> Medicine. 2020 September 28;1(5):175-185. doi: 10.37871/jbres1140.
-
Subject area(s):
- Antiretrovirology
- Virology
- Infectious Diseases
- Antivirology
Biophysical Aspects of Interactions at the Bionanointerface between Viruses and Metal and Metal Oxide Nanomaterials
Lahir YK*
- Bionanointerface
- Metal and metal oxide nanoparticles
- Virus, Surface of a virus
- Uptake of virus by a host cell
- Interface between metal and metal oxide nanoparticle and host cell
Viruses are at the threshold of living and nonliving entities. Virus particles exhibit life-activities when are within their respective hosts and act as non-living when present outside their hosts. This feature is very interesting and the related investigations can help to understand the differences between the functionalities at bionanointerfaces under living and nonliving phases. Metal and metal oxide nanomaterials occur naturally and are synthesized as per the need to meet the set targets. These nanosized materials have specific physicochemical properties such as high volume to area ratio, ability to get functionalized as per the need. These ubiquitous materials have multifaceted applications in almost all fields of sciences, industries, medical, clinical diagnostics, and remedial operations; these occupy an omnipresent status in our day to day life. Since these nanomaterials are a major integral part of industries and human life; these interact with the abiotic and biotic components of the environment. Viruses are the active entities of both these aspects of our environment. The interactions between metal and metal oxide nanomaterials and viruses are obvious and complex interactive phenomena. These complex interactions take place between nanomaterials and viruses within their respective hosts. The profiling of such interactions helps to optimize the resultant impacts and enhances the degree of de novo designing, in vivo, and in vitro performances.
At biointerface, inorganic and organic molecules communicate at the molecular level with biomolecules and biomaterials. The effective biointerface is suitable for biomolecular, cellular modulations, topographic, mechano-and chemo-structural modifications. These mechano-chemical adjustments help the responses of prokaryotic and eukaryotic biosystems that help the ongoing biointeractions [1]. One of the prime reasons for applications of nanomaterials in biological, medical, and pharmacological fields is their dimensions which are quite similar to the biomolecules like nucleic acids, proteins, and many conjugated biomolecules. The interactions between nanomaterials and biomolecules result in the formation of appropriate biointerface between them. Such interfaces have synergetic properties and specific functional aspects. The investigations in these interactions have resulted in the development of an offshoot of applied science and referred to as bioelectrochemistry. This interdisciplinary offshoot focuses on designing and formulating the potential bionanointerfaces that have a higher ability to fathom the intricacies of biological, medical, pharmacological, and nanosciences [2]. The interactions between nanomaterial and biomolecules, cell organelles establish bionanointerfaces and this establishment is related to the colloidal, and biophysicochemical forces. These interactions result in the formation of the corona, wrapping of particles, cellular uptake, biocatalytic function, enzyme technology, tissue engineering and cause either biocompatible or bioadverse outcomes [3]. Nanobiointeractions can affect phase transformations, entropy, and enthalpy, rearrangement of biochemical structural aspects, or dissolution at the site of interaction. Furthermore, these interactions permit the predictions of the relationship between surface and activity that concern with size, shape, surface chemistry, topography, and coating. One of the most essential aspects of interaction at the bionanointerface reflects on the safe or misuse of nanomaterials [4].
Recent investigations related to biointerfaces have shown manifold characteristics that reflect on its diverse modes of interaction in spite of it being a seemingly simple entity. The biomolecules, either interact at a specific site of biosystem or biological surface with alien molecules at biointerface. Biomolecules and the biointerfaces follow the principles of materials science, classical surface science and their functionalities depend on the basic principles of physics, chemistry, biological sciences, molecular science, and biophysics [5].
The biointerface incriminate biomolecules and a specific surface; this surface is the exact location of interaction. Such surfaces are ubiquitous in nature. In recent times, a good number of lab-generated biointerfaces are available for studying the concerning interactions. All these efforts have developed into an interdisciplinary science. This science is paving a path for understanding the intricacies of biointerface science while dealing with biointerface science the characteristics of biomolecules where the concerned surface acts as integral components. Quite a good number of molecules have been synthesized and these are different from the biological molecules. The biomolecules need water for their interaction; they have a relatively larger and fragile molecular structure with sophisticated functionalities and are the products of limited groups of their respective precursors. The ambient environment affects the activity of biomolecules, and it is feasible to understand their mode of activity during their interactions. Biomolecules like proteins show a greater degree of adaptability as per the need and these molecules undergo unfolding, folding, transformations like inactive to active forms [5].
The cell membrane is the prototypical example of a natural design of a dynamic biointerface. The components of cell membrane-like lipid bilayers, receptors, and channels, embedded and attached molecules permit fluctuations, elastoplasticity, functional limits of deformation, and compression. This natural biointerface has limited permeability, charge, surface specificity and it is capable of conducting to and fro inter and intracellular signals. This cellular surface offers a suitable degree of adhesion and cohesion to ensure its structural and functional integrity. There is a pertinent position of receptors and it engages helices of membrane-spanning ensuring optimization of their functions. The membrane-spanning motifs and other embedded components show lateral diffusion with the zone of the cell membrane; as a result, the cell membrane ensures rapid and spatial remodeling as per the requirement. The cell membrane is suitably adapted to act as a time-bound spatial and temporal dynamic system so that the switch on and off mechanism regulates the extra and intracellular signaling system. The application of electrical potential changes the conformation of surface constrained monolayers, like hydrophobic phase and hydrophilic phase; this aspect is applicable in lab-on-chip designs [6]. While investigating cellular and materials components, during the engineering microsystem, there is a need to develop a dynamic substrate that can harmonize the cell which adheres. This aspect plays a significant role in understanding the basic and applied aspects of cell biology. There are strategies like electrical, photochemical, thermal, and mechanical forces, to design the active participation during cell signaling and behavior of adherent cells [7]. The interactive surface molecules show physical, chemical, or electrostatic forces. Some of these forces correspond to the adsorption, covalent bonding, or van der Waals forces [8].
Nanomaterials occur naturally and are synthesized. In nature, these fine materials are the product of combustion, volcanic eruption as volcanic ash, water and soap bubbles, oceanic waves, and water springs as the invisible fine water spray. The nanomaterials are also the product of anthropogenic activities such as forest fire, electric arc, welding process, vehicular exhaust, demolishing old buildings, and domestic dust. Nanomaterials have specific physicochemical properties like high volume to area ratio, ability to get functionalized as per the need, and have at least one dimension within 1-100 nm range. Nanotechnology and nanoscience deal with the formation, characterization, classification, and applications of nanomaterials. These nanomaterials have multifaceted applications in almost all fields of sciences, industries, medical, clinical diagnostics, and remedial operations; these occupy a ubiquitous place in our day to day life. Since the nanomaterials are an integral part of industries and human life, these interact with the biosystems. During these interactions, the biomolecules and nanomaterials interact and their sites of interactions are significant and are called biointerfaces and bionanointerfaces. The basic principles of materials science and surface science regulate the functionalities of biomolecules and nanomaterials interfaces. There are myriads of parameters involved during the interactions between biointerfaces and nanomaterials like wettability, hydrophobicity, hydrophilicity, surface topography, and surface chemistry [3].
