Medicine Group . 2022 April 22;3(4):373-384. doi: 10.37871/jbres1451.
miR-497-5p Enhances the Chemosensitivity of Non-Small Cell Lung Cancer Cells to Cisplatin via Targeting of the CDCA4 Gene
Nasir Azam1, Shuo Yang1, Khalil Ur Rahman1, Jiawen Yu3, Chunhui Zhao2* and Bin Feng1*
2College of Life Sciences, Liaoning Normal University, Dalian, China
3Department of Hematology, the First Affiliated Hospital of Dalian Medical University, Dalian, China
- miR-497-5p
- Chemosensitivity
- Non-small cell lung cancer
- Cisplatin
- CDCA4
Abstract
Non-Small Cell Lung Cancer (N-SCLC) accounts for almost 85% of all diagnosed lung cancer and the prognosis remains poor usually because of assimilated drug resistance including cisplatin. The miR-497-5p family has been discovered to play a significant role in regulating biological functions in N-SCLC. The purpose of this study was to investigate the molecular mechanism of miR-497-5p and its target gene on modulating cisplatin chemosensitivity in N-SCLC cells. The enhanced chemosensitivity effect of miR-497-5p to cisplatin in A549 and H1299 cells was detected by MTT method. Dual luciferase reporter assay, quantitative Real-Time PCR (qRT-PCR) and Western blotting were performed to demonstrate that miR-497-5p directly targets CDCA4 to reduce the expression. Transwell, colony formation and flow cytometry assays showed that combination of miR-497-5p and cisplatin exerted stronger effects on inhibiting N-SCLC cells proliferation, migration and invasion as well as promoting apoptosis and G1 phase arrest than miR-497-5p and cisplatin alone. The same tendency was observed in the upregulation of apoptosis-related protein Bax and Cytochrome-C and downregulation of cycle-related proteins CyclinB1 and CDK1. Our results indicate that upregulation of miR-497-5p targets CDCA4 directly and may function as an important modifier to sensitize N-SCLC cells to cisplatin.
Introduction
Lung cancer is the foremost malignant tumor globally and remains the leading cause of cancer death. Although there have been ground-breaking advances in initial diagnosis and treatment, metastasis-mediated progression represents the main cause of cancer-related mortality. Pathologically, lung cancer can be divided into two main types: Small Cell Lung Cancer (SCLC) and Non-Small Cell Lung Cancer (N-SCLC). NSCLC accounts for almost 85% of all diagnosed lung cancer and the prognosis remains poor [1]. Patients at level IV are mostly treated with chemotherapy to get rid of symptoms, with only 2% 5-year survival rate [2]. Many different chemotherapeutic agents have recently been used to target N-SCLC. So far, cisplatin is one of the most effective platinum-containing first-line drugs and can inhibit progression of N-SCLC [3]. However, because of regular administration of cisplatin, its efficacy is usually compromised by assimilated drug resistance [4]. Therefore, advanced and effective approaches are necessary to increase cisplatin sensitivity and reduce the mortality rate of NSCLC.
As noncoding RNAs, miRNAs (20-24 nucleotides) regulate the expression of multiple gene mRNAs by binding with complementary 3' untranslated regions (3'-UTRs). It is predicted that miRNAs can bind with over 50% of all the human coding genes. miRNAs play different regulatory roles in various physiological and developmental procedures such as cell apoptosis, proliferation, development and differentiation through interfering with expression of mRNA at transcriptional or post-transcriptional levels [5,6]. The irregularly expressed miRNAs, such as miR-1254, miR-1253, miR-504, miR-26a-5p and miR-574-5p, are recognized as biomarkers in the diagnosis and subcategorization of N-SCLC [7-9]. miRNAs such as miR-224 and miR-211 are considered as the key regulators in the pathology of lung cancer and inhibit NSCLC progression [10,11]. Some miRNAs contribute to drug sensitivity of human cancers. Inhibition of miR-328 enhances the chemosensitivity of NSCLC to cisplatin [12]. It is reported that miR-497-5p may serve as a tumor suppressor in different human cancers. Chen et al. found that miR-497-5p inhibits cell proliferation and invasion of angiosarcoma by targeting an oncogenic potassium channel KCa3.1 gene [13]. miR-497-5p also decreases several malignant activities in melanoma cells [14] in human glioma cells miR-497 enhances temozolomide resistance [15]. Therefore, to investigate the molecular mechanisms of cisplatin resistance and increase its efficacy synergistically with miRNA may support clinicians to improve drug efficacy and diagnosis of NSCLC.
Cell Division Cycle‑Associated protein 4 (CDCA4), also called SEI‑3/Hematopoietic Progenitor Protein (HEPP), has a distinctive role in cell cycle regulation and represses E2F‑dependent transcriptional activation and cell proliferation through the E2F/Rb protein pathway [16]. CDCA4 is also involved in the spindle formation and distribution in prometaphase, chromosomal mid‑mitotic stages, segregation, and cytoplasmic division [17]. Thus, more studies of this family of proteins, including CDCA4, could offer a better understanding of their role in cancer and provide a novel target for miR-497-5p in clinical treatment of N-SCLC. In the present study, we investigated the effect of miR-497-5p and the target gene CDCA4 on enhancing the chemosensitivity of N-SCLC cells to cisplatin.
Materials and Methods
Cell culture and reagents
The human lung cancer cells A549 and H1299 were obtained from the Chinese Academy of Sciences (Shanghai, China). Cells were cultured with RPMI 1640 (Hyclone, Logan, UT, USA) medium containing 10% fetal bovine serum (FBS; Invitrogen Gibco, Carlsbad, CA, USA), and penicillin (100 U/L) and streptomycin (100 mg/L) at 37°C in an incubator with 5% CO2. miR-497-5p mimics, inhibitor and Native Control (NC) were purchased from Shanghai GenePharma Co. Ltd. (China). Cell transfection was performed with Lipofectamine 2000 (Invitrogen).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
A549 and H1299 cells were plated at 104 cells per well in 96-well plates and cultured overnight. Attached cells were transfected with miR-497-5p mimics of 400 pM concentration using Lipofectamine 2000. After 6 h incubation, minimal essential medium was replaced with complete 10% RPMI 1640 containing cisplatin 2-12 μM, and incubated for 24 and 48 h, then replaced with 180 µl fresh complete Dulbecco’s modified Eagle’s medium followed by 20 µl MTT solution to measure cell viability. After incubation for 4 h, MTT medium was replaced with 150 µl dimethyl sulfoxide and mixed for 10 min. A microplate reader was used to measure the absorbance at 492 nm and a dose-response curve was used to calculate IC50 of cisplatin.
Immunofluorescence analysis of Ki67
A549 cells were cultured in 24-well plates overnight, treated with miR-497-5p mimics, inhibitor, NC and cisplatin, and miR-497-5p mimics combined with cisplatin. After 24 h, the cells were washed twice with Phosphate-Buffered Saline (PBS), fixed with 4% paraformaldehyde and treated with 0.2% Triton X-100 for 10 min. After each treatment, cells were washed twice with PBS and blocked with 1% bovine serum albumin for 1 h. Cells were incubated with anti-Ki67 primary antibody (Abcam, Cambridge, MA, USA) at 4°C overnight, and goat anti-rabbit Alexa-Fluor-594-conjugated secondary antibody (Life Technologies, Carlsbad, CA, USA). The nuclei were stained for 20 min with 4',6-diamidino-2-phenylindole and an Olympus IX71 fluorescence microscope was used to visualize the fluorescence signals.
Total RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated using TRIzol reagent (Invitrogen) after A549 cells were treated with miR-497-5p mimics, cisplatin and cisplatin+miR-497-5p mimics for 24 h. Total RNA (1 µg) was used for cDNA synthesis with a First Strand cDNA Synthesis Kit (TaKaRa Biotechnology Co. Ltd., Dalian, China). qRT-PCR was performed using One Step SYBR Prime Script™ RT-PCR Kit II (TaKaRa). GAPDH gene was used as internal control and compared with CDCA4 gene expression. All the experiments were performed in triplicate and gene expression level was analyzed using the comparative threshold method (2−ΔΔCT).
