> Biology. 2020 June 11;1(2):011-020. doi: 10.37871/jels1114.
Effects of Irradiance and Different Nitrogen and Carbon Concentrations on the Minerals Accumulation in Scenedesmus obliquus Biomass
Jiménez-Veuthey Mariana1,2*, Zapata Luz Marina1, Vezzosi-Zoto Gina1, Sacks Natalia1, Flores Agustina1, Zampedri Patricia1 and Zampedri Carolina1
2ConsejoNacional de Investigaciones Científicas y Técnicas (CONICET), Buenos Aires, Argentina
- Irradiance
- Mineral
- Scenedesmus obliquus
- Sodium acetate
- Sodium nitrate
Microalgae are capable of absorbing and concentrating constituent elements that have a wide variety of applications in agriculture, food industry, and medicine. Microalgae chemical composition change according to internal and external factors. In this study, the effect of irradiance, sodium nitrate and sodium acetate concentration on the accumulation of essential minerals in Scenedesmus obliquus biomass were evaluated using 23 factorial screening designs. The simultaneous effect of the three experimental factors was studied using three levels for each parameter (irradiance: 36.71, 69.50, 102.30 μE m-2 s-1, sodium nitrate: 0.27, 44.00, 87.73 g L-1 and sodium acetate: 0.00, 2.50, 5.02 g L-1). The response variables were the minerals concentration of Na, K, Ca, Mg, Fe, Zn and Mn. Results show that each mineral has an optimal operation condition in order to improve its concentration in the microalgae biomass. A significant interaction between the variables was observed, which has direct effects on the minerals accumulation in the microalgae biomass. Under these conditions, the maximum concentration of K (1515.77 [mg (100gdw)-1]), Ca (2744.24 [mg (100gdw)-1]), Mg (9697.65 [mg (100gdw)-1]), Fe (2932.42 [mg (100gdw)-1]), Mn (38.48 [mg (100gdw)-1]), Zn (324.00 [mg (100gdw)-1]) and the minimum concentration of Na (5607.20 [mg (100gdw)-1]) were obtained from the microalga biomass. Thus, Scenedesmus obliquus biomass was characterized as good essential mineral source and confirmed to be potentially valuable ingredient for utilization in the food industry.
Microalgae biotechnology has gained relevance in the last two decades due to the wide range of applications from biomass production for food, feed, aquaculture, bio-fertilizer, until obtaining valuable products therapeutic or industrial. Microalgae absorb and concentrate constituent elements such as proteins, carbohydrates, lipids, pigments vitamins, and minerals, among others [1]. Microalgae chemical composition change according to the species, habitat, life cycle stage and environmental. These factors determine their nutritional value [2].
Minerals have different functions in the human body, such as muscle function, nerve stimulation, enzymatic and hormonal activities, and oxygen transport. For example, Sodium (Na) and Potassium (K) both are regulating osmotic pressure. Calcium (Ca) and Magnesium (Mg) are key functional components of the tissue and skeleton [3]. Iron (Fe), is an essential mineral that plays an important role in many metabolic processes such as synthesis of haemoglobin and myoglobin (oxygen transport proteins); oxidation-reduction processes [4]. Zinc (Zn) mainly participates in regulation of growth and differentiation cellular and it is an important co-factor in many enzymes [5]. Manganese (Mn) is a trace mineral of fundamental importance to human health, is an essential component of the antioxidant enzymes, involved in the formation of connective tissue, bones and nerve function [6]. Among mineral insufficiencies, deficiencies in Fe and Zn are reported as highly prevalent nutritional problems around the world, affecting mainly developing country. Iron and zinc deficiency may lead to slow cognitive and skeletal development and immunodeficiency disorders [7]. Similarly, low calcium intake is associated with chronic diseases such as osteoporosis, colon cancer, hypertension and obesity [8]. However, an excess in the intake of other minerals, such as sodium, may trigger various body disorders, mainly cardiovascular disease and stroke. So, a balanced consumption of minerals is important for the proper functioning of the body. In this sense, dietary variation and/or supplementation are essential. The enrichment of food with natural source with high mineral content, such as microalgae, can be useful in avoiding the intake of supplementation or pharmaceutical products.
Nowadays, microalgae have numerous commercial applications in food and animal feed. For example, in food, microalgae are incorporated in pasta, sweet snack bars and drinks, as a natural nutritional supplement, enhancing its nutritional value [9].
Microalgae production is highly sustainable and has certain advantages over the use of other microorganisms due to its high productivity, fast growth, CO2 absorption from the atmosphere, growth in extreme environmental conditions, and do not need agricultural land [10,11]. Microalgae biotechnology allows controlling cultivation conditions in order to production of many compounds of biological interest. There are several parameters that affect the microalgae growth and therefore its nutritional value: distribution and intensity of light within the bioreactor, temperature, nutritional composition of the culture media, among others [2,12]. According to the composition of the culture media, there are studies that refer the effect of sodium acetate, as a source of carbon, on the production microalgae biomass. Cordero, et al. [13], studied the effect of sodium acetate concentration on Chlorella sorokiniana biomass production. They observed a 20% increase in biomass when the sodium acetate concentration varied between 0 to 40 mM, while at higher levels of sodium acetate (50 and 60 mM), not observed changes in the biomass production. Nitrogen is another nutrient that affects the growth and composition of microalgae. Microalgae species differ in its nitrogen requirements and their assimilation kinetics [14].
Light is another factor that affects the microalgae production. Nutrient assimilation responds to the free energy gradient between inside and outside of cell. Thus, limitations in cellular energy can limit the assimilation of nutrients and consequently cell growth [14]. Changes in the nutritional composition of the culture medium as well as culture conditions induce stress in microalgae cultures, causing modifications in cell physiology and biochemical composition [15]. Therefore, it is important to determine both, nutritional composition optimum and culture conditions optimum for the achievement of high yields of microalgae with valuable metabolites.
This study was designed to determine the best combination of the variables sodium nitrate, sodium acetate and irradiance on the Scenedesmus obliquus culture in order to maximize and minimize the accumulation of essential minerals, and thus contribute to its possible application in formulated food in order to reduce the essential minerals deficiency in a natural form.
Microalga inoculum
The Scenedesmus obliquus microalga (IOAC081F) was isolated from Embalse Salto Grande, a region of North-East Argentina [16]. The inoculum was cultured in batch mode, using Allen & Arnon culture medium enriched with 0.850 g L-1 of NaNO3. The culture was maintained in exponential growth phase at temperature of 25 ± 1°C, aerated at 0.20 v v-1 min-1, a pH = 8.0 ± 0.1, a light intensity of 150 μE m-2 s-1 and a 12:12 h light/dark cycle.
Obtaining microalgal biomass: Culture media and operating conditions
The microalgal biomass obtaining was carrying out in two stages.
Stage 1: Exponential phase: Assays were performed at laboratory scale in 36 bubble-column type bioreactors. Each bioreactor was filled with 250 mL of Allen & Arnon culture medium (enriched with NaNO3) and 50 mL of microalga inoculum. The bioreactors were installed in an incubator chamber 400H (MGC, China) with control of temperature, photoperiod and Relative Humidity (RH). The operating conditions were: 25 ± 1°C, 65% RH, aeration of 0.20 v v-1 min-1 and pH of 8.0 ± 0.1. The cultures were artificially illuminated using eight 36 W fluorescent tubes (Osram Lumilux T8). Illumination was performed using a circadian cycle (12:12 light/dark). These operating conditions were maintained until reaching the stationary phase.
Stage 2: Stationary phase: Once the stationary phase was reached, the culture was operated in a semi-continuous mode. Under this condition, the sodium nitrate and sodium acetate concentrations of Allen & Arnon culture medium and the irradiance were modified according to the experimental design. In stationary phase, 20% of the volume daily was harvested and replaced it with fresh culture medium formulated (Table 1). The cultures were operated in semi-continuous mode during 21 days. Each 7 days, the biomass was harvested for minerals analysis.
Experimental design
The central composite design was performed using Statgraphics Centurion XVI software. The three studied factors were irradiance (μE m-2 s-1), sodium nitrate concentration (g L-1) and sodium acetate concentration (g L-1) which were evaluated at three different levels, using a 23 screening factorial design. The choice of factors levels was based on information from literature and preliminary experiments. Thirty-six experiments were carried out in randomized run order (2 blocks with 15 points of factorial design and three centre points to establish experimental errors) (Table 1). The response variables were the minerals concentration [mg (100gdw)-1] of Na, K, Ca, Mg, Fe, Zn and Mn.
