2025 July 01;6(7):858-861. doi: 10.37871/jbres2138.
Granulomatous Amebic Encephalitis Caused by Entamoeba Dispar
Weiwei Qi, Junjie Guo and XueXu*
Abstract
Entamoeba dispar, belonging to the Genus Entamoeba along with Entamoeba histolytica that causes amebiasis, was previously considered a non-pathogenic ameba and an ideal model for studying the pathogenesis of amebiasis. However, since its strains were isolated from symptomatic patients in Brazil in 1996, its pathogenic potential has been a subject of discussion. We report a case of multiple granulomatous encephalitis in a 4-year-old immunocompetent boy presenting with left eye esotropia. Sequences of E. dispar were detected in his cerebrospinal fluid through Next-Generation Sequencing (NGS). After three courses of metronidazole therapy, the brain and lung lesions showed significant reduction in subsequent examinations. This case expands the spectrum of diseases associated with E. dispar.
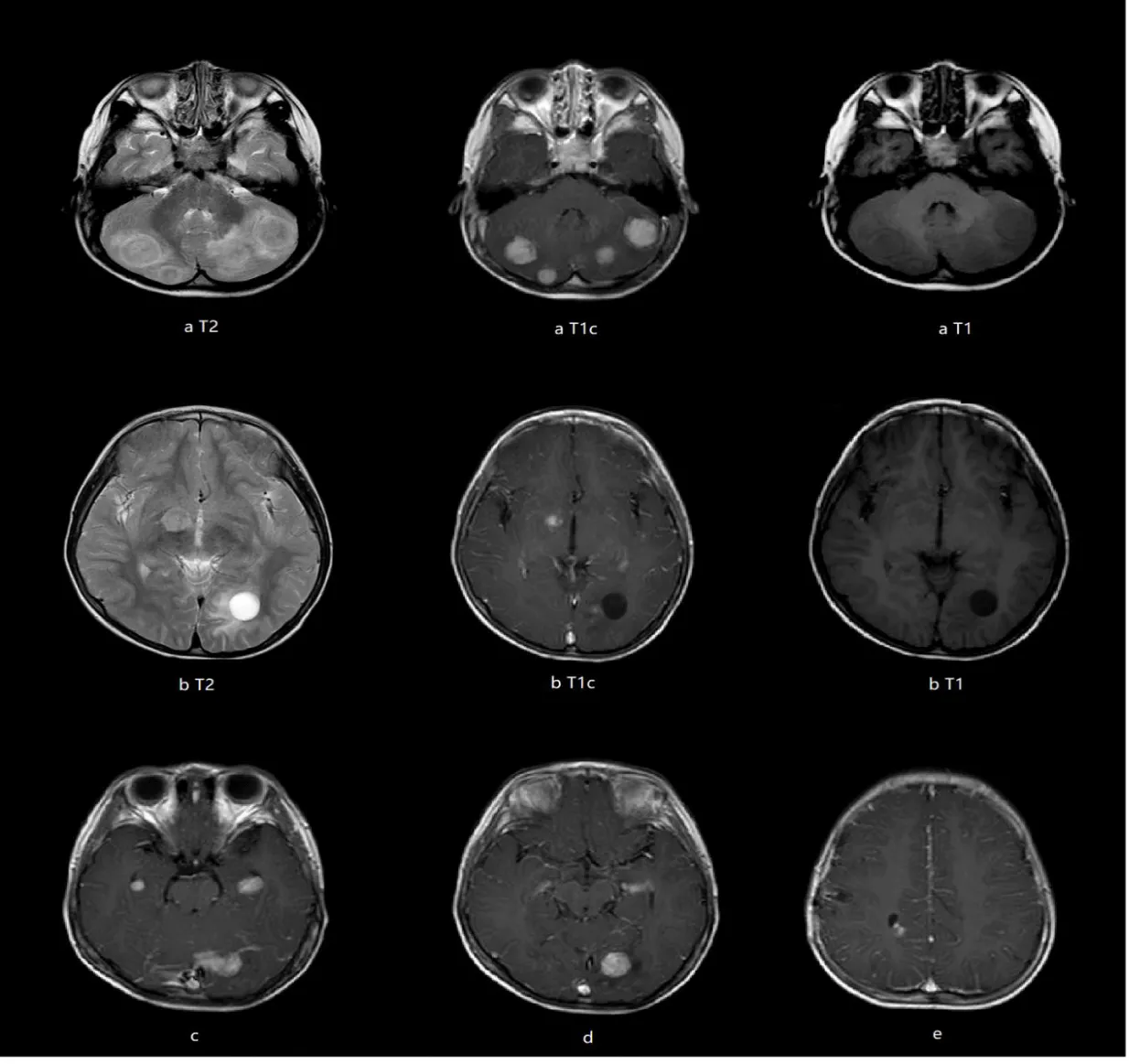
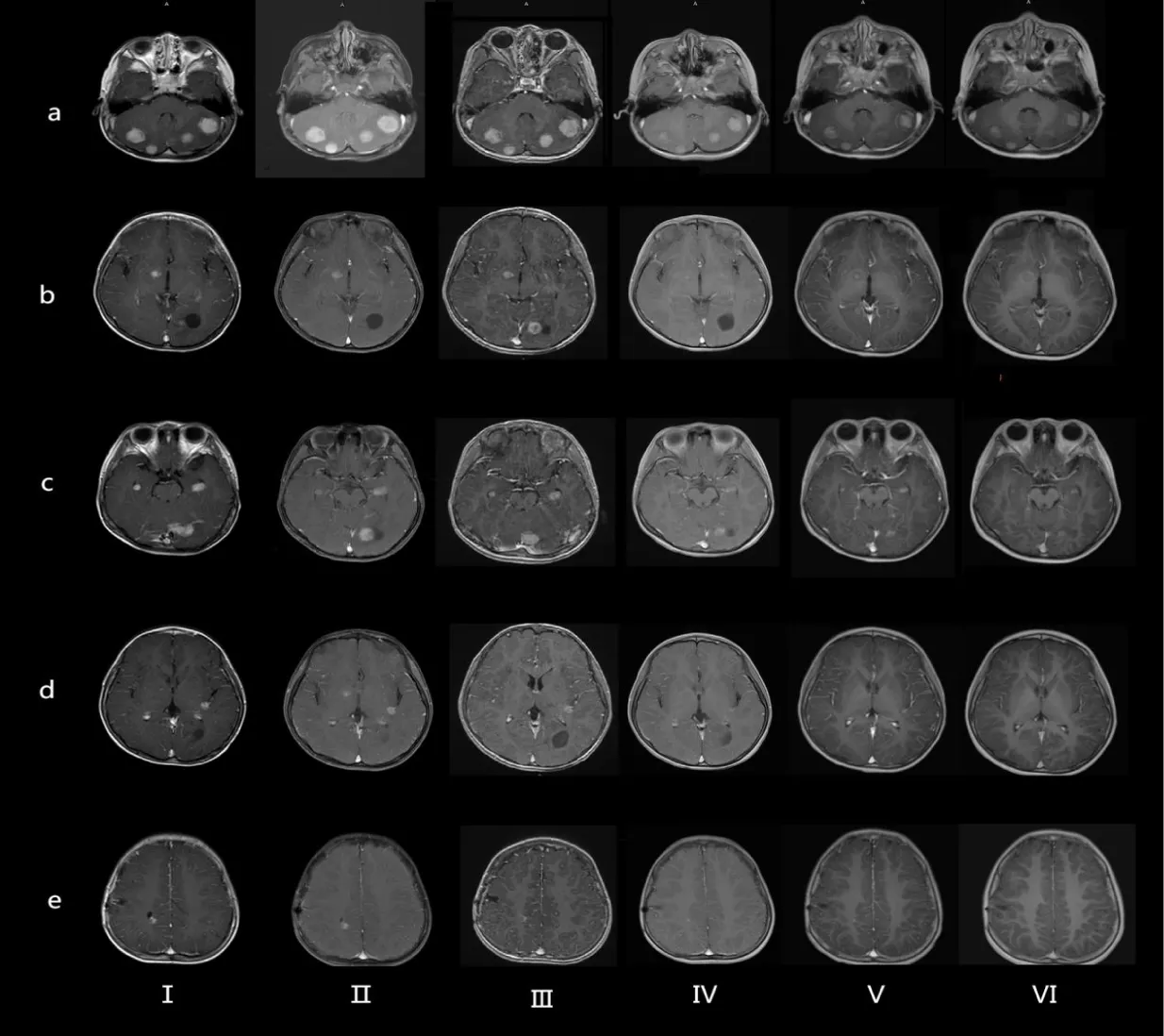
A previously healthy 4-year-old boy presented with a 2-year history of progressive left eye esotropia, without accompanying systemic symptoms such as headache, fever, or vomiting. His medical and family histories were unremarkable, with no exposure to contaminated water, undercooked meat, or pigeon feces. Neurological examination revealed subtle signs: slight limitation in left eye abduction and a positive Babinski sign on the left side. Initial laboratory tests showed normal hematologic parameters (WBC 7.76×10⁹/L, hemoglobin 130 g/L) and mildly elevated serum alkaline phosphatase (273 U/L) and calcium (2.31 mmol/L). Brain MRI demonstrated multiple heterogeneous lesions in bilateral cerebellar hemispheres, left occipital lobe, medial temporal lobes, right thalamus, and right frontal/parietal lobes. These lesions exhibited ring enhancement with perilesional edema and some with adjacent cystic components (Figures 1a-e).
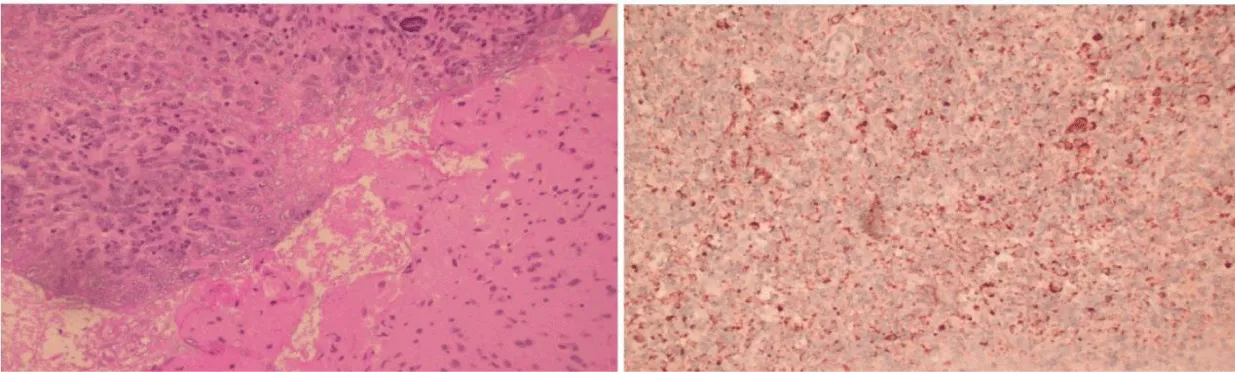
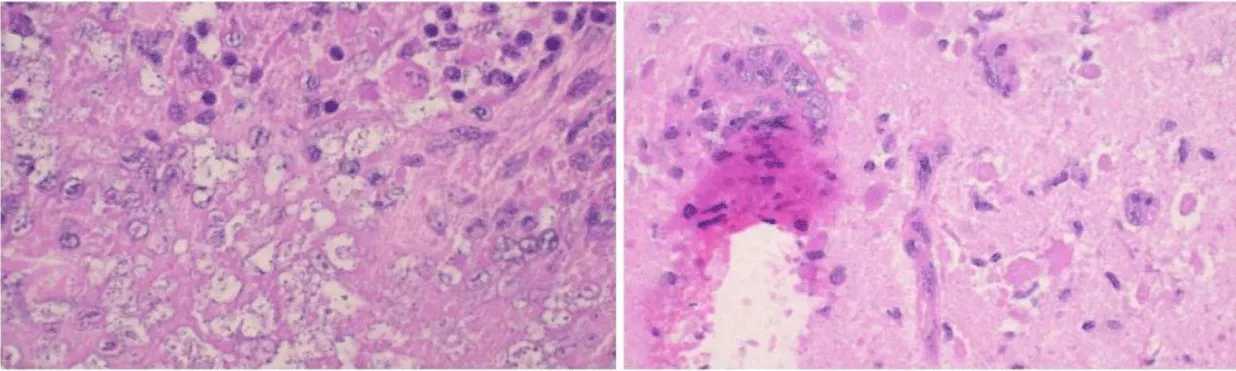
A stereotactic biopsy of the right frontal lobe lesion revealed granulomatous inflammation with focal necrosis, lymphocyte infiltration, multinucleated giant cells, and structures morphologically consistent with amoebic cysts and trophozoites (Figures 2,3).