Plasmid construction and dual-luciferase reporter assay
Targetscan (http://www.targetscan.org) and miRanda (http://www.microrna.org/microrna) bioinformatics tools were used to predict the target gene of miR-497-5p, and the 3'-UTR sequence of CDCA4 gene was amplified with primer 1 (5'-CCGGAGCTCGTCTGTTTGTAGATCACAGGCACCAG-3', underline sequences indicate SacI site) and primer 2 (5'-CCGGTCGACCAGCACCCATGACACTACAGATCAAG-3', underline sequences indicate SalI site). The amplified sequence was purified and introduced into the pGLO vector (Promega, Madison, WI, USA) to produce pGLO-CDCA4-WT. To generate mutated pGLO-CDCA4-MUT vector, the seed sequence of miR-497-5p was mutated with overlapping PCR method using primers (5'-ATAAGCAATGCTACTTCCTCACCCA-3') and (5'TGAGGAAGTAGCATTGCTTATGGATGAGAAACG-3') combined with the above two primers. The recombinant plasmids were confirmed by enzyme digestion and subjected to sequence analysis (Sangon Biotech, Shanghai, China).
293-T cells were cotransfected with pGLO-CDCA4-WT, pGLO-CDCA4-MUT and miR-497-5p mimics or NC mimics with Lipofectamine 2000 for 48 h. Then, the Ranilla and firefly activity was measured by a dual luciferase reporter assay system (Promega).
Transwell assay
A549 cells were transfected in six-well plates with miR-497-5p mimics, inhibitor and NC at a concentration of 400 pM and treated with 6 μM cisplatin combined with miR-497-5p mimics for 24 h. For cell migration analysis, Transwell inserts (Corning Inc., Corning, NY, USA) containing polycarbonate filters with pores of 8 μm were placed in 24-well plates. For cell invasion analysis, Transwell filters precoated with Matrigel matrix (BD Biosciences, San Jose, CA, USA) were inserted and 2 × 104 cells were seeded in the upper chamber with 200 μl 1% FBS, while the lower chamber was filled with 500 μl complete RPMI-1640 medium and incubated for 16 h. The cells were fixed with methanol on the lower surface of the filters, then migrating and invading cells were stained and counted under an inverted microscope in five random fields of view (20 × objective).
Cell cycle and apoptosis analysis by flow cytometry
To analyze the cell cycle, A549 cells were collected after 24 h treatment with miR-497-5p mimics, inhibitor, NC (400 pM) and cisplatin (6 µM) combined with miRNA mimics. The cells were washed with PBS, centrifuged and resuspended with ice-cold 70% ethanol and incubated at 4°C overnight in the dark. RNaseA was added and cells were incubated at 37°C for 30 min, stained with Propidium Iodide (PI) with the cell cycle detection kit (KeyGEN BioTECH, Shanghai, China) at 4°C for 30 min in the dark. Cell samples were kept on ice and the cell cycle was detected by a CytoFLEX flow cytometer (Beckman Coulter, Brea, CA, USA).
Apoptosis was measured in A549 and H1299 cells after 12 h treatment using the same concentration of miRNA mimics and cisplatin as for the cell cycle analysis, with untreated cells as a control group. EDTA-free trypsinization was performed to collect the cells, which were washed twice with ice-cold PBS. The cells were resuspended in binding buffer and stained with annexin V-fluorescein isothiocyanate and PI. The fluorescent intensity and the numbers of apoptotic cells were expressed as a percentage of the total number of cells in each treatment using flow cytometric analysis.
Colony formation assay
After A549 cells were treated for 24 h with miR-497-5p mimics, inhibitor, NC, cisplatin and cisplatin+miR-497-5p, the cells were collected and 500 cells per well were plated in six-well plates for 14 days of incubation at 37°C. The cells were washed twice with PBS and fixed with methanol for 20 min. Cells stained with 0.1% crystal violet were washed twice with PBS and the number of colonies was counted under an inverted microscope and representative images were photographed.
Western blotting
A549 and H1299 cells were treated with miR-497-5p mimics, NC, inhibitor, cisplatin and cisplatin+miR-497-5p for 24 h. After washing with PBS, the cells were lysed in RIPA buffer (Sigma, St. Louis, MO, USA) to extract protein. Equivalent amount of proteins were subjected to 12% SDS-PAGE and the separated proteins were transferred to polyvinylidene difluoride membranes followed by 2 h blocking in Tris-buffered saline–Tween (TBST) with 5% fat-free dried milk. Membrane strips were incubated with primary antibodies against Proliferating Cell Nuclear Antigen (PCNA), CDCA4, Bax, cytochrome-C, cyclin B1 and CDK4 (all rabbit polyclonal) from Proteintech (SanYing Biotechnology, Wuhan, China) overnight at 4°C, then washed with TBST and incubated with horseradish-peroxidase-conjugated secondary antibodies for 2 h at room temperature. Protein bands were imaged on a ChemiDoc MP imaging system (Bio-Rad, Hercules, CA, USA) with enhanced chemiluminescent substrate kit (Thermo Scientific, Rockford, IL, USA). Image-Pro Plus software was used to quantify the intensity of expressed signal of protein. β-Actin was used as an internal control.
Bioinformatics analysis
To analyze the expression level of the CDCA4 gene in cancer and normal tissues in N-SCLC patients, a web-based tool, UALCAN (http://ualcan.path.uab.edu), which explores and collects evidence of various genes from The Cancer Genome Atlas (TCGA), was used. The survival curve was analyzed in the website http://kmplot.com.
Statistical analysis
Student’s t-test and one-way analysis of variance in GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA) were used to determine significant differences between control and treated groups. Data were expressed as the mean ± SD and all the experiments were performed in triplicate. p < 0.05 was considered statistically significant.
Results
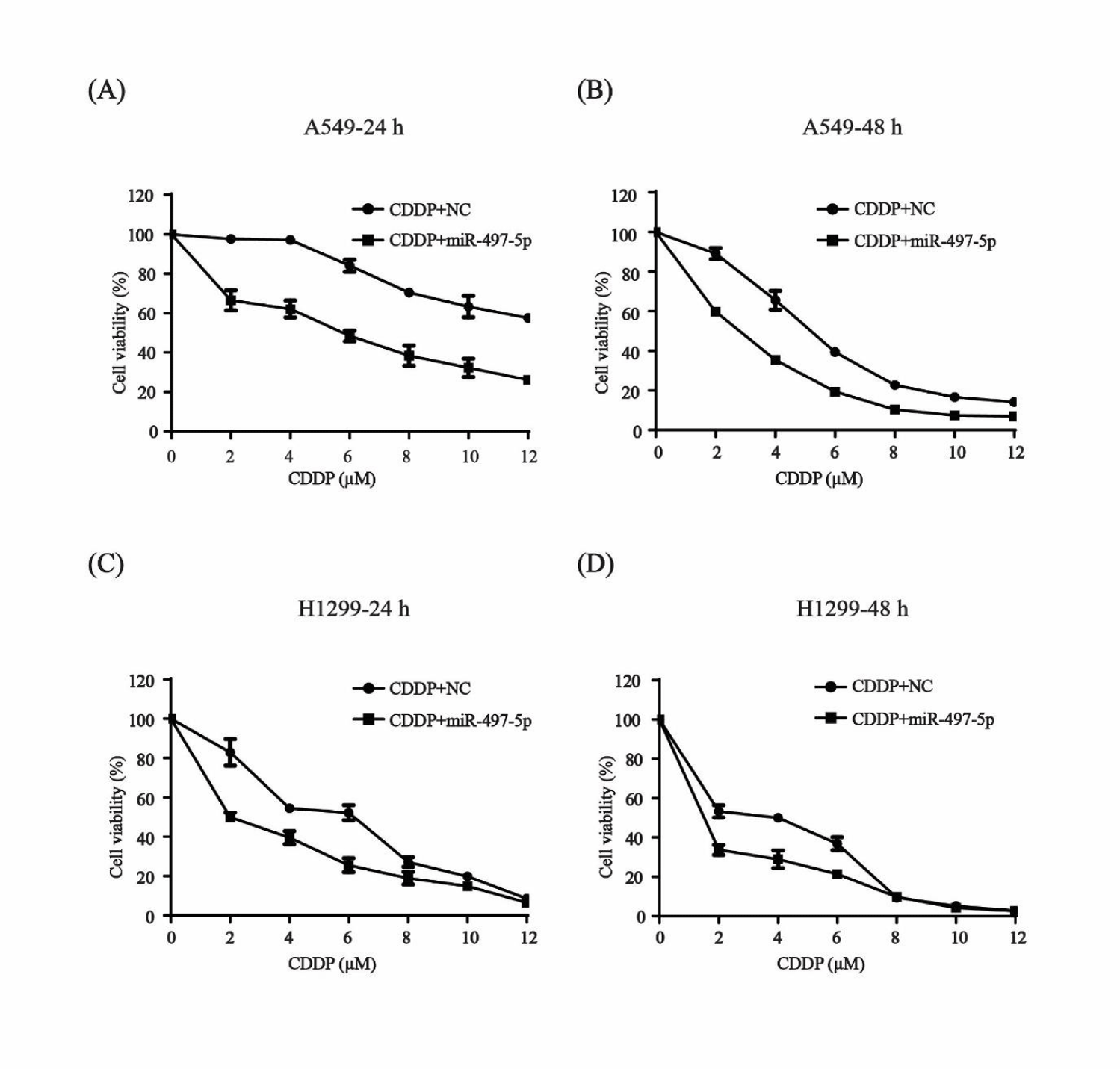
miR-497-5p increases the sensitivity of N-SCLC cells to cisplatin
Lung cancer cells A549 and H1299 were used to check whether miR-497-5p had a role in the sensitivity of N-SCLC cells to cisplatin. After transfection with miR-497-5p mimics and NC, the cells were exposed to 2-12 µM cisplatin for 24 and 48 h and an MTT assay was performed. Cell viability was significantly inhibited in both of the cell lines after combination treatment with miR-497-5p mimics and with increasing concentration of cisplatin compared to the control group treated with miR-NC and cisplatin (Figure 1). These results showed that miR-497-5p increased the sensitivity of N-SCLC cells to cisplatin.
