> Environmental Sciences. 2021 September 30;2(9):876-887. doi: 10.37871/jbres1326.
Recent Developments in Purification Techniques for Whey Valorization
Maham Aslam, Ansa Khalid, Ghanwa Tahir and Hamid Mukhtar*
- Whey proteins
- Fractionation
- Membrane processes
- Chromatography
- Food industry
- Pharmaceutical
- Chymosin
Abstract
Whey being a by-product of dairy industry, although is highly nutritive, was previously regarded as a waste but with time found its application in feedstock, pharmaceutical and food industry. Whey’s composition varies with respect to multiple factors such as source of milk, type of whey (acid or sweet whey) etc. Main challenge in whey utilization is that it has less quantity of whey constituents which need to be purified. Previously, the methods such as heat or acid treatment, precipitation and salting out were efficient only on laboratory scale and caused degradation of native protein structure making it difficult to understand its functional, nutritional and therapeutic properties, shifting focus towards innovative techniques which give product of high purity, are rapid, efficient, cost effective, eco-friendly and easy to be scaled up. Among such techniques, membrane separation and chromatography are widely employed ones. There is always a concern about purity and use of a single technique leads to compromise between purification level and overall purified product yield, shifting focus towards coupling of separation techniques. The following article is a comprehensive approach towards novel approaches for the isolation and separation of different whey constituents such as whey protein isolate and whey protein hydrolysate etc. along with their application in dairy, food and pharmaceutical industry and animal feedstock.
Introduction
During cheese and casein manufacturing; coagulation of casein proteins by chymosin; a by-product of great significance is obtained; named whey which is a multi-component protein [1]. It was discovered about 3000 years ago when the milk coagulation was observed during storage and transport of calves’ stomach (an old mean of milk storage) which is naturally rich in enzyme chymosin; leading to initiation of cheese and whey industry [2]. Whey is considered to be a diluted solution of lactose; minerals; proteins and fats. Whey proteins are about 0.7% (w/v) of the whey and can be classified into major and minor proteins. Table 1 shows the composition of whey among which first 4 are major proteins while last 2 are minor proteins [3].
| Table 1: Whey protein composition and characteristics [5]. | ||||
| Protein | Isoelectric point | Molecular mass (kDa) | Content % | Properties |
| β-lactoglobulin (β-Lg) | 5.2 | 18.4 | 30.0-55.0 | Antihypertensive and anticarcinogenic activity, emulsifying property and appetite enhancer |
| α-lactalbumin (α-La) | 4.5-4.8 | 14.2 | 20.0-25.0 | Antihypertensive, antioxidant activity and anti-obesity potential |
| Bovine serum albumin (BSA) | 4.7-4.9 | 66.5 | 5.0-10.0 | Anticarcinogenic and antioxidant activity. Foaming, emulsifying and gelling properties |
| Immunoglobulin’s (lg) | 5.5-8.3 | 150.0-1000.0 | 10.0 | Anti-cholesterol, antimicrobial and antiviral properties |
| Proteose-peptone | 3.7 | 4.0-22.0 | 12.0 | Enhances antibody production |
| Glycomacropeptide (GMP) | 4.3-4.6 | 6.8 | 10.0-15.0 | Anti-inflammatory and immunosuppressive properties. Inhibits colitis, and enhances cognitive development in animals |
| Lactoferrin (Lf) | 7.0-9.0 | 78.0 | 1.0-2.0 | Anti-obesity potential, anti-inflammatory and antimicrobial activity |
| Lactoperoxidase (Lp) | 9.5 | 89.0 | 0.5 | Bacteriostatic, bactericidal and antifungal activities |
Mainly whey is utilized in lactose production; animal feedstock; in production of individual whey proteins and whey powders. Although having wide range of applications; the main concern in whey usage is that it contains less concentration of these significant proteins [4]. Due to its vast application and exceptional properties; there is a high demand of whey fractionation. But the methods employed cause degradation of individual whey components during processing; making it difficult to understand it’s functional; nutritional and therapeutic properties. And that’s why there is a need of employing such protocols which will produce each whey component on pilot scale without harming its functional properties or structure [2].
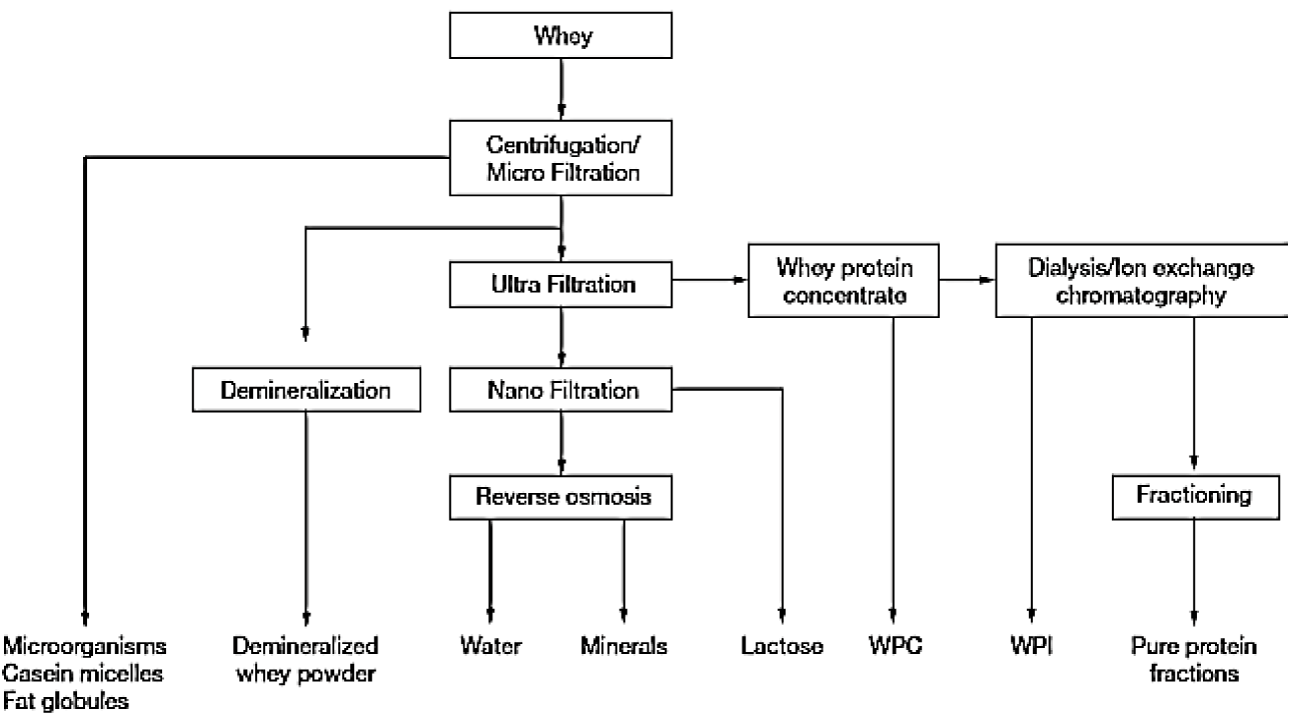
Whey fractionation is performed by various protein separation techniques such as; fractional precipitation; membrane separation; spray drying and chromatography (ion exchange chromatography; affinity chromatography) and combined methods; which result in a number of whey formulations such as shown in figure 1 [5].
Whey protein separation and valorization had always been an area of interest and for that purpose such methods should be employed which are cost effective but usually the protocols that are followed are >80% of the product cost. At industrial level; chromatography is one of the most commonly used methods but again faces the problem of being expensive [6]. So; an alternative; membrane filtration is a promising way of getting individual proteins and has got the attention for being energy efficient; easy to scale up and easy to be operated. Further; membrane chromatography techniques (affinity and ion exchange chromatography) are also being employed. Pressure driven membrane process; for example ultrafiltration and diafiltration are frequently used in food industry but face the drawback of fouling [7].
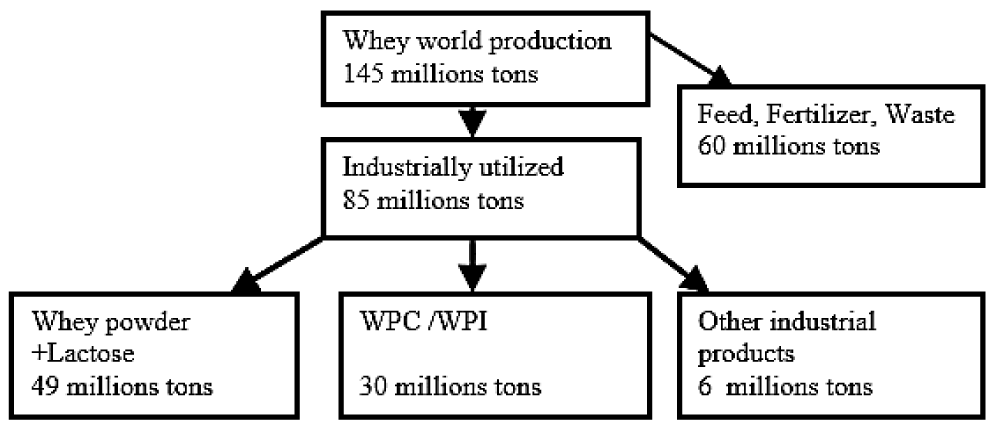
It is surprizing to know that for years; whey was considered as waste and was either dumped; used as fertilizer or dried as whey powder and used in animal feeds [1]. Whey proteins are frequently employed in pharmaceuticals and food industry as their constituent proteins have high nutritional value and versatile properties. Concentrated whey protein is widely used in growth medium of yeast; bacteria and algae and in animal feed. WPC can also be used as a human food source but is not appreciated due to its high lactose and salt concentration and low protein content (about 10%) [8]. WPI along with WPC can be used in food industry as they govern characteristics such as low sodium; calories and fat; high amino acid and protein content; no toxicity; biocompatibility and readily available and inexpensive products. Global utilization of whey is given in figure 2 [9].
