> Medicine Group. 2021 January 28;2(1):024-029. doi: 10.37871/jbres1182.
Measurement of Total Electronic Cross-Section, Total Atomic Cross-Section, Effective Atomic Numbers, Effective Electron Densities and Kerma for Some Br Compounds
Tekerek Saniye1* and Küçükönder Adnan2
2Departman of Physics, Kahramanmaraş Sutçu Imam Üniversity, Turkey
- Mass attenuation coefficient
- Effective atomic number
- Electron densitiy
- Kerma
The aim of this study is to calculate the experimental and theoretical the mass attenuation coefficient some Br compounds by using transmission method. Also using these values were determined the total electronic section, total atomic section, effective atomic number, effective electron density and Kerma. We performed the calculations of these values in attenuation by using direct excitation experimental geometry. The total attenuation cross sections of some halogene Br compounds were measured in a narrow beam good geometry using a high resolution Si(Li) detector in the energy with γ photons at 59.543 keV from Am-241 annular source. Theoretical mass attenuation coefficient values were computed from the XCOM data programme, based on mixture rule method. This study provide new insight into the literature since the values of effective atomic number, electron density and Kerma for some Br compounds have not been determined before. According to the results shown in mass attenuation coefficient, Zeff and Neff of Br compounds are closely associated with chemical structure. This research were undertaken to explore how Bromine compounds is gamma ray shielding material.
Defining the interplay between radiation and matter, mass attenuation coefficient have been widely used in the field of interaction of photon. While penetrating the compounds, the intensity of gamma photon reduces and mass attenuation coefficient measures the energy absorbed by the compounds. The gamma transmission method is used for measurement of photon atomic emission parameters. The mass attenuation coefficient depends on the atomic number of element and the chemical structure [1].
The mass absorption coefficients of materials are important in several applications of medical physics, nuclear physics, radiation shielding, radiotherapy, medical fields. Theoretically mass attenuation coefficient of compounds calculated by Bragg’s mixture rule [2], which represent that the total mass attenuation coefficient of any compound is the sum of fractional weighted of the individual elements. By using Lambert’s law and the rule of mixture, we can obtain to determine theoretically the mass attenuation coefficient. The correct experimental mass attenuation coefficient values are essential to determine exact effective atomic number.
For compounds has been called as effective atomic number Zeff, suggested. Also scientists was created theoretical expressions to evaluate effective atomic numbers for the separate photon interaction processes [3,4]. The experimental Zeff and Neff values are crucial for many fields. The electron density is formulates as the numbers of electrons per unit mass. In addition, effective atomic number and the electron density have a physical meaning that depends on the chemical structure. A large body of literatüre has determined the data of this parameter for different materials [5-8]. To understand the chemical structure, physical and biological properties of a compound, the knowledge of effective atomic number is crucial [9,10]. The tables of mass attenuation coefficient were published for element, mixture and compounds at various energy [11].
Photons interact with matter and lose their energy as a result of the three main processes which are, photoelectric effect, Compton scattering and pair-production. The attenuation coefficient is a measure of the average number of interactions between incident photon and matter that occur in a given mass per unit area thickness of the compounds encountered. The mass attenuation coefficient (μ/ρ) is an event dependent on the possibility of photon interaction with compound samples. The attenuation of gama rays are involved where the intensity is reduced to I after the photon beam of intensity I0 the passes through compound of mass per unit area thickness (t).
Also another important parameter calculated is Kerma. Kerma is kinetic energy released in air. In other words, Kerma is the kinetic energies of charged ionizing particules liberated by radiation. The numerical value of the kerma have defineted that of the absorbed dose [12,13]. The availability of accurate Kerma value is significant in dosimety and medical therapy. This study has been used in Bromine compounds and is important because of its wide use in applied fields such as radiation physics, health physics and medicine [14]. The purpose of this study is to determine molecular, atomic and electronic cross-section, effective atomic numbers, effective electron density for experimental measurement and theoretical calculated of Kerma by using mass attenuation coefficient for some compounds of bromine.
