Medicine Group 2025 March 28;6(3):297-308. doi: 10.37871/jbres2084.
Potential Use of Immunological Indices as Predictors of Bacterial Infections Following Cardiopulmonary Bypass Surgery in Elderly Patients
Piotr Sindera1, Barbara Zawidlak-Wegrzynska2* and Ewa Kucewicz-Czech3
2Department of Chemistry, Faculty of Medical Sciences, Academy of Silesia, Zabrze, Poland
3Department of Anaesthesiology and Intensive Care, Silesian Centre for Heart Diseases, Zabrze, Poland
- Cardiopulmonary bypass
- Leukocytes
- Bacterial infections
- Flow cytometry
- ELISA
Abstract
Cardiac surgery with Cardiopulmonary Bypass (CPB) induces postoperative immunological responses in patients. We aimed to determine changes in various immunological parameters in patients during and after cardiac surgery supported by CPB and to search for possible reliable predictors of postoperative bacterial infections. CD4, CD8, CD3, CD19, CD45, CD16+56+ and CD3+HLA-DR+ lymphocytes and CD14 monocytes were counted by flow cytometry in blood samples taken from 36 patients (21 women and 15 men divided into 3 age groups), three of whom were diagnosed with postoperative infections (fourth group). An increased percentage of B lymphocytes and a reduced percentage of cells with CD4 receptors (T-helper cells) were observed on the first postoperative day. These changes during this period correlated with the onset of bacterial infections.
Introduction
Cardiopulmonary Bypass (CPB) is a complex process that maintains proper blood circulation during cardiac surgery, protecting it from the negative consequences of reduced heart function. A mechanical pump forces the flow of blood, which is also oxygenated and free of excess CO2 and deleterious metabolites [1,2].
Despite the apparent benefits, the patient's blood is exposed to various surfaces of the system equipment during CBP, which can lead to Systemic Inflammatory Response Syndrome (SIRS). This syndrome is induced by several agents that alter the secretion of cytokines that activate the endothelial cells of blood vessels. This leads to damage to these cells and increased permeability [3,4]. The syndrome is also associated with cellular and humoral immune responses and the activation of the complement system, white blood cells and platelets. Adhesion and migration of neutrophils into the tissues, provoking a disseminated inflammatory response in different tissues, can eventually cause Multiple Organ Dysfunction Syndrome (MODS), sometimes with fatal results [3,5].
In addition, cardiac arrest during surgery can cause myocardial ischaemia, leading to low output syndrome. Reperfusion promotes transient cardiac dysfunction that can lead to cardiogenic shock and endotoxemia [6]. The use of CPB in patients may disrupt the physiological balance of inflammatory and anti-inflammatory mediators in the blood due to damage to blood cells and/or intravascular coagulation.[1,7,8].
It is generally accepted that reducing the inflammatory response that develops after cardiac surgery, supported by cardiopulmonary bypass, should limit postoperative complications. Therefore, the prophylactic use of anti-inflammatory drugs (steroids, statins, aprotinin) to alleviate postperfusion syndrome has been tested for many years [11,12].
The severity of SIRS associated with CBP-assisted surgery depends on many factors, such as the efficiency of the individual patient's homeostatic mechanisms, age, type of treatment and duration of CBP, and others [13-15].
Older patients are more susceptible to infection due to the effect of CPB on the immune system [16,17]. T-cell depletion and reduced affinity for antigens resulting from age-related thymus activity contribute to this [18,19].
Some authors report that the activity of Antigen Presenting Cells (APC) does not decrease with age [11], and others suggest that dendritic cells may partially compensate for the T cell deficit and thus maintain cell-mediated immunity [10,20]. Rinder et al. [11] state that this type of immune suppression post-surgery is not age-dependent; in older patients, it lasts longer than in younger ones.
Humoral immunity is also weakened in the elderly patients due to a decreased number of circulating B cells, which produce high-affinity antibodies and memory cells [21]. An increase in CD5 B lymphocytes, which produce antibodies with low affinity to antigens, has been observed. Elderly patients also have increased NK cells in the blood, though there's mixed evidence on whether their activity changes with age [22,23].
Systemic chronic low-intensity inflammation, probably resulting from prolonged stimulation by antigens, is common in the elderly patients [2]. This state is characterised by increased serum levels of inflammatory cytokines (IL-1, IL-6, TNF-α, produced by cells of the innate immune system) and is often more prevalent in patients with IHD [24].
The physiological disturbances and health consequences of CBP in elderly patients, especially those with postoperative infections, have been studied very little. However, with the increasing number of elderly patients in modern societies, the problem of postoperative bacterial infections caused by the use of CBP is gaining clinical and social importance [25].
This study aims to identify reliable immune response markers for predicting CBP-assisted surgery-related infections in the elderly patients.
Materials and Methods
The study was approved by the Bioethics Committee (KNW/0022/KB1/164/11) and conducted according to its guidelines. Adult patients selected for the study had EUROSCORE (European System for Cardiac Operative Risk Evaluation) ≤ 12 and EF (ejection fraction) ≥ 40% and were scheduled to undergo various CBP-assisted cardiac surgeries. Patients were excluded from the study if they had the following diagnoses: ongoing infection, chronic renal failure with GFR < 50 (mL*min-1*1.76 m-2), anaemia, chronic ulcer disease, peripheral atherosclerosis reaching the carotid arteries, Chronic Obstructive Pulmonary Disease (COPD), glycated haemoglobin (HbA1C) > 8.0 mmol/l, immunodeficiency or autoimmune disease, pregnancy, coagulopathy or liver failure. A total of 36 patients were included in the study (21 females and 15 males, divided into 3 age groups (Table 1); in the oldest group, a fourth group was established, consisting of 3 patients diagnosed with postoperative infections (Table 2).
| Table 1: Clinical data on patients with postoperative infections. AVR: Aortic Valve Replacement; MVR: Mitral Valve Replacement. | |||
| Age groups [years] | |||
| 18 - 39 | 40 - 69 | 70 - 83 | |
| Patients (males) | 10 (6) | 8 (1) | 15 (7) |
| Mean time of CPB ± SD [min] | 128 ± 72.5 | 109 ± 42.5 | 127 ± 48.9 |
| Mean aortic clamping time ± SD [min] | 88 ± 61.3 | 67 ± 26.2 | 81 ± 33.9 |
| Euro Score ± SD | 4 ± 1.69 | 6 ± 3.0 | 7 ± 1.62 |
| Table 2: Clinical data on patients with postoperative infections. AVR: Aortic Valve Replacement; MVR: Mitral Valve Replacement. | |||
| Patient 1 | Patient 2 | Patient 3 | |
| Surgery | aortic aneurysm, AVR | AVR | AVR, MVR |
| Sex | Male | Female | Female |
| Age | 73 | 80 | 77 |
| Inoculation test | E. coli in blood and urine | Klebsiella pneumoniae in blood and urine | E. coli in urine |
| Euroscore | 12 | 7 | 8 |
| CPB duration [min] | 368 | 100 | 147 |
| Aortal clamping [min] | 207 | 62 | 105 |
| Postoperative complications | bleeding | neurological | pleural exudate, renal complications: creatinine >200 |
| Reoperation | tamponade | no | tamponade, hemofiltration/ hemodiafiltration |
| Transfusion | erythrocyte mass/plasma | no | erythrocyte mass |
| Amount of blood product [units] | 15 | 0 | 8 |
| Duration of the first hospitalization (days) | 32 | 30 | 48 |
Sample collection and preparation
Blood samples (2.6 mL) were collected using EDTA syringes (SARSTEDT AG & Co.) according to the following schedule: immediately before CPB (K), at 60 minutes of CPB (60min), and 24 hours after the start of surgery (24h). No later than 4 hours after blood collection, 10 µL of labelled antibodies (Becton Dickinson) or isotype control were added to 50 µL of blood and incubated for 20 minutes in the dark at room temperature. Red blood cells were then lysed by adding 500 µL of Opti-lyse reagent (BeckmanCoulter®), followed by incubation in the dark for 10 min. Then 500 µL of PBS buffer (Gibco®) pH 7.4 was added and the samples were incubated for 25 min in the dark at 37 oC. The samples were then centrifuged (200 x g, 5 min) at room temperature. The supernatants were decanted and the sediment fraction was washed by centrifugation three times with 500 µL PBS (Gibco®) and finally resuspended in 500 µL PBS, pH 7.4.