Objectives
The main objective of this article was to review different purification techniques for whey valorization with main focus on widely employed techniques named membrane separation and chromatography. Article also reviews the vast applications of individual whey components.
Acid and sweet whey and way of their seperation
Composition of whey varies with the source of milk (bovine; sheep or cow); time and stage of lactation; type of whey (acidic or sweet); processing protocol and animal feed; but among all whey obtained from cow is of great significance due to being inexpensive and its production volume. Whey can be classified in two main categories; “acid whey obtained from acid coagulated cheese with pH of 3.57 - 4.341 and sweet whey obtained from rennet coagulated cheese with pH of 6.02 - 6.58” [8]. Another type is casein whey which is obtained during casein production when mineral acid is used for the purpose of precipitation [10]. Acid whey is a product (either supplemented or not) obtained by lowering the pH and coagulation of milk by lactic acid fermentation with help of different microbial cultures such as thermophiles; Lactobacillus acidophilus; Streptococcus salivarius subsp. Lactobacillus casei and Bifidobacterium sp. among other Lactic Acid Bacteria” [11].
Acid and sweet whey have their own unique way of separation. In the 1st method; rennet like enzyme (optimal pH 5.6) is used to improve the coagulation so that sweet whey is obtained [12]. In 2nd approach; Lactobacillus culture at pH 5.1 is used for acidification of milk to produce acid whey [13]. The specific percentage composition of each constituent of different types of whey is shown in table 2.
| Table 2: Composition of different types of whey [1]. | |||
| Composition | Sweet Whey | Acid Whey | Casein Whey |
| Total solids (%) | 94-98 | 93-97 | 93-98 |
| Lactose (%) | 69-76 | 65-69 | 64-68 |
| Proteins (%) | 11-14 | 7-9 | 9-11 |
| Fat (%) | 0.5-2 | - | - |
| Ash (mineral) (%) | 6-9 | 9-12 | 10-13 |
| pH | 6-6.8 | 4.2-5 | 4.0-4.8 |
Purification methods
Getting a purified product had always been a concern and same goes for whey. A number of methods were developed in past and are still being developed for this purpose. In past; most common approaches were coagulation of milk by heat or acid; precipitation and salting out but these were practical on laboratory scale only [2]. With the understanding of vast whey applications in food and pharmaceutical industry; there was a need of employing such methods which could be utilized on pilot scale; are rapid; efficient and simple and of high yield. With time different techniques were developed for getting highly purified whey protein sub products as shown in Figure 1 and are divided in 4 categories such as membrane filtration; chromatography; combined methods and other methods [4].
Since 1920s; efforts are being made to concentrate whey by heat drying methods but are disadvantageous due to being expensive; thus limiting their use on commercial scale. Later on a process named spray drying was employed which caused thermal degradation of the whey components and caught the attention due to being economical but being disadvantageous due to the fact that it denatures the protein [8]. In 1970s; for efficient concentration of whey protein fractions without harming the protein structure; a new method named membrane filtration rose to fame which took advantage of semipermeable membranes of well-defined pore sizes. To get different compositions and purities of whey protein fractions; combined filtration steps were used such as; filtration followed by spray drying gives product named WPC (35-80% protein) and ion exchange chromatography which gives product WPI (90% protein) [14].
For commercial scale up of whey protein fractions; several methods have been developed which fall mainly in two categories: membrane filtration and chromatography [15]. Conventional chromatography such as affinity; hydrophobic interaction and ion exchange chromatography; remain one of the most efficient protein separation techniques but present the disadvantage of complex process control systems; long cycle time and fouling [16]. But problem can be overcome by use of “macroporous membrane monolith columns” which not only reduce backpressure but also increase separation rate [17]. While membrane separation requires no phase change (evaporation); is quite an attractive approach for concentration of whey proteins [7]. Each process is discussed in detail below.
Membrane processes for whey protein separation
Membranes are defined as barriers which selectively restrict the transport of some compounds while letting others to pass through. Transport is mediated either by diffusion or convection or induced by pressure; electric field; temperature and concentration gradient. Semipermeable membranes divide the influent is permeate (filtrate) and retentate (concentrate) [18]. It readily became logical approach for whey purification as based on size; many whey components can be separated. It doesn’t allow phase transition thus making it widely employed [2]. Efforts are being made to improve membrane separation systems so that these methods can be employed in food and pharmaceutical industry.
Membrane separation is widely used in agro-food and bulk biotech markets. Ultrafiltration (UF); Microfiltration (MF); Reverse Osmosis (RO) and Nanofiltration (NF) are widely employed. While other technologies such as Electrodialysis (ED); Vapour Permeation (VP); Membrane Contactors (MCs) and Pervaporation (PV) are not used frequently on industrial scale [19]. The success of these membrane process can be linked to a number of advantage such as: are easy to operate; cost effectiveness; environmental friendly; production of high quality products; replacement of conventional distillation or filtration methods; flexibility in process development and efficiency [8].
The main reason of using membrane separation in whey purification is that; it maintains the nutritional; biological and functional properties of the whey as no high temperature is used leading to preservation of heat labile components of whey [20].
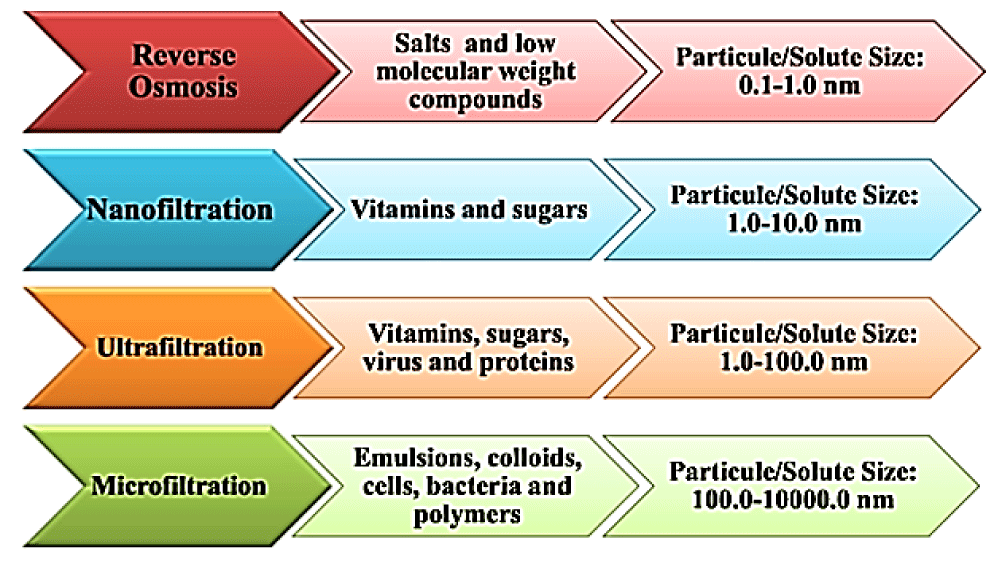
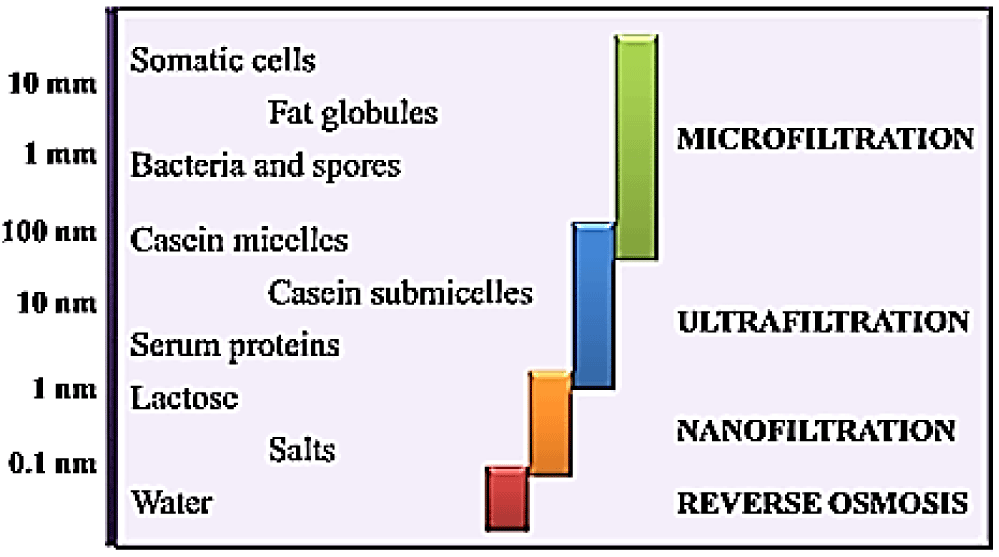
Pressure-gradient membrane separation: As the name shows; this kind of membrane separation takes advantage of pressure difference between outlet and inlet streams and includes NF; RO; MF and UF (Figure 3). All these processes differ on the basis of membrane pore size and are used for a specific purpose [21]. Figure 4 depicts the applications of such processes in dairy industry [8].
