2025 July 28;6(7):986-993. doi: 10.37871/jbres2154.
A Mutational Pathway Leading to Thyroid Tumors During Medication for Feline Hyperthyroidism
Ryunosuke Kikuchi1,3*, Yumiko Kagawa2 and Rosário Plácido Roberto da Costa3
2North Lab, Sapporo 003-0027, Japan
3CERNAS, Escola Superior Agrária, Coimbra 3045-601, Portugal
Abstract
Over 350 million cats worldwide live in homes with their human companions. An inexplicable phenomenon is affecting domestic cats (felis catus) - the global prevalence of feline hyperthyroidism has been increasing since the late 1970s. Feline hyperthyroidism is the result of a malignant thyroid carcinoma in ~2% and a benign functional thyroid adenoma in ~98% of diagnosed cases. Thyroid tumors remain palpable in cats after medication (e.g., methimazole, carbimazole), but few studies have reported such remaining tumors. Hence, we pathologically examined tissues that were sampled from 206 cats by thyroidectomy after such medication at 65 veterinary hospitals. Our results show carcinoma in 11.2% and adenoma in 83.4% of samples. Based on an increase in the carcinoma rate from ~2% at the diagnosis stage to 11.2% at the post-treatment stage, it can be inferred that a thyroid adenoma transforms into a thyroid carcinoma in a hyperthyroid cat through a multistep mutational pathway like the human adenoma-carcinoma sequence. It remains for future study to assess whether this feline phenomenon is somehow related to the human one in terms of the One Health approach; e.g., the incidence rate of thyroid cancers has been increasing in humans since the early 1980s (cf. this tendency cannot be explained only by over-diagnosis and detection technology).
Introduction
There is little in history to show any large numbers of animals having been kept as pets. Although the reasons that pet keeping has become a widespread cultural phenomenon are unclear, it is evident from the prevalence of the practice that companion animals are vitally important in human lives [1]. In Japan, the number of pet animals (~18.1 million) now significantly exceeds the number of children (~15.1 million under 15 years old), so how to live a comfortable life with pets is a major issue for consideration as a country with a shrinking birth rate and rising number of elderly citizens [2]. As people have come to treat pet animals like family members, their concern about their pet’s health/well-being has also grown over recent years (cf. 92% of pet owners in the US are as concerned about their pet’s health as their own health [3]). Pet healthcare is now a high priority for both owners and veterinary practices. Consequently, animal hospitals specializing in pets are increasing in number, and many owners have their pets on waitlists for medical treatment [4].
Looking at it from a different perspective, pets and wild animals have been used to predict adverse effects on the human population. In a modern example, canaries were used to detect the toxic gas sarin (isopropyl methylphosphonofluoridate) after it was released into the Tokyo subways by a terrorist cult in 1995 [5]. Animals suffer a similar spectrum of disease as humans, and they are sensitive indicators and provide an early warning signal for human health intervention [6]. The One Health approach indicates that human, animal and environment are closely interconnected, and this approach has gained worldwide attention in recent years.
The annually increasing incidence of malignant tumors is of great concern in medical treatment for pet animals [7,8], and pet-owning communities demonstrate an increasing expectation for advanced veterinary oncology services [9]. In addition, it is also necessary to consider current disease trends among animals and humans from the One Health viewpoint. Against this backdrop, we focus on a common disease in domestic cats to reconsider whether the well-known therapy for this disease is truly effective and to contemplate whether the pathogenetic mechanisms are somehow related to malignancy risk in pet animals and humans. Basic information is briefly reviewed first.
Feline Hyperthyroidism
It is estimated that over 350 million cats worldwide live in homes with their human companions [10]. In domestic cats (felis catus), the prevalence of hyperthyroidism has been constantly increasing worldwide since veterinary evidence for this feline disorder was first reported in 1979 [11,12], and it is now recognized worldwide as the most common feline endocrine disorder. Annual incidence rates indicate that ~10% of cats older than 9 years in the US [11], ~12% of cats older than 9 years in the UK [11], ~9% of cats older than 9 years in Japan [11], ~8% of cats older than 10 years in Spain [13] and ~11% of cats older than 8 years in Germany [14] suffer from hyperthyroidism.
- Symptoms: Hyperthyroidism can be caused by an increase in thyroid hormones (i.e., triiodothyronine T3 and thyroxine T4) that are produced in an enlarged thyroid gland near a cat’s pharynx [11,12] (Figure 1A). Enlargement of thyroid glands generally results from a non-cancerous tumor called an adenoma [11,12,15]. As thyroid hormones influence almost all organs, a thyroid disease frequently creates secondary problems such as cardiovascular disease and hypertension [16]. Excessive thyroid hormones stimulate an increased heartbeat and a stronger contraction of the heart muscle, resulting in left ventricular hypertrophy of the heart [15].
- Risk factors: A number of risk factors have been proposed as follows [11,12,15]: increased age, genetic mutation, nutritive imbalance (e.g., iodine uptake), cat litter, canned cat food, mimicking hormones, goitrogens, environmental chemicals, and improved diagnostic capabilities. However, a clear relation between these factors and the increasing prevalence remains difficult to prove. As the major trigger of feline hyperthyroidism is still unknown, it is unclear how to take measures against it [11,12,14,15].
