Medicine Group 2025 March 24;6(3):282-292. doi: 10.37871/jbres2082.
Expression and Function of Hypoxia Inducible Factor-1a and Vascular Endothelial Growth Factor in Rat Periodontal ligament under Orthodontic Stretch
Qinghang Zhao2, Huidong Zhao1, Zhengxuan Hu1, Zhiheng Ren1, Liujun Chen1, Zhenggang Chen3, Rongtao Yuan1 and Qingyuan Guo1*
2Huangdao District Central Hospital, Qingdao City, Qingdao, China
3The affiliated Yantai Stomatological Hospital, Binzhou Medical University, Yantai, China
- HIF-1a
- VEGF
- Orthodontic stretch
Abstract
Orthodontic tooth movement is considered as a biological response to the orthodontic stretch via the osteoblast and osteoclast processes. Orthodontic stretch leads to strain in the periodontal ligament, circulatory disturbances, and vascular changes of the periodontal tissue, which cause a hypoxic environment in local tissue. However, bone remodeling is not only induced by mechanical stress but also effected by hypoxic environment which regulated by numerous factors. Thus, we established a rat model of orthodontic tooth movement to investigate the expression and function of HIF-1α and VEGF in rat periodontal ligament under orthodontic stretch. The results showed that osteogenesis and vascular changes occurred in the tension site of alveolar bone during orthodontic tooth movement. Additionally, there were significant changes in the expression of HIF-1a and VEGF proteins under orthodontic force. Compared with control group, experimental group expressed significantly more HIF-1α and VEGF protein on the surface of the alveolar bone tension side during the rat tooth movement (p < 0.05). These data showed that the hypoxic environment could be induced by mechanical force. HIF-1a and VEGF may play an important role in maintaining the physiologic equilibrium of periodontal tissue reconstruction during orthodontic tooth movement.
Introduction
During orthodontic tooth movement, mechanical stress plays a crucial role in bone formation and absorption during orthodontic tooth movement via the bone remodeling as a result of osteoblast and osteoclast. This type of tooth movement is considered as a biological response to the physiologic equilibrium, when the dentofacial complex interfered by an externally applied force [1]. The external force generates two different strains in the Periodontal Ligament (PDL), compression and tension. At the compression site, the force generated from the root against the alveolar bone induces bone resorption. At the tension site, PDL fibers are stretched and bone tissue is formed [2,3]. Because of evoking strain in the loose connective tissue and deformation of blood vessels in the PDL, the oxygen levels in periodontium were relatively lower after orthodontic force loading [4].Hence, the alveolar bone remodeling may be not only cause by mechanical stress but also influenced by hypoxia environment.
It has been known that cells in the organism can sense and respond to hypoxia. On the negative effects, the previous studies had demonstrated that hypoxia could disrupt stability of internal environment and cause some disease and cancer [5]. On the other hand, present studies have showed that hypoxia induces positive effects on proliferation of cardiomyocyte in adult mammalian heart, suggesting a novel strategy in regenerative medicine [6]. Actually the hypoxia tolerance represented a process of the adaptability adjustment of low oxygen conditions [7]. Hypoxia inevitably lead to a strong angiogenic response in the hypoxic and injured tissue, which may secure the supply of oxygen, nutrients and growth factors for as much as possible to maintain the stability of internal environment [5,8,9].Moreover, according to previous study, we found that osteoblast proliferation in low ambient oxygen tension involved in response to hypoxic signal sens and transduction [10].
Hypoxia-Inducible Factor (HIF), the major regulator of oxygen homeostasis in all extant metazoan species [5], is a heterodimer consisting of an oxygen-regulated α-subunit (HIF-α) and a constitutively expressed β-subunit (HIF-β). Three distinct HIF-α subunits, HIF-1α, 2α, and 3α, exist in humans [7].Hypoxia-inducible factor-1α (HIF-1α), the essential transcription factor induced by hypoxia, activates 100–200 genes in response to a variety of hypoxic stimulus [5,10,11]. Vascular Endothelial Growth Factor-A (VEGF) is a classical target of HIF-1α, whose expression and activity are tightly involved with angiogenic response [12]. Recent studies showed that upregulating the expression of VEGF can promote both angiogenesis and osteogenesis [13]. It was found that cell apoptosis in the process of endochondral bone formation can be caused by loss of VEGF, which reduced the number of blood vessels in the surrounding soft tissue [10].Taken together, these data suggest that HIF/VEGF pathway plays an important role in bone formation.
Unfortunately, the orthodontics research have received less attention to influence of hypoxia in combined effects of orthodontic force and remodeling of periodontal tissues. Owing to the complicated effect of the bone remodeling during orthodontic treatment, it is still unclear how hypoxic stimulation influence osteoblastic differentiation under mechanical stress. In order to explore the influences of hypoxic stimulation in osteogenesis under orthodontic stretch, we established a rat tooth movement model for study. These observations may provide better understand the hypoxia stimulation under orthodontic stretch and explore the function of HIF-1a and VEGF in tooth movement.
Materials and Methods
Animals
The study design was submitted to and approved by the Animal Ethical Committee of Shandong University (number ECAESDUSM2015032).
Thirty 8-week-old male Wistar rats with Specific Pathogen Free (SPF), which obtained from the Central Animal Breeding House of Shandong University, were used in this study. The average weight of the rats was 200 ~ 220 g. The rats were fed with a standard diet (Vital River Laboratory Animal Company, Beijing, China) and mineral water ad libitum and housed with a 12:12hour artificial light cycle to avoid any discomfort after the orthodontic appliance inserting. Room temperature and humidity according to the National Research Council’s guide for the care and use of laboratory animals. The rats were randomly assigned to 5 groups (n = 6): five experimental groups for force application at left maxillary with 1d, 3d, 1w, 2w and 4w. The right maxillary was selected as self-comparison which received orthodontic appliance without force loading.
