2025 June 20;6(6):763-768. doi: 10.37871/jbres2128.
Anastomotic Renal Sinus Haemangioma - A Case Report
Mariella Izzo1, Francesco Negri2, Rosario Gnazzo1,2, Nicola Bertini1, Francesco Verrengia3, Simona Petrosino1, Simone Cilio4, Paolo Verze4,5 and Vincenzo Ciccone2*
2Radiology Unit, Azienda Ospedaliera Universitaria San Giovanni di Dio e Ruggi d’Aragona – Salerno, 84100 Italy
3Centro Radiologico Verrengia, Salerno, 84100 Italy
4Urology Unit, Azienda Ospedaliera Universitaria San Giovanni di Dio e Ruggi d’Aragona – Salerno, 84100 Italy
5Department of Medicine and Surgery, University of Salerno, Salerno, 84100 Italy
- Anastomotic hemangioma
- Kidney
- CT scan
- MRI
- Radiology
- Diagnosis
Abstract
Rationale: Vascular tumours of the kidney are a heterogeneous group of lesions showing common characteristics with malignant neoplasms, making it significantly challenging the pre-operative diagnosis. Among these lesions, Anastomotic Hemangioma (AH) is a recently recognised variant of hemangioma of the genito-urinary tract characterized by a complex vascular structure. This manuscript presents the case of a 85-year-old caucasian woman diagnosed with renal sinus AH.
Patient concerns: The man was admitted to the hospital with a 4-month history of a left renal mass discovered by ultrasound (US) of the abdomen performed for an elevation of transaminases, in the absence of significant genito-urinary symptoms. We performed radiological examinations through Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) that revealed a lesion showing apparently malignant characteristics. The multidisciplinary team decided to proceed with nephrectomy. The postoperative pathological examination revealed that the mass contained capillaries arranged in a characteristic anastomotic or confluent pattern commonly seen in AHs.
Outcomes: The mass was successfully removed. The follow-up examination at 7 months post-surgery showed that the patient recovered well, and no recurrence or metastasis was found.
Conclusion: Anastomotic renal sinus haemangioma is a rare benign vascular tumor. On imaging examinations, AHs appear as mostly heterogeneous masses with peripheral and/or pseudonodular enhancement. However, a definitive diagnosis can only be achieved through histopathological examination.
Introduction
Vascular tumours are a very small group of renal neoplasms with a mesenchymal origin [1,2].
In this category of tumor, the most frequent is the haemangioma which occurs frequently in the skin and subcutaneous tissues, whereas visceral forms are rare and are mainly found in the liver [3].
Anastomotic Hemangioma (AH), a unique variant of renal capillary hemangioma, was first identified by Montgomery and Epstein [4]. It's characterized by its peculiar anastomosing sinusoidal capillary vessels, resembling those found in the spleen. While primarily seen in the genitourinary system, especially the kidneys, AH can also appear in other locations like muscle, skin, and liver [2,5].
A significant challenge with AH is its resemblance to other vascular tumors, particularly angiosarcoma, a malignant neoplasm. Both can show an anastomosing vascular pattern, hobnail endothelial cells, and positive staining for endothelial markers, making differentiation difficult [6]. Misdiagnosing AH as angiosarcoma can lead to unnecessary aggressive treatments and patient distress, given angiosarcoma's tendency for early metastasis and the need for radical intervention.
Herein we describe a case of AH occurring in the kidney. Considering difficulties in differentiating malignant lesions with respect to AH, the aim of our study is to report the pre-operative features of AH of the renal sinus. Particularly, in the case we detail the radiological features, histological findings and relevant clinical information, in order to supplement the information already available to improve the pre-operative diagnosis.
Case Description
We report a case of an AH originating in the left kidney of an 85-year-old Caucasian man with a history of stage IIIB chronic renal disease, hypertension and mixed dyslipidaemia. The patient was incidentally diagnosed with a left renal mass during an ultrasound examination of the abdomen performed for an elevation of transaminases. He was admitted to our hospital for suspected kidney malignancy and hospitalized at the Urology unit. He showed no significant genito-urinary symptoms. We performed radiological examinations through Computed Tomography (CT) and Magnetic Resonance Imaging (MRI).