Harvesting and storing the microalgal biomass
After 21 days of starting stationary phase, cultures were stopped and cells harvested by centrifugation at 4000 rpm for 4 min (Boeco C-28 centrifuge, Germany). Biomass cakes of samples were washed with bi-distilled water in order to eliminate non-biological material (mineral salt precipitates). Then the biomass was lyophilized at -90ºC (Heto Drywiner), 5-7% moisture values. Samples were stored at −80°C until analyses.
Sample preparation and determination of minerals
Lyophilized samples (~100 mg) were weighed in triplicate and digested using a Kjeldahl digester. For digestion, 5 mL of HNO3 70% concentrated (Merck) was used, the temperature was maintained at 80 ºC for 30 min. After digestion, samples were diluted 5 to 500 times in bi-distilled water for subsequent quantification by microwave plasma atomic emission spectrometry (4210 Series MP-AES, Agilent Technologies, USA).
Minerals were measured using AccuStandard standard solutions for calibration. The microalgal biomass was analysed by following methods described by Association of Official Analytical Chemists [17].
The instrumental parameters used for the multi-mineral determination were with a radiofrequency generator of 40 MHz, a power of 1.2 kW, plasma gas flow rate of 15 L min−1, auxiliary gas flow rate of 0.5 L min−1, and nebulizer gas flow rate of 0.7 L min−1. The wavelength (nm) of the minerals analysed were: Na (589.592), K (766.491), Ca (317.933), Mg (285.213), Fe (238.204), Zn (213.857), and Mn (259.372). Mineral composition was expressed as [mg (100gdw)-1]. Samples were analysed in triplicate.
Statistical analysis
The analysis of Response Surface Methodology (RSM) for each mineral was performed using the statistical software Statgraphics Centurion, Version XV (StatPoint Technologies Inc., Warrenton, VA, USA). The experimental factors and interactions were studied by analysis of variance. Factors and interactions were considered significant if p <0.05. While if p >0.05, they were excluded from the analysis.
In order to maximize Ca, Mg, K, Fe, Mn and Zn concentrations and to minimize Na concentration, the level of each factor was optimized by the Desirability Function Approach (DFA).
Initial mineral concentration in the microalgae biomass
The initial mineral concentration in Scenedesmus obliquus cells is presented in table 2, being especially rich in Ca (2963.92 [mg (100gdw)-1]), Na (2290.45 [mg (100gdw)-1]), K (392.09 [mg (100gdw)-1]) and Mg (360 [mg (100gdw)-1]). Among the micro-minerals were found as follows: Fe, 321.00 [mg (100gdw)-1]; Mn, 22.13 [mg (100gdw)-1]; and Zn, 6.88 [mg (100gdw)-1]. Toxic heavy metal amounts were not detected. The Allen & Arnon culture medium used had a low amount of toxic elements, but the situation could be different with other culture media or water sources. These results are in agreement with Tokusoglu and Ünal [18], who reported the mineral concentration of Spirulina platensis, Chlorella vulgaris and Isochrisis galbana in Conway culture medium (Table 2). However, the Ca and Mn concentration in Scenedesmus obliquus were 3 times higher.
Once the stationary phase of the culture had begun, each bioreactor was operated in a semi-continuous mode, where 20% of the volume daily was harvested and replaced it with fresh culture medium formulated. Each culture medium was prepared according to the experimental design (Table 1) in which the concentration of nitrate and acetate sodium, as well as the irradiance, were changed. The results obtained for each mineral are indicated below. For all minerals, the highest values were achieved after 7 days of starting the stationary phase.
Table 1: Experimental design used to analysis the minerals concentration of microalga Scenedesmus obliquus. |
||||||
| Factors | Discretized Levels |
Experimental Levels |
Units | |||
| Low | Upper | Low | Centre points |
Upper | ||
| Irradiance | -1 | 1 | 36.71 | 69.50 | 102.30 | μE m-2 s-1 |
| sodium nitrate concentration | -1 | 1 | 0.27 | 44.00 | 87.73 | g L-1 |
| sodium acetate concentration | -1 | 1 | 0.00 | 2.50 | 5.02 | g L-1 |
| Response variable | Units | |||||
| Minerals concentration | [mg (100gdw)-1] | |||||
Table 2: Comparison of mineral concentration between 3 microalgae samples (Tokusoglu and Ünal [18]), and initial mineral concentration of Scenedesmus obliquus biomass grown in Allen & Arnon culture medium enriched with NaNO3. |
|||||||
| Microalga | Concentration [mg (100gdw)-1] | ||||||
| Ca | Na | K | Mg | Fe | Mn | Zn | |
| Spirulina platensis | 883.00 | 1897.30 | 1326.90 | 398.60 | 90.10 | 3.80 | 2.50 |
| Chlorella vulgaris | 593.70 | 1346.40 | 49.90 | 344.30 | 259.10 | 2.10 | 1.20 |
| Isochrisis galbana | 1081.00 | 1109.20 | 1193.20 | 688.60 | 228.40 | 5.70 | 2.70 |
| Scenedesmus obliquus | 2963.92 | 2290.45 | 392.09 | 360.72 | 321.00 | 22.13 | 6.88 |
Effect of experimental factors on the Potassium (K) concentration
The K concentration varied between 3.35 [mg (100gdw)-1] and 1515.77 [mg (100gdw)-1] according to the change in the experimental factors values.
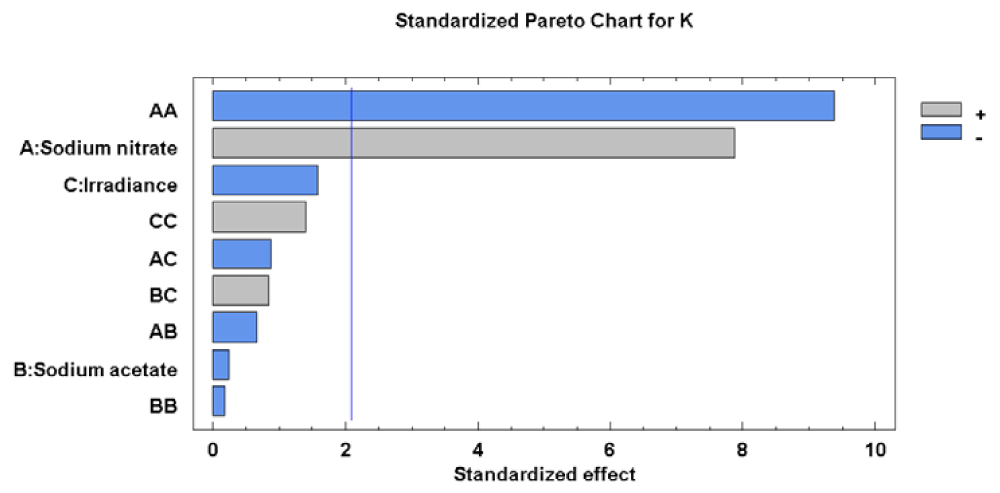
For the K concentration, the effects of sodium nitrate concentration (factor A) on its linear and quadratic component were significant. The effects of each factor and their interaction, as well as statistical significance, can be seen most clearly in the Pareto diagram (Figure 1).
Factor A, in its linear and quadratic component, had a significant effect on K levels in the microalgae biomass, which indicates that the factor A maximize the response. Therefore, it is clear that the level of K in cells of Scenedesmus obliquus is directly related to the sodium nitrate concentration in culture medium used. In conditions of relatively constant temperature and pH, this factor would control reactions related to the accumulation of K in the microalgae cells. The value of the R-squared statistic supports this statement because the results obtained explain 84.81% of the variability of the K concentration.
