> Biology. 2020 October 26;1(6):233-240. doi: 10.37871/jbres1148.
-
Subject area(s):
- Infectious Diseases
- Biology
- Virology
- Antiretrovirology
- Antivirology
In silico Screening of Approved Drugs to Describe Novel E. coli DNA Gyrase A Antagonists
Rakhi Chandran1, Archana Ayyagari2, Prerna Diwan3, Sanjay Gupta4 and Vandana Gupta3*
2Department of Microbiology, Swami Shraddhanand College, University of Delhi, New Delhi-110036, India
3Department of Microbiology, Ram Lal Anand College, University of Delhi, New Delhi-110021, India
4Independent Scholar (former Head and Professor, Department of Biotechnology, Jaypee Institute of Information Technology, Sector 62, Noida, UP-201309, India)
- ESBL
- Escherichia coli
- Antibiotics
- Anti-microbial resistance
- Docking
- Drug repurposing
- DNA gyrase A
- Cephalosporins
The alarming multiple drug resistance developed by Escherichia coli towards the routine conventional antibiotics owing to their non-judicious usage is fast becoming a tough menace. This necessitates the urgent unleashing of novel and diverse strategies and antibacterial compounds. Since finding a new antibiotic from the scratch, followed by endless clinical trials is exceedingly time-consuming, a powerful alternate strategy of CADD coupled with repurposing the available drugs could save precious time and money. DNA gyrases (topoisomerase II) of E. coli are among the promising new drug targets. The interface between the N-terminal domain of gyrA and C- terminal domain of gyrB which is targeted by most of the available inhibitory drugs, is of particular interest. Crucial active site residues within the N-terminal domain of gyrA were delineated through a literature search. FDA approved drugs were docked using FlexX on the receptors created around the co-crystallized reference ligand. Based on the docking scores and interactions with crucial residues, 12 leads were shortlisted, namely ceforanide, tetrahydrofolic acid, azlocillin, cefazolin, adenosine triphosphate, cefixime, dihydronicotinamide adenine dinucleotide, moxalactam, leucal, cromoglicic acid, cefotetan, and cedax. Surprisingly quinolones, which are approved inhibitors of gyrases were not picked up in the top leads, rather, the most dominant class of molecules that docked successfully was cephalosporin. Our results indicated that these cephalosporins, as well as the other shortlisted leads, could be further optimized and validated through in-vitro experiments for their potential as gyrase A antagonists. Hence the present study holds immense promise in combating MDR of human bacterial pathogens.
ADME: Absorption, Distribution, Metabolism, and Excretion; CADD: Computer-Aided Drug Discovery; ESBL: Extended Spectrum β-Lactamase; ESBL-Ec: ESBL Producing E. coli; MDR: Multiple Drug Resistance; NDM: New Delhi Metallo-β-Lactamase; SD8: Simocyclinone D8; UTI: Urinary Tract Infections
Escherichia coli, a common intestinal pathogen, is known to cause gastroenteritis and a variety of extra-intestinal diseases, such as Urinary Tract Infections (UTIs), meningitis among newborns, colisepticemia, and skin and soft tissue infections [1,2]. E. coli infection is also reported to be responsible for several post-operative abscesses and other complications such as neonatal sepsis [3,4]. It has been developing more and more resistance towards the available antibiotics. Extended-Spectrum Β-Lactamase (ESBL) producing E. coli (ESBL-Ec) has recently gained much importance as a common cause of contagious nosocomial and community acquired infections in India and abroad, because it resists treatment with almost all the β-lactam antibiotics including three generations of cephalosporins [5]. Multiple surveys recorded the highest rate of ESBL-Ec in India (80%), followed by China (60%), and less than 30% in East and Southeast Asia. In Europe, Australia and North America it ranges between 5-10%. Further resistance to the advanced antibiotics like carbapenems due to the production of carbapenemases/ New Delhi Metallo β-lactamases (NDM) among these pathogens has rendered treatment of such infections extremely challenging. During the last decade, the preventive measures followed to curb such infections have not proved to be adequate to prevent the rapid spread of resistant Gram-Negative Bacteria (GNB), particularly extended-spectrum β-lactamase producing Escherichia coli [6,7]. Approximately 67% of E. coli isolates from extra-intestinal infections are reported to be multidrug-resistant, of which up to 85% producing ESBL and 6% producing NDMs. These enzymes are responsible for inactivating β-lactam and carbapenem antibiotics commonly used in treating E. coli infections [1]. A single Extra-Intestinal Pathogenic strain Escherichia coli (ExPEC) clone, named Sequence Type (ST) 131, is the cause of millions of drug-resistant infections annually [1,8]. NDM was first described by Yong, et al. [9] in a Swedish national who fell ill with Klebsiella infection, acquired in New-Delhi. Similar trends were subsequently reported for other gram-negative pathogens including Proteus vulgaris, Serratia marcescens, Enterococcus, etc. Coupled with the increment in antibiotic-resistant bacteria, the extremely slow speed of newer approved antibiotics for treatment, several infectious diseases are not being addressed successfully [10].
