> Medicine. 2020 October 31;1(6):263-270. doi: 10.37871/jbres1153.
Repurposing Potential of Diminazene Aceturate as an Inhibitor of the E. coli DNA Gyrase B
Varsha Dwivedi1, Archana Ayyagari2, Rakhi Chandran3, Prerna Diwan1, Sanjay Gupta4 and Vandana Gupta1*
2Department of Microbiology, Swami Shraddhanand College. University of Delhi, New Delhi 110036, India
3Department of Biotechnology and Microbiology, School of Sciences, Noida International University,Yamuna Expressway, Gautam Budh Nagar, UP-203201, India
4Independent Scholar (former Head and Professor, Department of Biotechnology, Jaypee Institute of Information Technology, Noida, Sector 62, UP, India
- ESBL
- Escherichia coli
- Antibiotics
- Anti-microbial resistance
- Docking
- Drug repurposing
- DNA gyrase B
- NDM
Drug-resistant Escherichia coli (E. coli) has overburdened the healthcare facilities in recent years and is getting hard to combat, mandating search for novel therapeutics with a broad antibacterial spectrum and high chemotherapeutic index. The 24 kDa domain of DNA gyrase B that is involved in the ATPase activity has been reported to be a promising target for inhibitors. A PDB structure (1KZN) of the 24kD domain of gyrase B with the co-crystallized ligand clorobiocin was used for the docking studies to explore a library of 2924 FDA approved drugs from www.zinc.docking.org. FlexX docking module from Biosolve IT was used for receptor preparation and in silico docking experiments. Docking studies on the pocket created around the reference ligand clorobiocin revealed the best score with diminazene aceturate and it also demonstrated interactions with the crucial amino acids present within the pocket. Diminazene aceturate has been conventionally been used as an antiparasitic molecule in animals and it has also been demonstrated to exhibit repurposing potential in the treatment of disorders triggered due to overproduction of inflammatory cytokines, pulmonary hypertension, ischemia-induced cardiac pathophysiology, etc. among others. Findings from this study indicate the possibility of repurposing the age-old molecule diminazene aceturate into a DNA gyrase B antagonist to combat not just the drug-resistant E. coli but also other gram-negative ESKAPE pathogens. It may also aid in alleviating the inflammatory response induced in the body of the patients suffering from septicemia caused by a variety of Gram-negative bacterial pathogens.
ADME: Absorption Distribution Metabolism and Excretion; ESBL: Extended Spectrum b-Lactamase; ESBL-Ec: ESBL producing E coli; CADD: Computer-Aided Drug Discovery; MDR: Multiple Drug Resistance; NDM: New Delhi Metallo-Beta-Lactamase; UTI: Urinary Tract Infection
E. coli is a gram negative bacterium, normally a commensal inhabiting the human colon, and has indeed proved to be a great experimental organism of choice for all microbiology as well as gene cloning experiments for long. However, quite a few of its strains are known to cause various intestinal as well as extraintestinal diseases, owing to possession of a handful of virulence factors in some of its serotypes, which influence a number of metabolic processes [1]. Some of the problems caused by it include gastroenteritis, Urinary Tract Infections (UTI), bacterial meningitis, post-operative abscesses and neonatal sepsis. There are at least seven known pathotypes for enteric E. coli namely enteropathogenic, enterotoxigenic, enterohaemorrhagic, enteroaggregative, diffusely adherent, enteroinvasive and adherent invasive E. coli. Besides, there have been reported three pathotypes of E. coli which are extraintestinal in their effect, namely, uropathogenic, neonatal meningitis E. coli and avian pathogenic E. coli. Indiscriminate and improper usage of antibiotics has already resulted in an alarming multiple drug resistance among the pathogenic bacteria [2-5] and has become a global public health concern. Among many mechanisms of MDR, the predominant one is the plasmid-mediated synthesis of Extended–Spectrum Beta Lactamases (ESBLs), which breakdown the beta lactam antibiotics, including all the three generations of cephalosporins, penicillins and aztreonam particularly witnessed in E. coli and other Gram–ve EKAPE pathogens Enterococcus faecium, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species [6,7].
Considering the newly emerging and life threatening AMR E. coli, there is an urgent requirement of newer strategies, such as repurposing of older drugs, re-evaluation of many abandoned compounds using modern chemical synthesis methods, tools and technologies [5]. Another potential approach could be to explore unusual targets within the pathogen system, such as topoisomerases. DNA gyrase or topoisomerase type 2 is an important enzyme that controls DNA supercoiling as needed for processes like replication and transcription, and it is reported to be the target of some successful antibacterials [8-11]. A functional DNA gyrase is heterotetramer (A2B2), consisting of 2 subunits each of gyr A and gyr B, with 875 and 804 amino acids respectively [8]. GyrA creates single stranded nicks in the DNA and reseals them, whereas GyrB serves to provide energy for this process through ATP hydrolysis [9,10], which is essential for the unwinding of the DNA. Hence, DNA gyrases serve as the nanomachines that maintain the DNA in its appropriate topology throughout its replication and transcription [11]. Aminocoumarin antibiotics, quinolones and floroquinolones are the well-known catalytic inhibitors of the DNA gyrase [12-19]. An elegant evidence was provided by Gilbert and Maxwell [12] that a 24 kDa sub-domain located near the N-terminal of DNA gyrase B enzyme possessed the binding site towards coumarin antibiotics. They also suggested that the interaction of coumarins with the protein was predominantly hydrophobic in nature [14].
For carrying out meaningful and productive research on these lines, it is of paramount importance to explore the specific residues of Gyrase B which play a crucial role in maintaining its functionality and stability, and also to know which compounds disturb the same. This kind of approach would make it possible to target DNA gyrase of the drug resistant, pathogenic E. coli, that has become resistant to older antibiotics targeting their cell wall, nucleic acid or protein synthesis etc.
