> Biology. 2020 September 30;1(5):192-200. doi: 10.37871/jbres1143.
-
Subject area(s):
- Biomedical Engineering
- Biology
- Biomedical Science
- Biomedicine
- Bioengineering
In vitro Cytotoxicity Studies of Industrially Used Common Nanomaterials on L929 and 3T3 Fibroblast Cells
Madhulika Srikanth1, Waseem S. Khan2, Ramazan Asmatulu1, Heath E. Misak1, Shang-You Yang3 and Eylem Asmatulu1*
2Department of Mechanical Engineering, Fujairah Men’s College, Higher Colleges of Technology, Fujairah, UAE
3Department of Biological Sciences, Wichita State University, Wichita, KS, KS, 67260
- Common nanomaterials
- L929 and 3T3 cells
- Cytotoxicity
- Cell viability
The unique structures and properties of nanomaterials have attracted many engineers and scientists to these resources for different applications, including biomedical, electronics, manufacturing, transportation, energy, and defense. The increasing applications of nanomaterials have also caused some concern among the scientific community about their safety and cytotoxicity. To successfully use nanomaterials in different fields, their interaction with mammalian cells in vitro must be addressed before in vivo experiments can be carried out successfully. In this study, the cytotoxicity values of commonly known nanomaterials, such as 100-ply Carbon Nanotube (CNT) wires, graphene, CNTs, nanoclay, and fullerene, were investigated through in vitro tests on human L929 and mice 3T3 fibroblast cells and compared with each other. The effects of cytotoxicity on both cell types were similar in many ways, but not closely identical due to structural and morphological differences. Compared to mice fibroblast cells, human fibroblast cells have a larger surface area; therefore, the viability values of L929 cells at different dilutions and time durations vary over a larger range. Pristine 100-ply CNT wires were found to be the least cytotoxic, with an average viability of 86.9%, whereas materials with high aspect ratio (e.g., CNTs and graphene) had higher cytotoxicity values due to their potential to pierce through cell membranes.
A number of nanomaterials in the form of nanoparticles, nanotubes, nanofibers, nanowires, nanoflakes, nanorods, nanowhiskers, nanofilms, nanocomposites, and nanospheres have been produced using various methods. The materials used in this type of production can be polymers, metals and alloys, ceramics, composites, and their combinations [1-3]. Industrial applications of these synthetic and natural nanomaterials have exponentially increased in many fields, such as biomedical, cosmetics, electronics, energy, sensor, optics, automobile, aerospace, textile, and oil and gas industries [4-6]. Additionally, the applications of these materials in space, defense, micro and nanomanufacturing (microfluidics and nanofluidics) and Microelectromechanical Systems (MEMSs), and Nanoelectromechanical Systems (NEMSs) have been under extensive consideration in recent times [7].
As the structural size of the material is reduced, their properties are drastically changed (enhanced or reduced) and can potentially affect the health of animals and humans. Nanomaterials can enter the body by inhalation, skin penetration, or injection and ingestion processes, and they have a high potential to interact with intracellular structures and macromolecules for longer periods of time. Nanoparticles can be deposited in the respiratory systems via two major mechanisms: physical translocation and chemical clearance. Therefore, it is imperative to measure the cytotoxicity of these nanosized materials both in vitro and in vivo prior to their production, emission or use in various industrial applications [7-10]. Sato, et al. [8] studied the influence of particle length on cytotoxicity of multi-walled carbon nanotubes against the acute monocytic leukemia cell line THP-1 in vitro and subcutaneous tissue of rats in vivo. They reported that the degree of inflammation of 825-nm CNTs was stronger than that of 220-nm CNTs, since macrophages could envelop 220-nm CNTs more readily than 825-nm CNTs. One study on the cytotoxicity of graphene on bacteria has demonstrated that cell membrane damage to the bacteria caused by the extremely sharp edges of nanowalls is an effective mechanism in bacterial inactivation. Continuous cell lines, such as L929 and 3T3, are generally used for testing the cytotoxic properties of biomaterials because of their reproducible growth and biological response [9,10].
Human exposure to nanomaterials is unstoppable if nanomaterials are more widely used/handled in different locations (e.g., manufacturing, packaging, transportation, storage, handling, and consumption), and as a result, scientific research studies on nanotoxicity/cytotoxicity have been considered worldwide to eliminate the exposure and adverse side effects [1-3]. However, as the number of nanomaterial types and applications continue to increase, research studies to address their potential toxicity will be very difficult for many of the new generations of nanomaterials. In the medical field, nanomaterials are being used in diagnostic and therapeutic tools to detect and treat human diseases. Therefore, human contact with nanomaterials in the medical field is inevitable, and an understanding of their properties and effects on humans is critically important before clinical trials take place [10-14].
The objective of this work was to measure and compare the cytotoxicity of some nanomaterials that are commonly used in various industries, including pristine CNT wires, graphene nanoflakes, CNTs, nanoclays, and fullerenes using in vitro tests on L929 and 3T3 fibroblast cells. An overview has been provided for the in vitro and toxicological assessment of nanomaterials using MTT Assay and an attempt has been made to understand the correlation between the cytotoxicity effect with time and the morphology of the nanomaterials. The novelty of this work is that commonly used nanomaterials were tested at the same test conditions, and for the first time, their cytotoxicity values were determined in order to provide a better comparison. Test results indicate that the size, shape, and structure of nanomaterials are critically important to their toxicity. Also, the fundamental information obtained in this study will improve the materials-selection process for future industrial applications.
Materials
Cultured human L929 and mice 3T3 fibroblast cells were provided by the Department of Biological Science at Wichita State University. The nanomaterials used in this study include CNT wires (Nanocomp Technologies, Inc.), graphene nanoflakes (Angstron Materials, Inc.), multi-walled CNTs (SUNANO), nanoclay, (Southern Clay Products) and fullerene (US-Nano). MTT reagent (3-(4, 5-Dimethythiazol-2-yl)-2, 5-diphenyltetrazolium-bromide), trypsin, phosphate buffer saline (PBS), Dulbecco’s Modified Eagle Medium (DMEM), Sodium Dodecyl Sulfate (SDS), and Evans blue dye were purchased from Sigma-Aldrich. These materials were used in the experiments without further modifications and alterations.