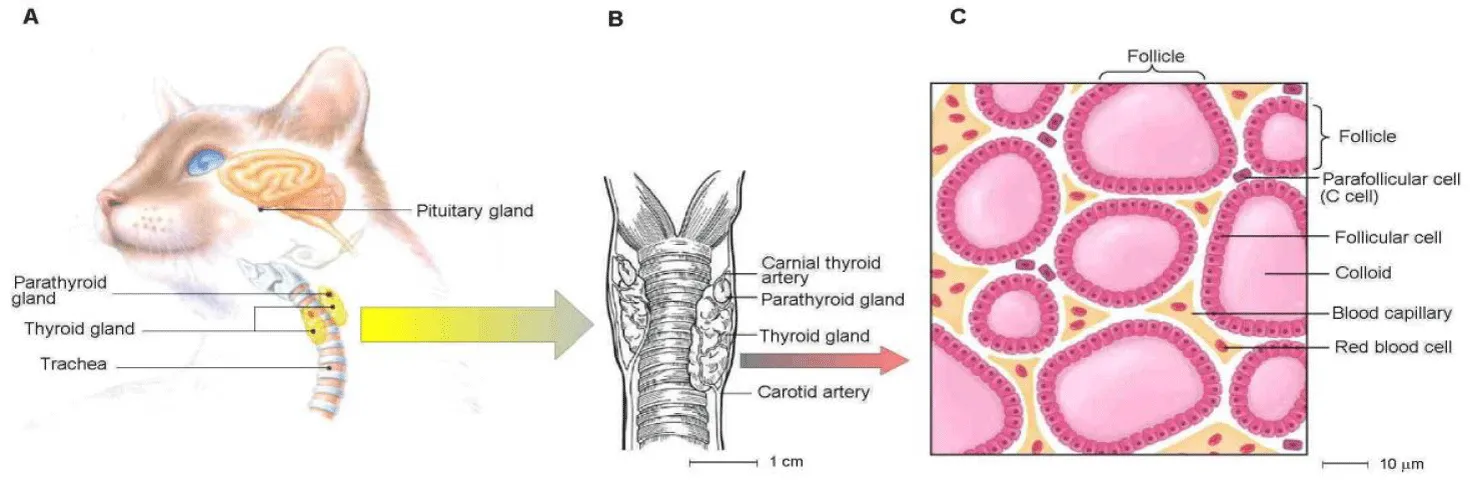
- Anatomical outline: The following anatomical outline is provided: the thyroid glands are two small elongated structures, located caudal to the larynx and lateral to the trachea on each side [17] (Figures 1A,B). The size of a single lobe in cats is approximately 2.0 cm in length and 0.3 cm in width [17], and the thyroid glands are each associated with four parathyroid glands (Figure 1B) that play a key role in maintaining calcium balance in the blood [17]. The thyroid contains two major populations of endocrine cells [18] (Figure 1C): (i) follicular cells - each major lobe of the thyroid consists of smaller lobules that are composed of sac-like follicles, and these cells synthesize and secrete iodine-rich hormones that regulate metabolism; and (ii) parafollicular cells - these are interspersed between the thyroid follicles and synthesize calcitonin.
Study Design
According to a review report on safety and efficacy assessments of anti-thyroid drugs such as methimazole and carbimazole [16], most of the medical treatments for feline hyperthyroidism have been borrowed from efficient treatments for human thyroid disease. Thyroid tumors remain palpable in cats after medication for controlling thyroid hormone concentrations [16,20]. A palpable thyroid abnormality suggests a possible presence of (i) thyroid dysfunction, (ii) obstruction or (iii) malignancy in humans, so it is recommended to conduct further tests for confirmation [21]. However, few studies have been reported on such remaining palpable tumors in cats. We therefore utilized thyroidectomy (a curative therapy option) to effectively sample thyroid tissues from hyperthyroid cats in collaboration with veterinary hospitals for the purpose of conducting a large-scale pathological observation of post-treatment tumors.
Typical treatments for feline hyperthyroidism are briefly reviewed as follows [11,12,15]. Medication (e.g., methimazole, thiamazole or carbimazole) is often the first line of therapy because the advantages of medication are that the drugs are readily available, simple and relatively inexpensive [11,12,15]. Although the medication using anti-thyroid drugs regulates thyroid hormone production [11,12,15], it cannot decrease the tumor size to cure the disease [16,22]. Furthermore, there is a possibility that some cats may experience side effects such as vomiting, anorexia, fever, anemia and lethargy [15].
It has been reported that hyperthyroid cats treated with long-term medication do not have a total Thyroxine (T4) level significantly different from untreated cats (reviewed in [23]). In the case that the demerits surpass the merits and/or a lack of efficacy continues in medical therapy over a prolonged period, curative treatments may be practical. They include (i) destruction of abnormal thyroid tissues through injection of radioactive iodine (iodine-131), and (ii) surgical removal of hyperthyroid glands [15]. The former has no serious side effects and avoids anaesthesia. This therapy provides a cure in ~95% of cases [15], but the handling/injection of a radioactive substance is not available to all practitioners [15]; e.g., there are 13 radioactive iodine facilities for veterinary services in the UK, in contrast, such facilities have not been legally licensed in Japan [24]. The latter is less common and is called thyroidectomy. It makes it possible to surgically resect hyper functioning thyroid glands [11,12,15]. From the viewpoint of our research approach, thyroidectomy is the only effective way of collecting a large number of tissue samples from hyperthyroid cats during their therapy.
