Medicine Group 2025 March 15;6(3):231-246. doi: 10.37871/jbres2078.
Can Ketamine Treat Schizophrenia?
Fikret Sahin*
Abstract
NMDAR antagonists, such as memantine and ketamine, have demonstrated efficacy in treating neurodegenerative diseases and major depression. Our recent findings reveal that these antagonists significantly enhance 20S proteasome activity, which is essential for degrading intrinsically disordered, oxidatively damaged, or misfolded proteins-key factors in neurodegenerative diseases like Alzheimer's and Parkinson's. Through the Ubiquitin-Independent 20S Proteasome pathway (UIPS), these drugs help maintain cellular protein homeostasis, a critical function that declines with age and contributes to protein aggregation and disease symptoms. Our findings provide a plausible explanation for how memantine alleviates symptoms of Alzheimer's and Parkinson's diseases by reducing or preventing protein aggregation in the brain. Furthermore, our data suggest that the dramatic changes in synaptic protein homeostasis induced by ketamine within 2 hours may contribute to its therapeutic effects in major depression.
The etiology and mechanisms of schizophrenia are not well understood, arising from complex interactions between genetic and environmental factors. Its pathophysiology reflects this complexity, with abnormalities in multiple brain functions. This review discusses the potential effects of NMDAR antagonists on the pathophysiology of schizophrenia and their therapeutic impact, supported by our data.
Introduction
N-Methyl-D-Aspartate Receptor (NMDAR)-Mediated Neurotransmission (NMDARMN) is fundamental to the development and plasticity of the Central Nervous System (CNS). Due to its critical role, both over-activation and under-activation of NMDARMN can significantly contribute to the development of CNS disorders. The involvement of NMDARMN has been demonstrated in various CNS disorders, including schizophrenia, depression, posttraumatic stress disorder, aging, cognitive impairment, and Alzheimer's dementia [1-4].
Schizophrenia is a complex psychiatric disorder characterized by a significant neurodevelopmental component [5,6]. It arises from a confluence of genetic and environmental risk factors that influence brain development. These factors can affect critical processes such as the maturation of interneurons and oligodendrocytes, both of which play vital roles in maintaining healthy brain function. Although there have been advances in understanding these developmental influences, such as deficiencies in NMDARMN, the precise neurobiological processes underlying schizophrenia remain poorly understood.
The glutamate hypothesis of schizophrenia
Despite the lack of consensus among the research community regarding the numerous proposed hypotheses, one of the most prominent suggests a disturbance in glutamatergic neurotransmission, particularly in the form of hypofunction of NMDAR-mediated neurotransmission [7-9]. Although this hypothesis has driven significant research, including numerous animal model studies, the primary evidence comes from direct human observations. For over two decades, it has been recognized that ketamine, MK801, and other NMDA receptor antagonists can induce symptoms similar to those of schizophrenia, encompassing positive, negative, and cognitive symptoms [9]. These observations formed the basis of what is now known as the glutamate hypothesis of schizophrenia. In addition, a systematic review and meta-analysis conducted by Beck, et al. [10] indicates that NMDAR antagonists, especially ketamine, were associated with schizophrenia-like or psychotomimetic symptoms with large effect sizes, underscoring the importance of ketamine for understanding schizophrenia pathophysiology.
In general terms, this hypothesis suggests that dysfunction in glutamatergic neurotransmission may play a role in the etiology of schizophrenia. However, direct evidence of changes in glutamate signaling in people with schizophrenia is still lacking. Structural studies measuring glutamate levels in the medial prefrontal cortex of both medication-naive and medication-free patients using magnetic resonance spectroscopy have produced mixed results [11,12].
Some studies of postmortem findings on glutamatergic measures in schizophrenia reveal evidence of structural changes in the dendrites of glutamatergic neurons; decreased dendrite length and complexity, as well as reduced dendritic spine density, have been reported in multiple brain regions [13]. In addition to these structural changes, research has explored genetic variations related to glutamate signaling. Initial studies suggested that some schizophrenia risk variants might be linked to genes involved in glutamate signaling, but these findings were not confirmed by larger studies [14].
A potential technical issue affecting postmortem studies that aim to identify the molecular correlates of structural alterations in glutamate neurons in schizophrenia brain tissue has been the focus on measuring mRNA expression. Some variability in molecular findings might also reflect the broader issue that mRNA levels account for only a minority of the variance in protein levels for many proteins. Unfortunately, to date, there has been limited analysis of glutamate receptor protein levels in schizophrenia, with too few evaluations to draw clear conclusions.