Each blood sample used for cytometry (Ortho Diagnostic, Beckman Coulter FC 500 MPL) consisted of five subsamples. A minimum of 8000 white cells were used for each assay. The lymphocyte or monocyte fraction was first gated by forward scatter and side scatter, then positive and negative areas were defined for a given fluorescence against an isotypic control. The addition of different fluorescence-labelled monoclonal antibodies allowed the percentage of cell subpopulations to be calculated (Cyflogic® - ©Perttu Terho & ©CyFlo Ltd). Each blood sample, even from the same patient, was gated independently.
CPB procedure
The priming solution used during CPB contained, in a total volume of approximately 1.252 L: 0.2 L 15 % mannitol, 0.5 L 6 % HAES (hydroxyetylated starch), 0.5 L Plasmalyte® (Baxter, Poland), 0.1 L 20 % MgSO4, 0.4 L 8.4 % NaHCO3 and 100 mg heparin. Approximately 800 mL of the solution was used to dilute the patient's blood and the remaining volume was used to prefill the CPB system.
CPB was initiated by injection of 3 mg - Kg-1 b.w. heparin to extend the activated clotting time above 480 s (preoperative ACT was 100-120 s). The right atrium and aorta were cannulated. If bicuspid, tricuspid or pulmonary valves were operated, inferior and superior caval veins were cannulated and connected. Cardiac arrest was induced by cold (4 oC) cardioplegia consisting of 450 mL Plasmalyte®, 60 mL 15% KCl and 50 mL 15% mannitol.
The circulation rate was maintained at 2.4 L/m2/min (constant flow) and the pressure at 8.0-9.3 kPa (10.7-12.0 kPa in elderly patients to maintain their higher blood pressure). Body temperature was in the range of 33-36 oC, depending on the type of surgical procedure.
Criteria of infection diagnosis
Patients enrolled in the study were monitored for infection throughout their postoperative hospital stay. When clinical symptoms were noted (pus from the bronchial tree, fever, pain and burning in the urethra), detailed laboratory tests (leukocytosis, leukopenia, procalcitonin level, urine analysis) and microbiological tests of body fluids (saliva, urine and blood) were performed. Infection was diagnosed in three of the patients in the oldest group (Table 8).
Statistical analysis
STATISTICA 10 software (StatSoft Polska) was used for statistical processing of the obtained results. Descriptive statistics were performed for each measured parameter. Kołmogorov-Smirnov, Lilliefors and Shapiro-Wilk tests for normality showed a lack of normal distribution of some measured parameters. Levene's test showed unequal variances in the experimental groups. Therefore, non-parametric tests were used for further analyses. Friedman's test was used to compare time groups, Kruskal-Wallis test was used to compare age groups, and Mann-Whitney U test was used to compare males and females (significance level p < 0.05 for each test).
Results
Changes of T lympocytes
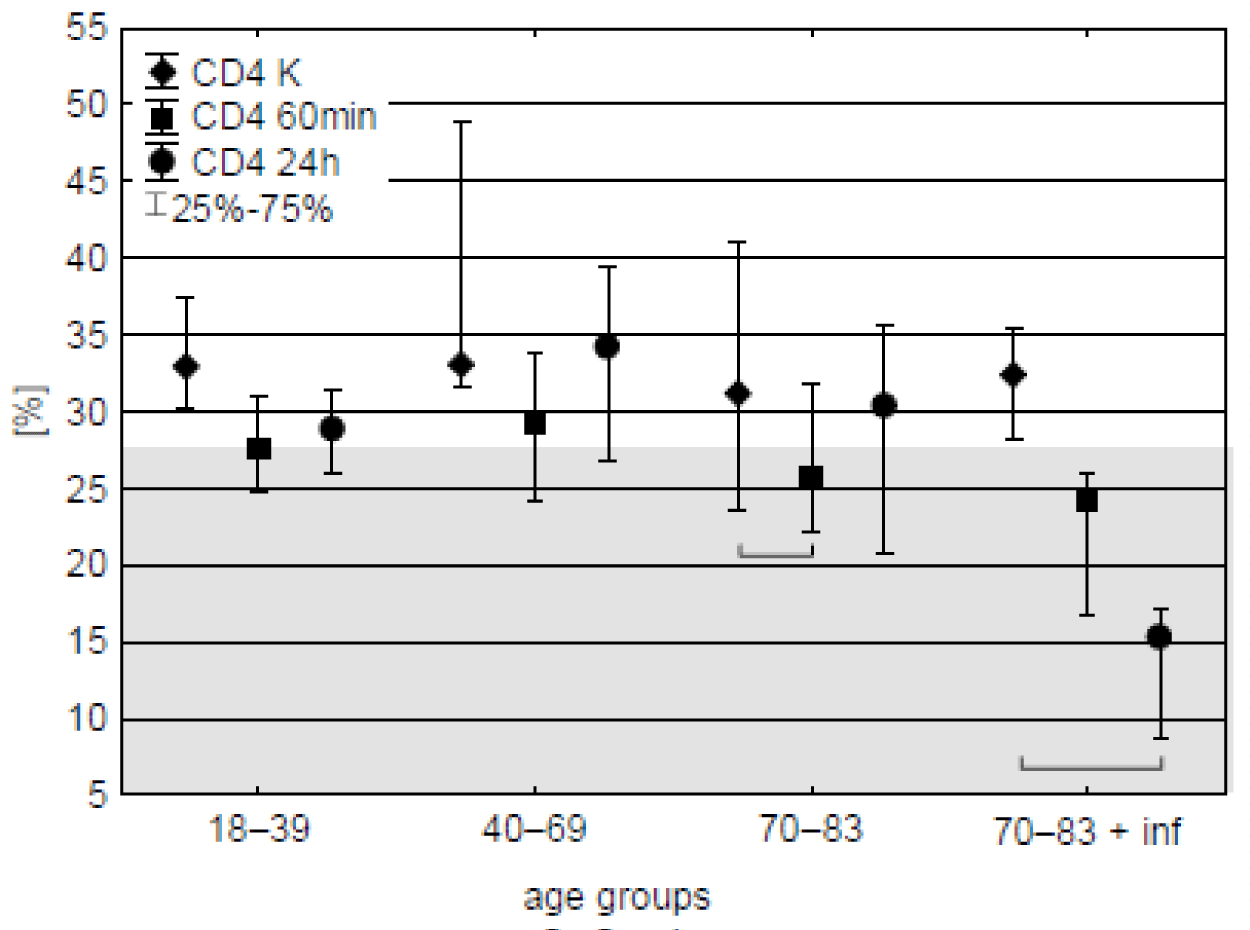
CD4 lymphocytes. Prior the operation CD4 cells amounted to 31-33 % of all lymphocytes. 60 minutes after start of CPB their percentage decreased in the oldest age-group but similar tendency was observed in all the groups. 24 h after the operation recovery was observed except of the patients with post-operative infections in which further decrease of CD4 occurred, reaching half or even nearly one-fourth of the initial value (Figure 1, table 3). With except of these patients median content of the lymphocytes remained within 95 % range for hematologically normal adults (BD Simultest TM IMK Plus, Becton, Dickinson and Company. ©2010 BD).