Methods
Each thyroid tissue was sampled from a diagnosed cat by thyroidectomy as a curative treatment, and the tissue sample was fixed with 10% neutral-buffered formalin immediately after resection. The fixed sample was put in an airtight plastic container and sent to a pathology laboratory. After that, sections of the mass and grossly normal small intestines in close proximity to the mass were prepared. The tissues were stained with hematoxylin and eosin to examine a tissue portion under a microscope to see the structure and appearance of cells.
Results
As stated above, radioactive iodine therapy is an effective and curative option for hyperthyroid cats. With the spread of radiation therapy, thyroidectomy will become much less common at local practices. If that is the case, large-scale sampling of thyroid tissues from thyroidectomy will inevitably become inaccessible in the near future. Before this happens, we carried out a sampling of thyroid tissues in cooperation with 65 animal hospitals located in the Hokkaido, Tohoku, Kanto, Tokai, Kansai, Chugoku and Kyushu regions of Japan during a 6-year period from 2019 to 2024, and we pathologically examined the tissues that were sampled from 206 cats by thyroidectomy after a certain period of medical therapy.
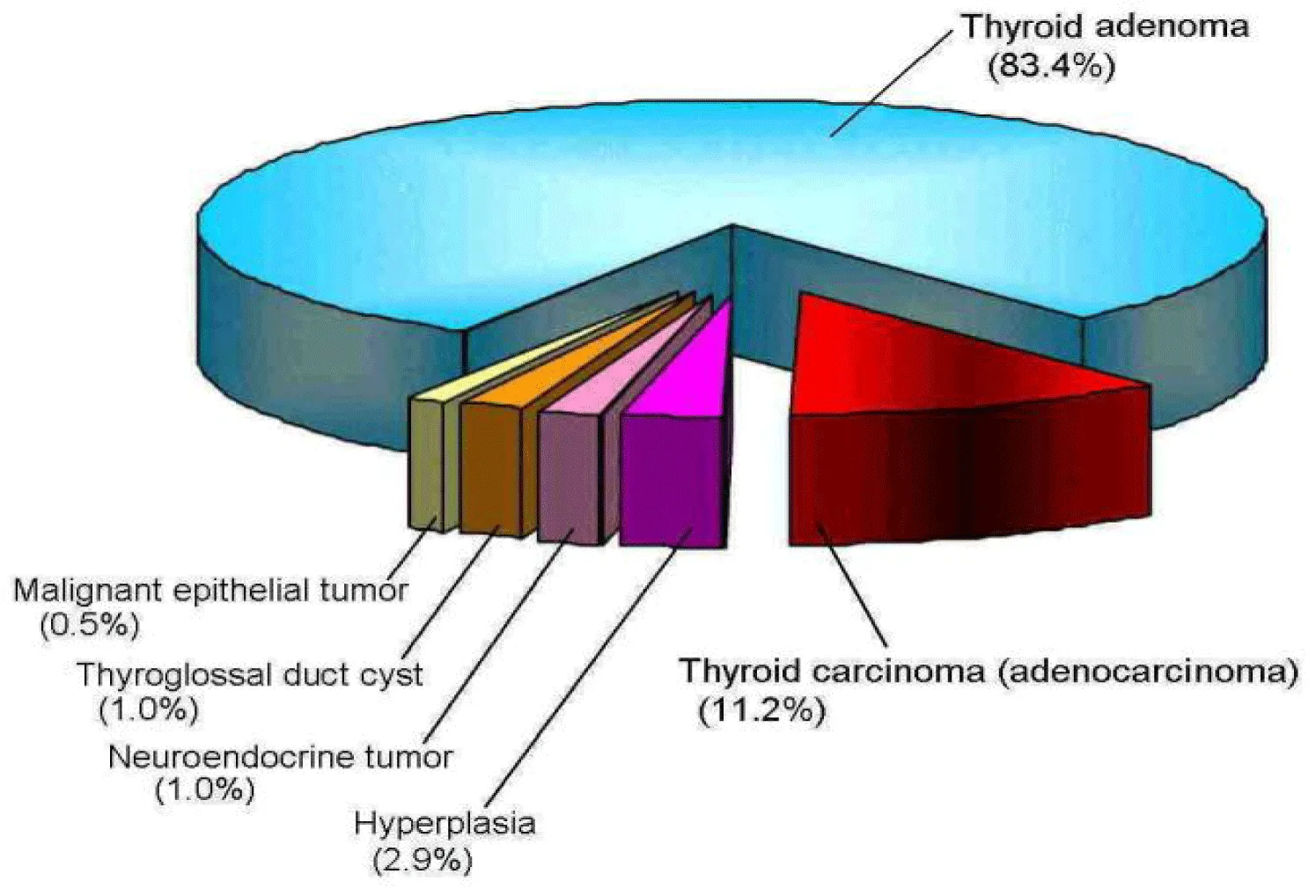
It is generally known that adenoma is a benign tumor (i.e., non-cancerous) arising from glandular tissue and carcinoma with metastasis ability is a malignant tumor derived from epithelial cells. Previous studies have reported that feline hyperthyroidism is the result of a malignant thyroid carcinoma (adenocarcinoma) in ~2% of diagnosed cases and a benign functional adenoma is responsible in ~98% of diagnosed cases [11,12]. By contrast, the results summarized in figure 2 show a comparatively high rate (~11%) of thyroid carcinoma and a comparatively low rate (~83%) of thyroid adenoma in the examined samples.