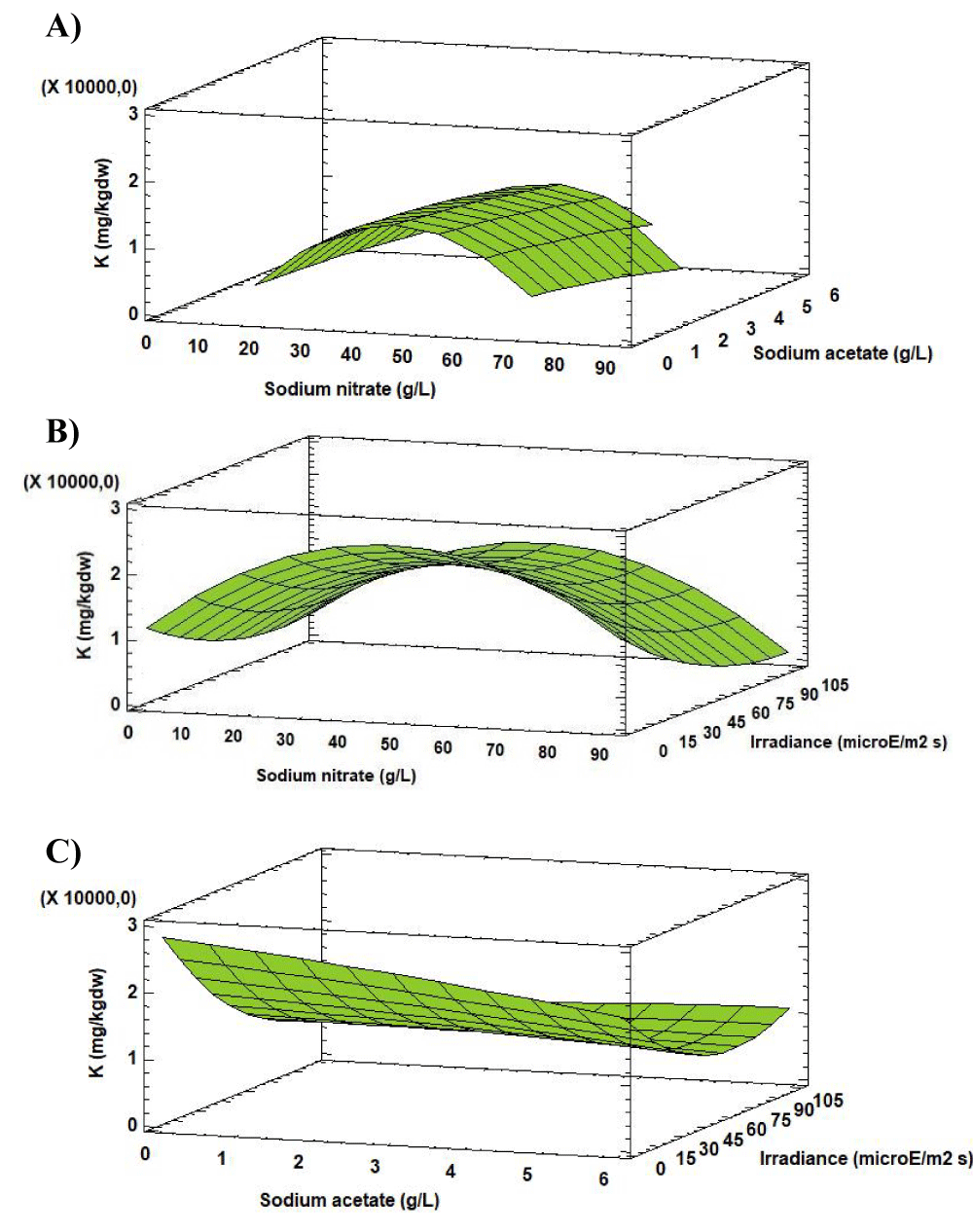
Analysis of the Response Surface Methodology (RSM) shows that the highest K concentration was 1515.77 [mg (100gdw)-1] (4 times higher than in the initial biomass), when the culture grown at 46.00 g L-1 of sodium nitrate, 0.00 g L-1 of sodium acetate and 36.71 µE m-2 s-1 (Figure 2). Under these conditions the K concentration in Scenedesmus obliquus is higher than that previously reported by Hernández, et al. [19] for Scenedesmus almeriensis growing in pig manure (810 [mg (100gdw)-1]).
An adult individual considered healthy requires 2000 mg of K per day, value recommended by the Food and Drug Administration [20], which could be achieved with a daily consumption of 132 g of Scenedesmus obliquus, produced under the indicated conditions. Otherwise, a daily intake of 510 g of Scenedesmus obliquus will be necessary. In other cases, a daily intake of approximately 150g of Spirulina platensis or 4000 g of Chlorella vulgaris will meet K requirements [18].
Effect of experimental factors on the Calcium (Ca) concentration
The Ca concentration varied between 11.48 [mg (100gdw)-1] and 2744.24 [mg (100gdw)-1] according to the change in the experimental factors values.
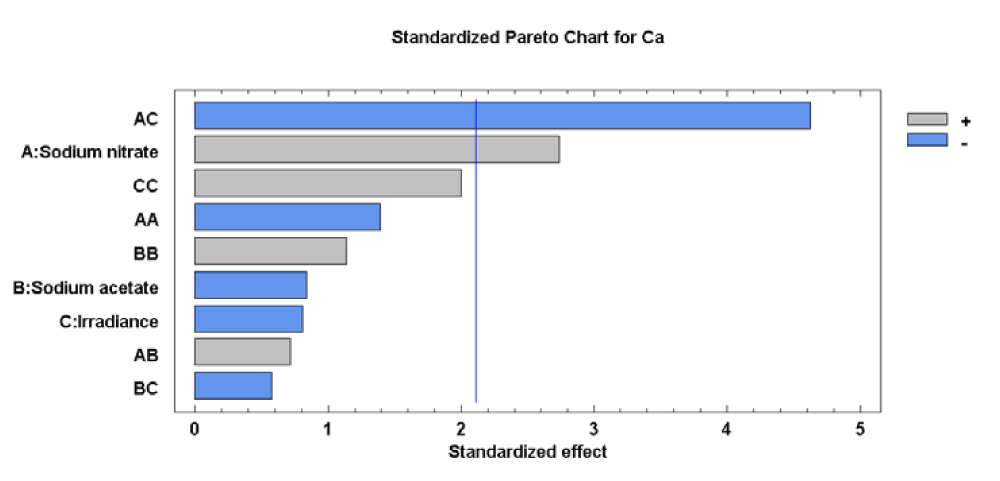
For the Ca concentration, the effects of sodium nitrate concentration (factor A) and the sodium nitrate concentration-irradiance interaction (factor AC) were significant. The effects of each factor and their interaction, as well as statistical significance, can be seen most clearly in the Pareto diagram (Figure 3).
The significance of sodium nitrate concentration (factor A) and the sodium nitrate concentration-irradiance interaction (Factor AC) shows that the both factors maximize the response. Therefore, it is clear that the level of Ca in cells of Scenedesmus obliquus is directly related to A and C factors used. In conditions of relatively constant temperature and pH, this factor would control reactions related to the accumulation of Ca in the microalgae cells. The value of the R-squared statistic supports this statement because the results obtained explain 80.51 % of the variability of the Ca concentration.