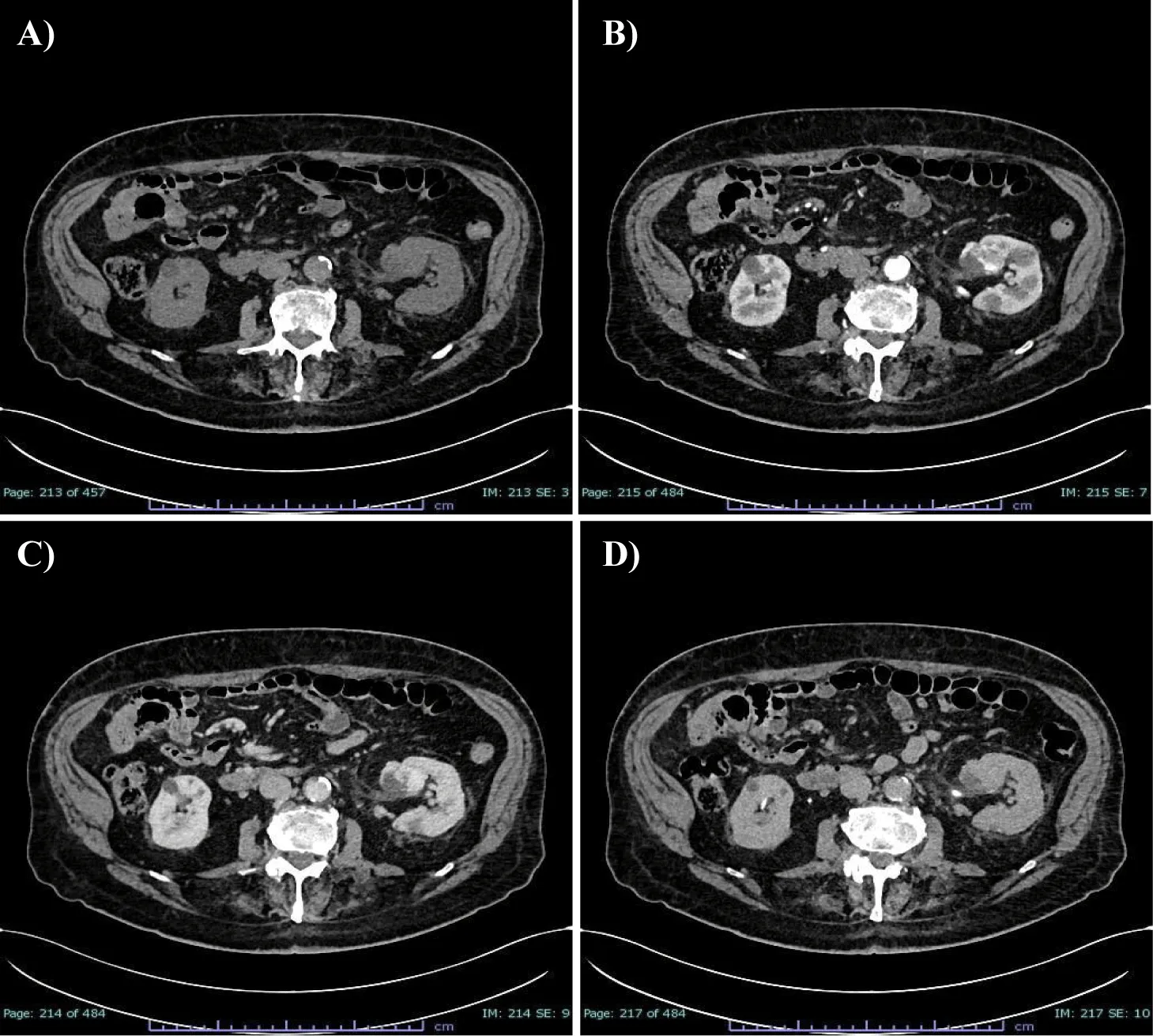
Following this finding, he underwent CT investigation without and with intravenous CM (Figure1). An axial CT scan showed an expansive formation with oval morphology in the left renal sinus outlining the organ with dimensions of about 32 x 30 mm, which tended to grow in the connective tissue of the renal sinus, slightly dislocating the inferior calyx group. In non-enhanced acquisition (Figure 1A) this formation presented a heterogeneous appearance with average values of HU of 19-20 HU, with negative values with adipose significance in some places, and peripheral areas values of 37-60 HU. In the arterial phase scans (Figure 1B), the periphery of the lesion showed a clearly annular and pseudonodular enhancement with a tendency to centripetal filling and densitometric values reaching 230 HU, thus demonstrating a highly representation of vascular tissue component. In the venous phase (Figure 1C), the lesion showed a tendency to homogeneous filling with average values up to 130 HU. The lesion showed homogeneously persistent enhancement in late scans (Figure 1D) with HU values between 50 and 70. Neither calico-pyelic dilatation was evident nor significant lymphadenopathy in the mesenteric, para-aortic and retroperitoneal stations.
