> Medicine Group. 2021 September 09;2(9):779-783. doi: 10.37871/jbres1310.
Medicare Coverage Improves Mortality Outcomes in Regions of Poverty in United States
Yana Puckett*
- Poverty
- Medicare coverage
- Mortality
Abstract
Objectives: Access to care and poverty have been associated with a higher risk of breast cancer, but their impact on breast cancer death has not been fully evaluated. We hypothesized that analysis of data from a large database would further elucidate the association between socioeconomic status and breast cancer mortality.
Methods: The Surveillance, Epidemiology, and End Results (SEER) database was used to identify cases of invasive ductal carcinoma diagnosed between 2006-2011, as well as data reflecting the presence or absence of a breast cancer death within five years. Two age groups, 40-64 year old women, and 65+ year old women, were analyzed. From the American Community Survey were acquired annual county level hospital rates, ambulatory care facility rates, nursing/residential care facility rates, rural business rates, population densities, and counts of women in the age groups of interest.
Results: With respect to poverty rates, incidence based mortality rates for 40-64 year old women were 13% (99% CI 3%, 25%) higher for counties in the third quartile and 19% (7%, 35%) higher for counties in the fourth quartile (p < 0.01) than for counties in the first quartile; counties in the second quartile did not show higher incidence mortality rates (p > 0.01). Mortality rates for 65+ year old women did not differ among poverty rate quartiles (p > 0.01 for each assessment). A 50% increase in hospitals per 100,000 persons was associated with 8% (5%, 11%) and 5% (1%, 8%) increases in mortality rates for 40-64 y and 65+ y women, respectively, likely reflecting better ascertainment of causes of death at hospitals. Impacts of differences in other rates and population density were not detected (p > 0.01 for each analysis).
Conclusion: Counties with higher poverty rates have increased breast cancer mortality rates for 40-64 y women, but not for 65+ y women. Universal coverage associated with Medicare is associated with the absence of an apparent effect of poverty upon breast cancer mortality.
Introduction
Breast cancer is still the most common cancer to occur in women in the United States (US) and is the second most-common cause of cancer mortality behind lung cancer for women [1-5]. Multitude of studies show a decline in mortality since 1989, however disparities exist [6,7].
The decline in breast cancer is largely attributed to early screening, utilization of genetic testing, and improved treatment options [8]. We hypothesized that poverty and lack of access to care is associated with higher mortality rate from breast cancer [9].
Poverty is defined as the state or condition in which a person or community lacks the financial resources and essentials for a minimum standard of living [10]. In the United States, poverty compares a person’s or family’s income to a set threshold or minimum amount of income needed to cover basic needs. The US divides the population equally based on these thresholds sets. For example, for a family of four, the poverty threshold reported in 2019 is $25,750 [11] the lower quartile is $6,437.50, the median quartile is $12,875, and the upper quartile is $19,312.50. These figures change by the year, as one might expect.
Poverty, and therefore access to healthcare, contributes greatly to women’s health and mortality. According to the World Health Organization, in many cases where women lack access to care, there is an upward trend of mortality rates, decreasing women’s life expectancy [12]. Lack of access to care is widely contributed by the lack of health insurance, specifically, in middle aged women (age 26-44), leading to unmet need of medical services and prescriptions. Lack of services leads to late diagnosis or no diagnosis at all which in turn leads to in some cases preventable mortality [13].
In 2017 alone, it was estimated that 252,710 new cases of invasive breast cancer were expected to be diagnosed in women in the United States, with 40,610 or approximately 16% of those expected to die. Approximately 12,000 of those cases were expected to be in women under the age of 40 (approximately 4.7%) [2].
The decline of breast cancer, and early diagnosis has improved and is largely attributed to early screening, utilization of genetic testing and improved treatment options [14,15]. Despite this, and the avid attempts to reduce the gaps in new cancer cases and deaths, there still is a high breast cancer mortality rate in certain rural geographic areas, where residents are more likely to live in poverty and forgo cancer screening [16,17].
Previous studies have shown that this high mortality rate can be attributed to county-level SES, racial/ethnic composition, density, availability, access to primary care providers, inadequate or no health insurance, and availability and access to screening [7,18]. We hypothesized that analysis of data from a large database would further elucidate the association between socioeconomic status and breast cancer mortality. Specifically, we aimed to evaluate poverty and breast cancer mortality between women aged 65 and older and those younger than 65.