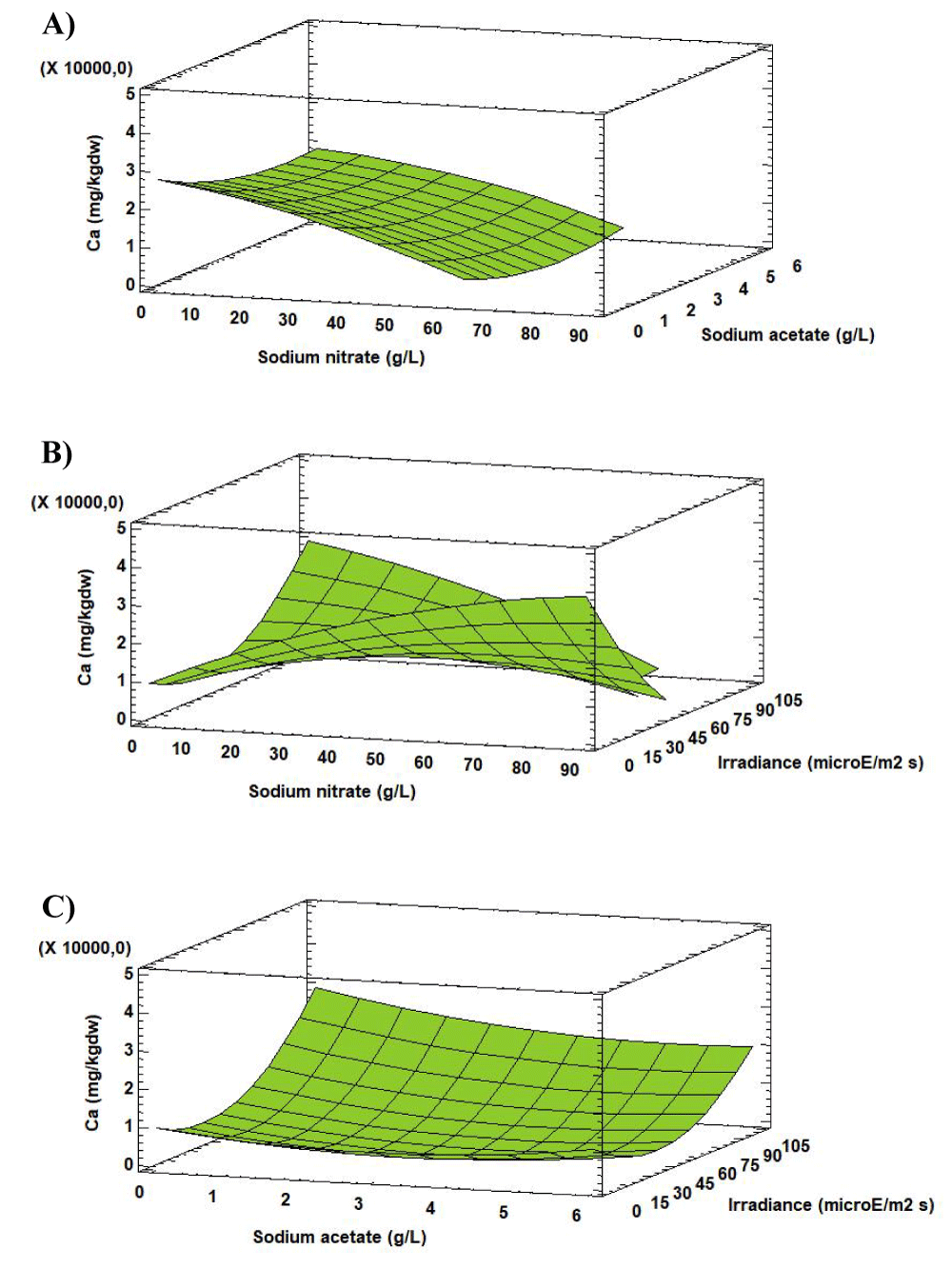
Analysis of the Response Surface Methodology (RSM) shows that the highest Ca was 2744.24 [mg (100gdw)-1],), when the culture grown at 0.27 g L-1 of sodium nitrate, 0.00 g L-1 of sodium acetate and 102.05 µE m-2s-1 (Figure 4). Under these conditions the Ca concentration in Scenedesmus obliquus is higher than that previously reported by Tokusoglu and Ünal [18] for Spirulina platensis (883.00 [mg (100gdw)-1]) Chlorella vulgaris (593.70 [mg (100gdw)-1]) and Isochrisis galbana (1081.00 [mg (100gdw)-1]) culturing in Conway standard medium.
The recommended daily intake for Ca is 1000 mg per day for adults [20]. Therefore, a daily intake of approximately 36 g of Scenedesmus obliquus, produced under the indicated conditions, will meet Ca requirements for an adult. Otherwise, a daily intake of 37 g of Scenedesmus obliquus will be necessary.
In other cases, a daily intake of approximately 113 g of Spirulina platensis or 168 g of Chlorella vulgaris will meet Ca requirements [18].
Effect of experimental factors on the Magnesium (Mg) concentration
The Mg concentration varied between 23.18 [mg (100gdw)-1] and 9697.65 [mg (100gdw)-1] according to the change in the experimental factors values.
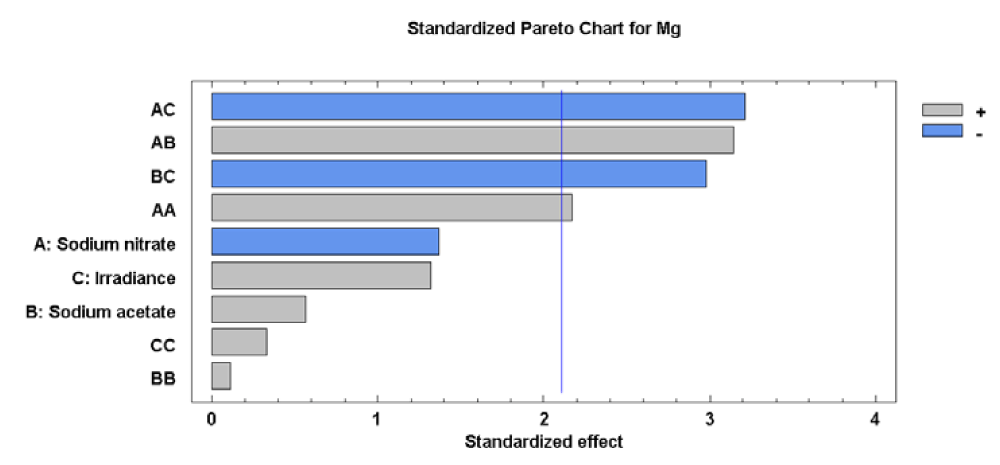
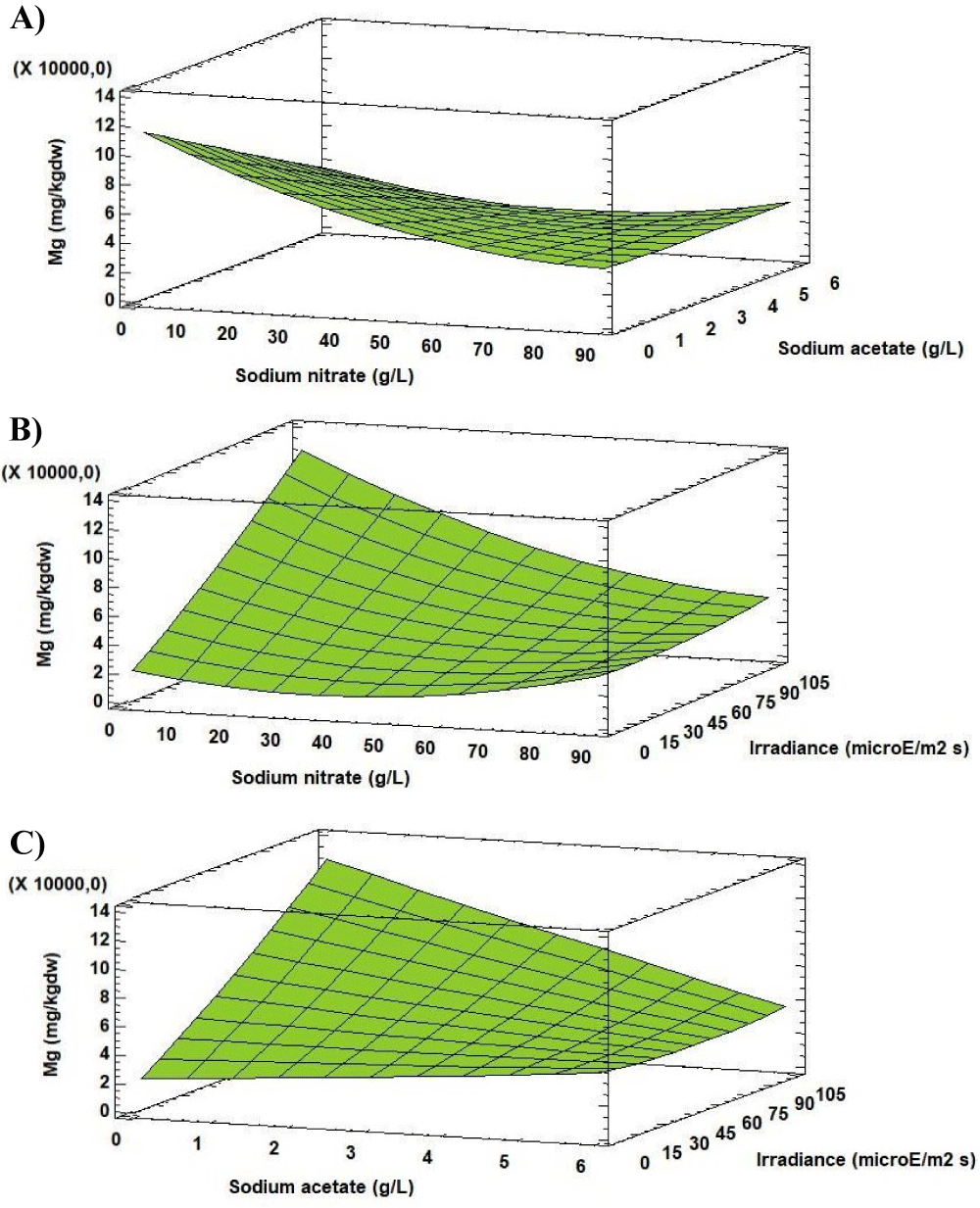
For the Mg concentration, the effects of sodium nitrate concentration on its quadratic component (factor AA), sodium nitrate concentration- sodium acetate concentration interaction (Factor AB), sodium nitrate concentration-irradiance interaction (Factor AC) and sodium acetate concentration-irradiance interaction (Factor BC) were significant. The effects of each factor and their interaction, as well as statistical significance, are reported in figure 5.