Experimental tooth movement
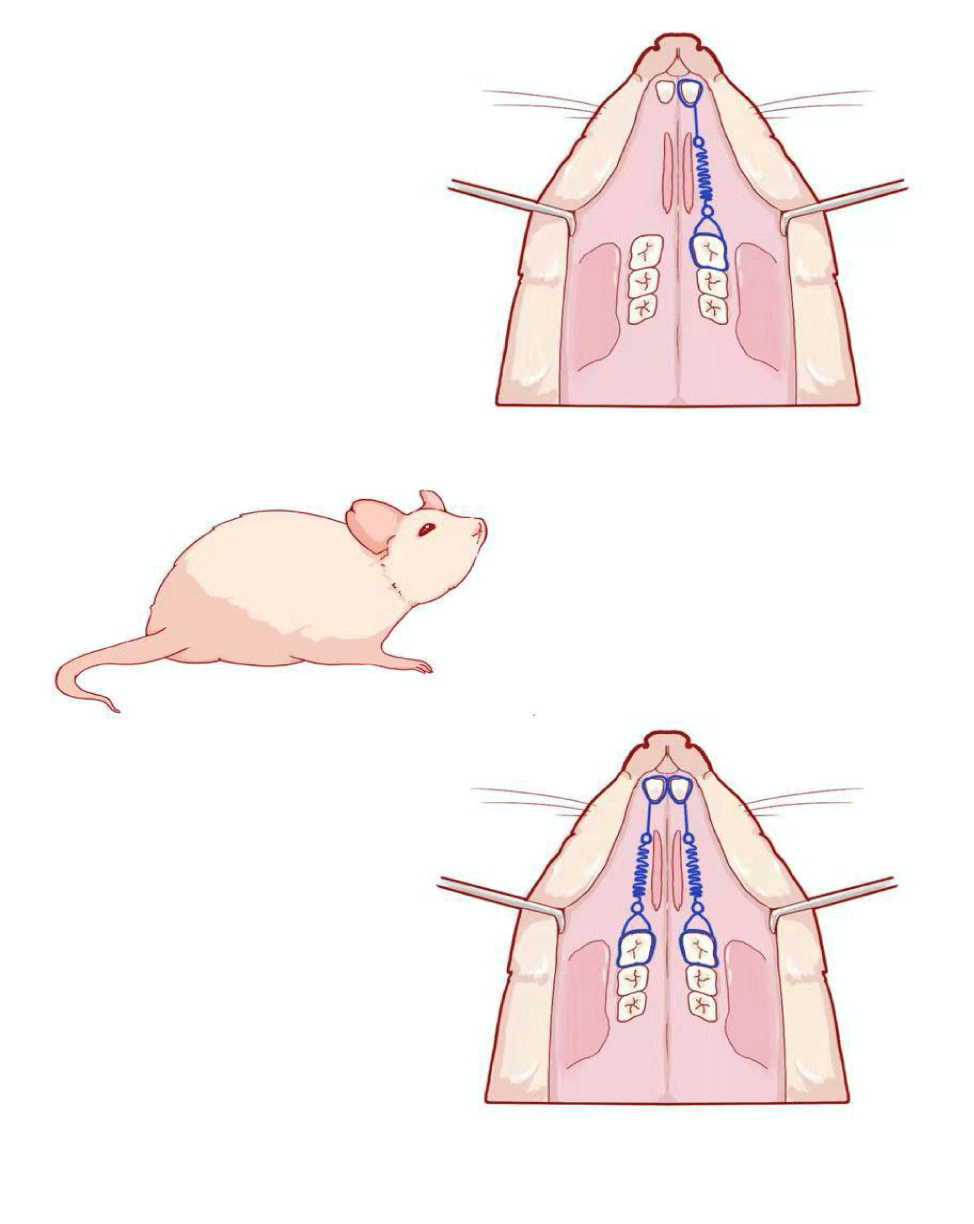
Orthodontic appliances were placed on the rats between the first upper molars and upper central incisors under general anesthesia with 1% Chloral hydrate. Experimental tooth movement was performed by using a method mentioned before [14], (Figure1) in which a closed-coil spring (inner diameter: 0.030’’, 3 M Unitek, CA, USA) was ligated to the left maxillary first molar cleat with a stainless steel ligature wire (wire size: 20#, OSU, Hangzhou, China). The other side of the coil spring was ligated with the holes in the maxillary incisors drilled laterally just above the gingival papilla with a round bur (#1/4). After be processed with composite resin [15] (Transbond XT, 3M Unitek, Monrovia, Calif), two upper incisors were attached together as Orthodontic anchorage. The left upper first molar was moved mesially by the closed coil spring at a force of 0.3 Newton [16,17]. The force was determined based on a preliminary study that showed that the upper first molar could be moved by orthodontic force without severe resorption [18], and the experiments were performed for a period of 1d, 3d, 1w, 2w and 4w.The right upper first molar was received orthodontic appliance without force application, as control group. (The study design was submitted to and approved by the Animal Ethical Committee of Shandong University (number ECAESDUSM2015032).
Histological analysis
To investigate the efficiency of bone remodelling under Orthodontic Stretch in different groups, specimens were harvested at 1d, 3d, 1w, 2w, 4w after surgery, fixed with 4% paraformaldehyde and were decalcified with 10% Ethylene Diaminete Traacetic Acid (EDTA) for 4 weeks. After that, the specimens were routinely embedded in paraffin and 5 mm thick paraffin-embedded sections were subjected to Hematoxylin and Eosin (H&E) staining for histological test. With the help of Image-Pro-Plus 6.0 software, the formation and organization of the related tissues were observed using light and polarized microscope (BX50, Olympus Optical). The relative osteogenic area was evaluated by at least 3 selected fields from each specimen randomly under microscope.
Immunohistochemistry
The sections were deparaffinized in xylene and rehydrated through a graduated alcohol series. Endogenous peroxidase activity was blocked with 3% H2O2 in methyl alcohol. Antigens were retrieved in pancreatin by incubating at 37°C for 20 min. After removal of excess retrieving reagent, sections were incubated with mouse anti-HIF-1α (1:200) and mouse anti-VEGF (1:500)( Abcam, USA) in 0.01 M PBS containing 1% Bovine Serum Albumin (BSA) and 0.3% Triton X-100 at 4℃ overnight. For negative controls, sections were incubated with 0.01 M PBS instead of primary antibody. After incubation with the primary antibody, sections were further incubated firstly with biotin-conjugated goat anti-mouse IgG (Cwbio, China) at room temperature for 30 min, according to the manufacturer’s instructions [19]. Antibody complexes were visualized by development with 0.1% 3-diaminobenzidine in PBS with 0.03% H2O2 for 1 min at room temperature. Each step was followed by three washes in 0.01 M PBS. Sections were dehydrated through a graded ethanol series, cleared with xylene, and mounted with neutral resins. Photomicrographs were taken using light and polarized microscopy (BX50, Olympus Optical).