NMDAR-mediated neurotransmission or glutamatergic neurotransmission activation requires two distinct agonists. Both co-agonist sites have more than one endogenous agonist; in addition to glutamate or aspartate, d-serine or glycine serve as obligatory co-agonists, with studies indicating that d-serine is the most potent in NMDAR activation [15]. d-Serine is converted from l-serine by Serine Racemase (SR), which is considered the primary source of endogenous d-serine. As a neurotransmitter, d-serine is crucial for the branching and spine density of pyramidal neurons in the cortex. Dysregulation of d-serine metabolism is associated with CNS pathology. For instance, deletion of SR leads to adaptive NMDAR supersensitivity. Genetically modified SR −/− mice exhibit reduced dendritic spines and neuronal branching, resembling human schizophrenia. At the molecular level, BDNF/Akt/mTOR signaling is also diminished in SR −/− mice [16].
The characteristics of SR −/− mice illustrate that NMDAR hypofunction can mirror various deficits observed in schizophrenia [17,18]. Although serine racemase is an intriguing candidate gene, no clear conclusion has been reached regarding its variants being linked to schizophrenia. While a definitive conclusion has not been established, numerous studies have indicated reductions in SR levels in schizophrenic patients. Recent research has revealed substantial amounts of serine racemase and d-serine in neurons in vivo, in brain slices, and other neuronal cultures, explaining the conversion of l-serine to d-serine in these neurons [19].
In light of our findings: The glutamate hypothesis in schizophrenia
Our recent discovery reveals that NMDAR antagonists enhance the activity of the 20S proteasome. While the 26S proteasome typically degrades ubiquitinated proteins, the 20S proteasome can directly degrade misfolded proteins, oxidatively damaged proteins, and intrinsically disordered protein regions without requiring ubiquitination [20]. Approximately 41% of the eukaryotic proteome is predicted to contain Intrinsically Disordered Regions (IDRs), suggesting a significantly large potential substrate pool for the 20S proteasome.
Additionally, the 20S proteasome plays a critical role in the selective recognition and degradation of oxidized proteins, handling roughly 90% of all intracellular oxidation-damaged proteins. Although it is generally believed that the 20S proteasome operates in an ubiquitin-independent manner, our findings indicate that it can also degrade ubiquitinated proteins, as well as intrinsically disordered, oxidatively damaged, and misfolded proteins, potentially with greater efficiency under certain conditions. This suggests that the substrate pool for the 20S proteasome may be much larger than previously thought.
These substrates are particularly significant because they include proteins that accumulate in neurodegenerative disorders, such as amyloid beta, tau, TDP-43, alpha-synuclein, Prion Protein (PrP), and polyglutamine repeats [20]. Our observations show that NMDAR antagonists, including ketamine and memantine, can reduce levels of proteins such as p21, p53, phosphorylated tau (pTau), and amyloid in as little as 10 minutes. Supported by extensive studies, we conclude that this effect is due to an increase in 20S proteasome activity, independent of the ubiquitin-dependent pathway (26S proteasome system). Furthermore, we observed that the effects of enhanced proteasome activity reach a maximum after approximately 2 hours.
Neurodegenerative diseases are characterized by a range of complex causes, with deficiencies in proteasome systems being a significant contributing factor to their development [21,22]. Proteasome function tends to decline with age, leading to the abnormal accumulation of intrinsically disordered proteins (IDPs) and misfolded proteins—both linked to the progression of neurodegenerative diseases. For nearly 50 years, NMDAR antagonists, such as memantine, have been effectively used in treating protein misfolding disorders, including Alzheimer’s disease, vascular dementia, and Parkinson’s disease [23]. Our findings contribute to a better understanding of how NMDAR antagonists may exert their therapeutic effects in treating neurodegenerative diseases.
Additionally, recent findings indicate that a single intravenous infusion of the NMDAR antagonist ketamine can rapidly alleviate symptoms of major depression, with effects lasting up to two weeks. While several hypotheses have been proposed to explain this phenomenon, the exact mechanisms underlying these effects have not been clarified in detail until our study [24].
Synaptic modifications and changes in synapse-associated proteins are implicated in the pathogenesis of various brain diseases. Prior research has shown that these alterations are relevant to mental disorders such as schizophrenia and bipolar disorder, as well as neurodegenerative conditions like Alzheimer’s Disease (AD), Parkinson’s Disease (PD), and Huntington’s Disease (HD) [25-26].