The significance of sodium nitrate concentration (factor A) with the other factors (B and C) on Mg levels in the microalgae biomass shows that the A, B and C factors have a significant influence to the response. Therefore, it is clear that the level of Mg in cells of Scenedesmus obliquus is directly related to A, B and C factors used. In conditions of relatively constant temperature and pH, this factor would control reactions related to the accumulation of Mg in the microalgae cells. The value of the R-squared statistic supports this statement because the results obtained explain 80.67% of the variability of the Mg concentration.
Analysis of the Response Surface Methodology (RSM) shows that the highest Mg concentration was 9697.65 [mg (100gdw)-1] (25 times higher than in the initial biomass), observed for the culture grown at 0.27 g L-1 of sodium nitrate, 0.00 g L-1 of sodium acetate and 95.28 µE m-2s-1 (Figure 6). Under these conditions the Mg concentration in Scenedesmus obliquus is higher than that previously reported by Hernández, et al. [19] for Scenedesmus almeriensis growing in pig manure (511 [mg (100gdw)-1]).
An adult individual considered healthy requires 420 mg of Mg per day, value recommended by the Food and Drug Administration [20], which could be achieved with a daily consumption of 4.33 g of Scenedesmus obliquus, produced under the indicated conditions. Otherwise, a daily intake of 116 g of Scenedesmus obliquus will be necessary. In other cases, a daily intake of approximately 105g of Spirulina platensis or 122 g of Chlorella vulgaris will meet Mg requirements [18].
Effect of experimental factors on the Sodium (Na) concentration
The Na concentration varied between 5607.20 [mg (100gdw)-1] and 17575.956 [mg (100gdw)-1] according to the change in the experimental factors values.
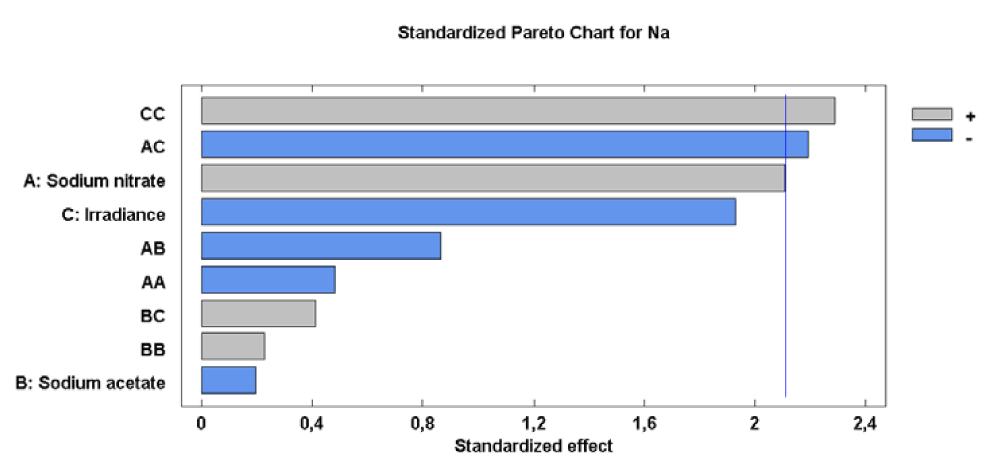
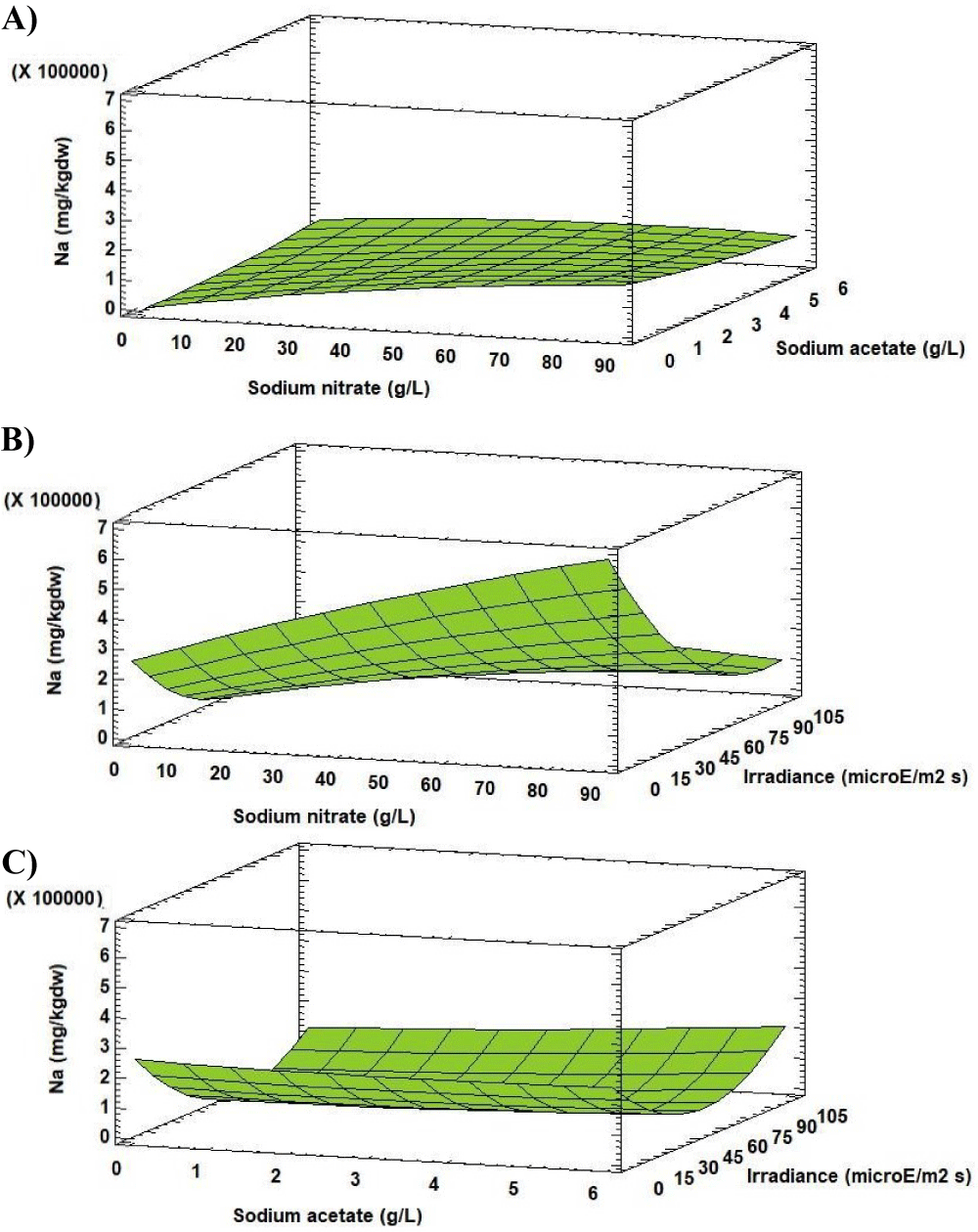
For the Na concentration, the effects of the sodium nitrate concentration-irradiance interaction (factor AC) and the irradiance on its quadratic component (factor CC) were significant. The effects of each factor and their interaction, as well as statistical significance, are reported in figure 7.