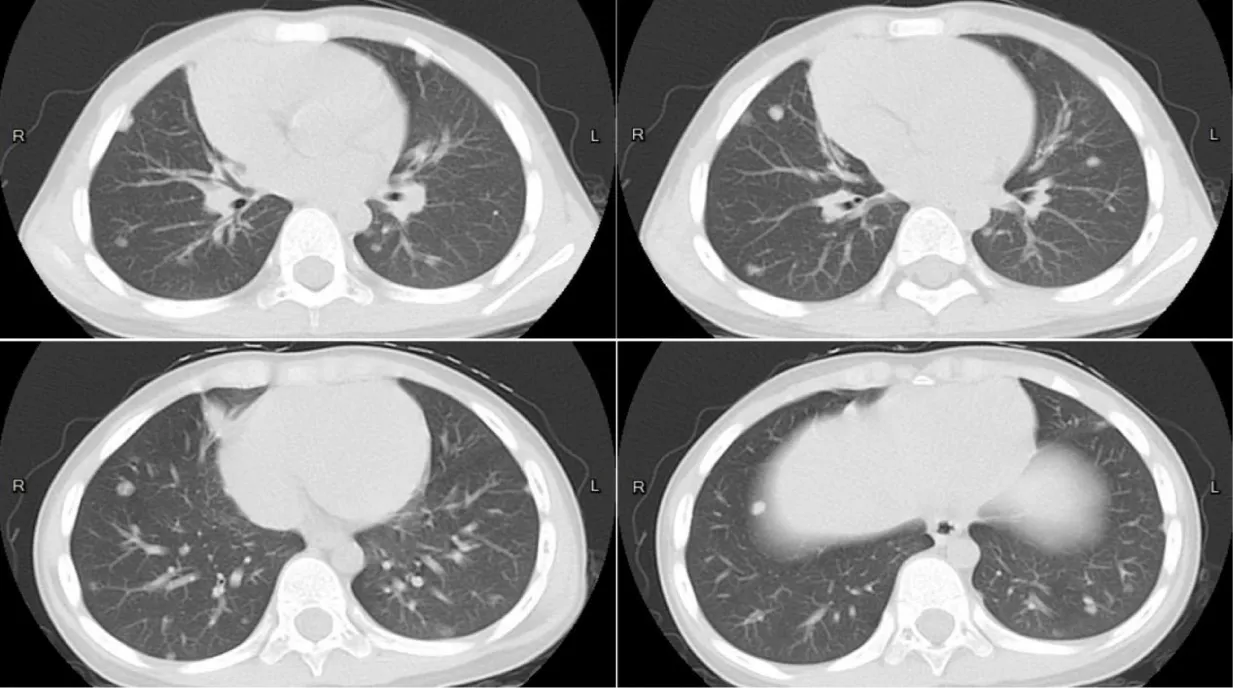
Lumbar puncture under general anesthesia showed elevated CSF pressure (>330 mm H2O), lymphocytic pleocytosis (7×10⁶/L, 89% lymphocytes), and elevated protein (634 mg/L), though glucose levels were normal (2.7 mmol/L). Next-Generation Sequencing (NGS) of CSF identified Entamoeba dispar (447 reads), while extensive serological tests for bacterial, fungal, and parasitic pathogens (including tuberculosis, toxoplasmosis, and neurocysticercosis) were negative. Chest CT revealed subpleural and mid-lung field nodules (Figure 4), suggesting possible pulmonary involvement. The patient received three 10-day courses of metronidazole (50 mg/kg/day) over five months, with intervals of 1-2 months between courses. Follow-up MRI of 4 years showed progressive resolution of brain lesions, including absorption of the cysts, reduction of lesion size, decreased enhancement and alleviation of the perilesional edema (Figures 5a-e).
