> Medicine. 2020 August 23;1(4):092-102. doi: 10.37871/jels1125.
-
Subject area(s):
- Neurological Disorders
- Neurology
- Neurosurgery
Ecological Dynamics: An Inspiration for Triggering Epilepsy
Ambreen Kanwal and Asima Tayyeb*
- Epilepsy
- Seizures
- Environmental factors
Epilepsy is a rigorous transmission of electrical impulses across neurons of the brain and reported more prevalent in lower-income countries. A systematic literature review has been performed to implicate the impact of environmental variables on the occurrence of epilepsy using the following keywords: Epilepsy or environmental risk factors or seizures. More than 500 genes have been reported to involve in epilepsy potentially. Moreover, family history with neurological disorders, sleep apnea, depression, alcohol, stress, diet, gestational period of mother, and social involvement are among the risk factors which can reliably predict onset and severity of the disease. Hence, minimizing these factors along with recommended therapies, counseling, and awareness could be a miracle in the life of epileptic patients and can improve societies.
Epilepsy refers to a group of disorders that result in eliciting the rapid discharge of electrical impulses across the motor, sensory, and associated brain neurons and leads to convulsions and unconsciousness. It is a neurological disorder in which the clusters of neurons transmit electrical impulses 500 times more sharply than the normal ones [1]. The rate of seizure incidence varies from two seizures in less than 24 hours to one seizure in two days [2]. In the world, more than 50 million people have suffered from epilepsy. About 90% epilepsy has been recognized in developing countries. The mentioned disease is more prevalent in Asia, as it has affected about 23 million Asians as compared to 3.3 million Africans and 1.2 million sub-Saharan Africans [3]. New cases of epilepsy have been reported among infants and elderly people. In the United States, one out of hundred has been diagnosed with epilepsy for which 75-80% can be treated with modern medicines and surgical techniques [4]. In France, 22.5% patients possess drug resistant epilepsy. In Pakistan, rural population is more affected (10/1000 per year) than the urban population [5].
A number of genetic defects (in almost 500 genes) have been found tied with epilepsy like mutations in Cystatin B gene, HNRNPU, IQSEC2, CACNA1A, CHD2, MTOR GABRB, GABRA1, GRIN2B, NEDD4L, PCDH7, GRIN1, ALG1, and FLNA genes. Some of these are involved in neuronal signaling like PCDH7 variants causes disruption of protocadherin 7, a molecule involved in neuronal cell-cell adhesion during synapse development. HCN1 variants also implicate ion channel disturbance in humans [6]. A number of other genes involved in ion flow are SCN1A (Generalized epilepsy with febrile seizures), SCN2A (Generalized epilepsy with febrile and a-febrile seizures), SCN1B (Generalized epilepsy with febrile seizures), KCNQ3 (Benign familial neonatal convulsion epilepsy), GABRA1 (Juvenile myoclonic epilepsy), GABRG2 where distorted pore forming alpha (α) subunits or accessory beta (β) subunits cause these channels to pass neuronal impulses inappropriately leading to seizures [7,8]. Another gene on X-chromosome, Fgf13 (fibroblast growth factor 13), codes for auxiliary protein of voltage-gated Na+ channels. Reduced expression in Fgf13 mRNA reveals decreased inhibitory and increased excitatory synapses in hippocampal neurons [9]. These genes run in families and produce a characteristic seizure. These are much more diverse than their similar ones. SLC2A1 gene which encodes glucose transporter 1 (GLUT1) is responsible for regulating the movement of glucose in the brain. A number of brain disorders like microcephaly, developmental delay, Non-Acquired Focal Epilepsy (NAFE), and generalized epilepsy are caused due to mutations in SLC2A1 gene. In July 2017, 200 patients were studied with NAFE out of which 126 were with temporal lobe epilepsy. Ten exons and their splice site regions were amplified by Polymerase Chain Reaction (PCR) and sequenced by Sanger Sequencing but no variants were detected, which concluded less contribution of GLUT1 mutation in NAFE [10]. Micro-deletion on gene CHRNA7 chromosome 8q, DOK5 on chromosome 20q13 and PCYTIB genes have been found responsible for Electrical Status Epilepticus (ESES) [11].
In January 2016, one of the girls in identical twins experienced nocturnal or night sleep seizures [12,13] and generalized seizures at the age of 6 years whereas her identical twin was found normal, which suggested that environmental factors are responsible to induce epilepsy [14]. Epigenetics have been reported to cause ESES in monozygotic twins resulting in 75% cases of epilepsy [15].
Epilepsy also develops as a result of other brain disorders like brain tumors, Alzheimer’s disease, sleep apnea, head injuries, lead poisoning and mal-development of the brain [16]. Conditions like hydrocephalus, AIDS, heart attacks, meningitis, strokes, viral encephalitis and other infectious diseases deprive the oxygen (O2) from the brain cells and lead to develop epilepsy with the passage of time [16] especially in older people [17]. Down’s Syndrome (DS), which is a main genetic cause of mental retardation, presents 1-13% patients with epilepsy. DS patients have better control on seizures as compared to infantile spasms when early treatment is started. Heart attacks and strokes have also been observed as a stimulus of epilepsy in the people of age over 45 years.
Neurocysticercosis (NCC) is a brain infection of humans and pigs that is caused by a parasite Taenia solium. Frequency of NCC among epileptic patients is estimated around 30% from 12 different studies conducted in America, Africa and South East Asia [18]. Autism Spectrum Disorder (ASD) is a group of disorders characterized by problems in social behaviors and communications in children. Epilepsy and ASD are associated with each other since a long time [19,20]. In ASD patients, prevalence of epilepsy is 10-30% as compared to the normal population where it is 2-3% [20]. Patients with ASD and epilepsy are more associated with social problems, incontinence, language problems and behavior disorders. Patients with cerebral palsy and ASD are at a greater risk for epilepsy [21]. Epileptic encephalopathies refer to conditions in which neurologic deterioration impairs sensory, motor and cognitive functions resulting paroxysmal epileptic activity [22].
In Dravet Syndrome, patient faces seizures from the age of six months to severe status epilepticus at the end. Neurologic deterioration has also been seen in Rassumen Syndrome due to epilepsy. In West Syndrome, Ohtahara Syndrome and Myoclonic Encephalopathy, continuous spike waves are discharged as a result of sub-continuous paroxysmal interictal activity [23].
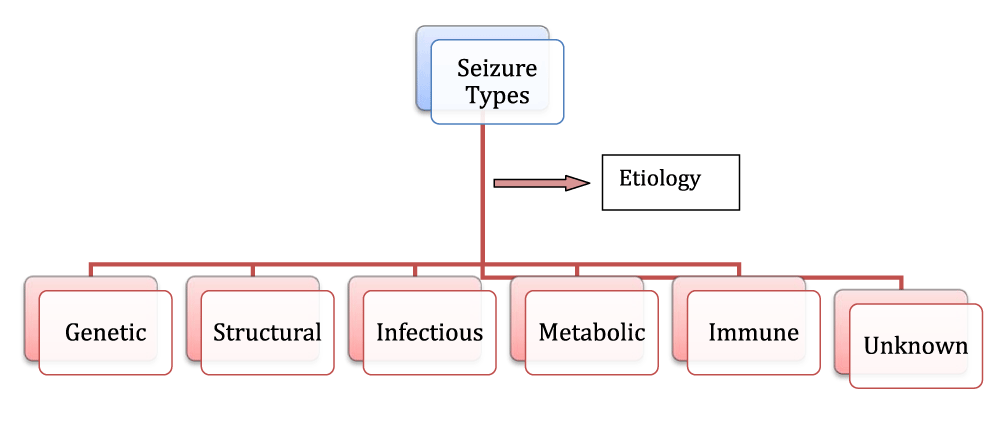
Seizures categories on the basis of Etiology
Some types of epilepsy are confined to particular stages of life but it cannot be considered as a single disorder because of its different symptoms with multiple seizures (Figure 1) all involving abnormal neuronal activity in brain [23].