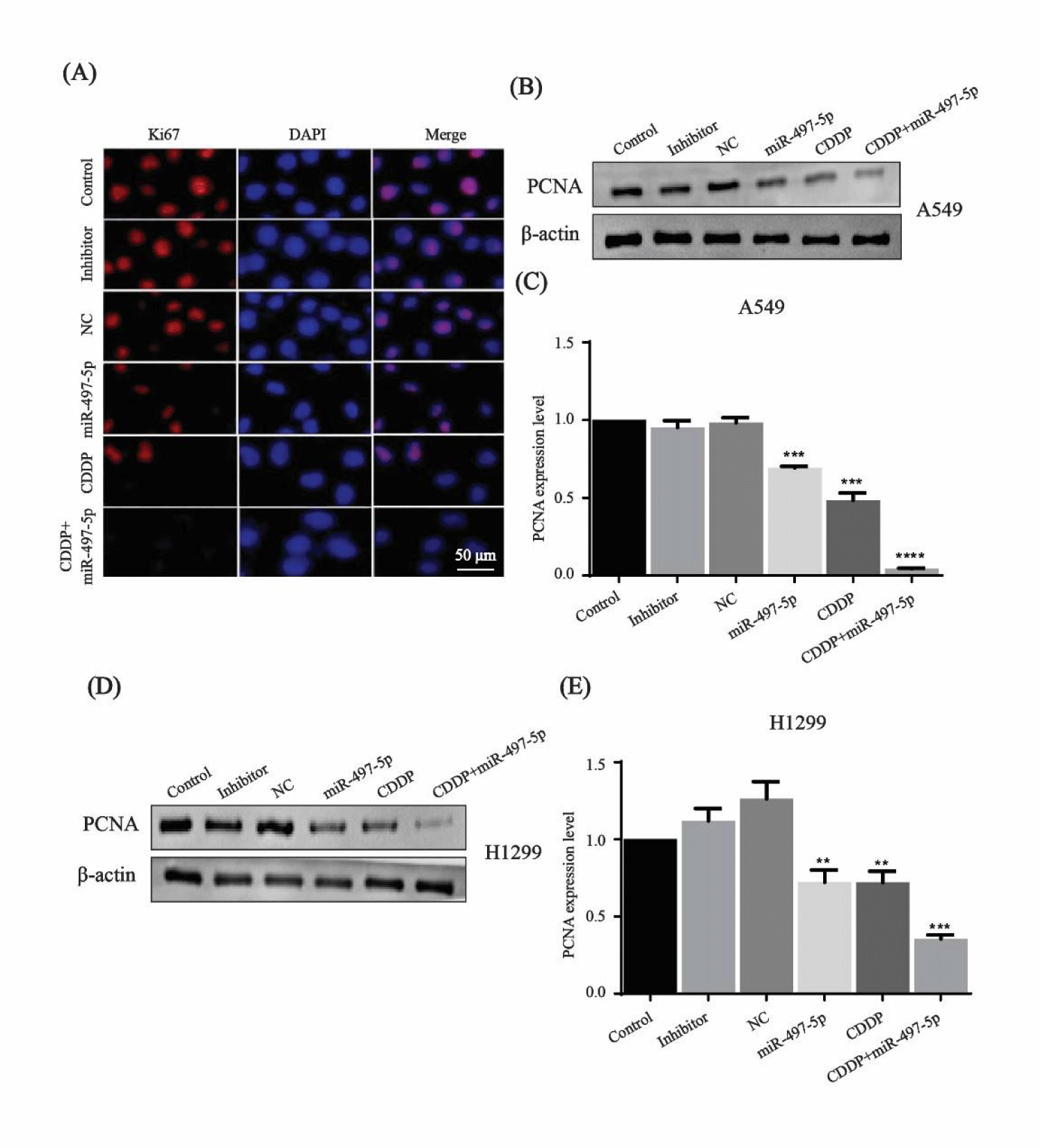
Effect of miR-497-5p and cisplatin combination on cell proliferation
The expression of Ki67 protein was observed with a fluorescence microscope after A549 cells were treated with miR-497-5p, cisplatin and their combination. The fluorescence intensity was significantly attenuated after treatment with miR-497-5p mimics, cisplatin and their combination as compared to that of control, inhibitor and NC groups (Figure 2A). Western blotting of PCNA expression level confirmed the increased antiproliferative effects of miR-497-5p, cisplatin and combined treatment in both of the cell lines (Figures 2B-E). Immunofl uorescence results of Ki67 and Western blotting analysis of PCNA indicated that miR-497-5p and cisplatin treatment both inhibited cell proliferation, while combined treatment had a greater effect than miR-497-5p or cisplatin alone.