The significance of the sodium nitrate concentration-irradiance interaction (factor AC) and the irradiance-irradiance interaction (factor CC) shows that the both interactions minimize the response. Therefore, it is clear that the level of Na in cells of Scenedesmus obliquus is directly related to A and C factors used. In conditions of relatively constant temperature and pH, this factor would control reactions related to the accumulation of Na in the microalgae cells. The value of the R-squared statistic supports this statement because the results obtained explain 85.68 % of the variability of the Na concentration.
Analysis of the Response Surface Methodology (RSM) shows that the minimum concentration of Na (5607.20 [mg (100gdw)-1]) was observed for the culture grown at 0.27 g L-1 of sodium nitrate, 0.00 g L-1 of sodium acetate and 62.23 µE m-2s-1 (Figure 8). Under these conditions the Na concentration in Scenedesmus obliquus is higher than that previously reported by Tokusoglu and Ünal [18] for Spirulina platensis (1897.30 [mg (100gdw)-1]), Chlorella vulgaris (1346.40 [mg (100gdw)-1]) and Isochrisis galbana (1109.20 [mg (100gdw)-1]) culturing in Conway standard medium.
The recommended daily intake for Na must be less than 2300 mg per day for adults [20]. Therefore, a daily intake of less 41 g of Scenedesmus obliquus, produced under the indicated conditions, will meet Na requirements for an adult. Sodium, normally found in foods in the form of Sodium Chloride (NaCl), is an essential mineral for the correct function of the human body. However, it is known that the high consumption of this mineral may trigger body disorders, as such cardiovascular disease and increased blood pressure [21].
Effect of experimental factors on the micro-minerals concentration: Fe, Zn and Mn
Among the micro-minerals, Fe was found in the largest amount, followed by Zn and Mn.
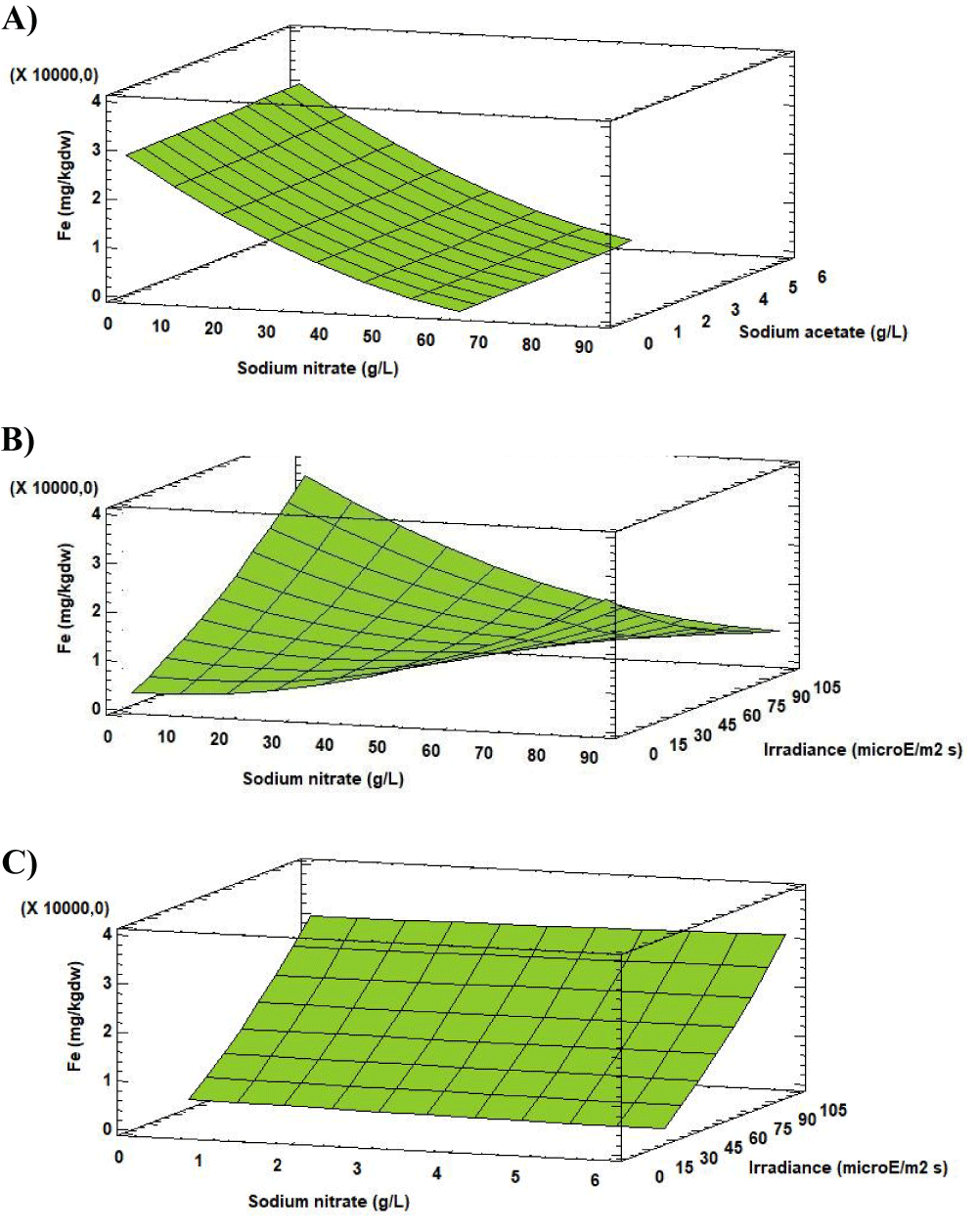
The Fe concentration varied between 18.02 [mg (100gdw)-1] and 2932.42 [mg (100gdw)-1] according to the change in the experimental factors values.
Analysis of the Response Surface Methodology (RSM) shows that the highest Fe concentration was 2932.42 [mg (100gdw)-1] (10 times higher than in the initial biomass), observed for the culture grown at 0.27 g L-1 of sodium nitrate, 5.00 g L-1 of sodium acetate and 102.05 µE m-2s-1 (Figure 9). Under these conditions the Fe concentration in Scenedesmus obliquus is higher than that previously reported by Tokusoglu and Ünal [18] for Spirulina platensis (90.10 [mg (100gdw)-1]), Chlorella vulgaris (259.10 [mg (100gdw)-1]) and Isochrisis galbana (228.40 [mg (100gdw)-1]) culturing in Conway standard medium.
Iron is an essential mineral nutrient which is present in many foods, taking as reference the food known worldwide as a source of iron, such as raw liver (5.60 [mg (100gdw)-1]), leaves of spinach (19.23 [mg (100gdw)-1]) and brindle raw beans (1860 [mg (100gdw)-1]) [22,23].
Adult males, children and infants requires 10 mg of Fe per day, while for an adult female the recommended daily intakes is 15 mg of Fe per day [20]. These values could be achieved with the consumption of 0.50 g day-1 of Scenedesmus obliquus, produced under the indicated conditions. Otherwise, a daily intake of 5 g day-1 of Scenedesmus obliquus will be necessary. Whereas about 6.6 g day-1 of Isochrisis galbana, 5.8 g day-1 of Chlorella vulgaris and 16.7 g day-1 of Spirulina platensis fulfils Fe requirements [18].