A virus is an infinitesimal entity that exhibits nonliving and living features; it behaves like nonliving when outside its respective host and as a living one when present within its host. A virus infects specific and different and life forms like bacteria, archaea, animals, and plants. These submicroscopic structures are of numerous types and present in most of the aspects of ecosystems of earth. The viruses easily move from one ecological niche to another [9,10]. The viruses exhibit variations in their sizes ranging from 2.5 nm to 400 nm [11]. Traditionally, viruses are looked upon as agents that cause diseases in plants and animals creating negative impacts; as a result, their elimination or control is the main target of a common individual. These viruses play beneficial roles and can be engineered to investigate some of the biochemical and molecular aspects like an expression of non-viral proteins, vectored vaccines, gene therapy, controlling bacterial infection, and cancer. The specifically engineered viruses control the population and may reflect information on the evolutionary aspect of a species. The engineered baculovirus is used during the expression of beneficial viral proteins and the non-viral proteins to facilitate the immunological uses; utilization of poxvirus and adenovirus to develop a vectored vaccine. The success of gene therapy involves a modified version of lentiviruses and adeno-associated viruses (basically these are parvoviruses) where these agents carry out the insertion of the targeted gene in the experimental cell. Bacteriophages play a significant role as remedial agents against bacterial infection. It is hypothesized that viruses are the potential agents for controlling selective tumor cells and also the potential to control cancer. Viruses are also effective in controlling the population of obnoxious insects that infest plants and other economical crops, e.g., baculovirus. The virus also causes avian influenza among poultry, so one has to be judicial while selecting the preferences.
The structural, topographic, and biochemical aspects of the surface of viruses
A virus is one of the quintessential entities that are supra-molecular complexes that need a specific living host for its survival. The virus particles propagate by hijacking the cellular mechanism of the host [12]. A virus is a nonliving entity when outside its respective host. This specific host can be bacteria, animals, or plants. Structurally viruses are the genetic material (DNA or RNA, never both together) ensheathed in a protective protein capsid (nucleic acid coat). In some viruses, there is an additional protective cover called an envelope or capsule covering the capsid. The capsid exhibits architectural assembly that resembles a crystal-like configuration. Commonly, capsid assembly shows icosahedral, rod-like, and helical arrangement; other variations are complex, spiral, brick-like, or non-specific. The capsid protects the virus from the adverse effects of changes in temperature, pH, radiations, other chemicals, and enzymes. It is also resistant to the cytological and other enzymes of the host cell and biosystem. The subunits of the capsid are the capsomeres; the capsid is very distinct in the different capsid assemblies [13,14]. The genetic material and capsid together are nucleocapsid. The nucleic acid is either single or double-stranded and these have the potential to form the copies of viron in a host cell [13].
The virus having an envelope or capsule is an enveloped virus. The capsule or envelope of the virus consists of a lipid bilayer, phospholipid, and proteins. Some of the proteins involve carbohydrates and are glycoproteins in nature. These extend as spike-like protrusion called peplomere. The viral glycoproteins ensure the linkage between the viral surface and the receptors present on the host cell membrane. The phospholipids bilayer is one of the major constituents of the envelope and these are similar to those present in the host plasma membrane. It also has a good number of viral glycoproteins. Although the envelope is a protective cover, it also facilitates cytological invasion of the virus in the host cell. It attaches to the cell membrane of the host cell and ensures the fusion between the virus and the cell membrane of the host cell [13,15]. Comparatively, the enveloped viruses are more congenial in the body fluids of the host.
The electron micrographic investigations suggest two primary geometrical orientations, helical and polyhedral orientations. In the helical orientation, the protein subunits assemble in helical sulci around RNA or DNA and it reflects a rod-like shape as seen in the case of the tobacco mosaic virus. In this case, the subunits assemble and form a discoid lock washer-like structure; these assemblies are arranged in a rod-like helical shell enclosing their respective genetic materials. The polyhedral assembly is the simplest and smallest; it is found the quasi-spherical viron also called isocahedral virus. As the name suggests, it has the symmetry with 20 faces (Sides). The three identical protein subunits of capsid constitute one triangular face of the quasi-spherical symmetry. The poliovirus and rhinovirus come under this category. The picornaviruses (Rhinovirus) have a capsid consisting of five proteins, i.e., VP1, VP2, VP3, and VP4 proteins. The VP4 protein is surrounded by VP1, VP2, and VP3 proteins. Most of the viruses having polyhedral orientation involve more than three subunits per face of the isocahedral assembly exhibiting quasi-equilateral contact. These subunits assemble in accordance with the concept of five-fold and six-fold symmetry [16-18]. There are clefts, also called canyons observed during the atomic resolution of the surface of the virus; these encircle each vertex present in the polyhedral assembly. These canyons interact with the receptors present on the cell membrane and facilitate the bonding between the virus and the host cell initiating the viral infection. The quaternary structure of proteins capsid may elude the identification by the immune system in the host. The shape and the locations of the receptor-binding sites on the surface of a virus help to their linking with the receptors present on the membrane of the host cell and expose the quintessential parasite within the host. The viruses exhibit mechanical properties like elasticity, deformability, brittleness, hardness, materials fatigue, resistance to osmotic stress, and act as soft physical materials; these are very suitable agents for bionanotechnology and nanomedicine [12].
Electron microscopy is one of the basic techniques that help the identification of the virus. This technique elucidates the topographic nature of the surface of viruses and their identification. There are chances of artifacts due to the disorientation of the cell organelles during handling the sample, like perichromatin granules may look like parvovirus, defectively fixed chromatin like paramyxovirus, and nucleocapsid, neurosecretory granules, and nuclear pores may appear as herpesvirus, etc. These techniques with modalities like immunoelectron microscopy, cryo-electron microscopy, and electron tomography provide a better understanding of the behavior of viruses, such as cellular bonding, the orientation of structural aspects of the virus, its association with the host cellular mechanisms, in a host. These types of observati0ns provide relevant information that helps treatment and vaccine development [19]. The electron microscopic investigations indicate the presence of spiky protrusions on the surface; these are made of hemagglutinin as trimers and neuraminidase proteins as tetramers. These trimers and tetramers are within the nucleocapsid of the virus [17,18].
The appearance of viruses under electron microscopic views is different and depends on the nature and the type of the sample. The diagnostic electron microscopy and nucleic amplification technique play a significant role in the diagnosis of viruses. The chickenpox virus shows hexagonal capsid (110nm) enclosed within a ruptured envelope. In the case of the herpes virus, there is a labile envelope with glycoprotein spikes and the capsid is thinner, these are visible after potassium phosphate tungstic acid negative staining. The capsid of herpes virus shows the occasional hexagonal outline, and electron-translucent zones along with the inner side, it consists of nucleoproteins that are associated with the DNA core. In the case of equine herpesvirus, there are fuzzy projections on the outer surface of the envelope, an ill-defined tegument underneath, and a tilted core. The genome appears as an electron-dense zone under thin-section transmission electron microscopy [20]. The electron microscopic technique enables the differentiation and identification of viruses. The prime parameters that help their identification include size, outer envelope, location, and structural features. This mode of viral identification should be authenticated using microbiological analysis. There are three groups of viruses depending on their size and the negative staining technique. There is a marker on the screen of an electron microscope that helps to determine the size of the virus sample under study. First groups with a size range within 22-35nm, include viruses like parvoviruses, calciviruses, and enteroiruses. The second group of viruses includes reoviruses and polyomavirus; these are within the size range of 40-55nm. The third group includes reoviruses, retroviruses, and adenoviruses, and their size varies between 70-90 nm ranges [21]. The viruses like orthomyxoviruses, paramyxoviruses, and coronaviruses have an outer envelope; this envelope exhibits relatively prominent surface projections and is visible during negative staining. Other viruses such as rubella virus, herpesviruses, and retroviruses also show fine or delicate out-growths and these are not detectable distinctly during negative staining. The envelope of the enveloped virus (specifically DNA enveloped virus) is a product during its budding off from the nuclear membrane or its movements through the cytoplasm. Even cytoplasmic vesicles and the plasma membrane also participate in the formation of the envelope of such viruses. The naked DNA viruses are seen within cytoplasm after nuclear desecration. Mostly, helical paramyxovirus nucleocapsid can also be seen in the nucleus partly, otherwise RNA viruses do not remain in the nucleus. In the case of enveloped RNA viruses, their envelope is a derivative of cytoplasmic binding or the plasma membrane [21].