Methods
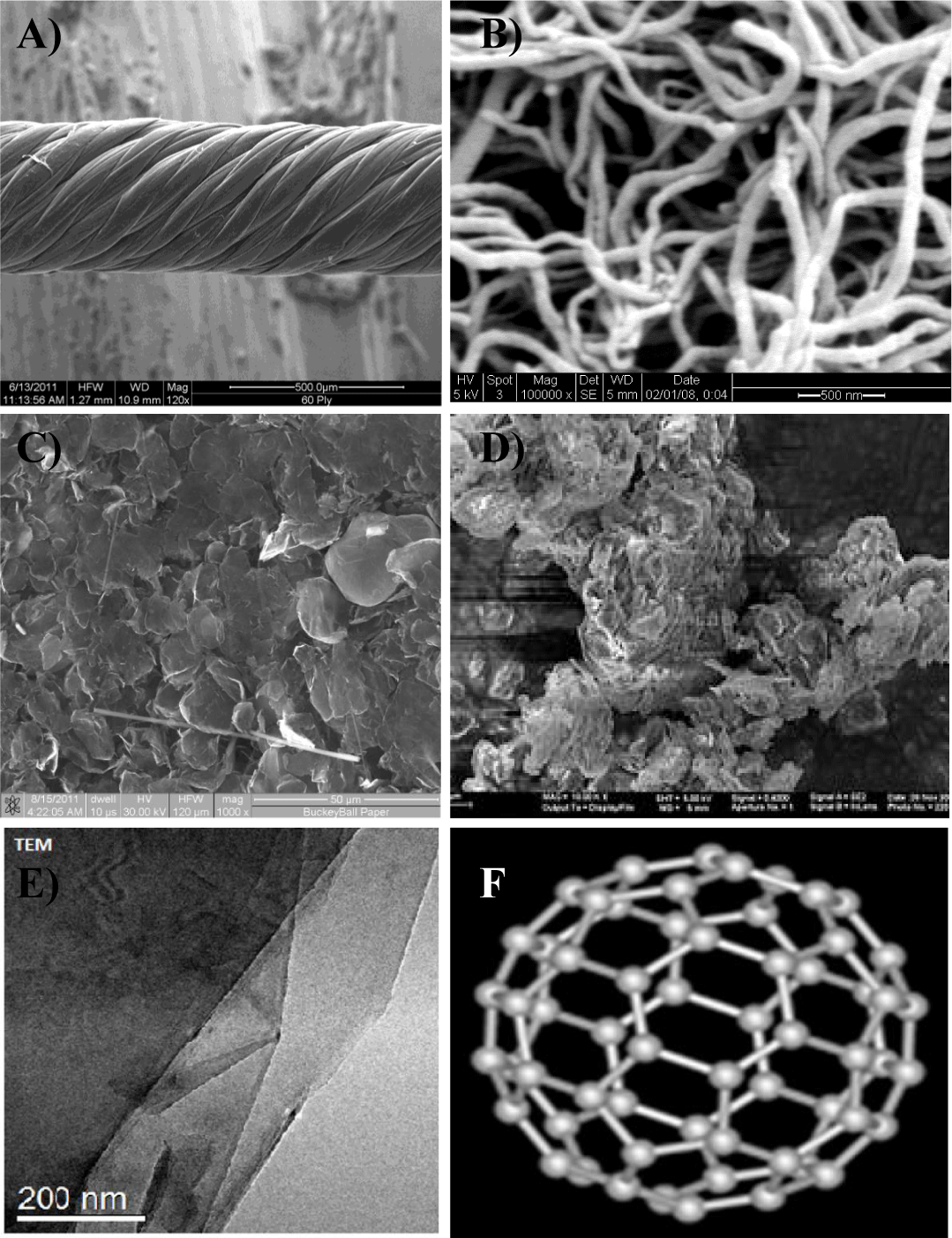
The cytotoxicity testing method used in this study was the MTT assay protocol [10] because this method is technologically proven and more sensitive than other methods, and non-harmful substances can be used with repeatable test results under the same test conditions. The cytotoxicity assay tests were conducted on five types of nanomaterials: pristine 100-ply CNT wires (413 nm diameter), graphene nanoflake powder (<10 nm in thickness), MWCNTs (10–20 nm diameter, 1–2 µm length, and > 99% pure), nanoclay (chemically functionalized Cloisite 30B), and fullerene (99.5% pure with a diameter of a few nm). Figure 1 shows Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), and schematic images of nanomaterials utilized in this cytotoxicity study. As can be noticed, all the sizes, shapes and structures of the selected nanomaterials are mainly different. The amount of material used per assay was according to the standard specifications for cytotoxicity testing [10]. The prepared material was mixed thoroughly into DMEM (medium) and Sodium Dodecyl Sulfate (SDS) using a vortex mixer, and then stored at 37oC in an incubator until further use. All selected fibroblast cells are compatible with the medium, but incompatible with SDS solutions at different concentrations. In other words, the SDS medium provides the lowest cell viability rates when compared to other prepared samples. Different specimen concentrations in the cell growth medium were prepared for each test sample. The contact surface per ml of DMEM was 6 cm2/ml, and for the powder materials, this was 10 mg/ml of DMEM [10]. The exposure time was varied from 1 to 10 days, and the concentration of the specimen in the cell growth medium was reduced from 1:1 to 1:128. All specimens were stored in a 96-well plate for further analysis. Table 1 shows the dilution rates and exposure times of the samples for the cytotoxicity studies.
Table 1: Dilution rates and exposure times of the samples in 96-well plate. |
|||||||||||||
| Samples |
|
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| Day 0-3 | A | 1:1 | 1:2 | 1:4 | 1:8 | 1:16 | 1:32 | 1:64 | 1:128 | SDS 1:1 | SDS 1:1 | Medium | Medium |
| Duplicate | B | 1:1 | 1:2 | 1:4 | 1:8 | 1:16 | 1:32 | 1:64 | 1:128 | SDS 1:2 | SDS 1:2 | Medium | Medium |
| Day 3-5 | C | 1:1 | 1:2 | 1:4 | 1:8 | 1:16 | 1:32 | 1:64 | 1:128 | SDS 1:4 | SDS 1:4 | Medium | Medium |
| Duplicate | D | 1:1 | 1:2 | 1:4 | 1:8 | 1:16 | 1:32 | 1:64 | 1:128 | SDS 1:8 | SDS 1:8 | Medium | Medium |
| Day 5-7 | E | 1:1 | 1:2 | 1:4 | 1:8 | 1:16 | 1:32 | 1:64 | 1:128 | SDS 1:16 | SDS 1:16 | Medium | Medium |
| Duplicate | F | 1:1 | 1:2 | 1:4 | 1:8 | 1:16 | 1:32 | 1:64 | 1:128 | SDS 1:32 | SDS 1:32 | Medium | Medium |
| Day 7-10 | G | 1:1 | 1:2 | 1:4 | 1:8 | 1:16 | 1:32 | 1:64 | 1:128 | SDS 1:64 | SDS 1:64 | Medium | Medium |
| Duplicate | H | 1:1 | 1:2 | 1:4 | 1:8 | 1:16 | 1:32 | 1:64 | 1:128 | SDS 1:128 | SDS 1:128 | Medium | Medium |
The MTT assay is a colorimetric assay method frequently employed for assessing cell metabolic activity and behavior under specific test conditions. MTT involves a yellow tetrazolium salt, which is metabolized by mitochondrial succinic dehydrogenase activity of proliferating cells to yield a purple formazan reaction product [10]. Six hours after it is introduced, the solution in the well plates turns to various shades of blue and purple. The plates are then read by measuring the optical density (absorbance) using a spectrophotometer (microtiter plate reader). Optical density is a logarithmic ratio of the radiation falling upon a material to the radiation transmitted through a material. The wavelength used in these tests was 590 nm (optical density OD 590). The deeper the blue color, the higher the absorbance. Similarly, the lighter the color, the higher the transmittance and the lower the value of absorbance. These absorbance values were taken periodically, and the graphs plotted to obtain a relative measure of cell viability values. All the experimental conditions were tested at the same conditions for all the selected cells and nanomaterials for a better comparison. Cell viability graphs (in percent) were plotted from the readings obtained from the spectrophotometer tests. At least, five experiments were conducted on each nanomaterial, and test readings were averaged. When needed, some of the experiments were repeated until reliable and reproducible data were obtained. Usually, a cell viability over 70% (in the safe side 80% or more) is acceptable for many cytotoxicity tests [10].