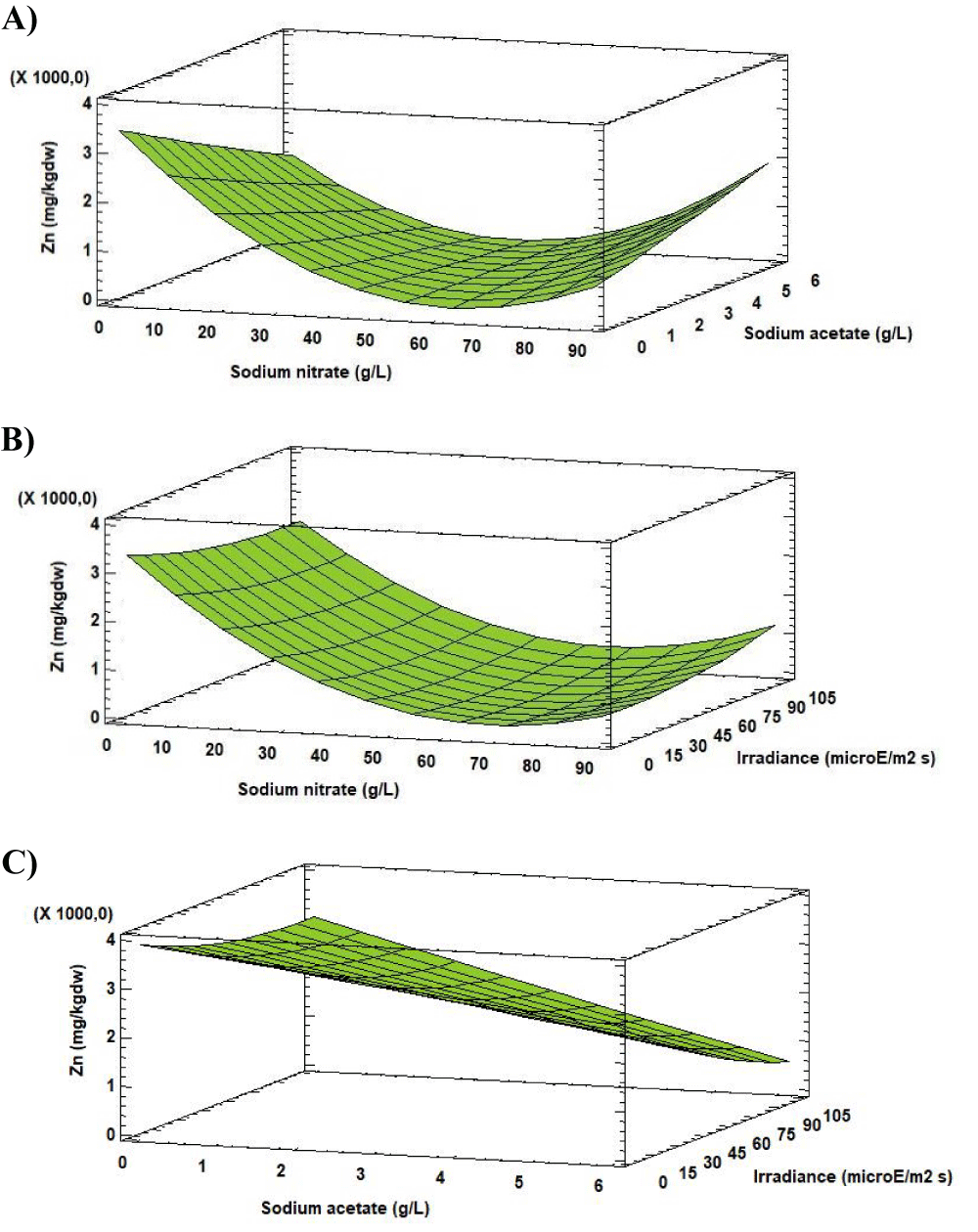
Regarding Zn, the concentration varied between 2.00 [mg (100gdw)-1] and 324 [mg (100gdw)-1] according to the change in the experimental factors values.
Analysis of the Response Surface Methodology (RSM) shows that the highest Zn concentration was 324 [mg (100gdw)-1] (50 times higher than in the initial biomass), observed for the culture grown at 0.27 g L-1 of sodium nitrate, 0.00 g L-1 of sodium acetate and 56.44 µE m-2s-1 (Figure 10). Under these conditions the Zn concentration in Scenedesmus obliquus is higher than that previously reported by Tokusoglu and Ünal [18] for Spirulina platensis (2.50 [mg (100gdw)-1]), Chlorella vulgaris (1.20 [mg (100gdw)-1]) and Isochrisis galbana (2.70 [mg (100gdw)-1]) culturing in Conway standard medium.
Biologically, Zn is a functional component of several metal-enzymes and metal-proteins, participates in many reactions of cellular metabolism, such as immune function, antioxidant defence and growth [6].
Zinc is present in much kind of foods, taking as reference the food known worldwide as a source of zinc, such as pork meat (1.39–2.80 [mg (100gdw)-1]), rice (1.53–5.84 [mg (100gdw)-1]) and dark chocolate (9.60 [mg (100gdw)-1]) [24].
The recommended daily intake for Zn is 11 mg per day for adults [20]. This value could be achieved with the consumption of 3.39 g day-1 of Scenedesmus obliquus, produced under the indicated conditions. Otherwise, a daily intake of 160 g day-1 of Scenedesmus obliquus will be necessary. Whereas about 407 g day-1 of Isochrisis galbana, 917 g day-1 of Chlorella vulgaris and 440 g day-1 of Spirulina platensis fulfils Zn requirements [18].
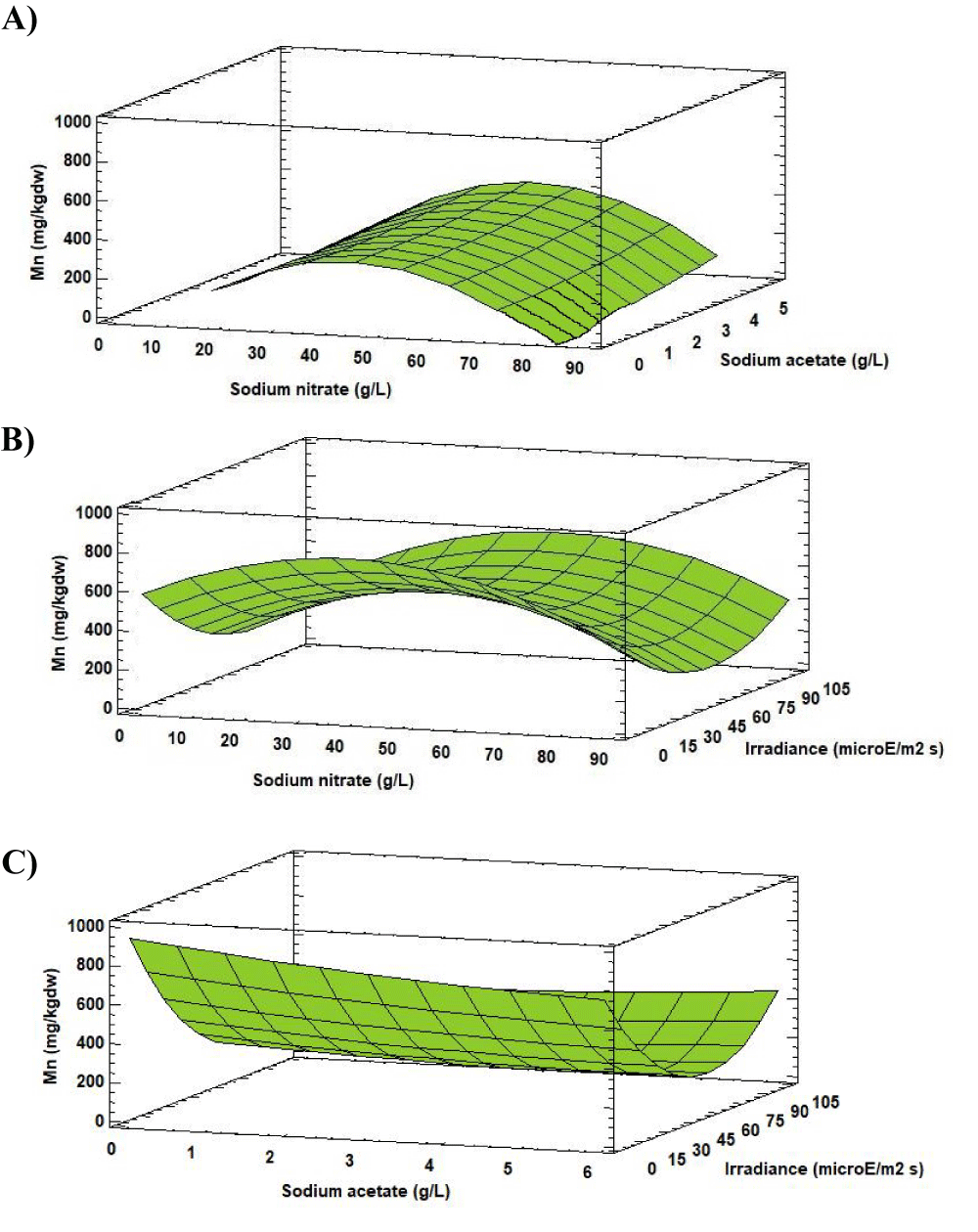
Manganese is another essential mineral. The Mn concentration varied between 0.09 [mg (100gdw)-1] and 38.48 [mg (100gdw)-1] according to the change in the experimental factors values.
