Medicine Group . 2022 May 24;3(5):585-587. doi: 10.37871/jbres1483.
Perfect is the Enemy of the Good: Outflow Tract Rupture During Self-Expandable Transcatheter Aortic Valve Implantation
Giuseppe Verolino1,2*, Andres Agustin Vecchia1, Luca Sandrelli1, Mario Fabbrocini1 and Paolo Cioffi1
2Invasive Cardiology, Istituto Auxologico Italiano, Milano, Italy
- TAVR; Annular rupture
- Surgical management
- Stand-by cardiac surgery
- LVOT calcifications
Abstract
Annular rupture is an ambiguous term that refers to a series of infrequent but life-threatening transcatheter aortic valve replacement complications associated with anatomical and clinical risk factors that often require urgent surgical management. We describe a case of annular rupture following self-expandable valve post-dilatation, successfully treated with urgent surgery.
Abbreviations
TAVR: Transcatheter Aortic Valve Replacement; LVOT: Left Ventricle Outflow Tract; RA: Rheumatoid Arthritis; TURB: Transurethral Resection of Bladder; ECG: Electrocardiogram; CT: Computed Tomography; PVL: Paravalvular Leak; BEV: Balloon Expandable Valve
Case report
An 84-year-old male with a recent diagnosis of severe Aortic Stenosis (AS) was admitted to our institution for progressive dyspnea that had worsened in the last couple of months. Transthoracic echocardiography showed a preserved left ventricular ejection fraction, concentric hypertrophy, and a severely calcified stenotic aortic valve (mean gradient 61 mmHg, vMax 4.52 m/sec, VTI AVA 0.6 cm2). After a multidisciplinary Heart Team discussion, TAVR was recommended due to the patient’s advanced age, comorbidities, and favorable femoral anatomy.
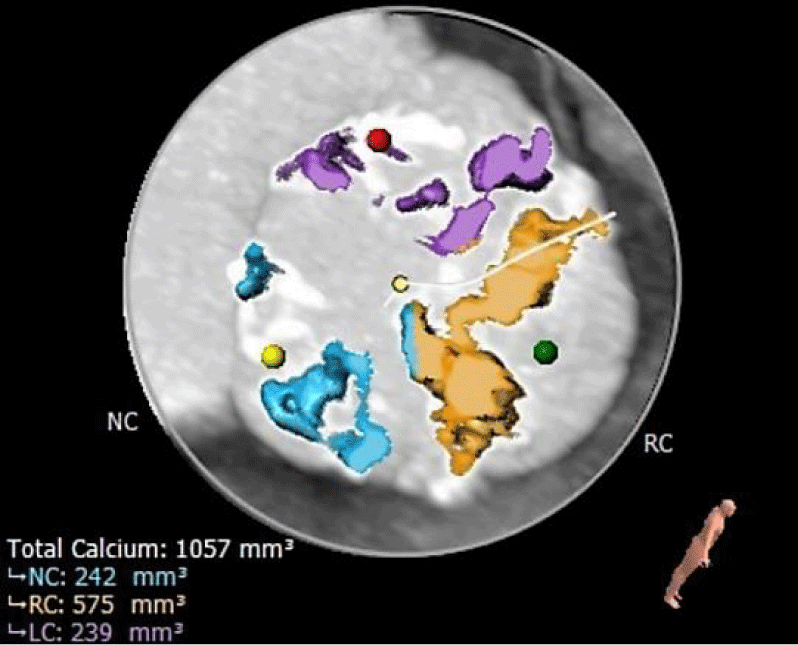
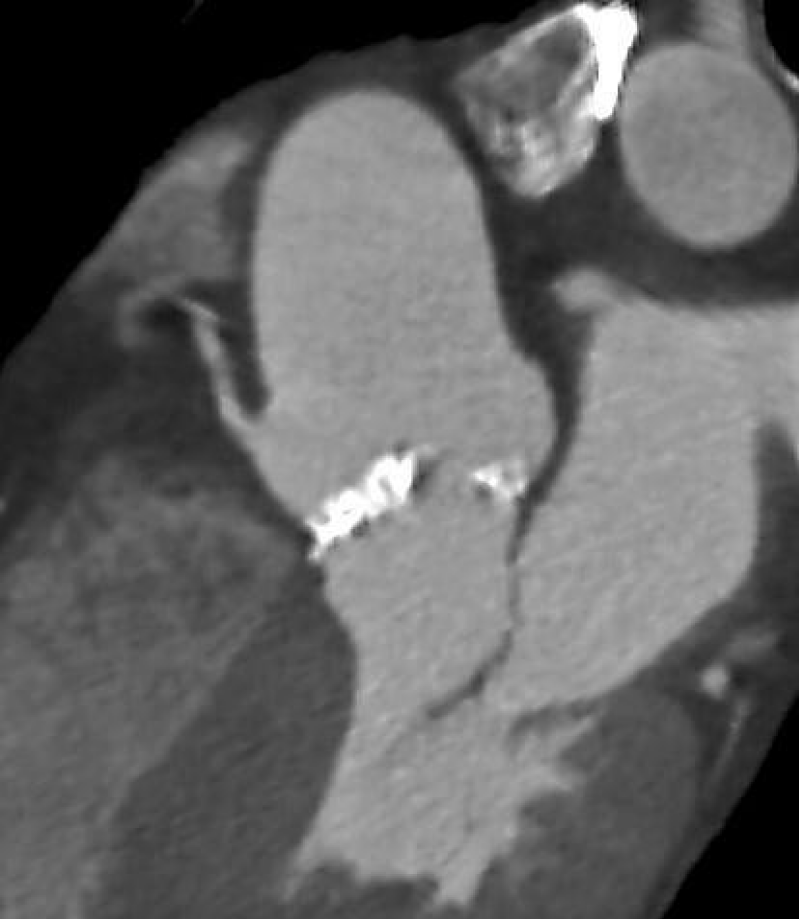
ECG-gated angio-CT showed a tricuspid aortic valve with moderate valvular calcifications); the right coronary cusp was particularly calcified (Figures 1,2). The annular perimeter was 82 mm, with a diameter derived perimeter of 26 mm. Other than a small 3 mm calcification under the non-coronary cusp, the LVOT was otherwise free from calcium (Figure 3). The mean diameter of the LVOT was 25 mm. Common femoral arteries were of adequate caliber, without significant tortuosity.