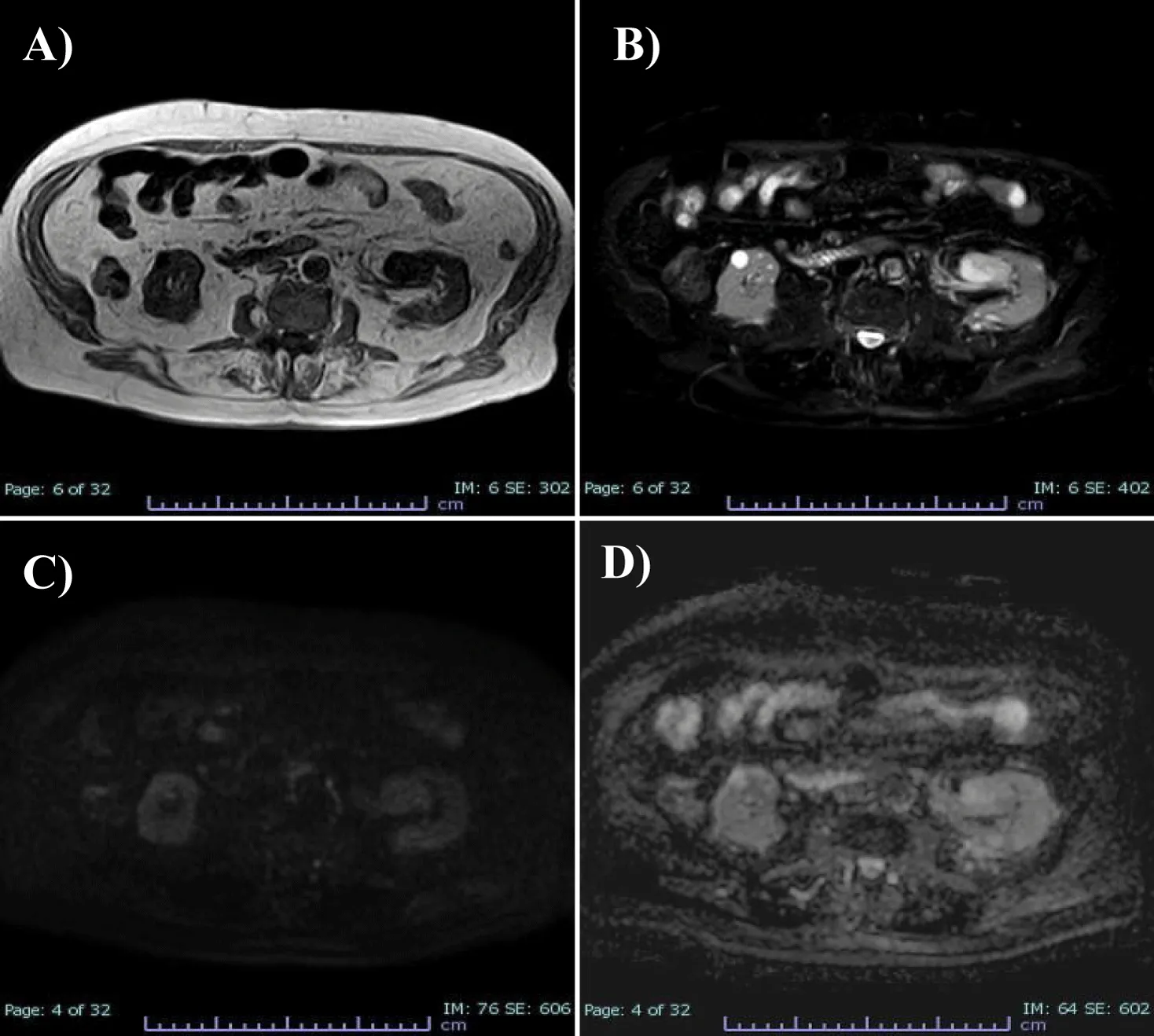
To better characterize the lesion, the patient underwent MRI examination of the upper and lower abdomen without and with intravenous CM (Figure 2).MRI images showed T1-dependent signal hypointensity (Figure 2A), inhomogeneous T2-dependent signal hyperintensity (Figure 2B), and appears to have poor diffusion-weighted signal restriction (Figures 2C,D). Overall, it is characterised by inhomogeneous contrast impregnation and has a greater axis of 32 x 23 x 26 mm (LL x AP x CC). This technique confirms that the heteroformation did not generate compressive effects on the renal pelvis or vascular invasion. No significant mesenteric, para-aortic or retroperitoneal lymphadenopathy was evident.
Considering the patient's clinical profile and given the localised nature of the lesion, with no extra-organic extension but located in the surgical risky region of the renal sinus, the decision of the multidisciplinary oncological group was to propose radical nephrectomy to the patient. The mass was succefully removed.