In our study, ketamine administration significantly altered the synaptic protein profiles in mouse brains, resulting in the downregulation of 372 known synaptic proteins and the upregulation of 145. Enrichment analysis revealed several factors associated with cognitive, emotional, and motor phenotypes, as well as various neurological and psychiatric disorders. Notably, the enrichment analysis of the synaptic proteins altered following ketamine infusion highlighted several factors linked to schizophrenia, bipolar disorder, Alzheimer’s disease and Parkinson’s disease, consistent with our expectations.
Furthermore, our data may elucidate how a single intravenous infusion of the NMDAR antagonist ketamine can rapidly alleviate symptoms of major depression, with effects lasting up to two weeks. This is supported by the significant changes in synaptic protein homeostasis in the brain induced by ketamine, which contribute to its therapeutic effects. Additionally, alterations in various pathways related to synaptic plasticity and potentiation affected by ketamine injection may help explain the duration of the treatment effects.
Ketamine effects on schizophrenia-related factors: Evidence from our study proteins
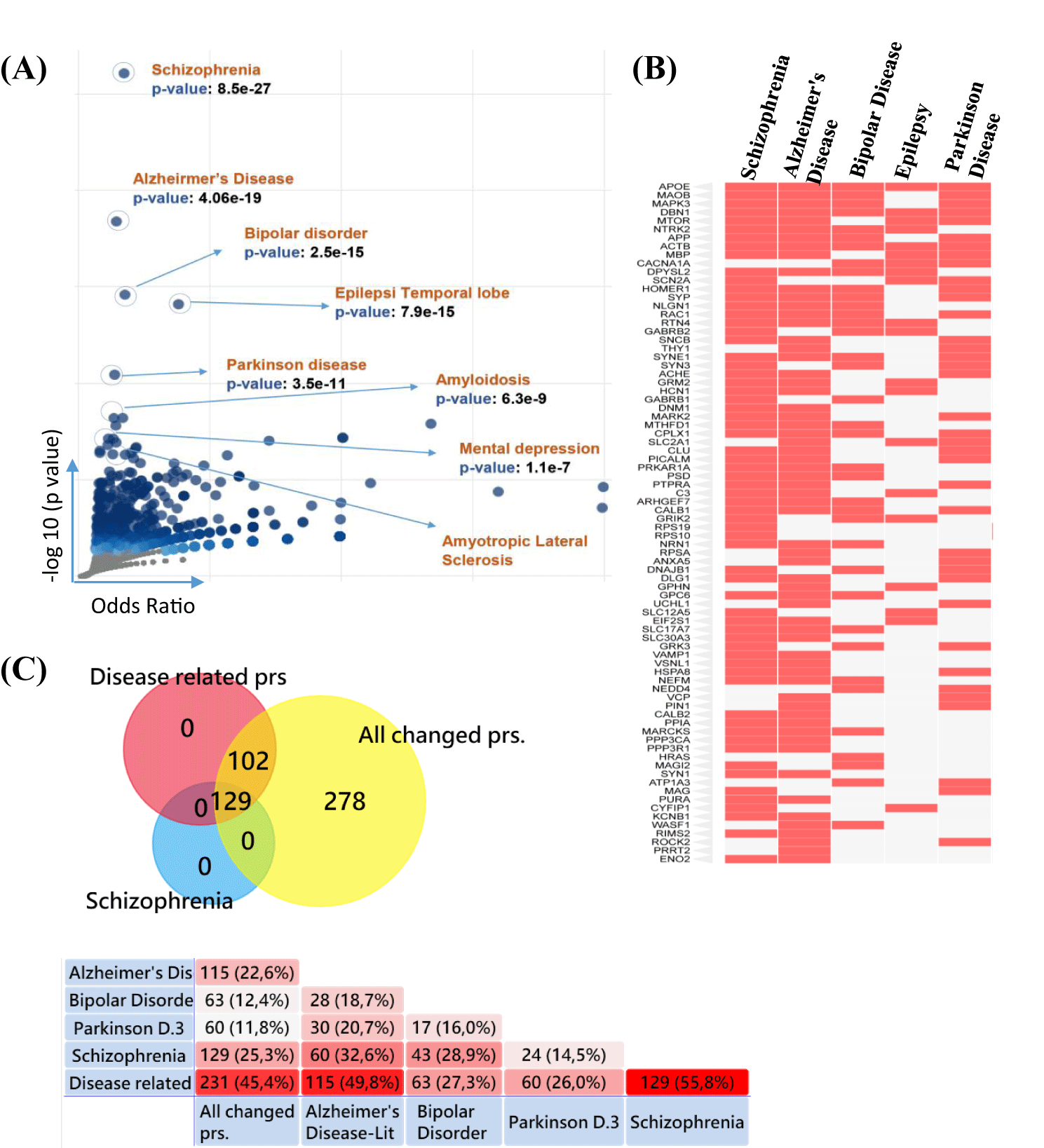
Ketamine administration significantly alters synaptic protein profiles, potentially explaining the effects of NMDAR antagonists on neurodegenerative diseases and major depression. A pivotal analysis revealed an unexpected finding: a volcano plot generated from 