| Table 3: Percentage of CD4 lymphocytes in individual patients with documented post-operative infections (CPB-aided cardio surgery). Sampling time: K – before CPB start, 60min –60 min after CPB start, and, 24h – 24 hours since the start of cardio surgery, % change – 24h sample relative to K. ↓ means overall decrease. | |||
| Blood sampling time | Percentage of CD4 cells among all lymphocytes | ||
| Patient 1 | Patient 2 | Patient 3 | |
| K | 35.5 | 32.46 | 28.29 |
| 60min | 24.11 | 16.82 | 25.97 |
| 24h | 17.11 | 8.7 | 15.33 |
| change [%] | 51.8 ↓ | 73.2 ↓ | 45.8 ↓ |
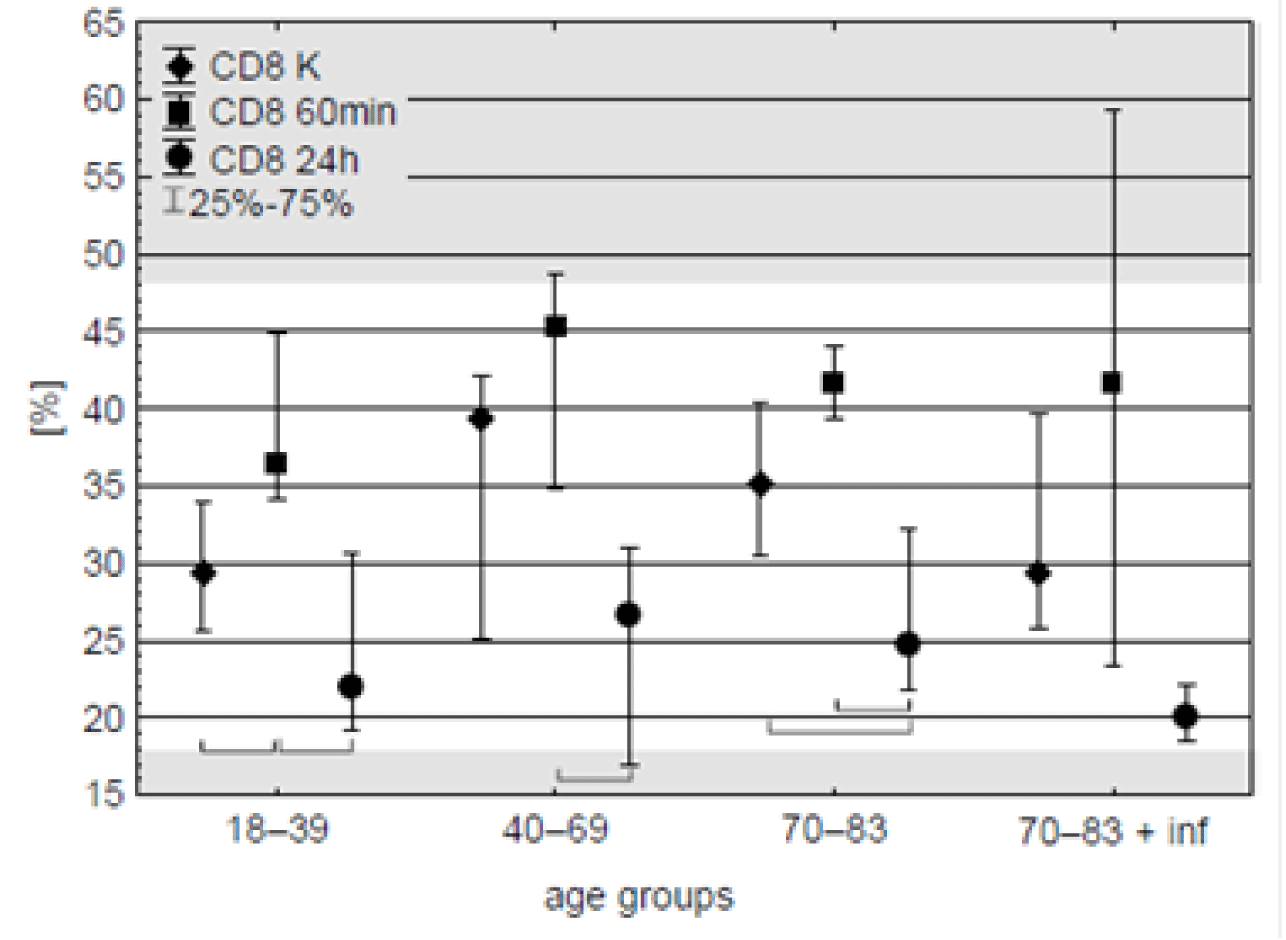
CD8 Lymphocytes. On the contrary to CD4 cells, one hour after CPB beginning median of CD8 lymphocytes content was about 20 % higher in comparison with pre-operation time in all the age groups but only in the youngest one the difference was significant.
Next day significant drop of CD8 content was observed in all the groups, with median of percent value lower than prior the operation in the oldest patients (Figure 2, table 4). Nevertheless, these final median values – as the all preceding’s - placed within the reference range for these cells (18.9-47.9 %, BD SimultestTM IMK Plus, Becton, Dickinson and Company. ©2010 BD).
| Table 4: Percentage of CD8 lymphocytes in individual patients with documented post-operative infections (CPB-aided cardio surgery). Sampling time as in table 3. | |||
| Blood sampling time | Percentage of CD8 cells among all lymphocytes | ||
| Patient 1 | Patient 2 | Patient 3 | |
| K | 29.45 | 39.76 | 25.84 |
| 60min | 41.57 | 59.28 | 23.42 |
| 24h | 22.12 | 20.12 | 18.54 |
Th/Tc (CD4/CD8) ratio. Increase of cytotoxic cells population resulted in decrease of CD4/CD8 ratio one hour after CPB start and full recovery next day in all the age-groups (Figure 3). Hence, only during CPB-aided cardio surgery in some cases the ratio fell much below the normal value - 1.3 (BD Simultest TM IMK Plus, Becton, Dickinson and Company. ©2010 BD). Although insignificant, the lowest ratio during and after the operation occurred in patients with post-operative infections (Figure 3, table 5).