Analysis of the Response Surface Methodology (RSM) shows that the highest Mn concentration was 38.48 [mg (100gdw)-1] (1.5 times higher than in the initial biomass), observed for the culture grown at 46.00 g L-1 of sodium nitrate, 5.00 g L-1 of sodium acetate and 102.05 µE m-2s-1 (Figure 11). Under these conditions the Mn concentration in Scenedesmus obliquus is higher than that previously reported by Tokusoglu and Ünal [18] for Spirulina platensis (3.80 [mg (100gdw)-1]), Chlorella vulgaris (2.10 [mg (100gdw)-1]) and Isochrisis galbana (5.70 [mg (100gdw)-1]) culturing in Conway standard medium.
Biologically, Mn is associated with the connective tissues and bones formation, carbohydrates and lipids metabolism, as well as growth and reproduction processes [24]. Rich sources of manganese include nuts (8.8 [mg (100gdw)-1]), leafy green vegetables, such as spinach (0.9 [mg (100gdw)-1]) and wheat germ (20 [mg (100gdw)-1]) [25].
The recommended daily intake for Mn is 2.3 mg per day for adults [20]. This value could be achieved with the consumption of 5.98 g day-1 of Scenedesmus obliquus, produced under the indicated conditions. Otherwise, a daily intake of 10.40 g day-1 of Scenedesmus obliquus will be necessary. Whereas about 40.35 g day-1 of Isochrisis galbana, 109.52 g day-1 of Chlorella vulgaris and 60.52 g day-1 of Spirulina platensis fulfils Mn requirements [18].
Thus, Scenedesmus obliquus biomass produced under the indicated conditions can be considered as a far more representative source of the micro-minerals Fe, Zn and Mn.
The present work provided information regarding the influence of the nitrate and acetate sodium concentrations and the irradiance in order to improve the minerals content in Scenedesmus obliquus biomass.
The model and the three-level-factorial a design give a reliable picture of the situation, and lets us know clear results for the decision making.
The microalgal biomass of Scenedesmus obliquus studied was characterized as a good source of essential minerals and confirmed to be a potentially valuable ingredient for the development of nutritional supplementation.
The authors acknowledge the financial support provided by the Universidad Nacional de Entre Ríos through the project Nº 8105: "Effect of light stress and nitrogen and carbon concentrations on the mineral composition of the cultivation of microalgae Scenedesmus obliquus".
- Gutiérrez-Cuesta R, González García KL, Valdés Iglesias OR, Hernández Rivera Y, Acosta Suárez Y. Algas marinas como fuente de compuestos bioactivos en beneficio de la salud humana: Un artículo de revisión. Revista de Ciencias Biológicas y de la Salud. 2016; 18: 20-27. https://bit.ly/37i9qiu
- Hira K, Tariq RM, Sultana V, Ara J, Ehteshamul-Haque S. Effect of seaweeds occurring at Karachi coast on mosquito larvae and liver function in rats. Pak J Pharm Sci. 2017; 30: 387-391. PubMed: https://pubmed.ncbi.nlm.nih.gov/28649061/
- Latham MC. In Nutrición humana en el mundo en desarrollo. 2002. FAO, New York, USA. https://bit.ly/2AUVXkS
- McDowell LR. In Minerals in Animal and human nutrition, 2nd Ed. 2003. Elsevier Science, Amsterdam.
- Salgueiro MJ, Zubilaga MB, Lysionek AE, Caro RA, Weill R, Boccio JR. The role of zinc in the growth and development of children. Nutrition. 2002; 18: 510-519. https://bit.ly/2ApnR8A
- Pedraza DF, Rocha ACD, Queiroz EO, Sousa CPC. Zinc nutritional status in children attending public daycare centers in the state of Paraíba, Brazil. J Nutr. 2011; 24: 539-552. https://bit.ly/3hkQXGH
- Kodoth-Prabhakaran N. Mineral deficiency and behavior vis-à-vis the central nervous system. In: food and human responses. Springer, Cham Food and Human Responses. 2020; 83-97.
- Pereira GAP, Genaro OS, Pinheiro MM, Szejnfeld VL, Martini LA. Dietary calcium: Strategies to optimize intake. Braz J Rheumatol. 2009; 49: 164-180. https://bit.ly/3dTDQdE
- Gómez AL, López JA, Rodríguez A, Fortiz J, Martínez LR, Apolinar A, et al. Producción de compuestos fenólicos por cuatro especies de microalgas marinas sometidas a diferentes condiciones de iluminación. Lat Am J Aquat Res. 2016; 44: 137-143. https://bit.ly/2UuyRIA
- Singh UB, Ahluwalia AS. Microalgae: A promising tool for carbon sequestration. Mitig Adapt Strategy Glob Change. 2013; 18: 3-95. https://bit.ly/30ygyWD
- Draaisma RB, Wijfels RH, Slegers PM, Brentner LB, Roy A, Barbosa MJ. Food commodities from microalgae. Curr Opin Biotechnol. 2013; 24: 169-177. PubMed: https://pubmed.ncbi.nlm.nih.gov/23084075/
- Ramírez-Mérida LG, Ragagnin de Menezes C, Queiroz-Zepka L, Jacob-Lopes E. Microalgas: Potencial para la producción de compuestos bioactivos nanoencapsulados. Ciência e Natura. 2015; 37: 7-17.
- Cordero BF, Obraztsova I, Couso I, León R, Vargs MA, Rodríguez H. Enhancement of lutein production in Chlorella sorokiniana (Chorophyta) by improvement of culture conditions and random mutagenesis. Mar Drugs. 2011; 9: 1607-1624. PubMed: https://pubmed.ncbi.nlm.nih.gov/22131961/
- González-González LM. Influencia de la deficiencia de nitrógeno y fósforo en las interacciones competitivas entre Chlorella vulgaris y Scenedesmus acutus. Tesis Magister. Universidad Nacional de Colombia. 2010. https://bit.ly/3cQZyNZ
- Ördög V, Stirk WA, Bálint P, Van Staden J, Lovász C. Changes in lipid, protein and pigment concentrations in nitrogen-stressed Chlorella minutissima cultures. J Appl Phycol. 2012; 24: 907-914. https://bit.ly/2UB5SmB
- Jiménez-Veuthey M, Vidal MN, Cabrera C, Páramo J, Bertoni M, Bordet HF, et al. A simple, efficient and economical method for isolation of Scenedesmus obliquus (Chlorophyceae) from freshwater sample (Embalse Salto Grande, Argentina). Asian J Microbiol Biotechnol Environ Sci. 2018; 20: 6-12. https://bit.ly/3dZnCji
- AOAC (Association of Official Analytical Chemistry). Official methods of analysis, 17th ed. 2000. Washington, DC: USA. https://bit.ly/2Ys0DGG
- Tokusoglu Ö, Ünal MK. Biomass nutrient profiles of three microalgae: Spirulina platensis, Chlorella vulgaris, and Isochrisis galbana. J Food Sci. 2003; 68: 1144-1148. https://bit.ly/30uOHXo
- Hernández D, Molinuevo-Salces B, Riaño B, Larrán-García AM, Tomás-Almenar C, García-González MC. Recovery of protein concentrates from microalgal biomass grown in manure for fish feed and valorization of the by-products through anaerobic digestion. Front Sustain Food Syst. 2018; 28: 1-11. https://bit.ly/3feVJng
- FDA (Food and Drug Administration). Researchers Accessed. 2019.
- Muniz Moreira L, Ribeiro AC, Duarte FA, Greque de Morais M, Almeida de Souza Soares L. Spirulina platensis biomass cultivated in Southern Brazil as a source of essential minerals and other nutrients. Afr J Food Sci. 2013; 7: 451-455. https://bit.ly/3cT7kqs
- Braga EO, Mendonça LG. Discution of the rational use of human diets, with focus to its main constituents: linseed and quinoa. Perspec Sci Technol. 2010; 2: 32-43.
- Karmakar K, Muslim T, Rahman M. Chemical composition of some leafy vegetables of Bangladesh. Dhaka Univ J Sci. 2013; 61: 199-201. https://bit.ly/2XPbziC
- Mafra D, Cozzolino SMF. The importance of zinc in human nutrition. J Nutr. 2004; 17: 79-87. https://bit.ly/2B1XGF6
- USDA. National nutrient database for standard reference. US Department of Agriculture, Agricultural Research Service, USDA Nutrient Data Laboratory. 2018, release 28: 9040. https://bit.ly/2UyYDve
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley