> Medicine Group. 2021 October 26;2(10):959-963. doi: 10.37871/jbres1339.
Exposure to Air Pollution Nanoparticles: Oxidative Stress and Neuroinflammation
Mojtaba Ehsanifar1,2*, Banihashemian SS3 and Masoud Ehsanifar3
2Anatomical Sciences Research Center, Kashan University of Medical Sciences, Kashan, Iran
3AJA University of Medical Sciences, Tehran, Iran
- Air pollution exposure
- Diesel exhausts nano-particles
- Airborne particulate matter
- Neuroinflammation
- Oxidative stress
Abstract
Urban air pollutants exposure is known as a source of neuroinflammation and oxidative stress that causes the Central Nervous System (CNS) and neuropathology disease. Transition metals, Particulate Matter (PM), including PM 2.5 (PM <2.5 μm) and PM 0.1 (PM <0.1μm), nitrogen oxides and ozone are of potent or oxidant capable of producing Reactive Oxygen Species (ROS). Redox-sensitive pathways can be caused by oxidative stress, leading to various biological processes, including inflammation and cell death. The incidence of Alzheimer's Disease (AD) and Parkinson's Disease (PD) and stroke are associated with exposure to air pollution. Some recent findings suggest that urban air pollutants reach the brain in addition to pulmonary and cardiovascular diseases and affect the CNS health too. While the underlying CNS pathology mechanisms induced air pollutants exposure are not well understood, recent studies show that changes in Blood Brain Barrier (BBB) and microglial activation are key components. In this work, we reviewed the new evidence of the mechanisms by which ambient air pollution reach the brain and activate innate immune response as a source of oxidative stress and neuroinflammatory factors.
Introduction
While a variety of environmental factors are involved in neuronal inflammation leading to CNS disease, air pollutants may be the most common source of environmental oxidative stress and inflammation. In the chronic nature and pathology of CNS disease, inflammation is recognized as a risk factor [1]. Ambient air contains a complex combination of toxins, including gases, benzene, and Particulate Matter (PM) that can be irritating. Chemical composition of particles varies widely depending on geographic, meteorological, and specific source variables [2]. In general, ambient particles include elemental and organic carbon, inorganic components (trace metals, nitrates, sulfates, chloride, and ammonium), biological components (pollens, bacteria, and spores), volatile and semi-disintegrating organic compounds [3]. Furthermore, when the ambient particles are mixed with atmospheric gases (carbon monoxide, sulfur, ozone, and nitric oxides), they can form airborne particles. Environmental particles are commonly characterized by aerodynamic properties and their size and defined as PM2.5 and PM10 with diameters of less than 2.5 and 10 μm: PM with an aerodynamic diameter of 2.5 to 10 μm (PM10), PM smaller than 2.5 μm (PM2.5) and very small PM less than 0.1 μm or ultrafine PM (UFPs; <100 nm). This particles are acceptable fractions from different sources such as agricultural dust, wood combustion, road, vehicles emission, tire wear propagation, construction, mining operations, and demolition work [4,5]. PM2.5 due to heavy metals absorbed in pores and particle surfaces produces more hydroxyl radicals, while larger particles (PM10) are mainly in the upper airways and it is purified by the mucosal system [6,7]. Diesel Exhaust Particles (DEPs), from vehicle emissions particularly diesel engines emission, are a main source of UFPs that may penetrate the home if poorly ventilated, where additional sources such as burning candles, cooking, chemical reactions, and tobacco smoke are also available [8]. However, attention has also been focused on nanoparticles less than 100 nm in diameter; UFPs are important because of their health effects such as chemical composition, high alveolar deposition fraction, large surface area, and ability to enter the bloodstream and cause inflammation [3,9]. The main route of air pollutant exposure is inhalation. The fine particles (PM2.5) are deposited in lungs, while the coarse particles (PM10) are filtered usually out by nose and the upper airways [3].
Recently, some findings support the involvement of neuroinflammation in pathogenesis of cognitive impairment and affective disorders [9,10]. For example, anxiety and depression in male mice and hippocampal inflammatory cytokine response and learning and memory disorder are related with DEPs exposure [10-12]. Traditionally, associated with an increased risk of cardiovascular and lung disease, now, air pollution exposure is also related to CNS diseases including stroke, Parkinson's, and Alzheimer's disease. Air pollutants are a multifaceted ambient poison that can attack the CNS through a variety of routes [3]. Now days, despite the varying chemical and physical properties of air pollution and their subsequent activation in multiple pathways, oxidative stress and inflammation are known to be the underlying mechanisms through which ambient air pollutants cause damage in the CNS [13]. In addition, while different types of cells in the brain are susceptible to air pollutants, new research suggests that capillaries and microglia may be important causes of cellular damage. This review corers the multifaceted mechanisms through which nanoparticles in the contaminated air affect the CNS as well as new mechanistic findings that contain chronic neuroinflammation and innate immunity in CNS disease caused by exposure to air pollutants.
Air pollution particles and health effects
Air pollution from traffic is a mixture that includes various components such as gases, Particle Matter (PM), organic compounds and metals [14]. Estimated that traffic pollution 20% to 70% of environmental pollution and 85% of ambient PM is related to traffic and resulting from vehicle combustion. So air pollution from traffic is one of the important sources of environmental pollution [7,15]. The association between exposure to urban air pollutant and mortality complications from respiratory and cardiovascular diseases is well established today, While new findings suggest that air pollutants exposure may also contribute to diseases of the CNS [12,16,17]. Some of the epidemiological surveys suggest that increased exposure to traffic air pollutant is associated with hearing and olfactory impairment, decreased cognitive function, as well as increased incidence of neurological pathology and depressive symptoms [18-20].
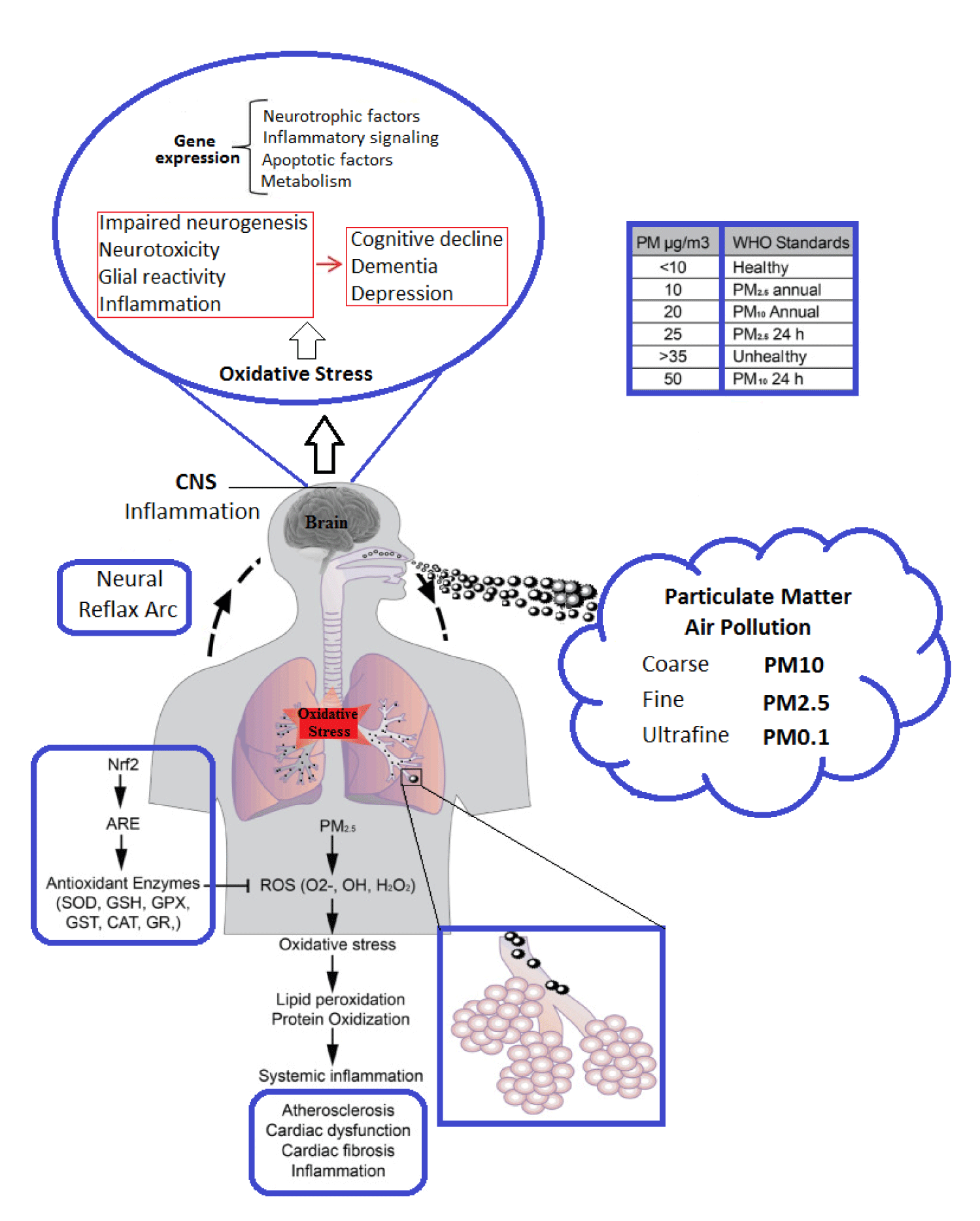
It is believed that exposure to PM is a very important threat between traffic air pollutant components and has been played a significant role in disease [17] (Figure 1). PM is broadly determined by size (aerodynamic diameter such as PM10 and PM2.5). UFPs is very worrying because these fine particles can easily enter bloodstream and after crossing the BBB, are transmitted to the brain and various organs [12,13]. Exhaust from diesel engines includes the complex mixture of hydrocarbons, gases, heavy metals, sulfur, particles and especially small sized PM produced in diesel fuel combustion [8,21]. Most DEPs are less than 1 micron in diameter and these particles are one of the main components of air pollutants [8]. DE is the main source of urban air pollution PM, which contains more than 40 toxic pollutants, especially UFPs. Some of the research to controlled Diesel Exhaust (DE) exposure has been investigated on human health. For example, following acute exposure to DEPs (300 µg/m3) in humans, it has been observed EEG changes [22]. DEPs contain many compounds that have potentially harmful effects on brain growth [23-25] and the immune system [26]. In 2013, DEPs was identified as a human carcinogen group 1 based on evidence of exposure to particles and lung cancer by the International Agency for Research on Cancer (IARC) [27,28].
As said, the mechanisms responsible for UFPs entry into brain are the main discussion topic, where active transport, BBB, and leakage and transmission along olfactory nerve into Olfactory Bulb (OB) have been proposed [12,29]. In spite of complex composition of urban air pollution, the classic surveys in cardiovascular system have shown that oxidative stress and inflammation are common mechanisms of air pollution injury [16,30,31]. Recent studies show that not only oxidative stress and inflammation are common concepts in neurodegenerative and CNS diseases, but current findings also point to a growing chain of evidence.