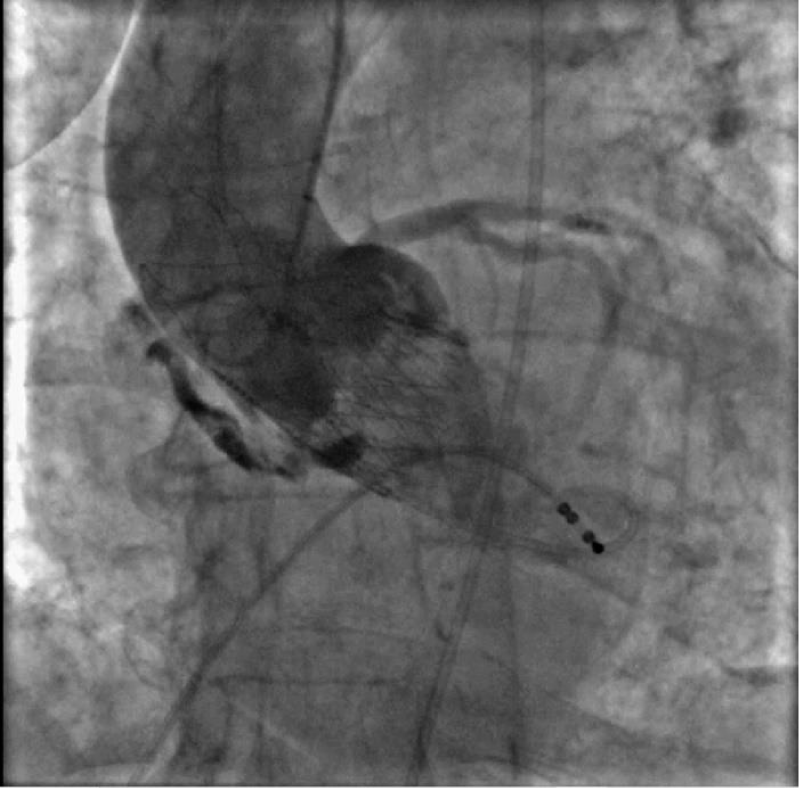
On the day of the procedure, echo-guided right femoral artery and left femoral vein accesses were obtained. The right radial artery was used as the secondary access for aortic angiography. After preclosure with two suture-based systems (ProGlide, Abbott), a 14F introducer (iSleeve, Boston Scientific) was positioned in the right femoral artery. Using an Amplatz-1 catheter, the aortic valve was crossed, gradients were obtained and the severity of the aortic stenosis was confirmed (LV-AO gradient of 60 mmHg). After placing a stiff guide, a pre-dilatation with a 22 mm valvuloplasty balloon was performed. After valve alignment in cusp overlap projection, a self-expandable Accurate Neo L valve was implanted according to protocol. Reevaluation of transvalvular gradients showed a residual 20 mmHg gradients and angiography revealed a moderate paravalvular leak. Given these findings, post-dilatation at nominal pressure with a 25 mm balloon under rapid ventricular pacing was performed. An acute hemodynamic deterioration was evident immediately after post-dilatation. Aortography showed contrast extravasation compatible with annular rupture (Figure 4). Echocardiogram showed pericardial tamponade and an urgent pericardiocentesis was performed. Approximately 1000 ml of blood was drained and auto-transfused through the left femoral vein, while the patient was transferred to the operating room for surgical repair. After sternotomy, a significant LVOT injury was evident at the level right/non-coronary commissure. The LVOT was reconstructed from the inside using an autologous pericardial patch through a transaortic approach. Aortic valve replacement with a Trifecta 19 mm valve was performed. The remaining surgery was uneventful.