There is a structural and architectural relationship in viruses and organisms and play essential roles in the development, differentiation, and organization of biological morphology. The formation, arrangement, symmetry of the subunits, and the three-dimensional aspects reflect on the internal and surface nature of the viruses and organisms. In the case of viruses, their surface consists of capsomere and spikes or the surface projection. The nature of the surface of the virus reveals the binding between RNA (Nucleic acid) and the oligomers and functional residues existing within the domains of the virus. These aspects are readily investigated with the help of crystallographic techniques [21-24]. The outer layer consisting of VP24-VP35 bridges and secures the in inner nucleocapsid along with RNA in the case of the helical Ebola virus. Its surface membrane has glycoproteins that function as receptor binding sites and clogs with the molecule. The main epitope exposes to the exterior surface which is near the viral envelope. The primary VP40 protein configures in a lattice pattern, the envelope enclosed this configuration and it communicates irregularly with the nucleocapsid [23]. The information on the physico-morphological features of the surface of the virus is useful in designing, and formulation of antiviral agents that facilitate in treating the viral infection.
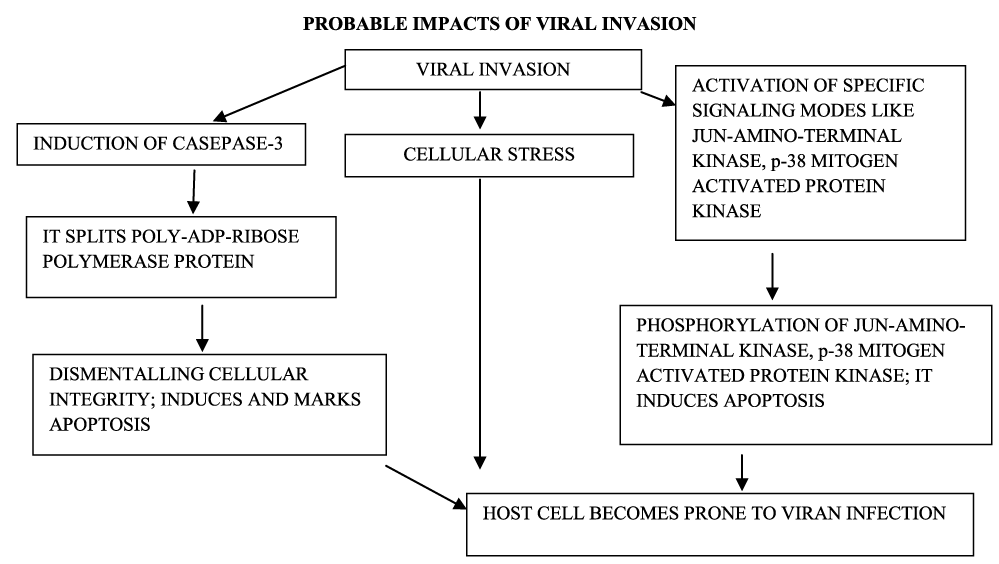
In addition to environmental stress, the invasion of viruses induces cellular stress, and Jun-Amino-Terminal Kinase and p38 Mitogen-Activated Protein Kinases signaling modes become activated. The phosphorylation of these kinases induces apoptosis. The role of Caspase-3 is one of the signatures for apoptosis and there is a possibility that caspases-3 gets induced during viral infection. This caspases-3 also splits Poly-ADP-Ribose-Polymerase Protein (PARP) - specific protein; this process of proteolytic cleavage enhances the dismantling of the cellular integrity and marks the apoptosis of the invaded host cell [25,26]. The degree of expression of Jun-Amino-Terminal Kinase (JNK) and p38 Mitogen-Activated Protein Kinases (MAPK) is higher in those cells which are not treated with zanamivir functionalized selenium nanoparticles in comparison to plain zanamivir and selenium nanoparticle. This indicates the possible inhibitory role of zanamivir functionalized selenium nanoparticles in the Madin Darby Canine Kidney Cell Line (MDCK- cell line) [26]. The surface of selenium nanoparticles modified with oseltamivir antiviral (used for HINI influenza) exhibits a higher degree of antiviral impacts than the only oseltamivir. There is a possibility that this formulation of selenium nanoparticles interrupts the hemagglutinin and neuraminidase activities in the infected host cells [27] (Figure 1).
Charge, PH, isoelectric point in case of virus and their roles
Charge on the surface of viruses plays a prime role during viral sorption, adsorption, adhesion, and movements of a virus onto the subsurface. The flocculation occurs during the treatment of water (Filtering Process) and it follows the electrostatic adsorption concept [28,29]. The charged microporous filters detect and concentrate the target virus from the large volume of the sample water because of the adsorption of the virus [30]. Viruses have a specific Isoelectric Point (IEP) and this correlates to the charge on their surface [31]. The nanowire arrays technique helps to find the charge on the virus during single viral detection [32].
The surface charge and the isoelectric point act as useful parameters and help to understand the behavior and its degree of sorption under different environments [33]. There is a formation of electrically charged surfaces of inter-facial organic and inorganic compounds in an aqueous medium because of their protonation; these protonated charged surfaces actively participate in environmental phenomena. The long-established concept of Darjaguin, Landau, Verway, and Overbeek (DLVO- concept) regulates the Van der Waal and electrostatic interactions occurring among these particles and their sorption behavior; these particles exhibit colloidal nature. The state of protonation in the cases of functional groups of proteins is related to a pH, mostly carboxyl and amino groups are the functional groups in protein and these two are related to H3O+ concentration. A change in the pH of the ambient environment affects this equilibrium. The net charge of a protein surface depends on the degree of protonation and non-protonation of the functional groups [33]. If there is a zero net charge of the colloid at a specific pH it is referred to as an electrically neutral state and is called isoelectric point. The same principle is applicable to the biological colloidal matter which includes medium containing bacteria, viruses, and proteins pH of biological colloids (Viral Colloids).
The surface charge on the virus is related to the pH of biological colloids (Viral Colloids) in an aqueous medium (Polar Solvents). The electrostatic charge influences the sorption behavior of viruses [33]. Most of the plant viruses (Isocahedral) show the isoelectric point within 3.6 to 6.3 while at neutral pH there is a negative charge on these viruses. In some cases the charge of the surface of the virus is uneven. There is a possibility that RNA containing viral nucleic acid gets activated during its genomic infection-induced transitional and may affect the distribution of charge on the viral surface [34]. Since the capsid of the virus is extensively heterogeneous the single-particle technique is more information concerning the surface charge. The single-particle technique is more favorable as it evaluates the adhesive forces that are functional due to the probe used during atomic force microscopic investigations. This is specifically more useful when the virus sample binds covalently with the surface. While investigating bulk sample zeta potential, and aqueous two-phase system cross-partitioning techniques are also useful and these also validate the finding on the isoelectric point of the surface of viruses [35]. The isoelectric point affects the solubility and electrical repulsion and at the isoelectric point these both features are the lowest while there is the maximum tendency to aggregate and precipitate. As far as viruses are concerned the isoelectric point reflects on the surface charge at their ambient environment [36].
There seems to be a possibility of least involvement of membrane charge or membrane potential during the attachment of the virus with the host cell or mucus layer; this process is helped by the equilibration between binding proteins; the binding to sialic acid on the cell surface facilitates the entry of influenza virus into the host [37].
The viability of these quintessential entities plays major roles during their transmission, and survival in different environments. Treating the sample viruses with effects of ultraviolet irradiation, photocatalysis by titanium oxide, and enriched coating of ferric chloride show derogative effects on the protein, damage of the genome. The effect on amino acids depends on the sensitivity of UV during photocatalysis the impact of hydroxyl radicals seems to be non-specific but definitely causes the damage of the capsid proteins. The amino acids adjacent to nucleotides get degraded; tyrosine plays a significant role during the activation of the virus. During physicochemical treatment does not affect the capsid composition and the size of the virus [38].
