present state and future projections. Clim Change. 2015;129(1):103-115. doi: 10.1007/s10584-014-1310-1.
- Silva-Inacio CL, Paiva AAP, Araújo JMG, Ximenes MFFM. Ecological relationships of Haemagogus spegazzinii (Diptera: Culicidae) in a semiarid area of Brazil. Rev Soc Bras Med Trop. 2020 Nov 25;53:e20200502. doi: 10.1590/0037-8682-0502-2020. PMID: 33263687; PMCID: PMC7723370.
- Ministério da Saúde (MS). Boletim epidemiológico 16: monitoramento dos casos de arboviroses até a semana epidemiológica 16 de 2022. 2022. https://tinyurl.com/28b6s5xr
- Garcia-Rejon JE, Navarro JC, Cigarroa-Toledo N, Baak-Baak CM. An updated review of the invasive Aedes albopictus in the Americas; geographical distribution, host feeding patterns, arbovirus infection, and the potential for vertical transmission of dengue virus. Insects. 2021 Oct 26;12(11):967. doi: 10.3390/insects12110967. PMID: 34821768; PMCID: PMC8621292.
- Nava A, Shimabukuro JS, Chmura AA, Luz SLB. The Impact of Global Environmental Changes on Infectious Disease Emergence with a Focus on Risks for Brazil. ILAR J. 2017 Dec 15;58(3):393-400. doi: 10.1093/ilar/ilx034. PMID: 29253158.
- Sharma R. Climate change impacts on human health in Bihar. Front Environ Microbiol. 2020 Sep;6(3):35-39. doi: 10.11648/j.fem.20200603.12.
- Loh EH, Zambrana-Torrelio C, Olival KJ, Bogich TL, Johnson CK, Mazet JA, Karesh W, Daszak P. Targeting transmission pathways for emerging zoonotic disease surveillance and control. Vector Borne Zoonotic Dis. 2015 Jul;15(7):432-7. doi: 10.1089/vbz.2013.1563. PMID: 26186515; PMCID: PMC4507309.
- Marcondes CB, Ximenes MF. Zika virus in Brazil and the danger of infestation by Aedes (Stegomyia) mosquitoes. Rev Soc Bras Med Trop. 2016 Feb;49(1):4-10. doi: 10.1590/0037-8682-0220-2015. Epub 2015 Dec 22. PMID: 26689277.
- Ali S, Gugliemini O, Harber S, Harrison A, Houle L, Ivory J, Kersten S, Khan R, Kim J, LeBoa C, Nez-Whitfield E, O'Marr J, Rothenberg E, Segnitz RM, Sila S, Verwillow A, Vogt M, Yang A, Mordecai EA. Environmental and Social Change Drive the Explosive Emergence of Zika Virus in the Americas. PLoS Negl Trop Dis. 2017 Feb 9;11(2):e0005135. doi: 10.1371/journal.pntd.0005135. PMID: 28182667; PMCID: PMC5300271.
- Althouse BM, Vasilakis N, Sall AA, Diallo M, Weaver SC, Hanley KA. Potential for Zika Virus to Establish a Sylvatic Transmission Cycle in the Americas. PLoS Negl Trop Dis. 2016 Dec 15;10(12):e0005055. doi: 10.1371/journal.pntd.0005055. PMID: 27977671; PMCID: PMC5157942.
- Ryan SJ, Carlson CJ, Tesla B, Bonds MH, Ngonghala CN, Mordecai EA, Johnson LR, Murdock CC. Warming temperatures could expose more than 1.3 billion new people to Zika virus risk by 2050. Glob Chang Biol. 2021 Jan;27(1):84-93. doi: 10.1111/gcb.15384. Epub 2020 Oct 28. PMID: 33037740; PMCID: PMC7756632.
- de Melo Ximenes MF, de Araújo Galvão JM, Inacio CLS, Macêdo E Silva VP, Pereira RLDN, Pinheiro MPG, de Medeiros Silva MM, Gomes CES. Arbovirus expansion: New species of culicids infected by the Chikungunya virus in an urban park of Brazil. Acta Trop. 2020 Sep;209:105538. doi: 10.1016/j.actatropica.2020.105538. Epub 2020 May 23. PMID: 32454032.