Seizures in the mature brain cause more cell death as compared to immature brain, triggering irreversible adverse effect on neuronal activity of the brain [24].
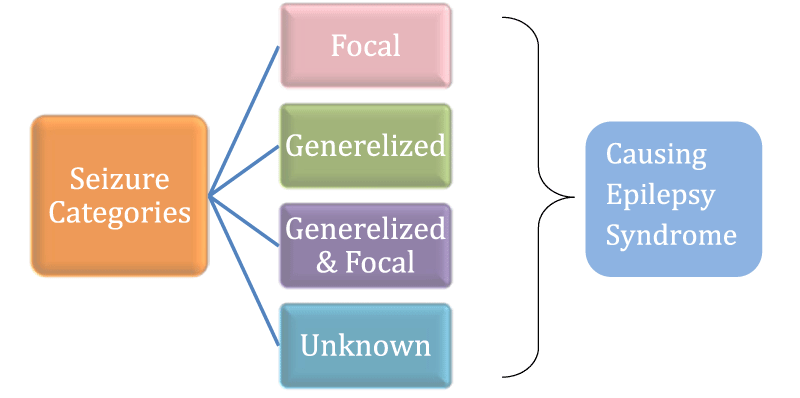
Seizure categories on the basis of the brain area
Seizures are categorized into four main types, generalized seizures, focal seizures, Unknown and focal to bilateral seizure (Figure 2) on the basis of area of the brain from which seizures begin [25-51] as given in table 1.
| Table 1: Categories of Seizures. | |||
| Generalized seizures Electrical impulses throughout the brain |
Characteristics | Recommended Treatment | References |
| Grand mal (Generalized tonic clonic) | Age dependent | Valproic acid or Levetiracetam | [26] |
| Typical absence | In childhood and adolescent | Ethosuximide and Valproic acid | [27] |
| Atypical absence | Children | Levetiracetam and Rufinamide | [28] |
| Myoclonic | Children | Sodium valproate | [29] |
| Myoclonic absence | Ictal automatisms | Valproate | [30] |
| Myoclonic atonic | Childhood | Valproate | [31] |
| Eyelid myclonia | Valproate | [32] | |
| Febrile or tonic clonic | Unknown eitology | Anakinra | [33] |
| Focal or Partial Seizures Produced in a specific area of brain |
Perampanel | [34] | |
| Occipital and parietal lobe seizures | Type of aura | Focal resection | [35,36] |
| Temporo parieto occipital junction seizures | Vertigo | Surgery | [36,37] |
| Focal motor seizures | Management dilemma | Botulinum toxin | [38] |
| Typical temporal lobe automatisms (mesial temporal lobe seizures) | Ablated volumes | Laser interstitial thermal therapy | [39] |
| Hyperkinetic automatisms | Kicking, rocking | [40] | |
| Focal negative myoclonus | Centrotemporal spikes | Valproate and Levetiracetam | [41] |
| Inhibitory motor seizures | Eyelid fluttering | [42] | |
| Gelastic seizures | Hypothalamus is epileptogenic zone | Surgery | [43] |
| Hemiclonic seizures | KCNQ3 mutation | Vaiproic acid | [44] |
| Secondarily generalized | Seizures in cortex | Surgery | [45] |
| Continuous seizure types | |||
| Generalized status epilepticus | Glutamate mediate cytotoxicity | [46] | |
| Generalized tonic-clonic status epilepticus | Cognitive impairment | Perampanel | [47] |
| Clonic status epilepticus | - | - | |
| Absence status epilepticus | - | - | |
| Tonic status epilepticus | - | - | |
| Myoclonic status epilepticus | Cognitive decline | ||
| Focal status epilepticus | Glycine receptor antibodies | [48] | |
| Epilepsia partialis continua of Kojevnikov | Transcranial magnetic stimulation | Cortical stimulation | [49] |
| Aura continua | Visual aura | Indomethacin and topiramate | [50] |
| Limbic status epilepticus (psychomotor status) | Mesial temporal lobes | AEDs | [51] |
In childhood, febrile seizures are the most common type occurring in 2-5% children in initial five years of age [52]. Febrile seizures usually occur before or after onset of fever and increase with the child’s temperature [53]. In identical twins generalized and febrile seizures are found more prevalent than partial ones [54].
Epilepsy is categorized on the basis of underlying causes as Idiopathic (genetic causes) or symptomatic (cause known) or cryptogenic (cause unknown). Different types of epilepsy are also known as generalized or partial on the basis of the brain area involved as listed in table 2.
| Table 2: Categories of Epilepsy. | |||
| Types of Epilepsy | Generalized Epilepsy | Partial Epilepsy | References |
| Idiopathic (genetic causes) | Childhood absence epilepsy, Juvenile myoclonic epilepsy, epilepsy with grand-mal seizures only | Benign focal epilepsy of childhood | [55,56] |
| Symptomatic (cause known) or cryptogenic (cause unknown) | West syndrome Lennox-Gastaut syndrome | Temporal lobe epilepsy, Frontal lobe epilepsy |
[57,58] |
Epilepsy can also be categorized as temporal and parietal depends on whether seizures originate from temporal lobe or parietal lobe of brain. Temporal Lobe Epilepsy (TLE) is one of most common types of partial onset epilepsies in adults [59]. TLE is one of the most common types of Partial Epilepsies (PE) which account for 60% adult epilepsy cases. In TLE patients, conflicts in processing and inhibition of response have been observed. In TLE patients, right hemisphere frontal lobe, right frontal junction, middle frontal, superior frontal and inferior frontal showed more activation as compared to control ones [60]. In these patients, neuro-cognitive effects have been observed when the disease onset was early and duration was long. Pathological state of hippocampus has been found linked to executive functions in 70% patients [61].
Extra temporal abnormalities have been observed in white matter and grey matter in TLE patients due to which hippocampus creates alterations in orbito-medical, fronto-striatal and tempo-frontal circuits as a result of which patients were no more able to use previously stored memory to use for future actions [61].
Status Epilepticus (SE) has been found acute symptomatic with bimodial distribution having peaks in children less than age of one year and elderly people. Short term mortality rate is 7.6-22% and long-term mortality rate is 43%. Aetiology and patients age are determinants of mortality [62]. Reflex Epilepsy (RE) is characterized in which generalized and myclonus convulsive and non-convulsive seizures and partial seizures are prevalent among children and adolescents [63].