Biophysicochemical approach involved at the interface between metal and metallic oxide nanoparticles and viruses
Evidently, the physicochemical properties of the reactants participating in a given interaction play an essential role. Some of the common physicochemical properties of metal and metal oxide nanoparticles include a large surface area to volume ratio, plasmon excitation, number of kinks present at the interface, and quantum confinement [39]. Metal and metal oxide nanoparticles exhibit more surface energy and plasmonic nature in comparison to their bulk form [3]. The available surface atoms are those that are present in the full-shell cluster. The arrangement of either protons or neutrons in a complete shell within the nucleus of the atom affects the available atom number. This number of atoms also depends on the size of the cluster; in a cluster, the atoms at the periphery envelop are the centrally placed atoms. These clusters may be isolated or merged with each other; the isolated clusters are dispersed while merged clusters get aggregated. The dispersed clusters exhibit a larger surface in comparison to the aggregated clusters. This factor influences the interaction and provides a larger area at the interface [3,40]. There are some flaws during the applications of metallic nanoparticles like their instability, impurities present in nanoparticles, probable toxic effects, tedious synthesis, and chances of explosion. Metal nanoparticles are within the region of high energy local minima, hence are thermodynamically unstable. This feature is responsible for their easy transformation resulting in a decline in their quality and degree of resistance to corrosion and retaining their structural integrity. There are many different types of impurities in metal nanoparticles that associate with them either during their synthesis or due to the impure ambient environment. These may lead to the formation of oxide, nitrate, nitrides, etc. When metal nanoparticles are synthesized in solution form they should be enveloped to retain their structural and chemical integrity. These encapsulated forms may cause difficulty during their interactions at the interface. There is a possibility that the nanoparticles undergo an explosion during exothermic reactions [40].
The nanoparticles of noble metals, like gold, and silver, are favorable antiviral agents because these nanomaterials undergo functionalization and readily break the disulfide bonds. The size of these metal nanoparticles plays a significant role; the small size of nanoparticles provides an increase in the number of surface atoms with unsaturated bonds. The gold and silver nanoparticles exhibit the state of atomic instability having higher energy and in this state, these nanomaterials provide many sites for adsorption, rendering their surface with favorable surface chemistry and more so in the biological medium. Such surfaces are suitable for lodging the target agents which are relatively stable in different biological media. The state of multi-valency of gold and silver nanoparticles depends on the branched ligands and this feature enhances the localized concentration of the target molecules [41].
Mechanism involved during entry or uptake of viruses in the host cell
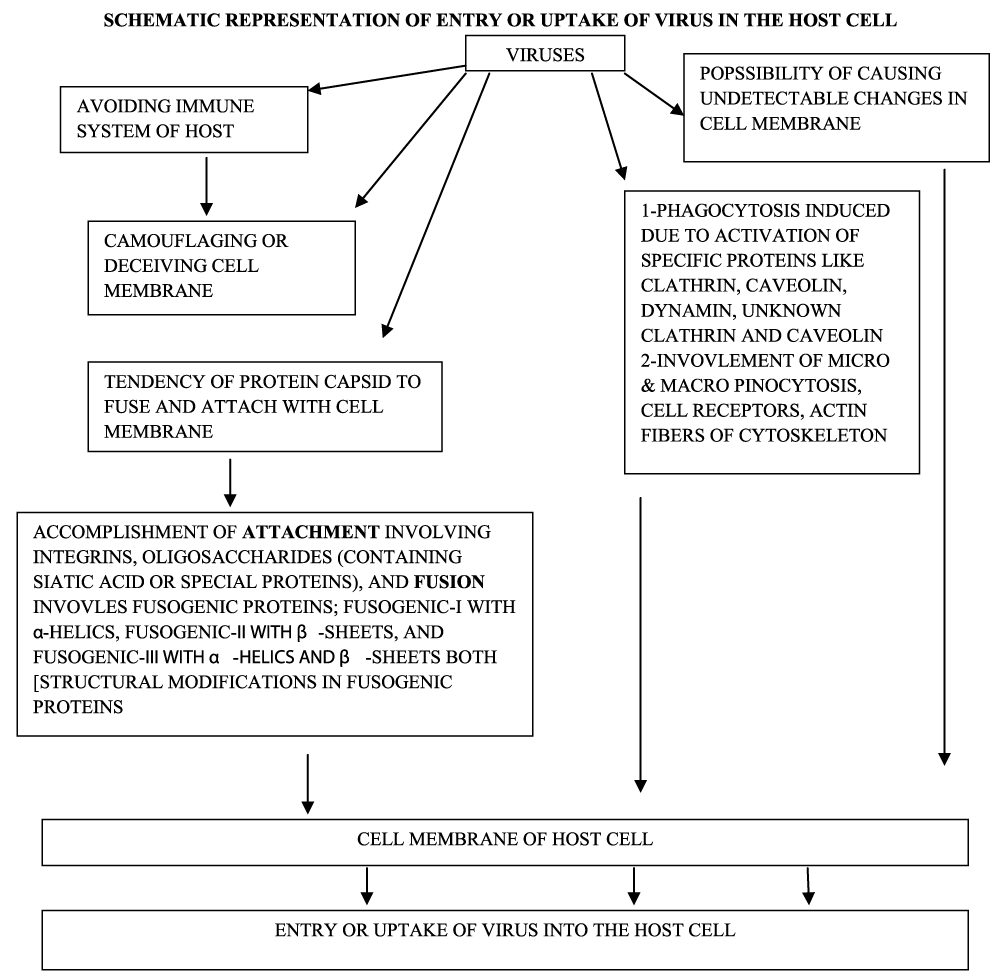
The entry of viruses may deceive the components of the immune system during their invasion. The entry point may get camouflaged and/or may not leave any structural evidence which can be detected cytologically. Basically, the cell membrane is impermeable and acts as a biological barrier for the intruders. Viruses are obligatory intracellular parasites and they have to move through an impermeable and formidable biological barrier- the cell membrane. The seemingly structurally simple virus particles are efficient in camouflaging and deceiving the host cell. These viruses are incapable of independent transportation but are able to disseminate the physicochemical features of the host [42]. The uptake or entry of viruses in the host cell involves processes like endocytosis, micropinocytosis, and phagocytosis. The viruses find entry to host cells due to their ability to fuse with the plasma membrane, cell to cell fusion, and syncytium. The surface topology of the surface of the virus, proteomes, and the type of the host cell plays an essential role during the cellular internalization of viruses. The specific proteins like caveolin, clathrin, dynamin, unknown non-clathrin, and non-caveolin induce endocytosis. During phagocytosis, micro and macropinocytosis, cell receptors, and cytoskeleton, specifically actin filaments participate more intensely.
The protein of viral capsid exhibits a high tendency to fuse with the plasma membrane of the potential host cell; this process utilizes fusogenic proteins. [In these proteins one group has more of α-helical coils (class-I fusogen), second group β-sheets (class-II fusogen), and the third group possesses both α-helical coils and β-sheets]. These proteins ensure the fusion between the virus and the cell membrane; there is a structural change at the site of fusion that results in the formation of a pore through which delivery of viral genome occurs. The pH of the host cell is one of the prime parameters for a successful viral entry during all these processes. Prior to fusion, there should be an appropriate attachment between the surface of the invading virus and the potential site on the host cell membrane. This attachment involves complements like integrins or oligosaccharides which possess sialic acid and /or specialized proteins. There are other varied forms of immunoglobulins and diverse ligands along with integrins or specific oligosaccharides; all these accomplish the basic cellular phenomena like cellular adhesion and immune responsiveness. The proteins of the spikes present on the surface of SARS-CoV-2 help in its internalization in the host cell. These spikes ligate with the membrane receptors involving receptor binding domain while human proteases activate this interaction [43-45]. There are practically no reports on the viruses outside the host and are called virus-like particles; their protein capsid and genome remains nonfunctional. Possibly this feature makes their study difficult (Figure 2).