Statistical analysis
All data were presented as mean ± SD and statistical significance was evaluated by analysis of variance analysis using SPSS 22.0 software. The statistical significance was considered at p ≤ 0.05.
Result
Tooth movement in different groups
PAs shown in table 1, there was continuous increase in distance between the first upper molar and upper central incisor, and none of orthodontic appliance lost during the entire study period. No significant differences in amount of tooth movement within each group (p > 0.05). The highest mean value was observed in 4W groups. It is suggesting that the Wistar rat model of orthodontic tooth movement was established successfully.| Table 1: Tooth movement in different groups (mm, mean ± SD). | ||||||
| experimental phase | 0d | 1 d | 3 d | 7 d | 2 w | 4 w |
| teeth transportation distance (mm) | 0 | 0.1 ± 0.02 | 0.20 ± 0.05 | 0.26 ± 0.06 | 0.50 ± 0.02 | 1.02 ± 0.03 |
Histological examination
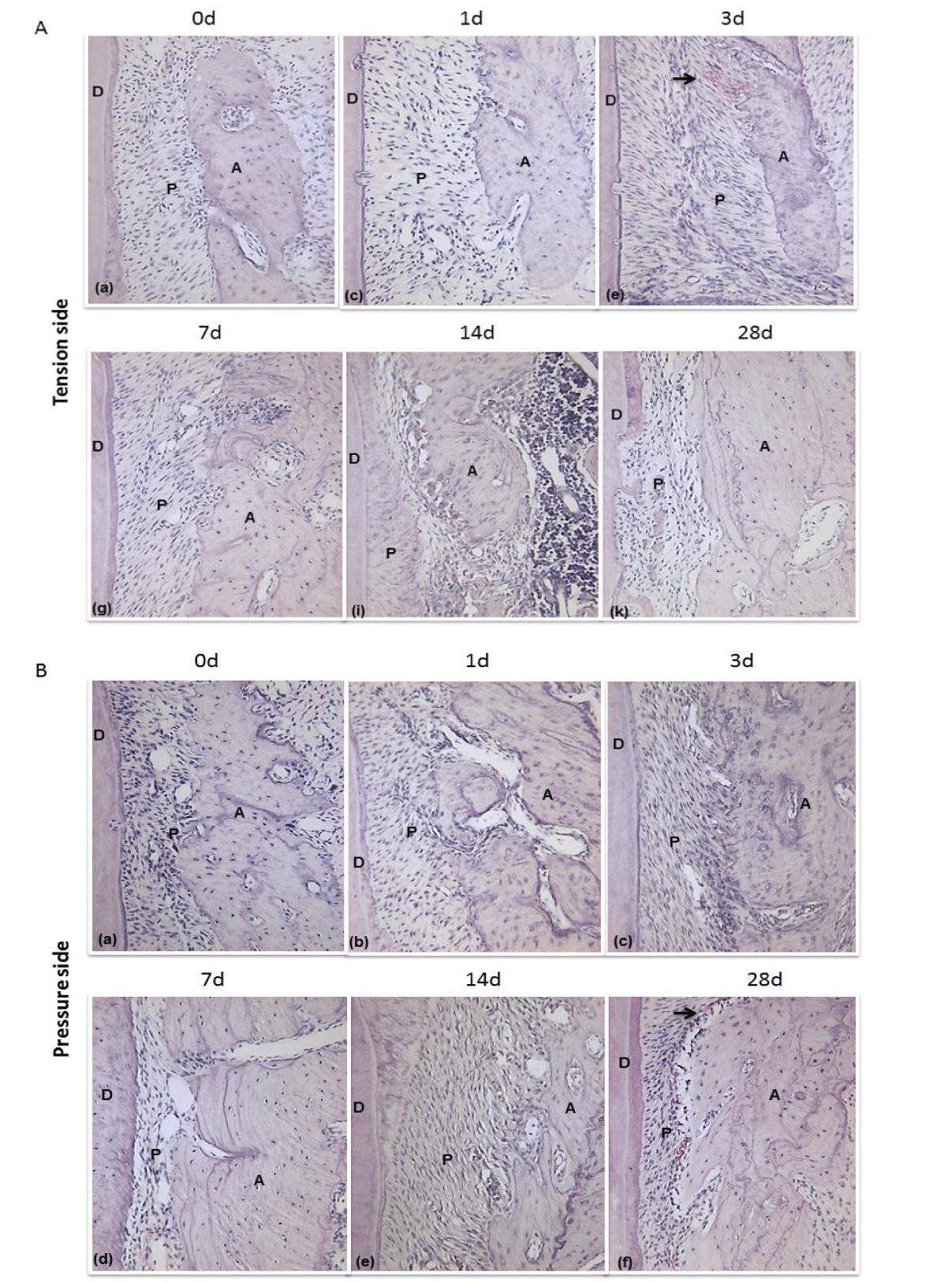
Histological examination showed that progressively changes in PDL, as demonstrated by cellular and structural events coupled withorthodontic force. Before the start of force loading, in the control group, periodontal ligament fibers arranged regularly and alveolar surface was flat. There were no significant signs of osteoblasting and osteoclasting. We could observe perfusion-fixed sections both at tension site and pressure site perfused by well-aligned PDL which interposed between the root and alveolar bone. The periodontal ligament fiber showed wavy configuration and well organized with a clearly defined interface and combined tightly between these layers, especially in the middle third of the PDL.