Methods
The Surveillance, Epidemiology, and End Results (SEER) Program research database was acquired for the years 2006-2011. Women with invasive breast cancer were categorized into two age groups: 40-64 y and 65+ y women. The seventh edition of the AJCC Cancer Staging Manual was used to define Stage 1, Stage 2, Stage 3, and Stage 4 invasive ductal carcinomas of the breast, all with histologic confirmation, none with autopsy only/death certificate only diagnoses, 0 months follow-up, or prior cancer diagnoses.
For each patient, the presence or absence of a breast cancer death within five years was ascertained to permit the calculation of incidence based mortality rates; counties of residence were also obtained. US Census American Community Survey (ACS) was used for the years 2006-2011 based on county-level data to define poverty: poor families, total families, total populations, numbers of 40-64 y and 65+ y women, land masses, hospitals (sector 222), nursing and residential care facilities (sector 223), ambulatory health care facilities (sector 221), and rural businesses (sector 11, agriculture, forestry, fishing and hunting establishments). Counties were included when both SEER and American Community Survey data were available (Table 1).
| Table 1: Summary of patient demographics. | |
| Patient Level Data | N (%) |
| Women 40-64 y | |
| Cancers diagnosed 2006-2011 | 100.677 (100%) |
| Stage I-II tumors | 83.969 (83.4%) |
| Stage III-IV tumors | 16.708 (16.6%) |
| Breast cancer deaths within 5 y | 8.495 (8.4%) |
| Women 65+ y | |
| Cancers diagnosed 2006-2011 | 58.763 (100%) |
| Stage I-II tumors | 50.676 (86.2%) |
| Stage III-IV tumors | 8.087 (13.8%) |
| Breast cancer deaths within 5 y | 6.294 (10.7%) |
| County Level Data (952 county year combinations) | Median (Interquartile Range) |
| Diagnosed with breast cancer deaths within 5 y* | |
| Per 100,000 40-64 y woman y | 11.2 (7.5, 16.2) |
| Per 100,000 65+ y woman y | 20.4 (13.5, 30) |
| Percent of tumors that were stage III-IV tumors | |
| 40-64 y women | 16.1% (12.8%, 20%) |
| 65+ y women | 13.4% (9.1%, 18.2%) |
| Percent of families in poverty | 9.5% (6.6%, 13.2%) |
| Hospitals per 100,000 persons | 1.8 (1.2, 2.7) |
| Ambulatory care facilities per 100,000 persons | 188 (150.0, 219) |
| Nursing/residential care facilities per 100,000 persons | 21.3 (15.5, 29.8) |
| Rural businesses per 100,000 persons | 4.2 (1.7, 10.6) |
| Persons per square kilometer | 126 (52.0, 344) |
| *Incidence based mortality rates | |
Chi-Square tests with Yates’ continuity correction evaluated differences among proportions. Poisson Regression was used to estimate Incidence based Mortality Rate Ratios (IRR), with logarithms of numbers of women 40-64 y and 65+ y taken as offsets. Entered into regression were year of diagnosis, quartiles of poverty rates, and percent of tumors that were stage III or IV, logarithms of hospital rates, ambulatory care rates, nursing/residential care rates, and population densities. Null hypotheses were rejected when p < 0.05; 0.25th and 97.5th percentiles of 15,000 bootstrap replicates were deemed 95% Confidence Interval (CI) limits. Differences between expected and observed values were evaluated by Cg tests. R-Programming Statistical Software Version 3.6.0, with the boot package was used to perform statistical calculations.
Results
From table 1, women 65 years or older were, compared with younger women, more likely to have lower stage tumors (Chi square with Yates’ correction 227, 1 df, p < 0.001) and more likely to have tumors with an associated breast cancer death within five years (Chi square with Yates’ correction 228, 1 df, p < 0.001).