Bromides are used as anticonvulsants in both veterinary and human medicine [14]. There are various kinds of bromides but in this study some particular bromides were taken into consideration. CaBr2 is an component in medication and food preservatives. The Sodium Bromide (NaBr) is used as a microbiocide in water treatment. Also known as sedoneural, sodium bromide has used in pharmaceutical as sedative. Sodium bromide is been used one of as antiepileptic drugs due to properties. Potassium bromide is used to strengthen the dough as a dough puff in bread making [15]. The CuBr compound is used in food packaging [16]. KBrO3 (Potassium Bromate) is used as a chemical in hair products, and it is a food additive used for the brewing of beer. KBrO3 has been used in bread making process [17-20]. C19H10Br4O5S (Bromophenol Blue) is a dye [21]. C6H6BrN (4-Bromoaniline) is employed as the pharmaceutial [22]. C21H16Br2O5S (Bromocresol Purple) is applied in laboratories to measure albuminin the blood [23]. In microbiology laboratory, it is used for staining dead cells and for the assaying of lactic acid bacteria [24].
The effective atomic number is a physical parameter informing us abouth the caracteristic properties of Br compounds. This paper presents the experimental measurement and theoretically calculate of the effective atomic numbers, effective electron density, molecular cross-section, atomic cross-section, electronic cross-section and Kerma for some Br compounds. Our present investigation of mass attenuation coefficients (μ/ρ) of some Br compounds for total photon interaction processes will generate fresh insight in to the field, since the response of medically used bromine compounds to radiation has not been studied before.
Theory
Theoretical procedures were applied to the data obtained as a result of the experiment. The molecular cross section, atomic cross section, effective atomic number and effective electron density can be determined using experimental results of the mass attenuation coefficients. The effective atomic number (Zeff) are calculated [25-27] as the ratio of atomic cross section (σta) to electronic cross section (σte) i.e. The following formulas described were used to calculations of the results.
Values of mass attenuation coefficients are then used to determine the total molecular cross section σtm by the following relation [28].
where is the molecular weight, Ai is the atomic weight of the ith element in a compound, ni is the number of atoms of the element in a compound. NA is Avogadro’s number. Total mass attenuation coefficient is calculated by following equation [29].
where (I) with absorber count incident photon, and (I0) without absorber count transmission photon intensity, ρ is the density of the material, x is the thickness of the sample (cm-1). ρx is the mass per unit area of the sample (g/cm2). Where m is the mass of the sample (g) and r is the radius of the compound powder sample (cm).
The equation below is particularly useful in calculating the total atomic cross section σta [28]:
where σta the total atomic cross section, total molecular cross section σtm, ni, total number of atoms in a compound, the total electronic cross section σte for the individual element is expressed by the following formula [28]:
fi; the fractional abundance of element i with respect to the number of element.
Ai is the atomic weight and Zi is the atomic number of element of the constituent element i, respectively.
The total atomic and electronic cross sections are closely to the effective atomic number (Zeff) through the following relation formula [28]:
The effective electron number or electron density, Neff can be derived from the formula [29] below:
where; is compounds mass attenuation coefficient and σte is the total electronic cross section.
Kerma is the product of the air mass attenuation coefficient and the compounds mass attenuation coefficient. Kerma of an compounds relative to air can be calculated as [30]:
In here; is mass attenuation coefficient of compounds and is air mass attenuation coefficient.