Interactions between metallic and metal oxide nanoparticles and viruses
The prime objective of the study of metal and metal oxide nanoparticles against virus infection is to provide know-how about their interaction with viruses. The main effort focuses on the ability of metal and metal oxide nanoparticles to elevate the degree of surface disinfection, the degree of survival among the host, blocking the viral outspreading, upraise the immunity of the patient or host, prevent uptake of viruses. All these intentions are heading towards effective antiviral infection [41]. The application of metal and metal oxide nanomaterials is mainly towards blocking those proteins which play a role during the uptake by the host cell, oxidation of proteins present in a viral protein capsid, mechanical destruction of viruses, impersonate the cell membrane [41]. The interactions between metallic and metal oxide nanoparticles and the surface of the virus follow the concepts metal oxide of the Monte-Carlo simulation method and Coulombic interactions. The concept of the Monte-Carlo method of simulation involves the randomness to find the solution that is mostly deterministic in nature. The Coulombic interactions concern with parameters like electric force, charges, and the distance of separation. These parameters play essential roles in maintaining the stability of given viral-like particles. The multivalent ions (cations and anions both) affect the stability of these quintessential entities [46]. The overall strategies involved during the interaction between metal nanomaterials and viruses are represented in figure 3.
The survey of the literature reveals the applications of gold and silver nanoparticles and metal oxide such as magnetite, zinc oxide titanium dioxide against virus-like particles. Gold nanoparticles within the size range 1-2 to 150 nm) are a good choice for the applications in medical, biochemical, and molecular sciences. These nanoparticles have better conductivity, flexibility for the modification of their surfaces, high biocompatibility, inert nature, and least or no toxicity to the biosystem. Gold nanoparticles are also versatile functionally as their photophysical features and linkage with the thiol group; these features are readily modified [47]. Gold nanoparticles provide a favorable interface that helps in binding with sulfated biological ligands. These nanoparticles enhance the degree of adhesion sites that immobilize different ligands [48]. The gold nanoparticles offer a suitable interface for the attachment and induce aggregation of sugars. The glycoconjugates of gold nanoparticles result in a synergistic impact on anti-gp120 interactions. Thus, the gold nanoparticles linked with antiviral glycoconjugates and N-linked high-mannose glycans exhibit a high degree of anti-gp 120 interactions, and possible effective agents against HIV therapy [49-52]. The gold nanoparticles provide greater sites for adhesion and help in immobilizing different ligands [48]. Gold nanoparticle synthesized by green chemistry from garlic extract (Allium sativa) Au-NPs-As) prevents the replication of the measles virus (ES50; 8.829 µg/mL) in the Vero cells. During this inhibition, these gold nanoparticles block the linking between virus particles and interacting with Vero cells [53].
Silver nanoparticles show a very good degree of conductivity, catalytic ability, and chemical firmness. Generally, these nanomaterials release silver ions which elevate the degree of antiviral impact and cause disruption of cell membrane and damage to the genetic material [47]. Silver nanoparticles, within non-cytotoxic concentrations, exhibit anti-viral impact specifically in the case of HIV. These nanoparticles influence the process of replication of the viral genome in the host cell and bind with the glycoproteins (gp120) of the envelope of the invading virus. As a result, these nanoparticles inhibit fusion, degree of infectivity, and binding that involve CD4 dependent. [CD4 is a cluster of differentiation 4. It is a glycoprotein present on the cell membrane of immune cells like T helper cells, monocytes, macrophages, and dendritic cells]. The specifically engineered silver nanoparticles are able to sustain and release drugs in s per the need, penetrate and reach deep tissues. Their antiviral impact is related to their physicochemical parameters like size, shape, surface chemistry, etc. These nanoparticles interact with the surface of viruses and influence the binding of viruses, prevent the viral uptake by the host cell, interfere with viral genome, and prevent its replication; as result impair the protein synthesis, assembly, and the delivery of newly formed viroins in the host [54].
Other metallic nanoparticles like titanium, zinc, and copper also act as effective antiviral agents. The platinum nanoparticles also exhibit a similar nature [47,55]. The core-shell nanoparticles have core particles of one metal and it is enclosed by a shell of other metal; the outer metallic shell may be monometallic or bimetallic in composition [47,56].
Metal nanoparticles, like gold, silver, magnetic nanoparticles, etc., are suitable agents for viral detection including the CORONA virus. During these detections, specifically fabrications like nanotraps, involving specific antibody conjugation, the formation of magnetic fluorescence complex, application of silver nanoparticles to improve analytic interactions that use complementary viral genome, and help to improve detection efficacy of polymerase chain reaction techniques, etc. The gold nanoparticles linked with graphene nanosheets, diphyllin enclosed in PEG-PLGA vesicles, are useful agents to act vaccines, as carriers of antiviral drugs, nanocages, etc., [57-61]. Graphene oxide loaded with silver and copper based nanoformulations act as effective viricidal agents against enveloped and non-enveloped viruses. The copper oxide nanoparticles functionalized with polypropylene are also useful as anti-influenza viral agents. Metal nanoparticles such as silver nanoparticles interact with the receptors of host cell and prevent viral uptake and inhibit the viral reproductive activities, specifically in the case of HIV-1 virus. The silver nanoparticles also interfere with the double strain RNA viruses and prevent its replication. The gold nanoparticles functionalized with sulfated ligands, silver nanoparticles and hybrid silver and copper nanoparticles bind with the glycoprotein gp120 present on the envelope of HIV and results in prevention of viral infection in the model dells.
The oxide nanoparticles like iron oxide, zinc oxide, and titanium oxide exhibit viricidal impacts. There are reports concerning the inactivation of MS2, a bacteriophage, using photocatalytic silver doped titanium dioxide. The comparative study of nano-sized silver/titanium dioxide and silver ions released from catalyst shows that the inactivation rate depends on the base TiO2 materials. When the silver contents increase the degree of inactivation also elevates. There is a production of free hydroxyl ions and these play an effective role during the inactivation of bacteriophages MS2 [62]. Biological matter, specifically viruses (within a size range 20-100 nm), are isolated from the test water samples using nanofilters coated with titanate salts; on exposure to UV illumination, these titanate materials liberate OH- and O2- radicals because of their photocatalytic behavior. The titanate nanosheets act as good adsorptive surfaces for the viruses present in the sample water and under UV irradiation; the radicals like OH- and O2- ambush the adsorbed viruses. In the absence of UV irradiation, this interaction does not take place [63].