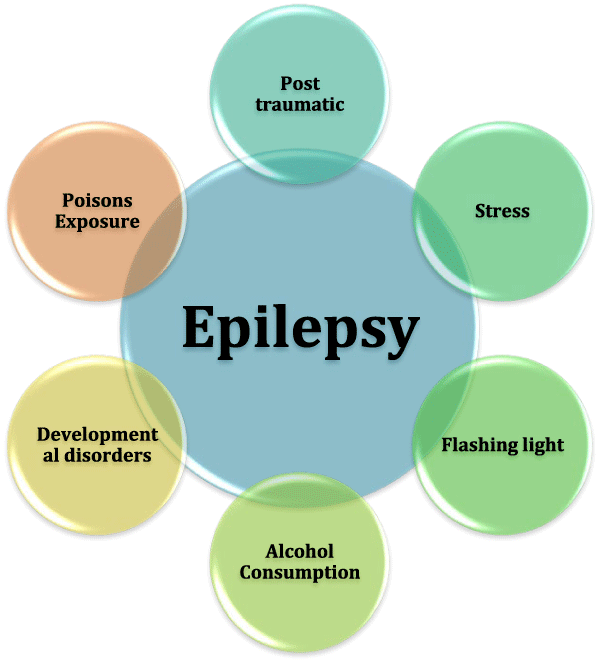
During a seizure, a person can perform a number of semi purposeful activities like wandering around aimlessly, lip smacking and television watching [64]. Epilepsy can be associated with a number of behavioral issues, social competence, emotional problems and disturbed academic achievements [65]. Factors that led to the onset of seizure or epilepsy are referred as “seizure triggers” or Triggering Factors (TF) (Figure 3). Imbalance of neurotransmitters causes epilepsy, which can be excitatory or inhibitory. Epileptogenesis is a process of converting normal brain to epileptic one by lesions or excite toxicity by some pesticides. One of the most studied inhibitory neurotransmitters is Gamma Amino Butyric Acid (GABA) [64]. It is the root cause of epilepsy in some patients; in others, it can accelerate the rate of seizures recurrence.
Some other recounted dynamics which can hasten the onset of seizures are listed below:
Alcohol consumption
Heavy intake of alcohol, use of morphine and caffeine are TFs for epilepsy (Figure 1). Nicotine in cigarettes excites Acetyl Choline neurotransmitter in the brain by increasing neuronal firing, which triggers the inward and outward movement of ions causing seizures [66].
Developmental disorders
Head traumas during an accident with other developmental disorders accelerate epilepsy to a greater extent [67]. Prenatal injuries are also found epileptogenic. When a mother is suffering from infection and mal nutrition, deficiency of oxygen leads to cerebral palsy which is a leading cause of seizures in 20% children [17]. Autism and Downs’s syndrome are also leading causes of epilepsy [68].
Flashing light
Flashing lights flickering in photosensitive epilepsy lead to tonic-clonic or myoclonic seizures. These seizures are triggered by watching TV, playing video games, riding during day light or driving [17].
Stress
Glutamate is excitatory neurotransmitter in brain increases in case of stress, mal-nutrition and sleep apnea, triggering neuronal signal transmission through inter neuronal junctions [17].
Post traumatic epilepsy
Post-traumatic seizure studies have become more prevalent in the last 40-50 years. Traumatic Brain Injury (TBI) is identified as a major cause of epilepsy in recent years [69]. It has been found that 4-53% seizures are posttraumatic. If a person has one seizure after TBI then 86% chances of second seizure are there in the next two years with 25-40% remission rates. Significant risk factors of developing seizures are acute intracerebral hematoma, especially subdural hematoma, severe brain injury, >65 years age and brain contusion [70].
Excessive exposure to lead, carbon monoxide, street drugs, and over-dose of anti-depressants are important TFs for seizures [17].
Localization related epilepsy
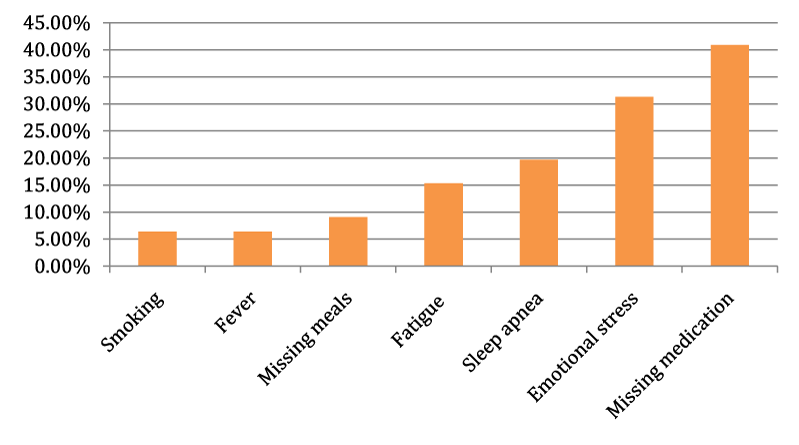
Simple partial seizures change to complex partial seizures and if it remains untreated, it may further evolve into generalized tonic-clonic seizures. This type of epilepsy referred as localization related epilepsy for which several epilepsy surgery is beneficial [71]. Most common TFs are smoking (6.4%), fever (6.4%), missing meals (9.1%), fatigue (15.3%), sleep apnea (19.7%), emotional stress (31.3%) and missing medication (40.9%) [72] (Figure 4).
Almost 25% epilepsy is characterized as symptomatic in which causes are head trauma, brain infections, injuries, and strokes [73].
Epilepsy and pregnant women>
Rate of epilepsy in untreated epileptic women’s offspring is not higher as compared to non-epileptic mothers, whereas mothers who receive Antiepileptic Drugs (AEDS) have more chances of malformations in their children than normal ones. Women With Epilepsy (WWE) taking AEDS have no risk of premature labor, delivery or premature contractions, but remaining seizure free prior nine months is essential [74].
Risk factors of febrile seizures include neonatal exclusion after 28 days, developmental delay, daycare attendance, vaccinations, iron zinc deficiencies, severe viral infections, and family history of febrile seizures [75].
For patients with first unprovoked seizures, routine parameters are used of which EEG is considered as a primary parameter. The most common and reliable method performed to check status of epilepsy is EEG [76]. Brain imaging like Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) are reported to be useful for 15% patients. Laboratory tests like Blood Hemoglobin (Hb) count, blood glucose level and level of electrolytes could be useful parameters for epilepsy [77].
In some of the cases, seizures stop when the disease is treated, but becoming seizure-free is uncertain and depends on affected brain area, the extent of brain damage, type of antiepileptic medication, and type of seizures [78].
Since biblical times, fasting was recommended as a safe treatment for epilepsy but with the advent of AEDs, it is much reduced [2]. Its hypotheses focus on detoxification of gut due to production of ketones which reduce seizure reoccurrence. Ketogenic diet was recommended in 4:1 fat carbohydrates ratio by Pfeifer and Thiele [79,80]. Effectiveness of ketogenic diet in children for the reduction of seizure onset has been reported but it may cause growth retardation, kidney stones, constipation and dehydration [80]. Statistical analysis has shown >50% seizure reduction in 1084 patients staying on the ketogenic diet. In others with the cessation of the diet, seizure reduction was<50%. Diet restriction was seen in 16.4% patients and side effects was observed in 13.2% patients [81,82].
Vagus Nerve Stimulator (VNS) under chest skin deliver short bursts of electrical discharges in the brain and reduces seizures onset by 20-40% but creates coughing, shortness of breath, muscle pain, hoarseness, and tingling along with throat pain. In refractory epilepsy, VNS therapy is found effective for the reduction of seizure frequency and seizure severity. Before and after VNS, data for EEG should be taken 3-5 day on Epilepsy Monitoring Unit (EMU) around ictogenic zones [83].