A case study of side effects
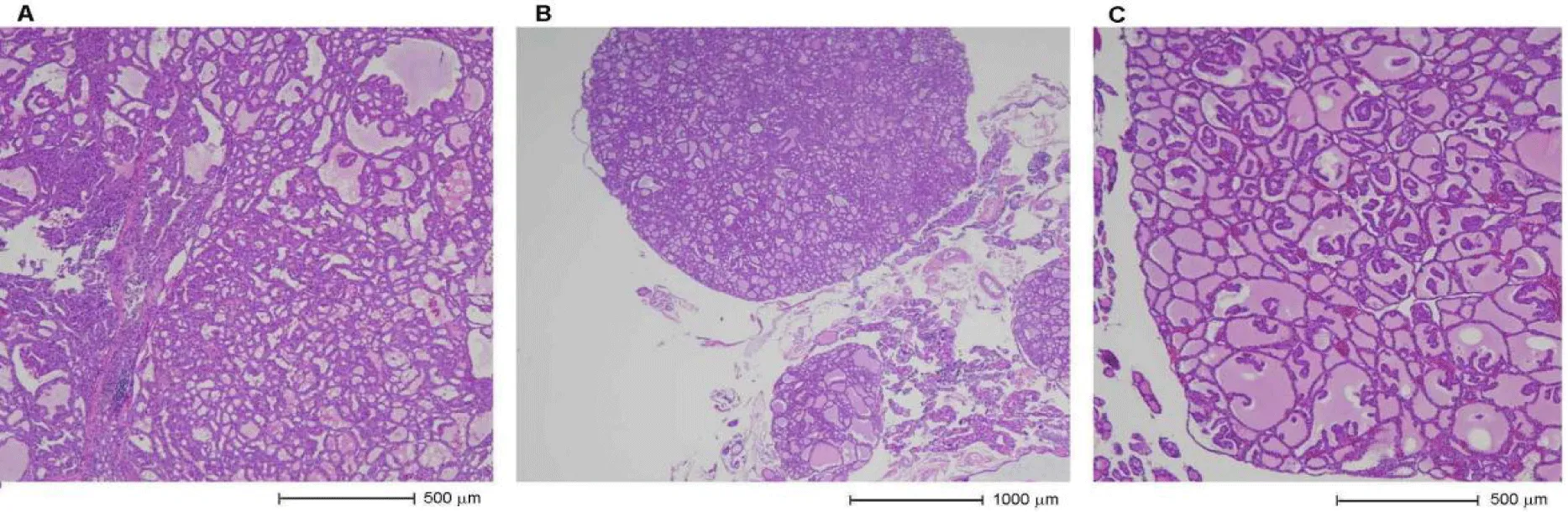
A cat with ID number BP20-1438 is characterized as a mixed breed, sterilized female of 15 years of age at the time. When she had a health check including a T4 blood test in October 2019, she was diagnosed with hyperthyroidism on the basis of 13.2 μg/dL T4 value (cf. reference range of 0.9‒3.7μg/dL). As treatment, 2.5 mg/day of mercazole (brand name methimazole) was administered to her every day, and she was not interested in any veterinary diets containing low levels of iodine; in consequence, the T4 value decreased only to 6.4μg/dL in November. The drug dose was increased to 4.0 mg/day, and the T4 value dropped to 2.7μg/dL in December; however, she had serious side effects during her medical therapy, in particular intense itchiness. She often scratched her face and ears because of her itchiness, and they were torn and bled. Finally, she had a thyroidectomy in January 2020. The thyroid tissues sampled from her thyroidectomy are shown in figure 3 - there were follicular-patterned lesions (Figure 3A) in multi-nodular form (Figure 3B) in the thyroid glands, and these lesions were well-circumscribed (Figure 3C) and comparatively inhomogeneous. Furthermore, they did not infiltrate through the surrounding normal tissues, so this cat was pathologically diagnosed as having a benign thyroid adenoma.