| Table 5: CD4/CD8 ratio in individual patients with documented post-operative infections (CPB-aided cardio surgery). Sampling time as in table 3. | |||
| Blood sampling time | CD4/CD8 ratio | ||
| Patient 1 | Patient 2 | Patient 3 | |
| K | 1.21 | 0.82 | 1.09 |
| 60min | 0.58 | 0.28 | 1.11 |
| 24h | 0.77 | 0.43 | 0.83 |
| Change [%] | 36.4 ↓ | 47.6 ↓ | 23.9 ↓ |
Changes of B lymphocytes
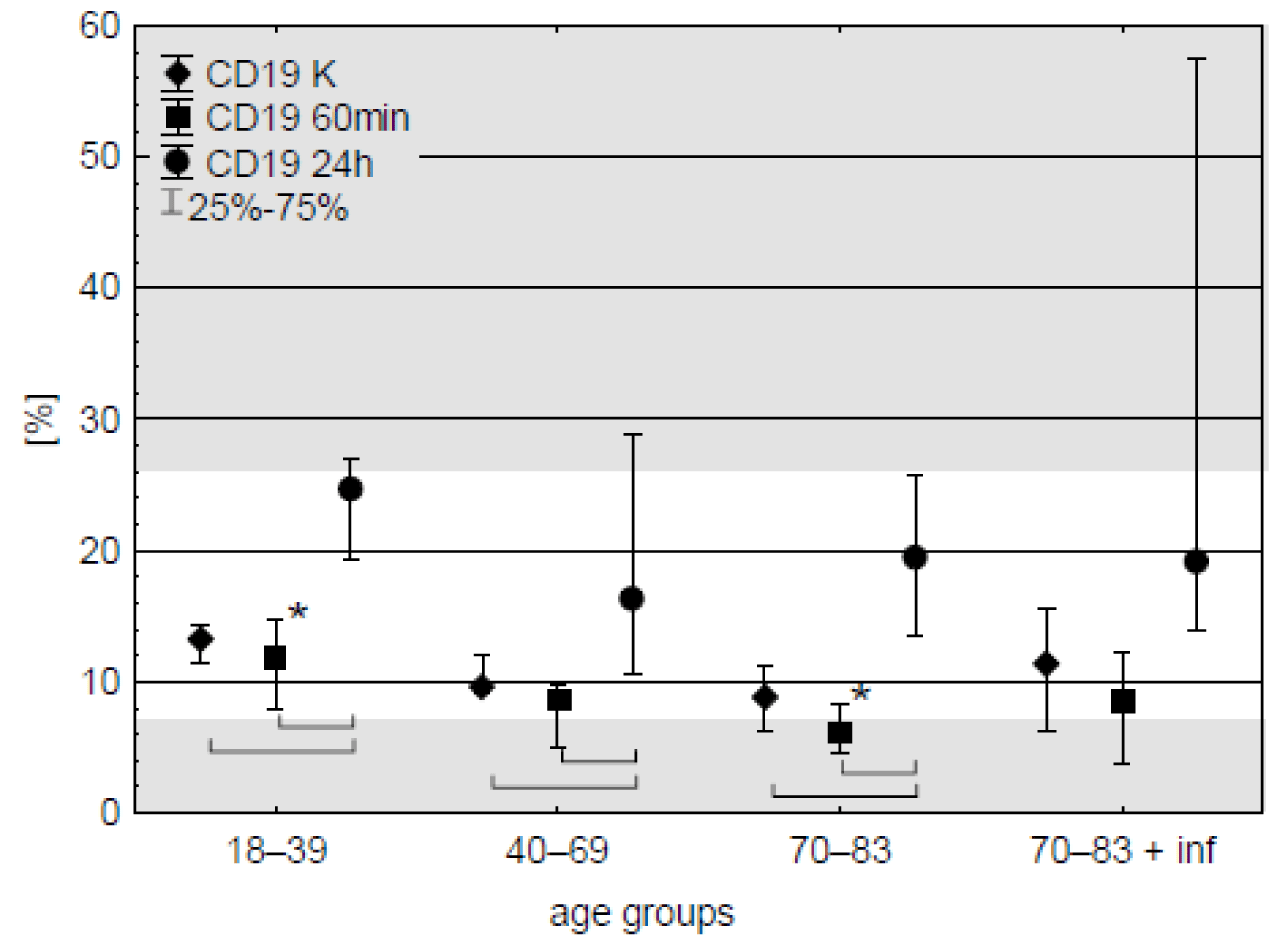
CD19 cells. Population of these lymphocytes accounted for about 8-13 % of all lymphocytes in the blood samples taken before the operation and did not change after an hour of CPB. Next day 2-2.5-fold increase of CD19 percentage was observed in all age groups. Except the old patients with post-operative infections this tendency was significant (Figure 4). Despite the increase, median of obtained results remained within reference range – 7.1 - 23.3 % (BD Simultest TM IMK Plus, Becton, Dickinson and Company. ©2010 BD). Changes in CD19 level in patients with post-operative infection are shown in table 6.
| Table 6: Changes of B (CD19) cells percentage in individual patients with documented post-operative infections (CPB-aided cardio surgery). Sampling time as in table 3. ↑ means overall increase. | |||
| Blood sampling time | Percentage of CD19 cells among all lymphocytes | ||
| Patient 1 | Patient 2 | Patient 3 | |
| K | 6.19 | 15.53 | 11.39 |
| 60min | 3.72 | 8.5 | 12.23 |
| 24h | 19.07 | 57.44 | 13.84 |
| change [%] | 208.1↑ | 269.9↑ | 21.5↑ |
Changes of NK lymphocytes
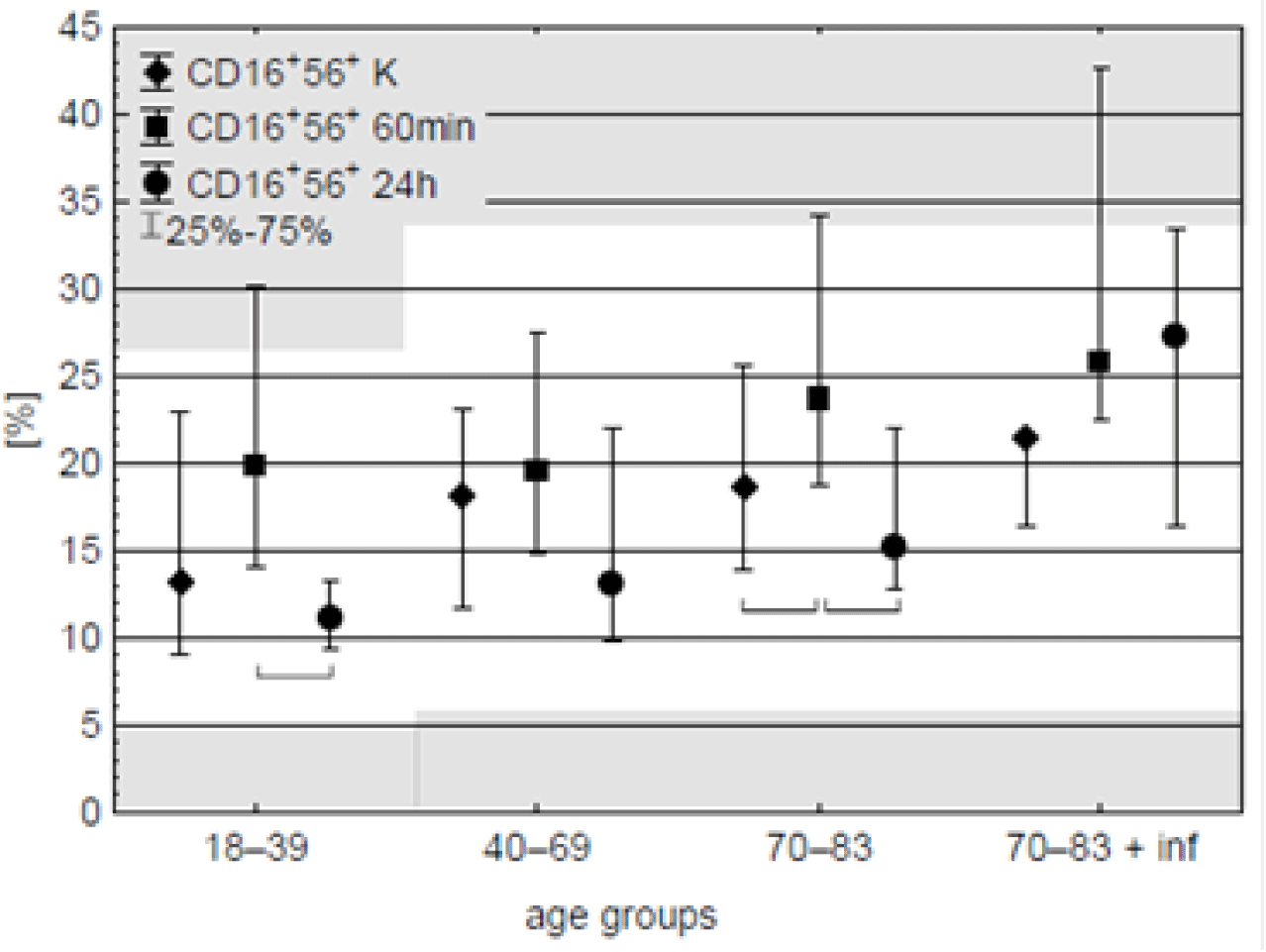
NK (CD16+56+) cells. Median percentage values of these cells were within the range of normal values – 4.8–33.5 % (BD Simultest TM IMK Plus, Becton, Dickinson and Company. ©2010 BD). After running of CPB elevation of NK cells was observed in the old-age patients, although similar (but insignificant) tendency was observed in all the age-groups. On the day after surgery, the percentage of NK cells returned to preoperative values in certain groups, except for the old patients with documented postoperative infections (Figure 5, table 7).
| Table 7: Changes of CD16+56+ cells percentage in individual patients with documented post-operative infections (CPB-aided cardio surgery). Sampling time as in table 3. ↑ means overall increase. | |||
| Blood sampling time | Percentage of CD16+56+ cells among all lymphocytes | ||
| Patient 1 | Patient 2 | Patient 3 | |
| K | 21.49 | 16.41 | 21.44 |
| 60min | 22.48 | 42.7 | 25.82 |
| 24h | 33.47 | 16.42 | 27.3 |
| change [%] | 55.7 ↑ | 0.0 | 27.3 ↑ |
Changes in other leukocytes
Counts of leukocytes with other phenotypes showed no differences - neither age nor time after cardiac surgery with CPB affected the levels of these cells in the blood (Table 8).
| Table 8: Changes of CD3, CD45, CD14, and CD3+HLA-DR+ cells in the blood of the patients selected for the study. Sampling time as in the Figure 3. | |||||||
| Age group | Time points | Leukocytes counts | |||||
| N | Min | Max | Q25 | Median | Q75 | ||
| CD3 | |||||||
| 18 – 39 | K | 10 | 44.93 | 72.39 | 59.60 | 60.92 | 68.33 |
| 60min | 10 | 46.21 | 72.42 | 54.08 | 58.97 | 70.41 | |
| 24h | 10 | 35.88 | 72.83 | 39.33 | 51.75 | 57.98 | |
| 40 - 69 | K | 7 | 53.44 | 75.83 | 54.85 | 67.25 | 75.60 |
| 60min | 8 | 51.99 | 77.66 | 54.38 | 64.90 | 74.95 | |
| 24h | 8 | 24.73 | 79.17 | 44.84 | 57.29 | 67.83 | |
| 70 - 83 | K | 15 | 40.23 | 74.86 | 47.74 | 63.26 | 67.99 |
| 60min | 15 | 36.89 | 74.19 | 50.51 | 63.50 | 67.84 | |
| 24h | 15 | 27.28 | 63.45 | 35.89 | 49.89 | 58.84 | |
| 70 - 83 + inf |
K | 3 | 43.22 | 66.41 | 43.22 | 55.83 | 66.41 |
| 60min | 3 | 37.59 | 64.72 | 37.59 | 44.71 | 64.72 | |
| 24h | 3 | 15.92 | 39.15 | 15.92 | 23.06 | 39.15 | |
| CD45 | |||||||
| 18 - 39 | K | 10 | 92.98 | 99.76 | 98.50 | 98.96 | 99.46 |
| 60min | 10 | 95.47 | 99.48 | 98.14 | 99.09 | 99.41 | |
| 24h | 10 | 85.60 | 99.37 | 94.72 | 98.00 | 98.73 | |
| 40 - 69 | K | 7 | 92.13 | 99.53 | 97.93 | 99.00 | 99.44 |
| 60min | 8 | 96.64 | 100.00 | 98.16 | 99.13 | 99.76 | |
| 24h | 8 | 71.41 | 98.50 | 94.94 | 96.77 | 97.93 | |
| 70 - 83 | K | 15 | 82.41 | 99.53 | 97.07 | 98.17 | 99.03 |