Until now, curing the epilepsy is not possible but thanks to medication by which its effects can be minimized [84]. In 30% epilepsy cases, seizures cannot be controlled even with the best available AEDs. The drug resistance mechanism is variable and multifactorial according to cause, drug type, drug mode of action, drug site of action, age of the patient, and patient health factor. Seizure free rate is more in older people than younger ones [5]. Resistance against AEDs is due to over-expression of genes and proteins particularly p-glycoprotein [85]. Anticonvulsant medications are the most reliable treatment for epilepsy. Some patients take medicine throughout their life and these medicines possess much influence on their quality of life. Their effectiveness, way of action and side effects vary according to the type of epileptic seizures. Toxicology and clinical pharmacology showed chlordiazepoxide, nitrazepam, clonazepam, flurazepam, oxazepam and diazepam as effective AEDs out of which flurazepam has not been tested experimentally. Benzodiazepines possess no effect on focal epilepsy but effective in stopping generalized seizures like myoclonic, infantile, absence, alcohol causing, and photosensitive. Sodium Di-Propylacetate (DPA) has found the most effective in the treatment of absence seizures. With its antiepileptic structure, this branched-chain carboxylic acid is used in the treatment of generalized tonic-clonic seizures and partial seizures [86-100]. Grand-mal is aggravated by these AEDs, but focal and partial seizures are reduced to some extent. Status epilepticus and ecliptic convulsions are reduced by the use of AEDs. Benzodiazepines are found most effective but orally their effectiveness is reduced and tolerance may also develop. Some of the treatments prescribed for different seizures are given in table 3.
Table 3: Treatments prescribed for different seizures. |
|||||
| Type | Cause | Symptoms | Age | Treatment | References |
| Pyridoxine dependent | Illness or high fever (Pipecolic acid elevation) | Intractable seizures | Newborn | B6 supplementation | [87] |
| Awakening grand mal | Sleep apnea | Tonic clonic grand mal seizures | 20-60y | Sleep awake cycle | [88] |
| Reflex epilepsy | Idiopathic, visual stimuli | Myoclonic generalized convulsive or non-convulsive seizures | 30y | With or without AEDs | [89] |
| Juvenile Absence (JAE) | Idiopathic generalized | Absence seizures, tonic clonic seizures | Prepubertal adolescence (3Hz) | Successful treatment by AEDs | [90] |
| Juvenile Myoclonic (JME) | idiopathic | Rapid isolated jerks in muscles, myoclonus | Teenagers (4-6Hz) | Anti-convulsant medications | [90] |
| Benign | Benign infantile encephalopathy | Benign neonatal convulsions | Childhood | …. | [91] |
| Benign Rolandic (BRE)/ Benign Centro Temporal Lobe | Benign infantile encephalopathy | Jerking of face, limbs with memory loss and difficulty in phonologic processing | Children 3-13 years age | Regular sleep awake cycle | [91] |
| Status Epilepticus (SE) | …. | Continuous seizures | adults | Death | [62] |
| SUDEP | …. | Generalized tonic clonic seizures | …. | Heart arrhythmias and death | [92] |
| Childhood absence | …. | Rapidly blinking eyes, jerking arms | Before puberty | …. |
|
| Temporal Lobe (TLE) | Symptomatic | Focal seizures, Hippocampus shrinks | Late childhood and adolescence | Anticonvulsant medications, surgery | [81] |
| Neocortical | Brain cortex damages | Visual hallucinations, muscle spasms | Adults | Surgical treatment | [93] |
| Lennox-Gastaut Syndrome (LGS) | idiopathic, symptomatic or cryptogenic | Tonic seizure, drop attacks, tonic seizures | Children (2Hz slow spike waves) (2-18y) | Anticonvulsants are not successful | [94] |
| Autosomal dominant nocturnal frontal lobe | idiopathic | Frontal lobe seizures, hand clenching, arm raising | Childhood | Carbamazepine | [95] |
| Benign Occipital (BOEC) | idiopathic | Scotoma, fortifications | 3-10 years | CBZ, VAP | [96] |
| Catamenial (CE) | idiopathic | Sublte chewing, eye blinking | 4-12 years | No specific treatment | [97] |
| Severe myclonic epilepsy of infancy(SMEI)/ dravets syndrome | Idiopathic (mutations in SCNA1 gene) | Unilateral convulsions | Starts in 1st year and remains throughout life | No treatment | [98] |
| Female epilepsy without mental retardation | PCDH19 mutations | Tonic clonic, tonic, atonic seizures | 6-36 months | …. | [99] |
| Frontal lobe epilepsy | Symptomatic or cryptogenic | Seizures in frontal lobe |
|
Surgery | [100] |
Valproate, phenytoin, phenobarbital and other AEDS should be avoided during pregnancy, especially during the first trimester to avoid congenital malformations [74]. Remifentanil works in dose dependent manner and affects cortical spikes [101]. Mono-therapy in pregnancy possesses epileptic patient on a higher risk of congenital malformation. European and International Registry of Antiepileptic Drugs and Pregnancy (EURAP) show effects of four common AEDs phenobarbital, lamotrigine, valproic acid and carbamazepine up to 12 months after birth. Assessment is according to dose at the time of conception [4,5,61]. Lowest rates of congenital malformation are observed with lamotrigine (dose: less than 300 mg per day) whereas risks of malformations are higher with barbital, carbamazepine (dose: 400 mg per day) and valproic acid [102].
AEDs dose during prenatal exposure should be determined particularly due to teratogenicity associated with them [103]. If dose is increased gradually, then other side effects like drowsiness, ataxia and toxicity are minimized [61]. Depression, anxiety, fatigue, loss of coordination, dizziness, loss of bone density, inflammation to liver, pancreas and suicide are ultimate side effects of medication [85].
In some cases of brain damages, surgery is performed to avoid epileptic seizures. Surgical removal of ictogenic zones is necessary for patients with refractory epilepsy [104]. In the United States, there are 4 x 105 to 6 x 105 patients with refractory epilepsy and only 2-3% epileptic patients are offered surgery [105,106]. In 97-98% patients, it is difficult to localize ictogenic zone [83] whereas 66% patients are found seizure free after temporal lobe resections, 27% after frontal lobe resections and 46% after occipital and parietal resections [65]. With left sided temporal surgery, verbal memory was reduced to 44%, and verbal fluency was improved in 27% patients [107].Today our basic need is to improve epilepsy surgery by making it cost effective and using non-invasive biomarkers. High Frequency Neuromagnetic Signals (HFNS) and spikes are potential biomarkers for the localization of ictogenic zones and have improved seizure freedom rate [108]. The number of daily seizures is correlated with spikes in HFNS [109]. A patient in which ablative surgery is not recommended, bilateral hippocampal stimulation is useful [110].
Epileptic persons are at a higher risk for thinking and learning capabilities, attention, memory, skills, emotional and behavioral difficulties along with perception problems. National Institute for Health and Care Excellence (NICE) recommended that epileptic patients should have a regular medical checkup. To cope with epileptic after effects, this review should be performed on yearly basis for adults and children. This gives a chance to discuss seizures, their effects, treatment and any other questions [111]. Psychotherapy which includes group work, oriented therapy and cognitive behavior therapy is helpful with antidepressant medication. In 2008 a campaign was started to create awareness in people regarding epilepsy and named as a Purple Day on March 26 [52,112].
The severity of seizures, stigma, and other variables like self-esteem, self-efficacy, coping style which are the determinants of employment may cause difficulties for epileptic patients. It is very difficult to maintain regular employment in epileptic patients [113]. Rate of underemployment and unemployment are much higher in epileptic patients especially with severe seizures. Employment impacts much to the quality of normal life [114]. In society, earning and acceptance by others give us confidence of living life [114]. Employment also helps gaining self-confidence. From more than three decades employment is considered a significant problem for epileptic persons [115]. In developing countries, epileptic persons are ignored and stigmatized. Epileptic persons are more prone to sexual difficulties and non-acceptance of self. Epilepsy can be associated with several behavioral problems, social competence, emotional disturbance and disturbed academic achievements even after surgery [116]. Inadequacy, social impairment, sporadic illness and recalcitrance increased above average in children with epilepsy [117].