Compounds belonging to three families namely quinolones such as norfloxacin, cyclothialidines and caumarins (eg. Clorobiocin and novobiocin) have been reported to inhibit gyrB (14). A number of other compounds are also reported in the literature, which inhibit Gyrase B. These include Pyridoxal 5’-diphospho-5’adenosine (PLP-AMP) [9], tyrosine-based diamides [20], 1H-pyrrole-2-carboxamide moieties [21], N-phenylpyrrolamides [22], N-phenyl 4,5-dibromo-pyrrolmides [23], and (1,2,4)triazole (4,3-a)quinoxaline derivatives of different heteroaromatization members [24], cinodine, clerocidin, albicidin, GSK299423, a series of tetrahydroindazoles, quinazoline-2,4-diones PD0305970 and PD0326448, QPT-1, quinolone based drug NXL-101 [25] etc. In addition to gyrB, even gyrA possesses a pocket which is capable of binding to coumarin or aminocoumarin, suggesting that any inhibitor like simocyclinone binding to gyrB may bind to gyrA. This is expected to enhance the effectiveness of the drug used [25].
Important amino acids for the ATP hydrolysis in E. coli gyrB as reported in the literature include Tyr5, Ile10, His38, Glu42, Asn46, Glu50, Asp73, Arg76, Gly77, I78, P79, K103, Arg136, Thr165, Asp426 and Lys447 [26-28]. Tyr5 and Ile10 help the process of dimerization in gyrB so to increase binding affinity and also to double the length of DNA site bound protein as well as to increase binding specificity. Some dimer interface residues are important and mutations of these residues result in loss of enzyme catalysis [27]. Glu42 helps in hydrolysis process and His38 in ATPase reaction, acting to orient and polarize glutamate residue in gyrase B [10]. Substitutions with Alanine at Glu42, Asn46, Glu50, Asp73, Arg76, Gly77, Ile78 resulted in decreased or not demonstrable ATPase activity, signifying their importance in ATP hydrolysis [26]. Pro79 and Lys103 are reported to be important in the coupling of ATP hydrolysis and DNA unwinding and mutations in Asp73, Gly77, Ile78, and Thr165 lead to resistance to novobiocin [28]. Lastly, Asp426 and Lys447 are described to be the part of a quinolone-binding pocket of gyrB [26].
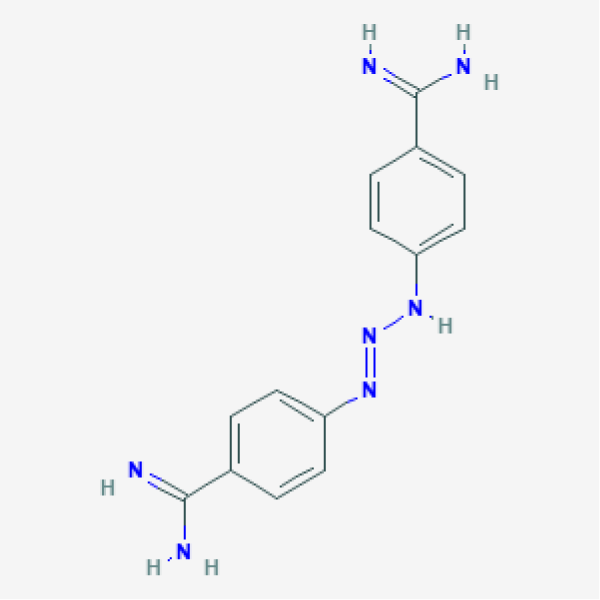
Diminazene aceturate / diminazene / dize / 4,4’-(1-Triazene-1,3-diyl) bis (benzenecarboximidamide) sold with several trade names such as Pirocide, Berenil, Azidin, Ganasag, etc. is an anti-parasitic drug. Though it primarily targets pathogenic protozoans including Trypanosoma, Babesia, and Cytauxzoon, it is also reported to be active against some bacteria namely Brucella and Streptococcus. The potential of dize in the treatment of inflammatory cytokine triggered disorders [29], pulmonary hypertension, ischemia-induced cardiac pathophysiology possibly through the activation of angiotensin converting enzymes 1-7 as studied in the rats appears promising [30,31]. It upturns ACE2 and AT2 receptor expression in the kidney cells in the type1 diabetes rat model and inhibits nephropathy [32]. It has also been reported to completely inhibit the topoisomerase I in Caenorhabditis elegans, though at high concentration of 125 µM [33] and was reported to dock successfully with Chikungunya Virus RNA polymerase [34]. To further support its therapeutic potential in different conditions Oliveira, et al. [35] reported improved activity of diminazene aceturate on Trypanosoma evansi when encapsulated in the liposomes.
Drug repurposing is now viewed as a faster and efficient solution to discover novel therapeutics with a goal to address a number of health issues concerning infectious diseases, cancers etc. This could equip healthcare industry to serve the society better in obtaining a viable solution in fighting the dreaded problem of MDR posed by pathogenic bacteria. Computer-Aided Drug Discovery (CADD) is one of the most powerful approach to investigate drugs that would act upon the novel targets of pathogenic bacteria [36-38]. Correctly termed “Drug Repurposing”, this strategy is indeed proving to be very productive, quicker, and more practical towards fighting the menace of multiple drug resistant pathogenic bacteria in general [39]. This in silico study was undertaken to analyse the interactions of the GyrB subunit towards a number of inhibitors, in an attempt to find a potential lead against MDR E. coli.