Exposure to air pollution: Oxidative stress and inflammation
In recent decades, due to the traffic of vehicles and other combustion processes, much attention has been given to exposure to air pollution. Gas and PM pollutants are very important factors in the urban areas, and various mechanisms have been proposed to explain the side effects of exposure to them on human health [32]. Although any air pollutant directly through the activation of intracellular oxidant pathways can exert its toxicity on cardiovascular and respiratory systems, particulates, ozone, and nitrogen oxides all have the same property of being strong oxidants, either through their direct effect on proteins and lipids [30,33-35]. Oxidative stress is in fact a biochemical imbalance that in which ROS production exceeds the capacity of the normal antioxidant. The biochemical imbalance can be caused by exposure to prooxidant air pollutants in the body. Uncontained ROS, in presence of oxidative stress, causes dysfunction and tissue damage by attacking and functional molecules (proteins, lipids, carbohydrates, RNA, DNA, NO, etc.) and denaturing structural, by modulating activities of redox-sensitive transcription factors [36,37].
ROS can generate by the particle surface where Polycyclic Aromatic Hydrocarbon (PAH) and nitro PAH are adsorbed, except for the transition metals (copper, iron, vanadium, and chromium) that catalyze the Fenton (Fe2++ H2O2 + H+ → Fe3++ OH• + H2O) reaction [38,39]. Numerous researchers reported that some of the metals such as iron in the transition from the particles or through their presence on the particle surfaces are involved in production of ROS in the biological systems [37]. Besides, it should be noted that nitrogen dioxide and ozone are oxidative DNA damage that they are usually associated with particles in ambient air [40,41]. Furthermore, photochemical oxidants (peroxyl acetate nitrate and ozone), secondary pollutants caused by sunlight in the atmosphere that contain reactive hydrocarbons and NOx, are involved in increasing oxidation stress. Then, if excess ROS is formed, mitochondrial damage occurs by induction of the NADPH Oxidase 4 (NOX4) isoform, together with activation of inflammatory cells (monocytes, neutrophils, and eosinophils) and an increase in the number of macrophages capable of ROS and reactivity nitrogen production [37,42-44]. At first, when oxidative stress is relatively low, for the formation of protection against undesirable biological consequences, various transcription factors, such as red blood cell Nuclear Factor (Nrf2), induce a series of antioxidant and detoxifying enzymes (catalase, glutathione S-transferase, and superoxide dismutase) that neutralize ROS [45-47]. Secondly, if the protective antioxidant response fails or is insufficient to counteract increased ROS production, it results in a pro-inflammatory state with various effects on cytotoxicity [48]. These effects are mediated by cascades of Mitogen-Activated Protein Kinase (MAPK) and NF-κB, which are responsible for expression of inflammatory biomarkers such as cytokines, chemokines, and adhesion molecules [47,48].
Essential pathways in response to toxic pollutants related to antioxidant enzymes and the proteins involved in phase II detoxification is first line of defense against oxidative stress. These responses are regulated by the Nuclear Factor-Erythroid 2-Related Factor 2 (Nrf2)/Antioxidant-Response Element (ARE) pathway. When oxidative and electrophilic chemical signals modify Kelch-like ECH associated protein 1 to release Nrf2, the pathway is activated, which then travels to nucleus and activates expression of antioxidant and phase II genes with ARE [36,47]. This pathway is important in mitigating oxidative stress-induced endothelial dysfunction [49]. Cytotoxic effects could occur at high concentration of pollutant exposure [47,50]. When these defense mechanisms are overwhelmed at a high dose of pollutants exposure induced oxidative stress, proinflammatory effects lead to via the activation of NFκB. The NFκB activation is increases transcription of chemokines, cytokines, and acute-phase proteins [51].
Findings show linkages of depressed antioxidant capacity and oxidative stress with the risk of hypertension [52], cardiovascular [30,53], and kidney disease [54]. Oxidative stress can play an important role in cardiovascular and respiratory effects of exposure to air pollution through the effects of the immune system and its ability to thrombogenic activity and initiate inflammatory process [16]. Experimental findings show that redox-active UFPs components following oxidative stress lead to ROS production in various cells in vascular tissues, blood, and the lungs. This can cause increased systemic inflammation, airway inflammation, and adverse cardiovascular reactions after overcoming antioxidants [50,55].
Epidemiological studies data that directly support this empirical evidence are limited, but there is indirect support for studies that have examined modification of responses to the exposure to air pollutants with a variety of genes associated with oxidative stress [56-61]. Also, some epidemiological studies reported that exposure to PM significantly increases the oxidative stress biomarkers in the blood, but surveys are limited for the populations exposed to air pollutants. Epidemiological data include panel investigation of healthy individuals with repeated criteria [62-65] and survey of workers exposed to smoke and aerosols of combustion [61,66-71]. Exposure to urban air pollution increases ox-LDL in mice [72] and impairs the anti-inflammatory capacity of the High-Density Lipoprotein (HDL) in the mice [73]. The secondhand tobacco smoke similar urban air pollution may have to carry redox-active components. It has been observed that low-density lipoprotein oxidation (ox-LDL) has increased among the people this smoke exposed [74]. Epidemiological studies show that the risk of atherosclerotic lesions is increased among the subjects living near heavy traffic [9,75-77]. Recent advances and findings have provided key insights into how exposure to air pollutants has harmful and dangerous effects on the brain. In particular, it is believed that air pollution exposure affects the brain in multiple pathways. Air pollutants are a mixture of toxin that causes a variety of CNS damage through multiple interconnected mechanisms that may cause CNS disease. While some of the air pollution effects have been attributed to the specific components of CNS, no specific pathway responsible for the CNS pathology has yet been identified. As said, given the complex nature of air pollutants, CNS pathology is most likely caused by the synergistic interactions of multiple pathways and causes air pollution to become an important challenge for mechanistic inquiry.
Oxidative stress responses, the importance of particle size and composition
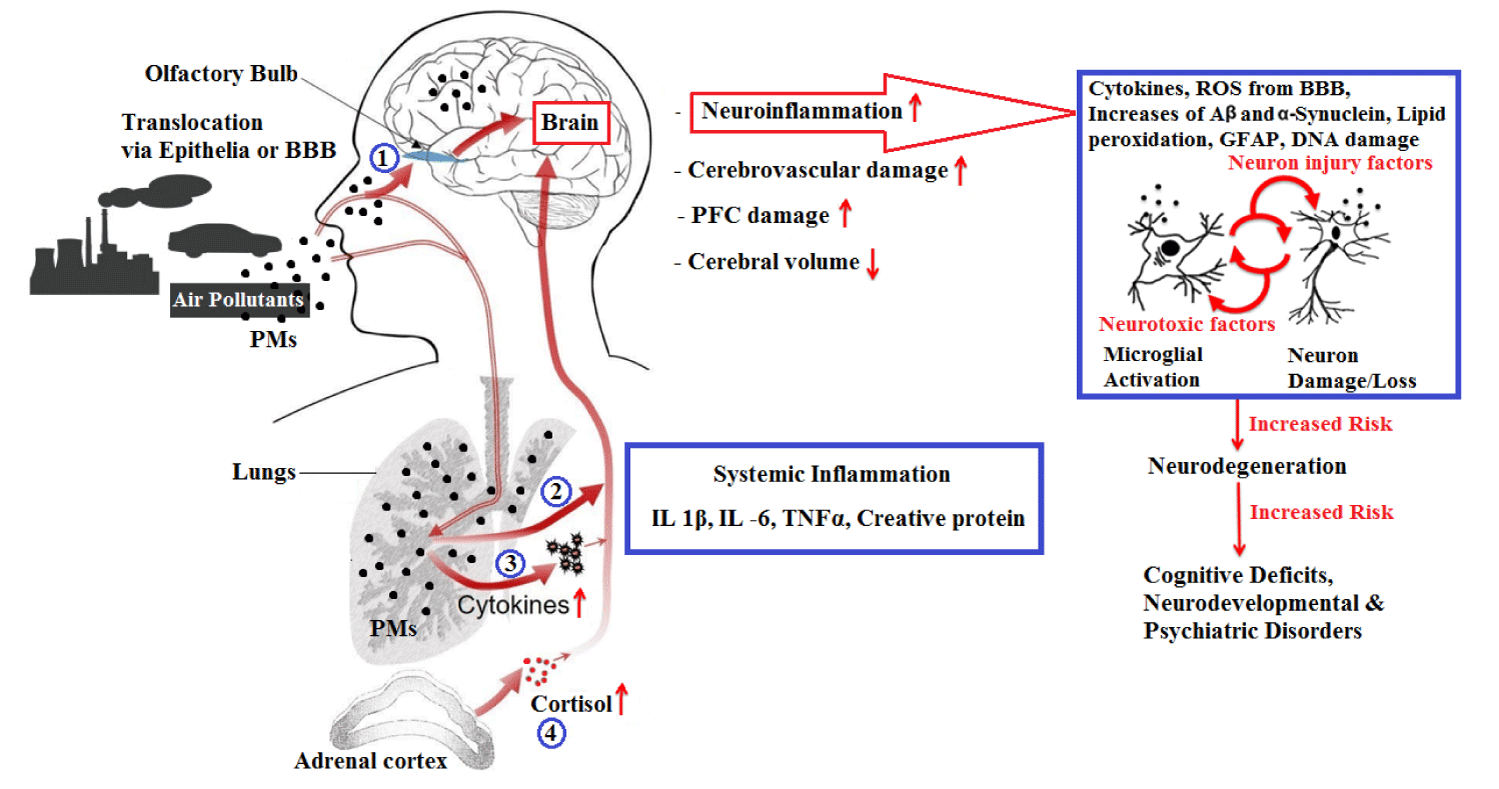
In vitro results show that toxin compounds in PM such as transition metals and organic compounds (e.g., Cu, Fe, Zn, and Ni) are capable to produce ROS directly [50,61,78,79] or, as a result, their capacity to activate alveolar macrophages, respiratory endothelial and epithelial cells, and neutrophils or other leukocytes. Transmission metals through Fenton reactions have known the potential to stimulate oxidative stress. The ROS cellular generation has been demonstrated following exposed to PM mixtures using an invitro system of the rat alveolar macrophages [80,81]. Responsible for most of the PM emissions mobile-source are automobile exhaust [7,82], which produces nearly the complete set of pollutants [83]. One of the causes of oxidative stress is the important reactive chemicals, which may include PM2.5 organic components such as quin ones, which are PAHs or oxidized PAH species that are converted to biotransformation using cytochrome P-450 1A1 to quinones [84,85]. DEPs are enriched in these redox cycling components [86]. Experimental findings show that PAH and themselves-oxidizing (e.g., quinones) in DEPs promote the generation of ROS, which leads to oxidative stress and inflammatory response resulting from NFkB activation [85,87,88]. Exposure to DEPs also leads to changes in the expression of antioxidant enzymes as shown in laboratory data [89] and studies of airway responses in humans [90] (Figure 2). These effects may underlie epidemiological findings of an increase in circulating inflammatory markers and excessive vascular hypercoagulability markers about exposure to ambient air pollutants in cross-sectional studies [91,92] and cohort panel studies [93-95].
