> Biology. 2020 August 26;1(4):105-113. doi: 10.37871/jels1127.
-
Subject area(s):
- Microbiology
- Biology
A Review on the Possible Leakage of Electrons through the Electron Transport Chain within Mitochondria
Nafisa Tabassum1, Ilora Shabnam Kheya2, Syed Abdullah Ibn Asaduzzaman2, Syeda Muntaka Maniha2, Abrar Hamim Fayz2, Amayna Zakaria2 and Rashed Noor2*
2Department of Microbiology, School of Environment and Life Sciences (SELS), Independent University Bangladesh (IUB), Plot 16, Block B, Aftabuddin Ahmed Road, Bashundhara, Dhaka- 1229, Bangladesh
- Electron transport
- Reactive Oxygen Species (ROS)
- Electron/proton leakage
- Mitochondrion
The finding of electron leakage during the electron transport within the mitochondrial membrane (in eukaryotes) or in the cell membrane of the prokaryotes is an important issue for the accumulation of the Reactive Oxygen Species (ROS) in the cytosol which in turn induce the probable aging of cells. In eukaryotes, mitochondrion is known to be the major site of the ROS generation in different pathological processes which may further cause cell damages as evident through the ischemia-reperfusion (I/R) injury, respiratory diseases, cell apoptosis, and even the onset of cancer. Thus, the mitochondrial leakage and the physiological effect of leaked protons and electrons grow up with future interest in energy metabolism. Current review focused on the physiological impact of electron/ proton leakage particularly in the eukaryotic cells based on the previous reports; emphasized on the prospects of the eukaryotic mitochondrion as a modulator of proton and electron leakage; and finally attempted to assess the regulatory mechanisms of such electron/ proton leakage.
The respiratory chain or electron transport chain
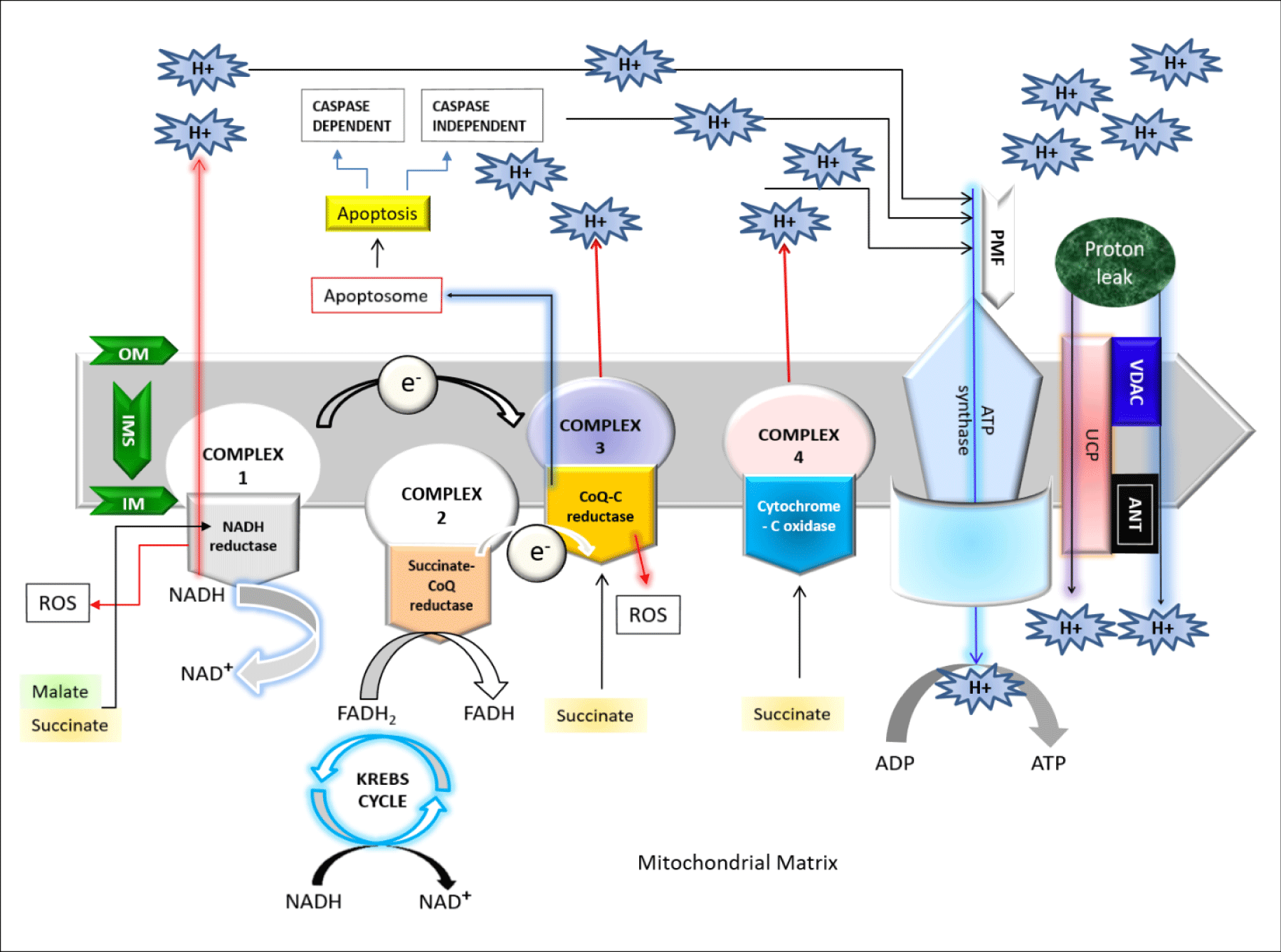
The respiratory chain or electron transport chain uses electron flow to create a proton gradient (proton motive force) that is used to drive Adenosine Tri-Phosphate (ATP) synthesis. Mitchell P, proposed the theory of coupling of phosphorylation to electron and hydrogen transfer by a chemiosmotic type of mechanism [1]. This chain is widely known to consist of five functional units or complexes embedded in the inner mitochondrial membrane [1,2]. Complex I, NADH:ubiquinone oxidoreductase consists of 25-28 different polypeptides, seven of which are encoded by mitochondrial DNA (mtDNA), carry reducing equivalents from NADH to the Co-Enzyme Q (CoQ); complex II (the succinate- CoQ reductase) contains five polypeptides, including the Flavin Adenine Dinucleotide (FAD)-dependent succinate dehydrogenase and a few non-heme-iron-sulfur centers, carry the reducing equivalents from the reduced flavin adenine di-nucleotide (FADH2) to CoQ [1]. Complex III (Coenzyme Q: Cytochrome C-Oxidoreductase,) containing 11 subunits helps to transfer electrons from CoQ to cytochrome c; while the complex IV (Cytochrome-C Oxidase), composed of two cytochromes (a and a3), two copper atoms, and 13 different protein subunits, catalyzes the transfer of reducing equivalents from cytochrome c to molecular oxygen [1,2]. Indeed, the respiratory chain catalyzes the oxidation of fuel molecules such as malate or succinate by oxygen which serves as the terminal electron acceptor in the chain of oxidation-reduction reactions that occur in the inner mitochondrial membrane [1]. Electrons are transferred to oxygen via the energy-transducing complexes for the concomitant energy transduction into ATP: complexes I, III, and IV for are used for succinate dehydrogenase; complexes III and IV for FADH2 derived from the oxidation pathway via the Electron-Transfer Flavoprotein (ETF) and the ETF-CoQ oxidoreductase system; CoQ (a lipoidal quinone) and cytochrome c (a low molecular-weight hemoprotein) act as major shuttles between the complexes. The electron flux is coupled to the translocation of protons (H+) into the intermembrane space at three coupling sites (complexes I, III, and IV) which create a transmembrane gradient. Complex V (ATP synthase) allows protons to flow back into the mitochondrial matrix by using proton motive force and uses the released energy to synthesize ATP [1-3]. As shown in figure 1, the proton movement through the F1FoATP synthase couples the energy-releasing reactions of oxidation to the energy-storing reaction of phosphorylation [1,2]. The energetic function of the isolated mitochondria can be assessed by polarographic measurement of oxygen consumption under various conditions which in turn confirm that the addition of Adenosine Di-Phosphate (ADP) to the isolated mitochondria increases the electron flow and oxygen consumption until the excess ADP is phosphorylated to ATP [2].