These processes are of great interest on industrial level for removal of mineral salts from the whey. NF being permeable to organic compounds and monovalent salts; is widely used for demineralization of whey and has the advantage of being cost effective in terms of production and waste disposal. MF is used to remove residual lipids and microorganisms in order to reduce membrane fouling phenomenon and RO is used for pre-concentration of whey [22].
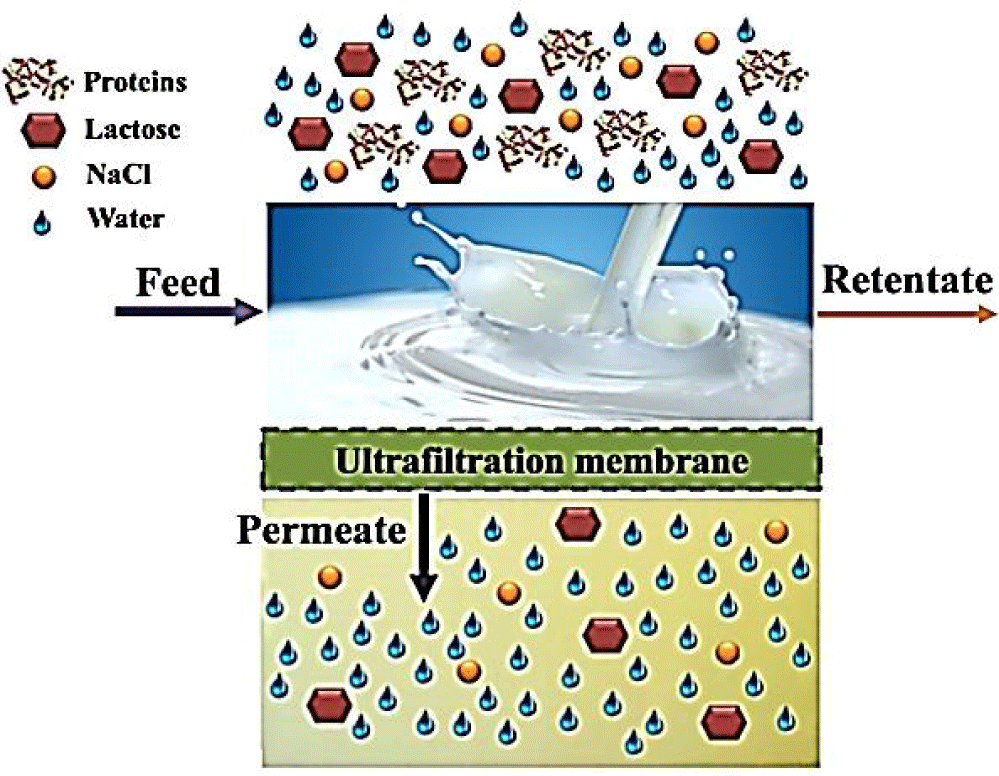
Ultrafiltration (UF): A Membrane Separation Process (MSP) for particles of 0.01-1.0μm and pressure applied is of 100-500kPa. UF is widely used in dairy industry due to no requirement of heat induced phase change; its ability to retain macromolecules and for being cost effective [3]. UF is used for separation; concentration and fractionation of whey components through selective permeation of lower molecular weight minerals; waters and lactose and by retaining proteins. A general depiction of this process is given in figure 5.
Commercially; WPI; WPC and Whey Protein Hydrolysate (WPH) are available. UF is mainly used for separation of WPC using membranes made of polymeric polysulfonate. The reason of using these membranes is that; it doesn’t alter the proteins’ native structure and has good thermal and mechanical stability [10]. Performance is dependent on membrane’s ability to retain proteins and let the permeate flow and the effect of temperature; pressure and recycle flow on the overall yield. But if process is run for longer duration of time; it becomes less efficient in delivering the purified product due to fouling [23].
Fouling always affects the UF efficiency but this problem can be solved by usage of enzyme catalysts as described by [24]. The enzyme Transglutaminase (TG) is used to enhance functional properties and texture of foods such as noodles; seafood and dairy products. TG was used prior to UF and its effect on Relative Permeation Flux (RPF); whey protein rejection rate; Whey Protein Recovery Rate (WPRR) and Lactose Rejection Rate (LRR) was observed. It was noted that at optimal process conditions there was an increase of 30-40% in RPF; 15-20% increase in WPRR and a decrease of 10% in LRR along with a significant decrease in membrane resistance due to GF catalysis [24]. Due to limitations of UF processes; there is a need of monitoring the overall process especially total protein and solid concentration in the product stream. If deviation from the desired requirement is observed; proper adjustments are made [10].
There are several limitations of UF for whey purification such as concentration polarization; inability to separate proteins of same size and low selectivity. Concentration polarization reduces the membrane flux rate by reducing the efficiency of membranes leading to increased cost of overall purification process [8]. Furthermore UF is operated along with DF in order to get whey fractions of greater purity by removing maximum ash from the whey [3].
Diafiltration: Ultrafiltration technique is used to enhance the yield and purity of the protein. It is run in the mode of diafiltration in which deionized water or buffer is constantly used to wash the retentate. Energy consumption can be reduced by using this technique [19]. In 1999; a study was conducted by Muller in which they used three altered approaches of UF (continuous; discontinuous concentration and diafiltration) to segment the Alpha-lactalbumin (ALA) from acid casein whey [25]. By combining these different approaches of ultrafiltration; the yield and purity of ALA was enhanced up to 90%. Almecija [26] used a tubular ceramic membrane of 300kDa in continuous diafiltration technique for the fractionalization of whey. The pH effects were also studied by observing the flux time features and determining the yield of ALA and BLG (Beta-lactoglobulin) in the retentate and permeate. By increasing the pH; the yield of BLG was increased (67% at the pH of 3 to 100% at the pH of 4). But in contrast to BLG; the yield of ALA in the retentate was increased by decreasing the pH (43% at the pH of 9 and 100% at the pH of 4). Baldasso [10] utilized the ultrafiltration technique combined with discontinuous diafiltration to purify the whey protein. In the course of their study; the effects of VCF (volumetric concentration factor); number of diafiltration steps; and the water volume of diafiltration were observed. It was concluded that the ultrafiltration technique is appropriate for the concentration of whey protein. About more than 70% of the yield of protein by weight was achieved.
In 2016; Slukova [27] examined the production of barley sourdough from cheese whey. They exploited the diafiltration technique to reduce the amount of lactose in whey. Holzmuller and Kulozik [28] exploited repetitive diafiltration operations to isolate the milk fat globule membrane from the butter-milk whey. Diafiltration technique has an advantage that pH does not change significantly throughout the whole process so the sample protein is not degraded during the time of the experiment. But there are some limitations in diafiltration i.e. the issue of concentration polymerization and difficulty in separating the same sized proteins. Due to these limitations; the life span of the diafiltration membrane was reduced which automatically increased the cost of the experiment.
High-performance tangential flow filtration (HPTFF): HPTFF is a newly developed technology derived from the conventional cross-flow filtration and used to expand the purification process by enhancing the yield and purity of the product. It also reduced the problem of membrane fouling and also improved the life span of the membrane. It works on the principle of change in size and charge between different protein molecules. Those protein components which differ from each other in the size of even less than three-fold can be separated by using this technique [29]. By incorporating the charges on the ultrafiltration membranes; allow the usage of a membrane with larger pore size. Ultrafiltration membrane with larger pore size offers greater fluxes and no obstruction in protein recapturing.
In 2014; Arunkumar and Etzel [30] used tangential flow ultrafiltration technology having positively charged 300kDa cellulose membrane to purify the ALA and BLG proteins from bovine milk. In their research; they utilized membrane at different staged configurations (i.e. one; two; and three staged) to enhance the separation of the desired product (ALA and BLG). It was concluded that the three-staged membrane system gave a higher yield of ALA and BLG (87% and 83%; respectively). Cheang and Zydney [31] also used this tangential flow filtration technique with a charged membrane. They reported high retention of BLG and a large permeation of ALA by using a double staged tangential flow filtration system. The membrane series of 30 and 100kDa were used for the purification purpose. A yield of 90% of ALA protein was obtained with a 10 fold output but the yield of BLG was low.