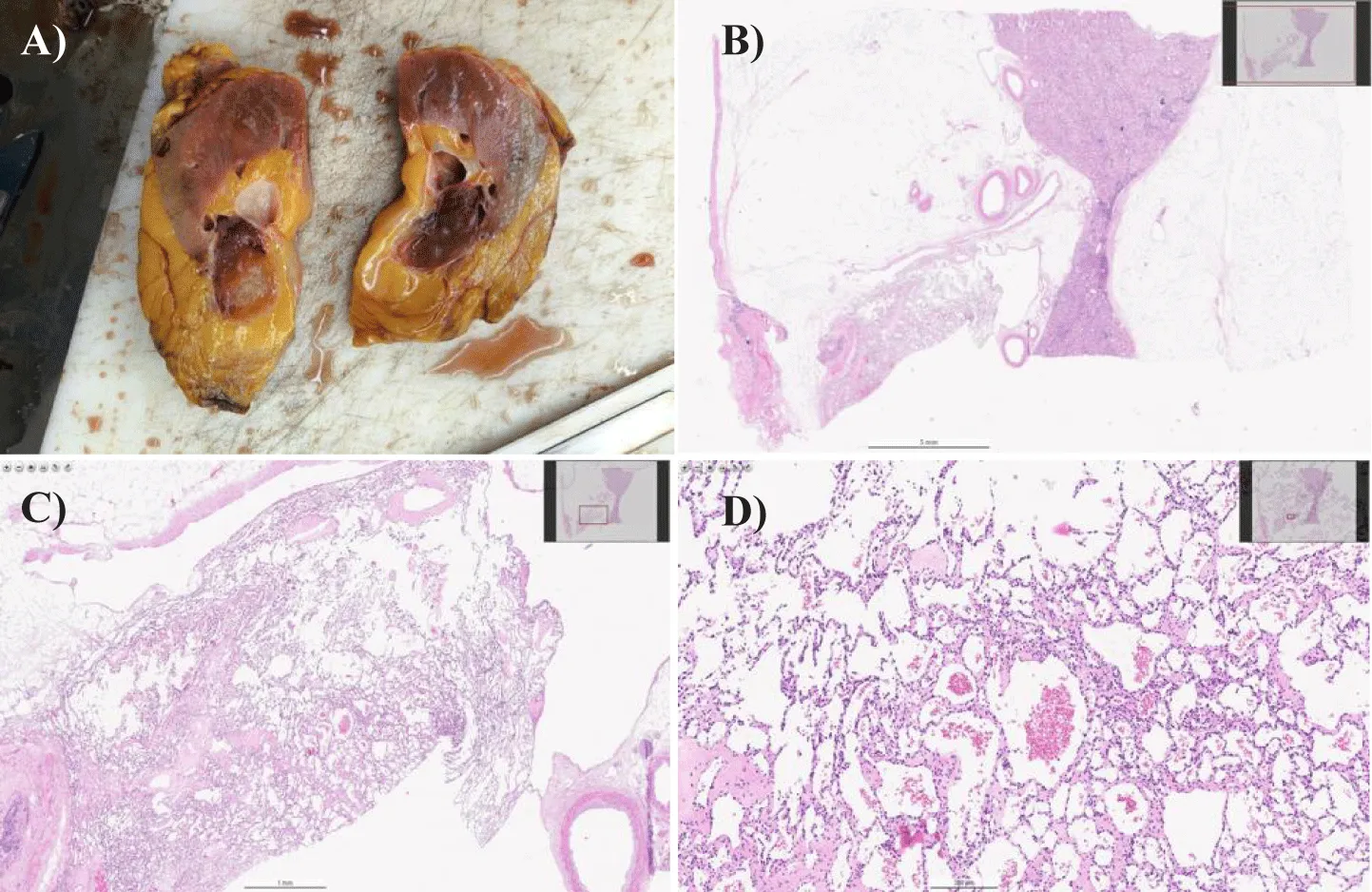
Figure 3 describes the macroscopical and microscopical characteristics of the operatic specimen. The gross pathological examination revealed a nodule of 3.5×2.5x2.5 cm located at the renal sinus. It resulted to be circumscribed but without a recognizable capsule and with a translucent red cut surface, which was extensively sampled (Figure 3A). The described sinus nodule had the histological features of AH and its epicentre was in the renal sinus. Microscopic contiguity with the apex of a renal pyramid is observed. The examination of the tissue under the microscope revealed a nodule with fairly well-defined borders. At lower magnifications (Figure 3B), the capillaries within the sinusoids showed a distinct interconnected, "traffic-like" pattern. We also noted some loose areas with swelling and the presence of a fibrin clot. Shifting to high magnification (Figures 3C,D) the tumor was seen to be composed of a single, plump layer of endothelial cells. A striking feature was how the nuclei of these cells protruded into the vessel cavity, giving them a distinctive "shoenail" appearance. Importantly, we didn't find any other significant abnormalities in the nuclei, such as atypical features or mitotic figures. Finally, a fibrous connective tissue separated the vascular components in most areas, and some adipose tissue was also observed.
Immunohistochemistry of the left kidney lesion confirmed positivity for CD34 and CD31. Immunostains for smooth muscle antibody and CD34 highlighted the orderly vasoformative architecture of this lesion. Positivity CD31 confirmed the presence of endothelial cells in immunohistological tissue sections. The specimen cells did not express D2-40, CK5/6, PAX8, desmin, inhibin, calretinin, CD99, WT1, CD10, catenin-β, EMA, or CK7; Ki67 proliferation index was nearly 5%. Based on the histopathological findings, the patient was diagnosed with an AH of the left renal sinus.
The patient was reviewed after 7 months. The follow-up examinations showed that the patient recovered well after the operation, and the imaging examinations revealed no evidence of recurrence or metastasis.