Discussion
Annular rupture during TAVR is an infrequent but life-threatening complication that occurs in less than 1% of procedures [1]. The most important risk factors associated with annular damage are aggressive valve/balloon oversizing and calcium distribution. Oversizing is considered a condition that is necessary but not sufficient in it to determine an annular rupture [2]. A normal annulus tolerates overexpansion fairly well; in an animal model, annular dilatation with balloons less than or equal to 1.7 times the aortic annulus produced no relevant damage [3]. However, calcified annuluses which are frequently present in TAVR patients are more prone to rupture. In a recently published study, the annular rupture was 10 times more frequent in patients with moderate or severe LVOT calcifications and with the use of BEV in this group of patients [4]. The LVOT in our patient was fairly free from calcium except from a small 3 mm zone underneath the non-coronary cusp (Figure 3). Given that the rupture was near this zone we believe it may have contributed to the outcome.
Conclusion
Annular rupture is an infrequent but feared complication of TAVR. Although sometimes unpredictable, knowing the associated risk factors may help reduce its incidence. Further, as our case clearly exemplifies, surgical standby is fundamental to successfully managing landing zone injuries during TAVR.
References
- Pineda AM, Harrison JK, Kleiman NS, Rihal CS, Kodali SK, Kirtane AJ, Leon MB, Sherwood MW, Manandhar P, Vemulapalli S, Beohar N. Incidence and outcomes of surgical bailout during TAVR: Insights from the STS/ACC TVT registry. JACC Cardiovasc Interv. 2019 Sep 23;12(18):1751-1764. doi: 10.1016/j.jcin.2019.04.026. PMID: 31537276.
- Barbanti M, Yang TH, Rodès Cabau J, Tamburino C, Wood DA, Jilaihawi H, Blanke P, Makkar RR, Latib A, Colombo A, Tarantini G, Raju R, Binder RK, Nguyen G, Freeman M, Ribeiro HB, Kapadia S, Min J, Feuchtner G, Gurtvich R, Alqoofi F, Pelletier M, Ussia GP, Napodano M, de Brito FS Jr, Kodali S, Norgaard BL, Hansson NC, Pache G, Canovas SJ, Zhang H, Leon MB, Webb JG, Leipsic J. Anatomical and procedural features associated with aortic root rupture during balloon-expandable transcatheter aortic valve replacement. Circulation. 2013 Jul 16;128(3):244-253. doi: 10.1161/CIRCULATIONAHA.113.002947. Epub 2013 Jun 7. PMID: 23748467.
- Moore JW, Laird JR, White CJ, Kirby WC, Ramee SR, Banks AK, Ross TC, Graeber GM, Wahl RC. Tolerance of normal aorta to oversized dual balloon valvuloplasty. Observations in a swine model: technical note. Cardiovasc Intervent Radiol. 1990 Apr-May;13(2):107-110. doi: 10.1007/BF02577363. PMID: 2143690.
- Okuno T, Asami M, Heg D, Lanz J, Praz F, Hagemeyer D, Brugger N, Gräni C, Huber A, Spirito A, Räber L, Stortecky S, Windecker S, Pilgrim T. Impact of left ventricular outflow tract calcification on procedural outcomes after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2020 Aug 10;13(15):1789-1799. doi: 10.1016/j.jcin.2020.04.015. PMID: 32763071.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.