His esotropia resolved completely by the fifth month of treatment, with no residual neurological deficits at one-year follow-up.
Both Entamoeba dispar and Entamoeba histolytica belong to the Genus Entamoeba. Unlike E. histolytica, which causes amebiasis, E. dispar was long considered a non-pathogenic ameba and an ideal model for studying the pathogenesis of amebiasis. However, the isolation of E. dispar strains from symptomatic patients in Brazil in 1996 [1] and the detection of its DNA sequences in samples from patients with amebic liver abscess [2] suggest that E. dispar may also contribute to the development of lesions in the human intestine and liver. Yet, no cases of Granulomatous Amebic Encephalitis (GAE) caused by E. dispar have been reported.
The diagnosis of GAE relies on pathological evidence: the presence of trophozoites and cysts in brain tissue is necessary. Moderate granulomatous inflammation with prominent vascular involvement is typically observed on brain biopsy. Additionally, radiological findings and CSF examinations are crucial. MRI often shows single or multiple space-occupying lesions with ring enhancement. CSF parameters usually reveal mild pleocytosis with lymphocytic predominance, high protein concentration, and low or normal glucose concentration. Rarely, Acanthamoeba trophozoites may be seen on Giemsa stain of the CSF sediment. With technological advancements, diagnosis can also be made using laboratory testing for the parasite's nucleic acid in CSF, biopsy, or tissue specimens [3,4].
The imaging findings of this case revealed multiple nodular enhancing lesions, with cystic changes in some of the lesions. The Cerebrospinal Fluid (CSF) pressure was significantly elevated, with mild elevation of CSF protein levels, while the CSF glucose and chloride levels were essentially normal. The diagnosis of GAE was established through the detection of Entamoeba dispar sequences in the CSF by next-generation sequencing. Follow-up after treatment showed gradual reduction in the size of the lesions, which supports the correct diagnosis and effective treatment. This case has expanded our understanding of the pathogenic potential of Entamoeba dispar.
References
- de Martinez AM, Gomes MA, Viana Jda C, Romanha AJ, Silva EF. Isoenzyme profile as parameter to differentiate pathogenic strains of Entamoeba histolytica in Brazil. Rev Inst Med Trop Sao Paulo. 1996 Nov-Dec;38(6):407-12. doi: 10.1590/s0036-46651996000600004. PMID: 9293086.
- Ximénez C, Cerritos R, Rojas L, Dolabella S, Morán P, Shibayama M, González E, Valadez A, Hernández E, Valenzuela O, Limón A, Partida O, Silva EF. Human amebiasis: breaking the paradigm? Int J Environ Res Public Health. 2010 Mar;7(3):1105-20. doi: 10.3390/ijerph7031105. Epub 2010 Mar 16. PMID: 20617021; PMCID: PMC2872301.
- Pana A, Vijayan V, Anilkumar AC. Amebic Meningoencephalitis. 2023 Jan 21. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 28613505.
- Kalra SK, Sharma P, Shyam K, Tejan N, Ghoshal U. Acanthamoeba and its pathogenic role in granulomatous amebic encephalitis. Exp Parasitol. 2020 Jan;208:107788. doi: 10.1016/j.exppara.2019.107788. Epub 2019 Oct 21. PMID: 31647916.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.