The results of the chemical mass equilibrium model using the source tracer show that most PAHs come from vehicular sources. The biomarkers level of systemic inflammation were significantly associated with concentrations of both outdoor and indoor home of the low, medium, and high molecular weight PAHs [85,96,97]. Another study found that associations between plasma homocysteine levels with black carbon, and urban air pollutants [98,99]. Mechanisms have been suggested to explain these findings, including the inactivation of enzymes involved in homocysteinere methylation or involvement of pollutant-generated ROS. These results are consistent with the extensive literature review that has concluded that PM2.5 black carbon standard should be considered in line with national standards for ambient air quality standards in the United States, and "vehicular emissions are a main ambient factor in cardiovascular complications and mortality in united states" [61,100].
Also, the particle size has a vitally important determinant role of the chemical dose of redox-active, which is delivered to target organs. The UFPs (diameter <0.1 micrometers) and accumulation modes (PM2.5) make up the total mass of fine particles, which is regulated by United States Environmental Protection Agency (EPA). UFPs are expected to induce larger responses per unit mass than coarse particles that dominate PM2.5 mass. This is attributed to greater deposition and retention in lungs, the ability to escape phagocytosis by surface and large macrophages, and a higher number and surface area of fine particle, than larger particles, thus transferring and delivering more concentrations of toxic components to the lungs [50,61,101-104]. Due to this surface area, UFPs contain redox-active organic chemicals (such as PAH), and transport metals at concentrations much higher than coarse PM [105]. Even for low organic contents soot particles, the UFPs cell interactions may be an important the pro-oxidant mechanism that stimulates inflammatory responses, particularly in the lungs, possibly due to the carbon extensive reactive surface area [103,106]. Thus, the components of toxic particles and the effective surface area are not well-represented by EPA-regulated PM10 and PM2.5. However, some studies of cohort panels still showed associations of oxidative stress biomarkers with PM10 or PM2.5 mass [62,63,107,108].
Oxidative DNA damage (mainly 8-oxodG) in the lymphocytes following exposure to the high level of UFPs [64] and PM of metals [65] have been shown in two small panel cohort studies. However, the sample size was somewhat small due to the difficulty of performing 8-oxodG test on newly isolated lymphocytes. Besides, there is a few evidence of epidemiological study in humans that PM exposure is causing an increase in oxidative stress biomarkers in the blood. However, the movement of UFPs in the circulation have been demonstrated in several research, and despite its very low overall velocity, which is probably ≤1%, due to the high surface area and long shelf life, its effect on target organs may be significant [103,104] High retention of UFPs in the lungs, suggests that the effect of PM transfer through to the circulation is much greater [109]. This could be lead to lasting effects through the gradual transfer of redox-active component sin to circulation over a long time. And may be especially crucial for chemicals such as PAH that require biotransformation through phase I enzymes [110]. The results of a cohort panel study for UFPs showed that only pseudo-UFPs <0.25 micrometers (PM0.25) were significantly related with systemic inflammation biomarkers [TNFα and IL-6] [111] and are measured by ischemic ST-segment depression with outpatient electrocardiography [112].
PM itself contains ROS as well as active components of redox oxidation that can lead to the production of ROS by interaction with samples of biological origin. The capacity of inhaled PM to cause cell damage through oxidative reactions is called oxidative potential and can be measured using cell-free methods (Table 1).
| Table 1: Biomarkers and methods of assessing oxidative stress. | ||
| Oxidative-stress assays | ||
| ROS oxidative potential | ERS, HRP/DCFH, DTT | |
| Oxidative damage | Proteins | Oxidation level |
| Lipids | Peroxidation level | |
| DNA | Deoxyguanosine modification | |
| Detoxifying proteins | MAPK, Nrf2, PTEN, PI3K | |
| Antioxidant defense | GPx, SOD1, SOD2, XO, NOX | |
| Abbreviations: Cytp450: Cytochrome P450; CAT: Catalase; DNA: Deoxyribonucleic Acid; ERS: Electron Spin Resonance; DTT: Dithiothreitol; HPRT/DCFH: Horseradish Peroxidase/2070-Dichlorodihydrofluorescein; Gpx: Glutathione Peroxidase; NADPH: Nicotinamide Adenine Dinucleotide Phosphate; MAPK: Mitogen-Activated Protein Kinase; Nrf2: Nuclear Factor Erythroid 2-Related Factor 2; NOX: Nitric Oxidase; PTEN: Phosphatase and Tens in Homolog Protein; PI3K: Phosphoinositide 3- Kinase; XO: Xanthine Oxidase; SOD1: Superoxide Dismutase 1; SOD2: Superoxide Dismutase 2. | ||
Potential evaluation of PM components oxidation
Exposed to airborne particles, macrophages are first line of defense against lung damage. After phagocytizing the particles, following the cytokine cascades, the macrophages create ROS in an oxidative burst, leading to additional ROS production and inflammation. In this assay, the activity of cell-based ROS induced by aqueous particles extracts has been associate with organic compound concentration and the metal transition [2,80,113-115]. Also, in another study, the activity of ROS for PM0.25 was shown higher than that of larger particle fractions [115]. The association of the systemic inflammatory responses and the airway to the potential of collected PM to induce cellular ROS production was evaluated with both plasma NO and IL-6 measurements for 12 weeks in the sixty elderly subjects [116]. As described to evaluate the oxidative potential of PM, ROS production has been measured. Both IL-6 and NO exhalations were positively associated with macrophage ROS generation levels. The relationship of NO exhalation with ROS activity is not reflected by the mass concentration of PM0.25. But PM0.25 co-regression models with ROS production show that the oxidative potential of IL-6 has the highest association with PM0.25 mass [116]. In conclusion, the systemic biological indicators of inflammation are related to the ability of particles to induce ROS production by macrophages, and this relationship is nominally reflected by the mass concentration of the particles.
Cellular mechanisms of neuronal inflammation
Besides, recent studies, to understanding how air pollutants exposure affects the brain, surveyed the cell types that mediate the CNS pathology following air pollution exposure.
Astroglia
Astroglia plays an important role in BBB integration, maintains glia-neuron contact, maintains ionic homology, buffer excess neurotransmitters, and secrete nerve factors [117]. Storm activation occurs in response to a variety of CNS damage [118]. Accordingly, astroglia has been reported to be activated in humans that exposure to high doses of air pollutants, which is evident with increased Glial Fibrillary Acidic Protein (GFAP) [119,120]. Exposure to ozone in animal studies showed that local astrocytes near the brain capillaries increased the expression of TNFα and IL-6 [37,121]. Moreover, ozone exposure astrocytes In vitro result in the death of astrocytes [122]. Nevertheless, does astroglia respond to urban air pollutants, to oxidative stress and inflammation produced by other cell types or cell damage? It is unclear how astrology is activated in the brain.
Microglia
Microglia are activated in response to pathogenic proteins in the body (eg. water and synuclein), cytokines, neurotoxicity, and environmental toxins (eg. paraquat and rotenone) [1,10], including air pollutants [123-125]. Microglia is of the brain innate immune cells that actively monitor the brain environment [126] and are activated in neurological diseases such as PD and AD [127,128]. Findings show that microglia mediate the neuronal damage because mixed cultures of neurons and moths treated with DEPs show selective dopaminergic neurotoxicity which occurred only in the presence of microglia [123]. It has been shown that microglia respond to the titanium nanoparticles by ROS production that is neurotoxic [129]. Also, it has been shown that microglia respond to PM in a laboratory study using DEPs. DEPs-treated cultures showed the microglial activation, as indicated by changes in the morphology and increased the production of superoxide, although PGE2, Nitric Oxide (NO), and TNFα were not detected [88,123]. Interestingly, exposure to concentrated air pollutants In vitro microglia, shows an increase in the mRNA expression for inflammatory cytokines, such as TNFα and IL-1b [10,12,124], suggesting that exposure to some forms of PM induces cytokine production. Besides, metal sin air pollutants activate microglia because microglia are In vitro activated by manganese [130], a component of industrial-induced air pollution. Further to neural death, disease proteins, and environmental stimuli such as air pollutants, microglia are also activated by the cytokines produced in response to the systemic inflammation, with catastrophic neurological consequences [131,132] and brain injury.
While most microglia activation is useful, activated microglia can be a chronic source of oxidative stress (●NO, H2O2, O●2—, ONOO●—/ONOOH) and inflammatory factors (TNFα, PGE2, and IFNg) in the brain [1]. Exposure to air pollutants can contribute to activation of the toxic microglial by activating the reactive microglia cycle through three mechanisms: (I) Air pollution components may directly activate microglia. (II) Microglia activated by cytokines arising from peripheral systemic inflammatory response. (III) PM, sorbents, or cytokines from margin may directly damage nerve cells to activate reactive microglia. Therefore, components of air pollutants may be interpreted as pathogens by microglia and lead to oxidative stress, chronic inflammation, brain vascular damage, and neurotoxicity.
Blood and brain barrier
Blood vessels throughout body show a wide range of different phenotypes that vary in function, gross structure, cellular structure, and blood exchange properties [133], which may be unique in their responses to air pollutants. Compared to most peripheral "leaky" vessels, brain microscopes (3 to 8 mm in diameter) are separated from many vessels because of a large barrier to macromolecules, small organic drugs, various toxins, and ions are. Therefore, these small vessels with in brain parenchyma from BBB [134]. The BBB is a physical and chemical barrier that protects different cell types, metabolic enzymes, and carrier proteins and protects brain from external insults. PM has been identified in both the human capillaries and brain parenchyma [120,135], indicating the ability to interact with both BBB-forming cells and to move within BBB through mechanisms still unknown. Recent findings have suggested that aluminum nanoparticles can increase oxidative stress, decrease the viability of human brain microvascular endothelial cells, alter mitochondrial potential, and reduce tight junction protein expression, suggesting that nanoparticles can endothelial cells damage and damage to BBB [136].
Exposure to air pollution is associated with increased ICAM and VCAM injury to brain endothelial cells [120]. Further, In vitro surveys using rat capillaries show that particle treatment induces the production of the cytokines and the ROS and that these signal caused changes in the expression and function of transmitters (eg: P-glycoprotein and resistance) [137]. Therefore, brain capillaries detect air pollutants and respond to these by regulating the function of the chemical and physical barrier and by producing inflammatory signals. Also, this response may act as an inflammatory sensor and finally contribute to the distribution of ROS, cytokines, and particles in the brain parenchyma, more likely in CNS pathology. Therefore, these findings are dependent on CNS drug therapy in neurodegenerative diseases. In particular, PM rearrangement induced by circulating transporters (multidrug resistance protein and p-glycoprotein in the BBB) in BBB may be have significant consequences for drug access to brain parenchyma for people living in heavily infected cities, be it [138-144].