Mitochondria controlling the electron transport process
Being an extraordinary complex organelle, mitochondria contains their own DNA, a dual membrane: Outer Mitochondrial Membrane (OMM) and Inner Mitochondrial Membrane (IMM) which gives rise to two compartments; [1] the Inter Membrane Space (IMS) located between OMM and IMM, and [2] the Matrix (M) located in the internal space created by the IMM itself. Mitochondria, the cellular power house, are the major contributor to oxidative stress being the site of oxygen consumption [1,3,4]. The oxidative phosphorylation helps to remove electrons from biological fuels such as glucose, fatty acids etc which further go through a series of electron carrier system named the Mitochondrial Electron Transport Chain (ETC) located in IMM, generating energy in the form of ATP; and ultimately these electrons reduce the molecular oxygen to water [3]. It’s well known that electron transfer and ATP synthesis are indirectly coupled by a transmembrane electrochemical proton gradient which in turn simply proposed that the energy produced by electron transfer through ETC during oxidation metabolism, used to establish a proton gradient across the IMM that drives mitochondrial ATP production [1]. The proton gradient established by pumping protons against their electrochemical gradient across the IMM, can induce a proton gradient (chemical) across the membranes which is known as a Protonmotive force (ΔP) and electrical gradient known as mitochondrial membrane potential (ΔΨm) [1-4]. Scientists proposed a number of theories on this electron transport system, chemical coupling hypothesis for ATP synthesis, oxidative phosphorylation etc [1]. Most recently, a mechano-chemiosmotic model, discussed by Kasumov (2015) explaining the coupling of the electron transfer along ETC, proton transfer, transport of cations, cyclic low-amplitude swelling-shrinkage and ATP synthesis. This model uses the chemiosmotic model of Peter Mitchell as its basis, and supplements it by dynamic properties characteristic of biological structures with particular attention to a regulatory role of a low-amplitude swelling-shrinkage of organelles [1].
The Reactive Oxygen Species (ROS)
Mitochondria are the most significant reactive oxygen species ROS producers in the cell under non-pathological condition. Due to oxidative metabolism and oxidative damage, Mitochondria produce superoxide as a by-product which are a major source of intracellular reactive Oxygen Species (ROS) production as well as greatly dependent on proton leakage pathway which may cause numerous degenerative disorders contributing to the aging [4-7]. The primary (ROS) generated by ETC are known as superoxides. ROS, at higher amount are detrimental to cells, therefore, superoxide once produced get rapidly dismutated by Superoxide Dismutase (SOD) to hydrogen peroxide as a protective mechanism [8]. ROS at lower amount, works signaling molecules in various biological pathways. However, cell membrane bound Nicotinamide Adenine Dinucleotide Phosphate (NADPH) oxidase may contribute to total cellular ROS production during certain pathological conditions [9].
Mitochondrial proton leakage and electron leakage coupling to the oxidative phosphorylation and the ROS production
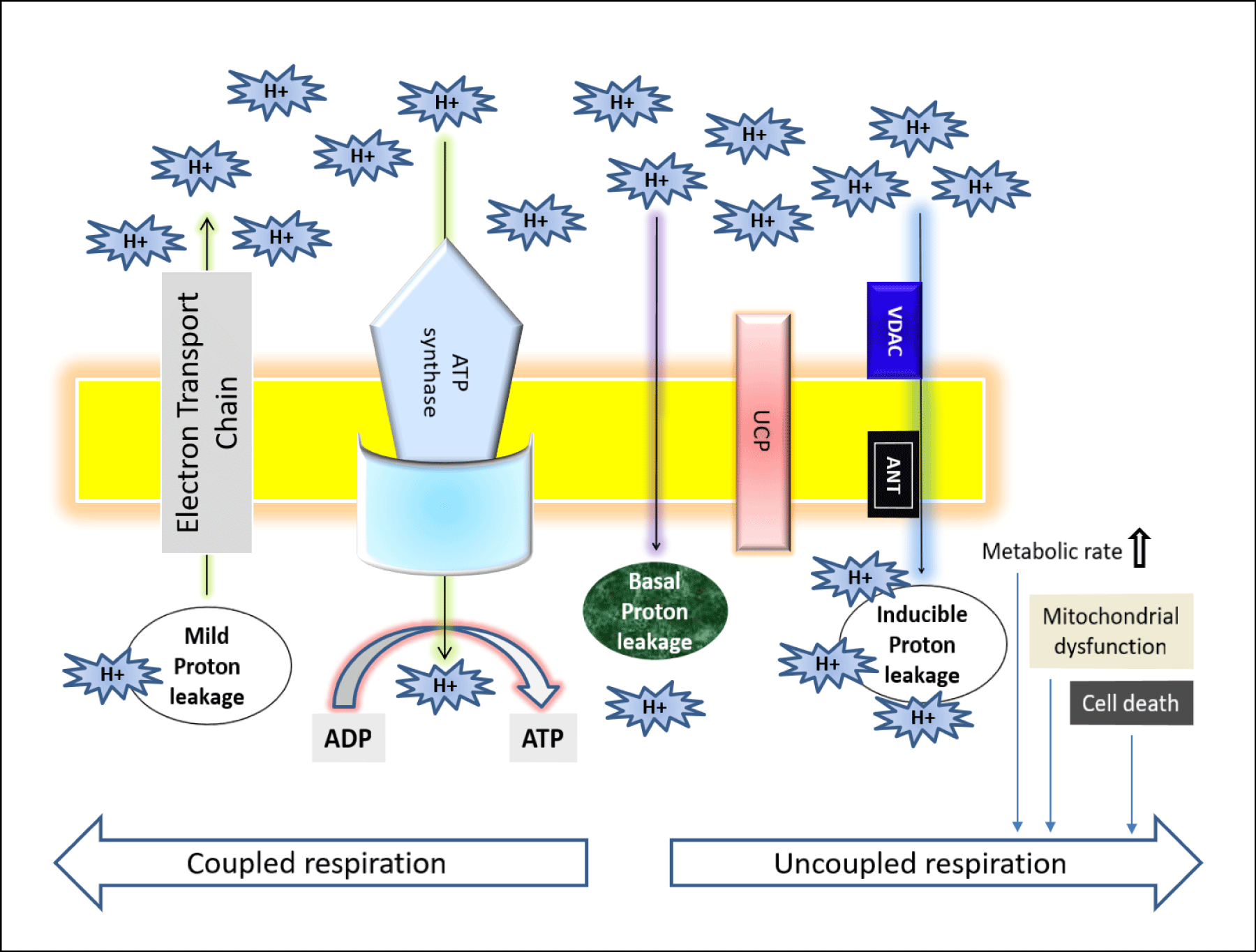