Discussion
This case-report is an unusual case: the patient was not symptomatic, and he incidentally discovered the kidney lesion during a routine abdomen US. Once admitted to our hospital to characterize the lesion before treatment decision, the TC and RMI imaging obtained showed features with a tendency towards heterogeneity: both methods lean towards a pre-operative diagnosis of malignant lesions. At the CT scan without CM, an inhomogeneous hypointense lesion is evident in the left renal pelvis, at the level of the lower third, which shows uneven density in all its points, varying from punctiform areas with adipose-like densitometric coefficients (from -10 HU to -2 HU), to more frankly solid areas (49-20 HU) and to sections suggesting the presence of vascular components (37-60 HU). These findings are confirmed in subsequent scans enhanced with CM and, in particular, the lesion shows a frank enhancement in the more caudal peripheral portion in the arterial phase (up to 230 HU). In the subsequent portal phase a more homogeneous contrastographic filling of the entire lesion, with values of around 130 HU, was noted confirming the presence of an important hypervascular tissue component. However, the lesion shows no frank signs of invasiveness or compression of the surrounding structures, in the absence of dilation upstream and downstream of the lesion. Furthermore, there are no signs of necrosis or calcifications within the lesion.
MRI images also show characteristics of inhomogeneous signal intensity, reflecting its vascular nature, revealing signal hypointensity in T1 sequences, inhomogeneous signal hyperintensity in T2 and poor signal restriction in DWI, indicative of poor cellularity. The final histopathological result was Anastomotic hemangioma, a benign entity associated with an excellent prognosis [8]. There are no reports of metastasis or recurrence after nephrectomy. However, it is still controversial whether total nephrectomy is necessary for such benign lesion [8].
Montgomery and Epstein [4] firstly identified a particular variant of renal capillary haemangiomas, which had intermediate features of the sinusoidal and the hobnail hemangioma, which usually occurs in the skin and soft tissues. This neoplasm was named Anastomotic Hemangioma (AH) because of its peculiar histological structure showing anastomotic sinusoidal capillary vessels similar to splenic sinusoids. Although AH is considered characteristic of the genitourinary system, with a specific predilection for the kidneys, it has also been reported in other sites, including muscle, skin, adrenal gland, liver and gastrointestinal tract [2,5].
The similarity of AH with other vascular neoplasms is a crucial aspect to consider potentially leading to misdiagnosis and overtreatment. Indeed, the differential diagnosis of AH of the kidney mainly includes malignant vascular tumours such as angiosarcoma, intravascular Papillary Endothelial Hyperplasia (PEH), Kaposi's sarcoma and angiomyolipoma [6].
The most frequent malignant neoplasm showing similarities with AH is angiosarcoma, occurring most frequently between the sixth and seventh decade of life [7]. This tumour has a strong tendency to early metastasize, mainly involving liver, lungs and bones. This tumour often shows an anastomising vascular pattern, hobnail endothelial cells and immunopositivity to endothelial cell markers, features similar to those of AH. In addition, the high cellularity and entrapment of renal tubules could be misinterpreted. Since it is a malignant neoplasm, angiosarcoma requires an early and radical approach. Thus, an inadequate management of an AH, misdiagnosed as an angiosarcoma, can lead to unnecessary aggressive treatment and psychological stress for the patient.
Renal AH presents a distribution over a wide age range and a predilection for the male sex. Clinically, the manifestations are variable and mostly insignificant; occasionally haematuria or flank pain have been reported [5], which are common symptoms in the presence of malignant lesions. Overall, AH is usually incidentally detected by radiographic investigations performed for other unrelated reasons [5]. In the evaluation of masses in the genitourinary tract, Computed Tomography (CT) imaging, possibly supported by magnetic resonance imaging (MRI), plays a crucial role. CT images with and without Contrast Medium (CM) can provide valuable information on the vascularisation and architecture of the lesion. Thus, it is important to analyse how the AH presents radiologically, as the radiological features may be useful to guide the differential diagnosis.