L929 cell viability of nanomaterials
Several tests were performed on different nanomaterials to determine the effects of their cytotoxicity and the cell viability values. The preliminary test results were compared with the cell growth medium and SDS at different dilution rates from 1:1 to 1:128 for each cell as a function of time duration. Cell viability tests were carried out on L929 fibroblast cells with the addition of CNT wires, graphene, CNTs, nanoclay, and fullerene, due to their current consumptions and enormous potential for several different industrially applications [15-19]. These selected nanomaterials must be tested for future studies and applications. The medium provides the highest viability values (about 100%), while the SDS solutions show the lowest viability (closer to 0%) at higher concentrations. The medium shows many visible L929 cells; however, the SDS solutions provide very few (or none) visible L929 cells at higher concentrations. Table 2 gives the OD 590 readings of 100-ply CNT wire, graphene nanoflakes, CNTs, nanoclay, and fullerene on L929 cells at different dilution rates and time durations.
Table 2: OD 590 readings of 100-ply CNT wire, graphene, CNTs, nanoclay, and fullerene on L929 cells at different dilution rates and times. |
|||||||||
| OD 590 | L929 | 1:1 | 1:2 | 1:4 | 1:8 | 1:16 | 1:32 | 1:64 | 1:128 |
| 100-Ply CNT Wire | Day 3 | 0.69 | 0.75 | 0.78 | 0.78 | 0.75 | 0.89 | 0.80 | 0.81 |
|
Day 5 | 0.58 | 0.52 | 0.56 | 0.65 | 0.69 | 0.73 | 0.75 | 0.68 |
|
Day 7 | 0.51 | 0.51 | 0.56 | 0.60 | 0.60 | 0.60 | 0.67 | 0.66 |
|
Day 10 | 0.49 | 0.53 | 0.55 | 0.61 | 0.60 | 0.62 | 0.70 | 0.64 |
|
SDS | 0.04 | 0.04 | 0.04 | 0.04 | 0.90 | 0.90 | 0.86 | 0.87 |
|
Medium | 0.93 | 0.94 | 0.84 | 0.74 | 0.72 | 0.68 | 0.73 | 0.70 |
| Graphene | Day 3 | 0.04 | 0.04 | 0.74 | 0.83 | 0.97 | 1.02 | 0.85 | 0.86 |
|
Day 5 | 0.40 | 0.58 | 0.74 | 0.87 | 0.88 | 0.92 | 0.82 | 0.80 |
|
Day 7 | 0.48 | 0.61 | 0.65 | 0.69 | 0.68 | 0.69 | 0.86 | 0.75 |
|
Day 10 | 0.53 | 0.67 | 0.74 | 0.78 | 0.78 | 0.76 | 0.85 | 0.84 |
|
SDS | 0.04 | 0.04 | 0.04 | 0.04 | 0.58 | 0.58 | 1.04 | 1.09 |
|
Medium | 1.04 | 1.04 | 0.95 | 0.98 | 0.94 | 0.89 | 0.95 | 1.06 |
| Carbon Nanotubes | Day 3 | 0.50 | 0.60 | 0.62 | 0.70 | 0.81 | 0.79 | 0.87 | 0.90 |
|
Day 5 | 0.50 | 0.61 | 0.66 | 0.76 | 0.81 | 0.91 | 1.04 | 1.11 |
|
Day 7 | 0.51 | 0.53 | 0.77 | 0.66 | 0.70 | 0.71 | 0.80 | 0.66 |
|
Day 10 | 0.54 | 0.59 | 0.59 | 0.60 | 0.65 | 0.72 | 0.74 | 0.80 |
|
SDS | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 1.28 | 1.04 | 1.13 |
|
Medium | 1.11 | 1.07 | 0.96 | 1.00 | 0.95 | 1.00 | 0.93 | 1.12 |
| Nanoclay | Day 1 | 0.04 | 0.30 | 0.69 | 1.07 | 1.31 | 1.31 | 1.42 | 1.38 |
|
Day 3 | 0.04 | 0.24 | 0.59 | 0.93 | 1.26 | 1.36 | 1.42 | 1.33 |
|
Day 5 | 0.04 | 0.33 | 0.71 | 1.13 | 1.37 | 1.44 | 1.46 | 1.32 |
|
Day 7 | 0.05 | 0.28 | 0.57 | 0.83 | 1.10 | 1.22 | 1.37 | 1.34 |
|
SDS | 0.04 | 0.04 | 0.04 | 0.04 | 0.65 | 0.65 | 1.06 | 1.56 |
|
Medium | 1.48 | 1.50 | 1.40 | 1.49 | 1.42 | 1.41 | 1.54 | 1.63 |
| Fullerene | Day 1 | 0.98 | 1.02 | 1.22 | 1.29 | 1.40 | 1.44 | 1.48 | 1.39 |
|
Day 3 | 1.61 | 1.51 | 1.25 | 1.50 | 1.66 | 1.45 | 1.26 | 1.37 |
|
Day 5 | 1.30 | 0.80 | 1.01 | 1.09 | 1.12 | 1.35 | 1.64 | 1.51 |
|
Day 7 | 0.99 | 0.88 | 0.97 | 1.13 | 1.10 | 1.31 | 1.32 | 1.16 |
|
SDS | 0.045 | 0.046 | 0.038 | 0.040 | 0.65 | 0.65 | 1.06 | 1.46 |
|
Medium | 1.682 | 1.726 | 1.521 | 1.694 | 1.530 | 1.594 | 1.597 | 1.834 |
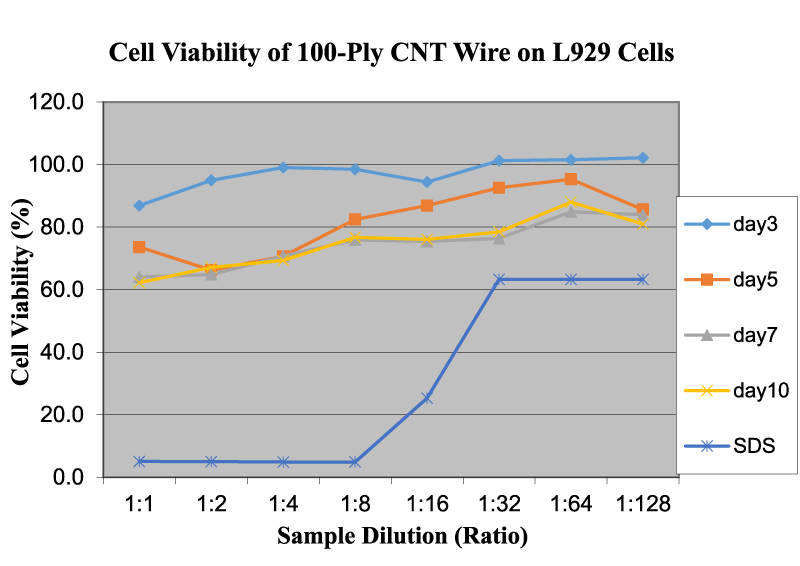
Figure 2 shows the cell viability of 100-ply CNT wire on L929 cells as a function of sample dilution and time. As shown, the OD readings of 100-ply CNT wire increase only slightly but remain almost the same with the increase in dilution rate. However, with an increase in time duration, the OD readings decrease, which indicates that the cytotoxicity also increases to some degree. The average OD readings on day 3 are higher than the readings on day 10. This can be attributed to the fact that CNT wire becomes weaker/flimsy in the liquid medium and begins to disperse in the prepared medium solution. Our study clearly indicated that individual CNTs might be more toxic than the bundled CNTs in CNT wires.
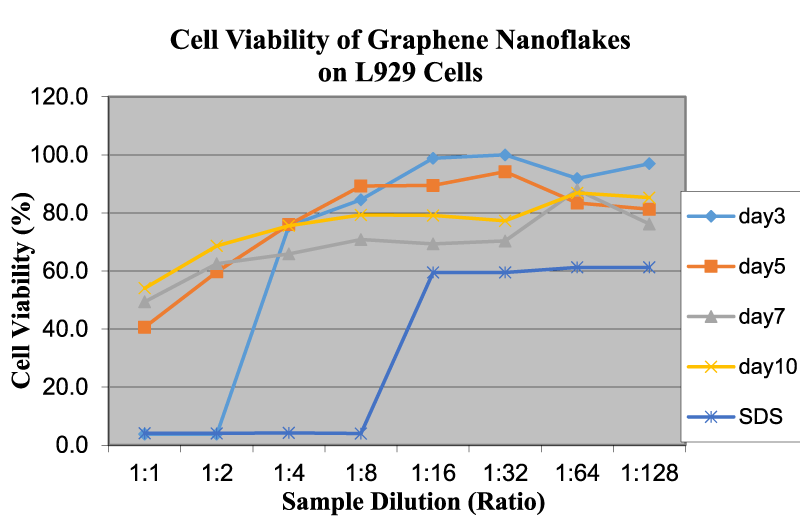
Figure 3 shows the cell viability of graphene nanoflakes on L929 cells as a function of sample dilution and time. As far as graphene is concerned, at higher concentrations, the OD readings of these nanoflakes are considerably low, which may indicate the high cytotoxicity. The OD readings for graphene increase with the increase in dilution rate. Graphene is compatible with L929 cells below a concentration of 0.58 mg/ml. The effect of time duration at a 1:1 concentration is almost the same as with other cells mentioned in previous studies [4,10]. The viability of graphene increases as the time duration increases. Liao, et al. [11] demonstrated that at the smallest size, graphene oxide exhibited the greatest hemolytic activity, whereas aggregated graphene sheets exhibited the lowest hemolytic activity. Our studies are agreed well with this funding using different cells and nanomaterial structures.
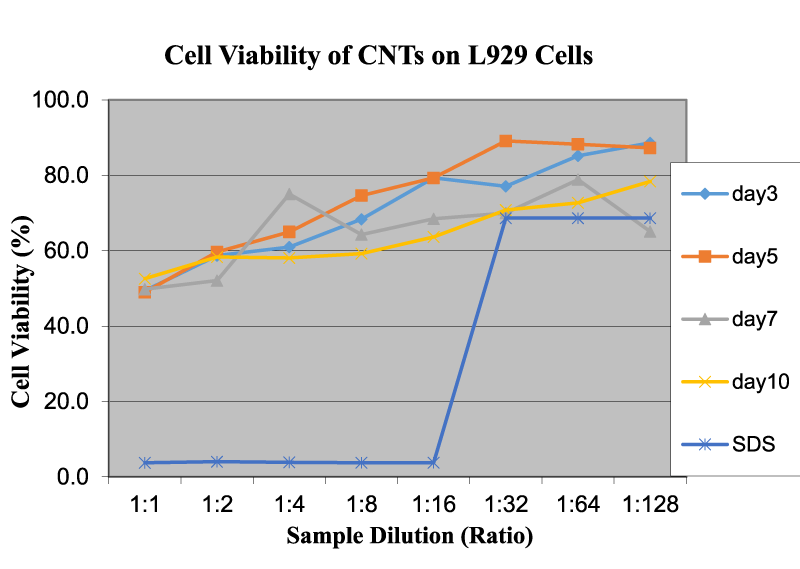
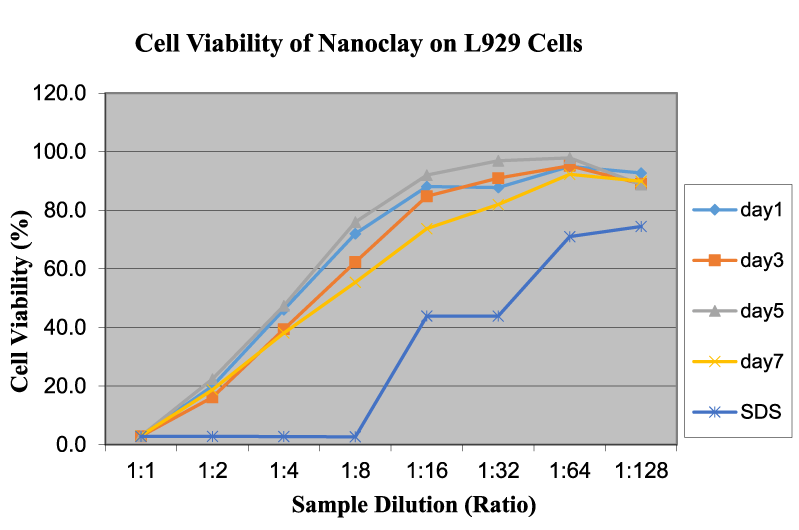
Figure 4 reveals the cell viability of CNTs on L929 cells as a function of sample dilution and time. In this CNT test, OD readings increase with the increase in dilution rate of the solution/dispersion. The cytotoxicity is less at lower concentrations and increases as dilution increases. The time duration shows a mixed trend on the toxicity value. Bottini et al. demonstrated that hydrophobic pristine CNTs were less toxic when compared to oxidized CNTs [12]. The increased toxicity of oxidized CNTs could be due to better dispersion in an aqueous solution, thereby reaching higher concentrations of free CNTs at similar weight-per-volume values. Test results closely agreed with our experiments and approaches although the authors used different cells and experimental procedures. Figure 5 exhibits the cell viability of nanoclay on L929 cells as a function of sample dilution and time. The cell viability is close to 50% at a 1:1 dilution and increases to around 90% at 1:32 and 11:64 dilution levels. This indicates that nanoclay may be used in the vicinity of live cells over a dilution of 0.58 mg/ml. The time duration does not show any permanent trend or significant changes, and there is no notable difference between the curves for days 1, 2, 5, and 7. This behavior is similar to graphene and other related nanomaterials. Nanoclay possess a high aspect ratio, in which the thickness of the platelet is approximately 1 nm and surface dimensions are around 300–600 nm [10]. Because of this feature, nanoclay can slice through healthy live cell membranes and may destroy them in the long term at higher concentrations. Brownian motion can be effective for cutting the cell membrane and for cell damage and leakage [4]. Additionally, nanoclay is highly hydrophilic, so it can disperse readily in the medium and easily interact with cell membranes to slow down or stop their functions [4]. The effect of time duration is similar to that in the graphene tests. The behavior and potency of nanoclay is very similar to graphene, both of which have layered structures. The high cytotoxicity could also be assigned to high surface area, sharp edges, color of solution, and powdery structures with various sizes and shapes.
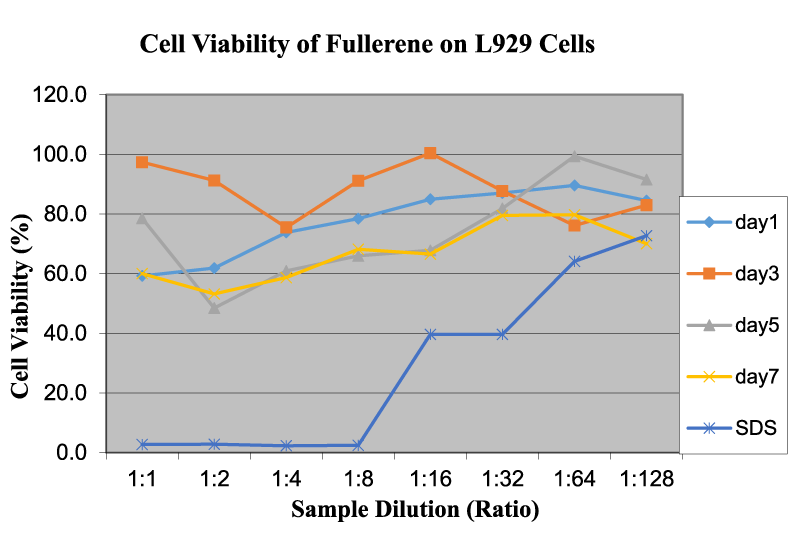
Figure 6 reveals the cell viability of fullerene on L929 cells as a function of sample dilution and time. Test results indicate that fullerene displays a wide range of OD readings. There is a small increase in OD readings with an increase in the dispersion dilution. However, this increase in OD readings is not very significant and could be attributed to the fact that on a macroscopic scale fullerene is present in clusters almost immiscible in the cell medium, which makes it difficult to control precisely the concentration levels. Due to the very low solubility of fullerene and negative surface charges, it is difficult to keep dispersed fullerene in the medium for a prolonged period of time in order to obtain a reasonable relationship between OD readings and time duration. Also, fullerenes become clustered through van der Walls interaction and surface hydrophobicity, thereby acting like larger particles. This behavior of the fullerene structure is synchronous with other studies conducted in the same field [15]. Partha, et al. [15] studied that the low cytotoxicity of fullerene can be attributed to its chemical nature. Viability is over a safe range (> 80%), beyond 1:8 dilution, for example below 1.10 mg/ml. Jia, et al. [13]. performed cytotoxicity tests on single-walled nanotubes, multi-walled nanotubes, and fullerene and observed profound cytotoxicity in single-walled nanotubes in an alveolar macrophage. No profound toxicity was noticed for C60 up to a dose of 226.00 μg/cm2. Our studies using the L929 cells indicated that the smaller sizes with higher surfaces areas might be accumulated together to reduce the overall toxicity of the cells.
3T3 cell viability of selected nanomaterials
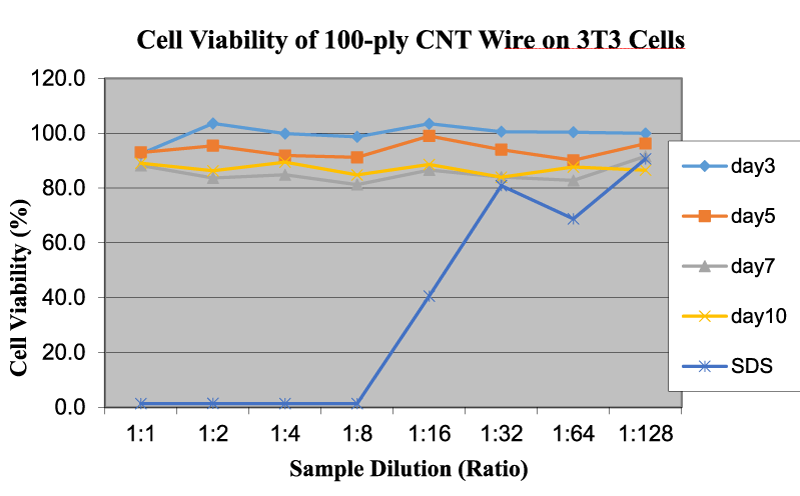
Similar cell viability tests were also carried out on 3T3 cells with the addition of CNT wires, graphene, and CNTs, due to their enormous potential for several different industrial applications. Likewise, the medium provides the highest viability on 3T3 cells, while SDS solutions show the lowest viability, as discussed in the previous section. Table 3 shows the OD590 values for cytotoxicity testing of 100-ply CNT wire, graphene and CNTs on 3T3 cells at various dilution rates and time intervals. The cell viability values of fullerene and nanoclay on 3T3 cells were not added here because of the inconsistences and lack of test results. Figure 7 shows the cell viability of CNT wire on 3T3 cells as a function of sample dilution and time. As can be seen, the higher OD values correlate to a higher viability percentage and lower cytotoxicity, as was observed in the cell viability of L929 cells.
Table 3: OD 590 readings of 100-ply CNT wire, graphene, and CNTs on 3T3 cells at different dilution rates and times. |
|||||||||
| OD 590 | 3T3 | 1:1 | 1:2 | 1:4 | 1:8 | 1:16 | 1:32 | 1:64 | 1:128 |
| 100-Ply CNT Wire | Day 3 | 2.63 | 2.94 | 2.84 | 2.80 | 2.94 | 2.86 | 2.85 | 2.84 |
|
Day 5 | 2.64 | 2.71 | 2.61 | 2.59 | 2.81 | 2.67 | 2.56 | 2.73 |
|
Day 7 | 2.51 | 2.38 | 2.41 | 2.31 | 2.46 | 2.39 | 2.35 | 2.60 |
|
Day 10 | 2.53 | 2.45 | 2.54 | 2.41 | 2.52 | 2.38 | 2.49 | 2.46 |
|
SDS | 0.04 | 0.04 | 0.04 | 0.04 | 1.15 | 2.30 | 1.95 | 2.57 |
|
Medium | 2.93 | 2.98 | 2.79 | 2.80 | 2.99 | 3.09 | 2.26 | 2.91 |
| Graphene | Day 3 | 0.04 | 0.04 | 1.04 | 1.59 | 1.93 | 2.26 | 2.37 | 2.42 |
|
Day 5 | 0.13 | 1.33 | 1.75 | 2.15 | 2.23 | 2.54 | 2.94 | 3.01 |
|
Day 7 | 0.85 | 1.54 | 1.84 | 2.15 | 2.41 | 2.71 | 2.89 | 2.81 |
|
Day 10 | 1.00 | 1.52 | 1.79 | 2.03 | 2.25 | 2.17 | 2.62 | 2.48 |
|
SDS | 0.04 | 0.04 | 0.04 | 0.04 | 0.53 | 2.29 | 2.35 | 2.28 |
|
Medium | 2.66 | 3.05 | 3.03 | 3.18 | 3.23 | 3.26 | 3.23 | 2.63 |
| Carbon Nanotubes | Day 3 | 1.29 | 1.44 | 1.68 | 1.88 | 1.96 | 2.21 | 2.26 | 2.57 |
|
Day 5 | 1.13 | 1.70 | 1.86 | 2.26 | 2.11 | 2.87 | 2.83 | 3.09 |
|
Day 7 | 1.17 | 1.39 | 1.88 | 2.11 | 1.70 | 2.58 | 2.71 | 3.06 |
|
Day 10 | 1.27 | 1.50 | 1.43 | 1.69 | 2.21 | 2.29 | 2.38 | 2.53 |
|
SDS | 0.04 | 0.04 | 0.04 | 0.04 | 1.05 | 2.73 | 2.41 | 2.23 |
|
Medium | 2.60 | 3.37 | 2.97 | 2.61 | 2.86 | 2.92 | 3.02 | 2.46 |
For 100-ply CNT wire, the OD values are almost the same and do not change with an increase in dilution rate. With the increase in exposure time, the viability is reduced marginally, but remains higher than 80% at any time, which is sufficient to claim good cell viability. Microscopically, CNT nanowires are tightly wound structures of CNTs. With increased exposure time, the structural integrity of the wire might decrease, whereby some nanotubes could be potentially introduced into the medium and act as individual CNTs instead of a bundle structure. In turn, this could lead to an increase in the cytotoxicity. The overall viability of the nanowire is approximately 87%, which makes it compatible with the 3T3 cells. Comparable test results were also observed in L929 and other cells using the similar nanomaterials.
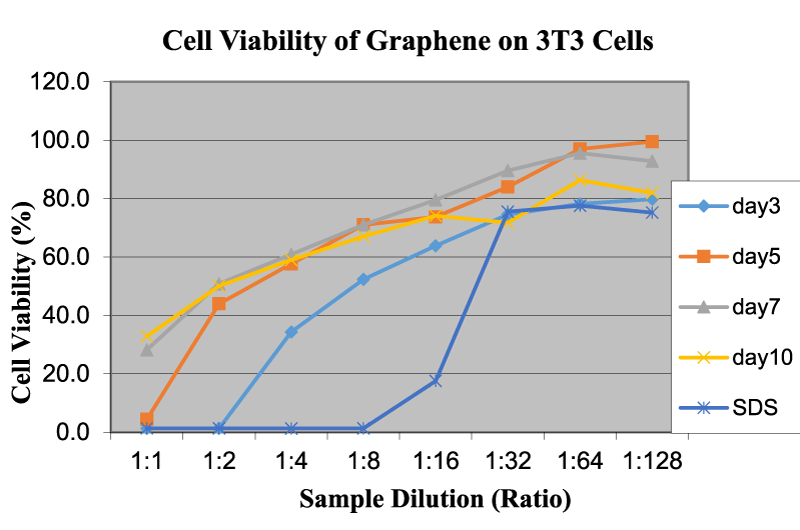
The same tests were conducted on graphene nanoflakes, which showed a different trend. Figure 8 reveals the cell viability of graphene on 3T3 cells as a function of sample dilution and time. Graphene showed very low OD (low viability percent) values at the initial concentration. In this sample of graphene, the percentage of a single layer is significantly high, which makes the specimen more impactful from a morphological standpoint, because its layers are capable of slicing through the cell membrane and structure at that scale. As the concentration is reduced by dilution, the cytotoxicity level of graphene is also reduced significantly. Generally, the percentage of a single layer present in graphene is approximately 80%; therefore, contact with the medium is very close. At a 1:16 dissolution level and above, the viability levels are at about 80% and above. This means that the concentration of graphene must be at a maximum of 0.58 mg/ml to be considered safe use for future studies. The present study also closely agreed well with other published articles in the same field [4,10,25,26].
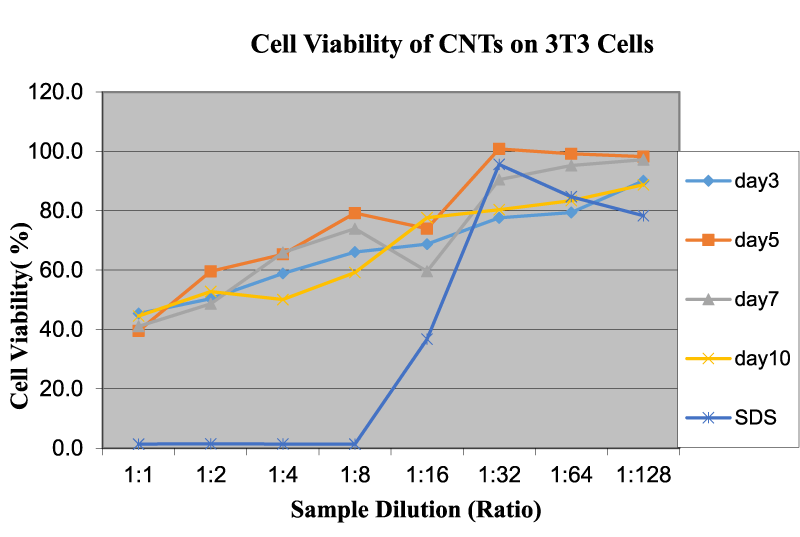
Figure 9 shows the OD 590 readings of CNTs on 3T3 cells at different dilution rates and time intervals. CNTs displayed intermediate OD values, which means that their viability is lower than CNT nanowires and higher than graphene. CNTs have a tube-like structure with sharp tips; therefore, the surface interaction with the cell-medium is less than that of graphene (slicing effects), which has a higher surface area and sharper edges. The general trend of the OD values (viability percent) increased with the increase in dilution. The exposure time does not seem to have a significant impact on the nominal values of the cell viability. Authors have stated that nanomaterials with sharp edges and tips could have more risk for cytotoxicity than their counterparts [4,22].
Asmatulu et al. reported that surface chemistry, surface potential, surface area, and particle size are dominant factors in the toxicity of many nanomaterials [1,4,16]. Since the properties of nanomaterials are different, their toxicity will also be different. Some studies on toxicity have also illustrated that particle size less than 100 nm induces toxicity in many cell-cultured human and animal cell models [16-19]. CNTs possess hydrophobic properties, and as a result, their level of cytotoxicity is generally lower than that of graphene and other layered materials with different oxidation levels and properties. The shape and size of carbon-based nanomaterials also play an important role in determining their cytotoxicity. Karakoti, et al. [21] and O’Brien and Cummins [22] reported that smaller particles diffuse faster into cells thereby causing cell membrane damages, in comparison to larger cells. This effect has also been observed in this study with graphene and nanoclay layered materials. This effect was more apparent in graphene due to its wide range of particle distributions. The recorded cytotoxicity of graphene was the highest on day 1 and the lowest on day 10, for both 3T3 and L929 cell cultures. Overall, nanoflakes and sharp-tip structures result in higher cytotoxicity, which may be because of the chemical structure, size, shape, surface area, surface functionality, solution conditions, and oxidative stress levels [20-26]. Thus, more scientific studies are needed to be conducted in vivo to determine the real cytotoxicity of these commonly used industrial nanomaterials.
Imai, et al. [27] examined the differentiation capacity of mouse embryonic stem cells cultured on C60 fullerene and cell viability effects, as well. Their results demonstrated small effects on differentiation assay and no influence on cell viability was observed. Sakai, et al. [28] used fullerene (C60) in cytotoxicity test. According to their studies, C60 was cytotoxic in BALB/3T3 cells under irradiated visible light. C60 acted as an initiating agent for cell transformation but did not act as a complete transforming agent. Uscátegui, et al. [29] evaluated cytotoxicity using the ISO 10993-5 (MTT) method with mouse embryonic fibroblasts L-929 (ATCC® CCL-1) in direct contact with the PUs and with NIH/3T3 cells (ATCC® CRL-1658) in indirect contact with the polyurethanes. Their results demonstrated that the cytotoxicity of the polyurethanes in direct contact with L-929 mouse fibroblasts revealed that the PUs were associated with greater than 70% cellular viability, which indicates that these polyurethanes are suitable for use as biomaterials. Additionally, polymer extracts did not appear to be toxic to NIH/3T3 fibroblasts. To study in vitro cell cytotoxicity of synthesized hydrogel, Banerjee, et al. [30] used a cell proliferation assay against NIH 3T3 fibroblast cells. From the MTT assay results, it was observed that cell proliferation was dependent on the quantity of the OMMT clay. They observed that with the increase in the OMMT content from 5 wt. % to 7 wt. %, cell viability reduced.
The cytotoxicity levels of five different nanomaterials (100-ply CNT wire, graphene nanoflakes, CNTs, nanoclay, and fullerene) were tested on L929 and 3T3 fibroblast cells. The effects of cytotoxicity on both cell types were very similar, but not completely identical. L929 fibroblast cells have a large surface area compared to 3T3 cells, and therefore the viability values of L929 cells at different dilutions and time durations varied over a wider range. In all five nanomaterials, pristine 100-ply carbon nanowire was found to be the least toxic, with an average viability value of 86.9%. The concentration of CNT nanowires that showed this high value of viability (86.9%) was 3 cm2/ml, and it weighed 18.52 mg/ml. The second material that displayed a higher viability value was fullerene, with a viability value of 75.2%. The cell viabilities of other nanomaterials were CNTs at 69.75%, graphene nanoflakes at 67.58%, and nanoclay at 61.34%. The morphology and structure of nanomaterials play a very crucial role in their cytotoxic effect on fibroblast cells. For example, layer and needle-like structures had more distractive effects on the cell membranes because the sharp tips and edges of graphene and nanoclay, and CNTs may cause cell membrane leak and death. A larger surface area and higher aspect ratio increase the cytotoxic effect, whereas a smaller contact surface availability and hydrophilic character reduce the cytotoxicity. This study may be useful for future applications of these commonly used nanomaterials in different industries.
- Asmatulu R, Khan WS. Synthesis and Applications of Electrospun Nanofibers. Elsevier: Amsterdam; 2018. https://bit.ly/3kZSpiL
- Groneberg DA, Giersig M, Welte T, Pison U. Nanoparticle-based diagnosis and therapy. Curr Drug Targets. 2006 Jun;7(6):643-8. doi: 10.2174/138945006777435245. PMID: 16787165.
- Jeong SC, Lee DH, Lee JS. Production and characterization of an anti-angiogenic agent from saccharomyces cerevisiae K-7. Journal of Microbiology and Biotechnology. 2006; 16(12):1904-1911. https://bit.ly/2Gcb8cf
- Asmatulu R. Nanotechnology Safety, Elsevier, Amsterdam; August 2013. https://bit.ly/3jjbCvr
- Wang B, Feng WY, Wang TC, Jia G, Wang M, Shi JW, Zhang F, Zhao YL, Chai ZF. Acute toxicity of nano- and micro-scale zinc powder in healthy adult mice. Toxicol Lett. 2006 Feb 20;161(2):115-23. doi: 10.1016/j.toxlet.2005.08.007. Epub 2005 Sep 13. PMID: 16165331.
- Yin H, Too HP, Chow GM. The effects of particle size and surface coating on the cytotoxicity of nickel ferrite. Biomaterials. 2005 Oct;26(29):5818-26. doi: 10.1016/j.biomaterials.2005.02.036. Epub 2005 Apr 15. PMID: 15949547.
- Patil US, Adireddy S, Jaiswal A, Mandava S, Lee BR, Chrisey DB. In Vitro/In Vivo Toxicity Evaluation and Quantification of Iron Oxide Nanoparticles. Int J Mol Sci. 2015 Oct 15;16(10):24417-50. doi: 10.3390/ijms161024417. PMID: 26501258; PMCID: PMC4632758.