- de Carvalho JA, Teixeira SRF, de Carvalho MP, Vieira V, Alves FA. Doenças emergentes: uma análise sobre a relaçăo do homem com o seu ambiente. Rev. Práx. 2009 Jan;1(1):19-23. doi: 10.47385/praxis.v1.n1.539.
- Pignatti MG. Saúde e ambiente: as doenças emergentes no Brasil. Ambient. Soc. 2004;7(1):133-147. doi: 10.1590/S1414-753X2004000100008.
- Otshudiema JO, Ndakala NG, Mawanda EK, Tshapenda GP, Kimfuta JM, Nsibu LN, Gueye AS, Dee J, Philen RM, Giese C, Murrill CS, Arthur RR, Kebela BI. Yellow Fever Outbreak - Kongo Central Province, Democratic Republic of the Congo, August 2016. MMWR Morb Mortal Wkly Rep. 2017 Mar 31;66(12):335-338. doi: 10.15585/mmwr.mm6612a5. PMID: 28358796; PMCID: PMC5657954.
- Ortiz-Martínez Y, Patiño-Barbosa AM, Rodriguez-Morales AJ. Yellow fever in the Americas: the growing concern about new epidemics. F1000Res. 2017 Mar 30;6:398. doi: 10.12688/f1000research.11280.2. PMID: 28529708; PMCID: PMC5414809.
- Ribeiro M, Antunes CM. Febre amarela: estudo de um surto. Rev Soc Bras Med Trop. 2009 Sep-Oct;42(5):523-31. doi: 10.1590/s0037-86822009000500009. PMID: 19967234.
- Soper FL, Penna H, Cardoso E, Serafim J. Jr, Frobisher M. Jr, Pinheiro J. Yellow fever without aedes aegypti. Study of a rural epidemic in the valle do chanaan, espirito santo, Brazil, 1932. Am J Epidemiol. 1933;18(3):555-587. doi: 10.1093/oxfordjournals.aje.a117967.
- Cunha MS, Faria NR, Caleiro GS, Candido DS, Hill SC, Claro IM, da Costa AC, Nogueira JS, Maeda AY, da Silva FG, de Souza RP, Spinola R, Tubaki RM, de Menezes RMT, Abade L, Mucci LF, Timenetsky MDCST, Sabino E. Genomic evidence of yellow fever virus in Aedes scapularis, southeastern Brazil, 2016. Acta Trop. 2020 May;205:105390. doi: 10.1016/j.actatropica.2020.105390. Epub 2020 Feb 7. PMID: 32044285.
- Marcondes CB, Alencar J. Revisão de mosquitos Haemagogus Williston (Diptera: Culicidae) do Brasil. Rev Biomed. 2010;21(3):221-238. https://tinyurl.com/36u2fyv4
- Medeiros AS, Marcondes CB, De Azevedo PR, Jerônimo SM, e Silva VP, Ximenes MF. Seasonal variation of potential flavivirus vectors in an urban biological reserve in northeastern Brazil. J Med Entomol. 2009 Nov;46(6):1450-7. doi: 10.1603/033.046.0630. PMID: 19960696.
- Inácio CLS, Silva JHT, Freire RCM, Gama RA, Marcondes CB, Ximenes MFFM. Checklist of Mosquito Species (Diptera: Culicidae) in the Rio Grande do Norte State, Brazil-Contribution of Entomological Surveillance. J Med Entomol. 2017 May 1;54(3):763-773. doi: 10.1093/jme/tjw236. PMID: 28399203.
- Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ. West Nile virus. Lancet Infect Dis. 2002 Sep;2(9):519-29. doi: 10.1016/s1473-3099(02)00368-7. PMID: 12206968.
- Castro-Jorge LA, Siconelli MJL, Ribeiro BDS, Moraes FM, Moraes JB, Agostinho MR, Klein TM, Floriano VG, Fonseca BALD. West Nile virus infections are here! Are we prepared to face another flavivirus epidemic? Rev Soc Bras Med Trop. 2019 Mar 28;52:e20190089. doi: 10.1590/0037-8682-0089-2018. PMID: 30942263.