Genetics, as well as environmental factors play crucial role in the development of epilepsy. Therefore, current literature review highly recommend to adjust these environmental triggering factors to minimize the severity and occurrence of seizure attacks in epileptic patients.
- Robert SF, J Helen C, Jacqueline AF, Norimichi H, Edouard H, Floor EJ, et al. Operational classification of seizure types by the international league against epilepsy: Position paper of the ilae commission for classification and terminology. 2017; 58: 522-530. DOI: 10.1111/epi.13670
- Chang BS, DHJN Lowenstein. Practice parameter: Antiepileptic drug prophylaxis in severe traumatic brain injury Report of the Quality Standards Subcommittee of the American Academy of Neurology. 2003; 60: 10-16. https://tinyurl.com/y5hahbc8
- Chetan AK, Jeffery WS, Carl RS, Joseph MA. Synthesis, characterization and evaluation of urethane derivatives of Bis-GMA. Dent Mater. 2003; 19: 584-588. DOI: 10.1016/s0109-5641(02)00108-2.
- Kwan P, Brodie M. Early identification of refractory epilepsy. 2000; 342: 314-319. DOI: 10.1056/NEJM200002033420503
- Kwan P, SC Schachter, MM Brodie. Drug-resistant epilepsy. 2011; 365: 919-926.
- Allen AS, Samuel FB, Patrick C, Norman D, Dennis D, Evan EE, et al. De novo mutations in epileptic encephalopathies. Nature. 2013; 501: 217. DOI: 10.1038/nature12439
- Asra S, Reinhold K, Michael EW, Ulrich B, Alice S, David BG, et al. Association of multidrug resistance in epilepsy with a polymorphism in the drug-transporter gene ABCB1. N Engl J Med. 2003; 348: 1442-1448. DOI: 10.1056/NEJMoa021986
- Hubner CA, TJJHmg J. Ion channel diseases. Human Molecular Genetics. 2002; 11: 2435-2445. DOI: 10.1093/hmg/11.20.2435
- Ram SP, Xiao PH, Lijun Y, Tri L, Wonjo J, Catherine WR, et al. Disruption of Fgf13 causes synaptic excitatory-inhibitory imbalance and genetic epilepsy and febrile seizures plus. J Neurosci. 2015; 35: 8866-8881. DOI: 10.1523/JNEUROSCI.3470-14.2015
- Alexander P, John AD, Susannah TB, Ingrid ES, Samuel FB, Saul AM, et al. Evaluation of GLUT1 variation in non-acquired focal epilepsy. Epilepsy Research. 2017; 133: 54-57. DOI: https://doi.org/10.1016/j.eplepsyres.2017.04.007
- Sanchez Fernandez, Tobias L, JM Peters, Sanjeev VK. Electrical status epilepticus in sleep: Clinical presentation and pathophysiology. Pediatr Neurol. 2012; 47: 390-410. DOI: https://doi.org/10.1016/j.pediatrneurol.2012.06.016
- St Louis EK. Sleep and Epilepsy: Strange Bedfellows No More. Minerva Pneumologica. 2011; 50: 59-176. https://tinyurl.com/y4c5qfrn
- Bazil CW. Nocturnal seizures. Semin Neurol. 2004; 24: 293-300. DOI: 10.1055/s-2004-835071
- Khan S, RAl Baradie. Epileptic encephalopathies: An overview. Epilepsy Res Treat. 2012; 2012: 403592. DOI: 10.1155/2012/403592
- Boel M, PJN Casaer. Continuous spikes and waves during slow sleep: A 30 month’s follow-up study of neuropsychological recovery and EEG findings. Neuropediatrics. 1989; 20: 176-180. DOI: 10.1055/s-2008-1071287
- Masel BE, DS DeWitt. Traumatic brain injury: A disease process, not an event. J Neurotrauma. 2010; 27: 1529-1540. DOI: 10.1089/neu.2010.1358
- Antebi D, J Bird. The facilitation and evocation of seizures. TBJP. 1992; 160: 154-164. DOI: https://doi.org/10.1192/bjp.160.2.154
- Patrick CN, Helene C, Christine MB, Hai N, Ying JQ, Elizabeth R, et al. A systematic review of the frequency of neurocyticercosis with a focus on people with epilepsy. PLoS Negl Trop Dis. 2010; 4: e870. DOI: 10.1371/journal.pntd.0000870
- Buckley AW, GL Holmes. Epilepsy and autism. 2016; 4: a022749. DOI: 10.1101/cshperspect.a022749
- Mannion A, G Leader. Comorbidity in autism spectrum disorder: A literature review. RASD. 2013; 7: 1595-1616. DOI: https://doi.org/10.1016/j.rasd.2013.09.006
- Suren, P, Inger JB, Camilla Stoltenberg. Autism spectrum disorder, ADHD, epilepsy, and cerebral palsy in Norwegian children. American Academy of Pediatrics. 2012; 130: e152-e158. DOI: 10.1542/peds.2011-3217
- Annalisa GS, Emily KM, Kristin L, Saadet MA, Weizhen Ji, et al. De novo pathogenic variants in Neuronal Differentiation Factor 2 (NEUROD2) cause a form of early infantile epileptic encephalopathy. J Med Genet. 2019; 56: 113-122. DOI: 10.1136/jmedgenet-2018-105322
- Nabbout R, Dulac. Epileptic encephalopathies: A brief overview. OJCN. 2003; 20: 393-397 https://tinyurl.com/y4l7otgb
- Holmes GL, Ben-Ari. The neurobiology and consequences of epilepsy in the developing brain. JPR. 2001; 49: 320. https://tinyurl.com/y6gjak9w
- Bhasin H, S Sharma. The New International League Against Epilepsy (ILAE) 2017 classification of seizures and epilepsy: What pediatricians need to know! Indian J Pediatr. 2019; 86: 569-571. DOI: 10.1007/s12098-019-02910-x
- Roberto C, Sebastian S, Lucas B, Agustin Calvo, Ricardo C. Childhood-only epilepsy with generalized tonic-clonic seizures: A well-defined epileptic syndrome. Epilepsy Research. 2019. 153: 28-33. DOI: 10.1016/j.eplepsyres.2019.03.017
- Brigo F, SC Igwe, S Lattanzi. Ethosuximide, sodium valproate or lamotrigine for absence seizures in children and adolescents. CDSR. 2019. DOI: https://doi.org/10.1002/14651858.CD003032.pub3
- Virdziniha T, Georgina F, Roger DT, Miles AW, Stephen P Hall. Levetiracetam and Rufinamide are effective at supressing spike and wave seizure activity in an in vitro model of absence epilepsy. bioRxiv. 2018: 298711. DOI: https://doi.org/10.1101/298711
- Gonçalo C, Joana P, Shahidul M, Sanjay M S, Josemir WS. Juvenile myoclonic epilepsy refractory to treatment in a tertiary referral center. Epilepsy & Behavior. 2018; 82: 81-86. DOI: https://doi.org/10.1016/j.yebeh.2018.03.002
- Myers, KA and IE Scheffer, Myoclonic absence seizures with complex gestural automatisms. European Journal of Paediatric Neurology. 2018; 22: 532-535. DOI: https://doi.org/10.1016/j.ejpn.2017.12.003
- Katie A, Krista E, Garnett S, Charuta J, Scott D. Genetic testing in a cohort of patients with potential epilepsy with myoclonic-atonic seizures. Epilepsy research. 2019; 150: 70-77. DOI: 10.1016/j.eplepsyres.2019.01.008
- Jessica G, Serena M, Laura M, Elisa F, Lucio G. Childhood absence epilepsy evolving to eyelid myoclonia with absence epilepsy. Seizure. 2018; 61: 1-3. DOI: https://doi.org/10.1016/j.seizure.2018.07.009
- Daniel LKJ, Annamaria V, Robert JK, Reghann GL, France C, Mai‐Lan Ho, et al. Febrile infection related epilepsy syndrome treated with anakinra. Annals of neurology. 2016; 80: 939-945. DOI: https://doi.org/10.1002/ana.24806