A membrane having silver and copper stocked TiO2 nanowires render bacteriophages MS2 inactive under dark and U V illumination. There is a synergic effect of stuffed silver and copper ions on the said membrane along with the combined impact of copper and silver ions present in the water sample under study. This inactivation of the bacteriophages is the result of exposure of UV irradiation (254 nm) and elimination of bacteriophages to the tune of 4.02 (log per sample) [64]. Chlorine, bromine, iodine interact with some of the metal oxides like CeO2, Al2O3, and TiO2 and form thermostable solid adducts. These adducts are efficient biocides in an open environment. These metal oxides have abrasive features and are effective oxidants. Although the exact mechanism is quite unclear there is an opinion that these halogenated metal oxides are able to kill viruses like bacteriophage MS2, the phi X 174(Φx174), bacteriophage, (single-stranded DNA (ssDNA) virus that infects Escherichia coli), (Φx174), PRD-1-a phage, (tectiviridae phage PRD1, with a lipid bilayer between the icosahedral capsid shell and the internal dsDNA). These halogenated metal oxides prevent the attachment of these viruses with the host cells [65]. Pristine zinc oxide and PEGylated zinc oxide are not as effective as viricide against the H1N1infuenza virus directly at any concentration. Their antiviral impact is seen only when the virus is in the host cell, there are a significant antiviral effect of ZnO nanoparticle (75 µg/ml) and PEGylated ZnO nanoparticles (75,100, and 200 µg/ml; non-cytotoxic range). The antiviral effect is expressed as a decline in viral titer values. These nanoparticles suppress the multiplication of the virus in the host cell. During the experimentation, the parameter of surface hydrophilicity increases and causes a decline in cellular uptake of these nanoparticles [66]. The quantitative Polymerase Chain Reaction (PCR) is one of the convenient techniques to evaluate the transcriptional level of viral RNA (or viral genetic material) due to a reactant and investigate its viricidal impact. The iron oxide nanoparticles (10-15 nm; 2pg/ml; incubation period 72 h) reduce the viral RNA (influenza virus) eight folds. This impact is size dependent involving binding with the virus under study [67-74].
Virus particles exhibit life-activities like replication of their genome when are within their respective hosts and act as non-living when present outside their hosts. The investigation in these conditions can help to understand the differences between the functionalities at bionanointerfaces under the living and nonliving phases. Metal and metal oxide nanomaterials occur naturally and as-synthesized forms; the engineered forms meet the set targets. Biointerface is a site where an appropriate molecular communication gets established. This effective site is a suitable interacting place for biomolecular and cellular modulation, topographic, mechano-and chemo-structural modifications. These mechano-chemical adjustments help the responses of prokaryotic and eukaryotic biosystems that help the ongoing biointeractions. The cell membrane is suitably adapted and provides a time-bound spatial and temporal dynamic systems so that the switch on and switch off mechanism regulates the extra and intracellular signaling system. The functionalized metal nanoparticles, nanotraps, and bimetallic agents enhance the biocompatibility, biodistribution, and attain targeted functionalities. These functionalities include drug delivery, imaging, and localization techniques. Such multiplexing is helpful during infection, treatment, recovery, and recapitulates phases, even for biomedical and biochemical research fields. The use of PEGylated metal and metal oxide nanoparticles facilitates the investigation of non-specific intermolecular interactions, immune responses, like regulated antigen-antibody interaction, immunostimulation or immunosuppression, related adjuvants, during detection, treatment, viral infection. Such studies are also helpful in designing, formulation of pharmaceutical agents and their applications for the current and future.
- Hutmatcher D, Chrazanowaski W. Biointerfaces: Where material meets biology. Royal Society of Chemistry. Cambridge. 2015. doi: 10.1039/9781782628453
- Chen D, Wang D, Li J. Interfacial bioelectrochemistry fabrication: Properties, and applications of functional nanostructured biointerface, American Chemical Society. J Phys Chem C. 2007 Dec14;11(6):2351-2367. doi: 10.1021/jp065099w
- Lahir YK, Avti P. Nanomaterials and their interactive behavior with biomolecules, cell, and tissues, (Bentham Books) Bentham Science Publishers, Singapore; 2020. doi: 10.2174/97898114617811200101.
- Nel AE, Mädler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009 Jul;8(7):543-557. doi: 10.1038/nmat2442. Epub 2009 Jun 14. PMID: 19525947.
- Chilkoti A and Hubbell JA. Biointerface Science. MRS Bulletin. 2005;30(3):175-179. doi: 10.1557/mrs2005.48
- Lahaan J and Langer R. Smart materials with dynamically controlled surfaces. MRS Bulletin. 2005;30(3):185-188. doi: 10.1557/mrs2005.50
- Mrkisch M. Dynamic substrates for cell biology. MRS Bulletin. 2005;30(3):180-184. doi: 10.1557/mrs2005.49
- Lahir YK. Interactions at the interface between nanomaterials and biofilm: A General Survey. Advances in Clinical toxicology-med win publishers. 2020;5(3): doi: 10.23803/act-16000192
- Breitbart M, Rohwer F. Here virus, there virus, everywhere the same virus, Trend in Microbiology. 2005;13(6):273-284. doi: 10.1016/j.tim.2005.04.003
- Edwards RA, Rohwer F. Viral metagenomics. Nat Rev Microbiol. 2005 Jun;3(6):504-510. doi: 10.1038/nrmicro1163. PMID: 15886693.
- Leland DS. Clinical Virology, Saunders, New York. 1996
- Buzon P, Maity S, Roos WH. Physical virology: From virus self-assembly to particle mechanics. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020 Jul;12(4):e1613. doi: 10.1002/wnan.1613. Epub 2020 Jan 20. PMID: 31960585; PMCID: PMC7317356.
- Sunnen GV. A Virology Primer: with special reference to ozone.
- Capsid. Definition, function and structure. 2017 Aug.
- Envelope virus. Wikipedia, Wikipedia. 2017 Aug.
- Smith TJ, Chase ES, Schmidt TJ, Olson NH, Baker TS. Neutralizing antibody to human rhinovirus 14 penetrates the receptor-binding canyon. Nature. 1996 Sep 26;383(6598):350-354. doi: 10.1038/383350a0. PMID: 8848050; PMCID: PMC4167671.
- Lodish H, Berk A, Zipursky SL, Matsudaria P, Baltimore D, Darnell J. Molecular cell Biology, (4th Ed) New York, Scientific American Books. 2000.
- Alberts B, Johnson A, Lewis I, Morgan D, Raff M, Roberts K, and Walter P. Molecular Biology of Cell (16th Ed), Garland Science, ISBN 978-1-317-56375-4. 2014.
- Goldsmith CS, Miller SE. Modern uses of electron microscopy for detection of viruses. Clin Microbiol Rev. 2009 Oct;22(4):552-563. doi: 10.1128/CMR.00027-09. PMID: 19822888; PMCID: PMC2772359.
- Gelderblom HR, Madeley D. Rapid Viral Diagnosis of Orthopoxviruses by Electron Microscopy: Optional or a Must? Viruses. 2018 Mar 22;10(4):142. doi: 10.3390/v10040142. PMID: 29565285; PMCID: PMC5923436.
- Zhang Y, Hung T, Song J, He J. Electron microscopy: Essentials for viral structure, morphogenesis and rapid diagnosis. Sci China Life Sci. 2013 May;56(5):421-430. doi: 10.1007/s11427-013-4476-2. Epub 2013 May 1. PMID: 23633074; PMCID: PMC7089233.
- Rossmann MG. Crystallography, evolution, and the structure of viruses. J Biol Chem. 2012 Mar 16;287(12):9552-9. doi: 10.1074/jbc.X112.348961. Epub 2012 Feb 8. PMID: 22318719; PMCID: PMC3308792.
- Beniac DR, Melito PL, Devarennes SL, Hiebert SL, Rabb MJ, Lamboo LL, Jones SM, Booth TF. The organisation of Ebola virus reveals a capacity for extensive, modular polyploidy. PLoS One. 2012;7(1):e29608. doi: 10.1371/journal.pone.0029608. Epub 2012 Jan 11. PMID: 22247782; PMCID: PMC3256159.
- Carter SD, Surtees R, Walter CT, Ariza A, Bergeron É, Nichol ST, Hiscox JA, Edwards TA, Barr JN. Structure, function, and evolution of the Crimean-Congo hemorrhagic fever virus nucleocapsid protein. J Virol. 2012 Oct;86(20):10914-10923. doi: 10.1128/JVI.01555-12. Epub 2012 Aug 8. PMID: 22875964; PMCID: PMC3457148.