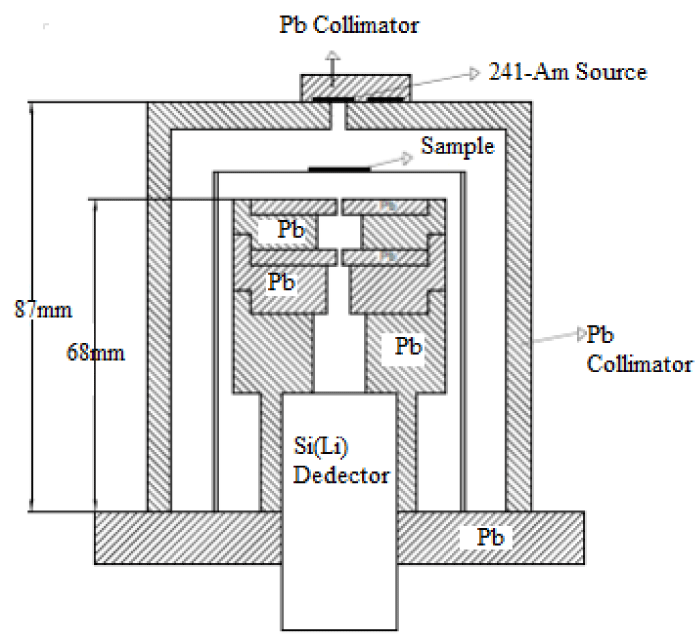
Experimental detail
Total mass attenuation coefficients were measured through transmission with narrow beam geometry method. It is a fact that gamma attenuation information dependent on photon energy is required for material characterization of compounds. In the present experiment, a Si(Li) detector with multi channel analyzer was used for detection of γ-rays. The target samples were been used 59.543 keV photons from a 241Am radiative source and were detected by Si(Li) detector. Detector with a high resolution were used to count the photon intensity transistted from the compounds and the spectra were recorded. The data of this spectrum was analysed.
For each powder compound samples were sieved to 200 mesh. Sample mass thickness affect gama ray emission. Prepared by spreading powder sample compound on a mylar film at 0.0224-0.1975 g/cm2 mass thickness, the experimental setup used in the present study is shown in figure 1.
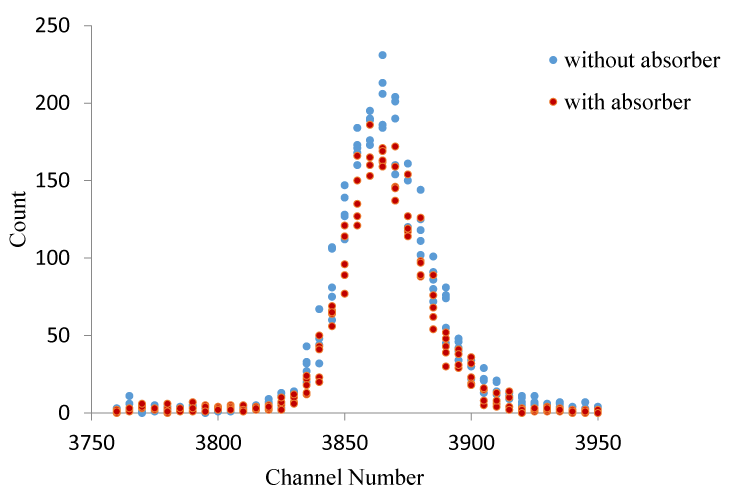
The mass attenuation coefficient for Br compounds were calculated at 59.543 keV. The net record and count with (I) absorber and without (I0) absorber were obtained for the same time duration and under the same experimental conditions. Incident photon unattenuation intensity and transmission photon attenuation intensity for CaBr2 is shown in figure 2 & 3.
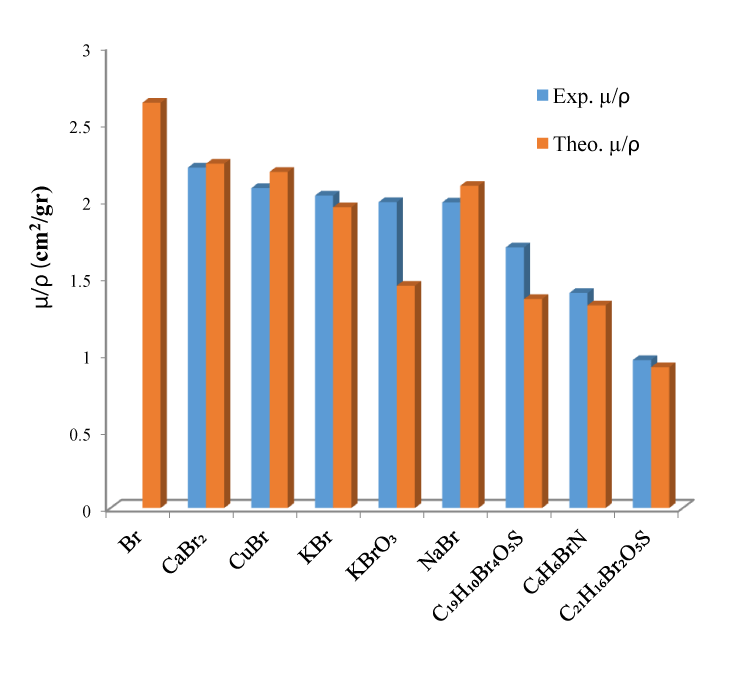
The experimental and theoretical data from this study for bromine compounds have been listed in tables 1-3. The results obtained for Br compounds are pure Br for μ/ρ it ranges from 15.92% to 63.17% has been observed.