These astonishing global health threats call for urgent accelerated research into finding novel and more effective therapeutic options for such infections. Available options for the same encompass either newer antibacterials or newer strategies to target such drug-resistant microorganisms. Given this challenging scenario, there is an urgent need to look for either newer target components of the bacterial cells or find novel inhibitors of the older ones. Conventionally, all anti-infection agents are used to target certain pathways within the pathogenic bacteria, such as cell wall production, nucleic acid synthesis, protein synthesis, and folate synthesis [11]. However, over a span of time, indiscriminate, excessive, and improper usage of these has led to astonishing unresponsiveness of these bacteria towards the same antibiotics, translating into the emergence of drug resistant mutant bacteria [12,13]. This very fact necessitates a paradigm shift in our focus towards unconventional targets presents within bacterial machinery. DNA gyrase is one such important enzyme that introduces negative supercoils in DNA, classified as topoisomerase type 2 that controls DNA topology in proper form during its replication and transcription as well as during cell division. The native DNA gyrase (370kD protein) comprises two types of subunits gyrase A (gyrA) and gyrase B (gyrB) with 875 and 804 residues respectively [14]. Its active form is made up of a hetero-tetramer complex A2B2. It possesses various molecular interfaces named N-gate, DNA gate, and C-gate which assist in strand passage and DNA binding in a specific manner. GyrA function is to break and re-join DNA and GyrB function is to hydrolyze ATP to provide energy for the DNA unwinding. DNA gyrase is known to be targeted by catalytic inhibitors such as aminocoumarins, or ‘poison’ such as quinolones. Several citations suggest that the known inhibitors of DNA gyrase mostly dock onto amino acids located near the amino-terminal of the gyrA. Gepotidasin, a novel inhibitory antibiotic is demonstrated to interact with the complete E. coli DNA gyrase nucleoprotein complex [15]. Similarly inhibition of gyrA by 7-oxy-4methyl coumarinyl amino alcohol derivatives 17 and 18 [16], 4,5,6,7-tetrahydrobenzeno (1,2-d) thiazole-2,6 diamine, 2-(2-aminothiazol-4-yl)acetic acid and benzol (1,2,-d) thiazole-2,6-diamine) [17], fluoroquinolones with alkylamine, alkylpnthalimide, and alkylphenyl groups introduced at N-1 position [18] and 4,5,6,7 tetrahydrobenzo(d)thiazole [19] has been reported.
Residues crucial to the activity of gyrA as reported in the literature include Lys42, Val44, His45, His78, Pro79, His80, Gly81, Asp82, Ser83, Ala84, Asp87, Arg91, Lys103, Lys110, Tyr122 Asn169, Gly170, Ser171, Ser172, and Asp424. Alanine substitutions at Asp87 and Ser83 lead to DNA cleavage, DNA supercoiling, and resistance to different quinolones [14,20,21], Lys42 stabilize interaction with ciprofloxacin [14], His45 and Arg91 are involved in binding to nalidixic acid [22], deletion of Tyr122 leads to the change in the conformation of the active site of enzyme affecting its interaction with neighboring amino acids and binding of ciprofloxacin to gyrase [23] while Asp424 is crucial to the DNA cleavage at the active site of gyrA and is involved in a conformational change, while others are either active site residues or are involved in binding to simocyclinone D8 [14].
The in silico research is very promising in this direction and also is much quicker. Further, to make it more rewarding and fruitful for successfully dealing with the emergence of AMR in the bacterial pathogens, these studies could be combined with the stimulating possibility of repurposing the already available drugs, indicated for other ailments [24]. Various computational tools may be used for in silico screening of libraries of these approved drugs. Scaffold hopping of the top leads from each protein without altering the ADME properties significantly helps in further optimizing these leads. Molecular docking paves the way quite effectively towards establishing a quantitative structure-activity relationship between the antibacterial and the target enzyme of infecting pathogens [25].
This particular study was planned by carrying out an extensive literature search on the bacterial gyrases concerning their active sites and inhibitor binding residues, and an attempt was made to explore the potential of existing FDA Approved drugs to be repurposed as DNA gyrase inhibitors. Such a piece of work certainly holds a paramount potential for competent healthcare and well-being of the society at large, and therefore, is worth attempting.
To accomplish the stated goals, an extensive literature search was carried out and many target proteins were delineated and explored. The suitable 3D structures of the target enzymes DNA GyrA were retrieved from the PDB database. From the available PDB structures of E. coli DNA GyrA, 4CKL [14] was selected. It is a 55kD N-terminal domain of gyrA in complex with the antibiotic Simocyclinone D8 (SD8) obtained from Streptomyces antibioticus. The library of 2924 molecules FDA approved drugs was downloaded from www.zincdocking.org in mol2 file format. FlexX docking and scoring module from BiosolveIT was used for docking. It is an effective flexible docking module as it flexibly docks every molecule on the target site in up to 2000 iterations while using the graphic user interface. It ranks each pose and gives the output as the best-docked pose score for all the compounds in the library and rank them based on docking score and lower the score better is the binding. Receptor IntelligenceTM is included in FlexX includes, which is a fundamentally simple and different way to design and perform a docking. It helps in accurately predicting the geometry of the protein-ligand complex within a few seconds.