Generally, human, animal, and cell culture research have shown that exposure to air pollutants causes CNS oxidative stress, nerve inflammation, nerve damage, BBB changes, abnormal filamentous protein reinforcement, and brain injury and pathways. This indicates through which air pollution is affected in the pathology of CNS disease. While empirical evidences are compelling, given nature of chronic air pollution exposure in humans, the effects of the CNS are likely to reflect exposure to pollutants throughout human life length, including critical growth and development periods. It is noteworthy that these chronic effects are not harmful by laboratory methods and short-term exposure to animals (Table 2). However, these important and empirical studies have provided the foundation needed to identify the air pollutants toxic components and opportunity to the address their role in the CNS disease and pave the way for rigorous empirical investigation at the epidemiological level.
| Table 2: Effects of exposure to traffic air pollution particulate matter on brain. | ||||
| Exposure Model of Air Pollution |
Experimental Model |
Pro-inflammatory Markers & Neuroinflammation | Behavior Changes& Neuropathology |
References |
| Chronic Exposure to Urban Air Pollution |
Human Dog | IL1-b, COX2, iNOS, & CD14 Increase NFkb, iNOS, & CD14 Increase |
White Matter Lesions, Diffuse AbPlaques, BBB Damage, a Synuclein Aggregation, Cognitive Deficits, & DNA Damage White Matter Lesions Diffuse Ab Plaques a Synuclein Aggregation, DNA Damage, & BBB Damage |
[119,120,136,140-142] [141,143,144] |
| Exposure to Nanoparticles |
Cell Culture
Cell Culture Mouse
Mouse Mouse |
Microglial Activation Superoxide Production N/T N/T | DA Neuron Damage Lower Tight Junction Expression Oxidative Stress (Brain) Lipid Peroxidation, HBMEC Toxicity |
[145,146] [136] [12,147-149] |
| Exposure to Particulate Matter | Mouse | N/T | DA Neuron Damage in the | [150] |
| Substantia Nigra | ||||
| Mouse | TNFa, IL1-b, and INFg | Change in | [10,12,151] | |
| increase in OB | Neurotransmitters | |||
| Mouse | Cytokine Production, JNK | N/T | [152,153] | |
| Activation, Enhanced NFkb | ||||
| Expression | ||||
| Mouse | N/T | Changes in | [154] | |
| Neurotransmitters | ||||
| Rat | N/T | Lipid Peroxidation, | [155] | |
| Decrease in Exploratory | ||||
| Behavior | ||||
| Cell Culture | Microglial Activation | DA Neuron Damage | [123] | |
| Superoxide Production | ||||
| Cell Culture | Microglial Activation | N/T | [124] | |
| IL-6 & TNFaProduction | ||||
| Brain Capillary | TNFa & ROS | P-GP & MRP2 Increase | [137] | |
| Culture | Production | Tight Junction Protein | ||
| c-Jun Phosphorylation | Decrease | |||
| Abbreviations: N/T: Not Tested; IL-1b: Interleukin 1b; DA: Dopamine; c-JNK: c-Jun N Terminal Kinase; INFg, Interferon g; TNFa: Tumor Necrosis Factor a; IL-6: Interleukin 6; NFkb: Nuclear Factor kb; P-GP: P-Glycoprotein; OB: Olfactory Bulb; ROS: Reactive Oxygen Species; BBB: Blood-Brain Barrier; MRP2: Multidrug Resistance Associated Protein-2; COX2: Cyclooxygenase 2; HBMEC: Human Brain Microvascular Endothelial Cells. | ||||
Conclusion and Future Perspective
The air pollutants effects are transmitted from the periphery to the brain through systemic inflammation and the movement of UFPs to the brain, where both the physical properties of the particles and the toxic compounds absorbed in the PM can cause damage. Cerebral capillaries, astroglia, and particularly microglia respond to the air pollutants components by oxidative stress, chronic activation, and inflammation. Exposure to urban air pollutants at high concentrations is problematic given the suggested association between air pollution exposure and neurodegenerative diseases such as AD or dementia. In addition, exposure to air pollutants in the workplace is usually low, but very worrying, given that even short-term exposure can cause the biochemical changes associated with such diseases. In general, other studies are needed to better describe the effects of exposure to traffic air pollutants on the CNS, its role, and its underlying mechanisms in the development of neurodegenerative and neurodevelopmental diseases. In particular, due to the higher prevalence of neurodevelopmental (such as AD) and neurological disorders (such as PD) in males, gender may be affected by exposure to air pollution [145-155]. In any case, according to recent findings, the higher emissions of diesel engines than the ones mentioned above have raised major concerns that should be addressed with further studies on the health effects of exposure to DEPs, so experimental studies and the epidemiological link between the DEPs and the CNS of the disease is of particular importance.
Acknowledgment
We thank Dr. Ehsanifar Lab. Tehran, Iran, for the support of this work.
References
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: Uuncovering the molecular mechanisms. Nat Rev Neurosci. 2007 Jan;8(1):57-69. doi: 10.1038/nrn2038. PMID: 17180163.
- Haghani A. Toxicity of urban air pollution particulate matter in developing and adult mouse brain: Comparison of total and filter-eluted nanoparticles. Environment International. 2020;136:105510. https://tinyurl.com/6ufet9n6
- Forman HJ, Finch CE. A critical review of assays for hazardous components of air pollution. Free Radic Biol Med. 2018 Mar;117:202-217. doi: 10.1016/j.freeradbiomed.2018.01.030. Epub 2018 Jan 31. PMID: 29407794; PMCID: PMC5845809.
- Garcidueñas L, Reynoso-Robles R, Maciel A. Combustion and friction-derived nanoparticles and industrial-sourced nanoparticles: The culprit of Alzheimer and Parkinson's diseases. Environ Res. 2019 Sep;176:108574. doi: 10.1016/j.envres.2019.108574. Epub 2019 Jul 5. PMID: 31299618.
- Slechta DA, Sobolewski M, Marvin E, Conrad K, Merrill A, Anderson T, Jackson BP, Oberdorster G. The impact of inhaled ambient ultrafine particulate matter on developing brain: potential importance of elemental contaminants. Toxicol Pathol. 2019 Dec;47(8):976-992. doi: 10.1177/0192623319878400. Epub 2019 Oct 14. PMID: 31610749; PMCID: PMC6911038.
- Slechta DA, Allen JL, Conrad K, Marvin E, Sobolewski M. Developmental exposure to low level ambient ultrafine particle air pollution and cognitive dysfunction. Neurotoxicology. 2018 Dec;69:217-231. doi: 10.1016/j.neuro.2017.12.003. Epub 2017 Dec 13. PMID: 29247674; PMCID: PMC5999548.
- Jafari A, Ehsanifar. The share of different vehicles in air pollutant emission in tehran, using 2013 traffic information. Caspian Journal of Health Research. 2016;2(2):28-36. https://tinyurl.com/a3wfkfry
- Reis H, Reis C, Sharip A, Reis W, Zhao Y, Sinclair R, Beeson L. Diesel exhaust exposure, its multi-system effects, and the effect of new technology diesel exhaust. Environ Int. 2018 May;114:252-265. doi: 10.1016/j.envint.2018.02.042. Epub 2018 Mar 7. PMID: 29524921.
- Russ TC, Reis S, van Tongeren M. Air pollution and brain health: defining the research agenda. Curr Opin Psychiatry. 2019 Mar;32(2):97-104. doi: 10.1097/YCO.0000000000000480. PMID: 30543549.
- Ehsanifar M, Jafari AJ, Nikzad H, Zavareh MS, Atlasi MA, Mohammadi H, Tameh AA. Prenatal exposure to diesel exhaust particles causes anxiety, spatial memory disorders with alters expression of hippocampal pro-inflammatory cytokines and NMDA receptor subunits in adult male mice offspring. Ecotoxicol Environ Saf. 2019 Jul 30;176:34-41. doi: 10.1016/j.ecoenv.2019.03.090. Epub 2019 Mar 25. PMID: 30921694.
- Ehsanifar MYZ, Karimian M, Behdarvandi M. Anxiety and depression following diesel exhaust nano-particles exposure in male and female mice. Journal of Neurophysiology and Neurological Disorders. 2020;6(101):1-8. https://tinyurl.com/2ya8fype
- Ehsanifar M, Tameh AA, Farzadkia M, Kalantari RR, Zavareh MS, Nikzaad H, Jafari AJ. Exposure to nanoscale diesel exhaust particles: Oxidative stress, neuroinflammation, anxiety and depression on adult male mice. Ecotoxicol Environ Saf. 2019 Jan 30;168:338-347. doi: 10.1016/j.ecoenv.2018.10.090. Epub 2018 Nov 2. PMID: 30391838.
- Elwood E, Lim Z, Naveed H, Galea I. The effect of systemic inflammation on human brain barrier function. Brain Behav Immun. 2017 May;62:35-40. doi: 10.1016/j.bbi.2016.10.020. Epub 2016 Nov 1. PMID: 27810376; PMCID: PMC5380128.
- Orellano P, Quaranta N, Reynoso J, Balbi B, Vasquez J. Effect of outdoor air pollution on asthma exacerbations in children and adults: Systematic review and multilevel meta-analysis. PLoS One. 2017 Mar 20;12(3):e0174050. doi: 10.1371/journal.pone.0174050. PMID: 28319180; PMCID: PMC5358780.
- Lanki T, de Hartog JJ, Heinrich J, Hoek G, Janssen NA, Peters A, Stölzel M, Timonen KL, Vallius M, Vanninen E, Pekkanen J. Can we identify sources of fine particles responsible for exercise-induced ischemia on days with elevated air pollution? The ultra study. Environ Health Perspect. 2006 May;114(5):655-660. doi: 10.1289/ehp.8578. PMID: 16675416; PMCID: PMC1459915.
- Rao X, Zhong J, Brook RD, Rajagopalan S. Effect of particulate matter air pollution on cardiovascular oxidative stress pathways. Antioxid Redox Signal. 2018 Mar 20;28(9):797-818. doi: 10.1089/ars.2017.7394. Epub 2017 Dec 12. PMID: 29084451; PMCID: PMC5831906.
- Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roque P. Neurotoxicants are in the air: Convergence of human, animal, and In vitro studies on the effects of air pollution on the brain. Biomed Res Int. 2014;2014:736385. doi: 10.1155/2014/736385. Epub 2014 Jan 12. PMID: 24524086; PMCID: PMC3912642.
- Garcidueñas L, Kavanaugh M, Block M, D'Angiulli A, Delgado-Chávez R, Torres-Jardón R, González-Maciel A, Reynoso-Robles R, Osnaya N, Villarreal-Calderon R, Guo R, Hua Z, Zhu H, Perry G, Diaz P. Neuroinflammation, hyperphosphorylated tau, diffuse amyloid plaques, and down-regulation of the cellular prion protein in air pollution exposed children and young adults. J Alzheimers Dis. 2012;28(1):93-107. doi: 10.3233/JAD-2011-110722. PMID: 21955814.
- Guxens M, Sunyer J. A review of epidemiological studies on neuropsychological effects of air pollution. Swiss Med Wkly. 2012 Jan 3;141:w13322. doi: 10.4414/smw.2011.13322. PMID: 22252905.
- Weuve J, Puett RC, Schwartz J, Yanosky JD, Laden F, Grodstein F. Exposure to particulate air pollution and cognitive decline in older women. Arch Intern Med. 2012 Feb 13;172(3):219-227. doi: 10.1001/archinternmed.2011.683. PMID: 22332151; PMCID: PMC3622279.