- Julian O and Well JA. Caspases and their substrates, Cell Death and Differentiation, 2017;24(8): 1380-1389. doi.10.1038//cdd.2017.44
- Lin Z, Li Y, Guo M, Xiao M, Wang C, Zhao M, Xn T, Xia Y, Zh B. Inhibition of HINI influenza virus by selenium nanoparticles loaded with zanamivir through p38 and JNK signaling pathways, RSC Adv. 2017 July;7:35290-35295. doi: 10.1039/C7RA06477B
- Li Y, Lin Z, Guo M, Xia Y, Zhao M, Wang C, Xu T, Chen T, and Zhu B, (2017) Inhibitory activity of selenium nanoparticles functionalized with oseltamivir on H1N1 influenza virus, International Journal of Nanomedicine, 2017 July;12:5733-5743. doi.10.2140.S140939
- Schijven JF, Hassanizadeh SM. Removal of viruses by soil passage: an overview of modeling, process and parameters. Crit Rev Env Tech. 2000;30:49-127. doi: 10.1080/10643380091184174
- Matsushita T, Matsui Y, Shirasaki N. Analysing mass balance of viruses in a coagulation-ceramic microfiltration hybrid system by a combination of the Polymerase Chain Reaction (PCR) method and the Plaque Forming Units (PFU) method. Water Sci Technol. 2006;53(7):199-207. doi: 10.2166/wst.2006.225. PMID: 16752782.
- Cashdollar JL, Dahling DR. Evaluation of a method to re-use electropositive cartridge filters for concentrating viruses from tap and river water. J Virol Methods. 2006 Mar;132(1-2):13-17. doi: 10.1016/j.jviromet.2005.08.016. Epub 2005 Sep 27. PMID: 16194574.
- Brorson K, Shen H, Lute S, Pérez JS, Frey DD. Characterization and purification of bacteriophages using chromatofocusing. J Chromatogr A. 2008 Oct 17;1207(1-2):110-121. doi: 10.1016/j.chroma.2008.08.037. Epub 2008 Aug 15. PMID: 18778829.
- Patolsky F, Zheng G, Hayden O, Lakadamyali M, Zhuang X, Lieber CM. Electrical detection of single viruses. Proc Natl Acad Sci U S A. 2004 Sep 28;101(39):14017-14022. doi: 10.1073/pnas.0406159101. Epub 2004 Sep 13. PMID: 15365183; PMCID: PMC521090.
- Michen B, Graule T. Isoelectric points of viruses. J Appl Microbiol. 2010 Aug;109(2):388-397. doi: 10.1111/j.1365-2672.2010.04663.x. Epub 2010 Jan 22. PMID: 20102425.
- Arkhipenko MV, Nikitin NA, Baranov OA, Evtushenko EA, Atabekov JG, Karpova OV. Surface charge mapping on virions and virus-like particles of helical plant viruses. Acta Naturae. 2019 Oct-Dec;11(4):73-78. doi: 10.32607/20758251-2019-11-4-73-78. PMID: 31993237; PMCID: PMC6977955.
- Mi X, Bromley EK, Joshi PU, Long F, Heldt CL. Virus isoelectric point determination using single-particle chemical force microscopy. Langmuir. 2020 Jan 14;36(1):370-378. doi: 10.1021/acs.langmuir.9b03070. Epub 2019 Dec 31. PMID: 31845814.
- Christin S, Finja K, Robert M, Isabel A, Maria G-M, Hermann W. Physicochemical properties of SARSCoV2 for drug targeting, virus inactivation and attenuation, vaccine formulation and quality control, Electrophoresis, 2020 May;41(13-14):1137-1151. doi: 10.1002/elps.202000121
- Michael DV, Fletcher DA. Influenza-A-virus surface proteins are organized to help penetrate host mucus, (Microbiology and Infectious Disease), elife. 2019;8:e43764. doi: 10.7554/elife.43764
- Mayer BK, Yang Y, Gerrity DW, Morteza A. The impact of capsid proteins on virus removal and inactivation during the water treatment process, Microbiology Insight. 2015 Nov;8(Supp-2): 15-28. doi:10.4137/MBI.S31441
- Campelo JM, Luna D, Luque R, Marinas JM, Romero AA. Sustainable preparation of supported metal nanoparticles and their applications in catalysis. ChemSusChem. 2009;2(1):18-45. doi: 10.1002/cssc.200800227. PMID: 19142903.
- Harish KK, Nagasamy V, Himangshu B, Anuttam K. Metallic Nanoparticles: A review. BJSTR. 2018;4(2): 3765- 3775. doi: 10.26717/BSTR.2018.04.001011
- Reina G, Peng S, Jacquemin L, Andrade AF, Bianco A. Hard nanomaterials in time of viral pandemics. ACS Nano. 2020 Aug 25;14(8):9364-9388. doi: 10.1021/acsnano.0c04117. Epub 2020 Jul 22. PMID: 32667191; PMCID: PMC7376974.
- AE, Helenius A. How viruses enter animal cells. Science. 2004 Apr 9;304(5668):237-242. doi: 10.1126/science.1094823. PMID: 15073366.
- Dermody TS, Kirchner E, Guglielmi KM, Stehle T. Immunoglobulin superfamily virus receptors and the evolution of adaptive immunity. PLoS Pathog. 2009 Nov;5(11):e1000481. doi: 10.1371/journal.ppat.1000481. Epub 2009 Nov 26. PMID: 19956667; PMCID: PMC2777377.
- Bhella David. The role of cellular adhesion molecules in virus attachment and entry. Phil Trans R Soc. 2015;B37020140035. doi: 10.1098/rstb.2014.0035
- Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, Li F. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020 May 26;117(21):11727-11734. doi: 10.1073/pnas.2003138117. Epub 2020 May 6. PMID: 32376634; PMCID: PMC7260975.
- Javidpour L, Bozic AL, Podornik R, and Naji A. Role of metallic core for stability of virus like-particle in strongly coupled electrostatics, Scientific Reports. 2019 Mar; 3881. doi: 1038/s41598-019-39930-8
- Singh L, Kruger HG, Maguire GEM, Govender T, Parboosing R. The role of nanotechnology in the treatment of viral infections. Ther Adv Infect Dis. 2017 Jul;4(4):105-131. doi: 10.1177/2049936117713593. Epub 2017 Jul 5. PMID: 28748089; PMCID: PMC5507392.
- Yadavalli T, Shukla D. Role of metal and metal oxide nanoparticles as diagnostic and therapeutic tools for highly prevalent viral infections. Nanomedicine. 2017 Jan;13(1):219-230. doi: 10.1016/j.nano.2016.08.016. Epub 2016 Aug 26. PMID: 27575283; PMCID: PMC5237416.
- Di Gianvincenzo P, Marradi M, Martínez-Avila OM, Bedoya LM, Alcamí J, Penadés S. Gold nanoparticles capped with sulfate-ended ligands as anti-HIV agents. Bioorg Med Chem Lett. 2010 May 1;20(9):2718-2721. doi: 10.1016/j.bmcl.2010.03.079. Epub 2010 Mar 25. PMID: 20382017.
- Arnáiz B, Martínez-Ávila O, Falcon-Perez JM, Penadés S. Cellular uptake of gold nanoparticles bearing HIV gp120 oligomannosides. Bioconjug Chem. 2012 Apr 18;23(4):814-825. doi: 10.1021/bc200663r. Epub 2012 Mar 30. PMID: 22433013.
- Di Gianvincenzo P, Chiodo F, Marradi M, Penadés S. Gold manno-glyconanoparticles for intervening in HIV gp120 carbohydrate-mediated processes. Methods Enzymol. 2012;509:21-40. doi: 10.1016/B978-0-12-391858-1.00002-2. PMID: 22568899.