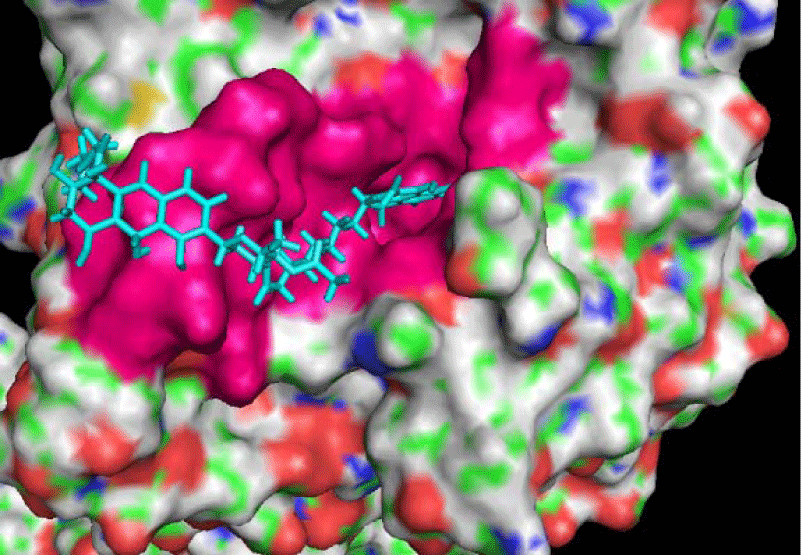
The active site residues of E. coli DNA gyrA were delineated after an extensive literature search. Gyrase A crystal structure 3CKL has a co-crystallized ligand (SM8) which was used as a reference ligand to create a binding pocket. The active site pocket was further modified to include some of the remaining crucial conserved residues delineated by the literature search while some of the non-conserved residues from the pocket were removed without disturbing the integrity of the core binding pocket so that less specific or non-specific interactions can be avoided. We used PyMOL for visualizing the pocket created automatically by the lead IT software and all the changes in residues were made after confirming the position of each residue in the surface view using PyMOL. DNA Gyrase A pocket was created with the residues Gly40, Leu41, Lys42, Val44, His45, Arg47, Ile74, His78, Pro79, His80, Gly81, Asp82, Ser83, Ala84, Asp87, Thr88, Arg91, Met92, Phe96, Ser97, Leu98, Arg99, Leu102, Asn165, Asn169, Gly170, Ser171, Ser172, Gly173, Ile182, Tyr266, and Gln267. The lead selection was done based on the docking score obtained from Flex X. The binding of the leads in comparison to the reference ligand and the details of the interaction of leads with the residues within the docking pocket was analyzed using the PyMOL and the Discovery Studio Visualizer platforms.
The crystal structure of the DNA gyrA N-terminal domain 4CKL is a 55kD dimer with the co-crystallized ligand SD8. The structure is resolved at 2.05 Å. As the interface between the N-terminal of gyrase A and C-terminal of gyrase B in a functional tetrameric DNA gyrase is the site involved in DNA cleavage and strand passage, 4CLK was considered to be a suitable structure for the docking studies. Moreover, it also has a co-crystallized ligand to be used as a reference point in docking studies. DNA Gyrase A (4CKL) docking pocket created (Figure 1) with the residues Gly40, Leu41, Lys42, Val44, His45, Arg47, Ile74, His78, Pro79, His80, Gly81, Asp82, Ser83, Ala84, Asp87, Thr88, Arg91, Met92, Phe96, Ser97, Leu98, Arg99, Leu102, Asn165, Asn169, Gly170, Ser171, Ser172, Gly173, Ile182, Tyr266, Gln267 include 16 of the crucial functional residues reported in the literature (in bold), their significance is summarized in table 1. Lys42 forms polar contacts and helps in protein stability in