After the start of tooth movement on the 1 day, broadened PDL gap, elongated cellular elements and vasodilation were visible on the tension site. In contrast to the observations in pressure site, periodontal space has obviously constricted. Three days after orthodontic force loading, the above changing structure in periodontal tissue became more obviously. Periodontal fiber arrangement appeared to be more disorganized than 1 day group. The cell density in the pressure side was higher than the tension side. Companying with force loading at 7 days, PDL was further elongated and regenerated bone with positive osteoblasts was recognized on the alveolar bone tension site. On contrast, the bone resorption was observed in pressure side, where there were plenty of osteoclasts in bone lacuna. On the 14th day of retention, bone resorption and newly deposited bone matrix became more obvious to detect in periodontal tissue. At 28 d of tooth movement, regenerated bones were thickened, the cell density of osteoblasts was reduced, and calcification of the newly formed bones enhanced on the surfaces of the proper alveolar bones on the tension site. Significantly absorption of alveolar bone, expanded blood vessels and increased periodontal ligament fibroblasts were noted on the pressure site (Figures 2A,B).
In selected images of PDL, the blood vessels were increased in the PDL after 7 days of experimental groups. Vascularization in the pressure side was concentrated in the zone of the bone absorption, characterized by the presence of capillaries. In the tension side, vascularization was also detected along the new bone accordingly, but less obviously. After 14 days of experimental groups, the blood vessels were significantly accumulated and angiogenesis was more accelerated compared with the former period. The vessels were still regeneration at 28 days with the lower growth rate and displayed more normal morphology, suggesting the processes of PDL reconstruction was almost accomplished.
It is suggesting that the Wistar rat model of orthodontic tooth movement was established successfully (Figures 2A,B).
Light micrographs of PDL (Periodontal Ligament) tissue on the tension site (A) and pressure site (B) in rat on days 0, 1, 3, 7, 14 and 28 after tooth movement. (A: Alveolar; P: Periodontal ligament; D: Dentin; Arrowheads: Artery; Magnification, 200×).
Immunohistochemistry examination
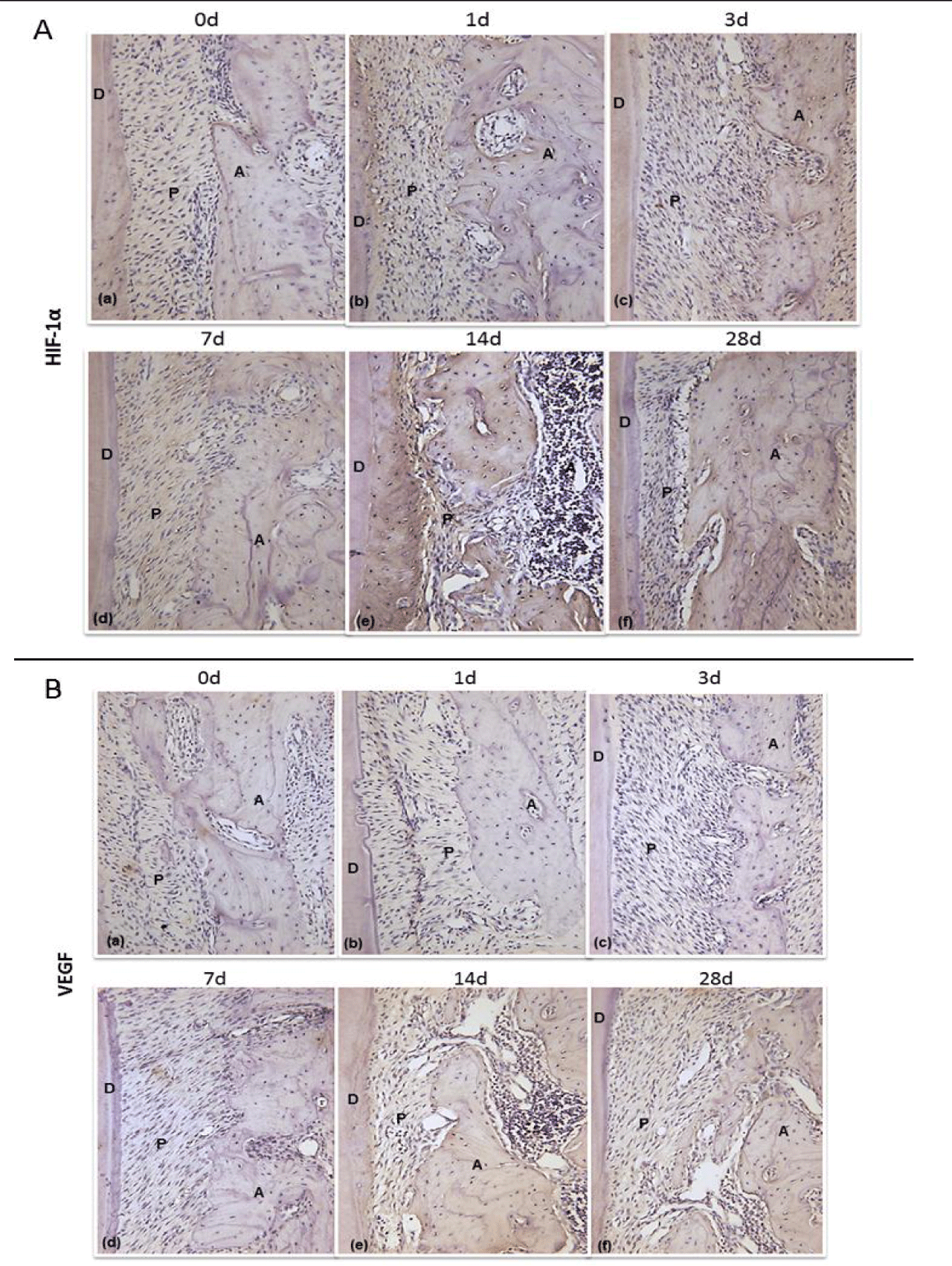
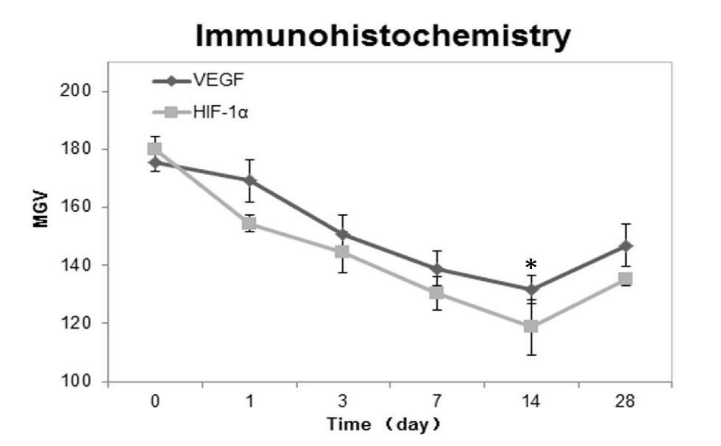
HIF-1α and VEGF protein expression both in PDL tension site and pressure site was investigated using immunohistochemical analysis. On the 1st day after the beginning of tooth movement, several HIF-1α and VEGF positive cells were observed on the surfaces of the alveolar bone. On the 3rd, 7th, and 14th days, more and more HIF-1α and VEGF protein appeared on the tension site. The expression of HIF-1a and VEGF in the PDL during the whole experiment was peaked at 14th day group. Compared with control groups (p < 0.05), experimental groups expressed significantly moreHIF-1α and VEGF protein on the surface of the alveolar bone tension surface side during the rat tooth movement (Figures 3A,B). Immunohistochemical analysis revealed the same changing tendency of HIF-1α and VEGF on the alveolar bone. The MGV (Mean Gray Value) of HIF-1α positive cells was lower than that of the VEGF positive cells. This finding indicated that HIF-1α was expressed to a higher level than VEGF (white = 255, black = 0). 28 days after the initiation of the tooth movement, the regenerated bone with HIF-1α and VEGF positive cells was recognized on the surface of the alveolar bone at tension side. In our quantitative evaluation, the MGV of HIF-1α and VEGF positive cells was significantly decreased on the 1st, 3rd, 7th and 14th days and reached nadir on the 14th days, and then increased on the 28th day (Figure 4). It indicated that difference was statistically significant. (Figures 3A,B)
Immunohischemistry results of HIF-1α and VEGF on the surface of the alveolar bone tension surface side at days 0, 1, 3, 7, 14 and 28 after the start of tooth movement. (A: Alveolar; P: Periodontal ligament; D: Dentin; Magnification, 200×) figure 4.
Discussion
In this article, we have shown that tooth movement and remodeling of periodontal tissues were closely related to orthodontic force loading. However, we observed the dynamic changes of hypoxia inside the PDL as a response to orthodontic force. Moreover, we have demonstrated that the HIF-1α expression and production was affected by the traction strain during the osteoblastogenesis process in vivo.