Discussion
The difference in the carcinoma rate between our study (~11%) and previous studies (~2%) is considered to be attributable to the timing of sample collection because there is no methodological difference between our pathological examination and previous ones - the sample collections (i.e., removal of thyroid gland by thyroidectomy) were carried out almost immediately after diagnosis and preoperative preparation for anaesthesia in previous studies [11,12]; on the other hand, our sampling was done after a certain period of medication. We could not gather enough information about the medication period from cooperating veterinary hospitals (cf. medical treatment ranging from 4 months to 7 years, with an average of 15.8 months according to published referral records [25]). Nevertheless, it is rational to consider that the previous studies have shown the tumor features at the initial stage of hyperthyroidism (i.e., the time of diagnosis) [11,12], and our results have shown those at the post-treatment stage (i.e., after a certain period of medication, at least a few months). Accordingly, the combination of previous studies [11,12] with our results (Figure 2) leads to speculation that the carcinoma rate increases from ~2% to ~11% of cases during medication.
Sequence theory
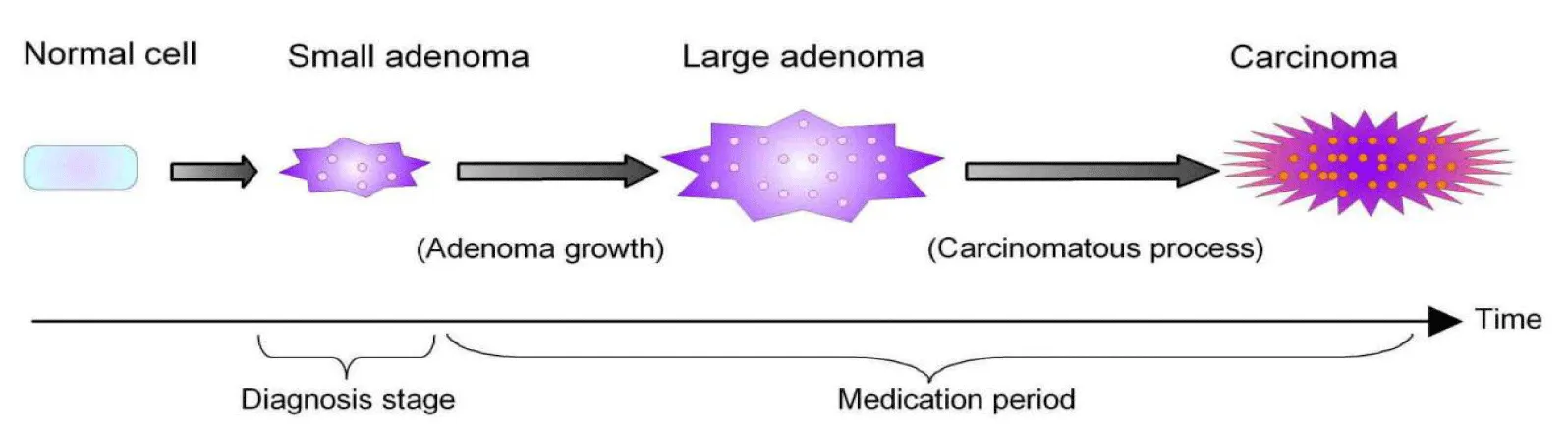
A cross-section study based on 2,096 hyperthyroid cats has hypothesized that the prevalence of “suspected” thyroid carcinoma increases with increasing duration of medication [26]. This hypothesis is consistent with the above-mentioned speculation, so the following theory is built to interpret an obvious increase in the carcinoma rate (Figure 4): (i) anti-thyroid medication such as methimazole does not decrease tumor size [16,22], (ii) adenomatous tissues continue to grow over time despite medical therapy, and (iii) this growth predisposes the initiation of a carcinomatous process. The above-mentioned steps are illustrated in figure 4 to facilitate understanding of the proposed theory. It is broadly accepted that the adenoma-carcinoma sequence represents the process by which most colorectal cancers arise in humans (reviewed in [27,28]). A simplified version of this sequence is basically the conversion of normal colonic mucosa → (i) benign adenoma → (ii) advanced adenoma → (iii) ultimately progressing to carcinoma (reviewed in [27]). Thus, the feline sequence in thyroid carcinoma development seems to be similar to the human adenoma-carcinoma sequence in colorectal cancers (Figure 4).
There is a difference in terms of affected area between feline thyroid tumors (pharynx) and human colorectal cancers (colon), so doubt remains as to whether the adenoma-carcinoma sequence (i.e., the known mechanism of human colorectal cancer) occurs in thyroid tumors. The adenoma-carcinoma sequence is an enigmatic and debatable issue in thyroid tumors (reviewed in [29]). There are indirect evidences such as mixed simultaneous tumors, hostage theory and collision hypothesis to justify this sequence. Benign adenomas tend to have a higher prevalence of RAS gene mutations (a biomarker for the classical type of thyroid cancer), and papillary thyroid cancer tends to have a higher prevalence of BRAF gene mutations (a biomarker for the aggressive type of thyroid cancer), respectively [30]. A genetic study in human cases shows that similar N-RAS (a RAS gene family member) and BRAF mutations are prevalent in both benign and malignant thyroid nodules, so it is suggested that there is a possibility of adenoma-carcinoma sequence in thyroid tumors [31].