- Hesterberg TW, Long CM, Sax SN, Lapin CA, McClellan RO, Bunn WB, Valberg PA. Particulate matter in New Technology Diesel Exhaust (NTDE) is quantitatively and qualitatively very different from that found in traditional diesel exhaust (TDE). J Air Waste Manag Assoc. 2011 Sep;61(9):894-913. doi: 10.1080/10473289.2011.599277. PMID: 22010375.
- Crüts B, van Etten L, Törnqvist H, Blomberg A, Sandström T, Mills NL, Borm PJ. Exposure to diesel exhaust induces changes in EEG in human volunteers. Part Fibre Toxicol. 2008 Mar 11;5:4. doi: 10.1186/1743-8977-5-4. PMID: 18334019; PMCID: PMC2329662.
- Fujimaki H, Kurokawa Y, Yamamoto S, Satoh M. Distinct requirements for interleukin-6 in airway inflammation induced by diesel exhaust in mice. Immunopharmacol Immunotoxicol. 2006;28(4):703-714. doi: 10.1080/08923970601067433. PMID: 17190745.
- Suzuki T, Oshio S, Iwata M, Saburi H, Odagiri T, Udagawa T, Sugawara I, Umezawa M, Takeda K. In utero exposure to a low concentration of diesel exhaust affects spontaneous locomotor activity and monoaminergic system in male mice. Part Fibre Toxicol. 2010 Mar 23;7:7. doi: 10.1186/1743-8977-7-7. PMID: 20331848; PMCID: PMC2853486.
- Cancer, IAfRo, WHO. Outdoor air pollution a leading environmental cause of cancer deaths. World Health Organization. 2013. https://tinyurl.com/57464y54
- Takano H, Yanagisawa R, Ichinose T, Sadakane K, Inoue K, Yoshida S, Takeda K, Yoshino S, Yoshikawa T, Morita M. Lung expression of cytochrome P450 1A1 as a possible biomarker of exposure to diesel exhaust particles. Arch Toxicol. 2002 Apr;76(3):146-151. doi: 10.1007/s00204-002-0323-0. Epub 2002 Feb 20. PMID: 11967619.
- Danysh HE, Mitchell LE, Zhang K, Scheurer ME, Lupo PJ. Traffic-related air pollution and the incidence of childhood central nervous system tumors: Texas, 2001-2009. Pediatr Blood Cancer. 2015 Sep;62(9):1572-1578. doi: 10.1002/pbc.25549. Epub 2015 May 11. PMID: 25962758.
- Filippini T, Heck JE, Malagoli C, Del Giovane C, Vinceti M. A review and meta-analysis of outdoor air pollution and risk of childhood leukemia. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2015;33(1):36-66. doi: 10.1080/10590501.2015.1002999. PMID: 25803195; PMCID: PMC4586078.
- Ajmani GS, Suh HH, Pinto JM. Effects of ambient air pollution exposure on olfaction: A review. Environ Health Perspect. 2016 Nov;124(11):1683-1693. doi: 10.1289/EHP136. Epub 2016 Jun 10. PMID: 27285588; PMCID: PMC5089874.
- Møller KL, Brauer C, Mikkelsen S, Bonde JP, Loft S, Helweg-Larsen K, Thygesen LC. Cardiovascular disease and long-term occupational exposure to ultrafine particles: A cohort study of airport workers. Int J Hyg Environ Health. 2020 Jan;223(1):214-219. doi: 10.1016/j.ijheh.2019.08.010. Epub 2019 Sep 4. PMID: 31492618.
- Hamanaka RB, Mutlu GM. Particulate matter air pollution: Effects on the cardiovascular system. Front Endocrinol (Lausanne). 2018 Nov 16;9:680. doi: 10.3389/fendo.2018.00680. PMID: 30505291; PMCID: PMC6250783.
- Schnelle-Kreis J, Küpper U, Sklorz M, Cyrys J, Briedé JJ, Peters A, Zimmermann R. Daily measurement of organic compounds in ambient particulate matter in Augsburg, Germany: New aspects on aerosol sources and aerosol related health effects. Biomarkers. 2009 Jul;14 Suppl 1:39-44. doi: 10.1080/13547500902965997. PMID: 19604057.
- Møller P, Loft S. Oxidative damage to DNA and lipids as biomarkers of exposure to air pollution. Environmental health perspectives. 2010;118(8):1126-1136. https://tinyurl.com/4chhjyrt
- Valavanidis A, Fiotakis K, Vlachogianni T. Airborne particulate matter and human health: Toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2008 Oct-Dec;26(4):339-362. doi: 10.1080/10590500802494538. PMID: 19034792.
- Jeng HA. Chemical composition of ambient particulate matter and redox activity. Environ Monit Assess. 2010 Oct;169(1-4):597-606. doi: 10.1007/s10661-009-1199-8. Epub 2009 Nov 10. PMID: 19902370.
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89-116. doi: 10.1146/annurev.pharmtox.46.120604.141046. PMID: 16968214.
- Leni Z, Künzi, Geiser M. Air pollution causing oxidative stress. Current Opinion in Toxicology. 2020;20:1-8. https://tinyurl.com/933xseru
- Andersson H, Piras E, Demma J, Hellman B, Brittebo E. Low levels of the air pollutant 1-nitropyrene induce DNA damage, increased levels of reactive oxygen species and endoplasmic reticulum stress in human endothelial cells. Toxicology. 2009 Jul 28;262(1):57-64. doi: 10.1016/j.tox.2009.05.008. Epub 2009 May 19. PMID: 19460413.
- Park JH, Troxel AB, Harvey RG, Penning TM. Polycyclic aromatic hydrocarbon (PAH) o-quinones produced by the aldo-keto-reductases (AKRs) generate abasic sites, oxidized pyrimidines, and 8-oxo-dGuo via reactive oxygen species. Chem Res Toxicol. 2006 May;19(5):719-728. doi: 10.1021/tx0600245. PMID: 16696575; PMCID: PMC2366214.
- Uzma N, Kumar BS, Hazari MA. Exposure to benzene induces oxidative stress, alters the immune response and expression of p53 in gasoline filling workers. Am J Ind Med. 2010 Dec;53(12):1264-1270. doi: 10.1002/ajim.20901. PMID: 20886531.
- Tachibana K, Takayanagi K, Akimoto A, Ueda K, Shinkai Y, Umezawa M, Takeda K. Prenatal diesel exhaust exposure disrupts the DNA methylation profile in the brain of mouse offspring. J Toxicol Sci. 2015 Feb;40(1):1-11. doi: 10.2131/jts.40.1. PMID: 25560391.
- Baulig A, Garlatti M, Bonvallot V, Marchand A, Barouki R, Marano F, Baeza-Squiban A. Involvement of reactive oxygen species in the metabolic pathways triggered by diesel exhaust particles in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2003 Sep;285(3):L671-679. doi: 10.1152/ajplung.00419.2002. Epub 2003 May 2. PMID: 12730081.
- Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang M, Oberley T, Froines J, Nel A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003 Apr;111(4):455-460. doi: 10.1289/ehp.6000. PMID: 12676598; PMCID: PMC1241427.
- Amara N, Bachoual R, Desmard M, Golda S, Guichard C, Lanone S, Aubier M, Ogier-Denis E, Boczkowski J. Diesel exhaust particles induce matrix metalloprotease-1 in human lung epithelial cells via a NADP(H) oxidase/NOX4 redox-dependent mechanism. Am J Physiol Lung Cell Mol Physiol. 2007 Jul;293(1):L170-181. doi: 10.1152/ajplung.00445.2006. Epub 2007 Apr 20. PMID: 17449795.
- Kang KW, Lee SJ, Kim SG. Molecular mechanism of nrf2 activation by oxidative stress. Antioxid Redox Signal. 2005 Nov-Dec;7(11-12):1664-1673. doi: 10.1089/ars.2005.7.1664. PMID: 16356128.
- Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci. 2009 Apr;34(4):176-188. doi: 10.1016/j.tibs.2008.12.008. Epub 2009 Mar 25. PMID: 19321346.
- Kim JS, Oh JM, Choi H, Kim SW, Kim SW, Kim BG, Cho JH, Lee J, Lee DC. Activation of the Nrf2/HO-1 pathway by curcumin inhibits oxidative stress in human nasal fibroblasts exposed to urban particulate matter. BMC Complement Med Ther. 2020 Mar 30;20(1):101. doi: 10.1186/s12906-020-02886-8. PMID: 32228565; PMCID: PMC7106591.
- Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009 Sep 15;78(6):539-552. doi: 10.1016/j.bcp.2009.04.029. Epub 2009 May 4. PMID: 19413999; PMCID: PMC2730638.
- Mann GE, Niehueser-Saran J, Watson A, Gao L, Ishii T, de Winter P, Siow RC. Nrf2/ARE regulated antioxidant gene expression in endothelial and smooth muscle cells in oxidative stress: implications for atherosclerosis and preeclampsia. Sheng Li Xue Bao. 2007 Apr 25;59(2):117-127. PMID: 17437032.
- Ayres JG, Borm P, Cassee FR, Castranova V, Donaldson K, Ghio A, Harrison RM, Hider R, Kelly F, Kooter IM, Marano F, Maynard RL, Mudway I, Nel A, Sioutas C, Smith S, Baeza-Squiban A, Cho A, Duggan S, Froines J. Evaluating the toxicity of airborne particulate matter and nanoparticles by measuring oxidative stress potential--a workshop report and consensus statement. Inhal Toxicol. 2008 Jan;20(1):75-99. doi: 10.1080/08958370701665517. PMID: 18236225.
- Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004 Dec 16;351(25):2599-2610. doi: 10.1056/NEJMoa040967. PMID: 15602020.
- Vaziri ND, Rodríguez-Iturbe B. Mechanisms of disease: Oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol. 2006 Oct;2(10):582-593. doi: 10.1038/ncpneph0283. PMID: 17003837.
- Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000 Jun;18(6):655-673. doi: 10.1097/00004872-200018060-00002. PMID: 10872549.
- Vaziri ND. Oxidative stress in uremia: Nature, mechanisms, and potential consequences. Semin Nephrol. 2004 Sep;24(5):469-473. doi: 10.1016/j.semnephrol.2004.06.026. PMID: 15490413.
- Utell MJ, Frampton MW, Zareba W, Devlin RB, Cascio WE. Cardiovascular effects associated with air pollution: potential mechanisms and methods of testing. Inhal Toxicol. 2002 Dec;14(12):1231-1247. doi: 10.1080/08958370290084881. PMID: 12454788.
- Alexeeff SE, Litonjua AA, Wright RO, Baccarelli A, Suh H, Sparrow D, Vokonas PS, Schwartz J. Ozone exposure, antioxidant genes, and lung function in an elderly cohort: VA normative aging study. Occup Environ Med. 2008 Nov;65(11):736-742. doi: 10.1136/oem.2007.035253. Epub 2008 Jun 4. PMID: 18524839; PMCID: PMC2575239.
- Chahine T, Baccarelli A, Litonjua A, Wright RO, Suh H, Gold DR, Sparrow D, Vokonas P, Schwartz J. Particulate air pollution, oxidative stress genes, and heart rate variability in an elderly cohort. Environ Health Perspect. 2007 Nov;115(11):1617-1622. doi: 10.1289/ehp.10318. PMID: 18007994; PMCID: PMC2072834.