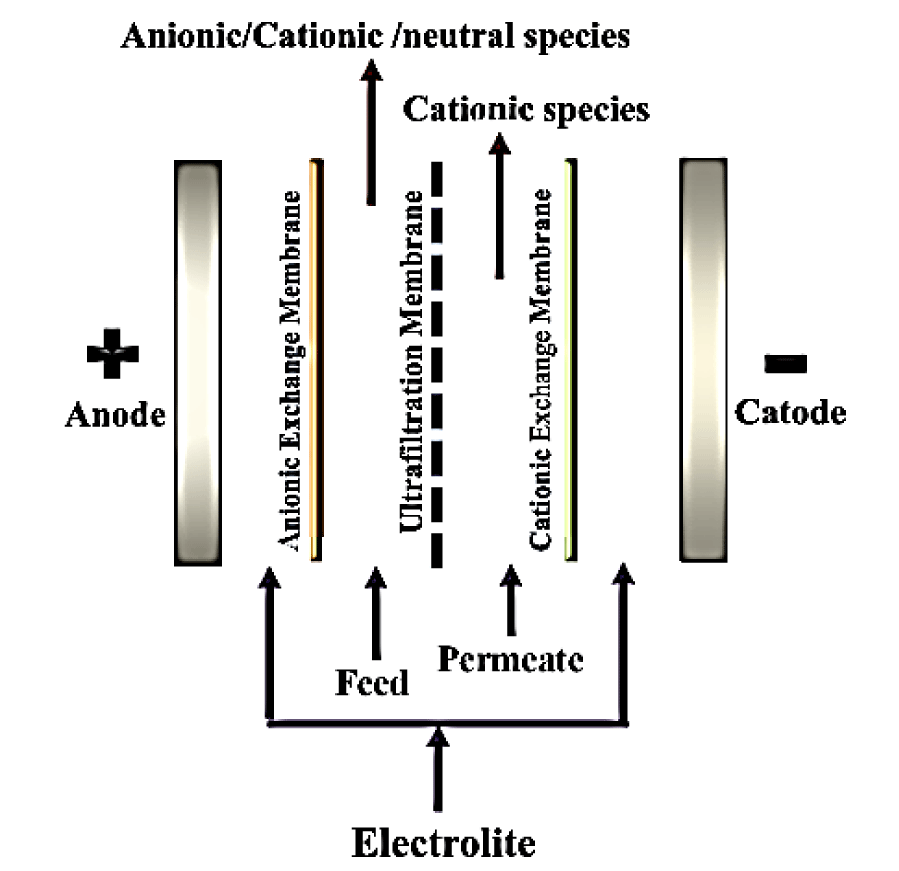
The electrically modified membrane system: The electrically modified membrane system has a synergistic effect of the electric field and conventional filtration system. This technique has a pressure-driven procedure connected with the constant source of electric current which automatically improves the purity and yield of proteins. There are two common electrically modified membrane systems that are commonly used for protein fractionation i.e. Electro-Ultrafiltration (EUF) and electro-dialysis combined with UF [7]. Figure 6 is showing EDUF technology. The EUF membrane system is used to concentrate protein solutions under the effect of the electric field. A membrane of a higher Nominal Molecular Weight Cut Off (NMWCO) as compared to the protein of interest is utilized in this technique. EUF has an advantage that it prevents fouling because of the constant supply of electric current; charged developed on the membrane which repelled the foulants. Due to electrophoresis; a polarity of an electric field generated by which charged particles move away from the membrane. For the high yield of protein; the isoelectric point of protein should be different [32]. EUF is an efficient method to filter highly concentrated samples at low crossflow [33].
A separation process was designed in which they utilized charged membranes for the purification of minor proteins (bovine serum albumin and lactoferrin) present in milk whey. Bu using a positively charged membrane at a pH of 5.0; they obtained a quite high separation flux of BSA (30.31g/m2h1). Galier and Balmanna [34,35] obtained fractions of whey protein (BLG and ALA) by using Electrophoretic Membrane Contactor (EMC) with a100kDa cellulose acetate membrane and a pH of about 4.8. Ndiaye [36] used electrodeposition ultrafiltration membranes to purify LF from whey. In the course of their study; it was concluded that at pH 3 maximum migration yield (15%) of LF was attained.
Chromatography
Chromatography is a biophysical technique which is designed for the isolation; identification; and purification of a combination of components by distributing them between the stationary and mobile phase. We can separate proteins based on their size; charge; shape; hydrophobicity; and binding capacities with the stationary phase [37]. The principle of chromatography is based on different interactions of a mixture of compounds within the two phases; stationary phase and mobile phase. The combination of molecules is applied either onto the surface or into the solid and fluid stationary phases distancing from each other while moving with the assistance of a mobile phase. The factors affecting the purification process include molecular characteristics associated with adsorption; partition; affinity; or difference between the molecular weight of molecules. For the purification of whey protein; affinity chromatography and ion exchange techniques are commonly applied.
Affinity chromatography: It is a selective form of chromatography in which specific interactions developed between the analyte and immobilized affinity ligand attach to the stationary phase. Affinity chromatography has been utilized for the separation of antibodies; hormones; nucleic acid; enzymes; and other specific proteins. For the purification of proteins; the ligand binds with the matrix of the column to which the specific protein binds [38]. The specific protein makes a strong interaction with ligand and attaches to the matrix while other free proteins are vacant in the column. Then by changing the ionic strength of specific proteins or by the addition of any salt solution; we can elute the specific protein [39]. Affinity chromatography has some disadvantages i.e. it gives relatively low productivity; the volume of the sample is limited; and nonspecific adsorption can occur. Table 3 is showing affinity chromatography techniques for whey protein fractionation.
| Table 3: Affinity chromatography techniques for fractioning of whey protein. | ||
| Adsorbent | Protein | Reference |
| Polyclonal immunochromatographic | BLG | Puerta, et al. [42] |
| D-Sepharose | LF | Wolman, et al. [43] |
| Heparin | Minor proteins | Ounis, et al. [44] |
| Yellow HE-4R attached to chitosan mini-sphere | LF | Baieli, et al. [45] |
| NHS sephrose | BSA | Besselink, et al. [41] |
| Chitosan beads | Glycomacropeptide | Baieli, et al. [83] |
| Reactive Red 4-Sepharose matrix | Bovine lactoperoxidase | Urtasun, et al. [40] |
Urtasun [40] applied the dye-based affinity chromatography for the isolation of bovine lactoperoxidase from whey. In the course of their study; they compared the Red 4-sepharose matrix and packed bed column for their purification yield; purification factor; and if they need any pre-treatment. It was concluded that a larger purification factor and yield (i.e. 46.1 ± 1.1 and 86.5 ± 3.8%; respectively) were obtained without any pretreatment; by using the Red 4-sepharose matrix. Besselink [41] isolated BSA (bovine serum albumin) from whey by using immunoaffinity chromatography. They used antibodies (llama antibody fragment) as affinity ligands for the adsorption of BSA. In the course of their study; it was concluded that with the use of immunoaffinity chromatography; a high yield of BSA was obtained. The same technique was used in in 2002 by Puerta [42]; to adsorb β-lactoglobulin on polyclonal immune-chromatographic support.
Wolman [43] separated LF (lactoferrin) from bovine milk serum by using affinity membrane chromatography. In this technique different membranes i.e. Diethylamino Ethanol-Seraphose (D-Sepharose) and Diethylamino Ethanol-Hollow Filter (D-HF) 1 or II were used to create hollow fibers having large adsorption capacity. It was concluded that a high adsorption and elution percentage of LF (i.e. 91 and 99%; respectively) were obtained by using a combination of Red HE3B dye and D-HF-II membranes. In 2008 a study was conducted by Ounis [44]; in which they used heparin affinity chromatography to purify the minor cationic proteins and growth factors from whey protein isolates. Baieli [45] isolated Lactoferrin (LF) from whey by dye-affinity chromatography with yellow HE-4R attached to chitosan mini-spheres. They used low cost chromatographic procedure for the purification of LF from sweet whey. The yellow dye (HE-4R) was immobilized as a ligand and chitosan was utilized as mini spheres. About 95% of LF was adsorbed by chitosan mini spheres and more than 80% of absorbed LF was eluted. Baieli [40] purified casein glyco-macropeptide; a peptide chain with an amino acid length of 46; from bovine whey by using affinity chromatography.
Ion exchange chromatography: Ion exchanged chromatography is used for the separation of proteins and ionic compounds. This analytical technique can separate large protein molecules; amino acid; and also small nucleotide molecules [46]. This technique is based on the ionic interactions between the ionic and polar analytes. It separates ions and polar molecules on the bases of their affinity to the ion exchanger. Ion exchanger can have a negative charge or a positive charge on it. The ionized charged molecules are present on the immobilized matrix of the stationary phase [47]. These inert charged molecules interact with the exchangeable analyte ions of oppositely charged molecules in the solution. The ionized protein molecules which are to be separate compete with these exchangeable counter ions to bind with the immobilized inert charges present in the stationary phase. Those molecules which do not bind or weakly bind with immobilized charged ions on the stationary phase are washed away earlier [48]. Ion exchange chromatography can be done in the form of anion exchange chromatography; cation exchange chromatography; or a combination of these methods.