Timing and probability of the carcinomatous process
There seems to be a difference in the sequence timing between cats and humans. It takes between 10 and 15 years (a long term) for an adenoma to develop into a carcinoma in human colon cancers [28], whereas the growth rate of a feline tumor can vary and malignant tumors are often characterized by fast growth. A malignancy analysis of feline neoplasms has reported that malignant tumors tend to occur 8 months after benign ones at a mean age of 9.8 years in Portuguese cats [32]. Based on our findings, the average duration of medical therapy [25] and the onset timing of malignancy [32], it can be inferred that adenomatous thyroid tissues (benign tumors) transform into carcinomatous ones (malignant tumors) through a multistep mutational pathway similar to the human adenoma-carcinoma sequence in ~10% of hyperthyroid cats that are medically controlled, presumably in one to two years after the diagnosis time.
Future study for the one health approach
Thyroid adenomas are mainly classified into (i) an active (functional) type that produces excessive thyroid hormones and (ii) an inactive (non-functional) type that does not produce thyroid hormones [33]. Most thyroid adenomas are active in cats [11,12], but inactive in humans, so they do not often cause any symptoms [33]. With all that said, the diagnosis rate of low-risk thyroid cancers has been increasing in humans since the early 1980s (e.g., an increase rate of ~3% per year in the US [34,35]), and this increasing tendency of thyroid cancer in humans cannot be explained only by over-diagnosis and detection technology [36]. Cats are defined as sentinel species that can indicate health threats and potential risks to humans [37]; therefore, it remains for a future task to carefully assess whether an increasing rate of hyperthyroidism in cats since the late 1970s [11,12] is somehow related to the increasing rate of thyroid cancers in humans since the early 1980s [34,35].
Conclusions and Perspectives
A wide range of side effects has been reported in the use of anti-thyroid drugs (reviewed in [11,23]). Our observation of the hyperthyroid cat with ID number BP20-1438 is consistent with this report, so the importance of dose adjustments should be noted in pharmacological management of hyperthyroid cats. Furthermore, our findings suggest that thyroid nodules require careful follow-up for exclusion of malignancy in the presence of feline hyperthyroidism. As stated in the introduction, nowadays the annually increasing incidence of malignant tumors is of great concern in veterinary medicine for companion animals. This malignancy trend implies that a multistep mutational pathway like the human adenoma-carcinoma sequence may be becoming increasingly common in veterinary medicine without our realizing it.
Even if an increasing rate of hyperthyroidism in cats since the late 1970s is related to the increasing rate of thyroid cancers in humans since the early 1980s, it will be difficult to maintain awareness of oncogenic risk because human thyroid adenoma is often inactive and asymptomatic in the face of its malignant potential [33]. The above chronological order is reminiscent of Minamata disease, which is a typical example of peculiar health damage in Japan. The outbreak of the disease in humans was preceded by abnormal behaviors (drooling, spinning in circles, etc.) in local cats in the 1950s [38]. This disease was coined “Dancing Cat Syndrome” in its early days; however, the syndrome was a precursor to severe neurological disorders that subsequently developed in a large number (~100 thousand cases) of human residents of Minamata Bay [39]. Considering that cats are categorized as sentinel species, the prevalence of feline hyperthyroidism potentially associated with the adenoma-carcinoma sequence may be a sign of what will next emerge in human beings on a global scale.
Acknowledgments
We thank 206 cats, their owners and the 65 cooperating veterinary hospitals for supplying tissue samples, Yu Animal Hospital for clinical records, Dr. N. Yoshida of Kyoto Prefectural University of Medicine for helpful suggestions, and Ms. C. Lentfer for English review. Special thanks are given to a Portuguese cat Mimi for providing us with response data for assessing medication effects.
Author’s Contributions
RK developed the conceptualization and aided in interpreting the results. YK performed the pathology examination and the data processing, and RPRC reviewed and edited the manuscript from the veterinary viewpoint. All authors provided critical feedback.
Funding
No funding was received for conducting this research.
Data Availability
The datasets are available from the corresponding author upon proper request.
Ethics Approval and Consent to Participate
Sample collections were approved by all the pet owners and the cooperative veterinary hospitals.
Competing Interests
The authors declare no competing interests.
References
- Herzong H. The impact of pets on human health and psychological well-being fact, fiction or hypothesis? Current Directions in Psychological Science. 2005;20(4):236-239. doi: 10.1177/0963721411415229.
- Living with cats and dogs surveys 2021- Toward a rewarding life with cats and dogs amid a changing society. Tokyo: Benesse Inc; 2021.
- Williams A, Williams B, Hansen CR, Coble KH. The Impact of Pet Health Insurance on Dog Owners' Spending for Veterinary Services. Animals (Basel). 2020 Jul 9;10(7):1162. doi: 10.3390/ani10071162. PMID: 32659934; PMCID: PMC7401533.
- High demand for animal clinics as Japan’s pets live longer. Tokyo: Yomiuri Shinbun; 2022.
- National Research Council of the National Academies. Sensor systems for biological agent attacks: Protecting buildings and military bases. Washington DC: The National Academies Press; 2005. p.208.