- Islam T, McConnell R, Gauderman WJ, Avol E, Peters JM, Gilliland FD. Ozone, oxidant defense genes, and risk of asthma during adolescence. Am J Respir Crit Care Med. 2008 Feb 15;177(4):388-395. doi: 10.1164/rccm.200706-863OC. Epub 2007 Nov 29. PMID: 18048809; PMCID: PMC2258440.
- Islam T, Berhane K, McConnell R, Gauderman WJ, Avol E, Peters JM, Gilliland FD. Glutathione-S-transferase (GST) P1, GSTM1, exercise, ozone and asthma incidence in school children. Thorax. 2009 Mar;64(3):197-202. doi: 10.1136/thx.2008.099366. Epub 2008 Nov 6. PMID: 18988661; PMCID: PMC2738935.
- Ren C, Park SK, Vokonas PS, Sparrow D, Wilker E, Baccarelli A, Suh HH, Tucker KL, Wright RO, Schwartz J. Air pollution and homocysteine: more evidence that oxidative stress-related genes modify effects of particulate air pollution. Epidemiology. 2010 Mar;21(2):198-206. doi: 10.1097/EDE.0b013e3181cc8bfc. PMID: 20110814; PMCID: PMC3939788.
- Xu F, Shi X, Qiu X, Jiang X, Fang Y, Wang J, Hu D, Zhu T. Investigation of the chemical components of ambient fine particulate matter (PM2.5) associated with In vitro cellular responses to oxidative stress and inflammation. Environ Int. 2020 Mar;136:105475. doi: 10.1016/j.envint.2020.105475. Epub 2020 Jan 31. PMID: 32007923.
- Liu L, Ruddy TD, Dalipaj M, Szyszkowicz M, You H, Poon R, Wheeler A, Dales R. Influence of personal exposure to particulate air pollution on cardiovascular physiology and biomarkers of inflammation and oxidative stress in subjects with diabetes. J Occup Environ Med. 2007 Mar;49(3):258-265. doi: 10.1097/JOM.0b013e31803220ef. PMID: 17351511.
- Chuang KJ, Chan CC, Su TC, Lee CT, Tang CS. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med. 2007 Aug 15;176(4):370-376. doi: 10.1164/rccm.200611-1627OC. Epub 2007 Apr 26. PMID: 17463411.
- Vinzents PS, Møller P, Sørensen M, Knudsen LE, Hertel O, Jensen FP, Schibye B, Loft S. Personal exposure to ultrafine particles and oxidative DNA damage. Environ Health Perspect. 2005 Nov;113(11):1485-1490. doi: 10.1289/ehp.7562. PMID: 16263500; PMCID: PMC1310907.
- Sørensen M, Schins RP, Hertel O, Loft S. Transition metals in personal samples of PM2.5 and oxidative stress in human volunteers. Cancer Epidemiol Biomarkers Prev. 2005 May;14(5):1340-1343. doi: 10.1158/1055-9965.EPI-04-0899. PMID: 15894700.
- Kim MK, Oh S, Lee JH, Im H, Ryu YM, Oh E, Lee J, Lee E, Sul D. Evaluation of biological monitoring markers using genomic and proteomic analysis for automobile emission inspectors and waste incinerating workers exposed to polycyclic aromatic hydrocarbons or 2,3,7,8,-tetracholrodedibenzo-p-dioxins. Exp Mol Med. 2004 Oct 31;36(5):396-410. doi: 10.1038/emm.2004.52. PMID: 15557812.
- Lai CH, Liou SH, Lin HC, Shih TS, Tsai PJ, Chen JS, Yang T, Jaakkola JJ, Strickland PT. Exposure to traffic exhausts and oxidative DNA damage. Occup Environ Med. 2005 Apr;62(4):216-222. doi: 10.1136/oem.2004.015107. PMID: 15778253; PMCID: PMC1740998.
- Rossner P Jr, Svecova V, Milcova A, Lnenickova Z, Solansky I, Santella RM, Sram RJ. Oxidative and nitrosative stress markers in bus drivers. Mutat Res. 2007 Apr 1;617(1-2):23-32. doi: 10.1016/j.mrfmmm.2006.11.033. Epub 2007 Feb 4. PMID: 17328930.
- Rossner P Jr, Svecova V, Milcova A, Lnenickova Z, Solansky I, Sram RJ. Seasonal variability of oxidative stress markers in city bus drivers. Part I. Oxidative damage to DNA. Mutat Res. 2008 Jul 3;642(1-2):14-20. doi: 10.1016/j.mrfmmm.2008.03.003. Epub 2008 Mar 25. PMID: 18436263.
- Rossner P Jr, Svecova V, Milcova A, Lnenickova Z, Solansky I, Sram RJ. Seasonal variability of oxidative stress markers in city bus drivers. Part II. Oxidative damage to lipids and proteins. Mutat Res. 2008 Jul 3;642(1-2):21-27. doi: 10.1016/j.mrfmmm.2008.03.004. Epub 2008 Mar 25. PMID: 18436262.
- Singh R, Sram RJ, Binkova B, Kalina I, Popov TA, Georgieva T, Garte S, Taioli E, Farmer PB. The relationship between biomarkers of oxidative DNA damage, polycyclic aromatic hydrocarbon DNA adducts, antioxidant status and genetic susceptibility following exposure to environmental air pollution in humans. Mutat Res. 2007 Jul 1;620(1-2):83-92. doi: 10.1016/j.mrfmmm.2007.02.025. Epub 2007 Mar 3. PMID: 17445838.
- Soares SR, Carvalho-Oliveira R, Ramos-Sanchez E, Catanozi S, da Silva LF, Mauad T, Gidlund M, Goto H, Garcia ML. Air pollution and antibodies against modified lipoproteins are associated with atherosclerosis and vascular remodeling in hyperlipemic mice. Atherosclerosis. 2009 Dec;207(2):368-373. doi: 10.1016/j.atherosclerosis.2009.04.041. Epub 2009 May 13. PMID: 19486979.
- Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, Nel AE. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res. 2008 Mar 14;102(5):589-596. doi: 10.1161/CIRCRESAHA.107.164970. Epub 2008 Jan 17. PMID: 18202315; PMCID: PMC3014059.
- Panagiotakos DB, Pitsavos C, Chrysohoou C, Skoumas J, Masoura C, Toutouzas P, Stefanadis C; ATTICA study. Effect of exposure to secondhand smoke on markers of inflammation: The ATTICA study. Am J Med. 2004 Feb 1;116(3):145-150. doi: 10.1016/j.amjmed.2003.07.019. PMID: 14749157.
- Hoffmann B, Moebus S, Möhlenkamp S, Stang A, Lehmann N, Dragano N, Schmermund A, Memmesheimer M, Mann K, Erbel R, Jöckel KH; Heinz Nixdorf Recall Study Investigative Group. Residential exposure to traffic is associated with coronary atherosclerosis. Circulation. 2007 Jul 31;116(5):489-496. doi: 10.1161/CIRCULATIONAHA.107.693622. Epub 2007 Jul 16. PMID: 17638927.
- Künzli N, Jerrett M, Mack WJ, Beckerman B, LaBree L, Gilliland F, Thomas D, Peters J, Hodis HN. Ambient air pollution and atherosclerosis in Los Angeles. Environ Health Perspect. 2005 Feb;113(2):201-206. doi: 10.1289/ehp.7523. PMID: 15687058; PMCID: PMC1277865.
- Cho AK, Sioutas C, Miguel AH, Kumagai Y, Schmitz DA, Singh M, Eiguren-Fernandez A, Froines JR. Redox activity of airborne particulate matter at different sites in the Los Angeles Basin. Environ Res. 2005 Sep;99(1):40-7. doi: 10.1016/j.envres.2005.01.003. PMID: 16053926.
- Cho AK, Sioutas C, Miguel AH, Kumagai Y, Schmitz DA, Singh M, Eiguren-Fernandez A, Froines JR. Redox activity of airborne particulate matter at different sites in the Los Angeles Basin. Environ Res. 2005 Sep;99(1):40-47. doi: 10.1016/j.envres.2005.01.003. PMID: 16053926.
- Ntziachristos L, Froines JR, Cho AK, Sioutas C. Relationship between redox activity and chemical speciation of size-fractionated particulate matter. Part Fibre Toxicol. 2007 Jun 7;4:5. doi: 10.1186/1743-8977-4-5. PMID: 17555562; PMCID: PMC1899517.
- Verma V. Redox activity of urban quasi-ultrafine particles from primary and secondary sources. Atmospheric Environment. 2009;43(40):6360-6368. https://tinyurl.com/3ea49mdu
- Verma V, Polidori A, Schauer JJ, Shafer MM, Cassee FR, Sioutas C. Physicochemical and toxicological profiles of particulate matter in Los Angeles during the October 2007 southern California wildfires. Environ Sci Technol. 2009 Feb 1;43(3):954-960. doi: 10.1021/es8021667. PMID: 19245042.
- Gertler AW.Does PM from diesel or gasoline vehicles dominate in the US? Atmospheric Environment. 2005;39(13):2349-2355. https://tinyurl.com/y62baez9
- Phuleria H. Roadside measurements of size-segregated particulate organic compounds near gasoline and diesel-dominated freeways in Los Angeles, CA. Atmospheric Environment. 2007;41(22):4653-4671. https://tinyurl.com/4z3cxuwu
- Bonvallot V, Squiban A, Baulig A, Brulant S, Boland S, Muzeau F, Barouki R, Marano F. Organic compounds from diesel exhaust particles elicit a proinflammatory response in human airway epithelial cells and induce cytochrome P450 1A1 expression. Am J Respir Cell Mol Biol. 2001 Oct;25(4):515-521. doi: 10.1165/ajrcmb.25.4.4515. PMID: 11694458.
- Yan D, Wu S, Zhou S, Tong G, Li F, Wang Y, Li B. Characteristics, sources and health risk assessment of airborne particulate PAHs in chinese cities: A review. Environ Pollut. 2019 May;248:804-814. doi: 10.1016/j.envpol.2019.02.068. Epub 2019 Feb 27. PMID: 30851590.
- Shinyashiki M. Electrophilic and redox properties of diesel exhaust particles. Environmental research. 2009;109(3):239-244. https://tinyurl.com/sk6sv3yz
- Riedl M, Diaz-Sanchez D. Biology of diesel exhaust effects on respiratory function. J Allergy Clin Immunol. 2005 Feb;115(2):221-228; quiz 229. doi: 10.1016/j.jaci.2004.11.047. PMID: 15696072.
- Wang X. An overview of physical and chemical features of diesel exhaust particles. Journal of the Energy Institute. 2019;92(6):1864-1888. https://tinyurl.com/3t8szhn2
- Li N, Kim S, Wang M, Froines J, Sioutas C, Nel A. Use of a stratified oxidative stress model to study the biological effects of ambient concentrated and diesel exhaust particulate matter. Inhal Toxicol. 2002 May;14(5):459-486. doi: 10.1080/089583701753678571. PMID: 12028803.
