> General Science. 2021 June 30;2(6):532-537. doi: 10.37871/jbres1272.
The Effect of Different Light Conditions on Antimicrobial Activity of the Microalgae Chlorella sp. Ethanolic Extract Against Streptococcus mutans
Elmira Ameri1, Farshid Pajoum Shariati1 and Hossein Delavari Amrei2*
2Department of Chemical Engineering, Faculty of Engineering, University of Bojnord, Bojnord, Iran
- Antimicrobial activity
- Extract
- Chlorella sp
- Light conditions
Abstract
Finding new antimicrobial agents from natural compounds with less side effects has been considered by number of researchers in the world. It is important to achieve efficient and up-to-date results in order to identify a substance with antimicrobial properties and achieve operational methods to increase these traits in a society where the prevalence of various diseases has been increased. In fact, the purpose of this study was to achieve compounds from microalgae with antimicrobial properties to be used in food and pharmaceutical industries that can have good consequences for human health. Therefore, in the current study antimicrobial activity of ethanolic extract of microalgae Chlorella sp., that was cultivated under different light conditions, was investigated. For this purpose, microalgae Chlorella sp. was separately cultivated under red, blue, green and white lights with intensity of 109 (μmol-photon m-2 s-1) and antimicrobial potential of the microalgae extracts investigated against the activity of Streptococcus mutans. In addition, Minimal Inhibitory Concentration (MIC) of the extracts determined. Based on the results, the wet extracts indicated more average antimicrobial activity than dried ones. Furthermore, the wet extract of microalgae cultivated under the red light showed a stronger antimicrobial activity compared to extract obtained under the other light spectrum with the minimum inhibitory of that was 10 mg/ml. Also, extract obtained under white light had no significant antibacterial activity against the bacterial strain.
Introduction
Owing to the alarming increase of drug resistance among microbial pathogens, many efforts have been made to find out and characterize new antimicrobial agents [1,2]. The products of marine microorganisms have shown many interesting activities, such as antimicrobial, cytotoxic, anticancer, antidiabetic, antifungal, anticoagulant and other pharmacological activities [3,4]. Algae are a wealthy source for pharmaceuticals on the basis of the presence of biologically active primary and secondary metabolic compounds [5].
Antimicrobial activities of compounds derived from microalgae have also been extensively studied by several other researchers [6-8]. The antimicrobial activity of microalgae has been attributed to compounds belonging to several chemical classes including indoles, terpene, acetogenins, phenols, fatty acids and volatile halogenated hydrocarbons [5,9].
Physical and chemical conditions of culture media affect the biochemical compositions in algal cultures. Manipulation of the culture condition is a possible way to enhance biological activities of microalgae. In recent years, researchers have identified the invaluable increment of microalgae as a possible source of strange antibacterial agents [10,11]. Several studies documented the antibacterial activity of Chlorella species [12-14]. However, few attempts were performed to manipulate the antimicrobial activity of green microalgae based on the culture conditions. Asadi, et al. [15] investigated antimicrobial effects of the Chlorella vulgaris extracts and the supernatant against Gram-negative bacterial foodborne pathogens. Acetone and Ethanol were used as solvent extracts, and antimicrobial activity of that was determined using well plate and disk diffusion methods. Extracts of Chlorella vulgaris and supernatant showed acceptable antibacterial properties against Proteus mirabilis, Salmonella enterica, E. coli, Shigella dusentriae, and Pseudomonas aeruginosa. The maximum inhibition zone diameters in 2 methods were 36.8 and 35.4 mm respectively, which related to the bacterium Proteus mirabilis. Also, using the agar disk diffusion method Ghasemi, et al. [16] observed that the supernatant and methanolic extract of Chlorella vulgar formed inhibition zones equivalent to 14, 15, 15, 14, 13 and 16 mm against the bacteria, Pseudomonas aeruginosa, Staphylococcus epidermidis, Bacillus subtilis, Escherichia coli, Salmonella typhi, and Staphylococcus aureus, respectively. The results indicated that methanolic extract of Chlorella vulgaris had a high antimicrobial activity against gram-positive bacteria.