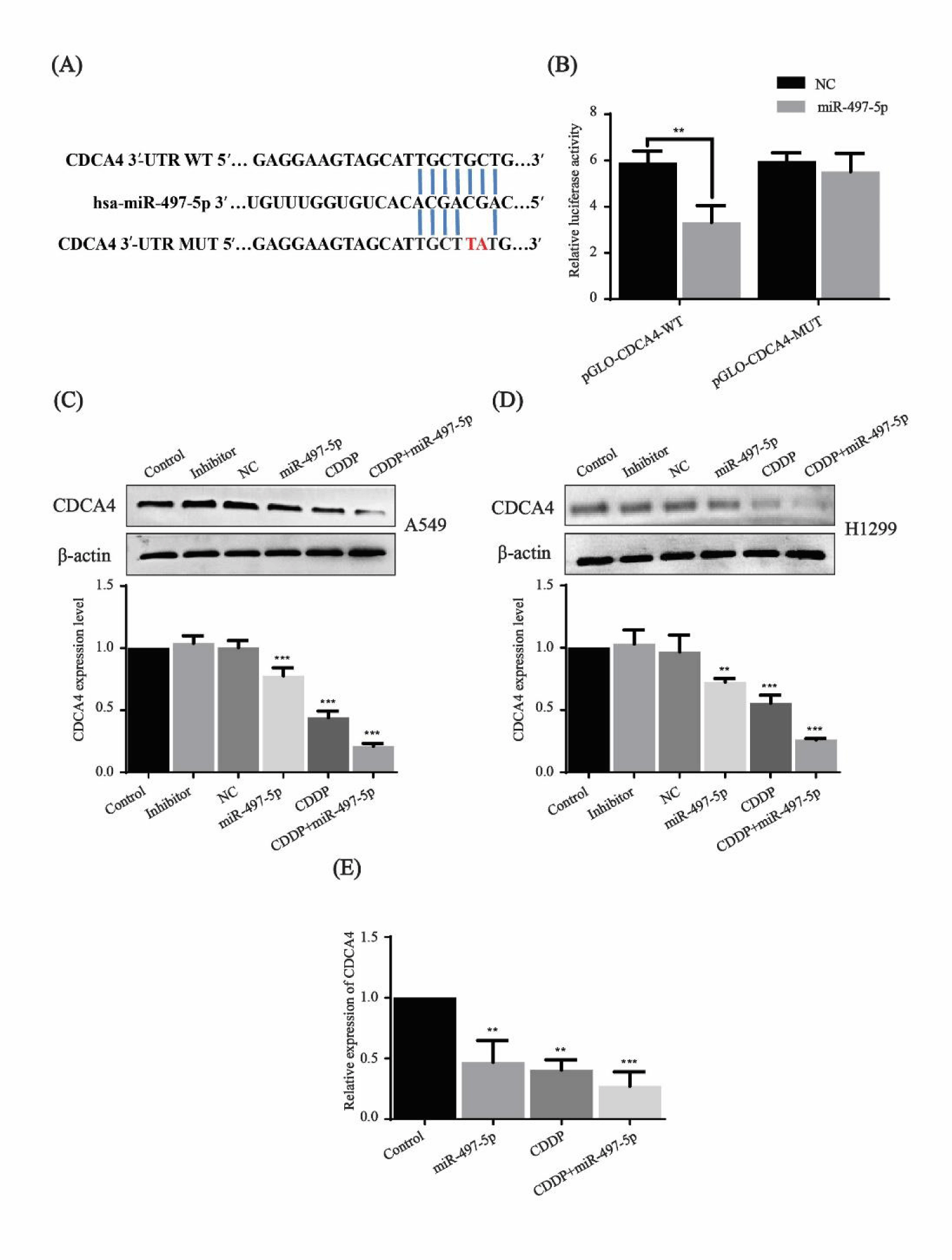
CDCA4 is a direct target gene of miR-497-5p and inhibited by miR-497-5p and cisplatin
miRNA target prediction algorithm TargetScan revealed that the CDCA4 gene may be the potential target of miR-497-5p (Figure 3A). To identify whether miR-497-5p directly bound to the 3′-UTR of the CDCA4 gene, the seed region TGCTGCT was mutated by TGCTTAT, and 293T cells were used to perform the dual luciferase reporter assay. As shown in figure 3B, cotransfection of miR-497-5p and pGLO-CDCA4-WT caused a significant decrease in fluorescence intensity compared to the control groups.
The effect of miR-497-5p and cisplatin on the CDCA4 expression level was further examined. Compared with the control, inhibitor and NC groups, CDCA4 protein expression was downregulated in both of the cell lines A549 and H1299 after treatment with miR-497-5p mimics, cisplatin and their combination, while combined treatment had a greater effect than miR-497-5p and cisplatin alone (Figures 3C,D). qRT-PCR also showed that the relative mRNA expression level of CDCA4 decreased significantly with miR-497-5p and cisplatin treatment, with combination treatment showing a greater effect (Figure 3E). The above results suggest that CDCA4 mRNA is the direct target of miR-497-5p and regulated at both mRNA and protein levels.
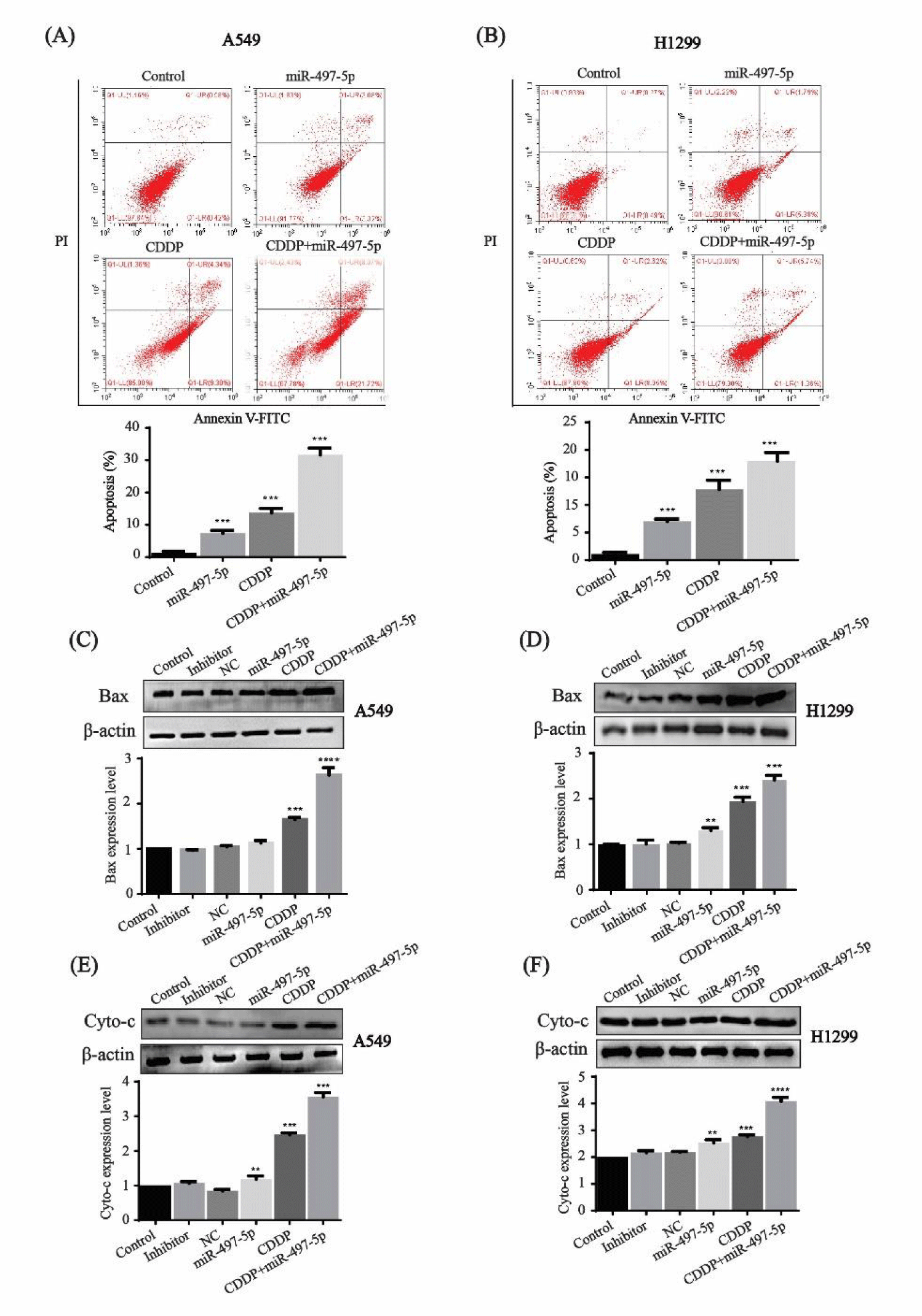
miR-497-5p increases cisplatin-induced apoptosis in N-SCLC cells
The flow cytometric analysis showed that the percentage of apoptotic cells after miR-497-5p and cisplatin treatment was 6.4% and 13.64%, respectively, while combined with miR-497-5p treatment, the percentage of apoptotic cells was significantly higher at 29.79% in A549 cells (Figure 4A). In H1299 cells treated with miR-497-5p or cisplatin, the percentage of apoptotic cells was 7.17% and 11.57%, respectively, while the percentage increased to 17.1% in the combination treatment (Figure 4B).
The expression level of apoptosis-related proteins Bax and Cytochrome-C was tested in both of the cell lines. Bax expression was increased significantly in miR-497-5p- or cisplatin-treated cells compared with control, inhibitor and NC groups, while combination treatment had a greater effect on A549 and H1299 cells (Figures 4C,D). Cytochrome-C expression showed a similar tendency, in which combination treatment exerted the highest expression on the two cell lines (Figures 4E,F). These results show that overexpression of miR-497-5p enhances cisplatin-induced apoptosis in N-SCLC cells.