| 60min | 15 | 95.62 | 99.61 | 97.90 | 98.49 | 99.33 | |
| 24h | 15 | 85.08 | 99.10 | 90.23 | 91.96 | 98.24 | |
| 70 - 83 + inf |
K | 3 | 98.67 | 99.22 | 98.67 | 99.07 | 99.22 |
| 60min | 3 | 49.17 | 99.67 | 49.17 | 97.73 | 99.67 | |
| 24h | 3 | 88.14 | 96.41 | 88.14 | 89.55 | 96.41 | |
| CD14 | |||||||
| 18 - 39 | K | 10 | 4.59 | 13.47 | 5.36 | 7.57 | 8.48 |
| 60min | 10 | 0.10 | 9.87 | 2.73 | 3.58 | 7.29 | |
| 24h | 10 | 6.82 | 15.92 | 8.22 | 9.19 | 10.41 | |
| 40 - 69 | K | 7 | 4.16 | 9.77 | 4.20 | 6.79 | 8.58 |
| 60min | 8 | 0.27 | 6.02 | 0.68 | 1.78 | 2.69 | |
| 24h | 8 | 3.82 | 12.43 | 5.36 | 6.59 | 8.60 | |
| 70 - 83 | K | 15 | 3.34 | 10.23 | 4.44 | 5.73 | 8.74 |
| 60min | 15 | 0.40 | 5.95 | 2.68 | 4.06 | 4.67 | |
| 24h | 15 | 3.40 | 16.76 | 4.44 | 6.70 | 10.53 | |
| 70 – 83 + inf | K | 3 | 6.32 | 12.47 | 6.32 | 6.51 | 12.47 |
| 60min | 3 | 2.02 | 5.29 | 2.02 | 2.65 | 5.29 | |
| 24h | 3 | 2.13 | 8.89 | 2.13 | 6.73 | 8.89 | |
| CD3+HLA-DR+ | |||||||
| 18 - 39 | K | 10 | 2.64 | 13.95 | 3.50 | 5.54 | 7.02 |
| 60min | 10 | 2.43 | 12.68 | 3.26 | 5.38 | 8.44 | |
| 24h | 10 | 2.39 | 9.27 | 3.01 | 4.18 | 6.21 | |
| 40 - 69 | K | 7 | 2.73 | 20.48 | 5.32 | 8.87 | 18.12 |
| 60min | 8 | 2.71 | 30.10 | 6.23 | 11.14 | 16.56 | |
| 24h | 8 | 1.86 | 20.48 | 4.35 | 6.73 | 11.01 | |
| 70 - 83 | K | 15 | 2.49 | 20.88 | 4.23 | 8.17 | 14.44 |
| 60min | 15 | 2.14 | 22.04 | 4.77 | 9.43 | 14.52 | |
| 24h | 15 | 2.19 | 22.82 | 3.78 | 7.27 | 9.88 | |
| 70 - 83 + inf |
K | 3 | 7.88 | 11.23 | 7.88 | 10.72 | 11.23 |
| 60min | 3 | 8.79 | 12.93 | 8.79 | 9.40 | 12.93 | |
| 24h | 3 | 2.82 | 5.20 | 2.82 | 4.68 | 5.20 | |
Discussion
Despite significant advances in medical practice, bacterial blood infections remain a common complication of major surgical procedures. Recent studies have shown a significant decrease in mortality from these infections, but the infection rate associated with cardiac surgery has not decreased in the same way [26]. The authors also indicated that the gram-negative bacteria E. coli, K. pneumoniae and P. aeruginosa were responsible for most of the infections, which were mainly caused by healthcare procedures and occurred mainly in the first month after surgery. Patients are also at risk of infection with drug-resistant strains of Staphylococcus aureus. E. coli was found in the blood and urine of patients with postoperative infections in our study.
According to the current literature [27], the investigated postoperative markers are not sufficient. As mentioned above, the incidence of infection after CPB surgery is still high, even when CRP, procalcitonin, and immature/total neutrophil ratio are used as markers of infection. Procalcitonin is the most promising, but its use in the hospital setting allows the detection of infection two days after surgery [28]. Since the late 1980s, attempts have been made to use the lymphocyte population as a marker [28]. These authors claimed that the lack of mobilisation of cellular reserves (e.g. NK cells) could serve as an early marker for the development of infection after CPB surgery.
The mechanism of CPB-induced immune dysfunction and immune cell depletion is not fully understood. Possible causes include physical damage to blood cells by roller pumps during perfusion blood dilution and intravascular-extravascular flow of body fluids [29].The decrease in the percentage of circulating lymphocytes during the use of CPB may be caused by lymphocytes entering other tissues, loss of cells during intraoperative and postoperative bleeding, adhesion of cells to the surface of the cardiopulmonary bypass apparatus, mutual phagocytosis of immunocompetent cells, damage to membrane receptors leading to cell dysfunction, reduced ability of monocytes to present antigens. The deterioration of immune function after CPB may be due to the degradation of complement system components and high levels of pro-inflammatory cytokines [29,30]. These factors may cause lymphocytes remaining in the bloodstream after extracorporeal circulation to be functionally compromised and to respond poorly to mitogenic and alloantigenic stimulation in vitro.