Atomic, moleculer and electronic cross sections, effective atomic number, effective electron densities and Kerma values of for some Br compounds were calculated by using mass attenuation coefficients which were obtained from experimentally. These conclusions derived from the experimental results were compared to theorically calculated values. It is concluded that the effective atomic number (Zeff), effective electron density and Kerma depend on the chemical environment of a compound under investigation. When the results for Br compounds were compared with the result for pure Br, it was observed that for Kerma it ranged from 15.93% to 63.15%. The chemical environment of a compound represent a rapport between element and ligand. It is observed that the atomic weight of the element possess compound is bound to the increase of the μ/ρ values on the contrary Neff values decrease. C6H6BrN has owned the high electron density values with 10.665x10+23. Measured values of Zeff for Br (Br = 35) and compounds are given table 1. From the present results, it is found that the compound KBrO3 having maximum Zeff values of 39.928. Besides, it is clear that C21H16Br2O5S maximum is 45.3%. The minimum KBr variation is 3.96 % in the Zeff, which may be explcined by its composition whith potassium element. Depending on the effective atomic number, the values of the electron density also depend on the atomic number of the elements that make up the compound. In general, it has been observed that the effective electron density of compounds consisting of elements with small atomic numbers has lower values. The overall error in the experimental parameters were the sum of the uncertainties in diffrent factors, as the evaluation of peak areas (2%-6%) and target mass thickness (1.45%-3.20%). Although the differences in the experimental and theoretical values observed as a result of the cross section were consistent within the error limits, some deviations were observed in some values. The reason for these deviations is assumed to be that inter molecular interactions are ignored when applying the mixing rule. Hence, these studies may help us to understand the occurrence of the absorption in compounds chemical structure and physical behavior of the compound under investigation. It is clear that all these parameters are affected with the change in chemical nature of the given elements due to its nearby lying ligands.
| Table 1: The mass attenuation coefficient µ/ρ (cm2/g), total moleculer cross section σtm (cm2/molecul), total atomic cross section σta (cm2/atom) and effective atomic number theoretically and experimentally values for Br compounds. | ||||||||
| Compounds | µ/ρ (cm2/gr) | σtm(cm2/molecul) | σta (cm2/atom) | Zeff | ||||
| Experimental | Theorical | Experimental | Theorical | Experimental | Theorical | Experimental | Theorical | |
| Br | 2.637 | 35 | ||||||
| CaBr2 | 2.217 ± 0.006 | 2.242 | 73.609 x 10-23 | 74.440 x 10-23 | 24.536 x 10-23 | 24.813 x 10-23 | 33.115 | 33.489 |
| CuBr | 2.084 ± 0.006 | 2.189 | 49.658 x 10-23 | 52.160 x 10-23 | 24.829 x 10-23 | 26.080 x 10-23 | 31.202 | 32.774 |
| KBr | 2.036 ± 0.005 | 1.960 | 40.246 x 10-23 | 38.744 x 10-23 | 20.123 x 10-23 | 19.372 x 10-23 | 33.612 | 32.357 |
| NaBr | 1.991 ± 0.013 | 2.099 | 34.030 x 10-23 | 35.876 x 10-23 | 17.015 x 10-23 | 17.938 x 10-23 | 31.528 | 33.238 |
| KBrO3 | 1.993 ± 0.005 | 1.452 | 55.287 x 10-23 | 40.297 x 10-23 | 11.057 x 10-23 | 8.055 x 10-23 | 39.928 | 29.089 |
| C19H10Br4O5S | 1.700 ± 0.007 | 1.365 | 189.182 x 10-23 | 151.902 x 10-23 | 4.855 x 10-23 | 3.894 x 10-23 | 30.979 | 24.852 |
| C6H6BrN | 1.405 ± 0.021 | 1.325 | 40.163 x 10-23 | 37.860 x 10-23 | 2.868 x 10-23 | 2.704 x 10-23 | 23.092 | 21.767 |
| C21H16Br2O5S | 0.971 ± 0.036 | 0.9245 | 87.207 x 10-23 | 82.962 x 10-23 | 1.937 x 10-23 | 1.843 x 10-23 | 19.136 | 18.205 |
| Table 2: σte (cm2/electron), Neff (electron/gram) atomic parameters theoretically and experimentally values for Br compounds. | ||||
| Compounds | σte | Neff | ||
| Experimental | Theorical | Experimental | Theorical | |
| CaBr2 | 0.740 x 10-23 | 0.740 x 10-23 | 2.992 x 10+23 | 3.025 x 10+23 |
| CuBr | 0.795 x 10-23 | 0.795 x 10-23 | 2.618 x 10+23 | 2.750 x 10+23 |
| KBr | 0.598 x 10-23 | 0.598 x 10-23 | 3.400 x 10+23 | 3.273 x 10+23 |
| NaBr | 0.539 x 10-23 | 0.539 x 10-23 | 3.689 x 10+23 | 3.889 x 10+23 |
| KBrO3 | 0.276 x 10-23 | 0.276 x 10-23 | 7.196 x 10+23 | 5.243 x 10+23 |
| C19H10Br4O5S | 0.156 x 10-23 | 0.156 x 10-23 | 10.847 x 10+23 | 8.709 x 10+23 |
| C6H6BrN | 0,124 x 10-23 | 0.124 x 10-23 | 11.314 x 10+23 | 10.665 x 10+23 |
| C21H16Br2O5S | 0.101 x 10-23 | 0.101 x 10-23 | 9.596 x 10+23 | 9.129 x 10+23 |
| Table 3: Kerma theoretically and experimentally values for Br compounds. | ||
| Compounds | Kerma (K) | |
| Experimental | Theorical | |
| CaBr2 | 11.855 | 11.989 |
| CuBr | 11.144 | 11.705 |
| KBr | 10.887 | 10.481 |
| NaBr | 10.647 | 11.224 |
| KBrO3 | 10.657 | 7.764 |
| C19H10Br4O5S | 9.091 | 7.299 |
| C6H6BrN | 7.513 | 7.085 |
| C21H16Br2O5S | 5.196 | 4.943 |
The present results constitute the first measurement so it was not possible to compare the findings reported in the literature. The obtained teoric results were not parallel with elements and its compounds. Additionally, experimental uncertainties can not be explained by theoretical values. The effective electron density decreases or increases depending on the type of bonding compound molecules. It is concluded that the mass attenuation coefficient effective atomic number and electron density parameters depend on the environment of compound material. This result may be attributed to understand chemical and molecular environment of present halogen compounds. It is clear that mass attenuation coefficient and total atomic cross section depend on different interactions between central atom and ligands in the chemical compounds. This result of effective atomic number be used for compound gamma ray mass shield. These results of the present study may be used for medical applications of these compounds. The compound with a minimum Kerma value of 5.196 is C21H16Br2O5S where is shown in table 3.
Atomic, moleculer and electronic cross sections, effective atomic number and electron densities, Kerma values of for some Br compounds were calculated by using mass attenuation coefficients. This study provide new insight into the literature since effective atomic number experimental values of some Br compounds have not been determined before. This research has thrown up many questions in need of further suggestion. Since Br has an unfilled 4p shell, they are sensitive to radiation interaction effects. According to the results shown in mass attenuation coefficient, Zeff and Neff of Br compounds are closely associated with chemical structure. Br molecules have different interatomic bond distances between ligands and central atom. As indicated the results of the present study useable for medical applications of bromine compounds. In this study, it is thought that the bromine compounds studied could be used as gamma ray shielding material due to the high values of the mass attenuation coefficient. It has also been found that the mass attenuation coefficient of some bromine compounds tends to increase with increasing Br concentration. In the light of this information fluorescence yield and jump ratio can be calculated for bromine compounds via the values in this publication. This study is a reference for future researchers due to the nature of manuscript the values obtained from the compounds studied in this values can be used as a reference in future studies. It is thought that bromine compounds can be alternative as protective material in terms of having high molecular weight. As indicated the results of the present study useable for medical applications of these compounds.
- Menesguen Y, Lepy MC. Mass attenuation coefficients in the range 3.8 ≤ E ≤ 11 keV, K fluorescence yield and Kβ/Kα relative X-ray emission rate for Ti, V, Fe, Co, Ni, Cu and Zn measured with a tunable monochromatic X-ray source. Nuclear Instruments and Methods in Physics Research Section B. 2010 August;268(16):2477-2486. doi: 10.1016/j.nimb.2010.05.044
- Deslattes RD. Estimates of X-ray Attenuation Coefficients for the Elements and Their Compounds. Acta Crystallogr. 1969 Jan;A25:89-93. doi: 10.1107/S0567739469000118
- Hine GJ. The effective atomic numbers of materials for various gamma interactions. Physics Review. 1952; 85:725-737.
- Murty RC. Effective Atomic Numbers of Heterogeneous Materials. Nature. 1965 July 24;207:398-399. doi: 10.1038/207398a0
- Manohara SR, Hanagodimath SM, Gerward L. The effective atomic numbers of some biomolecules calculated by two methods: a comparative study. Med Phys. 2009 Jan;36(1):137-41. doi: 10.1118/1.3030952. PMID: 19235382.
- Içelli O, Erzeneoǧlu S. Effective atomic numbers of some vanadium and nickel compounds for total photon interactions using transmission experiments. J Quant Spectrosc Radiat Transf. 2004 May 1;85(2):115-124. doi: 10.1016/S0022-4073(03)00197-3
- Polat R, Icelli O. Measurement of the effective atomic numbers of compounds with cerium near to the absorption edge. Nucl Instruments Methods Phys Res Sect A Accel Spectrometers, Detect Assoc Equip. 2010 April 1;615(2):201-210. doi: 10.1016/j.nima.2010.01.039
- Polat R, Yaln Z, Içelli O. The absorption jump factor of effective atomic number and electronic density for some barium compounds. Nucl. Instruments Methods Phys Res Sect A Accel Spectrometers, Detect Assoc Equip. 2011 Feb; 629:185-191. doi: 10.1016/j.nima.2010.11.001
- Hassan M, Nakayama M, Salah M, Akasaka H, Kubota H, Nakahana M, Tagawa T, Morita K, Nakaoka A, Ishihara T, Miyawaki D, Yoshida K, Nishimura Y, Ogino C, Sasaki R. A Comparative Assessment of Mechanisms and Effectiveness of Radiosensitization by Titanium Peroxide and Gold Nanoparticles. Nanomaterials (Basel). 2020 Jun 7;10(6):1125. doi: 10.3390/nano10061125. PMID: 32517328; PMCID: PMC7353194.
- Mann KS, Singla J, Kumar V, Sidhu GS. Verification of some building materials as gamma-ray shields. Radiat Prot Dosimetry. 2012 Aug;151(1):183-95. doi: 10.1093/rpd/ncr455. Epub 2012 Jan 5. PMID: 22223719.
- Hubbell JH, Berger MJ. Photon attenuation in: Engineering compendium on radiation shielding. Edited by RG Jaerger (IAEA, Vienna, Austria) 1968;1(4):198. https://bit.ly/3ogglQk
- Attix FH. The partition of kerma to account for bremsstrahlung. Health Phys. 1979 Mar;36(3):347-54. doi: 10.1097/00004032-197903000-00012. PMID: 489286.