Dental caries is a biofilm-induced oral disease with S. mutans performing an important function in the development of virulent cariogenic biofilms [17]. Streptococcus mutans (bacterial species) plays an important role in dental caries [18,19]. This bacterium is a gram-positive cocci, facultative anaerobic which is generally found in the human oral cavity. S. mutans is regarded as the main factor for tooth decay due to the production of lactic acid with binding to tooth surfaces in the presence of fermentable sugars, which contributes to corrosion of enamel [18-20]. Therefore, one of the basic biological goals in preventing dental caries is to decrease the bacterial burden of the oral cavity. Due to the increase of antibiotic resistance and side effects of some antimicrobials on one hand and the safety, availability and proportionately low costs of natural products on the other hand, for prevention of caries, various natural products have been assessed as well as incorporated into dental products. The increase of drug-resistant pathogens produces challenges to the successful treatment of microbial diseases, including tooth decay.
To combat disease caused by antimicrobial-resistant microorganisms, there is an immediate need to discover novel products which have antimicrobial properties. Thus, our goal in this study was to investigate the effect of the light quality spectrum (red, blue, green and white) on antimicrobial potential of microalgae Chlorella sp. against Streptococcus mutans, while Minimal Inhibitory Concentration (MIC) of the extracts was obtained.
Materials and Methods
Microalgae and Bacterial strain, culture, photobioreactor and light condition
The microalgae Chlorella sp. (PTCC 6010, Persian Type Culture Collection, Tehran, Iran) was used in this work. This microalgae species was provided by the Iranian Research Organization for Science and Technology (IROST) and pre-cultivated in Rudic medium (pH~8) [21]. This medium contained (mg/l): NaNO3: 300, MgSO4.7H2O: 10, K2PHO4: 80, NaCl: 20, KH2PO4: 20, CaCl2: 47, FeCl3.6H2O:17, Na2.EDTA:7.5, H3BO3: 0.3, MnSO4. H2O: 1.5, ZnSO4.7H2O: 0.1, (NH4)6MO7O24.4H2O: 0.3, CuSO4.5H2O: 0.08, Co (NO3)2. H2O: 0.02. Also, the Streptococcus mutans bacterium was obtained from Pasteur Institute of Iran.
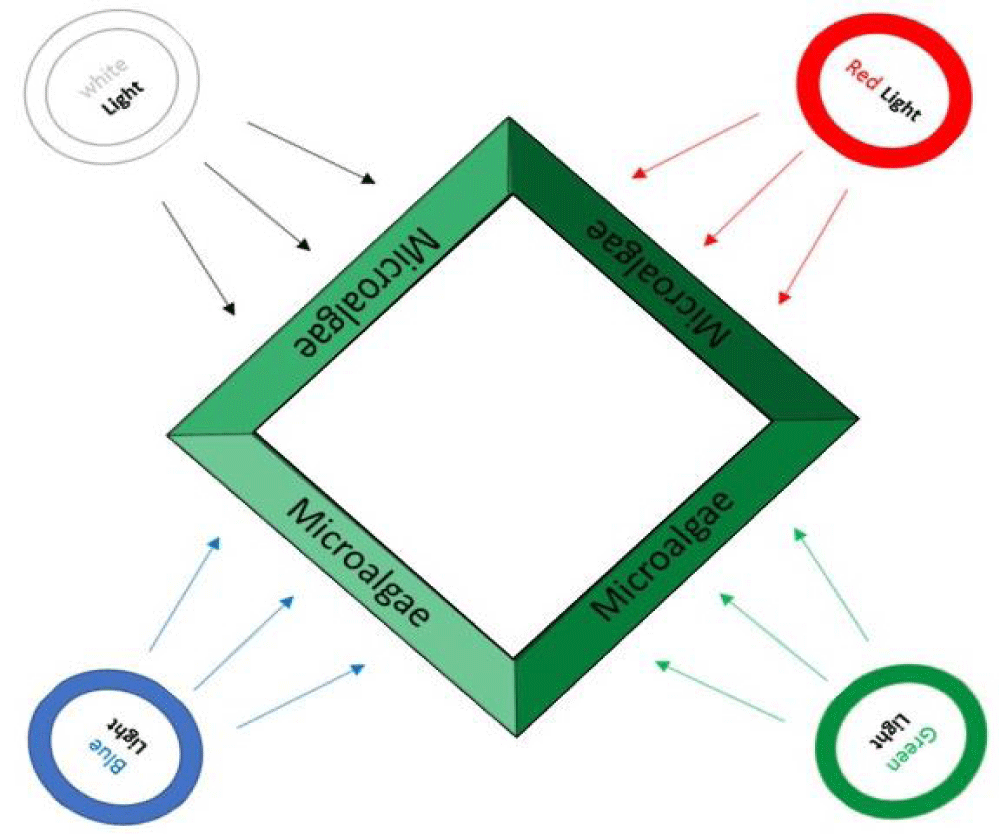
The microalgae was cultivated in pyramid photobioreactor [22]. In this study, a continuous 24 h; red, blue, green and white LEDs with the illumination of 109 µmol-photon m-2 s-1 were used (Table 1). Microalgae were exposed to red (R, 620-720 nm), blue (B, 450-495 nm), green (G, 495-570 nm), and white (W, 400-700 nm) light with extra white light in the first 8 days and wet biomass of these samples was used for extraction (W1, B1, R1, and G1). Then in 14 next day white lights were removed and only the colored lights stayed and both wet (W2, B2, R2, and G2) and dried (W3, B3, R3, and G3) biomass of these samples were used for extraction. The power consumption of all lamps was equal to 18 watts. Also, schematic of different light radiation to the reactor are presented in figure 1.
| Table 1: Description of sample ID. | |
| Sign | Description |
| W | White light |
| B | Blue light |
| G | Green light |
| R | Red light |
| 1 | Colored light + white light, extraction from wet biomass |
| 2 | Only colored light, extraction from wet biomass |
| 3 | Only colored light, extraction from dried biomass |
Extraction method
Extraction process was done in two stages. Once, on the 8th day from the beginning, as though the extra white lights turned off. The second time was at the end of the experiment (the end of the logarithmic phase). Those algal cells from different growth modes were harvested using centrifugation at 2500×g for 10 min.
The Extraction method was performed in two points/times (from dried and wet biomass). For wet biomass- active (W1, B1, G1, and R1, W2, B2, G2, and R2), a solvent technique extraction was performed using 99.8% ethanol (Merck, Germany). After 48 h in dark and room temperature, they were suspended in solvent (10:1 v/w), then sonicated (20 KHz) (Wise clean, China) and centrifuged (SIGMA 3-30 K, Germany) for 5 min at 2500×g, respectively. Supernatants were filtered and concentrated in rotary evaporator at 45ºC (Heidolph4002, Germany). The extracts were refrigerated at 4°C for the next steps of the experiment. In order to produce dried biomass-deactive (W3, G3, B3, and R3), the biomass was harvested and frozen in the freeze dryer (CHRIST, Germany) at -50°C for 72 h. Biomass powders were collected and the algal powders were suspended in the solvent in the ratio of 10:1 (v/w). Other steps were done like the previous extraction method [23].
Preparation of bacterial inoculums
To activate the bacterial strains before inoculation, bacteria inoculums were prepared by transferring a huge number of bacterial strains from fresh culture plates to tubes containing 10 ml of BHI (Brain Heart Infusion) broth (Merck) and incubating for 72 h at 37ºC (with 5% CO2). The tubes were shaken occasionally for growth promotion and better aeration. These cell suspensions were diluted with sterile BHI broth to provide initial cell count of about 108 CFU/ml.
Antimicrobial activity of algal extract
Antimicrobial activity of different algal extracts against microbial pathogen S. mutans was studied using paper disc diffusion assay [24]. Generally, the fresh culture of microbial strain was prepared and a suspension of that microorganism with the approximate cell population of 1.5×108 CFU/ml was prepared in BHI broth. The petri dish plates were prepared with 20 ml of sterile BHI agar (Merck, Germany). The microbial strain was inoculated uniformly onto the surface of BHI agar using sterile cotton swabs. The crude extracts were prepared and led on sterile discs which were placed on the surface of the solidified agar medium. The plates were incubated at 37ºC for 72 h (with CO2). The assay was performed in triplicates and the diameter of the zone of inhibition formed around the disc was determined using Vernier caliper. The extraction solvent was used as negative controls and disc with anti-bacterial agent Gentamycin (10 µg- PadtanTeb) was used as positive controls [25].
Minimal Inhibitory Concentration (MIC)
The Minimal Inhibitory Concentration (MIC) was determined for algal extracts using broth microdilution method to give a concentration between 1000 and 10 mg/ml [26,27]. Briefly, S. mutans from culture in sterile 96-well microtiter plates, 100 µl of algae extract were diluted with broth and placed into the well containing 100 µl of bacterial suspension in broth in order to prepare microbial inoculant, the prepared culture media for 48 h at 37ºC under anaerobic condition were incubated, Then the growth of Streptococcus Mutans was estimated Appropriate controls of medium culture with the microorganism or each extract were included. Triplicate samples were performed for each test concentration.
Results and Discussion
Antimicrobial activity
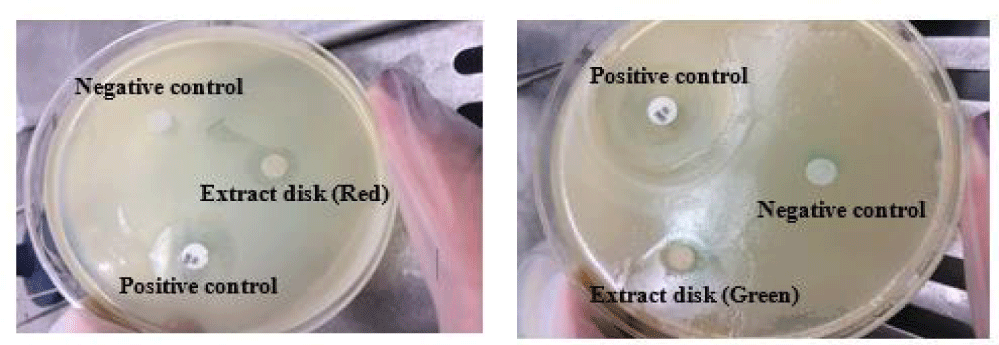
In our study, the antimicrobial activity of ethanolic extract of Chlorella sp. that cultivated in different wavelengths and methods of extraction (active/deactive) were assayed against the human pathogen by evaluating the inhibition zones, zone diameter, and MIC values. Algal extracts were tested for antibacterial activity against S. mutans. An example of results from the paper disc diffusion test can be seen in figure 2.
The sizes of the zone of inhibition in respect to the inhibitory effect of the microalgae extracts are presented in table 2. As presented in this table, the high inhibition zones were seen around the paper disc of the algal extract of sample R2. In fact, the extract obtained from wet biomass of microalgae that had been grown under red light (without extra white light) showed the highest antimicrobial activity against S. mutans. Also, the antimicrobial activity of the algae grown under green light (with extra light, G1) was remarkable. Furthermore, the samples grown under white light radiation had no activity. This phenomenon happened for both dried and wet biomass.
| Table 2: The size of the zone of inhibition (mm) of Chlorella sp. extracts against S. mutans. | ||||
| Ethanolic Extract | Red | Green | Blue | White |
| wet-(in 8th day) | R1 = 12 ± 0.5 | G1 = 14.13 ± 0.5 | B1 = 7.1 ± 0.2 | W1 = 6.3 ± 0.2 |
| wet-(end of log phase) | R2 = 18.64 ± 0.5 | G2 = 11.5 ± 0.5 | B2 = 7 ± 0.2 | W2 = 6.6 ± 0.2 |
| dried-(end of log phase) | R3 = 12.6 ± 0.5 | G3 = 10.5 ± 0.5 | B3 = 7 ± 0.2 | W3 = 6.8 ± 0.2 |
| (ethanol = 7, Gentamycin = 20) | ||||
Generally, in all Chlorella sp. extracts a noticeable effect has been found which is impressive against the bacterial strain. Less inhibitory effects were recorded for all extract of Chlorella that grown under white lights in all modes. It is obvious that the antimicrobial spectra of the active marine bacteria are different [28].
The organic extracts of Chlorella sp. differ in wavelengths under which they are grown. This difference comes from the variation of the ecological conditions of the environment of the microorganism [29]. Based on our results, it can be expressed that the antimicrobial activity of Chlorella sp. extracts was affected by a variety of light wavelengths positively and negatively. However, sometimes no significant effect was observed. Thus, it is clear that red light can lead to the production of antibacterial compounds in Chlorella sp. and the highest amount of these compounds were achieved in wet extraction method. The result gives an indication of the presence of promising antimicrobial compounds in ethanol extract of marine water algal species under our study. Further phytochemical investigations are needed to clarify the efficacious components in antimicrobial activity of these extract against bacteria.
Microalgal species and the solvents used for bioactive compounds extraction are the important factors in antimicrobial activity [30,31]. Antibiotics are natural or synthetic chemical compounds that can suppress the growth and destroy the microorganism [32,33]. The active components of various algae and cell extracts have been demonstrated to have antibacterial activity in vitro versus gram-negative and gram-positive bacteria [34].
The results of this research seem similar to the previous results of evaluating the antimicrobial properties of Chlorella sp. which may increase or decrease in different growth conditions. The present results agreed with the previous results about antimicrobial properties of green microalgae Chlorella sp. against bacterial strains. Several studies documented antibacterial activity of Chlorella species [12-14]. However, few attempts were performed to manipulate the antimicrobial activity based on the culture conditions. As an example of antimicrobial studies, Salem et al. examined the antibacterial activity of microalgae such as Chlorella vulgaris, against four Gram-positive bacteria (S.aureus, Sarcina lutea, B.subtilis, and B.megaterium) and one Gram-negative bacteria (Klebsiella pneumonia) were tested with agar well diffusion method [35]. The antimicrobial activity was tested using methanol and acetone extracts alga spices. The methanol extract was more effective against the studied bacterial strains. Chlorella vulgaris extract showed antimicrobial activity against B.subtilis, S.aureus and K.pneumonia with inhibition zone 17.5, 17 and 14.5 mm, respectively.
Syed, et al. [14] tested green microalgae Chlorella vulgaris against four bacterial strains (Escherichia coli, Klebsiella species, and Bacillus and Pseudomonas species) with acetone, ethanol, and chloroform extracted from microalgae, using the agar disk diffusion method. The observed highest inhibition zone was 13 mm in Chlorella vulgaris extracted with ethanol against Klebsiella sp. Alwathnani, et al. [36] studied the effect of Chlorella vulgaris extracts on morphological changes in human pathogens and antimicrobial activity. They evaluated the antimicrobial potential of Chlorella vulgaris extract against Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, Streptococcus pyogenes, and Bacillus subtilis. Extracts were prepared using methanol, chloroform, diethyl ether solvents. The finding revealed that the extracts could suppress the growth of tested pathogens. The maximum zone inhibition of diethyl ether extract was recorded against E.coli (28.6 mm) and 19.1 mm against Streptococcus pyogenes. Donal Mc Gee, et al. [37], extracted from 80 isolated marine and freshwater microalgae strains were tested for antimicrobial activity against 6 pathogens. These researchers reported, indicating a significantly higher bioactivity under blue light for the chlorophytes and red light for the diatom Stauroneis sp.
The results obtained from the present work concerning the antimicrobial properties of ethanolic extract of green microalgae that grown under different light conditions against human pathogen. It was concluded that the diameter of the inhibition zone depends mainly on the type of extraction modes (active/deactive) and type of wavelength of lights. Also, it was found that the Chlorella sp. grew under the red light and the extract in the active mode performed the highest antimicrobial activity against Streptococcus mutans (18.64 mm inhibition zone). According to the results we could test influence of red light on antimicrobial activity of algae in other different conditions. Till the results indicate an effective and beneficial effect, use it in various parts, including medicine. Based on research the red spectra were good an agent in increment activity of microalgae cell, growth rate, reproduction, and etc. This was what caused it the good antibacterial potential.
Minimum inhibitory concentrations
The Minimum Inhibitory Concentration (MIC) of extracted from Chlorella sp. was grown under various light conditions is presented in table 3. As presented in this table, the ethanolic extract of Chlorella sp. grown under red light had the minimum inhibitory concentration of 10 mg/ml. However, the extract of microalgae grown under green light with helping white light in the first 8 days has 50 mg/ml MIC. In contrast with these results, other findings showed that different wavelength can be modified to show highly potent antimicrobial properties against the bacterial strain. This study confirmed that the green microalgae Chlorella sp. possess biological active substances.
| Table 3: Minimum inhibitory concentration of microalgae Chlorella sp. grown under red light and green light in 2 periods. | ||||
| 10 mg/ml | 50 mg/ml | 100 mg/ml | 1000 mg/ml | Sample |
| + | + | + | + | R2 |
| - | + | + | + | G1 |
| (+ means have antimicrobial properties, - means do not have) | ||||
Conclusion
This study showed the antimicrobial activity of microalgae Chlorella sp. when grown under both white and red lights were more than that grown under red light alone. Also, the average antimicrobial activity of wet extracts was more than dried ones. In fact, the results of our work and mention previously research showed that microalgae Chlorella sp. is an appropriate candidate for health and medical purposes. Furthermore, S. mutans showed more sensitivity to the antibiotic Gentamycin; after that, ethanol extract of microalgae Chlorella grown under red light showed the highest zone of inhibition of the bacteria.
Acknowldgment
This work was supported by the Hourateb Co. (Tehran, Iran) and the authors thank the Dr S.H. Alisobhani and Dr S.K. Alisobhani for their kind assistance and valuable comments. Also the effort of Zahra Khoobkar in conducting experiment is gratefully acknowledged.
References
- Mala R, Sarojini M, Saravanababu S, UMADEVI G. Screening for antimicrobial activity of crude extracts of Spirulina platensis. Journal of Cell and Tissue Research. 2009;9(3):1951. https://bit.ly/3AogpEK
- Perry JJ, Staley JT, Lory S. Microbial life: Sinauer Associates Incorporated. 2002.
- Gul W, Hamann MT. Indole alkaloid marine natural products: an established source of cancer drug leads with considerable promise for the control of parasitic, neurological and other diseases. Life Sci. 2005 Dec 22;78(5):442-53. doi: 10.1016/j.lfs.2005.09.007. Epub 2005 Oct 19. PMID: 16236327; PMCID: PMC4918921.
- Mayer AM, Hamann MT. Marine pharmacology in 2001--2002: marine compounds with anthelmintic, antibacterial, anticoagulant, antidiabetic, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action. Comp Biochem Physiol C Toxicol Pharmacol. 2005 Mar-Apr;140(3-4):265-86. doi: 10.1016/j.cca.2005.04.004. PMID: 15919242; PMCID: PMC4928201.
- Diker K, Akan M, Hascelik G, Yurdakok M. The bactericidal activity of tea against Campylobacter jejuni and Campylobacter coli. Letters in Applied Microbiology. 1991;12(2):34-35. https://bit.ly/3jBnCeH
- Rajasulochana P, Dhamotharan R, Krishnamoorthy P, Murugesan S. Antibacterial activity of the extracts of marine red and brown algae. J. Am Sci. 2009;5(3):20-25. https://bit.ly/3wcu4LN
- Salvador Soler N, Gómez GA, Lavelli L, Ribera MA. Antimicrobial activity of Iberian macroalgae. Scientia Marina. 2007;71:101-113. https://bit.ly/3wedmvC
- Seenivasan R, Indu H, Archana G, Geetha S. The antibacterial activity of some marine algae from south east coast of India. J Pharm Res. 2010;8:1907-1912.
- Cardozo KHM, Guaratini T, Barros MP, Falcão VR, Tonon AP, Lopes NP, Campos S, Torres MA, Souza AO, Colepicolo P, Pinto E. Metabolites from algae with economical impact. Comp Biochem Physiol C Toxicol Pharmacol. 2007 Jul-Aug;146(1-2):60-78. doi: 10.1016/j.cbpc.2006.05.007. Epub 2006 Jun 29. PMID: 16901759.
- Prashantkumar P, Angadi S, Vidyasagar G. Antimicrobial Activity of Blue-Green and Green Algae. Indian journal of pharmaceutical sciences. 2006;68(5).
- Varfolomeev S, Wasserman L. Microalgae as source of biofuel, food, fodder, and medicines. Applied Biochemistry and Microbiology. 2011;47(9):789-807.
- Najdenski HM, Gigova LG, Iliev II, Pilarski PS, Lukavsky J, Tsvetkova IV. Antibacterial and antifungal activities of selected microalgae and cyanobacteria. International Journal of Food Science & Technology. 2013; 48(7):1533-1540. https://bit.ly/3jCxNj8
- Sanmukh S, Bruno B, Ramakrishnan U, Khairnar K, Swaminathan S, Paunikar W. Bioactive compounds derived from microalgae showing antimicrobial activities. J Aquac Res Dev. 2014;5(3). https://bit.ly/3wheRcx
- Syed S, Arasu A, Ponnuswamy I. The uses of Chlorella vulgaris as antimicrobial agent and as a diet: the presence of bio-active compounds which caters the vitamins, minerals in general. Int J Biosci Biotechnol. 2015;7(1):185-190.
- Asadi Y, Doudi M, Darki BZ. An In-Vitro Investigation of the Antibacterial Effects of the Acetone and Ethanol Extracts and the Supernatant of the Algae Chlorella vulgaris CCATM-210-1 on Some of the Gram-Negative Bacterial Foodborne Pathogens. International Journal Of Advanced Biotechnology And Research. 2016;7:278-287. https://bit.ly/3qHhenI
- Ghasemi Y, Moradian A, Mohagheghzadeh A, Shokravi S, Morowvat MH. Antifungal and antibacterial activity of the microalgae collected from paddy fields of Iran: characterization of antimicrobial activity of Chroococcus dispersus. J Biol Sci. 2007;7:904-910. https://bit.ly/3qH8ZYS
- Jeon JG, Rosalen PL, Falsetta ML, Koo H. Natural products in caries research: current (limited) knowledge, challenges and future perspective. Caries Res. 2011;45(3):243-63. doi: 10.1159/000327250. Epub 2011 May 12. PMID: 21576957; PMCID: PMC3104868.
- Bowden GH. Microbiology of root surface caries in humans. J Dent Res. 1990 May;69(5):1205-10. doi: 10.1177/00220345900690051701. PMID: 2186069.
- Marsh PD. Microbiologic aspects of dental plaque and dental caries. Dent Clin North Am. 1999 Oct;43(4):599-614, v-vi. PMID: 10553246.
- Gavanji S, Larki B, Jalali Zand A, Mohammadi E, Mehrasa M, Taraghian AM. Comparative effects of propolis of honey bee on pathogenic bacteria. African Journal of Pharmacy and Pharmacology. 2012;6(32):2408-2412. https://bit.ly/3dAPQSX
- Rudic V, Dudnicenco T. Process for cultivation of green alga Haeamatococcus pluvialis (Flotow). 2000;154:2000.
- Khoobkar Z, Shariati FP, Safekordi AA, Amrei HD. Performance Assessment of a Novel Pyramid Photobioreactor for Cultivation of Microalgae Using External and Internal Light Sources. Food Technol Biotechnol. 2019 Mar;57(1):68-76. doi: 10.17113/ftb.57.01.19.5702. PMID: 31316278; PMCID: PMC6600305.
- Patil K, Patil A, Mahajan SR, Mahajan RT. Bio-activity of algae belonging to Bhusawal region, Maharashtra. Current Botany. 2011.
- Murray P, Baron EJ, Pfaller MA, Tenover FC, Yolke RH. Clostridium, Manual of Clinical Microbiology. ASM Press, Washington. 2003;1.
- Cockerill F. Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing: Twentieth Informational Supplement. 2012.
- Lalitha M. Manual on antimicrobial susceptibility testing. Performance standards for antimicrobial testing: Twelfth Informational Supplement, 2004;56238:454-456.
- Tsai TH, Tsai TH, Chien YC, Lee CW, Tsai PJ. In vitro antimicrobial activities against cariogenic streptococci and their antioxidant capacities: A comparative study of green tea versus different herbs. Food Chem. 2008 Oct 15;110(4):859-64. doi: 10.1016/j.foodchem.2008.02.085. Epub 2008 Mar 4. PMID: 26047271.
- Zheng L, Han X, Chen H, Lin W, Yan X. Marine bacteria associated with marine macroorganisms: the potential antimicrobial resources. Annals of Microbiology. 2005;55(2):119-124.
- El-Wahidi M, El-Amraoui B, Biard JF. Variation saisonnière et géographique de l’activité antifongique des extraits de deux éponges marines récoltées sur le littoral atlantique d’El Jadida, Maroc. Journal de Mycologie Médicale. 2011;21(1):28-32.
- Prakash JW, Marimuthu J, Jeeva S. Antimicrobial activity of certain fresh water microalgae from Thamirabarani River, Tamil Nadu, South India. Asian Pacific Journal of Tropical Biomedicine, 2011;1(2):170-173. https://bit.ly/3dFRAKL
- Radhika D, Veerabahu C, Priya R. Antibacterial activity of some selected seaweeds from the Gulf of Mannar Coast, South India. Asian journal of pharmaceutical and clinical research. 2012;5(4):89-90.
- DeLong EF. Microbial population genomics and ecology. Curr Opin Microbiol. 2002 Oct;5(5):520-4. doi: 10.1016/s1369-5274(02)00353-3. PMID: 12354561.
- Martinez A, Kolvek SJ, Yip CL, Hopke J, Brown KA, MacNeil IA, Osburne MS. Genetically modified bacterial strains and novel bacterial artificial chromosome shuttle vectors for constructing environmental libraries and detecting heterologous natural products in multiple expression hosts. Appl Environ Microbiol. 2004 Apr;70(4):2452-63. doi: 10.1128/AEM.70.4.2452-2463.2004. PMID: 15066844; PMCID: PMC383137.
- Tan LT. Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry. 2007 Apr;68(7):954-79. doi: 10.1016/j.phytochem.2007.01.012. Epub 2007 Mar 2. PMID: 17336349.
- Salem OM, Hoballah EM, Ghazi SM, Hanna SN. Antimicrobial activity of microalgal extracts with special emphasize on Nostoc sp. Life Science Journal. 2014;11(12):752-758.
- Alwathnani H, Perveen K. Antibacterial activity and morphological changes in human pathogenic bacteria caused by Chlorella vulgaris extracts. Biomed Res-India. 2017;28:1610-1614.
- Mc Gee D, Archer L, Smyth TJ, Fleming GTA, Touzet N. Bioprospecting and LED-based spectral enhancement of antimicrobial activity of microalgae isolated from the west of Ireland. Algal Research. January 2020;45:101704.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.