- Martins LC, Silva EVPD, Casseb LMN, Silva SPD, Cruz ACR, Pantoja JAS, Medeiros DBA, Martins Filho AJ, Cruz EDRMD, Araújo MTF, Cardoso JF, Cunha MACRD, Almada GL, Romano APM, Santos MGDP, Rodrigues GAP, Chiang JO, Quaresma JAS, Carvalho VL, Vasconcelos PFDC. First isolation of West Nile virus in Brazil. Mem Inst Oswaldo Cruz. 2019 Jan 17;114:e180332. doi: 10.1590/0074-02760180332. PMID: 30672980; PMCID: PMC6343470.
- Costa ÉA, Giovanetti M, Catenacci LS, Fonseca V, Aburjaile FF, Chalhoub FLL, Xavier J, Iani FCM, Vieira MACS, Henriques DF, Medeiros DBA, Guedes MIMC, Santos BSAS, Silva ASG, Maranhão RPA, Faria NRC, Siqueira RF, de Oliveira T, Cavalcante KRLJ, Oliveira de Moura NF, Romano APM, Albuquerque CFC, Feitosa LCS, Bayeux JJM, Teixeira RBC, Lobato OL, Silva SC, Bispo de Filippis AM, Cunha RV, Lourenço J, Alcantara LCJ. West Nile virus in Brazil. Pathogens. 2021 Jul 15;10(7):896. doi: 10.3390/pathogens10070896. PMID: 34358046; PMCID: PMC8308589.
- Anderson CR, Downs WG, Wattley GH, Ahin NW, Reese AA. Mayaro virus: a new human disease agent. II. Isolation from blood of patients in Trinidad, B.W.I. Am J Trop Med Hyg. 1957 Nov;6(6):1012-6. doi: 10.4269/ajtmh.1957.6.1012. PMID: 13487973.
- Mackay IM, Arden KE. Mayaro virus: a forest virus primed for a trip to the city? Microbes Infect. 2016 Dec;18(12):724-734. doi: 10.1016/j.micinf.2016.10.007. Epub 2016 Oct 27. PMID: 27989728.
- Mota MTO, Ribeiro MR, Vedovello D, Nogueira ML. Mayaro virus: a neglected arbovirus of the Americas. Future Virol. 2015;10(9):1109-1122. doi: 10.2217/fvl.15.76.
- Pauvolid-Corrêa A, Juliano RS, Campos Z, Velez J, Nogueira RM, Komar N. Neutralising antibodies for Mayaro virus in Pantanal, Brazil. Mem Inst Oswaldo Cruz. 2015 Feb;110(1):125-33. doi: 10.1590/0074-02760140383. Epub 2015 Feb 3. PMID: 25742272; PMCID: PMC4371226.
- Azevedo RS, Silva EV, Carvalho VL, Rodrigues SG, Nunes-Neto JP, Monteiro H, Peixoto VS, Chiang JO, Nunes MR, Vasconcelos PF. Mayaro fever virus, Brazilian Amazon. Emerg Infect Dis. 2009 Nov;15(11):1830-2. doi: 10.3201/eid1511.090461. PMID: 19891877; PMCID: PMC2857233.
- Ganjian N, Riviere-Cinnamond A. Mayaro virus in Latin America and the Caribbean. Rev Panam Salud Publica. 2020 Feb 11;44:e14. doi: 10.26633/RPSP.2020.14. PMID: 32051685; PMCID: PMC7008609.
- Jansen AM, Xavier SC, Roque AL. The multiple and complex and changeable scenarios of the Trypanosoma cruzi transmission cycle in the sylvatic environment. Acta Trop. 2015 Nov;151:1-15. doi: 10.1016/j.actatropica.2015.07.018. Epub 2015 Jul 19. PMID: 26200785.
- Gürtler RE, Cardinal MV. Reservoir host competence and the role of domestic and commensal hosts in the transmission of Trypanosoma cruzi. Acta Trop. 2015 Nov;151:32-50. doi: 10.1016/j.actatropica.2015.05.029. Epub 2015 Jun 5. PMID: 26051910.
- Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo AM, Teixeira MM, Schijman AG, Llewellyn MS, Lages-Silva E, Machado CR, Andrade SG, Sturm NR. The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect Genet Evol. 2012 Mar;12(2):240-53. doi: 10.1016/j.meegid.2011.12.009. Epub 2011 Dec 27. PMID: 22226704.
- Dias JCP. Human chagas disease and migration in the context of globalization: some particular aspects. J Trop Med. 2013;2013:789758. doi: 10.1155/2013/789758. Epub 2013 Mar 30. PMID: 23606862; PMCID: PMC3625591.
- Lima VS, Xavier SC, Maldonado IF, Roque AL, Vicente AC, Jansen AM. Expanding the knowledge of the geographic distribution of Trypanosoma cruzi TcII and TcV/TcVI genotypes in the Brazilian Amazon. PLoS One. 2014 Dec 31;9(12):e116137. doi: 10.1371/journal.pone.0116137. PMID: 25551227; PMCID: PMC4281250.
- World Health Organization. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rep. 2015;90(6):33-44. https://tinyurl.com/yc7fv6rj
- da Costa AP, Nunes PH, Leite BHS, Ferreira JIGDS, Tonhosolo R, da Rosa AR, da Rocha PA, Aires CC, Gennari SM, Marcili A. Diversity of bats trypanosomes in hydroeletric area of Belo Monte in Brazilian Amazonia. Acta Trop. 2016 Dec;164:185-193. doi: 10.1016/j.actatropica.2016.08.033. Epub 2016 Sep 12. PMID: 27633579.
- Patz JA, Daszak P, Tabor GM, Aguirre AA, Pearl M, Epstein J, Wolfe ND, Kilpatrick AM, Foufopoulos J, Molyneux D, Bradley DJ; Working Group on Land Use Change and Disease Emergence. Unhealthy landscapes: Policy recommendations on land use change and infectious disease emergence. Environ Health Perspect. 2004 Jul;112(10):1092-8. doi: 10.1289/ehp.6877. PMID: 15238283; PMCID: PMC1247383.
- Vaz VC, D'Andrea PS, Jansen AM. Effects of habitat fragmentation on wild mammal infection by Trypanosoma cruzi. Parasitology. 2007 Nov;134(Pt 12):1785-93. doi: 10.1017/S003118200700323X. Epub 2007 Jul 26. PMID: 17651530.
- Thompson RC, Kutz SJ, Smith A. Parasite zoonoses and wildlife: emerging issues. Int J Environ Res Public Health. 2009 Feb;6(2):678-93. doi: 10.3390/ijerph6020678. Epub 2009 Feb 13. PMID: 19440409; PMCID: PMC2672361.
- Xavier SC, Roque AL, Lima Vdos S, Monteiro KJ, Otaviano JC, Ferreira da Silva LF, Jansen AM. Lower richness of small wild mammal species and chagas disease risk. PLoS Negl Trop Dis. 2012;6(5):e1647. doi: 10.1371/journal.pntd.0001647. Epub 2012 May 15. PMID: 22616021; PMCID: PMC3352825.
- Jansen AM, Xavier SCDC, Roque ALR. Trypanosoma cruzi transmission in the wild and its most important reservoir hosts in Brazil. Parasit Vectors. 2018 Sep 6;11(1):502. doi: 10.1186/s13071-018-3067-2. PMID: 30189896; PMCID: PMC6127949.
- Porfirio GEO, Santos FM, de Macedo GC, Barreto WTG, Campos JBV, Meyers AC, André MR, Perles L, de Oliveira CE, Xavier SCDC, Andrade GB, Jansen AM, Herrera HM. Maintenance of Trypanosoma cruzi, T. evansi and Leishmania spp. by domestic dogs and wild mammals in a rural settlement in Brazil-Bolivian border. Int J Parasitol Parasites Wildl. 2018 Oct 17;7(3):398-404. doi: 10.1016/j.ijppaw.2018.10.004. PMID: 30370220; PMCID: PMC6199764.
- Lima MM, Sarquis O, de Oliveira TG, Gomes TF, Coutinho C, Daflon-Teixeira NF, Toma HK, Britto C, Teixeira BR, D'Andrea PS, Jansen AM, Bóia MN, Carvalho-Costa FA. Investigation of Chagas disease in four periurban areas in northeastern Brazil: epidemiologic survey in man, vectors, non-human hosts and reservoirs. Trans R Soc Trop Med Hyg. 2012 Mar;106(3):143-9. doi: 10.1016/j.trstmh.2011.10.013. Epub 2011 Dec 1. PMID: 22136953.