- Gregory LK, Emilio P, Patrick K, Elinor BM, Xue FW, Jerry JS, et al. Final safety, tolerability, and seizure outcomes in patients with focal epilepsy treated with adjunctive perampanel for up to 4 years in an open‐label extension of phase III randomized trials: Study 307. Epilepsia. 2018; 59: 866-876. DOI: 10.1111/epi.14044
- Yamamoto T, Tadashi H, Kazumichi Y, Hideo K. Improvement of visual field defects after focal resection for occipital lobe epilepsy: Case report. JNS. 2018; 128: 862-866. DOI: https://doi.org/10.3171/2016.12.JNS161820
- Peng FY, Yan ZJ, Qiao L, Zhen M, Zi QC, Zhi YZ, et al. Intractable occipital lobe epilepsy: Clinical characteristics, surgical treatment, and a systematic review of the literature. Acta neurochirurgica. 2015; 157: 63-75. DOI: 10.1007/s00701-014-2217-3
- Kim DW, JS Sunwoo, SK Lee. Incidence and localizing value of vertigo and dizziness in patients with epilepsy: Video-EEG monitoring study. Epilepsy Res. 2016; 126: 102-105. DOI: 10.1016/j.eplepsyres.2016.07.002
- Wei IL, Patrick WC, Andrew JH, John SA. Refractory focal motor seizures controlled with intramuscular botulinum toxin. Epilepsy research. 2017; 133: 93-97. DOI: https://doi.org/10.1016/j.eplepsyres.2017.04.009
- Joon YK, Chengyuan W, Joseph T, Matthew L, James E, Maromi N, et al. Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy. Epilepsia, 2016; 57: 325-334. DOI: 10.1111/epi.13284
- Tinuper P, Bisulli F. From nocturnal frontal lobe epilepsy to sleep-related hypermotor epilepsy: A 35-year diagnostic challenge. Seizure. 2017; 44: 87-92. DOI: https://doi.org/10.1016/j.seizure.2016.11.023
- Chen J, Guo Zheng, Hu Guo, Xiaopeng Lu, Chunfeng Wu, Xiaoyu Wang, et al. Epileptic negative myoclonus as the first and only symptom in a challenging diagnosis of benign epilepsy with centrotemporal spikes. Child Neurol Open. 2017; 4: 2329048X17715965. DOI: 10.1177/2329048X17715965
- Iris Unterberger, Eugen T, Peter WK, Gerald W, Gerhard L, Gerhard B. Generalized nonmotor (absence) seizures-What do absence, generalized, and nonmotor mean? Epilepsia. 2018; 59: 523-529. DOI: https://doi.org/10.1111/epi.13996
- Stjepana K, Beate D, Tim W, Chiara F, Nathan T, Matthew CW, John SD. Gelastic seizures: Incidence, clinical and EEG features in adult patients undergoing video‐EEG telemetry. Epilepsia. 2015; 56: e1-e5. DOI: 10.1111/epi.12868
- Myers KA, Scheffer IE. Myoclonic absence seizures in dravet syndrome. Pediatric Neurology. 2017; 70: 67-69. DOI: https://doi.org/10.1016/j.pediatrneurol.2017.01.004
- Louis EM, Omar JA, Kyle QL, Sydney SC, Mark AK. Slow spatial recruitment of neocortex during secondarily generalized seizures and its relation to surgical outcome. J Neurosci. 2015; 35: 9477-9490. DOI: 10.1523/JNEUROSCI.0049-15.2015
- Fountain NB, Joshi S. Neuropathology of generalized convulsive status epilepticus, in status epilepticus 2018. Springer. 2018; 123-130.
- Power KN, Gramstad A, Gilhus NE, Hufthammer KO, Engelsen BA. Cognitive dysfunction after generalized tonic‐clonic status epilepticus in adults. Acta Neurologica Scandinavica. 2018; 137: 417-424. DOI: https://doi.org/10.1111/ane.12898
- Chan DWS, Thomas T, Lim M, Ling S, Woodhall M, Vincent A. Focal status epilepticus and progressive dyskinesia: A novel phenotype for glycine receptor antibody-mediated neurological disease in children. European Journal of Paediatric Neurology. 2017; 21: 414-417. DOI: https://doi.org/10.1016/j.ejpn.2016.08.013
- Antonio V, Ismail U, Beverly C, Robert M, Richard S, Gonzalo A. Epilepsia partialis continua responsive to neocortical electrical stimulation. Epilepsia. 2015; 56: e104-e109. DOI: 10.1111/epi.13067
- Jacopo F, Koscica N, Zorzon M, Belluzzo M, Granato A. Hemicrania continua with visual aura successfully treated with a combination of indomethacin and topiramate. Neurol Sci. 2015; 36: 643-644. DOI: 10.1007/s10072-014-2036-6.
- Baoqiong Liu, Yan Zhou, Lingbin Meng, Holly Skinner. A survival case of super-refractory status epilepticus due to glutamic acid decarboxylase antibodies-associated limbic encephalitis. Cureus. 2018; 10: e3125. DOI: 10.7759/cureus.3125
- Greer F, Sicherer S, Burks AJP. American academy of pediatrics section on allergy and immunology effects of early nutritional interventions on the development of atopic disease in infants and children: The role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. 2008; 121: 183-191.
- David B Graves. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. Journal of Physics D: Applied Physics. 2012; 45: 263001. DOI: https://doi.org/10.1088/0022-3727/45/26/263001
- Berkovic SF, Howell RA, Hay DA, Hopper JL. Epilepsies in twins: Genetics of the major epilepsy syndromes. Ann Neurol. 1998; 43: 435-445. DOI: 10.1002/ana.410430405
- Sinha SS, Pramod MN, Dilipkumar S, Satishchandra P. Idiopathic generalized epilepsy: Phenotypic and electroencephalographic observations in a large cohort from South India. Ann Indian Acad Neurol. 2013; 16: 163-168. DOI: 10.4103/0972-2327.112455
- Shorvon SD. The etiologic classification of epilepsy. Epilepsia. 2011; 52: 1052-1057. DOI: https://doi.org/10.1111/j.1528-1167.2011.03041.x
- Azra Alajbegovic, Natasa Loga, Enra Suljic. Characteristics of symptomatic epilepsy in patients with brain tumours. Bosn J Basic Med Sci, 2009; 9: 81-84. DOI: 10.17305/bjbms.2009.2862
- Bazilevich SN. [Cryptogenic epilepsy in adults: “hidden problems of structural well-being”]. Zh Nevrol Psikhiatr Im SS Korsakova. 2013; 113: 10-19. https://tinyurl.com/y2a9gkmy
- Hans C, Thomas K, Ulrike G, Robert S, Horst U, Ingmar B, et al. Analysis of different types of resection for pediatric patients with temporal lobe epilepsy. Neurosurgery. 2004; 54: 847-860. DOI: 10.1227/01.neu.0000114141.37640.37
- McDonald CR, Delis DC, Norman MA, Wetter SR, Tecoma ES, Iragui VJ. Response inhibition and set shifting in patients with frontal lobe epilepsy or temporal lobe epilepsy. E&B: 2005; 7: 438-446. DOI: 10.1016/j.yebeh.2005.05.005
- TR Browne, JK Penry. Benzodiazepines in the treatment of epilepsy a review. Epilepsia. 1973; 14: 277-310. DOI: 10.1111/j.1528-1157.1973.tb03965.x
- Chin R, Neville B, Scott N. A systematic review of the epidemiology of status epilepticus. Eur J Neurol. 2004; 11: 800-810. DOI: 10.1111/j.1468-1331.2004.00943.x.