association with ciprofloxacin a fluoroquinolone gyrase inhibitor and mutation lys42Ala also results in resistance to SD8. His45 and Arg91 are involved in the interaction with nalidixic acid and SD8 [14,26]. Whereas Ser83, Ala84, and Asp87 are involved in drug binding and are considered as hot spots for quinolone resistance mutations [14,20,21]. Alanine substitution at these positions leads to DNA cleavage, DNA supercoiling, and resistance to different quinolones, hence implying the importance of these residues in the functioning of gyrA [20,21,26].
Table 1: Residues of the docking pocket and the significance of crucial residues in the pocket [14]. |
|||
| Residues involved in binding to SD8 in the co-crystal structure | Residues involved in quinolone/ fluoroquinolone resistance | Residues involved in SD8 resistance | Active site residues |
| Lys42, Val44, His45, His78, Pro79, His80, Gly81, Asp87, Arg91, Ser171, Ser172, | Lys42, His45, Ser83, Ala84, Asp87, Arg91, | Lys42, His45, His80, Gly81, Ser83, Ala84, Asp87, Arg91, | Lys42, Val44, His45, His80, Gly81, Asp82, Ser83, Arg91, Asn169, Gly170, Ser172 |
Table 2: List of top-scoring moleculeswith their Zinc Ids, common names, current indications, and Flex-X scores. |
|||
| S.no. | ZincID/ Compounds | Current Indications | Flex-X SCORE |
| 1 | zinc03830434 Ceforanide |
Second generation cephalosporin indicatedfor bacterial infections | -39.9848 |
| 2 | zinc13513942 Tetrahydrofolic acid |
Treat hematologic complications eg.macrocytic anaemia, topical sprue, and megaloblastic | -38.3155 |
| 3 | zinc03830262 Azlocillin |
Antibacterial, broad spectrum semisyntheticpenicillin | -37.6348 |
| 4 | zinc03830407 Cefazolin |
First generation cephalosporin indicatedfor bacterial infections | -37.4504 |
| 5 | zinc18456332 Adenosine triphosphate |
Seems to improve appetite and quality oflife in people with weight loss due to tumors or other etiologies | -37.1850 |
| 6 | zinc03830435 Ceforanide |
Second generation cephalosporin indicatedfor bacterial infections | -36.7278 |
| 7 | zinc03830410 Cefixime |
Third generation cephalosporin indicatedfor bacterial infections | -36.3434 |
| 8 | zinc53682927 1,4-Dihydronicotinamideadenine dinucleotide | To treat simple fatigue, energy sappingdisorders, chronic fatigue, and fibromyalgia | -35.5141 |
| 9 | zinc03871613 Adenosine triphosphate |
Seems to improve appetite, food intake, andquality of life in people with weight loss due to tumors or other etiologies | -35.3634 |
| 10 | zinc18456284 Leucal |
Chemically reduced derivative of folic acidto treat overdose of anti-neoplastic folic acid antagonist methotrexate | -35.1048 |
| 11 | zinc03831157 moxalactam (latamoxef) |
Oxacephem antibiotic grouped withcephalosporins indicated in bacterial infections | -34.8578 |
| 12 | zinc03871615 Adenosine triphosphate |
Neurotransmitter | -34.5817 |
| 13 | zinc03831159 moxalactam (latamoxef) |
Treat bacterial infections | -34.2649 |
| 14 | zinc04468778 Cefixime |
Third generation cephalosporin indicatedfor bacterial infections | -34.0983 |
| 15 | zinc03871612 Adenosine triphosphate |
Neurotransmitter | -33.8784 |
| 16 | zinc01530604 Cromoglicic acid |
Prevents inflammatory release fromeosinophil and mast cell and inhibits calcium influx | -33.7909 |
| 17 | zinc09212428 Leucal |
Chemically reduced derivatives of folicacid to treat overdose of anti-neoplastic folic acid antagonist methotrexate | -33.5305 |
| 18 | zinc03830433 Ceforanide |
Second generation cephalosporin indicatedfor bacterial infections | -33.3712 |
| 19 | zinc03830441 Cefotetan |
Second generation cephalosporin indicatedfor bacterial infections | -33.3351 |
| 20 | zinc03871967 Ceftibuten |
Third generation cephalosporin indicated inUTI and other bacterial infections | -33.1164 |
In silico studies revealed that the docking site created exhibited reasonable binding to ligands with the formation of polar as well as nonpolar contacts between the atoms of the ligand and the docking site residues that contribute significantly to the stability of docked conformations. Scores as low as -39.9848 were obtained. Analysis of the 20 top scoring zinc Ids for the compound name, binding score, and current therapeutic indications is summarized in table 2.
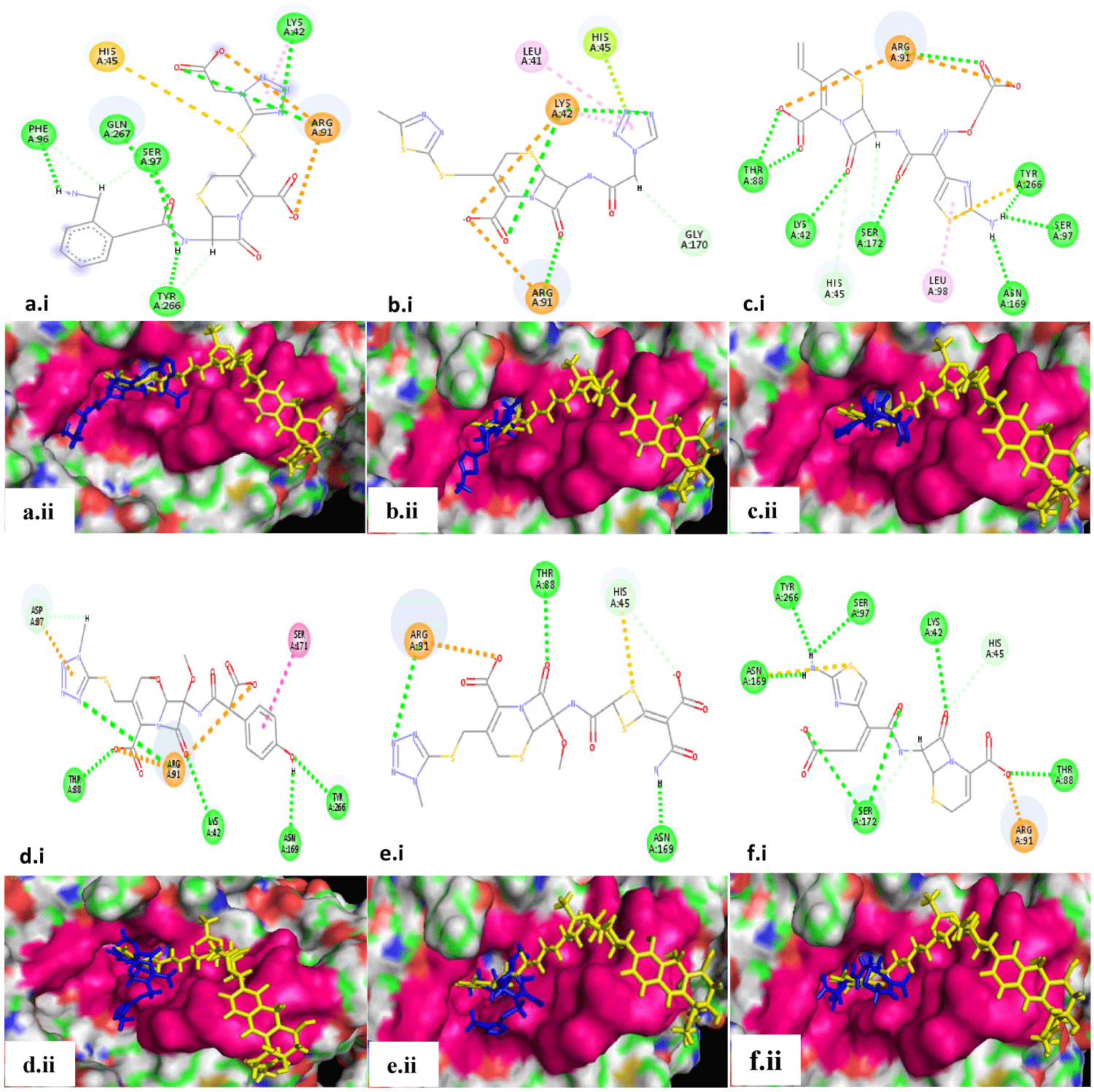
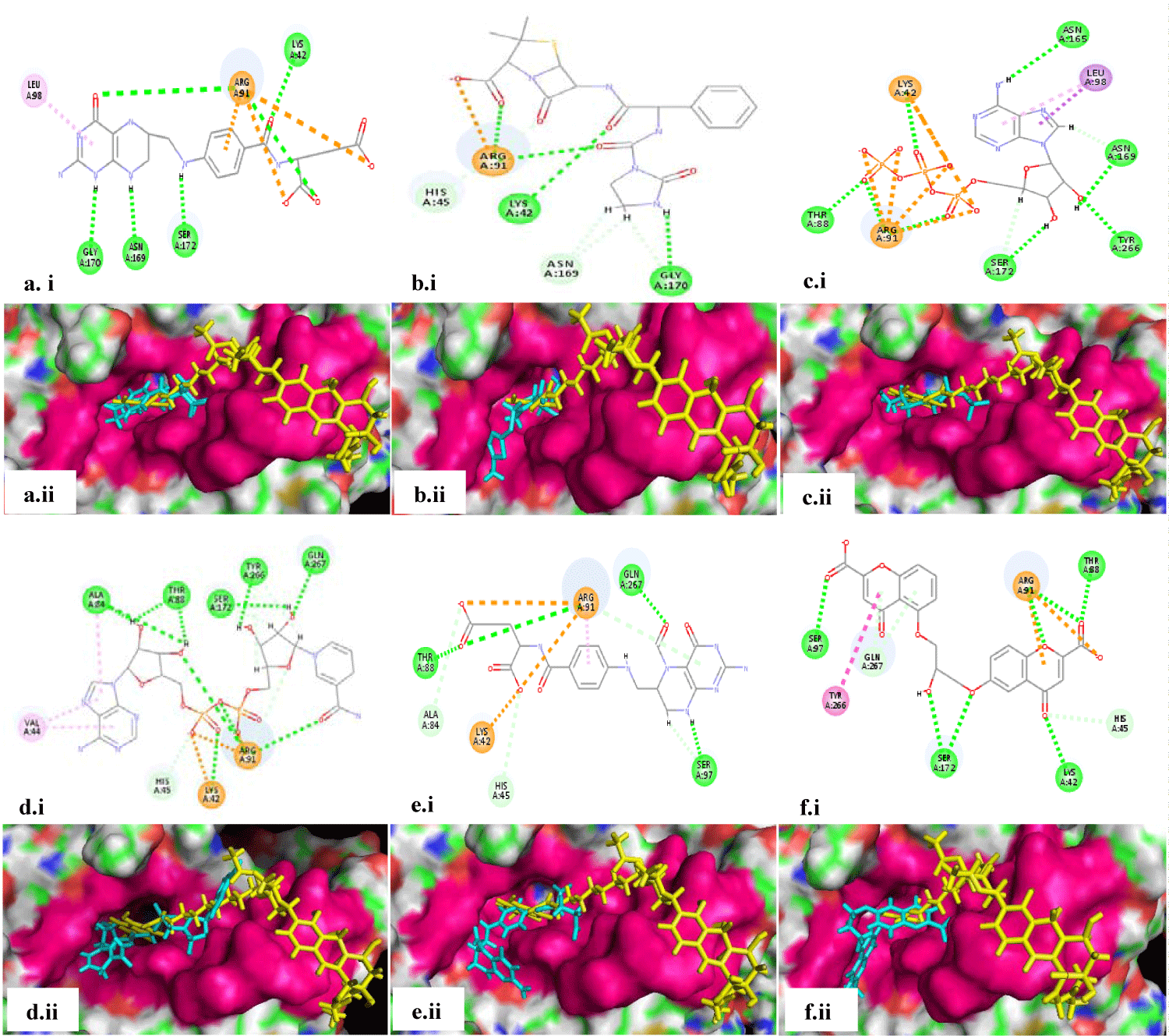
A list of top 20 leads was further sorted after removing the duplicated molecules as the compounds appearing again with lesser scores were eliminated. The shortlisted 12 leads along with the residues interacted with in the docking pocket are summarized in table 3. The interacting key residues are highlighted in bold font. Each lead has interacted with at least 3 to at most 5 crucial residues with multiple hydrogen bonds, salt bridges, and hydrophobic interactions (Figure 2 and 3).
Results obtained from these studies were very surprising and unexpected. Even though quinolones and fluoroquinolones are the known DNA gyrase inhibitors, they were not picked up among the top scoring leads. Rather, the cephalosporins appeared to be a dominant class of molecules binding to the DNA gyrase A, as out of the 12 top selected leads 6 are cephalosporins. The analysis of docked poses (with reference to the binding of reference ligand, as per the co-crystal structure) of cephalosporins (Figure 2), as well as the other lead molecules including tetrahydrofolic acid, azlocillin, ATP, 1,4-dihydronicotinamide adenine dinucleotide, leucal and cromoglicic acid (Figure 3), revealed that all of them bind within the deep seated pocket, where the aminocoumarin moiety of the reference ligand SD8 also binds. None of the selected leads docked at the pocket at the interface of the two monomers in this 55kD N-terminal domain homodimer of gyrA where the polyketide moiety of SD8 was reported to be binding [14]. The binding site and affinity of polyketide moiety change if the 55kD partial gyrA protein is replaced with 59kD partial gyrA protein, whereas the binding of aminocaumarin moiety remains essentially the same [14]. This signifies our results as in native tetrameric gyrA there is a high probability of the retention of the integrity of the aminocaumarin binding pocket, where all our lead molecules are apparently binding. Moreover, as the aminocaumarin moiety of SD8 interacted with Lys42, His45, Arg91, and Ser172, the leads molecules identified in our study also interacted with at least two to all four of these residues along with other crucial residues, signifying the distinction of our study.
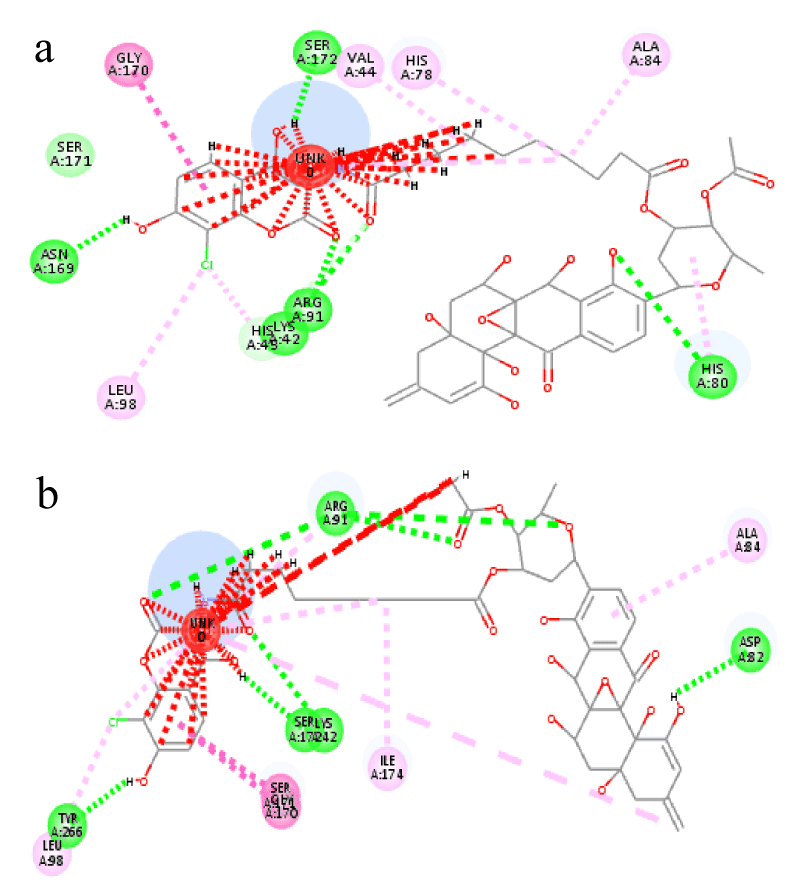
In comparison to the docking score of the reference ligand SD8 which is calculated to be -15.16 using Flex-X, lead molecules identified in our study demonstrated much lower scores and hence stable docking. The not so good docking score with the reference ligand could be attributed to the stearic bumps observed when we analyzed the co-crystallized structure 4CKL and the top scored docking pose we obtained with Flex-X (Figure 4a and b respectively). Recently in line with our results, Fois, et al. [27] demonstrated the efficacy of the hybrid molecule of DNA gyrase B (GyrB) inhibitor and ciprofloxacin against E. coli. They speculated that conjugation with ciprofloxacin facilitates the access of non-permeating GyrB inhibitors into the bacterial cells, facilitating their interaction with the fluoroquinolone binding site in the GyrA [27].
Further, to target ESBL-Ec, which are resistant to most of the generations of cephalosporins by virtue of production of ESBL, that can cleave the six-carbon beta-lactam ring in cephalosporins, we suggest detailed QSAR analysis of the selected leads. The leads can be optimized by fragment building and/or replacement to enhance their bioactivity and resistance to cleavage by ESBL. The addition of active groups from cephalosporins to non-cephalosporin leads, and also to the fluoroquinolones and vice versa to create a new library of chemically synthesizable molecules, and validation of these hybrid molecules will possibly lead to a newer class of gyrA inhibitors. Alternatively, simple in vitro validation using antibiotic sensitivity assays could be planned for different combinations of lead drugs to formulate newer concoctions in a pursuit to combat the drug-resistant E. coli.
CADD in conjunction with drug repurposing has already resulted in the worthwhile possibility of second medical use for many drugs, as is quite evident from the trends towards the discovery of effective therapeutics to SARS-CoV-2 to curtail the ongoing COVID-19 pandemic (reviewed by Singh and Gupta, unpublished) [28]. In this research, 12 repurposed drugs with reasonably good binding scores are reported. Out of these, six are cephalosporins and the rest are rather simple molecules like the antibiotic azlocillin, tetrahydrofolate, ATP, 1,4-dihydroNAD, and leucal. Hence, this study has widened the scope of developing promising leads in significantly less time and cost for the most part anti E. coli compounds. Accurate in vitro studies could be planned in the future based on CADD studies and their outcome may be expected to be fruitful to mankind.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors but we would like to acknowledge research grant No. RLA-202 (2013-14) received under the innovation project scheme of the University of Delhi for the purchase of docking software and other utilities. We acknowledge Ram Lal Anand College for providing all the support required in carrying out this work.
- Mellata M. Human and avian extraintestinal pathogenic Escherichia coli: infections, zoonotic risks, and antibiotic resistance trends. Foodborne Pathog Dis. 2013 Nov;10(11):916-32. doi: 10.1089/fpd.2013.1533. Epub 2013 Aug 20. PMID: 23962019; PMCID: PMC3865812.
- Alanazi MQ, Alqahtani FY, Aleanizy FS. An evaluation of E. coli in urinary tract infection in emergency department at KAMC in Riyadh, Saudi Arabia: retrospective study. Ann Clin Microbiol Antimicrob. 2018 Feb 9;17(1):3. doi: 10.1186/s12941-018-0255-z. PMID: 29422058; PMCID: PMC5806437.
- Hernaiz-Leonardo JC, Golzarri MF, Cornejo-Juárez P, Volkow P, Velázquez C, Ostrosky-Frid M, Vilar-Compte D. Microbiology of surgical site infections in patients with cancer: A 7-year review. Am J Infect Control. 2017 Jul 1;45(7):761-766. doi: 10.1016/j.ajic.2017.02.023. Epub 2017 Apr 3. PMID: 28385464.
- Xiao T, Chen LP, Liu H, Xie S, Luo Y, Wu DC. The Analysis of Etiology and Risk Factors for 192 Cases of Neonatal Sepsis. Biomed Res Int. 2017;2017:8617076. doi: 10.1155/2017/8617076. Epub 2017 Jul 3. PMID: 28758124; PMCID: PMC5512054.
- Pandey N, Cascella M. Beta Lactam Antibiotics. In: Stat Pearls. Treasure Island (FL): Star Pearls Publishing; 2020.
- Bassetti M, Poulakou G, Ruppe E, Bouza E, Van Hal SJ, Brink A. Antimicrobial resistance in the next 30 years, humankind, bugs and drugs: a visionary approach. Intensive Care Med. 2017 Oct;43(10):1464-1475. doi: 10.1007/s00134-017-4878-x. Epub 2017 Jul 21. PMID: 28733718.
- McDanel J, Schweizer M, Crabb V, Nelson R, Samore M, Khader K, Blevins AE, Diekema D, Chiang HY, Nair R, Perencevich E. Incidence of Extended-Spectrum β-Lactamase (ESBL)-Producing Escherichia coli and Klebsiella Infections in the United States: A Systematic Literature Review. Infect Control Hosp Epidemiol. 2017 Oct;38(10):1209-1215. doi: 10.1017/ice.2017.156. Epub 2017 Jul 31. PMID: 28758612.
- Pitout JD, DeVinney R. Escherichia coli ST131: a multidrug-resistant clone primed for global domination. F1000Res. 2017 Feb 28;6:F1000 Faculty Rev-195. doi: 10.12688/f1000research.10609.1. PMID: 28344773; PMCID: PMC5333602.
- Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, Walsh TR. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009 Dec;53(12):5046-54. doi: 10.1128/AAC.00774-09. Epub 2009 Sep 21. PMID: 19770275; PMCID: PMC2786356.
- Ribeiro da Cunha B, Fonseca LP, Calado CRC. Antibiotic Discovery: Where Have We Come from, Where Do We Go? Antibiotics (Basel). 2019 Apr 24;8(2):45. doi: 10.3390/antibiotics8020045. PMID: 31022923; PMCID: PMC6627412.
- Goodlet KJ, Nicolau DP, Nailor MD. Ceftolozane/tazobactam and ceftazidime/avibactam for the treatment of complicated intra-abdominal infections. Ther Clin Risk Manag. 2016 Dec 1;12:1811-1826. doi: 10.2147/TCRM.S120811. PMID: 27942218; PMCID: PMC5140030.
- Asadi A, Abdi M, Kouhsari E, Panahi P, Sholeh M, Sadeghifard N, Amiriani T, Ahmadi A, Maleki A, Gholami M. Minocycline, focus on mechanisms of resistance, antibacterial activity, and clinical effectiveness: Back to the future. J Glob Antimicrob Resist. 2020 Sep;22:161-174. doi: 10.1016/j.jgar.2020.01.022. Epub 2020 Feb 12. PMID: 32061815.
- Johura FT, Tasnim J, Barman I, Biswas SR, Jubyda FT, Sultana M, George CM, Camilli A, Seed KD, Ahmed N, Alam M. Colistin-resistant Escherichia coli carrying mcr-1 in food, water, hand rinse, and healthy human gut in Bangladesh. Gut Pathog. 2020 Jan 27;12:5. doi: 10.1186/s13099-020-0345-2. PMID: 32002025; PMCID: PMC6986151.