The mechanisms of proton and electron leakage are crucial to understand as they have a major physiological impact on mitochondrial coupling efficiency and production of reactive Oxygen Species (ROS) (Figures 2,3). The mitochondrial electron transport through complex I–IV create a proton motive force (ΔP) which will drive the protons back to matrix from IMS via ATP synthase [1]. However, proton migration towards the matrix independent of ATP synthase is usually called the “proton leak” which mostly occurs through the membrane proteins (e.g. uncoupling proteins, UCP) or by the non-specific transmembrane flux, depending on the lipid composition of the mitochondrial membrane [1,10]. Due to the proton leakage, high number of protons gather on one side of the membrane without producing ATP which causes the coupling of the substrate oxygen and hence the ATP generation remains incomplete [1]. Electron slip or electron leakage is the process where electrons are transported via ETC without pumping protons to IMS [1,10,11]. Thus, the term electron leakage refers to the exit of electrons from the ETC before they get reduced to water at Complex IV [1]. Therefore, electron slip results in disproportionate increase in oxygen consumption at high ΔP [11]. Energy coupling linked with oxidative phosphorylation, regulates metabolic homeostasis to maintain body function. Proton leakage due to this oxidative phosphorylation play functional role in thermogenesis by maintaining carbon flux despite low ATP demand, and modulating the nutrient response in glucose sensing cells [12]. The “uncoupling to survive” hypothesis explains the ubiquity of proton leakage, with regard to both cell type and phylogeny [13]. According to Kasumov’s mechano-chemiosmotic model, the ATP generation in mitochondria is a repeated cycling process which occurs in the presence of oxidative substrate, phosphate ions, ADP, and other essential ions. The cycling step sequences are as following:
- Polarization of inner membrane
- Movement of potassium ions to the matrix, and calcium ions to the intermembrane space
- Swelling of the mitochondrial matrix
- Shrinkage of intracristal space
- Reduction of Cyt c1
- Depolarization of inner membrane
- Movement of calcium ions into the matrix, and potassium ions to the intermembrane space
- Contraction of the mitochondrial matrix
- ATP synthesis
- Swelling of intracristal space
- Oxidation of Cyt c1
- Release of protons into the external medium (cytosol) [1].
Types of proton leakage
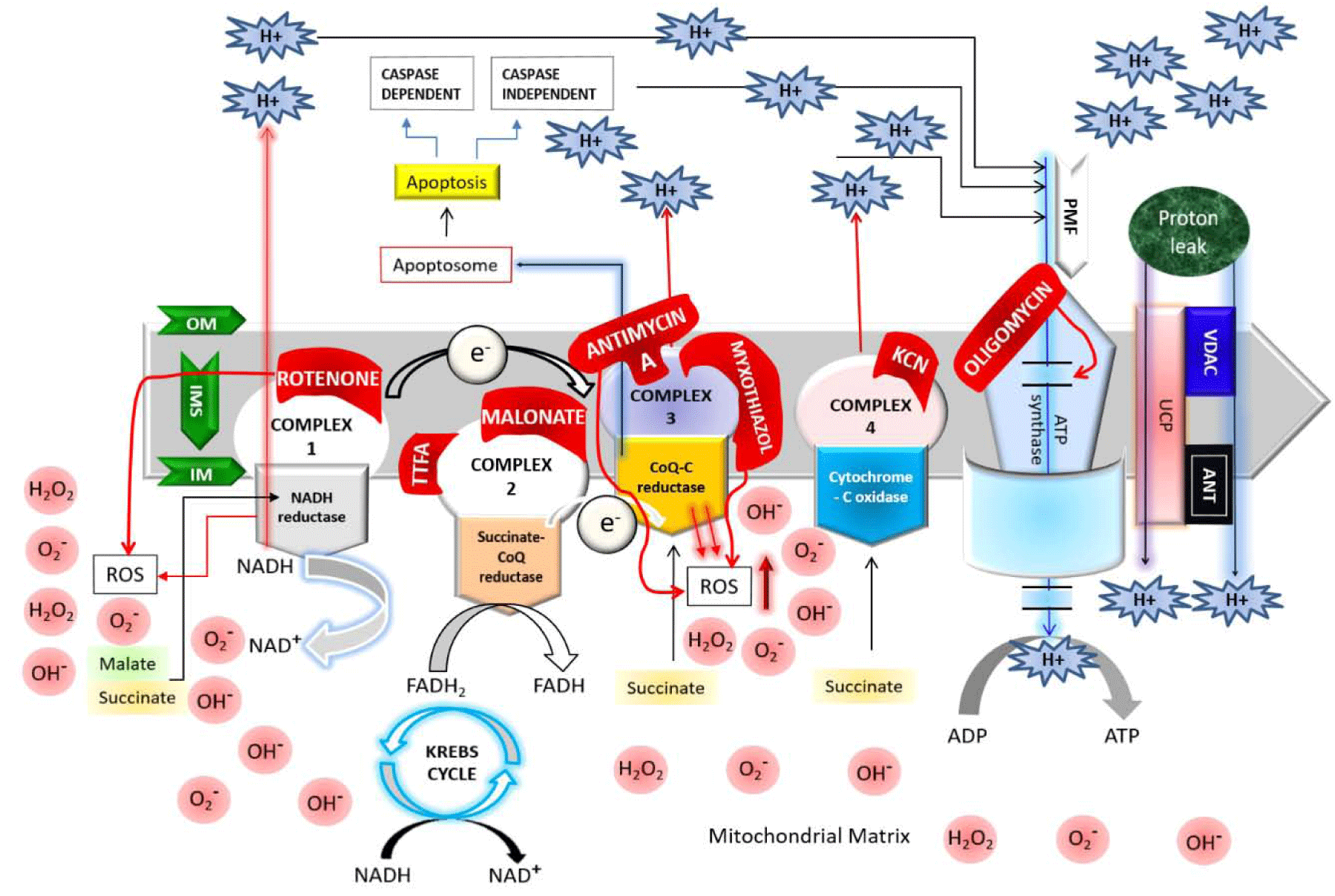
As shown in figure 2, the proton leakage pathway can be divided into two categories: Constitutive or basal proton conductance and the regulated or inducible proton conductance catalyzed by Uncoupling Proteins (UCPs). ROS produced via proton leakage are greatly dependent on the molecular nature of the basal and inducible proton leak pathways [14]. The major proportion of basal proton transition is regulated via mitochondrial inner membrane proteins and two-third of the basal proton conductance is correlated to the abundance of ANT (Adenine Nucleotide Translocase), which is a type of mitochondrial anion carrier protein found in the mitochondrial inner membrane [15]. So, it can be said that the basal leakage correlates with metabolic rate and a major proportion can be attributed to mitochondrial anion carriers, while the proton leak through the lipid bi-layer appears to be minor [14]. Although it’s known that the basal proton transition