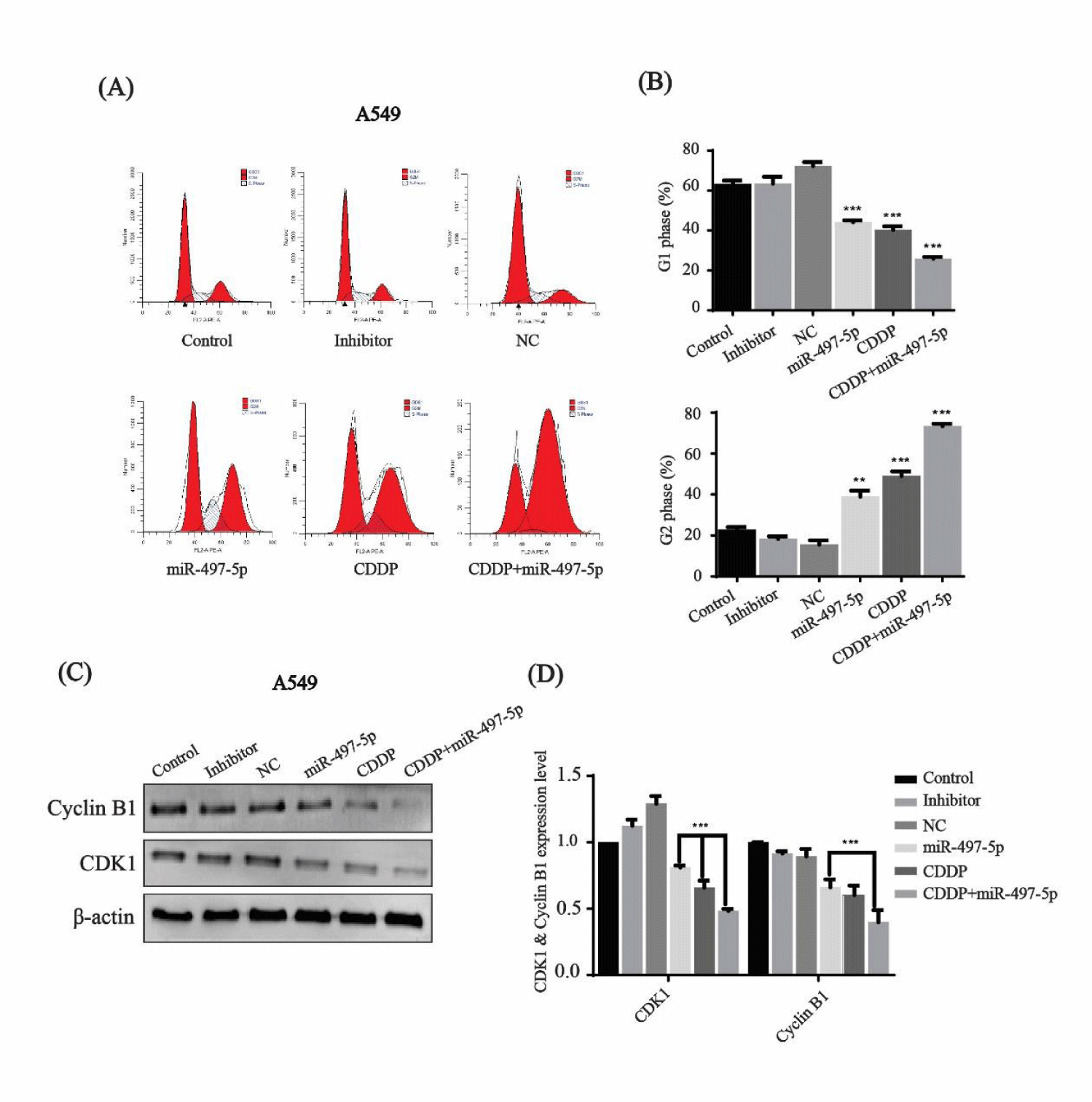
Effect of miR-497-5p and cisplatin on the cell cycle
The cell cycle percentage distribution in A549 cells was checked by flow cytometry after treatment with miR-497-5p, cisplatin, and their combination. The cell percentage in G1 phase decreased to 44.16 %, 40.68 %, and 24.24 %, while the number of cells in G2 phase increased up to 38.40%, 40.68% and 73.88%, respectively. In contrast, the cell percentage in G2 phase was 22.36%, 18.07% and 15.66 % in control, inhibitor and NC groups, respectively (Figures 5A,B). Thus, it is clear that the cell cycle was arrested in G2 phase after transfection with miR-497-5p and cisplatin treatment, and their combination showed the strongest inhibition.
Western blotting indicated that the expression of cell-cycle-related proteins, cyclin B1 and CDK1 was downregulated after miR-497-5p, cisplatin and miR-497-5p+cisplatin treatment as compared to control, inhibitor and NC groups (Figure 5C). Quantitative analysis revealed that the combined effect of miR-497-5p and cisplatin was greater than that of miR-497-5p or cisplatin treatment alone (Figure 5D).
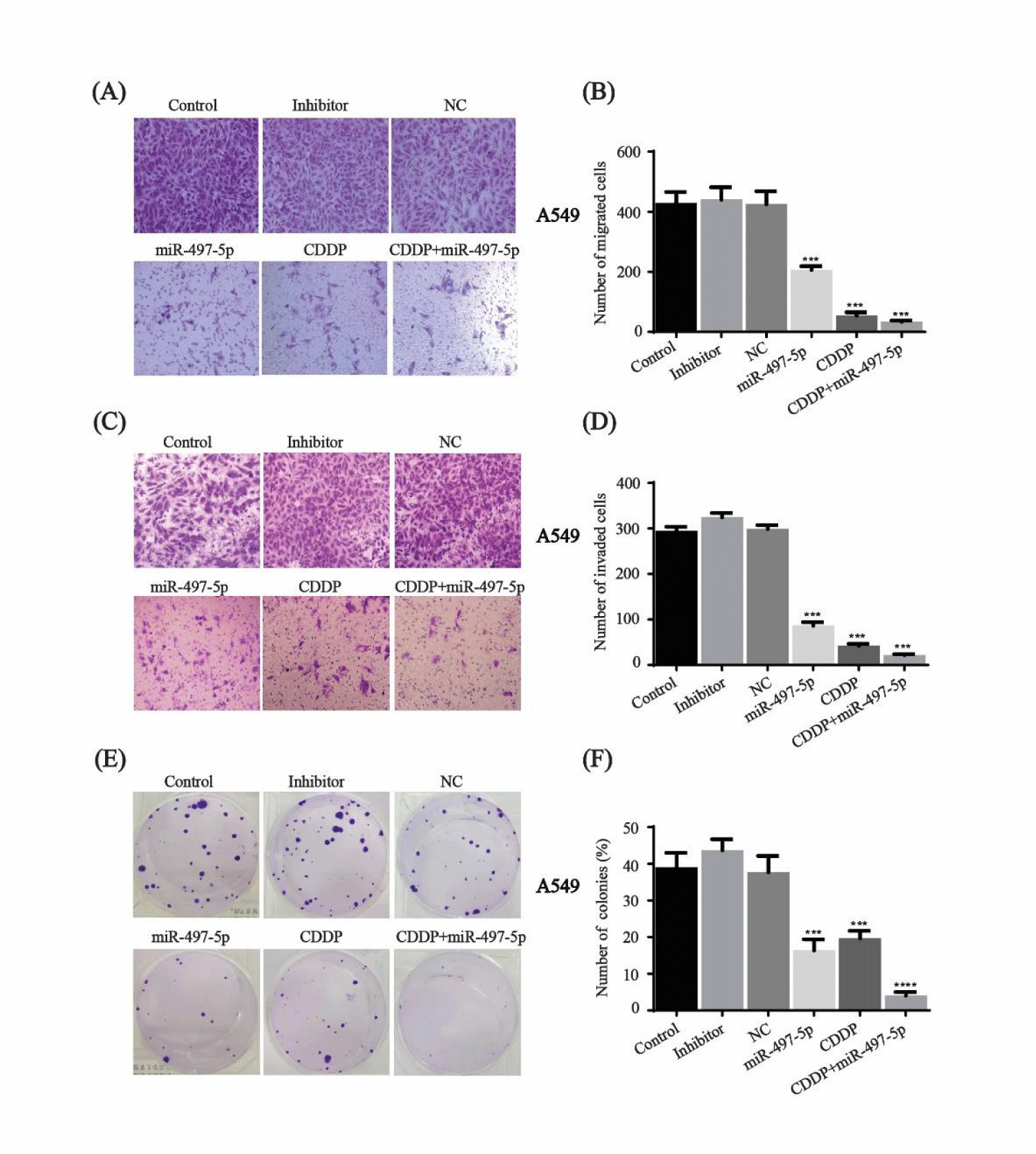
miR-497-5p increases the suppressive effect of cisplatin on migration, invasion and colony formation
Transwell migration and matrigel invasion assays were performed after miR-497-5p transfection, cisplatin and combination treatment in A549 cells. Compared to the control, inhibitor and NC groups, migration and invasion were inhibited in the cells treated with miR-497-5p or cisplatin. Moreover, the synergistic effect of cisplatin and miR-497-5p was greater than individual treatment (Figure 6A-D).