Anion exchange chromatography (AEC): In AEC; a positively charged ion exchange resin is utilized which has an affinity to bind with the negatively charged molecules. Table 4 is showing anion exchange chromatography for the fractioning of whey protein. Nakano Separated bovine κ- casein Glycomacropeptide (GMP) from sweet whey protein by using anion exchange chromatography [49]. In the course of their study; thermal treatment and acidification processes were utilized to precipitate the protein followed by Diethyl Aminoethyl (DEAE)-Sephacel AEC of the dissolved fraction obtained after the removal of precipitated proteins. A significant amount of peptide (7.5% of the overall dry weight of the sweet whey) was achieved.
| Table 4: Anion exchange chromatography for the fractioning of whey protein. | ||
| Adsorbent | Protein | References |
| Diethyl aminoethyl (DEAE)-Sephacel | bovine κ- casein glycomacropeptide (GMP) | Nakano, et al. [49] |
| pHEMA-DMAEMA cryogel | IgG | Dong, et al. [52] |
| Mono Q 5/50 GL column | BLG | Santos, et al. [51] |
| Quaternary ammonium diethyl-amine | BSA, ALA, and BLG | Goodall, et al. [50] |
Goodall [50] selectively separated major whey proteins by utilizing AEC. In the course of their study; they used certain parameters (the composition of protein in the eluted portion; measurement of binding capacity; and breakthrough curves) to determine the selectivity of membranes i.e. a strong quaternary ammonium membrane and a weak diethylamine membrane. The attained results showed that both membranes were selectively separate BLG with the elution containing less than 1% of ALA and BSA. A study was directed in 2012; in which 80% concentrated whey protein was utilized to fractionate the major whey protein; BLG by using AEC [51]. The Mono Q 5/50 GL column was used in anion exchange chromatography. Results obtained showed that about 60.5% BLG was isolated from 80% concentrated whey protein by using the combination of GL column and salt gradient elution technique. Dong [52] isolated IgG (immunoglobulin G) from bovine milk whey by using AEC. In the course of their study; they used pHEMA-DMAEMA cryogel to isolate IgG from bovine milk whey. It was concluded that by using proper chromatographic conditions; the maximum recovery of IgG about 94% can be obtained with high purity of >95%.
Cation Exchange Chromatography (CEC): In CEC; a negatively charged ion exchange resin is utilized which has an affinity to bind with the positive charge molecules. A limited study has been done on the purification of whey protein by using cation exchange chromatography. Table 5 is showing cation exchange chromatography for the fractioning of whey protein. A low-cost method was described for the fractioning of whey protein. Food grade adsorbent and buffers with high flow rates were used for the elution of protein. In the course of their study; they used different purification processes to achieve altered fractions of whey protein. High efficacy in yield and purity of whey protein were obtained by using cation exchange chromatography. El-Sayed and Chase [53] isolated BLG and ALA from WPC by using a cation exchanger (SP Sepharose FF). They used packed bed adsorption at an acidic pH of 3.7. Sodium Dodecyl (SDS) gel and size exclusion electrophoresis were used to analyze the analyte purities of fractioned eluents. Du purified lactoferrin in one step by using cation exchange (Fastline SP) expanded bed adsorption from crude sweet whey [54]. Results found showed that by using Fastline SP; high purity of LF about 88.5% and a high yield of 77.1% were obtained. Pan separated the LPO (lactoperoxidase) enzyme from milk whey by using a cation exchange composite cryogel implanted with macroporous cellulose beads [55]. The results showed that approximately 92% highest recapture of LPO was obtained.
| Table 5: Cation exchange chromatography for the fractioning of whey protein. | ||
| Adsorbent | Protein | References |
| Cryogel embedded with macroporous cellulose beads | Lactoperoxidase | Pan, et al. [55] |
| Fastline SP | Lactoferrin | Du, et al. [54] |
| SP Sepharose FF | BLG and ALA | El-Sayed and Chase [53] |
| SP-sepharose big beads | LF, ALA, and LPO | Doultani, et al. [15] |
Anion cation exchange chromatography: Anion cation exchange chromatography is used for process scale purification; research study; and for protein examination. This analytical system has high space and moderately low cost that’s for it imply mainly for the capturing of whey proteins. Table 6 is showing anion-cation exchange chromatography for the fractioning of whey protein. Voswinkel and Kulozik [56] utilized radical flow membrane adsorption chromatography to isolate all the whey protein from acidic whey. In that study; a 50 fold membrane was used at the pilot scale [57]. In the course of their study; a cation exchanger is used for the fractionation of LPO; LF; and IgG with ALA and an anion exchanger is used for the separation of BLG. It was concluded that about 90% purity of minor proteins and 80 to 97% yield of LF was achieved. The purified BSA (major whey protein) and LF (minor whey protein) from whey by using ion-exchange chromatography [58]. The results showed that 94% recovery of BSA and 80% recovery of LF was obtained.
| Table 6: Anion Cation exchange chromatography for the fractioning of whey protein. | ||
| Adsorbent | Protein | References |
| Sartobind S75 and Q75 MC device | LF, and BSA | Teepakorn, et al. [17] |
| Quaternary ammonium ligands and sulfonic acid | ALA, BLG, and minor proteins | Voswinkel and Kulozik [56] |
Combined Methods
The separation of useful proteins from whey could be made efficiently by coupling different usual techniques e.g. any chromatography combined with membrane filtration; or affinity adsorption combined with magnetic fishing. The combined methods were first implied for separation of GMP and IgG [57]. A polystyrene anion exchanger was used for adsorption of GMP and after that IgG was selectively concentrated by using ion exchange chromatography followed by membrane filtration. BLG was also purified by using two-stage membrane ultrafiltration followed by ion exchange chromatography [58]. The sweet whey was passed from UF membrane; on its permeate tryptic treatment was performed and finally diafiltration was performed on hydrolysate for isolation of ALA [59,60].
Magnetic fishing is a recent combination of techniques which utilizes specific immobilized ligand molecules firmly attached with magnetic particles. Ligand molecules can have affinity; hydrophobic interaction or ion exchange property for separation of desired protein component. The complexes formed in the process are separated by applying suitable magnetic field. After magnetic fishing super magnetic ion exchangers were introduced for separation of specifically whey proteins [61]. After completion of combined separation process; separated proteins are eluted by simple methods (e.g. salt elution). More recently an efficient fractionation method was used successfully which could also be used for scaling up procedures. This was affinity separation technique combined with magnetic field [62].
Another technique is Aqueous Two-Phase Separation (ATPS) for separation of materials in presence of water. It utilizes the differential behavior of two hydrophilic polymers in aqueous solution after a certain concentration point is reached. After ATPS; saline two-phase system containing potassium phosphate and poly-ethylene glycol has also been used for separation of whey proteins [63]. Further development introduced a two-phase system of polysaccharide hydroxypropyl methylcellulose and protein concentrate. After any two-stage purification chromatography strategy; further purification is accomplished by polishing proteins through Size Exclusion Chromatography (SEC) [64]. Table 7 shows usually used combined methods for fractionation of whey proteins.
| Table 7: Fractionation of whey protein by combined methods [26]. | |||
| Methods | Whey Protein | Yield | Purity |
| Electrophoresis and RP-HPLC | ALA, BLG | 23-89% | 83-90% |
| Electrophoretic membrane contactor | BLG, ALA | - | - |
| Electro-acidification | BLG | 44% | 98% |
| Gel-filtration chromatography | ALA, BLG | - | - |
| HMGMF | LF | 50% | - |
| Hydroxyapatite chromatography | BLG | 50-55% | 96% |
| MF, UF, ion exchange, reverse osmosis, and spray drying | Lactose | 74% | 99.8% |
| Mixed matrix membrane chromatography | ALA, BLG, BSA, LF | 69.23% | - |
| Two-stage UF/ion-exchange | BLG | - | 87.6% |
| UF/tryptic treatment | ALA, BLG | Low | 90-95% |
| ALA: Alpha-Lactalbumin; BLG: Beta-Lactoglobulin; LF: Lactoferrin; BSA: Bovine Serum Albumin; LPO: Lacto-Peroxidase. | |||
Other techniques for whey protein fractionation
Ceramic hydroxyapatite chromatography technique combined with a displacer salt like sodium fluoride has shown promising effects for separation of whey proteins [26]. Bipolar membrane electroacidification technique uses bipolar membranes and cation exchange membrane to acidify and demineralize whey proteins [65]. Low molecular weight components of whey obtained from milk could be separated by gel filtration chromatography. Electrophoretic membrane contactor is used to establish link between two separately flowing liquids [8]. The membranes are made up of cellulose acetate for separation of ALA and BLG fractions. Membranes made up of different matrices are used in chromatographic techniques and these versatile materials help in changing conditions of the chromatographic process and protein elution according to requirements [66].
Whey constituents and their applications
The nutritional value of different foods is increased by adding whey ingredients. High cost whey products are used in sea foods; infant’s food; meat; athlete’s food etc. Whey constituents do not provide only nutritional value; but they are also important in providing gelation property; foam production; and thermal stability to different foods. The development of industrial techniques has widened the applications of whey from food industry to pharmaceutical and cosmetic industry.