- Behndig AF, Mudway IS, Brown JL, Stenfors N, Helleday R, Duggan ST, Wilson SJ, Boman C, Cassee FR, Frew AJ, Kelly FJ, Sandström T, Blomberg A. Airway antioxidant and inflammatory responses to diesel exhaust exposure in healthy humans. Eur Respir J. 2006 Feb;27(2):359-365. doi: 10.1183/09031936.06.00136904. PMID: 16452593.
- Madrigano J, Baccarelli A, Wright RO, Suh H, Sparrow D, Vokonas PS, Schwartz J. Air pollution, obesity, genes and cellular adhesion molecules. Occup Environ Med. 2010 May;67(5):312-317. doi: 10.1136/oem.2009.046193. Epub 2009 Nov 2. PMID: 19884647; PMCID: PMC3229824.
- O'Neill MS, Veves A, Sarnat JA, Zanobetti A, Gold DR, Economides PA, Horton ES, Schwartz J. Air pollution and inflammation in type 2 diabetes: a mechanism for susceptibility. Occup Environ Med. 2007 Jun;64(6):373-379. doi: 10.1136/oem.2006.030023. Epub 2006 Dec 20. PMID: 17182639; PMCID: PMC2078522.
- Rückerl R, Ibald-Mulli A, Koenig W, Schneider A, Woelke G, Cyrys J, Heinrich J, Marder V, Frampton M, Wichmann HE, Peters A. Air pollution and markers of inflammation and coagulation in patients with coronary heart disease. Am J Respir Crit Care Med. 2006 Feb 15;173(4):432-441. doi: 10.1164/rccm.200507-1123OC. Epub 2005 Nov 17. PMID: 16293802.
- Yue W, Schneider A, Stölzel M, Rückerl R, Cyrys J, Pan X, Zareba W, Koenig W, Wichmann HE, Peters A. Ambient source-specific particles are associated with prolonged repolarization and increased levels of inflammation in male coronary artery disease patients. Mutat Res. 2007 Aug 1;621(1-2):50-60. doi: 10.1016/j.mrfmmm.2007.02.009. Epub 2007 Mar 1. PMID: 17603085.
- Delfino RJ, Staimer N, Tjoa T, Arhami M, Polidori A, Gillen DL, Kleinman MT, Schauer JJ, Sioutas C. Association of biomarkers of systemic inflammation with organic components and source tracers in quasi-ultrafine particles. Environ Health Perspect. 2010 Jun;118(6):756-762. doi: 10.1289/ehp.0901407. Epub 2010 Feb 2. PMID: 20123637; PMCID: PMC2898850.
- Arhami M, Minguillón MC, Polidori A, Schauer JJ, Delfino RJ, Sioutas C. Organic compound characterization and source apportionment of indoor and outdoor quasi-ultrafine particulate matter in retirement homes of the Los Angeles Basin. Indoor Air. 2010 Feb;20(1):17-30. doi: 10.1111/j.1600-0668.2009.00620.x. Epub 2009 Jul 31. PMID: 19874400; PMCID: PMC3781020.
- Delfino RN, Staimer, Tjoa. Airway and systemic inflammation are differently associated with primary and secondary organic aerosols in an elderly panel cohort. Epidemiol. 2010.
- Park SK, O'Neill MS, Vokonas PS, Sparrow D, Spiro A 3rd, Tucker KL, Suh H, Hu H, Schwartz J. Traffic-related particles are associated with elevated homocysteine: the VA normative aging study. Am J Respir Crit Care Med. 2008 Aug 1;178(3):283-289. doi: 10.1164/rccm.200708-1286OC. Epub 2008 May 8. PMID: 18467508; PMCID: PMC2542426.
- WHO. Health Effects of Black Carbon. Bonn: WHO regional office for Europe, the WHO European centre for environment and health. 2012. https://tinyurl.com/3z2tw4wa
- Grahame TJ, Schlesinger RB. Cardiovascular health and particulate vehicular emissions: A critical evaluation of the evidence. Air Qual Atmos Health. 2010 Mar;3(1):3-27. doi: 10.1007/s11869-009-0047-x. Epub 2009 Jun 30. PMID: 20376169; PMCID: PMC2844969.
- Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005 Jul;113(7):823-839. doi: 10.1289/ehp.7339. Erratum in: Environ Health Perspect. 2010 Sep;118(9):A380. PMID: 16002369; PMCID: PMC1257642.
- Mills NL, Donaldson K, Hadoke PW, Boon NA, MacNee W, Cassee FR, Sandström T, Blomberg A, Newby DE. Adverse cardiovascular effects of air pollution. Nat Clin Pract Cardiovasc Med. 2009 Jan;6(1):36-44. doi: 10.1038/ncpcardio1399. Epub 2008 Nov 25. PMID: 19029991.
- Schmid O, Möller W, Semmler-Behnke M, Ferron GA, Karg E, Lipka J, Schulz H, Kreyling WG, Stoeger T. Dosimetry and toxicology of inhaled ultrafine particles. Biomarkers. 2009 Jul;14 Suppl 1:67-73. doi: 10.1080/13547500902965617. PMID: 19604063.
- Geiser M, Kreyling WG. Deposition and biokinetics of inhaled nanoparticles. Part Fibre Toxicol. 2010 Jan 20;7:2. doi: 10.1186/1743-8977-7-2. PMID: 20205860; PMCID: PMC2826283.
- Sioutas C, Delfino RJ, Singh M. Exposure assessment for Atmospheric Ultrafine Particles (UFPs) and implications in epidemiologic research. Environ Health Perspect. 2005 Aug;113(8):947-955. doi: 10.1289/ehp.7939. PMID: 16079062; PMCID: PMC1280332.
- Lin CI. Instillation of particulate matter 2.5 induced acute lung injury and attenuated the injury recovery in ACE2 knockout mice. International journal of biological sciences. 2018;14(3):253. https://tinyurl.com/r488r9mc
- Sørensen M. Personal PM2. 5 exposure and markers of oxidative stress in blood. Environmental Health Perspectives. 2003;111(2):161-166. https://tinyurl.com/4ju9dxxn
- Frustaci A, Neri M, Cesario A, Adams JB, Domenici E, Dalla Bernardina B, Bonassi S. Oxidative stress-related biomarkers in autism: Systematic review and meta-analyses. Free Radic Biol Med. 2012 May 15;52(10):2128-2141. doi: 10.1016/j.freeradbiomed.2012.03.011. Epub 2012 Apr 18. PMID: 22542447.
- Möller W, Felten K, Sommerer K, Scheuch G, Meyer G, Meyer P, Häussinger K, Kreyling WG. Deposition, retention, and translocation of ultrafine particles from the central airways and lung periphery. Am J Respir Crit Care Med. 2008 Feb 15;177(4):426-432. doi: 10.1164/rccm.200602-301OC. Epub 2007 Oct 11. PMID: 17932382.
- Cho HY, Kleeberger SR. Nrf2 protects against airway disorders. Toxicol Appl Pharmacol. 2010 Apr 1;244(1):43-56. doi: 10.1016/j.taap.2009.07.024. Epub 2009 Jul 29. Erratum in: Toxicol Appl Pharmacol. 2010 Aug 1;246(3):186-187. PMID: 19646463.
- Delfino RJ, Staimer N, Tjoa T, Gillen DL, Polidori A, Arhami M, Kleinman MT, Vaziri ND, Longhurst J, Sioutas C. Air pollution exposures and circulating biomarkers of effect in a susceptible population: Clues to potential causal component mixtures and mechanisms. Environ Health Perspect. 2009 Aug;117(8):1232-1238. doi: 10.1289/ehp.0800194. Epub 2009 Apr 29. PMID: 19672402; PMCID: PMC2721866.
- Delfino RJ, Gillen DL, Tjoa T, Staimer N, Polidori A, Arhami M, Sioutas C, Longhurst J. Electrocardiographic ST-segment depression and exposure to traffic-related aerosols in elderly subjects with coronary artery disease. Environ Health Perspect. 2011 Feb;119(2):196-202. doi: 10.1289/ehp.1002372. Epub 2010 Oct 21. PMID: 20965803; PMCID: PMC3040606.
- Delfino RJ, Gillen DL, Tjoa T, Staimer N, Polidori A, Arhami M, Sioutas C, Longhurst J. Electrocardiographic ST-segment depression and exposure to traffic-related aerosols in elderly subjects with coronary artery disease. Environ Health Perspect. 2011 Feb;119(2):196-202. doi: 10.1289/ehp.1002372. Epub 2010 Oct 21. PMID: 20965803; PMCID: PMC3040606.
- Zhang Y, Schauer JJ, Shafer MM, Hannigan MP, Dutton SJ. Source apportionment of In vitro reactive oxygen species bioassay activity from atmospheric particulate matter. Environ Sci Technol. 2008 Oct 1;42(19):7502-7509. doi: 10.1021/es800126y. PMID: 18939593.
- Hu S. Redox activity and chemical speciation of size fractioned PM in the communities of the Los Angeles? Long Beach Harbor. 2008. https://tinyurl.com/44yhdrj5
- Landerman A. A macrophage-based method for the assessment of the oxidative stress activity of atmospheric Particulate Matter (PM) and application to routine (daily 24-hour) aerosol monitoring studies. Aerosol Sci Technol. 2008;42:946-957.
- Seifert G, Schilling K, Steinhäuser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006 Mar;7(3):194-206. doi: 10.1038/nrn1870. PMID: 16495941.
- Damiani CL, O'Callaghan JP. Recapitulation of cell signaling events associated with astrogliosis using the brain slice preparation. J Neurochem. 2007 Feb;100(3):720-726. doi: 10.1111/j.1471-4159.2006.04321.x. Epub 2006 Dec 14. PMID: 17176261.
- Garcidueñas L, Reed W, Maronpot RR, Roldán C, Delgado-Chavez R, Garcidueñas A, Dragustinovis I, Franco-Lira M, Flores M, Solt AC, Altenburg M, Jardón R, Swenberg JA. Brain inflammation and Alzheimer's-like pathology in individuals exposed to severe air pollution. Toxicol Pathol. 2004 Nov-Dec;32(6):650-658. doi: 10.1080/01926230490520232. PMID: 15513908.
- Garcidueñas L, Solt AC, Roldán C, Jardón R, Nuse B, Herritt L, Villarreal-Calderón R, Osnaya N, Stone I, García R, Brooks DM, González-Maciel A, Reynoso-Robles R, Delgado-Chávez R, Reed W. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol. 2008 Feb;36(2):289-310. doi: 10.1177/0192623307313011. Epub 2008 Mar 18. PMID: 18349428.
- Araneda S, Commin L, Atlagich M, Kitahama K, Parraguez VH, Pequignot JM, Dalmaz Y. VEGF overexpression in the astroglial cells of rat brainstem following ozone exposure. Neurotoxicology. 2008 Nov;29(6):920-7. doi: 10.1016/j.neuro.2008.09.006. Epub 2008 Sep 21. PMID: 18848842.
- Zhou NB, Fu ZJ, Sun T. Effects of different concentrations of oxygen-ozone on rats' astrocytes In vitro. Neurosci Lett. 2008 Aug 22;441(2):178-182. doi: 10.1016/j.neulet.2008.06.036. Epub 2008 Jun 18. PMID: 18577417.