Cell growth was evaluated using colony formation assays after treatment with miR-497-5p, cisplatin or miR-497-5p+cisplatin. The suppressive effect of combination treatment on cell growth was greater than that of individual treatment of miR-497-5p or cisplatin (Figures 6E,F). These results suggested that miR-497-5p increased the inhibitory effect of cisplatin in A549 cells.
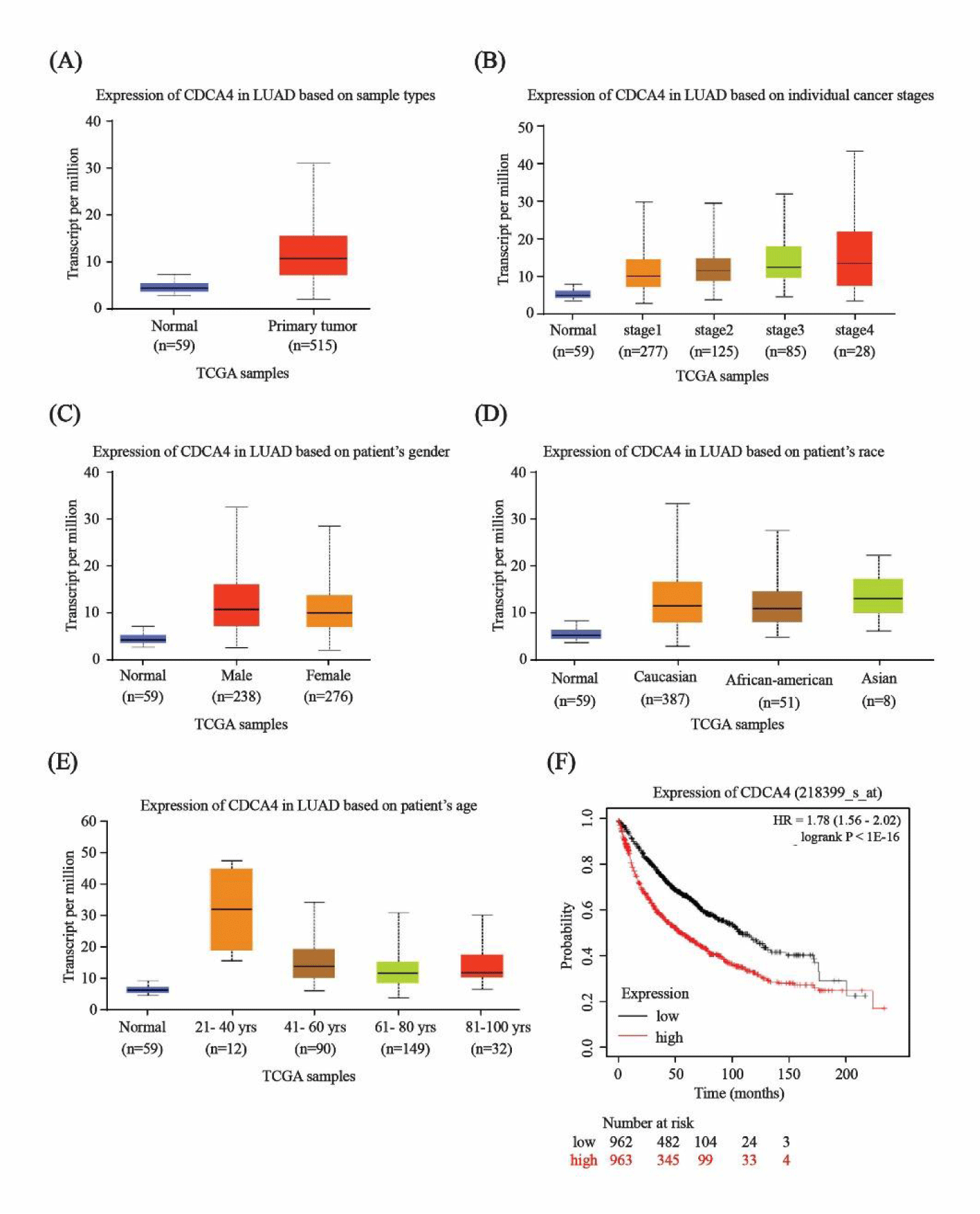
CDCA4 expression in N-SCLC
Based on GEPIA analysis, CDCA4 expression levels were significantly increased in lung cancer tissues at different tumor stages and in patients of different races, genders, and ages compared with normal tissues (Figures 7A-E). The survival time of patients with high CDCA4 expression were significantly lower than those with low CDCA4 expression (Figure 7F).
Discussion
The clinical application of cisplatin has become limited because of resistance of cancer cells, which is a major problem in the treatment of N-SCLC. As the first metal-based chemotherapeutic drug, cisplatin does not show its highest potential because of side effects and drug resistance [18]. Cisplatin resistance may be occurred at the process of blood-stream circulation, influx and efflux through cell membrane, presence in cytoplasm and post DNA binding. The DNA breaks developed from cisplatin-induced DNA damage stimulate DNA repair through Nuclear Excision Repair (NER). NER excises damaged nucleotides on both strands and then synthesizes DNA to reconstitute integrity of the gene [19]. Thus, NER is a way to remove DNA lesions to induce resistance of cisplatin and cells with overexpression with NER have low sensitivity to cisplatin [20].
Resistance to cisplatin may also be due to lower cellular uptake, decreased influx or increased efflux, detoxification by cellular thiols and altered drug targets. Increasing evidence has shown that drug-induced dysregulation of miRNAs is involved in resistance of tumor cells to anticancer drugs, and miRNAs play a different role in cisplatin resistance, which can be induced by alterations to many intracellular signaling pathways, including apoptosis, cell cycle, drug transport, or Epithelial-Mesenchymal Transition (EMT) [21,22]. As a tumor suppressor, miR-497 is lowly expressed in N-SCLC and its overexpression can inhibit the malignant phenotype via targeting different genes. For example, miR-497 suppresses cell proliferation, migration, EMT, induce apoptosis, and tumorigenesis by targeting KDR, FGFR1, SOX5, MTDH, YAP1, FGF2, and PLK1 [23-29]. miR-497 also modulates drug sensitivity, including gefitinib and cisplatin, via targeting insulin-like growth factor receptor and AKT2 [30,31]. Long non-coding RNA and circular RNA act as sponges or competing endogenous RNA to regulate the function of miR-497 in N-SCLC progression [32-35]. The target genes of miR-497 are also involved in cell cycle control. For example, cyclin E1 can regulate the transition of the cells from quiescence into S phase via activating CDK2 in lung cancer [36]. CDK6, which phosphorylates RB to release E2F transcription factors and activates the transcription of genes required for S-phase entry, inhibits tumor growth and migration in osteosarcoma [37]. In our work, CDCA4 was found to be an important target gene of miR-497-5p to inhibit cell proliferation and invasion, as well as induce apoptosis and arrest the cell cycle in N-SCLC cells. CDCA4, also known as HEPP/SEI‑3/TRIP‑Br3, was first identified and named HEPP by Abdullah, et al. [38] due to its preferential expression in hematopoietic progenitor cells and mature blood cells. CDCA4 belongs to the SEI gene family, which includes cyclin-A binding domain, C-terminal motif, SERTA domain, and PHD-bromine binding domain [39]. The relationship between the SEI family genes and tumorigenesis has been confirmed. For example, overexpression of SEI family genes, including CDCA4 can increase the transactivation activity of p53 gene, which leads to inhibition of p53‑independent growth in HeLa and U2OS cells [16]. As a downstream target gene of the transcription factor E2F, CDCA4 also regulates E2F-dependent transcriptional activation and cell proliferation [40]. Xu, et al. [41] showed that CDCA4 modulates cell proliferation and apoptosis in MCF-7/ADM breast cancer cells. Knockdown of CDCA4 expression inhibits triple negative breast cancer cell MDA‑MB‑231 proliferation in vitro and in vivo [39]. Recent studies have indicated that CDCA4 is downregulated by different miRNAs. For example, miR-15a and miR-29c-3p inhibit cell proliferation, causing cell cycle arrest and reduce the invasiveness of melanoma cells [42, 43].