- Attix FH. Fast neutron dosimetry. Progress report. July 1, 1978 June 30, 1979. Wisconsin Medical Physics report No. WMP-109. United States. 1979 Jan 1;249. doi:10.2172/5628655
- Adams SH. The Great American fraud. Press of the American Medical Association. 1905 Oct 7;29:14-15.
- Dagani MJ, Barda HJ, Benya TJ, Sanders DC. Bromine Compounds. Wiley-VCH. 2000 June 15. doi:10.1002/14356007.a04_405
- Copper(I) bromide. https://bit.ly/3t2r6sY
- Ribotta PD, Perez GT, Leon AE, Anon MC. Effect of emulsifiers and guar gum on microstructural, rheological and baking performance of frozen bread dough. Food Hydrocolloids. 2004 Mar;18(2):305-313. doi: 10.1016/S0268-005X(03)00086-9
- Kurokawa Y, Maekawa A, Takahashi M, Hayashi Y. Toxicity and carcinogenicity of potassium bromate-a new renal carcinogen. Environ Health Perspect. 1990 Jul;87:309-35. doi: 10.1289/ehp.9087309. PMID: 2269236; PMCID: PMC1567851.
- Expert Committee on Food Additives. Seventh Report on the Specifications for the Identity and Purity of Food Additives and Their Toxicological Evaluation: Emulsifiers, Stabilizers, Bleaching and Maturing Agents. WHO Tech Rep 1964;281:164.
- Food and Agriculture Organization-World Health Organization. Guide to the Safe Use of Food Additives, Second Series. World Health Organization, Geneva. 1979;60.
- Kreft S, Kreft M. Physicochemical and physiological basis of dichromatic colour. Naturwissenschaften. 2007 Nov;94(11):935-9. doi: 10.1007/s00114-007-0272-9. Epub 2007 May 30. PMID: 17534588.
- 4-Bromoaniline. https://bit.ly/3abR9oW
- Ito S, Yamamoto D. Mechanism for the color change in bromocresol purple bound to human serum albumin. Clin Chim Acta. 2010 Feb;411(3-4):294-5. doi: 10.1016/j.cca.2009.11.019. Epub 2009 Nov 20. PMID: 19932090.
- Lee HM, Lee Y. A differential medium for lactic acid-producing bacteria in a mixed culture. Lett Appl Microbiol. 2008 Jun;46(6):676-81. doi: 10.1111/j.1472-765X.2008.02371.x. Epub 2008 Apr 25. PMID: 18444977.
- Gowda S, Krishnaveni S, Yashoda T, Umesh TK, Gowda R, Pramana. Photon mass attenuation coefficients, effective atomic numbers and electron densities of some thermoluminescent dosimetric compounds. J Phys. 2004 Sep;63:529-541.
- El-Kateb AH, Hamid ASA. Photon attenuation coefficient study of some materials containing hydrogen, carbon and oxygen. Int. J. Appl. Radiat. Isotopes. 1991;42(3):303-307. doi: 10.1016/0883-2889(91)90093-G
- Conner AL, Atwater HF, Plassman EH, McCray JH. Gamma-Ray Attenuation-Coefficient Measurenmnts. Phys Rev. 1970;1:539.
- Singh K, Singh H, Sharma V, Nathuram R, Khanna A, Kumar R, Bhatti SS, Sahota H.S. Gamma-ray attenuation coefficients in bismuth borate glasses. Nuclear Instruments and Methods in Physics Research Section B. 2002; 194: 1-6. doi: 10.1016/S0168-583X(02)00498-6
- Gowda S, Krishnaveni S, Gowda R. Studies on effective atomic numbers and electron densities in amino acids and sugars in the energy range 30-1333 keV. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact with Mater Atoms. 2005;239:361-369. doi: 10.1016/j.nimb.2005.05.048
- Ervin B. Podgorsak, Ph.D. Review of Radiation Oncology Physics, A Handbook for Teachers and Students Department of Medical Physics. McGill University Health Centre Montréal, Québec, Canada Internatıonal Atomıc Energy Agency Vıenna, Austrıa. 2003.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.