- Hearnshaw SJ, Edwards MJ, Stevenson CE, Lawson DM, Maxwell A. A new crystal structure of the bifunctional antibiotic simocyclinone D8 bound to DNA gyrase gives fresh insight into the mechanism of inhibition. J Mol Biol. 2014 May 15;426(10):2023-33. doi: 10.1016/j.jmb.2014.02.017. Epub 2014 Mar 1. PMID: 24594357; PMCID: PMC4018983.
- Vanden Broeck A, Lotz C, Ortiz J, Lamour V. Cryo-EM structure of the complete E. coli DNA gyrase nucleoprotein complex. Nat Commun. 2019 Oct 30;10(1):4935. doi: 10.1038/s41467-019-12914-y. PMID: 31666516; PMCID: PMC6821735.
- Priyanka, Singh V, Ekta, Katiyar D. Synthesis, antimicrobial, cytotoxic and E. coli DNA gyrase inhibitory activities of coumarinyl amino alcohols. Bioorg Chem. 2017 Apr;71:120-127. doi: 10.1016/j.bioorg.2017.01.019. Epub 2017 Feb 1. PMID: 28196603.
- Tomašič T, Barančoková M, Zidar N, Ilaš J, Tammela P, Kikelj D. Design, synthesis, and biological evaluation of 1-ethyl-3-(thiazol-2-yl)urea derivatives as Escherichia coli DNA gyrase inhibitors. Arch Pharm (Weinheim). 2018 Jan;351(1). doi: 10.1002/ardp.201700333. Epub 2017 Dec 14. PMID: 29239018.
- Towle TR, Kulkarni CA, Oppegard LM, Williams BP, Picha TA, Hiasa H, Kerns RJ. Design, synthesis, and evaluation of novel N-1 fluoroquinolone derivatives: Probing for binding contact with the active site tyrosine of gyrase. Bioorg Med Chem Lett. 2018 Jun 1;28(10):1903-1910. doi: 10.1016/j.bmcl.2018.03.085. Epub 2018 Mar 30. PMID: 29661533; PMCID: PMC5938125.
- Lamut A, Skok Ž, Barančoková M, Gutierrez LJ, Cruz CD, Tammela P, Draskovits G, Szili PÉ, Nyerges Á, Pál C, Molek P, Bratkovič T, Ilaš J, Zidar N, Zega A, Enriz RD, Kikelj D, Tomašič T. Second-generation 4,5,6,7-tetrahydrobenzo[d]thiazoles as novel DNA gyrase inhibitors. Future Med Chem. 2020 Feb;12(4):277-297. doi: 10.4155/fmc-2019-0127. Epub 2020 Feb 11. PMID: 32043377.
- Yonezawa M, Takahata M, Banzawa N, Matsubara N, Watanabe Y, Narita H. Analysis of the NH2-terminal 83rd amino acid of Escherichia coli GyrA in quinolone-resistance. Microbiol Immunol. 1995;39(4):243-7. doi: 10.1111/j.1348-0421.1995.tb02196.x. PMID: 7651238.
- Barnard FM, Maxwell A. Interaction between DNA gyrase and quinolones: effects of alanine mutations at GyrA subunit residues Ser(83) and Asp(87). Antimicrob Agents Chemother. 2001 Jul;45(7):1994-2000. doi: 10.1128/AAC.45.7.1994-2000.2001. PMID: 11408214; PMCID: PMC90591.
- Mehla K, Ramana J. Structural signature of Ser83Leu and Asp87Asn mutations in DNA gyrase from enterotoxigenic Escherichia coli and impact on quinolone resistance. Gene. 2016 Jan 15;576(1 Pt 1):28-35. doi: 10.1016/j.gene.2015.09.063. Epub 2015 Sep 28. PMID: 26424597.
- Pourahmad Jaktaji R, Mohiti E. Study of Mutations in the DNA gyrase gyrA Gene of Escherichia coli. Iran J Pharm Res. 2010 Winter;9(1):43-8. PMID: 24363705; PMCID: PMC3869551.
- March-Vila E, Pinzi L, Sturm N, Tinivella A, Engkvist O, Chen H, Rastelli G. On the Integration of In Silico Drug Design Methods for Drug Repurposing. Front Pharmacol. 2017 May 23;8:298. doi: 10.3389/fphar.2017.00298. PMID: 28588497; PMCID: PMC5440551.
- Mehla K, Ramana J. Structural signature of Ser83Leu and Asp87Asn mutations in DNA gyrase from enterotoxigenic Escherichia coli and impact on quinolone resistance. Gene. 2016 Jan 15;576(1 Pt 1):28-35. doi: 10.1016/j.gene.2015.09.063. Epub 2015 Sep 28. PMID: 26424597.
- Fang Y, Lu Y, Zang X, Wu T, Qi X, Pan S, Xu X. 3D-QSAR and docking studies of flavonoids as potent Escherichia coli inhibitors. Sci Rep. 2016 Apr 6;6:23634. doi: 10.1038/srep23634. PMID: 27049530; PMCID: PMC4822154.
- Fois B, Skok Ž, Tomašič T, Ilaš J, Zidar N, Zega A, Peterlin Mašič L, Szili P, Draskovits G, Nyerges Á, Pál C, Kikelj D. Dual Escherichia coli DNA Gyrase A and B Inhibitors with Antibacterial Activity. ChemMedChem. 2020 Feb 5;15(3):265-269. doi: 10.1002/cmdc.201900607. Epub 2019 Dec 10. PMID: 31721445.
- Singh A, Gupta V. SARS-CoV-2 Therapeutics: How far do we stand from a remedy? Communicated work.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.