- One Health High-Level Expert Panel (OHHLEP); Adisasmito WB, Almuhairi S, Behravesh CB, Bilivogui P, Bukachi SA, Casas N, Cediel Becerra N, Charron DF, Chaudhary A, Ciacci Zanella JR, Cunningham AA, Dar O, Debnath N, Dungu B, Farag E, Gao GF, Hayman DTS, Khaitsa M, Koopmans MPG, Machalaba C, Mackenzie JS, Markotter W, Mettenleiter TC, Morand S, Smolenskiy V, Zhou L. One Health: A new definition for a sustainable and healthy future. PLoS Pathog. 2022 Jun 23;18(6):e1010537. doi: 10.1371/journal.ppat.1010537. PMID: 35737670; PMCID: PMC9223325.
- Mizuno T. Spontaneously occurring canine cancer as a relevant animal model for developing novel treatments for human cancers. Translational and Regulatory Sciences. 2021;3(2):51-59. doi: 10.33611/trs.2021-007.
- Grüntzig K, Graf R, Hässig M, Welle M, Meier D, Lott G, Erni D, Schenker NS, Guscetti F, Boo G, Axhausen K, Fabrikant S, Folkers G, Pospischil A. The Swiss Canine Cancer Registry: a retrospective study on the occurrence of tumours in dogs in Switzerland from 1955 to 2008. J Comp Pathol. 2015 Feb-Apr;152(2-3):161-71. doi: 10.1016/j.jcpa.2015.02.005. Epub 2015 Mar 29. Erratum in: J Comp Pathol. 2015 Jul;153(64-5). PMID: 25824119.
- Alshammari AH, Oshiro T, Ungkulpasvich U, Yamaguchi J, Morishita M, Khdair SA, Hatakeyama H, Hirotsu T, di Luccio E. Advancing Veterinary Oncology: Next-Generation Diagnostics for Early Cancer Detection and Clinical Implementation. Animals (Basel). 2025 Jan 30;15(3):389. doi: 10.3390/ani15030389. PMID: 39943159; PMCID: PMC11816279.
- Cat Population. Lancaster (PA): World Population Review; 2025.
- Carney HC, Ward CR, Bailey SJ, Bruyette D, Dennis S, Ferguson D, Hinc A, Rucinsky AR. 2016 AAFP Guidelines for the Management of Feline Hyperthyroidism. J Feline Med Surg. 2016 May;18(5):400-16. doi: 10.1177/1098612X16643252. PMID: 27143042; PMCID: PMC11132203.
- Peterson M. Hyperthyroidism in cats: what's causing this epidemic of thyroid disease and can we prevent it? J Feline Med Surg. 2012 Nov;14(11):804-18. doi: 10.1177/1098612X12464462. PMID: 23087006; PMCID: PMC11112171.
- Pérez Domínguez A, Santiago Tostado R, Feo Bernabe L, Priego Corredor A, Puig Prat J. Prevalence of feline hyperthyroidism in a laboratory-based sample of 27,888 cats in Spain. J Feline Med Surg. 2024 Dec;26(12):1098612X241303304. doi: 10.1177/1098612X241303304. PMID: 39713975; PMCID: PMC11672385.
- Köhler I, Ballhausen BD, Stockhaus C, Hartmann K, Wehner A. Prevalence of and risk factors for feline hyperthyroidism among a clinic population in Southern Germany. Tierarztl Prax Ausg K Kleintiere Heimtiere. 2016 Jun 16;44(3):149-57. English, German. doi: 10.15654/TPK-150590. Epub 2016 Feb 23. PMID: 26902958.
- Hyperthyroidism in Cats. Ithaca (NY): Cornell University College of Veterinary Medicine; 2017.
- Flanders JA. Surgical options for the treatment of hyperthyroidism in the cat. J Feline Med Surg. 1999 Sep;1(3):127-34. doi: 10.1016/S1098-612X(99)90201-2. PMID: 11919027; PMCID: PMC10832798.
- Volckaert V, Vandermeulen E, Daminet S, Saunders JH, Peremans K. Hyperthyreoïdie bij katten: anatomie, fysiologie, pathofysiologie, diagnose en beeldvorming (Hyperthyroidism in cats: anatomy, physiology, pathophysiology, diagnosis and imaging). Vlaams Diergeneeskd Tijdschr. 2016;85:255-264. doi: org/10.21825/vdt.v85i5.16325.
- Knight J, Andrade M, Weston ZB. Endocrine system 3: Thyroid and parathyroid glands. Nursing Times. 2021;117(7):46-50.
- Hayashi T, Kumano H, Ohta M, Sakai S, Takumi A, Niizuma A. The Illustrated encyclopedia of the Cat. 2nd ed. Kokyo: Kodansha Scientific; 2011. p.111.
- Vaske HH, Schermerhorn T, Armbrust L, Grauer GF. Diagnosis and management of feline hyperthyroidism: current perspectives. Vet Med (Auckl). 2014 Aug 20;5:85-96. doi: 10.2147/VMRR.S39985. PMID: 32670849; PMCID: PMC7337209.
- Argueta R, Whitaker MD. When a thyroid abnormality is palpable. What it means and what you should do. Postgrad Med. 2000 Jan;107(1):100-4, 109-10. doi: 10.3810/pgm.2000.01.806. PMID: 10649668.