The results obtained by researchers regarding changes in the percentage and number of B lymphocytes in patients undergoing surgery with the use of CPB are not consistent. De Palma, et al. [31] claim that the number of these cells decreases, whereas Lante, et al. [32] and Rinder, et al. [11] observed an increase in their concentration in the first postoperative days in all patients analysed. The results of my work indicate an increase in the percentage of B lymphocytes after surgery at the third measurement point (after 24 hours) in all age groups, both concerning the control and samples taken during surgery with the use of CPB. Furthermore, Rinder, et al. [11] observed differences in this parameter between patients under 60 and patients over 75. In the present study, the only difference between the different age groups was the increased percentage of B lymphocytes found during surgery in patients of the youngest age group (18-39 years) compared to the oldest group of operated patients (70-83 years). In addition, the synthesis of immunoglobulins and their secretion by B lymphocytes may be temporarily reduced after surgery with the use of CPB [1,12]. The above reports may indicate a periodic decrease in the body's ability to respond to antigens and the possibility of inducing antibody-dependent cellular immunity in lymphocytes.
The authors observed a decrease in the CD4 cell count during surgery in the oldest age group of patients compared to the control group. In contrast, the CD8 cell count increased during surgery in the youngest patients compared to the control group. Detailed studies of immune dysfunction have shown a post-operative decrease in the CD4+/CD8+ ratio [11] and we observed similar changes in all age groups of patients one hour after the start of surgery. The above changes resulted in a decrease in the CD4/CD8 ratio at 60 minutes of surgery with the use of CPB compared to the levels found in the control sample in the youngest and oldest patients. In addition, a reduced level of Th lymphocytes (CD4) was observed in patients who developed infections during the first postoperative day, compared with samples taken before CPB. In a group of age-matched patients without infections, no changes in the percentage of cells in question were observed between the level of this parameter before the start of cardiopulmonary bypass and the level on the first postoperative day. This may indicate that early postoperative analysis of the percentage of CD4 cells may identify individuals who are more susceptible to infection.
Some authors reported a decrease in the number and functionality of NK cells (CD16+56+) after extracorporeal circulation [33]. On the other hand, Rinder, et al. [11] reported an increase in the number of NK cells independent of the age of the patient. The authors' analyses of changes in the percentage of this lymphocyte population showed the greatest increase in the percentage of NK cells in the oldest age group 60 minutes after surgery compared with the values recorded in the control samples taken on the first postoperative day. Tajima, et al. [34] showed that a significantly reduced concentration of NK lymphocytes during CPB surgery in people with postoperative infection compared to people without infection may be a marker of infection. In the present study, a statistically significant increase in the percentage of NK lymphocytes during surgery was observed concerning the control sample (in the oldest group of subjects), but no differences in the level of this parameter were observed depending on the episode of postoperative infection.
This study also indicates the activation of the cellular immune response involving CD8, CD16+56+ cells during surgery and their suppression on the first post-operative day, often below the control concentration. A higher percentage of cells with the above phenotypes during surgery indicates a shift in the immune response towards cytotoxic reactions and may be caused by an increase in adrenaline levels in the blood. In contrast, the postoperative reduction of these leukocyte subpopulations may be caused by the effect of cortisol.