- Argolo AM, Felix M, Pacheco R, Costa J. Doença de chagas e seus principais vetores no Brasil. Rio de Janeiro: Imperial Novo Milênio. 2008;63. doi: 10.13140/2.1.1578.9449.
- Barbosa-Silva AN, Souza RCM, Diotaiuti L, Aguiar LMA, Câmara ACJD, Galvão LMDC, Chiari E. Synanthropic triatomines (Hemiptera: Reduviidae): infestation, colonization, and natural infection by trypanosomatids in the State of Rio Grande do Norte, Brazil. Rev Soc Bras Med Trop. 2019 Jul 18;52:e20190061. doi: 10.1590/0037-8682-0061-2019. PMID: 31340365.
- Belisário CJ, Pessoa GC, Silva EM, Rosa AC, Ferreira RE, Bedin C, Wilhelms T, de Mello F, Coutinho HS, Fonseca EL, Dos Santos RF, Rodrigues VL, Dias JC, Diotaiuti L. Genetic characterization of residual Triatoma infestans populations from Brazil by microsatellite. Genetica. 2017 Feb;145(1):105-114. doi: 10.1007/s10709-017-9949-y. Epub 2017 Jan 24. PMID: 28120213.
- Pan American Health Organization (PAHO). Leishmaniasis: epidemiological report in the Americas. 2020 Dec;9. https://tinyurl.com/5ev9xhrd
- Lainson R, Shaw JJ. Epidemiology and ecology of Leishmaniasis in Latin-America. Nature. 1978 Jun 22;273(5664):595-600. doi: 10.1038/273595a0. PMID: 351409.
- Costa CH, Pereira HF, Araújo MV. Epidemia de leishmaniose visceral no estado do Piauí, Brasil, 1980-1986 [Visceral Leishmaniasis epidemic in the State of Piauí, Brazil, 1980-1986]. Rev Saude Publica. 1990 Oct;24(5):361-72. Portuguese. doi: 10.1590/s0034-89101990000500003. PMID: 2101528.
- Werneck GL, Rodrigues L, Santos MV, Araújo IB, Moura LS, Lima SS, Gomes RB, Maguire JH, Costa CH. The burden of Leishmania chagasi infection during an urban outbreak of visceral Leishmaniasis in Brazil. Acta Trop. 2002 Jul;83(1):13-8. doi: 10.1016/s0001-706x(02)00058-x. PMID: 12062788.
- Werneck GL, Costa CH, Walker AM, David JR, Wand M, Maguire JH. Multilevel modelling of the incidence of visceral Leishmaniasis in Teresina, Brazil. Epidemiol Infect. 2007 Feb;135(2):195-201. doi: 10.1017/S0950268806006881. Epub 2006 Jul 7. PMID: 16824254; PMCID: PMC2870576.
- Ministério da Saúde (MS). Casos confirmados de leishmaniose visceral, Brasil, grandes regiőes e Unidades Federadas 1990 a 2013. 2014. https://tinyurl.com/2p8u6dzd
- Ministério da Saúde (MS). Departamento de informática do SUS (DATASUS). Epidemiológicas e morbidade. 2021. https://tinyurl.com/2p9bzs7f
- da Silva Santana Cruz C, Soeiro Barbosa D, Oliveira VC, Cardoso DT, Guimarães NS, Carneiro M. Factors associated with human visceral Leishmaniasis cases during urban epidemics in Brazil: a systematic review. Parasitology. 2021 May;148(6):639-647. doi: 10.1017/S0031182021000019. Epub 2021 Jan 12. PMID: 33431094.
- Jeronimo SM, Duggal P, Braz RF, Cheng C, Monteiro GR, Nascimento ET, Martins DR, Karplus TM, Ximenes MF, Oliveira CC, Pinheiro VG, Pereira W, Peralta JM, Sousa J, Medeiros IM, Pearsoni RD, Burns TL, Pugh EW, Wilson ME. An emerging peri-urban pattern of infection with Leishmania chagasi, the protozoan causing visceral Leishmaniasis in northeast Brazil. Scand J Infect Dis. 2004;36(6-7):443-9. doi: 10.1080/00365540410020451. PMID: 15307565.