526 proteins using disease-related data from the DisGeNET database within the Enrichr framework identified schizophrenia as the disease most closely associated with the observed protein alterations, demonstrating the strongest statistical significance (Figures 1A,B). This finding was further supported by Venn diagram analyses, which indicated that 55.8% of the disease-associated proteins (231) were linked to schizophrenia (Figure 1C). The disease-associated proteins obtained from the DisGeNET library included those previously described as related to schizophrenia, along with proteins linked to Alzheimer’s disease, bipolar disorder, and Parkinson’s disease.
Synaptic Changes
As indicated above, alterations in glutamatergic neurotransmission at the synapse have been implicated in the etiology of schizophrenia and serve as a main hypothesis. Additionally, studies suggest that depression and Alzheimer’s disease are also related to changes in glutamatergic neurotransmission at the synapse; however, Parkinson's disease (PD) is often excluded from this association [27-30].
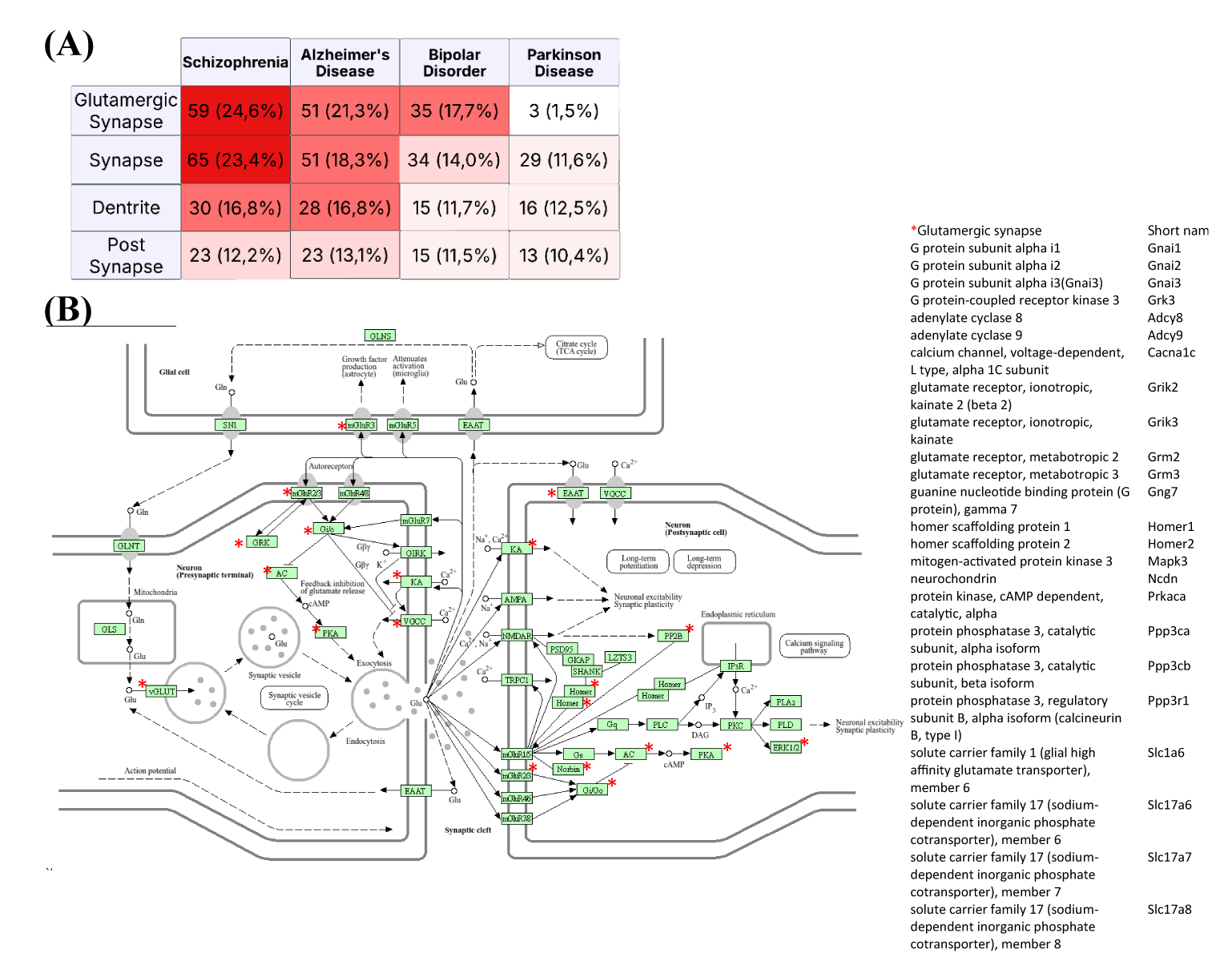
When we analyzed which components of the synapses were most affected by ketamine infusion, enrichment analyses of the synaptic proteins revealed that changes related to glutamatergic synapses exhibited the highest protein alterations (Figure 2A). Importantly, the altered glutamatergic synapse-related proteins were most closely associated with the pathology of schizophrenia compared to those related