in liver mitochondria is inversely proportional to body mass, correlating with thyroid status and phylogeny; however, still the relation between the changes in liver mitochondrial conductance and hepatocyte respiration across species with altered coupling efficiencies are not clear due to the changes in substrate oxidation and ADP phosphorylation [16-20]. The mechanism and the factors responsible for basal proton leak are not fully understood. However, previous research demonstrates the following factors controlling this mechanism: (1) although proton transportation is dependent on the fatty acyl composition of inner membrane phospholipids detergent-free liposome research explains that only a small percentage of constitutive leak is mediated by the lipid bilayer [17,18]; (2) proton abundance controls the proton leakage; (3) the activity of the Adenine Nucleotide Translocase (ANT) does not mostly affects the proton transportation [19]; (4) mitochondrial inner membrane proteins are not considered as the factors of proton conductance but mitochondrial solute carrier family may play some role in it [20]; mitochondrial carriers are responsible for metabolite transport across the inner membrane [21-23]; (5) in the recent years, there is a controversy that the Uncoupling protein 1 (UCP1), found in brown adipose tissue mitochondria at comparable levels to ANT [21] contributes to basal proton leak in this tissue [24,25]; (6) an organism with increased capacity for oxidative phosphorylation is able to raise the ANT concentration and thus free-radical production is increased within it, and hence these organisms maintains a greater degree of protection from ROS via increased “mild uncoupling,” slightly lowering the proton motive force to prevent ROS production without deleteriously lowering ATP synthesis [19].
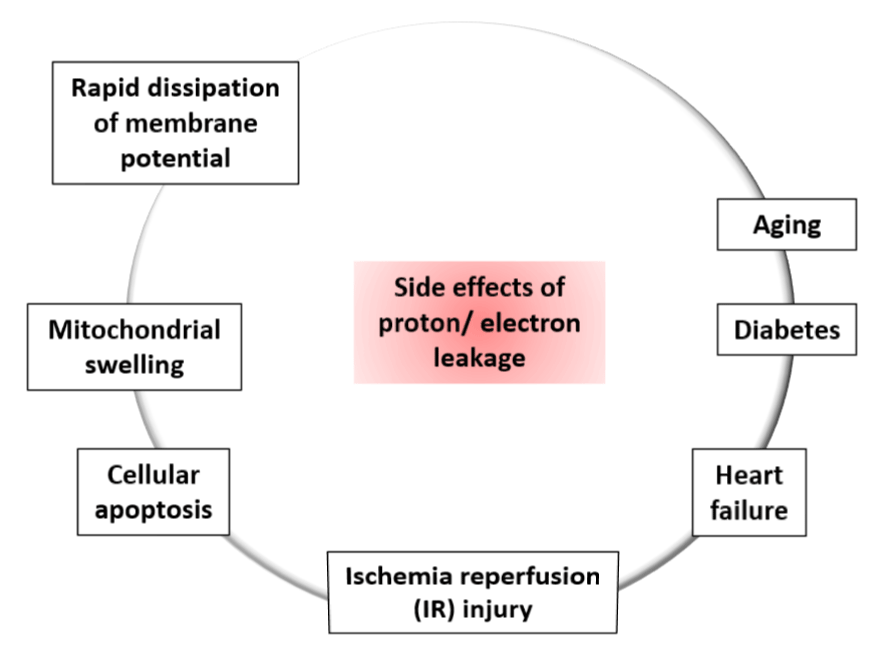
Inducible proton conductance catalyzed by specific mitochondrial inner membrane proteins such as the Uncoupling Proteins (UCPs) or the Nucleotide Translocase (ANT), is controlled on multiple levels, such as - molecular, transcriptional, translational, and proteolytic levels [26,27]. This type of leakage and changes due to this leakage may cause obesity, type 2 diabetes mellitus, the immune response, cancer, cardiovascular disease, and age-related disease caused by oxidative stress. Increased level of proton leakage is associated in mitochondria isolated from hearts subjected to IR [28]. Recent experiments suggested that intact rat neonatal ventricular myocytes exhibit exhaustion of the reserve capacity with an increased proton leak when exposed to 4 -Hydroxynonenal (HNE), which is a reactive lipid species accumulated in the heart during ischemia and heart failure [29]. Generally mild-moderate proton leak during IR injury is regulated by UCPs, whereas a larger proton leak is mediated by ANT [28]. The larger proton leakage mediated by the opening of Mitochondrial Permeability Transition Pore (MPTP) is detrimental to mitochondria and adversely influence to the cell viability as it causes rapid dissipation of the mitochondrial membrane potential (ΔΨm) leading mitochondrial swelling, and mediate cellular apoptosis or necrosis [30]. Therefore, according to these observations, the effects of proton-electron leakage within cell are predictive of several physiological dysregulation as presented in figure 3 [30].