In the majority of the animal experimental model in orthodontic studies, Wistar rat has showed much more adaptation to reveal the nature of orthodontic tooth movement, since the demonstration in previous studies. The appliance of orthodontic in our study is consistent with the theory of three elements: strength, direction and functional point. Compared with the elastics, the appliance that closed-coil spring between the first upper molar and upper central incisor has exhibited much longer exertion period and more precise force control [20]. To investigate direction of tooth movement in our study, the molar mesial moving along the maxillary arch is much more similar to orthodontic treatment. Moreover, bone amount at the mesial side is more adequate than the buccal side [21].Based on correlative references, we used 0.3N [17] as the optimum force for moving rat upper first molars. There was satisfactory changes in modeling of the alveolar bone and the obvious side effects have not been discovered in our study. Moreover, limitation of the teeth transportation distance at the mesial side and continuity of the maxillary incisors eruption is worth reminding in Wistar rat model. Thus, the longest experimental period in our studies should be no more than 4 weeks [16].
The bone remodeling cycle can be altered by mechanobiological stimulations [22]. It is known that alveolar bone osteocytes as mechanosensors which are sensitive to their mechanobiological environment [8]. During the process of tooth movement, loading the external mechanical force to tooth may stimulate periodontal tissues, and this may contribute to activate osteoblasts in surface-lining of alveolar bone which may lead to bone remodeling [23]. However, with the conduction of force, strain and pressure may lead to the oxygen level descending in local tissue. Thus it generates a hypoxic environment in PDL. In addition, local hypoxia between alveolar bone and the root was caused by mechanical stress during orthodontic tooth movement, and the local hypoxic microenvironment played a crucial role in starting bone remodeling [24]. Furthermore, previous study confirmed the fracture healing [25] and the skeletal development [10], is exposure to hypoxia in vitro and hypoxic signal transduction can regulate that process, which involved angiogenesis and bone mass. In our experiment, we observed that HIF-1α was over-expressed at tension site with time dependent tendency. Therefore, we hypothesized that hypoxic-related signal transduction may regulate osteogenesis at tension site in PDL. However, in orthodontic studies, the theory of hypoxia has not been paid sufficient attention in osteogenesis. The function and mechanism of hypoxia in osteogenesis at tension site during the process of orthodontic tooth movement require further investigation.
Although hypoxia is induced by external mechanical force, we think that HIF-1α can stimulate cells secrete active factor and regulate the expression levels of genes, in order to help tissue adaptive in hypoxia microenvironment [26,27]. Many studies have demonstrated that hypoxia is a common phenomenon in tumor growth, especially in solid tumors [28]. Over expression of HIF-1α is associated with more aggressive and invasive to metastasize [29]. Accordingly, HIF-1α silencing in tumor cells resulted in decreased growth of tumor [30]. This suggests that HIF-1α enhances the fitness or aggressiveness of tumor cells in vivo to sustain their homeostasis. On the other hand, sufficient oxygen and nutrient supply may depend on increasing angiogenesis, which improve the hypoxia stamina to reverse the disadvantaged situation [31]. VEGF, as a classical HIF-responsive gene product, also has positive function to promote the angiogenesis, which can regulate endothelial proliferation, remodel extracellular matrix, migrate and proliferate endothelial cell, secrete proteolytic enzymes, differentiate capillary [32]. It is demonstrated that HIF-1α/VEGF was also detected in fracture and heal bone defects because of poor diffusion of oxygen induced by vascular injury or avascular tissue. The disorder of the internal environment, inflammation induced by hypoxic, leads to an adaptive change in the damaged tissue, which will ultimately maintain homeostasis and survival [9]. In our experiment, low-level expression of HIF-1a and VEGF was detected in the control group. In the initial phase of experiment, HIF-1α and VEGF was obviously expressed in PDL at tension site when they are subjected to hypoxic stress. With the osteogenesis and angiogenesis of the alveolar bone appeared progressively active, more and more HIF-1α and VEGF positive cells appeared on the alveolar bone tension surface site within 1-14 days. At 14th day, the expression of HIF-1α and VEGF reached peak performance, and then the expression of HIF-1α and VEGF was significantly reduced on 28th day, when the periodontal tissue remodeling was stable relatively and new blood vessels forming enhances the adaption of low oxygen environment. Thus, the change of oxygen concentration in PDL went through the evolutionary process from hypoxic situation to normal condition. Our results are consistent with previous study, which indicated that HIF-1α/VEGF pathway may locally promote the process of osteogenesis. Because process of osteogenesis was always accompanied by angiogenesis, VEGF could be considered as a useful biological indicator to evaluate the progress of osteogenesis indirectly. However, further research shows that osteoblast proliferation and differentiation may associate with models of moderate-low oxygen tension, whereas more severe hypoxia dramatically suppresses osteoblastic formation [33]. Therefore, it reminds us that the differences in the oxygen level may lead to variability in cellular responses.