In this study, we identified that CDCA4 is a key target gene of miR-497-5p in N-SCLC cells, and miR-497-5p inhibits N-SCLC cells progression and increases cisplatin chemosensitivity by targeting CDCA4. Our results indicate that miR-497-5p plays a role as a tumor suppressor and CDCA4 as a molecular target in N-SCLC, providing the potential to treat N-SCLC in combination with cisplatin and miR-497-5p. Further study will clarify the molecular mechanism of CDCA4 and miR-497-5p in cisplatin resistance in N-SCLC.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant nos. 81371676, 81071248).
Conflict of Interest
The authors declare that they have no conflicts of interest to report regarding the present study.
References
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016 Mar-Apr;66(2):115-32. doi: 10.3322/caac.21338. Epub 2016 Jan 25. PMID: 26808342.
- Ferguson MK. Diagnosing and staging of non-small cell lung cancer. Hematol Oncol Clin North Am. 1990 Dec;4(6):1053-68. PMID: 1962775.
- Judson I, Kelland LR. New developments and approaches in the platinum arena. Drugs. 2000;59 Suppl 4:29-36; discussion 37-8. doi: 10.2165/00003495-200059004-00004. PMID: 10864228.
- Shen DW, Pouliot LM, Hall MD, Gottesman MM. Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev. 2012 Jul;64(3):706-21. doi: 10.1124/pr.111.005637. Epub 2012 Jun 1. PMID: 22659329; PMCID: PMC3400836.
- van Kouwenhove M, Kedde M, Agami R. MicroRNA regulation by RNA-binding proteins and its implications for cancer. Nat Rev Cancer. 2011 Aug 5;11(9):644-56. doi: 10.1038/nrc3107. PMID: 21822212.
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006 Nov;6(11):857-66. doi: 10.1038/nrc1997. PMID: 17060945.
- Foss KM, Sima C, Ugolini D, Neri M, Allen KE, Weiss GJ. miR-1254 and miR-574-5p: serum-based microRNA biomarkers for early-stage non-small cell lung cancer. J Thorac Oncol. 2011 Mar;6(3):482-8. doi: 10.1097/JTO.0b013e318208c785. PMID: 21258252.
- Landi MT, Zhao Y, Rotunno M, Koshiol J, Liu H, Bergen AW, Rubagotti M, Goldstein AM, Linnoila I, Marincola FM, Tucker MA, Bertazzi PA, Pesatori AC, Caporaso NE, McShane LM, Wang E. MicroRNA expression differentiates histology and predicts survival of lung cancer. Clin Cancer Res. 2010 Jan 15;16(2):430-41. doi: 10.1158/1078-0432.CCR-09-1736. Epub 2010 Jan 12. PMID: 20068076; PMCID: PMC3163170.
- Gasparini P, Cascione L, Landi L, Carasi S, Lovat F, Tibaldi C, Alì G, D'Incecco A, Minuti G, Chella A, Fontanini G, Fassan M, Cappuzzo F, Croce CM. microRNA classifiers are powerful diagnostic/prognostic tools in ALK-, EGFR-, and KRAS-driven lung cancers. Proc Natl Acad Sci U S A. 2015 Dec 1;112(48):14924-9. doi: 10.1073/pnas.1520329112. Epub 2015 Nov 16. PMID: 26627242; PMCID: PMC4672770.
- Cui R, Meng W, Sun HL, Kim T, Ye Z, Fassan M, Jeon YJ, Li B, Vicentini C, Peng Y, Lee TJ, Luo Z, Liu L, Xu D, Tili E, Jin V, Middleton J, Chakravarti A, Lautenschlaeger T, Croce CM. MicroRNA-224 promotes tumor progression in nonsmall cell lung cancer. Proc Natl Acad Sci U S A. 2015 Aug 4;112(31):E4288-97. doi: 10.1073/pnas.1502068112. Epub 2015 Jul 17. PMID: 26187928; PMCID: PMC4534291.
- Kang M, Shi J, Peng N, He S. MicroRNA-211 promotes non-small-cell lung cancer proliferation and invasion by targeting MxA. Onco Targets Ther. 2017 Nov 28;10:5667-5675. doi: 10.2147/OTT.S143084. PMID: 29238200; PMCID: PMC5713696.
- Wang C, Wang S, Ma F, Zhang W. miRNA‑328 overexpression confers cisplatin resistance in non‑small cell lung cancer via targeting of PTEN. Mol Med Rep. 2018 Nov;18(5):4563-4570. doi: 10.3892/mmr.2018.9478. Epub 2018 Sep 12. PMID: 30221716.
- Chen Y, Kuang D, Zhao X, Chen D, Wang X, Yang Q, Wan J, Zhu Y, Wang Y, Zhang S, Wang Y, Tang Q, Masuzawa M, Wang G, Duan Y. miR-497-5p inhibits cell proliferation and invasion by targeting KCa3.1 in angiosarcoma. Oncotarget. 2016 Sep 6;7(36):58148-58161. doi: 10.18632/oncotarget.11252. PMID: 27531900; PMCID: PMC5295420.
- Chai L, Kang XJ, Sun ZZ, Zeng MF, Yu SR, Ding Y, Liang JQ, Li TT, Zhao J. MiR-497-5p, miR-195-5p and miR-455-3p function as tumor suppressors by targeting hTERT in melanoma A375 cells. Cancer Manag Res. 2018 May 3;10:989-1003. doi: 10.2147/CMAR.S163335. PMID: 29760567; PMCID: PMC5937487.
- Zhu D, Tu M, Zeng B, Cai L, Zheng W, Su Z, Yu Z. Up-regulation of miR-497 confers resistance to temozolomide in human glioma cells by targeting mTOR/Bcl-2. Cancer Med. 2017 Feb;6(2):452-462. doi: 10.1002/cam4.987. Epub 2017 Jan 8. PMID: 28064447; PMCID: PMC5313645.
- Watanabe-Fukunaga R, Iida S, Shimizu Y, Nagata S, Fukunaga R. SEI family of nuclear factors regulates p53-dependent transcriptional activation. Genes Cells. 2005 Aug;10(8):851-60. doi: 10.1111/j.1365-2443.2005.00881.x. PMID: 16098148.
- Wang L, Zhu G, Yang D, Li Q, Li Y, Xu X, He D, Zeng C. The spindle function of CDCA4. Cell Motil Cytoskeleton. 2008 Jul;65(7):581-93. doi: 10.1002/cm.20286. PMID: 18498124.
- Ghosh S. Cisplatin: The first metal based anticancer drug. Bioorg Chem. 2019 Jul;88:102925. doi: 10.1016/j.bioorg.2019.102925. Epub 2019 Apr 11. PMID: 31003078.
- Wood RD, Araújo SJ, Ariza RR, Batty DP, Biggerstaff M, Evans E, Gaillard PH, Gunz D, Köberle B, Kuraoka I, Moggs JG, Sandall JK, Shivji MK. DNA damage recognition and nucleotide excision repair in mammalian cells. Cold Spring Harb Symp Quant Biol. 2000;65:173-82. doi: 10.1101/sqb.2000.65.173. PMID: 12760031.
- Selvakumaran M, Pisarcik DA, Bao R, Yeung AT, Hamilton TC. Enhanced cisplatin cytotoxicity by disturbing the nucleotide excision repair pathway in ovarian cancer cell lines. Cancer Res. 2003 Mar 15;63(6):1311-6. PMID: 12649192.
- Wu SG, Chang TH, Liu YN, Shih JY. MicroRNA in Lung Cancer Metastasis. Cancers (Basel). 2019 Feb 23;11(2):265. doi: 10.3390/cancers11020265. PMID: 30813457; PMCID: PMC6406837.
- Chen Y, Gao Y, Zhang K, Li C, Pan Y, Chen J, Wang R, Chen L. MicroRNAs as Regulators of Cisplatin Resistance in Lung Cancer. Cell Physiol Biochem. 2015;37(5):1869-80. doi: 10.1159/000438548. Epub 2015 Nov 17. PMID: 26584286.
- Xia Y, Hu C, Lian L, Hui K, Wang L, Qiao Y, Liu L, Liang L, Jiang X. miR‑497 suppresses malignant phenotype in non‑small cell lung cancer via targeting KDR. Oncol Rep. 2019 Jul;42(1):443-452. doi: 10.3892/or.2019.7163. Epub 2019 May 16. PMID: 31115562.
- Huang Q, Li H, Dai X, Zhao D, Guan B, Xia W. miR‑497 inhibits the proliferation and migration of A549 non‑small‑cell lung cancer cells by targeting FGFR1. Mol Med Rep. 2019 Oct;20(4):3959-3967. doi: 10.3892/mmr.2019.10611. Epub 2019 Aug 23. PMID: 31485617.
- Li G, Wang K, Wang J, Qin S, Sun X, Ren H. miR-497-5p inhibits tumor cell growth and invasion by targeting SOX5 in non-small-cell lung cancer. J Cell Biochem. 2019 Jun;120(6):10587-10595. doi: 10.1002/jcb.28345. Epub 2019 Feb 28. PMID: 30816573.
- Yin Q, Han Y, Zhu D, Li Z, Shan S, Jin W, Lu Q, Ren T. miR-145 and miR-497 suppress TGF-β-induced epithelial-mesenchymal transition of non-small cell lung cancer by targeting MTDH. Cancer Cell Int. 2018 Jul 27;18:105. doi: 10.1186/s12935-018-0601-4. PMID: 30065618; PMCID: PMC6062944.
- Huang C, Ma R, Yue J, Li N, Li Z, Qi D. MiR-497 Suppresses YAP1 and Inhibits Tumor Growth in Non-Small Cell Lung Cancer. Cell Physiol Biochem. 2015;37(1):342-52. doi: 10.1159/000430358. Epub 2015 Aug 24. PMID: 26316081.
- Huang X, Wang L, Liu W, Li F. MicroRNA-497-5p inhibits proliferation and invasion of non-small cell lung cancer by regulating FGF2. Oncol Lett. 2019 Mar;17(3):3425-3431. doi: 10.3892/ol.2019.9954. Epub 2019 Jan 21. PMID: 30867780; PMCID: PMC6396182.
- Xu C, Li S, Chen T, Hu H, Ding C, Xu Z, Chen J, Liu Z, Lei Z, Zhang HT, Li C, Zhao J. miR-296-5p suppresses cell viability by directly targeting PLK1 in non-small cell lung cancer. Oncol Rep. 2016 Jan;35(1):497-503. doi: 10.3892/or.2015.4392. Epub 2015 Nov 4. PMID: 26549165.
- Ma W, Feng W, Tan J, Xu A, Hu Y, Ning L, Kang Y, Wang L, Zhao Z. miR-497 may enhance the sensitivity of non-small cell lung cancer cells to gefitinib through targeting the insulin-like growth factor-1 receptor. J Thorac Dis. 2018 Oct;10(10):5889-5897. doi: 10.21037/jtd.2018.10.40. PMID: 30505497; PMCID: PMC6236188.
- Wang L, Ji XB, Wang LH, Qiu JG, Zhou FM, Liu WJ, Wan DD, Lin MC, Liu LZ, Zhang JY, Jiang BH. Regulation of MicroRNA-497-Targeting AKT2 Influences Tumor Growth and Chemoresistance to Cisplatin in Lung Cancer. Front Cell Dev Biol. 2020 Sep 8;8:840. doi: 10.3389/fcell.2020.00840. PMID: 33015042; PMCID: PMC7505950.
- Guo D, Wang Y, Ren K, Han X. Knockdown of LncRNA PVT1 inhibits tumorigenesis in non-small-cell lung cancer by regulating miR-497 expression. Exp Cell Res. 2018 Jan 1;362(1):172-179. doi: 10.1016/j.yexcr.2017.11.014. Epub 2017 Nov 11. PMID: 29133127.
- Li Z, Lu Q, Zhu D, Han Y, Zhou X, Ren T. Lnc-SNHG1 may promote the progression of non-small cell lung cancer by acting as a sponge of miR-497. Biochem Biophys Res Commun. 2018 Nov 30;506(3):632-640. doi: 10.1016/j.bbrc.2018.10.086. Epub 2018 Oct 25. PMID: 30454699.
- Qin S, Zhao Y, Lim G, Lin H, Zhang X, Zhang X. Circular RNA PVT1 acts as a competing endogenous RNA for miR-497 in promoting non-small cell lung cancer progression. Biomed Pharmacother. 2019 Mar;111:244-250. doi: 10.1016/j.biopha.2018.12.007. Epub 2018 Dec 24. PMID: 30590312.
- Xu SH, Bo YH, Ma HC, Zhang HN, Shao MJ. lncRNA LINC00473 promotes proliferation, migration, invasion and inhibition of apoptosis of non-small cell lung cancer cells by acting as a sponge of miR-497-5p. Oncol Lett. 2021 Jun;21(6):429. doi: 10.3892/ol.2021.12690. Epub 2021 Mar 30. PMID: 33868467; PMCID: PMC8045175.
- Han Z, Zhang Y, Yang Q, Liu B, Wu J, Zhang Y, Yang C, Jiang Y. miR-497 and miR-34a retard lung cancer growth by co-inhibiting cyclin E1 (CCNE1). Oncotarget. 2015 May 30;6(15):13149-63. doi: 10.18632/oncotarget.3693. PMID: 25909221; PMCID: PMC4537005.
- Tian ZS, Yan MJ, Li S, Cong D, Wang YY, Zhu QS. miR-497 inhibits tumor growth and migration of osteosarcoma by targeting plexinA4 and CDK6. Neoplasma. 2020 Sep;67(5):1122-1130. doi: 10.4149/neo_2020_200108N26. Epub 2020 Jul 2. PMID: 32614239.
- Abdullah JM, Jing X, Spassov DS, Nachtman RG, Jurecic R. Cloning and characterization of Hepp, a novel gene expressed preferentially in hematopoietic progenitors and mature blood cells. Blood Cells Mol Dis. 2001 May-Jun;27(3):667-76. doi: 10.1006/bcmd.2001.0434. PMID: 11482882.
- Pang S, Xu Y, Chen J, Li G, Huang J, Wu X. Knockdown of cell division cycle-associated protein 4 expression inhibits proliferation of triple negative breast cancer MDA-MB-231 cells in vitro and in vivo. Oncol Lett. 2019 May;17(5):4393-4400. doi: 10.3892/ol.2019.10077. Epub 2019 Feb 26. PMID: 30944632; PMCID: PMC6444385.
- Hayashi R, Goto Y, Ikeda R, Yokoyama KK, Yoshida K. CDCA4 is an E2F transcription factor family-induced nuclear factor that regulates E2F-dependent transcriptional activation and cell proliferation. J Biol Chem. 2006 Nov 24;281(47):35633-48. doi: 10.1074/jbc.M603800200. Epub 2006 Sep 19. PMID: 16984923.
- Xu Y, Wu X, Li F, Huang D, Zhu W. CDCA4, a downstream gene of the Nrf2 signaling pathway, regulates cell proliferation and apoptosis in the MCF‑7/ADM human breast cancer cell line. Mol Med Rep. 2018 Jan;17(1):1507-1512. doi: 10.3892/mmr.2017.8095. Epub 2017 Nov 15. PMID: 29257222; PMCID: PMC5780089.
- Alderman C, Sehlaoui A, Xiao Z, Yang Y. MicroRNA-15a inhibits the growth and invasiveness of malignant melanoma and directly targets on CDCA4 gene. Tumour Biol. 2016 Oct;37(10):13941-13950. doi: 10.1007/s13277-016-5271-z. Epub 2016 Aug 4. PMID: 27492455.
- Liu J, Tao G, Zhong C, Liu X. Upregulation of miR-29c-3p Hinders Melanoma Progression by Inhibiting CDCA4 Expression. Biomed Res Int. 2021 Aug 28;2021:7065963. doi: 10.1155/2021/7065963. PMID: 34497853; PMCID: PMC8419494.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.