- Lima ID, Lima ALM, Mendes-Aguiar CO, Coutinho JFV, Wilson ME, Pearson RD, Queiroz JW, Jeronimo SMB. Changing demographics of visceral Leishmaniasis in northeast Brazil: Lessons for the future. PLoS Negl Trop Dis. 2018 Mar 6;12(3):e0006164. doi: 10.1371/journal.pntd.0006164. PMID: 29509765; PMCID: PMC5839541.
- Cavalcante FRA, Cavalcante KKS, Florencio CMGD, Moreno JO, Correia FGS, Alencar CH. Human visceral Leishmaniasis: epidemiological, temporal and spacial aspects in Northeast Brazil, 2003-2017. Rev Inst Med Trop Sao Paulo. 2020 Feb 14;62:e12. doi: 10.1590/S1678-9946202062012. PMID: 32074215; PMCID: PMC7032011.
- Ximenes MF, Castellón EG, de Souza MF, Freitas RA, Pearson RD, Wilson ME, Jerônimo SM. Distribution of phlebotomine sand flies (Diptera: Psychodidae) in the state of Rio Grande do Norte, Brazil. J Med Entomol. 2000 Jan;37(1):162-9. doi: 10.1603/0022-2585-37.1.162. PMID: 15218921.
- Ximenes Mde F, Silva VP, Queiroz PV, Rego MM, Cortez AM, Batista LM, Medeiros AS, Jeronimio SM. Flebotomíneos (Diptera: Psychodidae) e leishmanioses no Rio Grande do Norte, Nordeste do Brasil: reflexos do ambiente antrópico [Phlebotomine (Diptera: Psychodidae) and Leishmaniasis in Rio Grande do Norte State Brazil: anthropic environment responses]. Neotrop Entomol. 2007 Jan-Feb;36(1):128-37. Portuguese. doi: 10.1590/s1519-566x2007000100016. PMID: 17420871.
- Ximenes MF, Castellón EG, de Souza MF, Menezes AA, Queiroz JW, Macedo e Silva VP, Jerônimo SM. Effect of abiotic factors on seasonal population dynamics of Lutzomyia longipalpis (Diptera: Psychodidae) in northeastern Brazil. J Med Entomol. 2006 Sep;43(5):990-5. doi: 10.1093/jmedent/43.5.990. PMID: 17017238.104. World Health Organization. Control of neglected tropical diseases. 2020a. https://tinyurl.com/2p9wv84j
- World Health Organization. Control of neglected tropical diseases. 2020a. https://tinyurl.com/2p9wv84j
- World Health Organization. Ending the neglect to attain the sustainable development goals: a road map for neglected tropical disesases 2021-2030. Overview. 2020b. https://tinyurl.com/5n8jxefc
- Ximenes Mde F, Castellón EG, De Souza Mde F, Menezes AA, Queiroz JW, Macedo e Silva VP, Jerônimo SM. Effect of abiotic factors on seasonal population dynamics of Lutzomyia longipalpis (Diptera: Psychodidae) in northeastern Brazil. J Med Entomol. 2006 Sep;43(5):990-5. PMID: 17017238.
- World Health Organization. Ending the neglect to attain the sustainable development goals: a road map for neglected tropical disesases 2021-2030. Overview. 2020b. https://tinyurl.com/5n8jxefc
- Pinheiro MP, Silva JH, Cavalcanti KB, de Azevedo PR, de Melo Ximenes MF. Ecological interactions among phlebotomines (Diptera: Psychodidae) in an agroforestry environment of northeast Brazil. J Vector Ecol. 2013 Dec;38(2):307-16. doi: 10.1111/j.1948-7134.2013.12045.x. PMID: 24581360.
- Dantas-Torres F. Canine leishmaniosis in South America. Parasit Vectors. 2009 Mar 26;2 Suppl 1(Suppl 1):S1. doi: 10.1186/1756-3305-2-S1-S1. PMID: 19426440; PMCID: PMC2679393.
- Macedo-Silva VP, Martins DR, De Queiroz PV, Pinheiro MP, Freire CC, Queiroz JW, Dupnik KM, Pearson RD, Wilson ME, Jeronimo SM, Ximenes MF. Feeding preferences of Lutzomyia longipalpis (Diptera: Psychodidae), the sand fly vector, for Leishmania infantum (Kinetoplastida: Trypanosomatidae). J Med Entomol. 2014 Jan;51(1):237-44. doi: 10.1603/me12131. PMID: 24605474; PMCID: PMC4277188.