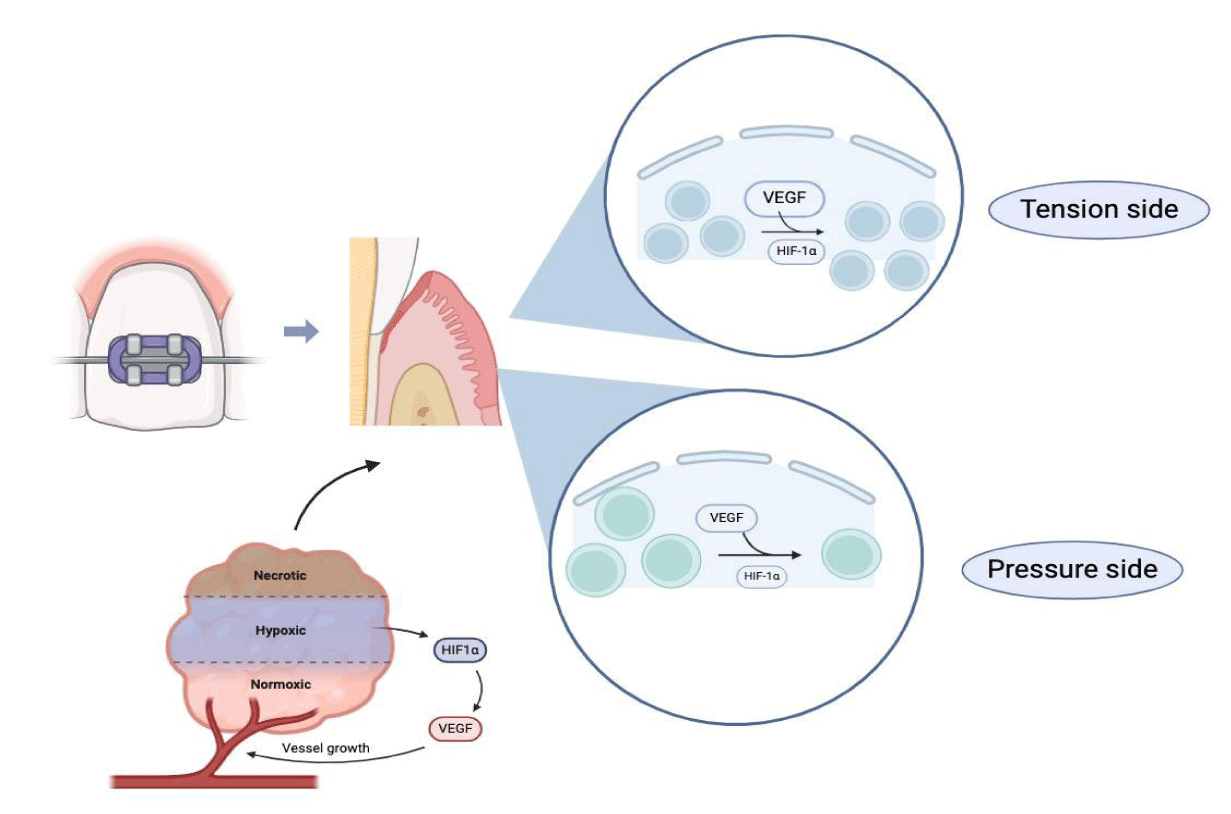
After satisfying orthodontic treatment, we expected that PDL was also able to maintain the stability in the microenvironment as same as before the treatment. On the basis of this viewpoint, we hypothesis, a possible mechanism (Figure 5), is that HIF pathway in PDL only regulates the process of reconstruction, a phase that contributes to improve the hypoxia and maintain homeostasis figure 5.
Firstly, mechanical forces are transmitted to periodontal tissues leading to deformation of periodontal membrane fiber. And then deformation of blood vessel in PDL may lead to blockages in blood flow and the local inflammation may increase the consumption of oxygen. These reasons may contribute to lead hypoxia in microenvironment. Recent studies have reported that HIF pathway may increase the process of cell differentiation to both osteoblast [34] and osteoclast [35].Our results are consistent with previous study, which indicated that high expression of HIF-1α was detected in processing of both alveolar regeneration and resorption. During the course of the experiment, we found that expression of HIF-1α and VEGF was significantly reduced on 14-28thd when the remodeling almost accomplished and microenvironment recovered from hypoxia. Hence, we conclude the function of HIF1-α during orthodontic treatment may not only simply contribute to improved osteogenesis but also regarded as the factor which was able to support favorable microenvironment in promoting remodelling of alveolar bone in hypoxia. On the other hand, mechanism of keeping homeostasis in hypoxia caused by various relevant factors. Magnitude and direction of force, the duration and timing of hypoxia, differences in the oxygen level and complex molecular interactions are all the reasons to explain that variability in cellular responses during the hypoxia. Therefore, whether the expression of HIF-1α and VEGF contributes to maintain homeostasis in promoting of tissue reconstruction in orthodontic force needs to be further gone into.
Conclusion
According to the theory of orthodontics, we have established the tooth movement model of rat to investigate the effect of hypoxia. In the current study, we have demonstrated the orthodontic force regulated the production of HIF-1α and VEGF in a time dependent manner. Our results suggested that HIF-1α plays an important role in bone remodeling induced by orthodontic force, which may support the production of HIF-1α by activating bone remodeling in vivo. HIF pathway seems to be able to maintain homeostasis in promoting of tissue reconstruction. A new perspective and conjecture may facilitate the research in orthodontic therapy.