Receptor preparation
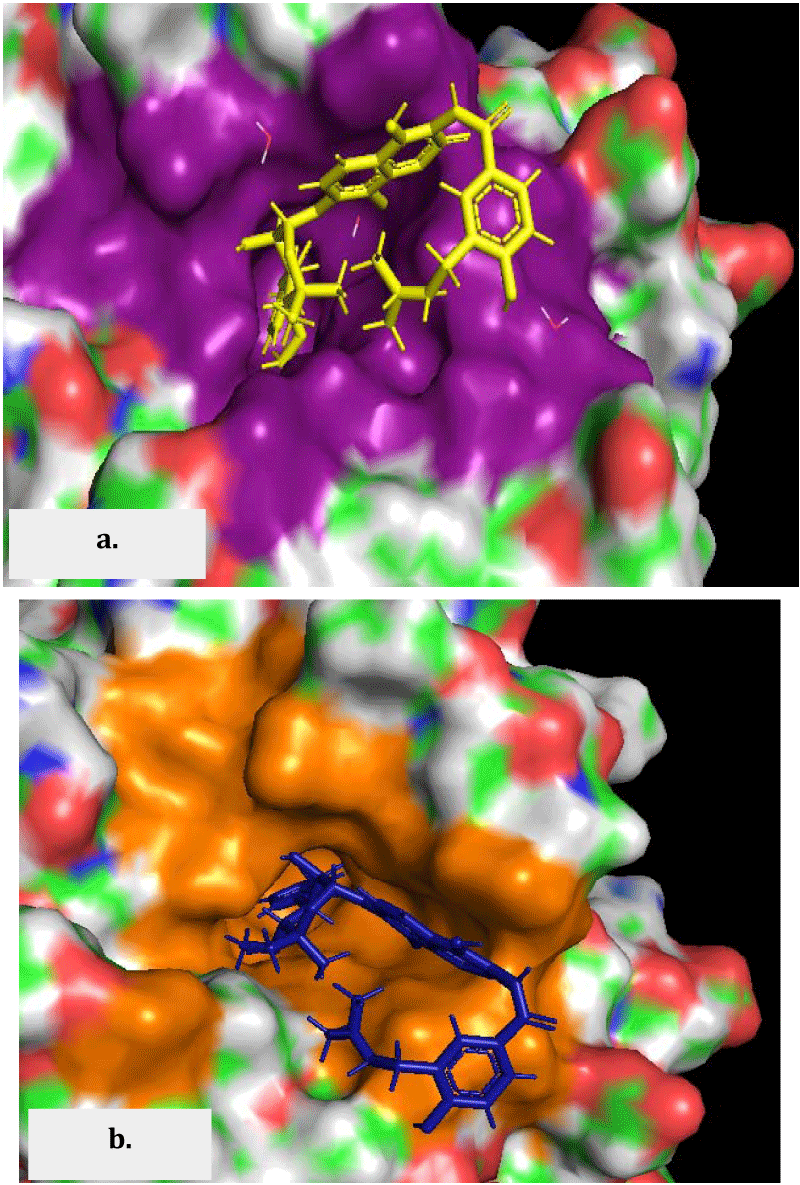
Target protein and important residues were described through the thorough literature search. The conservation of these residues in other gram negative ESKAPE bacteria was determined using Clustal omega. The appropriate 3D structures of the E. coli GyrB were downloaded in PDB format from the database: www.rcsb.org. 1KZN was selected from the available 3D structures of E. coli gyrB [14]. It is a 24 kD fragment of gyrB (Gly15-Glu219) with the co-crystallized antibiotic Clorobiocin (CBN). Docking pocket was created around the reference ligand CBN (referred to as pocket 1) with a diameter of 6.5 angstrom that included 30 residues namely Val43A, Val44A, Asp45A, Asn46A, Ala47A, Ile48A, Asp49A, Glu50A, Ile59A, Val71A, Gln72A, Asp73A, Gly75A, Arg76A, Gly77A, Ile78A, Pro79A, Thr80A, Gly81A, Ala86A, Ala87A, Ile90A, Met91A, Ala96A, Val120A, Arg136A, Gly164A, Thr165A, Met166A, Val167A (Figure 1a). An ‘A’ here with the residues signifies the chain A recognized in the PDB structure. Three water molecules (HOH1001, HOH1066, HOH1160) with at least 2 interactions were included in the receptor and were made freely rotatable.
Ligand selection, docking and lead selection
Docking protocol as per Ghildiyals, et al. [34] was followed for the initial screening of molecules with some modifications. Docking was performed with 500 solutions per iteration and 500 solutions per fragmentation for each molecule of the ZDD subset from zinc small molecule database that consists of a library of 2924 FDA approved drug molecules. Top 14 lead compounds were selected based on the FlexX docking score. The selected Zinc Ids were explored for their chemical nature, name and current therapeutic indications.
Refining docking
The autodetected docking pocket was altered by removing certain non-conserved residues and to take account of some more of the crucial conserved residues, without upsetting the core pocket’s integrity. We used PyMOL for visualizing the pocket created automatically by the lead IT software and all the changes in residues were made after confirming the position of each residue in the surface view using PyMOL. The modified pocket (pocket 2) included 24 residues i.e. Glu42, Val43A, Asp45A, Asn46A, Ala47A, Ile48A, Asp49A, Glu50A, Ile59A, Val71A, Gln72A, Asp73A, Gly75A, Arg76A, Gly77A, Ale78A, Pro79A, Met91A, Ala96A, Val120A, Arg136A, Thr165A, Met166A, and Val167A (Figure 1b). Further shortlisted top leads were docked for 2000 solutions per iteration and 2000 solutions per fragmentation in the auto-detected pocket as well as in the modified pocket. The details of the contacts between the leads and the residues of interest were examined using PyMOL and the Discovery Studio Visualizer platforms.
Among the available PDB structures 1KZN was considered suitable for carrying out in silico screening of drug molecules as this 24 kD C-terminal subdomain of the N-terminal of the DNA gyrase B encompasses the coumarin (gyrB inhibiting antibiotic) binding site. Bacteria overproducing this sub-domain exhibit resistance to coumarins, suggesting its role in in vivo interaction with the drug [12]. 1KZN is an X-ray diffraction determined structure with a resolution of 2.30Å along with a co-crystallized ligand clorobiocin an antibiotic belonging to the coumarin family [14]. Clorobiocin is used as a reference point in our docking studies. In silico studies revealed that the receptor prepared using clorobiocin as the reference ligand shaped a very deep seated and narrow pocket, exhibiting good binding scores (as low as -33) with the ligands. This pocket is referred to as pocket 1. Among the 30 residues present in the pocket 1 namely Val43, Val44, Asp45, Asn46, Ala47, Ile48, Asp49, Glu50, Ile59, Val71, Gln72, Asp73, Gly75A, Arg76, Gly77, Ile78, Pro79, Thr80, Gly81, Ala86, Ala87, Ile90, Met91, Ala96, Val120, Arg136, Gly164, Thr165, Met166, Val167, nine residues as depicted in bold font are fundamental to the functioning of the gyrB. This pocket was altered by removing certain non-conserved residues and to take account Glu42 which is a key conserved residue involved in ATP hydrolysis along with His38. A small part of the surface in continuation with the pocket 1 was contributed by Glu42. The position of His38 is on the other side of the pocket and was not continuous with the pocket 1 and hence was not included in the modified pocket. The modified docking pocket (pocket2) with 24 residues namely Glu42, Val43, Asp45, Asn46, Ala47, Ile48, Asp49, Glu50, Ile59, Val71, Gln72, Asp73, Gly75, Arg76, Gly77, Ale78, Pro79, Met91, Ala96, Val120, Arg136, Thr165, Met166, Val167 includes 10 key residues (bold font). But the docking on pocket 2 did not reveal any significant changes in the docking interactions and scores (data not shown).
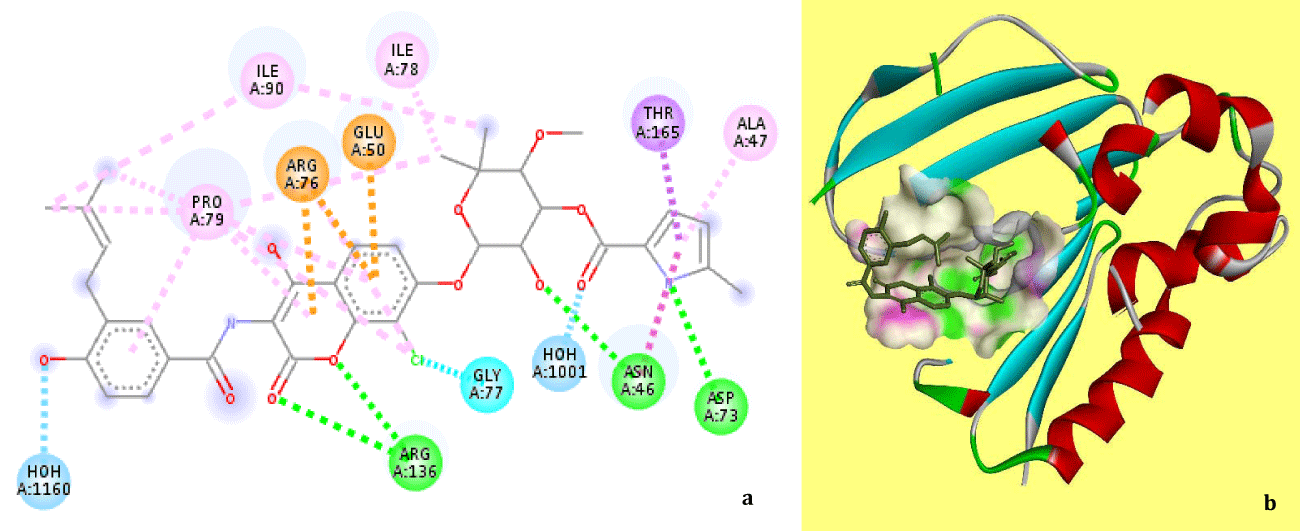
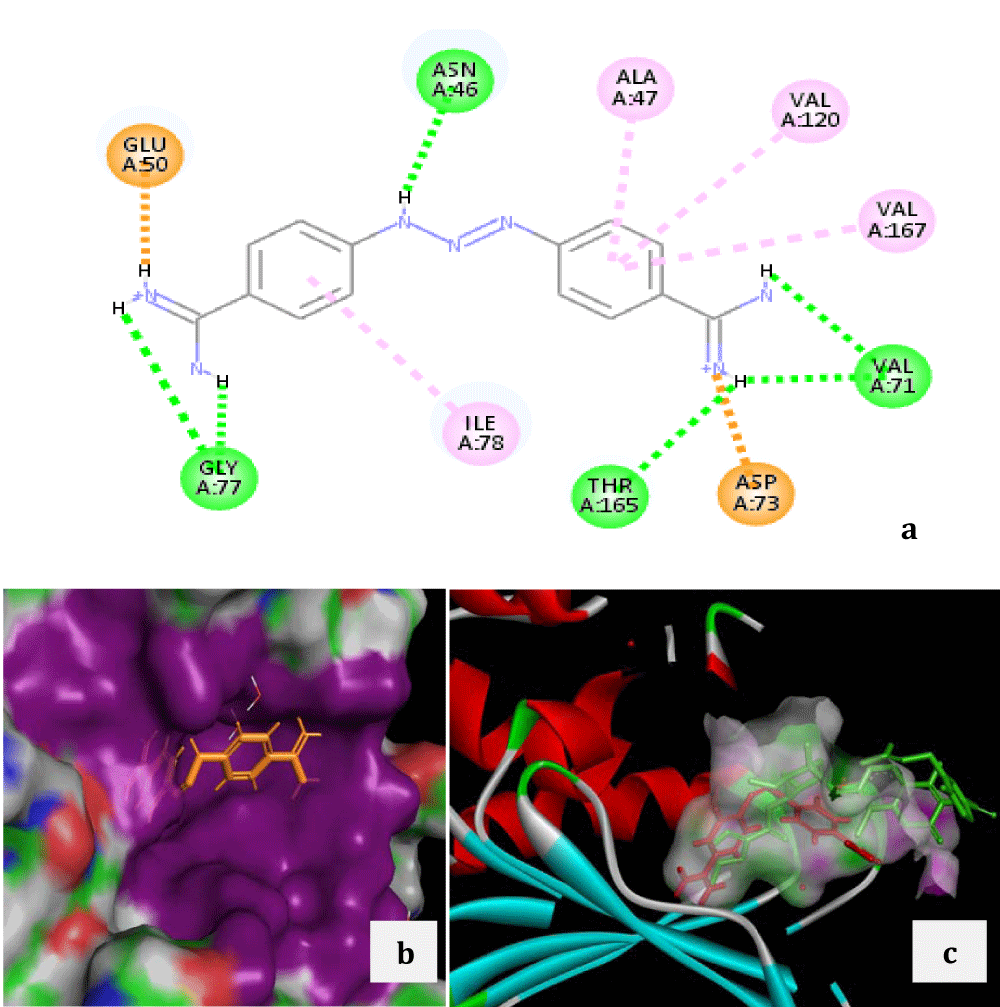
The Zinc IDs of selected top 14 lead compounds with a docking score less than -28, their FlexX docking score, chemical nature, name and current therapeutic indications are recorded in the table 1. On further analysis of these molecules with respect to the interaction with the key residues in the pocket and side effects, the top scoring molecule diminazene aceturate was recognized as a promising lead. Further docking of diminazene aceturate for 2000 solutions per iteration and 2000 solutions per fragmentation improved the docking score to -34.29. A comparative study of the binding of co-crystallized clorobiocin (Figure 2) and docked diminazene aceturate (Figure 3) revealed a noteworthy similarity in occupying the position within the coumarin binding pocket as well as interactions with the strategic residues.
| Table 1: List of top scoring ligands. | |||
| DNA GYRASE B (1KZN) | |||
| S.no | COMPOUNDS | CURRENT INDICATION | SCORE |
| 1 | zinc03830706 Diminazene | Antiparasitic trypanocidal drug | -33.79 |
| 2 | zinc03830435 Ceforanide | Antibacterial; Second generation cephalosporin | -33.30 |
| 3 | zinc00125031, Niflumic acid | Anti-inflammatory and analgesic agent used in the treatment of rheumatoid arthritis | -33.24 |
| 4 | zinc00537877, Ketanserin | Treat Severe Sepsis, Diabetic Foot Ulcer, Septic Shock | -31.96 |
| 5 | zinc35342789, Tolcapone | Antiparkinson drug and catechol O- methyltransferase inhibitor | -31.19 |
| 6 | zinc18456289, Folic acid | Treat and prevent folate deficiencies and megaloblastic anaemia | -30.76 |
| 7 | zinc01530747, Oxacillin | Antibacterial ,broad spectrum beta lactam antibiotic | -30.42 |
| 8 | zinc04676376, Cefmenoxime | Antibacterial; Third generation cephalosporin | -30.04 |
| 9 | zinc01481815, Exjade | Treat chronic iron overload due to blood transfusion | -29.40 |
| 10 | zinc18456286, Leucal | Treatment of overdose of methotrexate | -29.08 |
| 11 | zinc03830403, Cefatrizine | Antibacterial; Cephalosporin | -29.01 |
| 12 | zinc03830399, Cefamandole nafate | Antibacterial; Cephalosporin | -28.79 |
| 13 | zinc03812994, Flunitrazepam | Treat anxiety and sleep disorders | -28.49 |
| 14 | zinc03918453, Ertapenem | Treat wide variety of bacterial infections (bactericidal) | -28.41 |
Diminazene aceturate was found to interact with almost all the key residues with which the co-crystalized clorobiocin interacted, with a few exception (Arg76, Arg136, Pro79 and Ile90). Further details of these interactions with respect to the types and bond length are given in the supplementary table 1. Our results advocates the repurposing potential of this compound to target E. coli gyrB. Diminazene aceturate or simply dize is available in the market with several trade names such as pirocide, berenil, azidin, ganasag, etc. It is an anti-trypanosomal drug with the molecular formula C14H15N7 and chemical structure as shown in figure 4. Though the repurposing potential of this compound for various conditions has been explored widely [29-35], but still it is approved for use only in the animals due to the potential toxicity and low therapeutic index of the molecule[40]. More work is required in optimization of Dize to reduce its toxicity using fragment buiding and/or fragment replacement keeping the core structure of the molecule same.
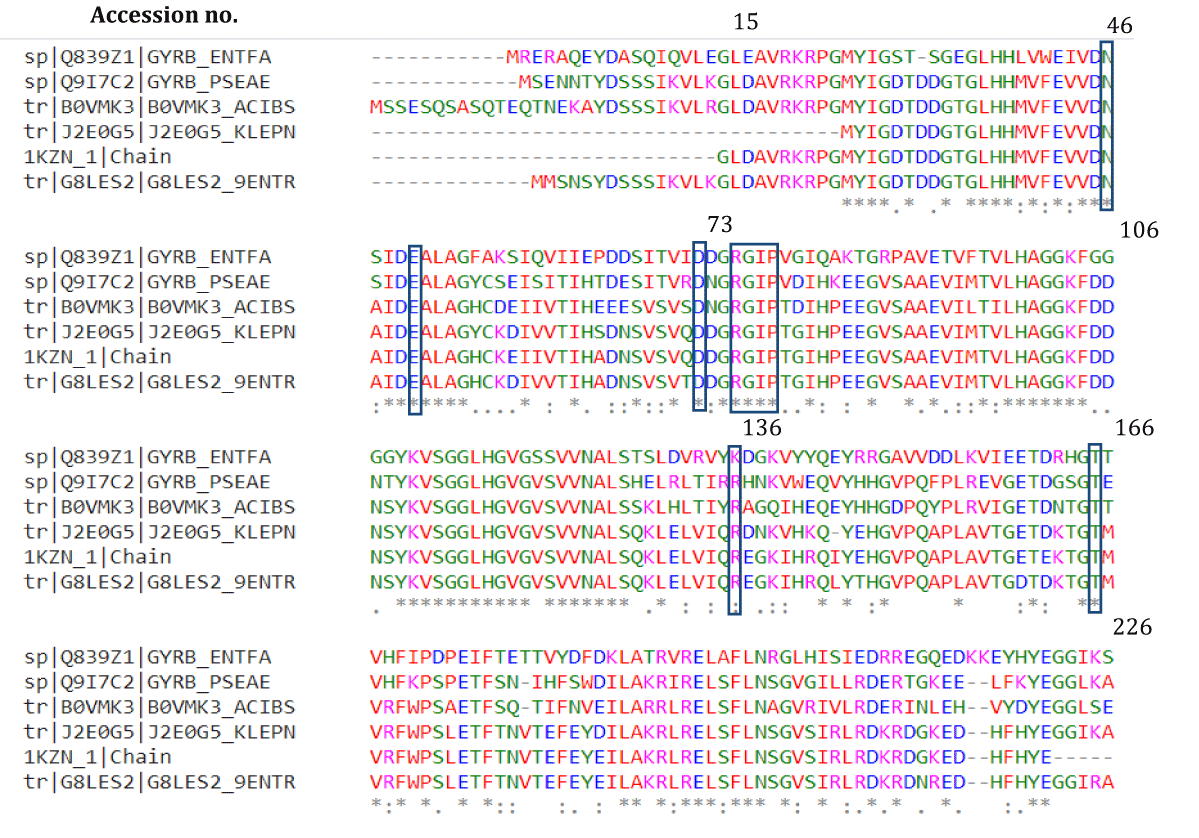
Alignment of the sequence of E. coli 24 kD domain with other Gram negative pathogens Enterococcus faecium, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species belonging to the ESKAPE group of nosocomial pathogens described by Infectious Diseases Society of America revealed highly conserved nature of the selected important residues and also the other interacting residues (Figure 5). This signifies our results as diminazene aceturate is predicted to have broad spectrum activity against gyrB including that of EKAPE gram negatives pathogens that are posing utmost challenges in the healthcare facilities because of their multidrug resistance.
Outcomes from our study specify the repurposing potential of a very simple molecule like diminazene aceturate as a DNA gyraseB antagonist. Our results indicate that dize may be developed into a broad spectrum antibacterial compound through the optimization process using scaffold hopping and pharmacokinetic analysis. It may also be helpful in easing the inflammatory responses in the patients suffering from Gram negative bacteria induced septicemia. Precise in vitro experiments could be deliberated in future, based on our CADD studies and their outcome is expected to be fruitful to mankind in terms of coping with MDR E. coli and the related EKAPE pathogens.
No specific grant was received from any funding agencies for this work. Nonetheless we would like to acknowledge the innovation project scheme of the University of Delhi for the purchase of docking software and other utilities under the grant number RLA-202 (2013-14). We acknowledge Ram Lal Anand College for providing all the support required in carrying out this work.
| Supplementary table 1: Details of interactions of clorobiocin and dize with the pocket 1 residues. | ||
| Residue | Distance (Angstorm) | Type of bond |
| 1. Clorobiocin | ||
| A:HOH1160 | 2.56596 | Conventional Hydrogen Bond |
| A:HOH1001 | 3.16293 | Conventional Hydrogen Bond |
| A:ARG136 | 3.06477 | Conventional Hydrogen Bond |
| A:ARG136 | 2.96011 | Conventional Hydrogen Bond |
| A:ASP73 | 2.81106 | Conventional Hydrogen Bond |
| A:ASN46 | 2.75973 | Conventional Hydrogen Bond |
| A:GLY77 | 3.19866 | Halogen (Cl, Br, I) |
| A:ARG76 | 3.37896 | Electrostatic Pi-Cation |
| A:ARG76 | 3.5633 | Electrostatic Pi-Cation |
| A:GLU50 | 4.35767 | Electrostatic Pi-Anion |
| A:THR165 | 3.89765 | Hydrophobic Pi-Sigma |
| A:ASN46; ALA47 | 4.5104 | Hydrophobic Amide-Pi Stacked |
| A:ILE78 | 4.37022 | Hydrophobic Alkyl |
| A:PRO79 | 5.08063 | Hydrophobic Alkyl |
| A:ILE90 | 3.98305 | Hydrophobic Alkyl |
| A:ARG76 | 4.80002 | Hydrophobic Alkyl |
| A:PRO79 | 4.72152 | Hydrophobic Alkyl |
| A:PRO79 | 3.79521 | Hydrophobic Alkyl |
| A:ILE90 | 4.47367 | Hydrophobic Alkyl |
| A:PRO79 | 4.79658 | Hydrophobic Alkyl |
| A:ALA47 | 4.54929 | Hydrophobic Pi-Alkyl |
| A:PRO79 | 5.38875 | Hydrophobic Pi-Alkyl |
| A:PRO79 | 4.80637 | Hydrophobic Pi-Alkyl |
| A:PRO79 | 4.42951 | Hydrophobic Pi-Alkyl |
| 2. Diminazine aceturate | ||
| A:GLU50 | 1.57609 | Hydrogen Bond;Electrostatic: Salt Bridge;Attractive Charge |
| A:ASP73 | 4.08169 | Electrostatic Attractive Charge |
| A:VAL71 | 2.03438 | Conventional Hydrogen Bond |
| A:THR165 | 2.41305 | Conventional Hydrogen Bond |
| A:GLY77 | 2.94042 | Conventional Hydrogen Bond |
| A:ASN46 | 1.95166 | Conventional Hydrogen Bond |
| A:VAL71 | 2.18623 | Conventional Hydrogen Bond |
| A:ALA47 | 5.36141 | Hydrophobic Pi-Alkyl |
| A:VAL120 | 5.48081 | Hydrophobic Pi-Alkyl |
| A:VAL167 | 5.44365 | Hydrophobic Pi-Alkyl |
| A:ILE78 | 4.96835 | Hydrophobic Pi-Alkyl |
- Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004 Feb;2(2):123-40. doi: 10.1038/nrmicro818. PMID: 15040260.
- Wanda C R. Antimicrobial Mechanisms of Escherichia coli, Editor Amidou Samie, Escherichia coli- Recent advances in physiology, pathogenesis and biotechnological applications, Submitted :April 27th 2016 Reviewed: December 27th 2016 Published: July 12th 2017, DOI:10.5772/67363
- Johura FT, Tasnim J, Barman I, Biswas SR, Jubyda FT, Sultana M, George CM, Camilli A, Seed KD, Ahmed N, Alam M. Colistin-resistant Escherichia coli carrying mcr-1 in food, water, hand rinse, and healthy human gut in Bangladesh. Gut Pathog. 2020 Jan 27;12:5. doi: 10.1186/s13099-020-0345-2. PMID: 32002025; PMCID: PMC6986151.
- Ventola C L. The antibiotic resistance crisis: part 1: Causes and threats. P T. 2015; 40(4):277-283.
- Ribeiro da Cunha B, Fonseca LP, Calado CRC. Antibiotic Discovery: Where Have We Come from, Where Do We Go? Antibiotics (Basel). 2019 Apr 24;8(2):45. doi: 10.3390/antibiotics8020045. PMID: 31022923; PMCID: PMC6627412.
- Li Q, Chang W, Zhang H, Hu D, Wang X. The Role of Plasmids in the Multiple Antibiotic Resistance Transfer in ESBLs-Producing Escherichia coli Isolated From Wastewater Treatment Plants. Front Microbiol. 2019 Apr 3;10:633. doi: 10.3389/fmicb.2019.00633. PMID: 31001218; PMCID: PMC6456708.
- Aguirre L, Vidal A, Seminati C, Tello M, Redondo N, Darwich L, Martín M. Antimicrobial resistance profile and prevalence of extended-spectrum beta-lactamases (ESBL), AmpC beta-lactamases and colistin resistance (mcr) genes in Escherichia coli from swine between 1999 and 2018. Porcine Health Manag. 2020 Apr 2;6:8. doi: 10.1186/s40813-020-00146-2. PMID: 32266079; PMCID: PMC7114809.
- Vanden Broeck A, Lotz C, Ortiz J, Lamour V. Cryo-EM structure of the complete E. coli DNA gyrase nucleoprotein complex. Nat Commun. 2019 Oct 30;10(1):4935. doi: 10.1038/s41467-019-12914-y. PMID: 31666516; PMCID: PMC6821735.
- O’Dea MH, Tamura JK, Gellert M. Mutations in the B subunit of Escherichia coli DNA gyrase that affect ATP-dependent reactions. J Biol Chem. 1996 Apr 19;271(16):9723-9. doi: 10.1074/jbc.271.16.9723. PMID: 8621650.
- Jackson AP, Maxwell A. Identifying the catalytic residue of the ATPase reaction of DNA gyrase. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11232-6. doi: 10.1073/pnas.90.23.11232. PMID: 8248233; PMCID: PMC47956.
- Reece RJ, Maxwell A. DNA gyrase: structure and function. Crit Rev Biochem Mol Biol. 1991;26(3-4):335-75. doi: 10.3109/10409239109114072. PMID: 1657531.
- Gilbert EJ, Maxwell A. The 24 kDa N-terminal sub-domain of the DNA gyrase B protein binds coumarin drugs. Mol Microbiol. 1994 May;12(3):365-73. doi: 10.1111/j.1365-2958.1994.tb01026.x. PMID: 8065258.
- Priyanka, Singh V, Ekta, Katiyar D. Synthesis, antimicrobial, cytotoxic and E. coli DNA gyrase inhibitory activities of coumarinyl amino alcohols. Bioorg Chem. 2017 Apr;71:120-127. doi: 10.1016/j.bioorg.2017.01.019. Epub 2017 Feb 1. PMID: 28196603.
- Lafitte D, Lamour V, Tsvetkov PO, Makarov AA, Klich M, Deprez P, Moras D, Briand C, Gilli R. DNA gyrase interaction with coumarin-based inhibitors: the role of the hydroxybenzoate isopentenyl moiety and the 5’-methyl group of the noviose. Biochemistry. 2002 Jun 11;41(23):7217-23. doi: 10.1021/bi0159837. PMID: 12044152.
- Lautenbach E, Strom BL, Bilker WB, Patel JB, Edelstein PH, Fishman NO. Epidemiological investigation of fluoroquinolone resistance in infections due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Clin Infect Dis. 2001 Oct 15;33(8):1288-94. doi: 10.1086/322667. Epub 2001 Sep 14. PMID: 11565067.
- Towle TR, Kulkarni CA, Oppegard LM, Williams BP, Picha TA, Hiasa H, Kerns RJ. Design, synthesis, and evaluation of novel N-1 fluoroquinolone derivatives: Probing for binding contact with the active site tyrosine of gyrase. Bioorg Med Chem Lett. 2018 Jun 1;28(10):1903-1910. doi: 10.1016/j.bmcl.2018.03.085. Epub 2018 Mar 30. PMID: 29661533; PMCID: PMC5938125.
- Barnard FM, Maxwell A. Interaction between DNA gyrase and quinolones: effects of alanine mutations at GyrA subunit residues Ser(83) and Asp(87). Antimicrob Agents Chemother. 2001 Jul;45(7):1994-2000. doi: 10.1128/AAC.45.7.1994-2000.2001. PMID: 11408214; PMCID: PMC90591.
- Heddle J, Maxwell A. Quinolone-binding pocket of DNA gyrase: role of GyrB. Antimicrob Agents Chemother. 2002 Jun;46(6):1805-15. doi: 10.1128/aac.46.6.1805-1815.2002. PMID: 12019094; PMCID: PMC127264.
- Moreno E, Prats G, Sabaté M, Pérez T, Johnson JR, Andreu A. Quinolone, fluoroquinolone and trimethoprim/sulfamethoxazole resistance in relation to virulence determinants and phylogenetic background among uropathogenic Escherichia coli. J Antimicrob Chemother. 2006 Feb;57(2):204-11. doi: 10.1093/jac/dki468. Epub 2006 Jan 3. PMID: 16390858.
- Fang Y, Lu Y, Zang X, Wu T, Qi X, Pan S, Xu X. 3D-QSAR and docking studies of flavonoids as potent Escherichia coli inhibitors. Sci Rep. 2016 Apr 6;6:23634. doi: 10.1038/srep23634. PMID: 27049530; PMCID: PMC4822154.
- Cotman AE, Trampuž M, Brvar M, Kikelj D, Ilaš J, Peterlin-Mašič L, Montalvão S, Tammela P, Frlan R. Design, Synthesis, and Evaluation of Novel Tyrosine-Based DNA Gyrase B Inhibitors. Arch Pharm (Weinheim). 2017 Aug;350(8). doi: 10.1002/ardp.201700087. Epub 2017 Jun 16. PMID: 28621824.
- Durcik M, Tammela P, Barančoková M, Tomašič T, Ilaš J, Kikelj D, Zidar N. Synthesis and Evaluation of N-Phenylpyrrolamides as DNA Gyrase B Inhibitors. ChemMedChem. 2018 Jan 22;13(2):186-198. doi: 10.1002/cmdc.201700549. Epub 2018 Jan 8. PMID: 29206345.
- Zidar N, Macut H, Tomašič T, Peterlin Mašič L, Ilaš J, Zega A, Tammela P, Kikelj D. New N-phenyl-4,5-dibromopyrrolamides as DNA gyrase B inhibitors. Medchemcomm. 2019 May 20;10(6):1007-1017. doi: 10.1039/c9md00224c. PMID: 31303999; PMCID: PMC6596384.
- Omar AM, Alswah M, Ahmed HEA, Bayoumi AH, El-Gamal KM, El-Morsy A, Ghiaty A, Afifi TH, Sherbiny FF, Mohammed AS, Mansour BA. Antimicrobial screening and pharmacokinetic profiling of novel phenyl-[1,2,4]triazolo[4,3-a]quinoxaline analogues targeting DHFR and E. coli DNA gyrase B. Bioorg Chem. 2020 Mar;96:103656. doi: 10.1016/j.bioorg.2020.103656. Epub 2020 Feb 10. PMID: 32062449.
- Collin F, Karkare S, Maxwell A. Exploiting bacterial DNA gyrase as a drug target: current state and perspectives. Appl Microbiol Biotechnol. 2011 Nov;92(3):479-97. doi: 10.1007/s00253-011-3557-z. Epub 2011 Sep 9. PMID: 21904817; PMCID: PMC3189412.
- Heddle J, Maxwell A. Quinolone-binding pocket of DNA gyrase: role of GyrB. Antimicrob Agents Chemother. 2002 Jun;46(6):1805-15. doi: 10.1128/aac.46.6.1805-1815.2002. PMID: 12019094; PMCID: PMC127264.
- Brino L, Urzhumtsev A, Mousli M, Bronner C, Mitschler A, Oudet P, Moras D. Dimerization of Escherichia coli DNA-gyrase B provides a structural mechanism for activating the ATPase catalytic center. J Biol Chem. 2000 Mar 31;275(13):9468-75. doi: 10.1074/jbc.275.13.9468. PMID: 10734094.
- Gross CH, Parsons JD, Grossman TH, Charifson PS, Bellon S, Jernee J, Dwyer M, Chambers SP, Markland W, Botfield M, Raybuck SA. Active-site residues of Escherichia coli DNA gyrase required in coupling ATP hydrolysis to DNA supercoiling and amino acid substitutions leading to novobiocin resistance. Antimicrob Agents Chemother. 2003 Mar;47(3):1037-46. doi: 10.1128/aac.47.3.1037-1046.2003. PMID: 12604539; PMCID: PMC149296.
- Qi Y, Zhang J, Cole-Jeffrey CT, Shenoy V, Espejo A, Hanna M, et al. Diminazene aceturate enhances angiotensin-converting enzyme 2 activity and attenuates ischemia-induced cardiac pathophysiology. Hypertension (Dallas), 2013, 62:746- 752 [PubMed:23959549]
- Rigatto K, Casali K R, Shenoy V, Katovich M J, Raizada M K. Diminazene aceturate improves autonomic modulation in pulmonary hypertension. Eur J Pharmacol. 2013, 713: 89-93 [PubMed:23665493]
- Kuriakose S, Uzonna JE. Diminazene aceturate (Berenil), a new use for an old compound? Int Immunopharmacol. 2014 Aug;21(2):342-5. doi: 10.1016/j.intimp.2014.05.027. Epub 2014 Jun 2. PMID: 24893117.
- Goru SK, Kadakol A, Malek V, Pandey A, Sharma N, Gaikwad AB. Diminazene aceturate prevents nephropathy by increasing glomerular ACE2 and AT2 receptor expression in a rat model of type1 diabetes. Br J Pharmacol. 2017 Sep;174(18):3118-3130. doi: 10.1111/bph.13946. Epub 2017 Aug 11. PMID: 28688122; PMCID: PMC5573423.
- Park SM, Koo HS. Purification of Caenorhabditis elegans DNA topoisomerase I. Biochim Biophys Acta. 1994 Sep 13;1219(1):47-54. doi: 10.1016/0167-4781(94)90245-3. PMID: 8086477.
- Ghildiyal R, Gupta S, Gabrani R, Joshi G, Gupta A, Chaudhary VK, Gupta V. In silico study of chikungunya polymerase, a potential target for inhibitors. Virusdisease. 2019 Sep;30(3):394-402. doi: 10.1007/s13337-019-00547-0. Epub 2019 Oct 26. PMID: 31803807; PMCID: PMC6864021.
- Oliveira CB, Rigo LA, Rosa LD, Gressler LT, Zimmermann CE, Ourique AF, DA Silva AS, Miletti LC, Beck RC, Monteiro SG. Liposomes produced by reverse phase evaporation: in vitro and in vivo efficacy of diminazene aceturate against Trypanosoma evansi. Parasitology. 2014 May;141(6):761-9. doi: 10.1017/S0031182013002114. Epub 2014 Jan 28. PMID: 24476993.
- Yu W, MacKerell AD Jr. Computer-Aided Drug Design Methods. Methods Mol Biol. 2017;1520:85-106. doi: 10.1007/978-1-4939-6634-9_5. PMID: 27873247; PMCID: PMC5248982.
- Batool M, Ahmad B, Choi S. A Structure-Based Drug Discovery Paradigm. Int J Mol Sci. 2019 Jun 6;20(11):2783. doi: 10.3390/ijms20112783. PMID: 31174387; PMCID: PMC6601033.
- Moitessier N, Englebienne P, Lee D, Lawandi J, Corbeil CR. Towards the development of universal, fast and highly accurate docking/scoring methods: a long way to go. Br J Pharmacol. 2008 Mar;153 Suppl 1(Suppl 1):S7-26. doi: 10.1038/sj.bjp.0707515. Epub 2007 Nov 26. PMID: 18037925; PMCID: PMC2268060.
- March-Vila E, Pinzi L, Sturm N, Tinivella A, Engkvist O, Chen H, Rastelli G. On the Integration of In Silico Drug Design Methods for Drug Repurposing. Front Pharmacol. 2017 May 23;8:298. doi: 10.3389/fphar.2017.00298. PMID: 28588497; PMCID: PMC5440551.
- Han D, Yoon W-K, Hyun C. Cerebellar encephalopathy from diminazene aceturate (beneril) toxicity in a dog. Korean J Vet Res, 2014 54(3) :193-196, doi:10.14405/kjvr.2014.54.3.193
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.