Contradictory reports on the linkage between ROS production and proton leakage: Increased level of ROS is proportional to increase proton leakage [30]. Previous research reported that the Mitochondrial RNA (mtRNA) is decreased in the presence of ADP as well as dissipation of ΔP, while few claims that uncoupling enhances the ROS production [30,31]. The differences in biological and experimental settings might be the reason behind these contradictory hypotheses. As uncoupling decreases mitochondrial ROS production, it may play a protective role by mitigating ROS production in cells for example cyto-protective role of uncoupling was observed in heart under conditions of oxidative stress such as Ischemia Reperfusion (IR) injury, aging and diabetes [31-33]. Peroxynitrite, a potent inducer of lipid oxidation, is known to increase the proton leak whereas superoxide can enhance the electron leak to a similar extent as peroxynitrite, and was shown to activate UCPs [34]. Additionally, UCPs and ANTs induce proton conductance in the presence of ROS. Thus, increased ROS generation activates mechanisms that promote proton leak; and the enhanced proton leak in turn reduce the ROS production limiting further damage to mitochondrial function [30,34].
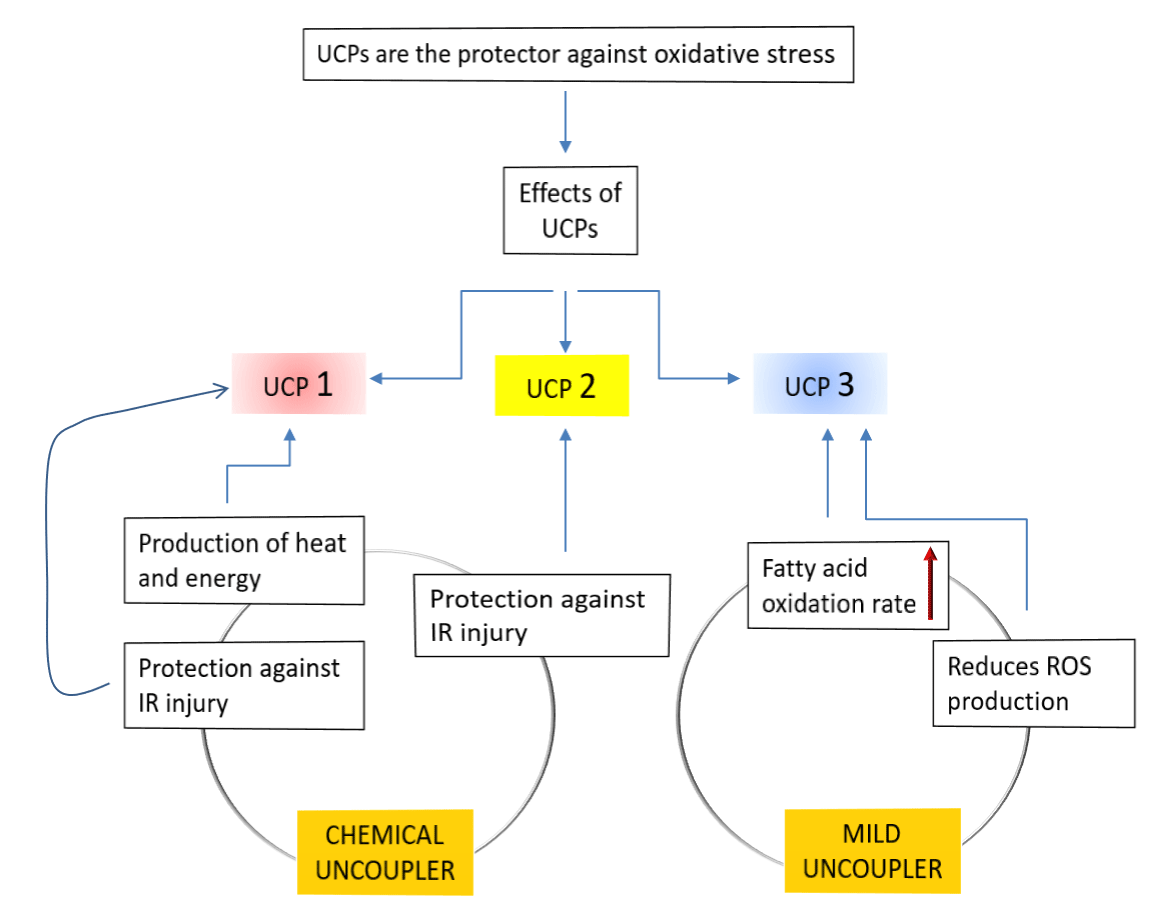
UCPs causing proton conductance: Generally, UCPs are a subfamily of the mitochondrial solute carrier family proteins. They induce transportation of various metabolites across the IMM. In mammals, five types of UCPs found, named as UCP1-5 among which UCP1 was the first to be identified in brown adipose tissue and had been extensively studied. According to the previous research data the physiological role of inducible leak through UCP1 in mammalian brown adipose tissue is heat production but the role of UCP2 and UCP3, are not yet resolved. Some data suggested that these two reduce the mitochondrial coupling efficiency, which is an evident that their role is largely focused on regulating Reactive Oxygen Species (ROS) levels rather that regulating thermogenesis [35,36]. Sometimes UCP1 and UCP2, and also chemical uncouplers offers protection against IR injury. Reportedly, during various cardiac pathologies increased levels of ROS are produced and these ROS mediate the breakdown of plasma membrane of cardiomyocytes, increasing the potential for arrhythmogenesis during a post-ischemic event and led to apoptosis of myocytes as well [37]. At this moment UCPs works as a protector against oxidative stress by suppressing ROS (Figure 4).
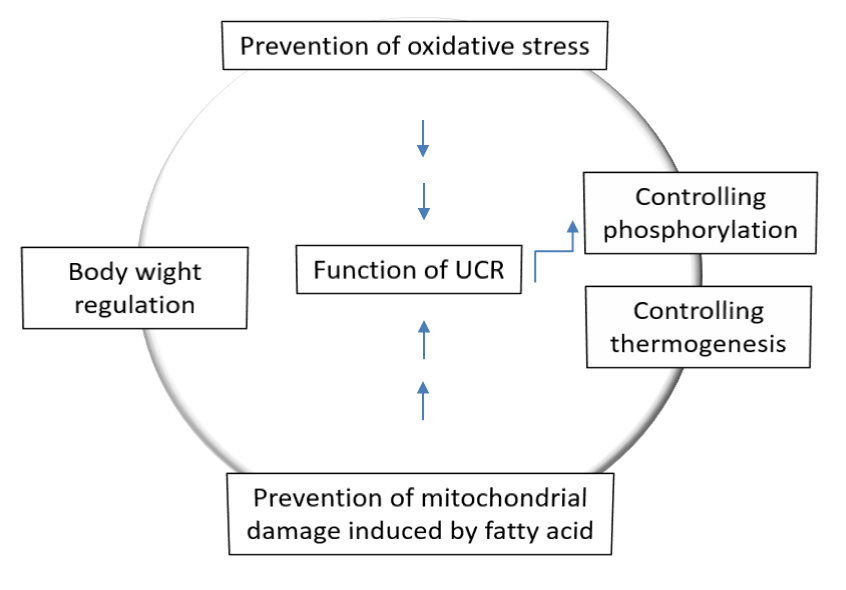
Transition from resting to exercise state of the body reduces proton leakage: Mitochondria and proton ‘leak’ respectively use proton gradients to make ATP and heat by increasing oxygen consumption (uncoupled respiration, UCR). According to Kent Sahlin the balance of these two consequences of fuel oxidation might be under physiological control but UCR alone may have, or may be associated with, important physiological functions e.g. thermogenesis and body weight regulation, prevention of oxidative stress, prevention of mitochondrial damage induced by Fatty Acids (FA) and control of oxidative phosphorylation (Figure 5). The energy will be dissipated as heat instead of being trapped as ‘useful energy’, i.e. ATP due to proton leakage (Figure 1). UCP1 promotes proton leak in brown adipose tissue to play a role in thermogenesis. UCP3, another homologous protein has been found in skeletal muscle but the significance of as a determinant of UCR and basal metabolic rate is under debate. UCP3 protects mitochondria from an overload of long chain Fatty Acid (FA) and its concentration is paralleled with plasma FA, both increases during fasting and high fat diets [38]. Additionally, UCP3 functions as a mild uncoupler and as such reduces formation of ROS. Indeed, UCR is an important factor in muscle energetics as well as a parameter under physiological control and can be both upregulated (overfeeding) and downregulated (exercise). Thus, proton leakage is reduced due to the transition from rest to exercise and futile proton cycling (pump and leak) may be a mechanism to control oxidative phosphorylation during rapid transitions in energy flux (Figure 4) [39].