to Alzheimer’s disease and bipolar disorder. Furthermore, the pathology of Parkinson's disease does not appear to be associated with glutamatergic synapses, as indicated in previous studies. Additionally, alterations in postsynaptic or dendritic site proteins were the most significant after those observed in glutamatergic synapses, correlating with changes noted in schizophrenia (Figure 2A). In figure 2B, KEGG pathway illustrates where the altered proteins are located within the glutamatergic transmission pathways [31].
Functional grouping of the changed proteins
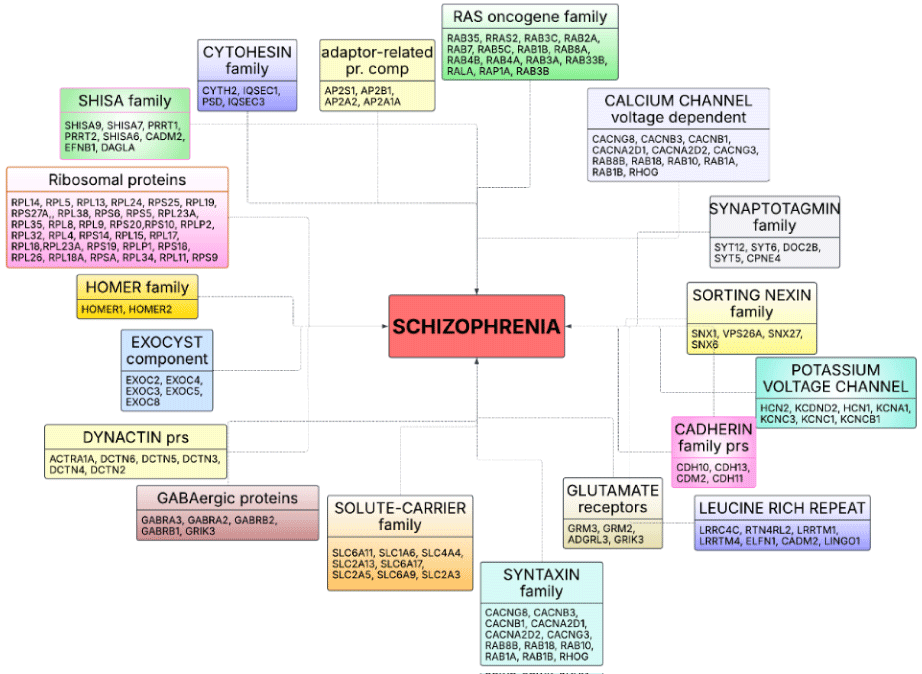
When we analyzed the altered proteins according to their functions, they were grouped and sorted based on their P values as follows: ribosomal proteins [32], Shisa family proteins [33], cytohesin and IQ motif and Sec7 domain-related proteins [34,35], exocyst complex component proteins [36], adaptor-related protein complexes [37], RAS oncogene family proteins [38], voltage-dependent calcium channel proteins [39], synaptotagmin family proteins, sorting nexin proteins [40,41], potassium voltage-gated channel proteins [42], cadherin family proteins [43], leucine-rich repeat proteins [43], glutamate receptors [44-46], solute-carrier family proteins [47], syntaxin family proteins [48], GABAergic proteins [49], HOMER proteins [50], and others (Figure 3). These proteins play significant roles and may contribute to the pathogenesis or etiology of schizophrenia, as previously described in various studies.