In summary, we can conclude that tooth movement leads to changes in pulp tissue and is accompanied by an inflammatory process. Mechanical force can enhance the expression of HIF-1α and VEGF, indicating that HIF-1α and VEGF may play an important role in maintaining pulp homeostasis during orthodontic movements. HIF-1α may become a global mediator of hypoxic angiogenesis response by inducing various angiogenic factors, including VEGF.
Author Disclosure Statement
The authors declare no competing financial interests in relation to the work described.
References
- Nanda R, Upadhyay M. Skeletal and dental considerations in orthodontic treatment mechanics: a contemporary view. Eur J Orthod. 2013 Oct;35(5):634-43. doi: 10.1093/ejo/cjs054. Epub 2012 Sep 5. PMID: 24068287.
- Wu Y, Zhang P, Dai Q, Fu R, Yang X, Fang B, Jiang L. Osteoclastogenesis accompanying early osteoblastic differentiation of BMSCs promoted by mechanical stretch. Biomed Rep. 2013 May;1(3):474-478. doi: 10.3892/br.2013.84. Epub 2013 Mar 20. PMID: 24648971; PMCID: PMC3917496.
- Patil AK, Shetty AS, Setty S, Thakur S. Understanding the advances in biology of orthodontic tooth movement for improved ortho-perio interdisciplinary approach. J Indian Soc Periodontol. 2013 May;17(3):309-18. doi: 10.4103/0972-124X.115648. PMID: 24049330; PMCID: PMC3768180.
- Niklas A, Proff P, Gosau M, Römer P. The role of hypoxia in orthodontic tooth movement. Int J Dent. 2013;2013:841840. doi: 10.1155/2013/841840. Epub 2013 Oct 21. PMID: 24228034; PMCID: PMC3818850.
- Kaelin WG Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008 May 23;30(4):393-402. doi: 10.1016/j.molcel.2008.04.009. PMID: 18498744.
- Nakada Y, Canseco DC, Thet S, Abdisalaam S, Asaithamby A, Santos CX, Shah AM, Zhang H, Faber JE, Kinter MT, Szweda LI, Xing C, Hu Z, Deberardinis RJ, Schiattarella G, Hill JA, Oz O, Lu Z, Zhang CC, Kimura W, Sadek HA. Hypoxia induces heart regeneration in adult mice. Nature. 2017 Jan 12;541(7636):222-227. doi: 10.1038/nature20173. Epub 2016 Oct 31. PMID: 27798600.
- Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011 Aug 11;365(6):537-47. doi: 10.1056/NEJMra1011165. Erratum in: N Engl J Med. 2011 Sep 8;365(10):968. PMID: 21830968.
- Krishnan V, Davidovitch Z. On a path to unfolding the biological mechanisms of orthodontic tooth movement. J Dent Res. 2009 Jul;88(7):597-608. doi: 10.1177/0022034509338914. PMID: 19641146.
- Stiers PJ, van Gastel N, Carmeliet G. Targeting the hypoxic response in bone tissue engineering: A balance between supply and consumption to improve bone regeneration. Mol Cell Endocrinol. 2016 Sep 5;432:96-105. doi: 10.1016/j.mce.2015.12.024. Epub 2016 Jan 6. PMID: 26768117.
- Schipani E, Mangiavini L, Merceron C. HIF-1α and growth plate development: what we really know. Bonekey Rep. 2015 Aug 12;4:730. doi: 10.1038/bonekey.2015.99. PMID: 26331009; PMCID: PMC4549921.
- Kaelin WG Jr, Ratcliffe PJ, Semenza GL. Pathways for Oxygen Regulation and Homeostasis: The 2016 Albert Lasker Basic Medical Research Award. JAMA. 2016 Sep 27;316(12):1252-3. doi: 10.1001/jama.2016.12386. PMID: 27622845.
- Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012 Feb 3;148(3):399-408. doi: 10.1016/j.cell.2012.01.021. PMID: 22304911; PMCID: PMC3437543.
- Song X, Liu S, Qu X, Hu Y, Zhang X, Wang T, Wei F. BMP2 and VEGF promote angiogenesis but retard terminal differentiation of osteoblasts in bone regeneration by up-regulating Id1. Acta Biochim Biophys Sin (Shanghai). 2011 Oct;43(10):796-804. doi: 10.1093/abbs/gmr074. Epub 2011 Aug 30. PMID: 21880603.
- Xue H, Zheng J, Cui Z, Bai X, Li G, Zhang C, He S, Li W, Lajud SA, Duan Y, Zhou H. Low-intensity pulsed ultrasound accelerates tooth movement via activation of the BMP-2 signaling pathway. PLoS One. 2013 Jul 23;8(7):e68926. doi: 10.1371/journal.pone.0068926. PMID: 23894376; PMCID: PMC3720872.
- Akhoundi MS, Sadegh AA, Ghazanfari R, Rezvaneh G, Etemad-Moghadam, S, Shahroo EM, Alaeddini M, Mojgan A, Khorshidian A, Azam K, Rabbani S, Shahram R, Shamshiri AR, Reza SA, Momeni N, Nafiseh M. Effect of supplementary zinc on orthodontic tooth movement in a rat model. Dental Press J Orthod. 2016 Mar-Apr;21(2):45-50. doi: 10.1590/2177-6709.21.2.045-050.oar. Erratum in: Dental Press J Orthod. 2019 Jan-Feb;24(1):109. doi: 10.1590/2177-6709.21.2.045-050.err.. Sadegh AA [corrected to Akhoundi MA], Rezvaneh G [coorected to Ghazanfari R], Shahroo EM [corrected to Etemad-Moghadam S], Mojgan A [corrected to Alaeddini M], Azam K [corrected to Khorshidian A], Shahram R [corrected to Rabbani S], Reza SH [corrected to. PMID: 27275614; PMCID: PMC4896281.
- Kirschneck C, Proff P, Fanghaenel J, Behr M, Wahlmann U, Roemer P. Differentiated analysis of orthodontic tooth movement in rats with an improved rat model and three-dimensional imaging. Ann Anat. 2013 Dec;195(6):539-53. doi: 10.1016/j.aanat.2013.08.003. Epub 2013 Oct 16. PMID: 24183941.