Proton or electron leakage as therapeutic drug: Mitochondria, the power House of cell, regulates ATP by controlling the production of proton and electrons that is essential to maintain health and survival. But mitochondrial functional damage causes the toxic superoxide or ROS production which is account for one third of the volume of cardiomyocytes [40]. Thus, the disruption in the dynamic function of mitochondria causes aging or age-related diseases such as cardiovascular diseases. Increased ROS can enhance proton leakage but increased proton leak reduces the ROS generation. This suggests the existence of a protective feedback that helps to heighten the detrimental effects caused by ROS on biological systems. Additionally, cellular stresses are also able to enhance proton leakage without affecting the overall energy efficiency in endothelial cells. Thus, proton leakage acts as an indirect mediator of signal transduction by fine tuning ROS production, which serves as a signaling molecule to enhance endothelial activation during early atherogenesis, and contribute to the progression of the disease. That is why the regulation of proton leak can be a potential therapeutic target for the treatment of many cardiovascular disorders [40].
Within an electron transport chain of a prokaryotic or eukaryotic cell, as electrons move through the various supply pathways upstream of the Electron Transport System (ETS) and subsequently flow down the ETS, some of them prematurely exit, or leak out, before reaching the terminal acceptor (oxygen) at the level of complex IV. The leakage or inability of electron transfer depends on the capacity of electronegative molecular species, such as Dioxygen (O2), to snatch electrons away from proteins in the ETS and the various electron supply routes (Krebs cycle, mGPDH, β-oxidation of lipids) [4]. Superoxide (O2-) and Hydrogen Peroxide (H2O2) are the two common ROS produced by the electron leakage of mitochondria. According to previous research, skeletal muscle mitochondria collected from the rat, have been extensively characterized for the mechanisms and sites of electron leak and the contribution of H2O2 to ROS metabolism [41,42].
Pathways of electron leakage
ATP production in mitochondria by the way of electron transfer of respiratory chain is always combined with the generation of O2- and H2O2 through any one specific the electron leak pathways among four in the mitochondria mentioned below [43-45]. Among the possible electron leakage pathways, two pathways are of the cytochrome c mediated pathway, which play a protective role by the way of down regulating the level of O2- and H2O2 in mitochondria [44,45]. The third pathway is O2-. + H+→HOO- which one is somehow related to maintain body temperature because the reaction of HOO with the double allyl hydrogen atom of the unsaturated fatty acid is a heat producing reaction [44]. The last and fourth pathway is O2- + NO→ONOO- where the ONOO- could pass through the membrane when it combines with H+ [43,45]. Indeed, superoxides are mainly produced from complexes I and III, but some mitochondrial proteins such as FAD-linked pyruvate and α-ketoglutarate dehydrogenase complexes are also known to generate mitochondrial superoxide [8].
Major effects of electron leakage
Firstly, the explosive generation of ROS by the electron leakage of respiratory chain plays a key role in the damage of ischemia reperfusion which indicates the pathological role of electron leakage of respiratory chain in disease [43-45]. Secondly, the ROS in mitochondria play as the signals to stimulate anti-oxidative function and the ability of damage repairing, it is considered as a dangerous factor causing oxidative damager and aging. Thirdly, the fourth pathway plays an important role in controlling the rate of ATP production in different physiological conditions. Because NO can be bind to the oxygen reactive center of complex IV to reduce ATP production of mitochondria, while the O2-. can release the bond NO to increase the ATP production of mitochondria [43]. Fourthly, the cytochrome c is a scavenger to dispose of O2- and H2O2 in the space of mitochondrial membranes. The major function of cytochrome c is to produce ATP in the respiratory chain depending on its location in the mitochondria and stimulate cell apoptosis in cytosol out of mitochondria. The O2-. generated in the electron leakage of respiratory chain plays a key role in driving cytochrome c migrating as well as cell apoptosis and aging [43-47]. It’s assumed by scientists that the levels of ROS increases with age so it is considered as a poison which causes chronic phenoptosis [47].
Aging and age-related diseases
As UCPs are involved in superoxide as well as ROS production, they are thought to cause age-related disease [45-47]. Depending on the “uncoupling to survive” hypothesis some previous research was able to build a positive correlation between increased lifespan and proton conductance in skeletal muscle mitochondria along with evidence for lifespan extension on dinitrophenol administration [48,49]. Additionally, a controversial explanation was found from the experiment on the effects of uncoupling proteins on lifespan in both flies and mice [50-52]. Furthermore, mild uncoupling may prove effective in treating pathologies caused by high ROS production. It is important to understand the effect of mild uncoupling at the cellular level as well as describe the physiological mechanism of UCP activation [51]. It is believed that the mitochondria in living cells, features a universal mechanism is completely able to prevent Mitochondrial Reactivate Oxygen Species (mROS) production as they have the potential to supply great deal of mROS. The mechanism causes mild depolarization of the inner mitochondrial membrane, decrease the membrane potential to a level sufficient to make ATP which might be insufficient to get mROS. Kasumov, et al. [53] assume that an mROS formation and aging are dependent on the cyclic low-amplitude swelling-shrinkage of mitochondria. According to the mechano-chemiosmotic mechanism an asymmetric contact of dimers of opposite cyt bc1 complexes is formed in the intracristae space during shrinkage of organelles, which is a mechanical regulator of electron transfer from the [2Fe-2S] cluster to heme c1. Hence, mROS is formed when the mitochondria are in a swollen state, and the amount of ROS depends on the time during which the mitochondria are in the swollen state. Reportedly, aging is caused by mild depolarization process in short-lived mice, leading to chronic poisoning of the organism with mROS whereas mild depolarization still functions for several years in long-lived naked mole rats and bats.