- Marradi M, Di Gianvincenzo P, Enríquez-Navas PM, Martínez-Ávila OM, Chiodo F, Yuste E, Angulo J, Penadés S. Gold nanoparticles coated with oligomannosides of HIV-1 glycoprotein gp120 mimic the carbohydrate epitope of antibody 2G12. J Mol Biol. 2011 Jul 29;410(5):798-810. doi: 10.1016/j.jmb.2011.03.042. Epub 2011 Mar 25. PMID: 21440555.
- Meléndez-Villanueva MA, Morán-Santibañez K, Martínez-Sanmiguel JJ, Rangel-López R, Garza-Navarro MA, Rodríguez-Padilla C, Zarate-Triviño DG, Trejo-Ávila LM. Virucidal Activity of Gold Nanoparticles Synthesized by Green Chemistry Using Garlic Extract. Viruses. 2019 Nov 30;11(12):1111. doi: 10.3390/v11121111. PMID: 31801280; PMCID: PMC6950311.
- A, Kafshdooz L, Razban Z, Dastranj Tbrizi A, Rasoulpour S, Khalilov R, Kavetskyy T, Saghfi S, Nasibova AN, Kaamyabi S, Kafshdooz T. An overview application of silver nanoparticles in inhibition of herpes simplex virus. Artif Cells Nanomed Biotechnol. 2018 Mar;46(2):263-267. doi: 10.1080/21691401.2017.1307208. Epub 2017 Apr 12. PMID: 28403676.
- Yang ZH, Zhuo Y, Yuan R, Chai YQ. An amplified electrochemical immunosensor based on in situ-produced 1-naphthol as electroactive substance and graphene oxide and Pt nanoparticles functionalized CeO2 nanocomposites as signal enhancer. Biosens Bioelectron. 2015 Jul 15;69:321-327. doi: 10.1016/j.bios.2015.01.035. Epub 2015 Jan 17. PMID: 25791337.
- Concha T, David B, M Arturo L. Core-Shell Nanocatalysts Obtained in Reverse Micelles: Structural and Kinetic Aspects. Journal of Nanomaterials. 2015;1-10. doi: 10.1155/2015/601617
- Al-Halifa S, Gauthier L, Arpin D, Bourgault S, Archambault D. Nanoparticle-based vaccines against respiratory viruses. Front Immunol. 2019 Jan 24;10:22. doi: 10.3389/fimmu.2019.00022. PMID: 30733717; PMCID: PMC6353795.
- Dhakal S, Renukaradhya GJ. Nanoparticle-based vaccine development and evaluation against viral infections in pigs. Vet Res. 2019 Nov 6;50(1):90. doi: 10.1186/s13567-019-0712-5. PMID: 31694705; PMCID: PMC6833244.
- Alphandéry E. The Potential of Various Nanotechnologies for Coronavirus Diagnosis/Treatment Highlighted through a Literature Analysis. Bioconjug Chem. 2020 Aug 19;31(8):1873-1882. doi: 10.1021/acs.bioconjchem.0c00287. Epub 2020 Jul 8. PMID: 32639742; PMCID: PMC7359670.
- Han Y, Král P. Computational Design of ACE2-Based Peptide Inhibitors of SARS-CoV-2. ACS Nano. 2020 Apr 28;14(4):5143-5147. doi: 10.1021/acsnano.0c02857. Epub 2020 Apr 16. PMID: 32286790; PMCID: PMC7163933.
- J, Wang MX, Ang IYH, Tan SHX, Lewis RF, Chen JI, Gutierrez RA, Gwee SXW, Chua PEY, Yang Q, Ng XY, Yap RK, Tan HY, Teo YY, Tan CC, Cook AR, Yap JC, Hsu LY. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel Coronavirus (2019-nCoV): A Systematic Review. J Clin Med. 2020 Feb 26;9(3):623. doi: 10.3390/jcm9030623. PMID: 32110875; PMCID: PMC7141113.
- Liga MV, Bryant EL, Colvin VL, Li Q. Virus inactivation by silver doped titanium dioxide nanoparticles for drinking water treatment. Water Res. 2011 Jan;45(2):535-544. doi: 10.1016/j.watres.2010.09.012. Epub 2010 Sep 19. PMID: 20926111.
- Soylemez E, de Boer MP, Sae-Ueng U, Evilevitch A, Stewart TA, Nyman M. Photocatalytic degradation of bacteriophages evidenced by atomic force microscopy. PLoS One. 2013;8(1):e53601. doi: 10.1371/journal.pone.0053601. Epub 2013 Jan 3. PMID: 23301095; PMCID: PMC3536765.
- Rao G, Brastad KS, Zhang Q. Rebecca R, Zhen H, Ying Li. Enhanced disinfection of Escherichia coli and bacteriophage MS2 in water using a copper and silver loaded titanium dioxide nanowire membrane. Front Environ Sci Eng. 2016 June. doi: 10.1007/s11783-016-0854-x
- Häggström J, Balyozova D, Klabunde KJ, Marchin G. Virucidal properties of metal oxide nanoparticles and their halogen adducts. Nanoscale. 2010 Apr;2(4):529-534. doi: 10.1039/b9nr00273a. Epub 2010 Feb 2. PMID: 20644755.
- Ghaffari H, Tavakoli A, Moradi A, Tabarraei A, Farah BS, Zehmatkeshan M, Vahid PM, Monavari SH, Angila AP. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: another emerging application of nanomedicine, Journal of Biomedical Sciences. 2019 Sep. doi: 10.1186/s12929-019-0563-4
- Kumar R, Nayak M, Sahoo GC, Pandey K, Sarkar MC, Ansari Y, Das VNR, Topno RK, Bhawna, Madhukar M, Das P. Iron oxide nanoparticles based antiviral activity of H1N1 influenza A virus. J Infect Chemother. 2019 May;25(5):325-329. doi: 10.1016/j.jiac.2018.12.006. Epub 2019 Feb 13. PMID: 30770182.
- Anthony von Fraunhofer. Adhesion and cohesion, International Journal of dentistry. 2012;1-8. doi: 10.1155/2012/951324
- Sobhy H. A comparative review of viral entry and attachment during large and giant dsDNA virus infections. Arch Virol. 2017 Dec;162(12):3567-3585. doi: 10.1007/s00705-017-3497-8. Epub 2017 Sep 2. PMID: 28866775; PMCID: PMC5671522.
- Terrettaz S, Vogel H. Investigating the functions of ion-channels in tethered membranes by impedance spectroscopy, MRS Bulletin. 2005;30(3):207-210. doi: 10.1557/mrs2005.54
- Chen CS, Jiang X, Whitesides GM. Microengineering the environment of mammalian cells in culture, MRS Bulletin. 2005;30(3): 194-201. doi: 10.1557/mrs2005.52
- Chen YN, Hsueh YH, Hsieh CT, Tzou DY, Chang PL. Antiviral activity of graphene-silver nanocomposites against non-enveloped and enveloped viruses. Int J Environ Res Public Health. 2016 Apr 19;13(4):430. doi: 10.3390/ijerph13040430. PMID: 27104546; PMCID: PMC4847092.
- Kerry RG, Malik S, Redda YT, Sahoo S, Patra JK, Majhi S. Nano-based approach to combat emerging viral (NIPAH virus) infection. Nanomedicine. 2019 Jun;18:196-220. doi: 10.1016/j.nano.2019.03.004. Epub 2019 Mar 21. PMID: 30904587; PMCID: PMC7106268.
- Sportelli MC, Izzi M, Kukushkina EA, Hossain SI, Picca RA, Ditaranto N, Cioffi N. Can Nanotechnology and materials science help the fight against SARS-CoV-2? Nanomaterials (Basel). 2020 Apr 21;10(4):802. doi: 10.3390/nano10040802. PMID: 32326343; PMCID: PMC7221591.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.