- Kumar R, Bunn PT, Singh SS, Ng SS, Montes de Oca M, De Labastida Rivera F, Chauhan SB, Singh N, Faleiro RJ, Edwards CL, Frame TCM, Sheel M, Austin RJ, Lane SW, Bald T, Smyth MJ, Hill GR, Best SE, Haque A, Corvino D, Waddell N, Koufariotis L, Mukhopadhay P, Rai M, Chakravarty J, Singh OP, Sacks D, Nylen S, Uzonna J, Sundar S, Engwerda CR. Type I Interferons Suppress Anti-parasitic Immunity and Can Be Targeted to Improve Treatment of Visceral Leishmaniasis. Cell Rep. 2020 Feb 25;30(8):2512-2525.e9. doi: 10.1016/j.celrep.2020.01.099. PMID: 32101732; PMCID: PMC7981274.
- Cardim MF, Rodas LA, Dibo MR, Guirado MM, Oliveira AM, Chiaravalloti-Neto F. Introduction and expansion of human American visceral Leishmaniasis in the state of Sao Paulo, Brazil, 1999-2011. Rev Saude Publica. 2013 Aug;47(4):691-700. doi: 10.1590/S0034-8910.2013047004454. PMID: 24346660.
- Walsh JF, Molyneux DH, Birley MH. Deforestation: effects on vector-borne disease. Parasitology. 1993;106 Suppl:S55-75. doi: 10.1017/s0031182000086121. PMID: 8488073.
- Patz JA, Campbell-Lendrum D, Holloway T, Foley JA. Impact of regional climate change on human health. Nature. 2005 Nov 17;438(7066):310-7. doi: 10.1038/nature04188. PMID: 16292302.
- World Health Organization and United Nations International Children’s Emergency Fund. Progress on sanitation and drinking water: 2015 update and MDG assessment. 2015. https://tinyurl.com/mt54hwm6
- Marengo JA, Torres RR, Alves LM. Drought in Northeast Brazil-past, present, and future. Theor Appl Climatol. 2017;129(3):1189-1200. doi: 10.1007/s00704-016-1840-8.
- Waldman EA, Sato AP. Path of infectious diseases in Brazil in the last 50 years: an ongoing challenge. Rev Saude Publica. 2016 Dec 22;50:68. doi: 10.1590/S1518-8787.2016050000232. PMID: 28099652; PMCID: PMC5152805.
- Paiva RFDPS, Souza MFDP. Associação entre condições socioeconômicas, sanitárias e de atenção básica e a morbidade hospitalar por doenças de veiculação hídrica no Brasil [Association between socioeconomic, health, and primary care conditions and hospital morbidity due to waterborne diseases in Brazil]. Cad Saude Publica. 2018 Feb 5;34(1):e00017316. Portuguese. doi: 10.1590/0102-311X00017316. PMID: 29412314.
- Lapola DM, Braga DR, Di Giulio GM, Torres RR, Vasconcellos MP. Heat stress vulnerability and risk at the (super) local scale in six Brazilian capitals. Clim Change. 2019;154(3):477-492. doi: 10.1007/s10584-019-02459-w.
- Sévêque A, Gentle LK, López-Bao JV, Yarnell RW, Uzal A. Human disturbance has contrasting effects on niche partitioning within carnivore communities. Biol Rev Camb Philos Soc. 2020 Dec;95(6):1689-1705. doi: 10.1111/brv.12635. Epub 2020 Jul 14. PMID: 32666614.
- Ellwanger JH, Veiga ABG, Kaminski VL, Valverde-Villegas JM, Freitas AWQ, Chies JAB. Control and prevention of infectious diseases from a One Health perspective. Genet Mol Biol. 2021 Jan 29;44(1 Suppl 1):e20200256. doi: 10.1590/1678-4685-GMB-2020-0256. PMID: 33533395; PMCID: PMC7856630.
- Lerner H, Berg C. A Comparison of Three Holistic Approaches to Health: One Health, EcoHealth, and Planetary Health. Front Vet Sci. 2017 Sep 29;4:163. doi: 10.3389/fvets.2017.00163. PMID: 29085825; PMCID: PMC5649127.