- Block ML, Wu X, Pei Z, Li G, Wang T, Qin L, Wilson B, Yang J, Hong JS, Veronesi B. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. FASEB J. 2004 Oct;18(13):1618-1620. doi: 10.1096/fj.04-1945fje. Epub 2004 Aug 19. PMID: 15319363.
- Sama P, Long TC, Hester S, Tajuba J, Parker J, Chen LC, Veronesi B. The cellular and genomic response of an immortalized microglia cell line (BV2) to concentrated ambient particulate matter. Inhal Toxicol. 2007 Oct;19(13):1079-1087. doi: 10.1080/08958370701628721. PMID: 17957548.
- Blasko I, Kountchev M, Robatscher P, Veerhuis R, Eikelenboom P, Grubeck-Loebenstein B. How chronic inflammation can affect the brain and support the development of Alzheimer's disease in old age: The role of microglia and astrocytes. Aging Cell. 2004 Aug;3(4):169-176. doi: 10.1111/j.1474-9728.2004.00101.x. PMID: 15268750.
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005 May 27;308(5726):1314-8. doi: 10.1126/science.1110647. Epub 2005 Apr 14. PMID: 15831717.
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988 Aug;38(8):1285-1291. doi: 10.1212/wnl.38.8.1285. PMID: 3399080.
- Butterfield DA, Halliwell B. Oxidative stress, dysfunctional glucose metabolism and alzheimer disease. Nat Rev Neurosci. 2019 Mar;20(3):148-160. doi: 10.1038/s41583-019-0132-6. PMID: 30737462.
- Long TC, Saleh N, Tilton RD, Lowry GV, Veronesi B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): Implications for nanoparticle neurotoxicity. Environ Sci Technol. 2006 Jul 15;40(14):4346-4352. doi: 10.1021/es060589n. PMID: 16903269.
- Zhang P, Hatter A, Liu B. Manganese chloride stimulates rat microglia to release hydrogen peroxide. Toxicol Lett. 2007 Sep 10;173(2):88-100. doi: 10.1016/j.toxlet.2007.06.013. Epub 2007 Jun 28. PMID: 17669604; PMCID: PMC2100035.
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007 Apr 1;55(5):453-462. doi: 10.1002/glia.20467. PMID: 17203472; PMCID: PMC2871685.
- Ling Z, Zhu Y, Tong Cw, Snyder JA, Lipton JW, Carvey PM. Progressive dopamine neuron loss following supra-nigral lipopolysaccharide (LPS) infusion into rats exposed to LPS prenatally. Exp Neurol. 2006 Jun;199(2):499-512. doi: 10.1016/j.expneurol.2006.01.010. Epub 2006 Feb 28. PMID: 16504177.
- Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007 Feb 2;100(2):158-73. doi: 10.1161/01.RES.0000255691.76142.4a. PMID: 17272818.
- Miller DS, Bauer B, Hartz AM. Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev. 2008 Jun;60(2):196-209. doi: 10.1124/pr.107.07109. Epub 2008 Jun 17. PMID: 18560012; PMCID: PMC2634288.
- Oppenheim HA, Lucero J, Guyot AC, Herbert LM, McDonald JD, Mabondzo A, Lund AK. Exposure to vehicle emissions results in altered blood brain barrier permeability and expression of matrix metalloproteinases and tight junction proteins in mice. Part Fibre Toxicol. 2013 Dec 17;10:62. doi: 10.1186/1743-8977-10-62. PMID: 24344990; PMCID: PMC3878624.
- Chen L, Yokel RA, Hennig B, Toborek M. Manufactured aluminum oxide nanoparticles decrease expression of tight junction proteins in brain vasculature. J Neuroimmune Pharmacol. 2008 Dec;3(4):286-295. doi: 10.1007/s11481-008-9131-5. Epub 2008 Oct 1. PMID: 18830698; PMCID: PMC2771674.
- Hartz AM, Bauer B, Block ML, Hong JS, Miller DS. Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the blood-brain barrier. FASEB J. 2008 Aug;22(8):2723-2733. doi: 10.1096/fj.08-106997. Epub 2008 May 12. PMID: 18474546; PMCID: PMC2493447.
- Roqué PJ, Dao K, Costa LG. Microglia mediate diesel exhaust particle-induced cerebellar neuronal toxicity through neuroinflammatory mechanisms. Neurotoxicology. 2016 Sep;56:204-214. doi: 10.1016/j.neuro.2016.08.006. Epub 2016 Aug 16. PMID: 27543421; PMCID: PMC5048600.
- Clougherty JE. A growing role for gender analysis in air pollution epidemiology. Environ Health Perspect. 2010 Feb;118(2):167-76. doi: 10.1289/ehp.0900994. PMID: 20123621; PMCID: PMC2831913.
- Thomson EM, Kumarathasan P, Garcidueñas L, Vincent R. Air pollution alters brain and pituitary endothelin-1 and inducible nitric oxide synthase gene expression. Environ Res. 2007 Oct;105(2):224-233. doi: 10.1016/j.envres.2007.06.005. Epub 2007 Jul 30. PMID: 17662977.
- Garcidueñas L, Tiscareño A, Ontiveros E, Gómez-Garza G, Mejía G, Broadway J, Chapman S, Valencia-Salazar G, Jewells V, Maronpot RR, Roldán C, Pérez-Guillé B, Jardón R, Herrit L, Brooks D, Brizuela N, Monroy ME, González-Maciel A, Reynoso-Robles R, Villarreal-Calderon R, Solt AC, Engle RW. Air pollution, cognitive deficits and brain abnormalities: A pilot study with children and dogs. Brain Cogn. 2008 Nov;68(2):117-127. doi: 10.1016/j.bandc.2008.04.008. Epub 2008 Jun 11. PMID: 18550243.
- Garcidueñas L, Villarreal-Calderon R, Valencia-Salazar G, Roldán C, -Castrellón P, Torres-Jardón R, Osnaya-Brizuela N, Romero L, Jardón R, Solt A, Reed W. Systemic inflammation, endothelial dysfunction, and activation in clinically healthy children exposed to air pollutants. Inhal Toxicol. 2008 Mar;20(5):499-506. doi: 10.1080/08958370701864797. PMID: 18368620.
- Garcidueñas L, Azzarelli B, Acuna H, Garcia R, Gambling TM, Osnaya N, Monroy S, DEL Tizapantzi MR, Carson JL, Villarreal-Calderon A, Rewcastle B. Air pollution and brain damage. Toxicol Pathol. 2002 May-Jun;30(3):373-389. doi: 10.1080/01926230252929954. PMID: 12051555.
- Garcidueñas L, Maronpot RR, Jardon R, Roldán C, Schoonhoven R, Ayala H, Calderón A, Nakamura J, Fernando R, Reed W, Azzarelli B, Swenberg JA. DNA damage in nasal and brain tissues of canines exposed to air pollutants is associated with evidence of chronic brain inflammation and neurodegeneration. Toxicol Pathol. 2003 Sep-Oct;31(5):524-538. doi: 10.1080/01926230390226645. PMID: 14692621.
- Long TC, Tajuba J, Sama P, Saleh N, Swartz C, Parker J, Hester S, Lowry GV, Veronesi B. Nanosize titanium dioxide stimulates reactive oxygen species in brain microglia and damages neurons In vitro. Environ Health Perspect. 2007 Nov;115(11):1631-1637. doi: 10.1289/ehp.10216. PMID: 18007996; PMCID: PMC2072833.
- Lovell MA, Markesbery WR. Oxidative damage in mild cognitive impairment and early Alzheimer's disease. J Neurosci Res. 2007 Nov 1;85(14):3036-40. doi: 10.1002/jnr.21346. PMID: 17510979.
- Rahman MF, Wang J, Patterson TA, Saini UT, Robinson BL, Newport GD, Murdock RC, Schlager JJ, Hussain SM, Ali SF. Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicol Lett. 2009 May 22;187(1):15-21. doi: 10.1016/j.toxlet.2009.01.020. Epub 2009 Jan 23. PMID: 19429238.
- Wang J, Chen C, Liu Y, Jiao F, Li W, Lao F, Li Y, Li B, Ge C, Zhou G, Gao Y, Zhao Y, Chai Z. Potential neurological lesion after nasal instillation of TiO(2) nanoparticles in the anatase and rutile crystal phases. Toxicol Lett. 2008 Dec 15;183(1-3):72-80. doi: 10.1016/j.toxlet.2008.10.001. Epub 2008 Oct 17. PMID: 18992307.
- Alekseenko AV, Waseem TV, Fedorovich SV. Ferritin, a protein containing iron nanoparticles, induces reactive oxygen species formation and inhibits glutamate uptake in rat brain synaptosomes. Brain Res. 2008 Nov 19;1241:193-200. doi: 10.1016/j.brainres.2008.09.012. Epub 2008 Sep 16. PMID: 18835382.
- Ehsanifar M, Jafari AJ, Montazeri Z, Kalantari RR, Gholami M, Ashtarinezhad A. Learning and memory disorders related to hippocampal inflammation following exposure to air pollution. J Environ Health Sci Eng. 2021 Jan 22;19(1):261-272. doi: 10.1007/s40201-020-00600-x. PMID: 34150234; PMCID: PMC8172730.
- Shwe, Mitsushima D, Yamamoto S, Fukushima A, Funabashi T, Kobayashi T, Fujimaki H. Changes in neurotransmitter levels and proinflammatory cytokine mRNA expressions in the mice olfactory bulb following nanoparticle exposure. Toxicol Appl Pharmacol. 2008 Jan 15;226(2):192-198. doi: 10.1016/j.taap.2007.09.009. Epub 2007 Sep 20. PMID: 17950771.
- Campbell A, Oldham M, Becaria A, Bondy SC, Meacher D, Sioutas C, Misra C, Mendez LB, Kleinman M. Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicology. 2005 Jan;26(1):133-140. doi: 10.1016/j.neuro.2004.08.003. PMID: 15527881.
- Ehsanifar M, Montazeri Z, Taheri MA, Rafati M, Behjati M, Karimian M. Hippocampal inflammation and oxidative stress following exposure to diesel exhaust nanoparticles in male and female mice. Neurochem Int. 2021 May;145:104989. doi: 10.1016/j.neuint.2021.104989. Epub 2021 Feb 12. PMID: 33582162.
- Zanchi AC, Venturini CD, Saiki M, Nascimento Saldiva PH, Tannhauser Barros HM, Rhoden CR. Chronic nasal instillation of residual-oil fly ash (ROFA) induces brain lipid peroxidation and behavioral changes in rats. Inhal Toxicol. 2008 Jul;20(9):795-800. doi: 10.1080/08958370802009060. PMID: 18645718.
- Zanchi AC, Venturini CD, Saiki M, Nascimento Saldiva PH, Tannhauser Barros HM, Rhoden CR. Chronic nasal instillation of residual-oil fly ash (ROFA) induces brain lipid peroxidation and behavioral changes in rats. Inhal Toxicol. 2008 Jul;20(9):795-800. doi: 10.1080/08958370802009060. PMID: 18645718.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.