- Zifkin BG, Kasteleijn D. Reflex epilepsy and reflex seizures of the visual system: A clinical review. Epileptic Disord. 2000; 2: 129-136. https://tinyurl.com/y2yt8m7b
- Shorvon SD, F Andermann, R Guerrini. The causes of epilepsy: Common and uncommon causes in adults and children. Cambridge University Press. 2011. https://tinyurl.com/yy8pgtje
- Tellez ZJF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: A systematic review and meta-analysis. Brain. 2005; 128: 1188-1198. DOI: 10.1093/brain/awh449
- Brathen. Alcohol and epilepsy. 2003; 123: 1536-1538.
- Amy LJ, Jeffrey WB, Melissa MB, Joseph EP, Gregory DC. Chronic traumatic encephalopathy in an epilepsy surgery cohort: Clinical and pathologic findings. Neurology. 2018; 90: e474-e478. DOI: 10.1212/WNL.0000000000004927
- Buckley AW, GL Holmes. Epilepsy and autism. Cold Spring Harb Perspect Med. 2016; 6: a022749. DOI: 10.1101/cshperspect.a022749
- Asikainen I, Kaste M, Sarna S. Early and late posttraumatic seizures in traumatic brain injury rehabilitation patients: brain injury factors causing late seizures and influence of seizures on long term outcome. Epilepsia. 1999; 40: 584-589. DOI: 10.1111/j.1528-1157.1999.tb05560.x.
- Jennifer AK, Emily B, Alexander CW, Andrew JC, Kevin JS, Sahar Z, et al. Epileptiform activity in traumatic brain injury predicts post traumatic epilepsy. Ann Neurol. 2018; 83: 858-862. DOI: 10.1002/ana.25211
- Floor E Jansen, Alexander CH, Ale A, Onno N. Epilepsy surgery in tuberous sclerosis: A systematic review. Epilepsia. 2007; 48: 1477-1484. DOI: 10.1111/j.1528-1167.2007.01117.x
- Balamurugan E, Meena A, Anurag L, Nitika D, Manjari T. Perceived trigger factors of seizures in persons with epilepsy. Seizure-European Journal of Epilepsy. 2013; 22: 743-747. DOI: https://doi.org/10.1016/j.seizure.2013.05.018
- WA Hauser, JF Annegers, LT Kurland. Prevalence of epilepsy in Rochester, Minnesota: 1940-1980. Epilepsia. 1991; 32: 429-445. DOI: 10.1111/j.1528-1157.1991.tb04675.x
- Shawn F, Eran K, Irena N, Thomas RE, Gideon K. Malformation rates in children of women with untreated epilepsy a meta-analysis. Drug Saf. 2004; 27: 197-202. DOI: 10.2165/00002018-200427030-00004
- Berg, Joyce, Dickhaut, John, McCabe, Kevin. Trust, reciprocity, and social history. Games and Economic Behavior. 1995; 10: 122-142. DOI: https://doi.org/10.1006/game.1995.1027
- Hirtz D, Ashwal S, Berg A, Bettis D, Camfield C, Camfield P, et al. Practice parameter: Evaluating a first nonfebrile seizure in children: report of the quality standards subcommittee of the American Academy of Neurology, The Child Neurology Society, and The American Epilepsy Society. Neurology. 2000; 55: 616-623. DOI: 10.1212/wnl.55.5.616
- Krumholz A, Wiebe S, Gronseth G, Shinnar S, Levisohn P, Ting T, et al. Practice parameter: Evaluating an apparent unprovoked first seizure in adults (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2007; 69: 1996-2007. DOI: 10.1212/01.wnl.0000285084.93652.43
- John H Stevens, William SP, Wesley D. Sterman, Hansen SGI. System and methods for performing endovascular procedures. Google Patents. 2000. https://tinyurl.com/y48ynen7
- Pfeifer HH, EA Thiele. Low-glycemic-index treatment: A liberalized ketogenic diet for treatment of intractable epilepsy. Neurology. 2005; 65: 1810-1812. DOI: 10.1212/01.wnl.0000187071.24292.9e
- Kossoff EH. More fat and fewer seizures: Dietary therapies for epilepsy. Lancet Neurol. 2004; 3: 415-420. DOI: 10.1016/S1474-4422(04)00807-5
- Beth H, Francis MF, Stephen CA, Joseph LL, Deirdre AC. Efficacy of the ketogenic diet as a treatment option for epilepsy: meta-analysis. J Child Neurol. 2006; 21: 193-198. DOI: 10.2310/7010.2006.00044.
- Keene DL. A systematic review of the use of the ketogenic diet in childhood epilepsy. Pediatr Neurol. 2006; 35: 1-5. DOI: 10.1016/j.pediatrneurol.2006.01.005.