- Kopenawa D, Albert B. A queda do céu: Palavras de um xamã Yanomami. 1st ed. São Paulo: Companhia das Letras; 2020. p.768.
- Krenak A. Ideias para adiar o fim do mundo. 1st ed. São Paulo: Companhia das Letras; 2019. p.64.
- Branco AFVC, Lima PVPSL, Medeiros Filho ES, Costa BMG, Pereira TP. Avaliação da perda da biodiversidade na Mata Atlântica. Ciência Florest. 2021 Nov 17;31(4):1885-909. doi: https://doi.org/10.5902/1980509853310.
- Mendoza-Roldan JA, Mendoza-Roldan MA, Otranto D. Reptile vector-borne diseases of zoonotic concern. Int J Parasitol Parasites Wildl. 2021 Apr 22;15:132-42. doi: 10.1016/j.ijppaw.2021.04.007. PMID: 34026483; PMCID: PMC8121771.
- Carnaval AC, Waltari E, Rodrigues MT, Rosauer D, VanDerWal J, Damasceno R, Prates I, Strangas M, Spanos Z, Rivera D, Pie MR, Firkowski CR, Bornschein MR, Ribeiro LF, Moritz C. Prediction of phylogeographic endemism in an environmentally complex biome. Proc Biol Sci. 2014 Oct 7;281(1792):20141461. doi: 10.1098/rspb.2014.1461. PMID: 25122231; PMCID: PMC4150330.
- Viani RAG, Barreto TE, Farah FT, Rodrigues RR, Brancalion PHS. Monitoring Young Tropical Forest Restoration Sites: How Much to Measure? Trop Conserv Sci. 2018;11:1-9. doi: 10.1177/1940082918780916.
- Safar NVH, Magnago LFS, Schaefer CEGR. Resilience of lowland Atlantic forests in a highly fragmented landscape: Insights on the temporal scale of landscape restoration. For Ecol Manage. 2020 Aug;470-471:118183. doi: 10.1016/j.foreco.2020.118183.
- Demange LHML. Ecological resilience: the role played by individuals, companies and the State. Rev Direito Ambient. 2016;82:17-35.
- Althoff TD, Menezes RSC, Carvalho ALD, Pinto ADS, Santiago GACF, Ometto JPHB, Randow C, Sampaio EVSB. Climate change impacts on the sustainability of the firewood harvest and vegetation and soil carbon stocks in a tropical dry forest in Santa Teresinha Municipality, Northeast Brazil. For Ecol Manage. 2016;360:367-375. doi: 10.1016/j.foreco.2015.10.001.
- Gomes VP, Galvíncio JD, Moura MS, Ferreira PS, Paz YM, Miranda RQ. 2016. Sensoriamento remoto hyperspectral aplicado para análise dos indicadores de resiliência e suscetibilidade do bioma caatinga frente às mudanças climáticas. Rev Bras Geogr Fís. 2016;9(4):1122-1136. doi: 10.26848/rbgf.v09.4.p1122-1136.
- Ministério do Meio Ambiente. Programa de ação nacional de combate à desertificação e mitigação dos efeitos da seca PAN-Brasil. 2005. https://tinyurl.com/drkxfa2n
- Barreto ML, Teixeira MG, Bastos FI, Ximenes RA, Barata RB, Rodrigues LC. Successes and failures in the control of infectious diseases in Brazil: social and environmental context, policies, interventions, and research needs. Lancet. 2011 May 28;377(9780):1877-89. doi: 10.1016/S0140-6736(11)60202-X. Epub 2011 May 9. PMID: 21561657.
- Kuiava VA, Perin AT, Chielle EO. Hospitalization and mortality rates by diarrhea in Brazil: 2000-2015. Ciênc. Saúde. 2019 Aug 02;12(2):e30022. doi: 10.15448/1983-652X.2019.2.30022.
- Doniec K, Dall'Alba R, King L. Brazil's health catastrophe in the making. Lancet. 2018 Sep 1;392(10149):731-732. doi: 10.1016/S0140-6736(18)30853-5. Epub 2018 Jul 20. PMID: 30037732.
- Fearnside PM. Will the next coronavirus come from Amazonia? Deforestation and the risk of infectious diseases (commentary). Mongabay-News & Inspiration from Nature’s Frontline. 2020. https://tinyurl.com/yntv9s7r
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.