- Ong CK, Walsh LJ, Harbrow D, Taverne AA, Symons AL. Orthodontic tooth movement in the prednisolone-treated rat. Angle Orthod. 2000 Apr;70(2):118-25. doi: 10.1043/0003-3219(2000)070<0118:OTMITP>2.0.CO;2. PMID: 10832999.
- Kohno T, Matsumoto Y, Kanno Z, Warita H, Soma K. Experimental tooth movement under light orthodontic forces: rates of tooth movement and changes of the periodontium. J Orthod. 2002 Jun;29(2):129-35. doi: 10.1093/ortho/29.2.129. PMID: 12114463.
- Shang F, Ming L, Zhou Z, Yu Y, Sun J, Ding Y, Jin Y. The effect of licochalcone A on cell-aggregates ECM secretion and osteogenic differentiation during bone formation in metaphyseal defects in ovariectomized rats. Biomaterials. 2014 Mar;35(9):2789-97. doi: 10.1016/j.biomaterials.2013.12.061. Epub 2014 Jan 15. PMID: 24439395.
- Ren Y, Maltha JC, Kuijpers-Jagtman AM. The rat as a model for orthodontic tooth movement--a critical review and a proposed solution. Eur J Orthod. 2004 Oct;26(5):483-90. doi: 10.1093/ejo/26.5.483. PMID: 15536836.
- Hikida T, Yamaguchi M, Shimizu M, Kikuta J, Yoshino T, Kasai K. Comparisons of orthodontic root resorption under heavy and jiggling reciprocating forces during experimental tooth movement in a rat model. Korean J Orthod. 2016 Jul;46(4):228-41. doi: 10.4041/kjod.2016.46.4.228. Epub 2016 Jul 25. PMID: 27478800; PMCID: PMC4965594.
- Saunders MM, Taylor AF, Du C, Zhou Z, Pellegrini VD Jr, Donahue HJ. Mechanical stimulation effects on functional end effectors in osteoblastic MG-63 cells. J Biomech. 2006;39(8):1419-27. doi: 10.1016/j.jbiomech.2005.04.011. Epub 2005 Jun 13. PMID: 15953606.
- Lohberger B, Kaltenegger H, Stuendl N, Payer M, Rinner B, Leithner A. Effect of cyclic mechanical stimulation on the expression of osteogenesis genes in human intraoral mesenchymal stromal and progenitor cells. Biomed Res Int. 2014;2014:189516. doi: 10.1155/2014/189516. Epub 2014 Apr 7. PMID: 24804200; PMCID: PMC3998000.
- Kitase Y, Yokozeki M, Fujihara S, Izawa T, Kuroda S, Tanimoto K, Moriyama K, Tanaka E. Analysis of gene expression profiles in human periodontal ligament cells under hypoxia: the protective effect of CC chemokine ligand 2 to oxygen shortage. Arch Oral Biol. 2009 Jul;54(7):618-24. doi: 10.1016/j.archoralbio.2009.03.010. Epub 2009 Apr 29. PMID: 19406381.
- Warren SM, Steinbrech DS, Mehrara BJ, Saadeh PB, Greenwald JA, Spector JA, Bouletreau PJ, Longaker MT. Hypoxia regulates osteoblast gene expression. J Surg Res. 2001 Jul;99(1):147-55. doi: 10.1006/jsre.2001.6128. PMID: 11421617.
- Riddle RC, Khatri R, Schipani E, Clemens TL. Role of hypoxia-inducible factor-1alpha in angiogenic-osteogenic coupling. J Mol Med (Berl). 2009 Jun;87(6):583-90. doi: 10.1007/s00109-009-0477-9. Epub 2009 May 5. PMID: 19415227; PMCID: PMC3189695.
- Gordillo GM, Sen CK. Revisiting the essential role of oxygen in wound healing. Am J Surg. 2003 Sep;186(3):259-63. doi: 10.1016/s0002-9610(03)00211-3. PMID: 12946829.
- Tafani M, Sansone L, Limana F, Arcangeli T, De Santis E, Polese M, Fini M, Russo MA. The Interplay of Reactive Oxygen Species, Hypoxia, Inflammation, and Sirtuins in Cancer Initiation and Progression. Oxid Med Cell Longev. 2016;2016:3907147. doi: 10.1155/2016/3907147. Epub 2015 Dec 20. PMID: 26798421; PMCID: PMC4699039.
- Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl). 2015 Dec 11;3:83-92. doi: 10.2147/HP.S93413. PMID: 27774485; PMCID: PMC5045092.
- Masoud GN, Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015 Sep;5(5):378-89. doi: 10.1016/j.apsb.2015.05.007. Epub 2015 Jun 6. PMID: 26579469; PMCID: PMC4629436.
- Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol. 2014;9:47-71. doi: 10.1146/annurev-pathol-012513-104720. Epub 2013 Aug 7. PMID: 23937437.
- Papandreou I, Powell A, Lim AL, Denko N. Cellular reaction to hypoxia: sensing and responding to an adverse environment. Mutat Res. 2005 Jan 6;569(1-2):87-100. doi: 10.1016/j.mrfmmm.2004.06.054. PMID: 15603754.
- Riddle RC, Leslie JM, Gross TS, Clemens TL. Hypoxia-inducible factor-1α protein negatively regulates load-induced bone formation. J Biol Chem. 2011 Dec 30;286(52):44449-56. doi: 10.1074/jbc.M111.276683. Epub 2011 Nov 12. PMID: 22081627; PMCID: PMC3248005.
- Wang T, Zhang X, Bikle DD. Osteogenic Differentiation of Periosteal Cells During Fracture Healing. J Cell Physiol. 2017 May;232(5):913-921. doi: 10.1002/jcp.25641. Epub 2016 Oct 26. PMID: 27731505; PMCID: PMC5247290.
- Knowles HJ. Hypoxic regulation of osteoclast differentiation and bone resorption activity. Hypoxia (Auckl). 2015 Nov 11;3:73-82. doi: 10.2147/HP.S95960. PMID: 27774484; PMCID: PMC5045091.a
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.