In fine, mitochondria can serve as a major site of oxygen consumption as well as a major source of endogenous oxidative stress. Researchers have performed a numerous research by using chemical inhibitors to identify site-specific defects in the respiratory chain where electrons leak and how ROS production increase [44]. In the aerobic eukaryotic cell, the mitochondrial electron transfer for oxidative ATP regeneration is responsible for the production of Reactive Oxygen Species (ROS) which is a dangerous factor causing oxidative damage and aging. ROS have physiological impact on the survivability of the eukaryotes as they can contribute to signaling as well as oxidative damage in cells (Figure 3). The regulation of the electron/ proton leakage through the mitochondrial electron transport chain has been schematically shown in the proposed models as presented by figures 4 and 5. Finally, in the proposed model through figure 6, the increased electron leakage has been shown to account for the mitochondrial dysfunction as well as the generation of oxidative stress.
The dynamic functions of mitochondria have built up a major impact on our modern science due to the growing number of age-associated pathogenesis caused by mitochondria. Similar to the previous reports, current review also incremented to the idea that when electrons leak from the electron transport chain due to any factor such as chemical inhibition or the site-specific defect, the normal respiration is hampered and an elevated level of the ROS is noticed which is indeed detrimental for the health. The novelty of this article lies under the proposed models of both the proton and electron leakage as well as the regulation scheme. However, new scientific methodology and research will be more applicable to clearly understand the response of electron transport process in response to the oxidative stress. Such mitochondrial dynamics will lead to new therapeutic approaches for the prevention or amelioration of age-associated degenerative diseases.
- Kasumov EA, Kasumov RE, Kasumova IV. A mechano-chemiosmotic model for the coupling of electron and proton transfer to ATP synthesis in energytransforming membranes: A personal perspective. Photosynthesis Research. 2015; 123: 1-22. DOI: 10.1007/s11120-014-0043-3
- Li X, Fang P, Mai J, Choi ET, Wang H, Yang XF. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J Hematol Oncol. 2013; 6: 19. DOI: 10.1186/1756-8722-6-19
- Rich PR, Marechal A. The mitochondrial respiratory chain. Essays Biochem. 2010; 47: 1-23. DOI: 10.1042/bse0470001
- Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry. 2005; 70: 200-214. DOI: 10.1007/s10541-005-0102-7
- Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol. 2010; 45: 466-472. DOI: 10.1016/j.exger.2010.01.003
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009; 417: 1-13. DOI: 10.1042/BJ20081386
- Lambert AJ, Brand MD. Superoxide production by NADH: Ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem J. 2004; 382: 511-517. DOI: 10.1042/BJ20040485
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009; 417: 1-13. DOI: 10.1042/BJ20081386
- Santulli G, Xie W, Reiken SR, Marks AR. Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci. 2015; 112: 11389-11394. DOI: 10.1073/pnas.1513047112
- Brand MD, Pamplona R, Portero-Otin M, Requena JR, Roebuck SJ, et al. Oxidative damage and phospholipid fatty acyl composition in skeletal muscle mitochondria from mice under expressing or overexpressing uncoupling protein 3. Biochem J. 2002;368, 597-603. DOI: 10.1042/BJ20021077
- Kadenbach B. Intrinsic and extrinsic uncoupling of oxidative phosphorylation. Biochim Biophys Acta. 2003; 1604: 77-94. DOI: 10.1016/s0005-2728(03)00027-6
- Sweetlove LJ, Lytovchenko A, Morgan M, NunesNesi A, Taylor NL, Baxter CJ, et al. Mitochondrial uncoupling protein is required for efficient photosynthesis. Proc Natl Acad Sci. 2006; 103: 19587-19592. DOI: https://doi.org/10.1073/pnas.0607751103
- Brand MD. Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp Gerontol. 2000; 35: 811-820. DOI: 10.1016/s0531-5565(00)00135-2
- Martin J, Ajit SD, Shona M, Jason R. Treberg, Martin DB. Mitochondrial proton and electron leaks. Essays Biochem. 2010; 47: 53-67. DOI: 10.1042/bse0470053
- Brand MD, Pakay JL, Ocloo A, Kokoszka J, Wallace DC, Brookes PS, et al. The basal proton conductance of mitochondria depends on adenine nucleotide translocase content. Biochem J. 2005; 392: 353-562. DOI: 10.1042/BJ20050890
- Brand MD. The efficiency and plasticity of mitochondrial energy transduction. Biochem Soc Trans. 2005; 33: 897-904. DOI: 10.1042/BST0330897
- Brand MD, Turner N, Ocloo A, Else PL, Hulbert AJ. Proton conductance and fatty acyl composition of liver mitochondria correlates with body mass in birds. Biochem J. 2003; 376: 741-748. DOI: https://doi.org/10.1042/bj20030984
- Hulbert AJ, Else PL. Membranes and the setting of energy demand. J Exp Biol. 2005; 208: 1593-1599. DOI: 10.1242/jeb.01482
- Brand MD, Pakay JL, Ocloo A, Kokoszka J, Wallace DC, Brookes PS, et al. The basal proton conductance of mitochondria depends on adenine nucleotide translocase content. Biochem J. 2005; 392: 353-362. DOI: 10.1042/BJ20050890
- Stuart JA, Cadenas S, Jekabsons MB, Roussel D, Brand MD. Mitochondrial proton leak and the uncoupling protein 1 homologues. Biochim Biophys Acta. 2001; 1504: 144-158. DOI: https://doi.org/10.1016/S0005-2728(00)00243-7
- Pebay PE, Dahout GC, Kahn R, Trezeguet V, Lauquin GJ, Brandolin G. Structure of mitochondrial ADP/ATP carrier in complex with carboxy atractyloside. Nature. 2003; 426: 39-44. DOI: 10.1038/nature02056
- Robinson AJ, Overy C, Kunji ER. The mechanism of transport by mitochondrial carriers based on analysis of symmetry. Proc Natl Acad Sci. 2008; 105: 17766-17771. DOI: https://doi.org/10.1073/pnas.0809580105