- Trepanier LA. Pharmacologic management of feline hyperthyroidism. Vet Clin North Am Small Anim Pract. 2007 Jul;37(4):775-88, vii. doi: 10.1016/j.cvsm.2007.03.004. PMID: 17619011.
- Hyperthyroidism in Cats part 2 – Treatment and owner advice. Peterborough: Vet Times; 2017.
- Radiotherapy for cats in UK. Tokyo: Vet Animall & AsaT; 2017.
- Hibbert A, Gruffydd-Jones T, Barrett EL, Day MJ, Harvey AM. Feline thyroid carcinoma: diagnosis and response to high-dose radioactive iodine treatment. J Feline Med Surg. 2009 Feb;11(2):116-24. doi: 10.1016/j.jfms.2008.02.010. Epub 2008 Oct 2. PMID: 18835538; PMCID: PMC10832792.
- Peterson ME, Broome MR, Rishniw M. Prevalence and degree of thyroid pathology in hyperthyroid cats increases with disease duration: a cross-sectional analysis of 2096 cats referred for radioiodine therapy. J Feline Med Surg. 2016 Feb;18(2):92-103. doi: 10.1177/1098612X15572416. Epub 2015 Feb 11. PMID: 25673019; PMCID: PMC11149013.
- Leslie A, Carey FA, Pratt NR, Steele RJ. The colorectal adenoma-carcinoma sequence. Br J Surg. 2002 Jul;89(7):845-60. doi: 10.1046/j.1365-2168.2002.02120.x. PMID: 12081733.
- Bortz JH, Friedrich-Nel H. The Adenoma-Carcinoma Sequence, Management, and Treatment of Colon Cancer. In: Bortz JH, Ramlaul A, Munro L, Editors. CT Colonography for Radiographers. Cham: Springer; 2023. pp. 209-220.
- Bhargav PRK. Enigma of adenoma-carcinoma sequence in thyroid gland: an interesting case report of multiple pathologies with literature review. World Journal of Endocrine Surgery. 2016;8(2):172-174. doi: 10.5005/jp-journals-10002-1185.
- Censi S, Cavedon E, Bertazza L, Galuppini F, Watutantrige-Fernando S, De Lazzari P, Nacamulli D, Pennelli G, Fassina A, Iacobone M, Casal Ide E, Vianello F, Barollo S, Mian C. Frequency and Significance of Ras, Tert Promoter, and Braf Mutations in Cytologically Indeterminate Thyroid Nodules: A Monocentric Case Series at a Tertiary-Level Endocrinology Unit. Front Endocrinol (Lausanne). 2017 Oct 16;8:273. doi: 10.3389/fendo.2017.00273. PMID: 29085338; PMCID: PMC5650698.
- Bangaraiahgari R, Panchangam RB, Puthenveetil P, Mayilvaganan S, Bangaraiahgari R, Banala RR, Karunakaran P, Md R. Is there adenoma-carcinoma sequence between benign adenoma and papillary cancer of thyroid: A genomic linkage study. Ann Med Surg (Lond). 2020 Dec 2;60:695-700. doi: 10.1016/j.amsu.2020.11.069. PMID: 33318795; PMCID: PMC7726453.
- Pinello K, Amorim I, Pires I, Canadas-Sousa A, Catarino J, Faísca P, Branco S, Peleteiro MC, Silva D, Severo M, Niza-Ribeiro J. Vet-OncoNet: Malignancy Analysis of Neoplasms in Dogs and Cats. Vet Sci. 2022 Sep 28;9(10):535. doi: 10.3390/vetsci9100535. PMID: 36288148; PMCID: PMC9611943.
- Mulita F, Anjum F. Thyroid adenoma. Treasure Island (FL): StatPearls Publishing; 2023. PMID: 32965923; Bookshelf ID: NBK562252.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018 Jan;68(1):7-30. doi: 10.3322/caac.21442. Epub 2018 Jan 4. PMID: 29313949.
- Lamartina L, Leboulleux S, Borget I, Schlumberger M. Global thyroid estimates in 2020. Lancet Diabetes Endocrinol. 2022 Apr;10(4):235-236. doi: 10.1016/S2213-8587(22)00048-1. Epub 2022 Mar 7. PMID: 35271820.
- Kim J, Gosnell JE, Roman SA. Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol. 2020 Jan;16(1):17-29. doi: 10.1038/s41574-019-0263-x. Epub 2019 Oct 15. PMID: 31616074.
- Bost PC, Strynar MJ, Reiner JL, Zweigenbaum JA, Secoura PL, Lindstrom AB, Dye JA. U.S. domestic cats as sentinels for perfluoroalkyl substances: Possible linkages with housing, obesity, and disease. Environ Res. 2016 Nov;151:145-153. doi: 10.1016/j.envres.2016.07.027. Epub 2016 Jul 29. PMID: 27479711.
- Harada M. The global lessons of minamata disease: An introduction to minamata studies. In: Takahashi T, Editor. Taking Life and Death Seriously - Bioethics from Japan (Advances in Bioethics, vol. 8). Leeds: Emerald Group Publishing; 2005. p. 299-335.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.