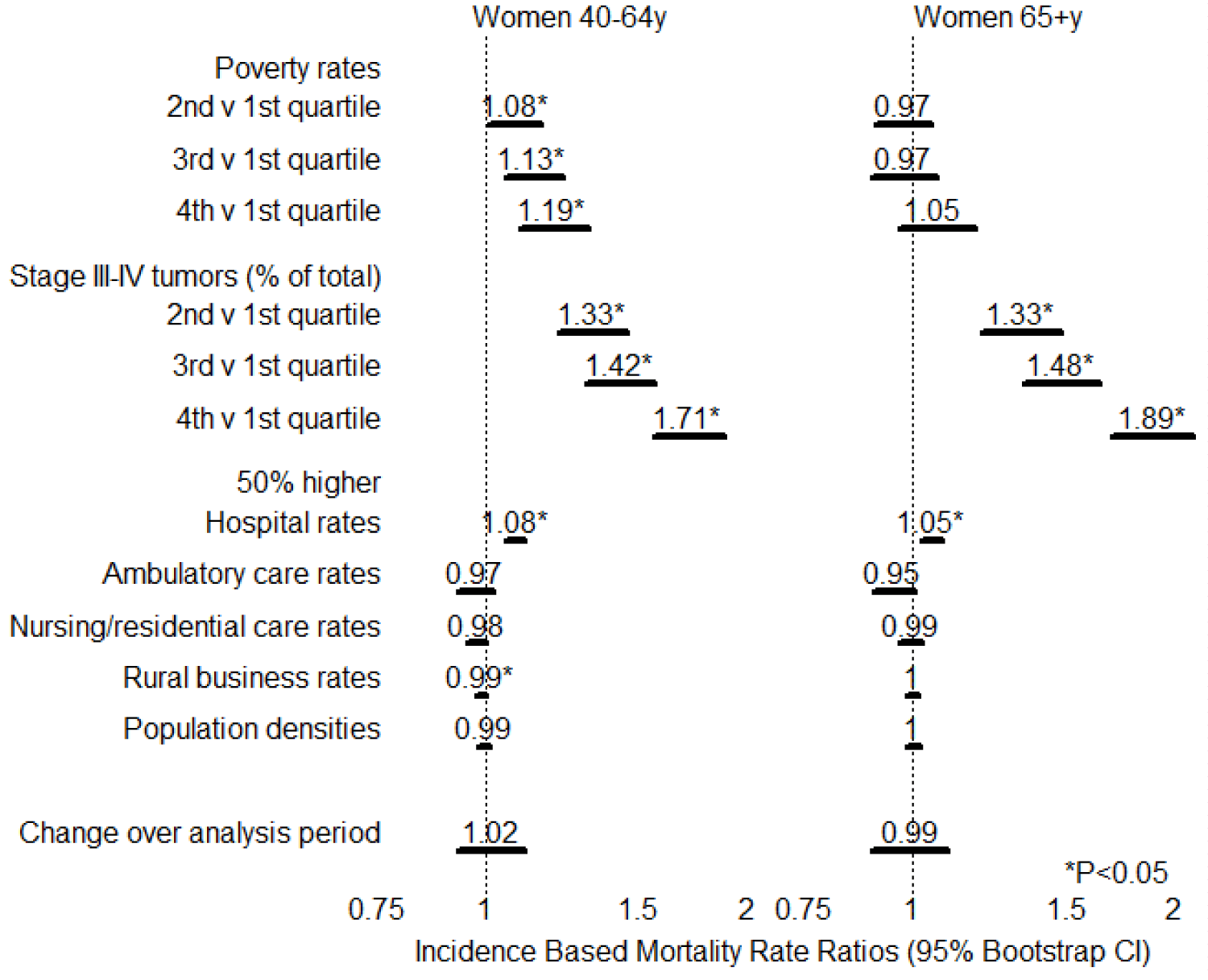
Results of regression are displayed in figure 1. For 40-64 year old women, compared with poverty rates in the first quartile, those in the second, third, and fourth quartiles were associated with 8%, 13% and 19% higher mortality rates (p < 0.05 for each comparison); by contrast differences among quartiles for 65+ year women might have been due to chance (p < 0.05 for each comparison). For 40-64 year old women, compared with Stage III-IV tumor proportions in the first quartile, those in the second, third, and fourth quartiles were associated with 33%, 42% and 71% higher mortality rates (p < 0.05 for each comparison); for 65+ y women, the corresponding figures were 33%, 48%, and 79% (p < 0.05 for each comparison).
A 50% increase in hospital rates was associated with an 8% increase in 40-64+ y mortality rates and a 5% increase in 65+ y mortality rates (p < 0.05 for each comparison; this may simply reflect better ability to discern causes of death at hospitals. A 50% increase in rural business rates was associated with a 1% decrease in mortality rates for 40-64 y women. Effects of differences in rural business rates for 40-64+ y women and of differences in ambulatory care rates, nursing/residential care rates, and population densities for both age groups were small and explicable by change (p > 0.05 for each comparison). No change in Odds Ratio was observed over the study period for either age group (p > 0.05 for each comparison).
Data were parsed into 16 groups by quartiles of poverty rates and quartiles of percent of tumors that were stage III or stage IV; differences between observed and expected counts might have been due to chance for 40-64 year old (Cg = 12.1, 15 df, p > 0.05) and for 65+ year old (Cg = 5.8, 15 df, p > 0.05) women.
Discussion
Our study hypothesized there was an association between socioeconomic status and breast cancer mortality. Specifically, we aimed to evaluate poverty and breast cancer mortality between women aged 65 and older and those younger than 65.
Our study shows that poverty imposed higher breast cancer mortality rates upon 40-64 year old women, but not 65+ year old women. This almost certainly reflects the lack of Medicare coverage for women less than 65 years old.
In a study conducted by C. DeSantis, et al. [2] it was found that from 2003 to 2007 the women in poor areas had a risk of breast cancer related death that was 7% greater than women not in poverty. This is in concordance with our findings. However, when the population is parsed by the age at which Medicare coverage begins, that risk is only observed among persons not covered by Medicare.
The explanation for why individuals in lower socioeconomic standings have higher rates of breast cancer has been speculated. Along with lack of access to treatment and screening methods, environmental factors have been shown to play a possible role in contributing higher mortality rates [9]. Mammography screening has been shown to decrease breast cancer mortality rates by almost 50% [19-21]. Medicare coverage for mammography in the US is preventative and covered 100%. Insurance coverage of preventive services in the US is almost always free [22,23]. As such, it can be speculated that the higher mortality rate observed in our study for women less than 65 years of age is due to lack of insurance coverage.
Less access to fresh fruits and vegetables and less physical activity has been linked to lower socioeconomic status, as well as an increased risk of partaking in cancer risky behaviors, such as smoking, due to marketing strategies that target these populations [24,25]. Obesity is a risk factor for higher breast cancer mortality [25]. Obesity rates are higher in the United States in areas of poverty and rural regions [25,26]. Unlikely, however, is it that women living in poverty have different environmental exposures and differing rates of obesity below and above 65 years of age.
The limitations within this review specifically include access to readily available research, and the interpretations of this research team. The scope of this research is also limiting as it focused on women in a specific area diagnosed with breast cancer from 2006 to 2010 over the age of 40 years old. In addition to this review’s own limitations, there are limitations within the data and state of the science itself. Although living in a rural geographic area has been identified in the relationship to breast cancer and breast cancer mortality, there are numerous other risk factors that may contribute, such as genetics.
Additionally, one must consider lack of care to seek medical attention and how this contributes to the mortality rate. The myriad of emotions and psychological stressors that accompany the diagnosis of breast cancer for any women, regardless of their geographical location cannot be understated. Because poverty rates differ for Black women, Hispanic women, and White women, and because this study, being one addressing the effects of poverty rates in general upon of incidence based mortality, could not disentangle the effects of race and poverty, analyses for Black women [27] and Hispanic women were not performed [28]; the questions of the specific influence of poverty on breast cancer mortality in minority populations require separate analyses which should be performed.
Conclusion
In summary, our study showed the important differences in survival and breast cancer as it relates to poverty in the United States. Further studies to elucidate why younger women living in poverty die at a higher rate than women over the age of 65 are needed. One suspicion for such a discrepancy is lack of healthcare coverage.
References
- Breast Cancer Epidemiology. SpringerReference. doi:10.1007/springerreference_178251
- Smith RA, DeSantis CE. Breast Cancer Epidemiology. Oxford Medicine Online. 2018. doi: 10.1093/med/9780190270261.003.0001.
- Li C. Breast Cancer Epidemiology. Springer Science & Business Media. 2009. https://bit.ly/3yVB69g
- Colditz GA. Epidemiology of breast cancer: Findings from the nurses’ health study. Cancer. 1993;1480-1489. doi: 10.1002/cncr.2820710413
- Zeleniuchjacquotte A. Epidemiology of Breast Cancer. Breast Cancer. 2005;3-14. doi:10.1016/b978-0-443-06634-4.50004-2
- Li CI. Abstract IA03: Breast cancer disparities: Progress, challenges, and opportunities. Cancer Disparities Research: 10 Years of Progress and Promise. 2018. doi: 10.1158/1538-7755.disp17-ia03
- Wallington SF, Brawley OW, Holmes MD. Socioeconomic Status and Breast Cancer Disparities. Toward the Elimination of Cancer Disparities. 2009;137-160. doi: 10.1007/978-0-387-89443-0_6
- Decline in Breast Cancer Incidence--United States. 1999-2003. PsycEXTRA Dataset. 2007. doi: 10.1037/e622402007-001
- Bartle-Haring S. Living in the context of poverty and trajectories of breast cancer worry, knowledge, and perceived risk after a breast cancer risk education session. Womens Health Issues. 2010 Nov-Dec;20(6):406-13. doi: 10.1016/j.whi.2010.06.008. Epub 2010 Aug 5. PMID: 20688528; PMCID: PMC2974806.
- Tomaskovic-Devey D. Poverty and Social Welfare in the United States. Poverty and Social Welfare in the United States. 2019;1-26. doi: 10.4324/9780429302787-1
- Howlader N, Ries LA, Stinchcomb DG, Edwards BK. The Impact of Underreported Veterans Affairs Data on National Cancer Statistics: Analysis Using Population-Based SEER Registries. JNCI Journal of the National Cancer Institute. 2009;533-536. doi: 10.1093/jnci/djn517
- Cock K de, de Cock K. Towards universal access: WHO’s role in HIV prevention, treatment and care. Bulletin of the World Health Organization. 2006;506-506. doi: 10.2471/blt.06.0339104
- Institute of Medicine, Committee on Monitoring Access to Personal Health Care Services. Access to Health Care in America. National Academies Press. 1993.
- Abe H. Current Status of “Dense Breast” Notification in US: Effects of Dense Breast in Breast Cancer Screening. Nihon Nyugan Kenshin Gakkaishi (Journal of Japan Association of Breast Cancer Screening). 2016;199-204. doi: 10.3804/jjabcs.25.199
- Breast, Cervical Cancer Screening for Millions of US Women. JAMA. 2014;1292. doi: 10.1001/jama.2014.11463
- McElfish PA, Su LJ, Lee JY, Runnells G, Henry-Tillman R, Kadlubar SA. Mobile Mammography Screening as an Opportunity to Increase Access of Rural Women to Breast Cancer Research Studies. Breast Cancer. 2019;13.
- Puckett Y, Abedi M, Dunn NA, Hayes A, Garcia B, Arentz C. Does Offering Free Breast Cancer Screenings Make a Difference? A Retrospective 3-Year-Review of a West Texas Free Breast Cancer Screening Program. Journal of Cancer Diagnosis. 2016. doi: 10.4172/2476-2253.1000101
- Kuehn BM. Breast Cancer Disparities. JAMA. 2012;2557. doi: 10.1001/jama.2012.150909
- Bleyer A, Baines C, Miller AB. Impact of screening mammography on breast cancer mortality. Int J Cancer. 2016 Apr 15;138(8):2003-12. doi: 10.1002/ijc.29925. Epub 2015 Dec 15. PMID: 26562826.
- Mayor S. Mammography screening nearly halves breast cancer mortality. BMJ. 2003;949-949. doi: 10.1136/bmj.326.7396.949
- Demb J, Abraham L, Miglioretti DL, Sprague BL, O’Meara ES, Advani S, et al. Screening mammography outcomes: risk of breast cancer and mortality by comorbidity score and age. J Natl Cancer Inst. 2019. doi:10.1093/jnci/djz172
- Insurance coverage does not guarantee access to high-quality health care services. PsycEXTRA Dataset. 2000. doi: 10.1037/e556792006-004
- Oecd, OECD. Health insurance coverage for a core set of services, 2014 (or nearest year). 2016. doi: 10.1787/health_glance_eur-2016-graph144-en
- Kortenkamp A. Breast cancer and environmental risk factors: an appraisal of the scientific evidence. Breast Cancer Research. 2008. doi: 10.1186/bcr1929
- Dal Maso L, Zucchetto A, Talamini R, Serraino D, Stocco CF, Vercelli M, Falcini F, Franceschi S; Prospective Analysis of Case-control studies on Environmental factors and health (PACE) study group. Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int J Cancer. 2008 Nov 1;123(9):2188-94. doi: 10.1002/ijc.23747. PMID: 18711698.
- Drewnowski A. Spatial Analyses of Obesity and Poverty. Insecurity, Inequality, and Obesity in Affluent Societies. 2012. doi: 10.5871/bacad/9780197264980.003.0005
- Yedjou CG, Tchounwou PB, Payton M, Miele L, Fonseca DD, Lowe L, Alo RA. Assessing the Racial and Ethnic Disparities in Breast Cancer Mortality in the United States. Int J Environ Res Public Health. 2017 May 5;14(5):486. doi: 10.3390/ijerph14050486. PMID: 28475137; PMCID: PMC5451937.
- Power EJ, Chin ML, Haq MM. Breast Cancer Incidence and Risk Reduction in the Hispanic Population. Cureus. 2018 Feb 26;10(2):e2235. doi: 10.7759/cureus.2235. PMID: 29713580; PMCID: PMC5919763.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.