Low fat food products
Recently; health awareness in people has resulted in increasing demand of low-fat products in market as high fat products cause cardiac diseases [67]. Fat imparts texture and flavor to certain foods; but decreased fat content does not attract many consumers [68]. Whey proteins have a good water binding capacity; and provide viscosity; emulsification and adhesiveness to foods [69]. Low fat containing yogurt was produced by adding whey proteins in microparticulate form. Effects of these small whey proteins developed positive impact on consumer acceptability [70].
Whey protein could also be used as substitute of fat in production of good quality low fat cheese. Low-fat products produced by substitution improvise fewer negative impacts on health. Other low-fat products include ice creams; salad dressings; variety of sauces and soups etc. [71]. The whey protein concentrates which are largely used on food industries include WPC80 and WPC34. The WPCs give best emulsification properties when the emulsion is prepared earlier than starting any industrial process [69]. The combination of carrageenan and WPC34 has helped in production of high-quality low-fat food products. Highest quality of silkiness and sweet aroma is developed in food (e.g. sausages) upon addition of low protein content WPCs [72].
Dairy and confectionary applications
Whey components are used to enhance the quality of dairy products as in a time of increased demand quality matters a lot [73]. The yogurt’s gel strength is increased due to WPCs which also increases viscosity. The gel structure of yogurt is destroyed due to low minerals which is then maintained by using hydrolysates of milk proteins. The creamy smoothness of the yogurt is further increased by adding whey protein. The texture and gel consistency of the yogurt significantly attract consumers by providing highly stable product [74].
The probiotic bacterial growth is stimulated in yogurt as well as in human intestine by the addition of those whey components which are bioactive. In a research it was observed that whey proteins increase the population of probiotic Bifidobacterium bifidum when used in yogurt formation [75]. Some major effects and benefits of whey and its products are presented in table 8. Whey components improve foaming in the ice-creams. The cost reduction of ice-cream production is a major goal of manufacturers. So; egg yolk and powdered milk like expensive components are also replaced by cheaper ingredients while maintaining quality of product [76]. The best quality ice-creams can be obtained by addition of WPC80; as it increases aeration; improves organoleptic conditions and decreases melting behavior.
| Table 8: Functions and benefits of the whey components in the manufacturing of ice-creams. | ||
| Functions | Effects on product | Benefits |
| Emulsification | The production of a stable emulsion | The potential to partially substitute casein |
| Gelation | enhancement of viscosity and formation of steady gel during heat treatment | Provide stable structure and help in resisting high temperatures |
| Taste and texture | Milky sweetness in flavor | Act as substitute of flavoring agents |
| Viscosity | Increased solidness | Reduced bubble formation and provide more creaminess in structure |
Cheese production
Whey components which are successful in cheese production are whey proteins; sweet whey and low lactose whey. Milk whey proteins are used for production of cheese and analogues [77]. The use of whey components in this product is cost effective as these are cheaper than other proteins. The emulsification imparting property of whey proteins is important during cooling and packages. The hardness of the product is increased by substituting casein with whey components [78]. Ricotta; giza; manouri; mysost; mizithra; gjetost and anthotyros are popular cheeses produced by addition of whey. The left-out whey of cheddar and mozzarella are used in the production of ricotta [79]. Table 9 shows some important industrial beneficial effects of whey components.
| Table 9: Industrial uses and benefits of whey components [58]. | ||
| Industrial uses | Whey components used | Beneficial effect |
| Ice cream | Phospholipid enriched WPCs | Cheap ingredient in confectionery |
| Cake | Whey proteins | Increased cake height and amino acid content |
| Infant growth formula | Demineralized whey, lactose and LF | Enhanced gut health and peristaltic movements |
| Supplements of protein | ALA | Improve muscle mass and strength |
| Confectionery and bakery | WPCs | Provide texture, aroma and improved whipping |
| Therapeutic\ pharmaceutical | WPCs | Improve growth, medications of cancer and diabetes, and provide essential amino acids |
As therapeutic food and pharmaceutical ingredient
Food products which are altered according to the need of its consumer are included in therapeutic foods. Recently soft drinks were prepared from whey and fruit concentrates. In these soft drinks different flavors (e.g. honey; vanilla; cocoa etc.) and sweeteners can be added. Many powdered formulations of whey drinks contain essential vitamins which are nutritionally beneficial [1]. Lactose is the most utilized whey component in pharmaceutical industry. Pure form of whey lactose has high demand due to its stability [80-85]. The chemically modified (e.g. succinylated) whey components are important drug carriers. Many aspects like bioavailability; drug release and pharmacokinetics are improved by whey component. Many commercially prepared inhalers contain their biologically active component attached with lactose particles. Whey preparations are also used for the improvement of solubility of many readily crystallizable substances in drug industry [86-90].
Concluding Remarks
Whey being enriched in functional and nutritional properties; has gained attention for the use of its individual constituents in purified form in pharmaceutical and food industry. For that aim; more efficient and advanced separation techniques have been developed. But still; membrane separation techniques and chromatography remains the methods of choice for whey fractionation. Novel improvements in fractionation techniques for whey valorization has gained popularity in dairy; food and pharmaceutical industry. But; usage of a single technique always leads to a compromise between either purity or overall yield. Therefore; combined methods by integration of two or more purification techniques has led to a product of greater yield and enhanced purity.
References
- Smithers GW. Whey and whey proteins - From “gutter-to-gold.” International Dairy Journal. 2008;18(7):695–704. https://tinyurl.com/xuxbk5dz
- El-Sayed MM, Chase HA. Trends in whey protein fractionation. Biotechnol Lett. 2011 Aug;33(8):1501-11. doi: 10.1007/s10529-011-0594-8. Epub 2011 Mar 19. PMID: 21424166.
- Argenta AB, Scheer. Membrane Separation Processes Applied to Whey: A Review. Food Reviews International. 2019. https://tinyurl.com/juh7kpuj
- Kaur N, Sharma S, Jaimni Kehinde Kaur. Recent developments in purification techniques and industrial applications for whey valorization: A review. Chemical Engineering Communications. 2019. https://tinyurl.com/xjbmzuww
- Chavan RS, Shraddha, Kumar, Nalawade. Whey based beverage: Its functionality, formulations, health benefits and applications. Journal of Food Processing and Technology. 2015;6:1-2. https://tinyurl.com/62m6b4cj
- Valino V, Roman R, Ibanez, Ortiz. Improved separation of bovine serum albumin and lactoferrin mixtures using charged ultrafiltration membranes. Separation and Purification Technology. 2014;25:163–169. https://tinyurl.com/3bccupcv
- Aguero RE, Bringas MF, San Roman, I Ortiz, R Ibanez. Membrane processes for whey proteins separation and purification. A Review. Current Organic Chemistry. 2017;21:1740-1752. https://tinyurl.com/trpvphh7
- Ramos OL, Pereira, Rodrigues, Teixeira, Vicente, Malcata. Whey and Whey Powders: Production and Uses. Encyclopedia of Food and Health. 2016;498-505. https://tinyurl.com/2yts32su
- Tsakali E, Petrotos, Allessandro, Goulas. A review on whey composition and the methods used for its utilization for food and pharmaceutical products. 6th International conference on simulation and modelling in the food and bio-industry. 2010. https://tinyurl.com/9ypnnwce
- Baldasso C, Lazzari, Scopel, Marczak and Tessaro. Concentration and purification of whey proteins by ultrafiltration. Desalination. 2011;278:381-386. https://tinyurl.com/4nfamuww
- Lievore P, Simoes KM, Silva NL, Drunkler AC. Chemical characterisation and application of acid whey in fermented milk. Journal of Food Science and Technology. 2013. doi: 10.1007/s13197-013-1244-z.
- Liu Y, Zhao X, Liu M, Zhao J. Cheese Manufacturing and Bioactive Substance Separation: Separation and Preliminary Purification of cAMP from Whey. Korean J Food Sci Anim Resour. 2018 Feb;38(1):52-63. doi: 10.5851/kosfa.2018.38.1.052. Epub 2018 Feb 28. PMID: 29725224; PMCID: PMC5932959.
- Pate SA, Parikh. Production of lactic acid from whey by Lactobacillus sp. isolated from local dairy products. International Journal of Current Microbiology and Applied Sciences. 2016;5:734-741. https://tinyurl.com/5pe5xhcs
- Schuck P. Spray drying of dairy products: state of the art. 2002:375-382. doi: 10.1051/lait:2002017. https://tinyurl.com/36h3zheh
- Doultani S, Turhan, Etzel. Fractionation of proteins from whey using cation exchange chromatography. Process Biochemistry. 2004;39:1737-1743. https://tinyurl.com/m4mxtrvj
- Plate K, Beutel S, Buchholz H, Demmer W, Fischer-Frühholz S, Reif O, Ulber R, Scheper T. Isolation of bovine lactoferrin, lactoperoxidase and enzymatically prepared lactoferricin from proteolytic digestion of bovine lactoferrin using adsorptive membrane chromatography. J Chromatogr A. 2006 Jun 2;1117(1):81-6. doi: 10.1016/j.chroma.2006.03.090. Epub 2006 Apr 17. PMID: 16616760.