- Chambers A, JM Bowen. Electrical stimulation for drug-resistant epilepsy: An evidence-based analysis. 2013; 13: 1-37. https://tinyurl.com/y69z99m7
- Robert SF, Carlos A, Alexis A, Alicia B, JH Cross, et al. ILAE official report: A practical clinical definition of epilepsy. Epilepsia. 2014; 55: 475-482. DOI: 10.1111/epi.12550
- Sisodiya SM. Mechanisms of antiepileptic drug resistance. Curr Opin Neurol. 2003; 16: 197-201. DOI: 10.1097/01.wco.0000063771.81810.6c
- Simon D, JK Penry. Sodium Di N Propylacetate (DPA) in the treatment of epilepsy: A review. Epilepsia. 1975; 16: 549-573. DOI: 10.1111/j.1528-1157.1975.tb04738.x
- Plecko B, Sylvia SI, Eduard P, Wolfgang E, Eduard AS, Cornelis J. Pipecolic acid elevation in plasma and cerebrospinal fluid of two patients with pyridoxine‐dependent epilepsy. Annals of neurology. 2000; 48: 121-125. DOI: https://doi.org/10.1002/1531-8249(200007)48:1<121::AID-ANA20>3.0.CO;2-V
- Gursahani R, Gupta N. The adolescent or adult with generalized tonic-clonic seizures. Ann Indian Acad Neurol. 2012; 15: 81-88. DOI: 10.4103/0972-2327.94988
- Zifkin BG, DKN Trenite. Reflex epilepsy and reflex seizures of the visual system: A clinical review. Epileptic disorders. 2000; 2: 129-136. https://tinyurl.com/y2pjzwgx
- Johanna D, Bela C, Szilvia P, Istvan F. Decrease of global current source density predicts successful treatment in absence and juvenile myoclonic epilepsies. Epilepsy research. 2017; 133: 1-5. DOI: https://doi.org/10.1016/j.eplepsyres.2017.03.006
- Northcott, E, Anne MC, Anna B, Mark S, Jenny M, Jane C, et al. The neuropsychological and language profile of children with benign rolandic epilepsy. Epilepsia. 2005; 46: 924-930. DOI: 10.1111/j.1528-1167.2005.62304.x
- Paulo AL, Leandro V, Isabela MB, Andre RB. A systematic review and meta analysis of heart rate variability in epilepsy and antiepileptic drugs. Epilepsia. 2012; 53: 272-282. DOI: 10.1111/j.1528-1167.2011.03361.x
- Sang KL, Seo YL, Kwang KK, Kkeun SH, Dong SL, Chun KC. Surgical outcome and prognostic factors of cryptogenic neocortical epilepsy. Ann Neurol. 2005; 58: 525-532. DOI: 10.1002/ana.20569
- Majoie HJ, Berfelo MW, Aldenkamp AP, Evers SM, Kessels AG, Renier WO. Vagus nerve stimulation in children with therapy-resistant epilepsy diagnosed as Lennox-Gastaut syndrome: Clinical results, neuropsychological effects, and cost-effectiveness. Journal of Clinical Neurophysiology. 2001; 18: 419-428. DOI: 10.1097/00004691-200109000-00006
- Combi R. Autosomal dominant nocturnal frontal lobe epilepsy. Journal of neurology. 2004; 251: 923-934. https://tinyurl.com/y4ooqlzr
- Chary P, B Rajendran. Benign occipital lobe seizures: Natural progression and atypical evolution. Ann Indian Acad Neurol. 2013; 16: 556-560. DOI: 10.4103/0972-2327.120465
- Alberto V, Claudia DE, Sergio A, Carla V, Piero P. Diagnosis and management of catamenial seizures: A review. Int J Womens Health. 2012; 4: 535. https://tinyurl.com/y42dtxfh
- Wolff M, Casse P, C Dravet. Severe myoclonic epilepsy of infants (Dravet syndrome): Natural history and neuropsychological findings. Epilepsia. 2006; 47: 45-48. DOI: 10.1111/j.1528-1167.2006.00688.x
- Kim Hynes, Patrick T, Leanne MD, Marta AB, Samuel FB, Raffaella S, et al. Epilepsy and mental retardation limited to females with PCDH19 mutations can present de novo or in single generation families. Journal of medical genetics. 2010; 47: 211-216. DOI: 10.1136/jmg.2009.068817
- Kellinghaus C, HO Luders. Frontal lobe epilepsy. Epileptic disorders. 2004; 6: 223-239. https://tinyurl.com/y4do7ge2
- Harden CL, Meador KJ, Pennell PB, Hauser WA, Gronseth GS, French JA, et al. Practice parameter update: Management issues for women with epilepsy-focus on pregnancy (an evidence-based review): Teratogenesis and perinatal outcomes report of the quality standards subcommittee and therapeutics and technology assessment subcommittee of the American academy of neurology and American epilepsy society. Neurology. 2009; 73: 133-141. DOI: 10.1212/WNL.0b013e3181a6b312
- Torbjorn T, Dina Battino, Erminio Bonizzoni, John Craig, Dick Lindhout, Anne Sabers, et al. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol. 2011; 10: 609-617. DOI: 10.1016/S1474-4422(11)70107-7
- Arpino C, Brescianini S, Robert E, Castilla EE, Cocchi G, Cornel MC, et al. Teratogenic effects of antiepileptic drugs: Use of an International Database on Malformations and Drug Exposure (MADRE). Epilepsia. 2000; 41: 1436-1443. DOI: 10.1111/j.1528-1157.2000.tb00119.x
- Yoshimi CH, Katsuaki K, Erik CB, Naoyuki M, Eishi A. Gamma activity modulated by naming of ambiguous and unambiguous images: Intracranial recording. Clin Neurophysiol. 2015; 126: 17-26. DOI: 10.1016/j.clinph.2014.03.034
- Dana C, Andrei B, Cristina M, Cristian D, Jean C, Ioana M. Presurgical evaluation and epilepsy surgery in MRI negative resistant epilepsy of childhood with good outcome. Turk Neurosurg. 2015; 25: 905-913. DOI: 10.5137/1019-5149.JTN.12093-14.0
- Enatsu R, Mikuni N. Invasive evaluations for epilepsy surgery: A review of the literature. 2016; 56: 221-227. DOI: 10.2176/nmc.ra.2015-0319
- Elisabeth MSS, Samuel W, Taryn BF, Jose TZ, Amy M, Lisbeth HR, et al. Neuropsychological outcomes after epilepsy surgery: systematic review and pooled estimates. Epilepsia. 2011; 52: 857-869. DOI: 10.1111/j.1528-1167.2011.03022.x
- Jing X, Yang L, Yingying W, Elijah GK, Rupesh K, Yangmei C, et al. Frequency and spatial characteristics of high-frequency neuromagnetic signals in childhood epilepsy. Epileptic Disord. 2009; 11: 113-125. DOI: 10.1684/epd.2009.0253
- Kimberly L, Jing X, Fawen Z, Jingping S, Lu T, Hongxing L, et al. Magnetoencephalography detection of high-frequency oscillations in the developing brain. Front Hum Neurosci. 2014; 8: 969. DOI: 10.3389/fnhum.2014.00969
- Cohen-Gadol AA, Brian G, Frederic C, Bradley J, Jeffrey W, Denise M, et al. Long-term outcome of epilepsy surgery among 399 patients with nonlesional seizure foci including mesial temporal lobe sclerosis. Journal of neurosurgery. 2006; 104: 513-524. DOI: https://doi.org/10.3171/jns.2006.104.4.513
- Demet K, Henrik P, Maiken IS, Erik TP, Mogens V, Merete JS, et al. Birth outcomes after prenatal exposure to antiepileptic drugs-A population based study. Epilepsia. 2014; 55: 1714-1721. DOI: 10.1111/epi.12758.
- Ivan Bielen, Ivana Zobic, Ana Sruk, Ana Ivakovic, Davor Dogan. Changes of attitudes toward epilepsy in college-preparatory high school students population: An indicator of global campaign successfulness? Seizure. 2012; 21: 775-779. DOI: https://doi.org/10.1016/j.seizure.2012.09.002
- Bishop. Determinants of employment status among a community-based sample of people with epilepsy: Implications for rehabilitation interventions. MJRCB. 2004; 47: 112-121. DOI: https://doi.org/10.1177/00343552030470020601
- Ann Jacoby, Joanne G, Gus AB. Employers’ attitudes to employment of people with epilepsy: Still the same old story? 2005 46: 1978-1987. DOI: https://doi.org/10.1111/j.1528-1167.2005.00345.x
- Bishop M. Barriers to employment among people with epilepsy: Report of a focus group. Journal of Vocational Rehabilitation. 2002; 17: 281-286. https://tinyurl.com/y4z6crnm
- Sang-Ahm Lee. What we confront with employment of people with epilepsy in Korea. Epilepsia. 2005; 46: 57-58. DOI: 10.1111/j.0013-9580.2005.461018.x
- Olga Braams, Renske Schappin, Joost Meekes, Peter CRijen, Onnovan N. Personality traits of children before and after epilepsy surgery. 2017; 133: 10-12. DOI: https://doi.org/10.1016/j.eplepsyres.2017.04.001
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.