- Roussel D, Harding M, Runswick MJ, Walker JE, Brand MD. Does any yeast mitochondrial carrier have a native uncoupling protein function? J Bioenerg Biomembr. 2002; 34: 165-176. DOI: 10.1023/a:1016027302232
- Parker N, Crichton PG, Vidal-Puig AJ, Brand MD. Uncoupling protein-1 (UCP1) contributes to the basal proton conductance of brown adipose tissue mitochondria. J Bioenerg Biomembr. 2009; 41: 335-342. DOI: 10.1007/s10863-009-9232-8
- Shabalina IG, Ost M, Petrovic N, Vrbacky M, Nedergaard J, Cannon B. Uncoupling protein-1 is not leaky. Biochim Biophys Acta. 2010; 1797: 773-784. DOI: 10.1016/j.bbabio.2010.04.007
- Parker N, Vidal PA, Brand MD. Stimulation of mitochondrial proton conductance by hydroxynonenal requires a high membrane potential. Biosci Rep. 2008; 28: 83-88. DOI: 10.1042/BSR20080002
- Azzu V, Brand MD. The on-off switches of the mitochondrial uncoupling proteins. Trends Biochem Sci. 2010; 35: 298-307. DOI: 10.1016/j.tibs.2009.11.001
- Quarrie R, Cramer BM, Lee DS, Steinbaugh GE, Erdahl W, Pfeiffer DR, et al. Ischemic preconditioning decreases mitochondrial proton leak and reactive oxygen species production in the postischemic heart. J Surg Res. 2011; 165: 5-14. DOI: 10.1016/j.jss.2010.09.018
- Nadtochiy SM, Tompkins AJ, Brookes PS. Different mechanisms of mitochondrial proton leak in ischaemia/reperfusion injury and preconditioning: Implications for pathology and cardio-protection. Biochem J. 2006; 395: 611-618. DOI: 10.1042/bj20051927
- Hill BG, Dranka BP, Zou L, Chatham JC, Darley-Usmar VM. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem J. 2009; 424: 99-107. DOI: 10.1042/BJ20090934
- Brookes PS. Mitochondrial H (+) leak and ROS generation: an odd couple. Free Radic Biol Med. 2005; 38: 12-23. DOI: 10.1016/j.freeradbiomed.2004.10.016
- Ganote CE, Armstrong SC. Effects of CCCP-induced mitochondrial uncoupling and cyclosporinE on cell volume, cell injury and preconditioning protection of isolated rabbit cardiomyocytes. J Mol Cell Cardiol. 2003; 35: 749-759. DOI: 10.1016/s0022-2828(03)00114-7
- Speakman JR, Talbot DA, Selman C, Smart S, McLaren JS, Redman P, et al. Uncoupled and surviving: Individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell. 2004; 3: 87-95. DOI: 10.1111/j.1474-9728.2004.00097.x
- Green K, Brand MD, Murphy MP. Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes. 2004; 53: S110-S118. DOI: 10.2337/diabetes.53.2007.s110
- Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, et al. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002; 415: 96-99. DOI: 10.1038/415096a
- Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet. 2000; 26: 435-439. DOI: 10.1038/82565
- Vidal-Puig AJ, Grujic D, Zhang CY, Hagen T, Boss O, Ido Y, et al. Energy metabolism in uncoupling protein 3 gene knockout mice. J Biol Chem. 2000; 275: 16258-16266. DOI: 10.1074/jbc.M910179199
- Azzu V, Brand MD. The on-off switches of the mitochondrial uncoupling proteins. Trends Biochem Sci. 2010; 35: 298-307. DOI: 10.1016/j.tibs.2009.11.001
- Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008; 88: 581-609. DOI: 10.1152/physrev.00024.2007
- Hoeks J, Hesselink MK, van Bilsen M, Schaart G, van der Vusse GJ, Saris WH, et al. Differential response of UCP3 to medium versus long chain triacylglycerols; manifestation of a functional adaptation. FEBS Lett. 2003; 555, 631-637. DOI: https://doi.org/10.1016/S0014-5793(03)01343-7
- Sahlin K, Tonkonogy M, Fernstrom M. The leaky mitochondria. Physiology News, 2004; 56: 27-28. DOI: https://doi.org/10.36866/pn.56.27
- Goffart S, von Kleist-Retzow JC, Wiesner RJ. Regulation of mitochondrial proliferation in the heart: Power-plant failure contributes to cardiac failure in hypertrophy. Cardiovasc Res. 2004; 64: 198-207. DOI: https://doi.org/10.1016/j.cardiores.2004.06.030
- Brand MD. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Rad Biol Med. 2016; 100: 14-31. DOI: 10.1016/j.freeradbiomed.2016.04.001
- Munro D, Treberg JR. A radical shift in perspective: mitochondria as regulators of reactive oxygen species. J Exp Biol. 2017; 220: 1170-1180. DOI: 10.1242/jeb.132142
- Jian-Xing X. The Electron Leak Pathways of Mitochondrial Respiratory Chain and its Potential Application in Medical Research. JSM Cell Dev Biol. 2015; 3: DOI: 1014. https://tinyurl.com/y2bkzyws
- Iqbal MD, Cawthon RF, WG Bottje. Lung mitochondrial dysfunction in pulmonary hypertension syndrome I. Site-specific defects in the electron transport chain. Poultry Sci. 2001; 80: 485-495. DOI: 10.1093/ps/80.4.485
- Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free RadicBiol Med 2007; 43: 477-503. DOI:10.1016/j.freeradbiomed.2007.03.034
- Sanz A, Pamplona R, Barja G. Is the mitochondrial free radical theory of aging intact? Antioxid Redox Signal. 2006; 8: 582-599. DOI: 10.1089/ars.2006.8.582
- Speakman JR, Talbot DA, Selman C, Smart S, McLaren JS, Redman P, et al. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell. 2004; 3: 87-95. DOI: 10.1111/j.1474-9728.2004.00097.x
- Caldeira da Silva CC, Cerqueira FM, Barbosa LF, Medeiros MH, Kowaltowski AJ. Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity. Aging Cell. 2008; 7: 552-560. DOI: 10.1111/j.1474-9726.2008.00407.x
- Andrews ZB, Horvath TL. Uncoupling protein-2 regulates lifespan in mice. Am J Physiol Endocrinol Metab. 2009; 296: E621-E627. DOI: 10.1152/ajpendo.90903.2008
- Johnson-Cadwell LI, Jekabsons MB, Wang A, Polster BM, Nicholls DG. ‘Mild uncoupling’ does not decrease mitochondrial superoxide levels in cultured cerebellar granule neurons but decreases spare respiratory capacity and increases toxicity to glutamate and oxidative stress. J Neurochem. 2007; 101: 1619-1631. DOI: 10.1111/j.1471-4159.2007.04516.x
- Kasumov EA, Kasumov RE, Kasumova IV. Mild depolarization of the inner mitochondrial membrane is a crucial component of the mechano-chemiosmotic mechanism of coupling. J Nov Physiother Phys Rehabil. 2020; 7: 033-035. DOI: 10.17352/2455-5487.000075
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.