- Teepakorn C. Optimization of lactoferrin and bovine serum albumin separation using ion-exchange membrane chromatography. Separation and Purification Technology. 2015;151:292-302. https://tinyurl.com/b4a8t6bd
- Qiong W, Chao, Fenga, Xia, Lin. Whey protein membrane processing methods and membrane fouling mechanism analysis. Food Chemistry. 2019;289:468-481. https://tinyurl.com/6zp9tsfy
- Saxena A, Tripathi BP, Kumar M, Shahi VK. Membrane-based techniques for the separation and purification of proteins: an overview. Adv Colloid Interface Sci. 2009 Jan 30;145(1-2):1-22. doi: 10.1016/j.cis.2008.07.004. Epub 2008 Aug 5. PMID: 18774120.
- Baldasso C, Lazzari, Scopel, Marczak and Tessaro. Whey fractionation through membranes separation process. Separation Science and Technology. 2016. https://tinyurl.com/2ckvzres
- Riera F, González P, Muro C. Whey cheese: membrane technology to increase yields. J Dairy Res. 2016 Feb;83(1):96-103. doi: 10.1017/S0022029915000679. PMID: 26869115
- Barukcic I, Bozanic, Kulozik. Influence of process temperature and microfiltration pre-treatment on flux and fouling intensity during cross-flow ultrafiltration of sweet whey using ceramic membranes. International Dairy Journal. 2016;51:1-7. https://tinyurl.com/444ytpb5
- Sprinchan EG. Optimization of technological regimes for obtaining protein-mineral concentrated products from secondary milk raw materials. Surface Engineering and Applied Electrochemistry. 2009;45:63-70. https://tinyurl.com/tdmucrde
- Qiong, Wei, Xue, Yi. Cheese whey protein recovery by ultrafiltration through Transglutaminase (TG) catalysis whey protein cross-linking, Food Chemistry. 2017;215:31-40. https://tinyurl.com/4bszj9tc
- Muller AG, Daufin, Chaufer. Ultrafiltration modes of operation for separation of a-lactalbumin from acid casein whey. Journal of Membrane Science. 1999;153:9-21. https://tinyurl.com/28y8y9ay
- Almecija MCR, Ibanez A, Guadix EM, Guadix. Effect of pH on the fractionation of whey proteins with a ceramic ultrafiltration membrane. Journal of Membrane Science. 2007;288:28-35. https://tinyurl.com/szpvh5dh
- Slukova M, Hinkova, Henke, Smrz, Lukacikova, Pour, Bubnık Z. Cheese whey treated by membrane separation as a valuable ingredient for barley sourdough preparation. Journal of Food Engineering. 2016;172:38–47. https://tinyurl.com/42pcmukb
- Holzmuller, Kulozik. Isolation of Milk Fat Globule Membrane (MFGM) material by coagulation and diafiltration of buttermilk. International Dairy Journal. 2016;63:88-91. https://tinyurl.com/ft2tvsrp
- Datta D, Bhattacharjee, Datta S. Whey protein fractionation using membrane filtration: A review. Journal of the Taiwan Institute of Chemical Engineers. 2008;89:45-50. https://tinyurl.com/22zvrv29
- Arunkumar A, Etzel. Fractionation of a-lactalbumin and b-lactoglobulin from bovine milk serum using staged, positively charged, tangential flow ultrafiltration membranes. Journal of Membrane Science. 2014;454:488-495. https://tinyurl.com/4hjcrvv3
- Cheang B, Zydney. A two-stage ultrafiltration process for fractionation of whey protein isolate. Journal of Membrane Science. 2004;231:159-167. https://tinyurl.com/5r242ha8
- Chen G, Song B, Qi J, Ghosh, Wan. Separation of protein mixtures by an integrated electro-ultrafiltration-electrodialysis process. Separation and Purification Technology. 2015;147:32-43. https://tinyurl.com/awwye9fv
- Enevoldsen AD, Hansen, Jonsson. Electro-ultrafiltration of industrial enzyme solution. Journal of Membrane Science. 2007;299(1-2):28-37. https://tinyurl.com/ysu5j6ft
- Valino V, Roman R, Ibanez, Benito, Escudero. Accurate determination of key surface properties that determine the efficient separation of bovine milk BSA and LF proteins. Separation and Purification Technology. 2014;135:145-157. https://tinyurl.com/3rdxy6k5
- Galier S, Balmann. The electrophoretic membrane contactor: A mass-transfer-based methodology applied to the separation of whey proteins. Separation and Purification Technology. 2011;77:237-244. https://tinyurl.com/5b4fr37h
- Ndiaye N, Pouliot L, Saucier L, Beaulieu, Bazinet. Electroseparation of bovine lactoferrin from model and whey solutions. Separation and Purification Technology. 2010;74:93-99. https://tinyurl.com/y9km7wk8
- Priyadarshini R, Gerard, Deepak. Chromatography - the essence of bioanalysis, European Journal of Biomedical and Pharmaceutical Sciences. 2016;3:366-377. https://tinyurl.com/n7j595x3
- Wilchek M, Chaiken. An overview of affinity chromatography in affinity chromatography–Methods and protocols. Humana Press. 2000;1-6.
- Firer MA. Efficient elution of functional proteins in affinity chromatography. J Biochem Biophys Methods. 2001 Oct 30;49(1-3):433-42. doi: 10.1016/s0165-022x(01)00211-1. PMID: 11694292.
- Urtasun N, Baieli, Hirsch, Martinez, Cascone, Wolman. Lactoperoxidase purification from whey by using dye affinity chromatography. Food and Bioproducts Processing. 2017;103:58–65. https://tinyurl.com/mayrvd7r
- Besselink T, Janssen, Boom. Isolation of bovine serum albumin from whey using affinity chromatography. International Dairy Journal. 2015;41:32-37. https://tinyurl.com/28dxmhx2
- Puerta A, Jaulmes A, De Frutos M, Diez-Masa JC, Vidal-Madjar C. Adsorption kinetics of beta-lactoglobulin on a polyclonal immunochromatographic support. J Chromatogr A. 2002 Apr 12;953(1-2):17-30. doi: 10.1016/s0021-9673(02)00124-3. PMID: 12058931.
- Wolman FJDG, Maglio, Grasselli Cascone. One-step lactoferrin purification from bovine whey and colostrum by affinity membrane chromatography. Journal of Membrane Science. 2007;288:132–138. https://tinyurl.com/kvf4e4a8
- Ounis WB, Gauthierm, Turgeon, Roufik, Pouliot Y. Separation of minor protein components from whey protein isolates by heparin affinity chromatography. International Dairy Journal. 2008;18:1043-1050. https://tinyurl.com/h2t8d66b
- Baieli MFN, Urtasun MV, Miranda O, Cascone, Wolman. Isolation of lactoferrin from whey by dye-affinity chromatography with yellow HE-4R attached to chitosan mini-spheres. International Dairy Journal. 2014;39:53-59. https://tinyurl.com/43asa483
- Fekete S, Beck A, Veuthey JL, Guillarme D. Ion-exchange chromatography for the characterization of biopharmaceuticals. J Pharm Biomed Anal. 2015 Sep 10;113:43-55. doi: 10.1016/j.jpba.2015.02.037. Epub 2015 Feb 26. PMID: 25800161.
- Jungbauer A, Hahn. Ion-exchange chromatography. Methods Enzymology. 2009;463:349-371.
- Lenhoff AM. Ion-exchange chromatography of proteins: the inside story, Mater. Today: Proceedings. 2016;3(10):3559-3567. https://tinyurl.com/rb6u7r3e
- Nakano T, Ozimek L, Betti M. Separation of bovine κ-casein glycomacropeptide from sweet whey protein products with undetectable level of phenylalanine by protein precipitation followed by anion exchange chromatography. J Dairy Res. 2018 Feb;85(1):110-113. doi: 10.1017/S0022029917000838. PMID: 29468990.
- Goodall S, Grandison AS, Jauregi PJ, Price J. Selective separation of the major whey proteins using ion exchange membranes. J Dairy Sci. 2008 Jan;91(1):1-10. doi: 10.3168/jds.2007-0539. PMID: 18096919.
- Santos MJ, Teixeira, Rodrigues. Fractionation of the major whey proteins and isolation of b-Lactoglobulin variants by anion exchange chromatography. Separation and Purification Technology. 2012;90:133-139. https://tinyurl.com/e7fznpaw
- Dong S, Chen L, Dai B, Johnson W, Ye J, Shen S, Yun J, Yao K, Lin DQ, Yao SJ. Isolation of immunoglobulin G from bovine milk whey by poly (hydroxyethyl methacrylate)-based anion-exchange cryogel. J Sep Sci. 2013 Aug;36(15):2387-93. doi: 10.1002/jssc.201300306. Epub 2013 Jul 8. PMID: 23720373.