Impaired mobilisation of the body's cellular reserves may be a marker for the early detection of infection after on-pump cardiac surgery [11]. In the present study, the patients with postoperative infections who have the lowest percentage of CD4 (Th) and CD3 (percentage of all T lymphocytes) lymphocytes on the first postoperative day. In addition, the percentage of Th lymphocytes in patients with postoperative infections was almost 2 to 4 times lower on the first postoperative day than before the start of cardiopulmonary bypass. In addition, all three patients with postoperative infections had a significantly lower percentage of Th lymphocytes on the first postoperative day compared to the control group. In addition, all three patients with postoperative infection had a significantly lower percentage of Th lymphocytes on the first postoperative day compared to the control group, while in the age-matched group of the oldest patients without infection, the percentage of these cells returned to a level similar to that before surgery on the first postoperative day.
It appears that, despite the current regime for performing surgery in extracorporeal circulation, which limits the time the blood is in contact with foreign material and significantly improves the biocompatibility of the components used in CPB, this surgical technique may affect the risk of postoperative infection. It should be noted, however, that the strict criteria for including and excluding patients from the studies meant that the study groups were not numerous, which limits the possibility of drawing full conclusions both on the role of gender in the immune response and on the risk of infection incidents. However, the results obtained may indicate the direction of further research in the search for an early marker of postoperative infection, which would allow early prediction and prevention of such complications.
Conclusion
The strict criteria used to select patients for these studies limited the size of the groups and consequently, the ability to conclude the role of gender in the immune response and risk of infection. An increase in the percentage of cytotoxic and NK lymphocytes during surgery and a decrease 24 hours after surgery, especially in the oldest patients, may indicate suppression of cytotoxic responses and may be a risk factor for postoperative infection. The results obtained about the CD4 marker, i.e. a reduced percentage of cells with the CD4 phenotype (Th lymphocytes) on the first postoperative day, may correlate with postoperative bacterial infections in the patients studied under cardiopulmonary bypass. This suggests a direction for further research in the search for early markers of post-operative infection that would allow the anticipation and prevention of infection in humans undergoing surgery using CPB.
Author Contributions
Conceptualization, P.S., and E.K.-C.; Methodology, P.S., and E.K.-C.; Validation, P.S., B.Z.-W., and E.K.-C.; Formal Analysis, P.S. and G.W.; Investigation, P.S.; Resources, P.S. and E.K.-C.; Data Curation, P.S. and E.K.-C.; Writing—Original Draft Preparation, B.Z.-W.; Writing—Review & Editing, P.S. and B.Z.-W.; Visualization, P.S., B.Z.-W., and E.K.-C.; Supervision, E.K.-C. Project administration, P.S.; Funding acquisition, P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Ministry of Science and Higher Education, as part of statutory research, grants for “Young Scientists”: Modern methods of studying selected immunological parameters in patients undergoing extracorporeal circulation during heart surgery. Funding amount: PLN 6100.
FundinInstitutional Review Board Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Local Bioethical Committee at the Medical University of Silesia 11.10.2011 (KNW/0022/KB1/164/11) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Statement
Informed consent was obtained from all individual participants included in the study. In this consent, the participants accepted to participate in the study and to publish their results.
Data Availability Statement
Data available on request due to restrictions privacy or ethical.
Acknowledgment
We would like to express our sincere gratitude to Grażyna Wilczek (Institute of Biology, Biotechnology and Environmental Protection, University of Silesia in Katowice, Katowice, Poland) for her invaluable support and guidance during this research. Her dedication and assistance were essential to the successful completion of this work. We thank Piotr Wilczek (Professor Zbigniew Religa Foundation of Cardiac Surgery Development, Zabrze, Poland) and Sławomir Zegle ´n (University of Opole, Poland) for their substantial assistance.
Disclosures
The authors declare that they have no conflict of interest.
References
- Ferreira LO, Vasconcelos VW, Lima JS, Vieira Neto JR, da Costa GE, Esteves JC, de Sousa SC, Moura JA, Santos FRS, Leitão Filho JM, Protásio MR, Araújo PS, Lemos CJDS, Resende KD, Lopes DCF. Biochemical Changes in Cardiopulmonary Bypass in Cardiac Surgery: New Insights. J Pers Med. 2023 Oct 18;13(10):1506. doi: 10.3390/jpm13101506. PMID: 37888117; PMCID: PMC10608001.
- Sarkar M, Prabhu V. Basics of cardiopulmonary bypass. Indian J Anaesth. 2017 Sep;61(9):760-767. doi: 10.4103/ija.IJA_379_17. PMID: 28970635; PMCID: PMC5613602.
- Edmunds LH Jr. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 1998 Nov;66(5 Suppl):S12-6; discussion S25-8. doi: 10.1016/s0003-4975(98)00967-9. PMID: 9869435.
- Squiccimarro E, Stasi A, Lorusso R, Paparella D. Narrative review of the systemic inflammatory reaction to cardiac surgery and cardiopulmonary bypass. Artif Organs. 2022 Apr;46(4):568-577. doi: 10.1111/aor.14171. Epub 2022 Jan 21. PMID: 35061922; PMCID: PMC9303696.
- Zhao X, Gu T, Xiu Z, Shi E, Yu L. Mild Hypothermia May Offer Some Improvement to Patients with MODS after CPB Surgery. Braz J Cardiovasc Surg. 2016 May-Jun;31(3):246-251. doi: 10.5935/1678-9741.20160048. PMID: 27737408; PMCID: PMC5062708.
- Gravlee GP, Davis RF, Kurusz M, Utley JR. Cardiopulmonary bypass: principles and practice. 2nd ed. Philadelphia (PA): Lippincott Williams & Wilkins; 2000.
- Cremer J, Martin M, Redl H, Bahrami S, Abraham C, Graeter T, Haverich A, Schlag G, Borst HG. Systemic inflammatory response syndrome after cardiac operations. Ann Thorac Surg. 1996 Jun;61(6):1714-20. doi: 10.1016/0003-4975(96)00055-0. PMID: 8651772.
- Hornick P, Taylor K. Pulsatile and nonpulsatile perfusion: the continuing controversy. J Cardiothorac Vasc Anesth. 1997 May;11(3):310-5. doi: 10.1016/s1053-0770(97)90100-2. PMID: 9161899.
- Dvirnik N, Belley-Cote EP, Hanif H, Devereaux PJ, Lamy A, Dieleman JM, Vincent J, Whitlock RP. Steroids in cardiac surgery: a systematic review and meta-analysis. Br J Anaesth. 2018 Apr;120(4):657-667. doi: 10.1016/j.bja.2017.10.025. Epub 2018 Feb 8. PMID: 29576107.
- Rinder CS, Mathew JP, Rinder HM, Tracey JB, Davis E, Smith BR. Lymphocyte and monocyte subset changes during cardiopulmonary bypass: effects of aging and gender. J Lab Clin Med. 1997 Jun;129(6):592-602. doi: 10.1016/s0022-2143(97)90193-1. PMID: 9178725.
- Gokalp O, Yesilkaya NK, Bozok S, Besir Y, Iner H, Durmaz H, Gokkurt Y, Lafci B, Gokalp G, Yilik L, Gurbuz A. Effects of age on systemic inflamatory response syndrome and results of coronary bypass surgery. Cardiovasc J Afr. 2018 Jan/Feb 23;29(1):22-25. doi: 10.5830/CVJA-2017-030. Epub 2017 May 23. PMID: 28556849; PMCID: PMC6002802.