- Sato Y, Yokoyama A, Shibata K, Akimoto Y, Ogino S, Nodasaka Y, Kohgo T, Tamura K, Akasaka T, Uo M, Motomiya K, Jeyadevan B, Ishiguro M, Hatakeyama R, Watari F, Tohji K. Influence of length on cytotoxicity of multi-walled carbon nanotubes against human acute monocytic leukemia cell line THP-1 in vitro and subcutaneous tissue of rats in vivo. Mol Biosyst. 2005 Jul;1(2):176-82. doi: 10.1039/b502429c. Epub 2005 Apr 20. PMID: 16880981.
- Akhavan O, Ghaderi E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano. 2010 Oct 26;4(10):5731-6. doi: 10.1021/nn101390x. PMID: 20925398.
- Srikanth M, In Vitro Cytotoxicity Tests of Nanomaterials on 3T3 and L929 Cells. M.S. Thesis, Wichita State University. May 2012. https://bit.ly/3n3Ilau
- Liao KH, Lin YS, Macosko CW, Haynes CL. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl Mater Interfaces. 2011 Jul;3(7):2607-15. doi: 10.1021/am200428v. Epub 2011 Jun 30. PMID: 21650218.
- Bottini M, Bruckner S, Nika K, Bottini N, Bellucci S, Magrini A, Bergamaschi A, Mustelin T. Multi-walled carbon nanotubes induce T lymphocyte apoptosis. Toxicol Lett. 2006 Jan 5;160(2):121-6. doi: 10.1016/j.toxlet.2005.06.020. Epub 2005 Aug 25. PMID: 16125885.
- Jia G, Wang H, Yan L, Wang X, Pei R, Yan T, Zhao Y, Guo X. Cytotoxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube, and fullerene. Environ Sci Technol. 2005 Mar 1;39(5):1378-83. doi: 10.1021/es048729l. PMID: 15787380.
- Misak HE, Asmatulu R, Gopu JS, Man KP, Zacharias NM, Wooley PH, Yang SY. Albumin-based nanocomposite spheres for advanced drug delivery systems. Biotechnol J. 2014 Jan;9(1):163-70. doi: 10.1002/biot.201300150. PMID: 24106002.
- Partha R, Mitchell LR, Lyon JL, Joshi PP, Conyers JL. Buckysomes: fullerene-based nanocarriers for hydrophobic molecule delivery. ACS Nano. 2008 Sep 23;2(9):1950-8. doi: 10.1021/nn800422k. PMID: 19206436.
- Asmatulu R. Toxicity of Nanomaterials and Recent Developments in Lung Disease. Chapter 6 in Bronchitis. In Tec, ed. P. Zobic; 2011. p. 95-108. https://bit.ly/3ihWcX9
- Asmatulu R, Asmatulu E, Yourdkhani A. Toxicity of Nanomaterials and Recent Developments in the Protection Methods. SAMPE Fall Technical Conference, Wichita, KS; October 19-22, 2009. p. 12. https://bit.ly/3n7nJOm
- Lyakhovich VV, Vavilin VA, Zenkov NK, Menshchikova EB. Active defense under oxidative stress. The antioxidant responsive element. Biochemistry (Mosc). 2006 Sep;71(9):962-74. doi: 10.1134/s0006297906090033. PMID: 17009949.
- Asmatulu R, Asmatulu E, Yourdkhani A. Toxicity of Nanomaterials and Recent Developments in Protection Methods. Proceedings of the Midwest Section Conference of the American Society for Engineering Education, Lawrence KS; 2010. p. 22-24. https://bit.ly/3n7nJO
- N. Nuraje, R. Asmatulu, and G. Mul. Green Photo-Active Nanomaterials: Sustainable Energy and Environmental Remediation. RSC Publishing: Cambridge, England; November 2015. https://rsc.li/3ieoP7C
- Karakoti S, Hench LL, Seal S. The potential toxicity of nanomaterials-the role of surfaces. JOM. 2006;58:77-82. https://bit.ly/2ELQe33
- N. O’Brien and E. Cummins. Nanomaterials: Risks and Benefits. Springer: The Netherlands; 2008. p. 161-178.
- Asmatulu R, Garikapati A, Misak HE, Song Z, Yang SY, Wooley PH. “Cytotoxicity of magnetic nanocomposite spheres for possible drug delivery systems,” ASME International Mechanical Engineering Congress and Exposition. 2012;911-918. doi: 10.1115/IMECE2010-40269
- Sanhes L, Tang R, Delmer A, DeCaprio JA, Ajchenbaum-Cymbalista F. Fludarabine-induced apoptosis of B chronic lymphocytic leukemia cells includes early cleavage of p27kip1 by caspases. Leukemia. 2003 Jun;17(6):1104-11. doi: 10.1038/sj.leu.2402895. PMID: 12764376.
- Li Y, Yuan H, von dem Bussche A, Creighton M, Hurt RH, Kane AB, Gao H. Graphene microsheets enter cells through spontaneous membrane penetration at edge asperities and corner sites. Proc Natl Acad Sci U S A. 2013 Jul 23;110(30):12295-300. doi: 10.1073/pnas.1222276110. Epub 2013 Jul 9. PMID: 23840061; PMCID: PMC3725082.
- Wang Y, Ma X, Wang J, Cheng S, Ren Q, Zhan W, Wang Y. Effects of Mercapto-functionalized Nanosilica on Cd Stabilization and Uptake by Wheat Seedling (Triticum aestivum L.) in an Agricultural Soil. Bull Environ Contam Toxicol. 2019 Dec;103(6):860-864. doi: 10.1007/s00128-019-02729-4. Epub 2019 Oct 11. PMID: 31605159.
- Imai K, Watari F, Nishikawa T, Tataka A, Tanoue A. “An attempt to study of the C60 Fullerence on differentiation of mouse ES cells” J-STaGE/ Nano Biomedicine. 2011;3(2):288-293. doi: 10.11344/nano.3.288
- Sakai A, Yamakoshi Y, Miyata N. Visible light irradiation of [60] fullerene causes killing and initiation of transformation in Balb/3T3 cells. Journal of Fullerenes Science and Technology. 1999;7(5):743-756. doi: 10.1080/10641229909351375
- Uscátegui YL, Arévalo FR, Díaz LE, Cobo MI, Valero MF. Microbial degradation, cytotoxicity and antibacterial activity of polyurethanes based on modified castor oil and polycaprolactone. J Biomater Sci Polym Ed. 2016 Dec;27(18):1860-1879. doi: 10.1080/09205063.2016.1239948. Epub 2016 Oct 11. PMID: 27654066.
- Banerjee SL, Swift T, Hoskins R, Rimmer S, Singha NK. A muscle mimetic polyelectrolyte-nanoclay organic-inorganic hybrid hydrogel: its self-healing, shape-memory and actuation properties. J Mater Chem B. 2019 Mar 7;7(9):1475-1493. doi: 10.1039/c8tb02852d. Epub 2019 Feb 11. PMID: 32255019.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.