In line with the literature, our experience confirms the complexity of discriminating against the dyskaryokinetic nature of such a complex lesion with CT investigations, even if supplemented by MRI acquisitions. Thus, even if there are many overlapping features among the entities placed in benign vs. malignant differential diagnosis from the anatomopathological perspective, this diagnostic difficulty appears even more evident to the radiologist's eye. Indeed, it is not surprising that AH is still treated with demolitive approaches, such as total or partial nephrectomy, or more rarely local excision, with the aim of clarifying the nature of the lesion. However, in support of the biologically indolent nature of AH, some limited evidence exists on a small group of patients brought to follow-up which showed no tendency for recurrence or metastasis [3,8].
Conclusion
Predicting the nature of such a complex rare renal lesion prior to a direct assessment is still a complex process. There is a clear need for careful analysis and a multidisciplinary approach in the management of renal masses, especially for those of rare onset such as AH. In this setting, our work takes part of this complex process by reporting imaging behaviour of an AH in order to enhance the awareness in the framing of such lesions. This could also stimulate improved imaging strategies and diagnostic guidelines, favouring more conservative approaches in suspected and eventually benignant lesions by avoiding demolishing treatments.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable on this case report.
Informed Consent Statement
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent form is available in his paper clinical medical record for review by the Editor-in-Chief of this journal.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interests.
References
- Brown JG, Folpe AL, Rao P, Lazar AJ, Paner GP, Gupta R, Parakh R, Cheville JC, Amin MB. Primary vascular tumors and tumor-like lesions of the kidney: a clinicopathologic analysis of 25 cases. Am J Surg Pathol. 2010 Jul;34(7):942-9. doi: 10.1097/PAS.0b013e3181e4f32a. PMID: 20534992.
- Mehta V, Ananthanarayanan V, Antic T, Krausz T, Milner J, Venkataraman G, Picken MM. Primary benign vascular tumors and tumorlike lesions of the kidney: a clinicopathologic analysis of 15 cases. Virchows Arch. 2012 Dec;461(6):669-76. doi: 10.1007/s00428-012-1333-9. Epub 2012 Oct 23. PMID: 23090628.
- Lin J, Bigge J, Ulbright TM, Montgomery E. Anastomosing hemangioma of the liver and gastrointestinal tract: an unusual variant histologically mimicking angiosarcoma. Am J Surg Pathol. 2013 Nov;37(11):1761-5. doi: 10.1097/PAS.0b013e3182967e6c. PMID: 23887160.
- Montgomery E, Epstein JI. Anastomosing hemangioma of the genitourinary tract: a lesion mimicking angiosarcoma. Am J Surg Pathol. 2009 Sep;33(9):1364-9. doi: 10.1097/PAS.0b013e3181ad30a7. PMID: 19606014.
- Tao LL, Dai Y, Yin W, Chen J. A case report of a renal anastomosing hemangioma and a literature review: an unusual variant histologically mimicking angiosarcoma. Diagn Pathol. 2014 Aug 8;9:159. doi: 10.1186/s13000-014-0159-y. PMID: 25102914; PMCID: PMC4149197.
- Omiyale AO. Anastomosing hemangioma of the kidney: a literature review of a rare morphological variant of hemangioma. Ann Transl Med. 2015 Jul;3(11):151. doi: 10.3978/j.issn.2305-5839.2015.06.16. PMID: 26244138; PMCID: PMC4499659.
- Leggio L, Addolorato G, Abenavoli L, Ferrulli A, D'Angelo C, Mirijello A, Vonghia L, Schinzari G, Arena V, Perrone L, Citterio F, Bonomo L, Rapaccini GL, Capelli A, Barone C, Gasbarrini G. Primary renal angiosarcoma: a rare malignancy. A case report and review of the literature. Urol Oncol. 2006 Jul-Aug;24(4):307-12. doi: 10.1016/j.urolonc.2005.10.002. PMID: 16818182.
- Perdiki M, Datseri G, Liapis G, Chondros N, Anastasiou I, Tzardi M, Delladetsima JK, Drakos E. Anastomosing hemangioma: report of two renal cases and analysis of the literature. Diagn Pathol. 2017 Jan 24;12(1):14. doi: 10.1186/s13000-017-0597-4. PMID: 28118845; PMCID: PMC5260082.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.