- El-Sayed MM, Chase HA. Simulation of the breakthrough curves for the adsorption of a-lactalbumin and b-lactoglobulin to SP sepharose FF cation-exchanger. Biochemical Engineering Journal. 2010;49:221–228. https://tinyurl.com/aymcd2xy
- Du QY, Lin, Xiong Yao SJ. One-step purification of lactoferrin from crude sweet whey using cation-exchange expanded bed adsorption. Industrial & Engineering Chemistry Research. 2013;52:2693-2699. https://tinyurl.com/y587awa3
- Pan M, Shen, Chen, Dai, Xu, Yun, Yao, Lin, Yao. Separation of lactoperoxidase from bovine whey milk by cation exchange composite cryogel embedded macroporous cellulose beads. Separation and Purification Technology. 2015;147:132-138. https://tinyurl.com/2f77tpzw
- Voswinkel L, Kulozik. Fractionation of all major and minor whey proteins with radial flow membrane adsorption chromatography at lab and pilot scale. International Dairy Journal. 2014;39:209-214. https://tinyurl.com/3bc3cu63
- Xu Y, R Sleigh, J Hourigan, R Johnson. Separation of bovine immunoglobulin G and glycomacropeptide from dairy whey. Process Biochemestry. 2000;36(5):393-399. https://tinyurl.com/99y6n6rr
- Bhattacharjee S, Bhattacharjee, Datta. Studies on the fractionation of beta-lactoglobulin from casein whey using ultrafiltration and ion exchange membrane chromatography. Journal of Membrane Science. 2015;275:141-150. https://tinyurl.com/79yumsuw
- Konrad G, Kleinschmidt. A new method for isolation of native alpha-lactalbumin from sweet whey. International Dairy Journal. 2008;18:47-54. https://tinyurl.com/5ykackjx
- Konrad G, Lieske, Faber. A large-scale isolation of native beta-lactoglobulin: characterization of physicochemical properties and comparison with other methods. International Dairy Journal. 2000;10:713-721. https://tinyurl.com/52kzfsf6
- Heebøll-Nielsen A, Justesen SF, Thomas OR. Fractionation of whey proteins with high-capacity superparamagnetic ion-exchangers. J Biotechnol. 2004 Sep 30;113(1-3):247-62. doi: 10.1016/j.jbiotec.2004.06.008. PMID: 15380659.
- Chen L, Guo, Guan, Liu. Isolation of lactoferrin from acid whey by magnetic affinity separation. Separation and Purification Technology. 2007;56:168–174. https://tinyurl.com/ajk9es8w
- Coimbra JD, Thömmes, Kula. Continuous separation of whey proteins with aqueous two-phase systems in a Graesser contactor. Journal of Chromatography A. 1994;668(1):85–94. https://tinyurl.com/54564cmt
- El-Sayed MM, Chase HA. Purification of the two major proteins from whey concentrate using a cation-exchange selective adsorption process. Biotechnol Prog. 2010 Jan-Feb;26(1):192-9. doi: 10.1002/btpr.316. PMID: 19927316.
- Bazinet L, Ippersiel D, Mahdavi B. Effect of conductivity control on the separation of whey proteins by bipolar membrane electroacidification. J Agric Food Chem. 2004 Apr 7;52(7):1980-4. doi: 10.1021/jf0348469. PMID: 15053539.
- Ganju S, Gogate. A review on approaches for efficient recovery of whey proteins from dairy industry effluents. Journal of Food Engineering. 2017;215:84-96. https://tinyurl.com/4hd99fu9
- Sandrou DK, Arvanitoyannis IS. Low-fat/calorie foods: current state and perspectives. Crit Rev Food Sci Nutr. 2000 Sep;40(5):427-47. doi: 10.1080/10408690091189211. PMID: 11029012.
- Sołowiej B. Textural, rheological and melting properties of acid casein reduced-fat processed cheese analogues. Milchwissenschaft Journal. 2012;67:9–13. https://tinyurl.com/ebzckfky
- Johnson BR. Whey protein concentrates in low-fat applications. U.S. Dairy export council, applications monograph. Low-fat applications. 2000;1-8. https://tinyurl.com/8dwescrn
- Madureira AR, Pereira AMP, Gomes ME. Pintado, Malcata FX. Bovine whey proteins - Overview on their main biological properties. International Food Research Journal. 2007;40(10):1197-1211. https://tinyurl.com/52z5peww
- Zhang T, McCarthy J, Wang G, Liu Y, Guo M. Physiochemical properties, microstructure, and probiotic survivability of nonfat goats’ milk yogurt using heat-treated whey protein concentrate as fat replacer. J Food Sci. 2015 Apr;80(4):M788-94. doi: 10.1111/1750-3841.12834. Epub 2015 Mar 21. PMID: 25808084.
- Gallardo-Escamilla FJ, Kelly AL, Delahunty CM. Sensory characteristics and related volatile flavor compound profiles of different types of whey. J Dairy Sci. 2005 Aug;88(8):2689-99. doi: 10.3168/jds.S0022-0302(05)72947-7. PMID: 16027181.
- Liu K, Y Tian, M Stieger, E Van der, Velde. Evidence for ball-bearing mechanism of microparticulated whey protein as fat replacer in liquid and semi-solid multi-component. Food Hydrocolloids. 2016;52:403-414. https://tinyurl.com/upn5vyep
- Sodini I, Montella, Tong. Physical properties of yogurt fortified with various commercial whey protein concentrates. Journal of the Science of Food and Agriculture. 2005;85(5):853-859. https://tinyurl.com/369y4xyy
- Lim SY, Swanson BG, Ross CF, Clark S. High hydrostatic pressure modification of whey protein concentrate for improved body and texture of lowfat ice cream. J Dairy Sci. 2008 Apr;91(4):1308-16. doi: 10.3168/jds.2007-0391. PMID: 18349223.
- Alfaifi MS, Stathopoulos. Effect of egg yolk substitution by sweet Whey Protein Concentrate (WPC), on physical properties of Gelato ice cream. International Food Research Journal. 2009;17:787-793. https://tinyurl.com/653xkp8u
- Philippopoulos CD, Papadakis. Current trends in whey processing and utilization in Greece. International Journal of Dairy Technology. 2001;54:14-19. https://tinyurl.com/y9f5c7nz
- Sołowiej B, Cheung, Chan. Texture, rheology and meltability of processed cheese analogues prepared using rennet or acid casein with or without added whey proteins. International Dairy Journal. 2014;37:87–94. https://tinyurl.com/w7dckkm2
- Sattin E, Andreani NA, Carraro L, Lucchini R, Fasolato L, Telatin A, Balzan S, Novelli E, Simionati B, Cardazzo B. A Multi-Omics Approach to Evaluate the Quality of Milk Whey Used in Ricotta Cheese Production. Front Microbiol. 2016 Aug 17;7:1272. doi: 10.3389/fmicb.2016.01272. PMID: 27582735; PMCID: PMC4987355.
- Durham RJ, Sleigh, Hourigan. Pharmaceutical lactose: a new whey with no waste. Australian Journal of Dairy Technology. 2004;59(2):138-141. https://tinyurl.com/5mvvbc8p
- Gunasekaran S, L Xiao. Use of whey proteins for encapsulation and controlled delivery applications. Journal of Food Engineering. 2007;83(1):31-40. https://tinyurl.com/44a8xbc5
- Bayizit A, T Ozcan, LY Ersan. Membrane processes in production of functional whey components. Mljekarstvo. 2010;4:282-288. https://tinyurl.com/y26r4euy
- Baieli MF, Urtasun N, Martinez MJ, Hirsch DB, Pilosof AM, Miranda MV, Cascone O, Wolman FJ. Affinity chromatography matrices for depletion and purification of casein glycomacropeptide from bovine whey. Biotechnol Prog. 2017 Jan;33(1):171-180. doi: 10.1002/btpr.2404. Epub 2016 Nov 29. PMID: 27897433.
- Aboumahmoud R, Savello P. Crosslinking of whey protein by transglutaminase. J Dairy Sci. 1990 Feb;73(2):256-63. doi: 10.3168/jds.S0022-0302(90)78668-7. PMID: 1970346.
- Jara F, Pilosof. Partitioning of alpha-lactalbumin and beta-lactoglobulin in whey protein concentrate/ hydroxypropyl methyl cellulose aqueous two-phase systems. Food Hydrocolloids. 2011;25(3):374-380. https://tinyurl.com/54u8sf8v
- Królczyk J, Dawidziuk E, Turak, Sołowiej. 2016. Use of whey and whey preparations in the food industry - a review. Polish Journal of Food and Nutrition Sciences. 2016;66(3):157-165. doi:10.1515/pjfns-2015-0052.
- Patel S. Emerging trends in nutraceutical applications of whey protein and its derivatives. J Food Sci Technol. 2015 Nov;52(11):6847-58. doi: 10.1007/s13197-015-1894-0. Epub 2015 Jun 9. PMID: 26884639; PMCID: PMC4744604.
- Salvatore E, Pes M, Falchi G, Pagnozzi D, Furesi S, Fiori M, Roggio T, Addis MF, Pirisi A. Effect of whey concentration on protein recovery in fresh ovine ricotta cheese. J Dairy Sci. 2014;97(8):4686-94. doi: 10.3168/jds.2013-7762. Epub 2014 May 23. PMID: 24856986.
- Sinha R, Radha, Prakash, Kaul. Whey protein hydrolysate: Functional properties, nutritional quality and utilization in beverage formulation. Food Chemistry. 2007;101:1484-1491.
- Steinhauer T, Schwing, Kraub, Kulozik. Enhancement of ultrafiltration-performance and improvement of hygienic quality during the production of whey concentrates. International Dairy Journal. 2015;45:8-14. https://tinyurl.com/ytvfu6j7
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.