- Lante W, Franke A, Weinhold C, Markewitz A. Immunoglobulin levels and lymphocyte subsets following cardiac operations: further evidence for a T-helper cell shifting. Thorac Cardiovasc Surg. 2005 Feb;53(1):16-22. doi: 10.1055/s-2004-830324. PMID: 15692913.
- Fulop T, Larbi A, Wikby A, Mocchegiani E, Hirokawa K, Pawelec G. Dysregulation of T-cell function in the elderly : scientific basis and clinical implications. Drugs Aging. 2005;22(7):589-603. doi: 10.2165/00002512-200522070-00005. PMID: 16038574.
- Castle SC. Clinical relevance of age-related immune dysfunction. Clin Infect Dis. 2000 Aug;31(2):578-85. doi: 10.1086/313947. Epub 2000 Sep 14. PMID: 10987724.
- Lazuardi L, Jenewein B, Wolf AM, Pfister G, Tzankov A, Grubeck-Loebenstein B. Age-related loss of naïve T cells and dysregulation of T-cell/B-cell interactions in human lymph nodes. Immunology. 2005 Jan;114(1):37-43. doi: 10.1111/j.1365-2567.2004.02006.x. PMID: 15606793; PMCID: PMC1782064.
- Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol. 2005 Jun 1;174(11):7446-52. doi: 10.4049/jimmunol.174.11.7446. PMID: 15905594.
- Steger MM, Maczek C, Grubeck-Loebenstein B. Morphologically and functionally intact dendritic cells can be derived from the peripheral blood of aged individuals. Clin Exp Immunol. 1996 Sep;105(3):544-50. doi: 10.1046/j.1365-2249.1996.d01-790.x. PMID: 8809147; PMCID: PMC2200540.
- Castle SC, Uyemura K, Crawford W, Wong W, Makinodan T. Antigen presenting cell function is enhanced in healthy elderly. Mech Ageing Dev. 1999 Mar 1;107(2):137-45. doi: 10.1016/s0047-6374(98)00141-9. PMID: 10220042.
- Shurin MR, Shurin GV, Chatta GS. Aging and the dendritic cell system: implications for cancer. Crit Rev Oncol Hematol. 2007 Nov;64(2):90-105. doi: 10.1016/j.critrevonc.2007.03.002. Epub 2007 Apr 18. PMID: 17446082; PMCID: PMC2084365.
- Yu Y, Lu C, Yu W, Lei Y, Sun S, Liu P, Bai F, Chen Y, Chen J. B Cells Dynamic in Aging and the Implications of Nutritional Regulation. Nutrients. 2024 Feb 8;16(4):487. doi: 10.3390/nu16040487. PMID: 38398810; PMCID: PMC10893126.
- Wardzyńska A, Kowalski ML. The ageing of the immune system and allergy in the elderly. Alergia Astma Immunologia. 2009;14:239-247.
- Camous X, Pera A, Solana R, Larbi A. NK cells in healthy aging and age-associated diseases. J Biomed Biotechnol. 2012;2012:195956. doi: 10.1155/2012/195956. Epub 2012 Nov 20. PMID: 23251076; PMCID: PMC3517269.
- Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000 Jun;908:244-54. doi: 10.1111/j.1749-6632.2000.tb06651.x. PMID: 10911963.
- Banks WA, Willoughby LM, Thomas DR, Morley JE. Insulin resistance syndrome in the elderly: assessment of functional, biochemical, metabolic, and inflammatory status. Diabetes Care. 2007 Sep;30(9):2369-73. doi: 10.2337/dc07-0649. Epub 2007 May 29. PMID: 17536070.
- Abbatecola AM, Ferrucci L, Grella R, Bandinelli S, Bonafè M, Barbieri M, Corsi AM, Lauretani F, Franceschi C, Paolisso G. Diverse effect of inflammatory markers on insulin resistance and insulin-resistance syndrome in the elderly. J Am Geriatr Soc. 2004 Mar;52(3):399-404. doi: 10.1111/j.1532-5415.2004.52112.x. PMID: 14962155.
- Alghamdi BA, Alharthi RA, AlShaikh BA, Alosaimi MA, Alghamdi AY, Yusnoraini N, Almashhor A. Risk Factors for Post-cardiac Surgery Infections. Cureus. 2022 Nov 7;14(11):e31198. doi: 10.7759/cureus.31198. PMID: 36505103; PMCID: PMC9728502.
- Prucha M, Zahorec R. Diagnosis of sepsis: which clinical and laboratory biomarkers are useful? Pol Arch Intern Med. 2024 Dec 19;134(12):16878. doi: 10.20452/pamw.16878. Epub 2024 Oct 29. PMID: 39470418.
- Nicolotti D, Grossi S, Palermo V, Pontone F, Maglietta G, Diodati F, Puntoni M, Rossi S, Caminiti C. Procalcitonin for the diagnosis of postoperative bacterial infection after adult cardiac surgery: a systematic review and meta-analysis. Crit Care. 2024 Feb 7;28(1):44. doi: 10.1186/s13054-024-04824-3. PMID: 38326921; PMCID: PMC10848477.
- Lesouhaitier M, Belicard F, Tadié JM. Cardiopulmonary bypass and VA-ECMO induced immune dysfunction: common features and differences, a narrative review. Crit Care. 2024 Jan;28(1):300. doi: 10.1186/s13054-024-05058-z.
- Tajima K, Yamamoto F, Kawazoe K, Nakatani I, Sakai H, Abe T, Kawashima Y. Cardiopulmonary bypass and cellular immunity: changes in lymphocyte subsets and natural killer cell activity. Ann Thorac Surg. 1993 Mar;55(3):625-30. doi: 10.1016/0003-4975(93)90265-j. PMID: 8452424.
- DePalma L, Yu M, McIntosh CL, Swain JA, Davey RJ. Changes in lymphocyte subpopulations as a result of cardiopulmonary bypass. The effect of blood transfusion. J Thorac Cardiovasc Surg. 1991 Feb;101(2):240-4. PMID: 1992233.
- Sindera P, Kucewicz-Czech E, Wilczek G. Assessment of selected immune parameters in patients undergoing cardiac surgery with the use of cardiopulmonary bypass: Aspects of age and sex—a pilot study. Biomedicines. 2023 Apr;11(4):1224. doi: 10.3390/biomedicines11041224.
- Sánchez IP, Leal-Esteban LC, Orrego-Arango JC, Garcés-Samudio CG, Gómez-Arias RD, Franco JL, Trujillo-Vargas CM. Variaciones en el número y función de los linfocitos asesinos naturales durante infecciones recurrentes o graves [Variation in NK cell number and function in individuals with recurrent or severe infections]. Biomedica. 2014 Jan-Mar;34(1):118-31. Spanish. doi: 10.1590/S0120-41572014000100015. PMID: 24967865.
- Tajima K, Yamamoto F, Kawazoe K, Hirata T, Kumon K, Tanaka K, Fujita T. [Effect of immune response during cardiopulmonary bypass on post-operative infections]. Nihon Kyobu Geka Gakkai Zasshi. 1989 Apr;37(4):671-5. Japanese. PMID: 2768943.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.