Biological processes related to changed proteins
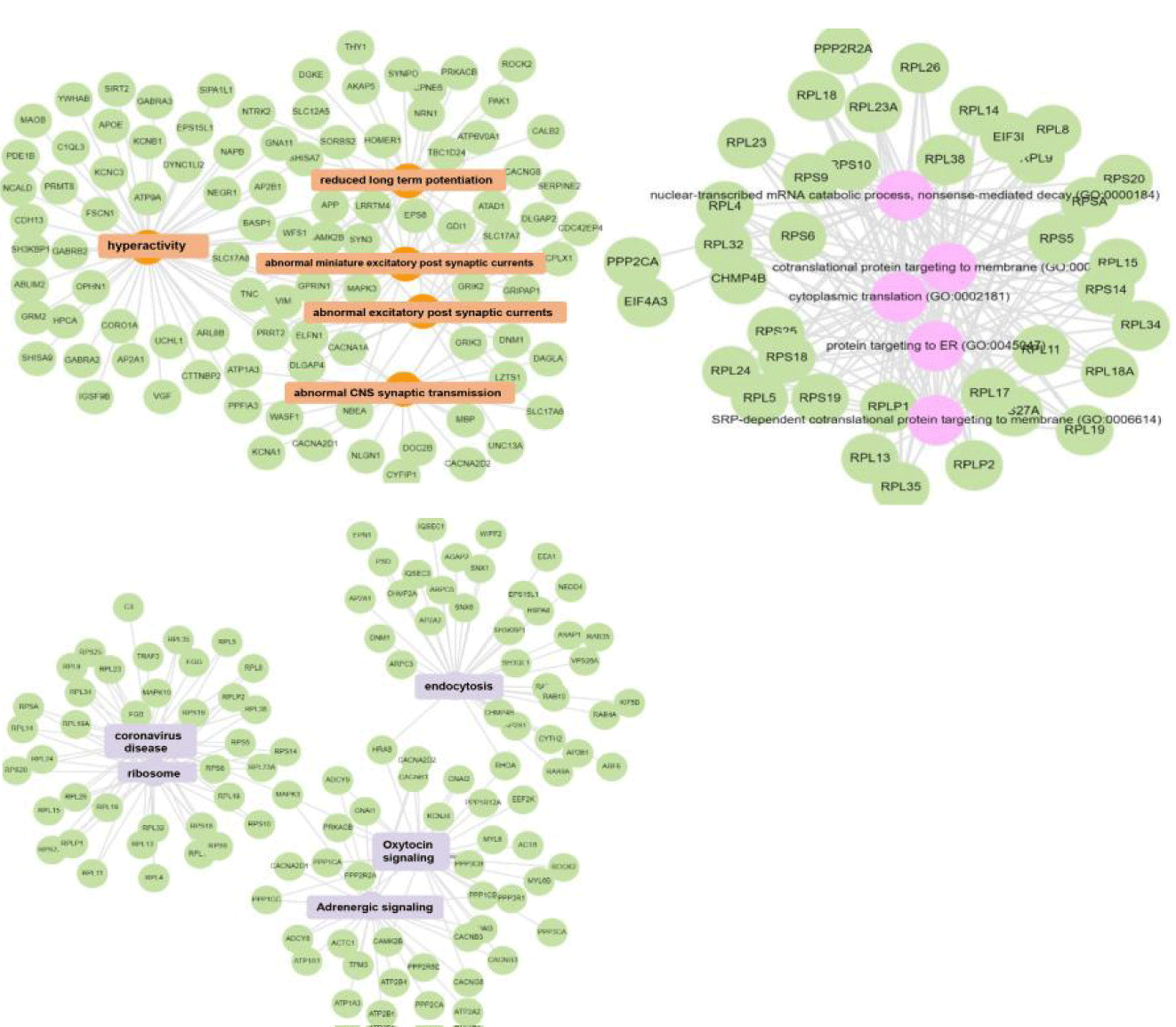
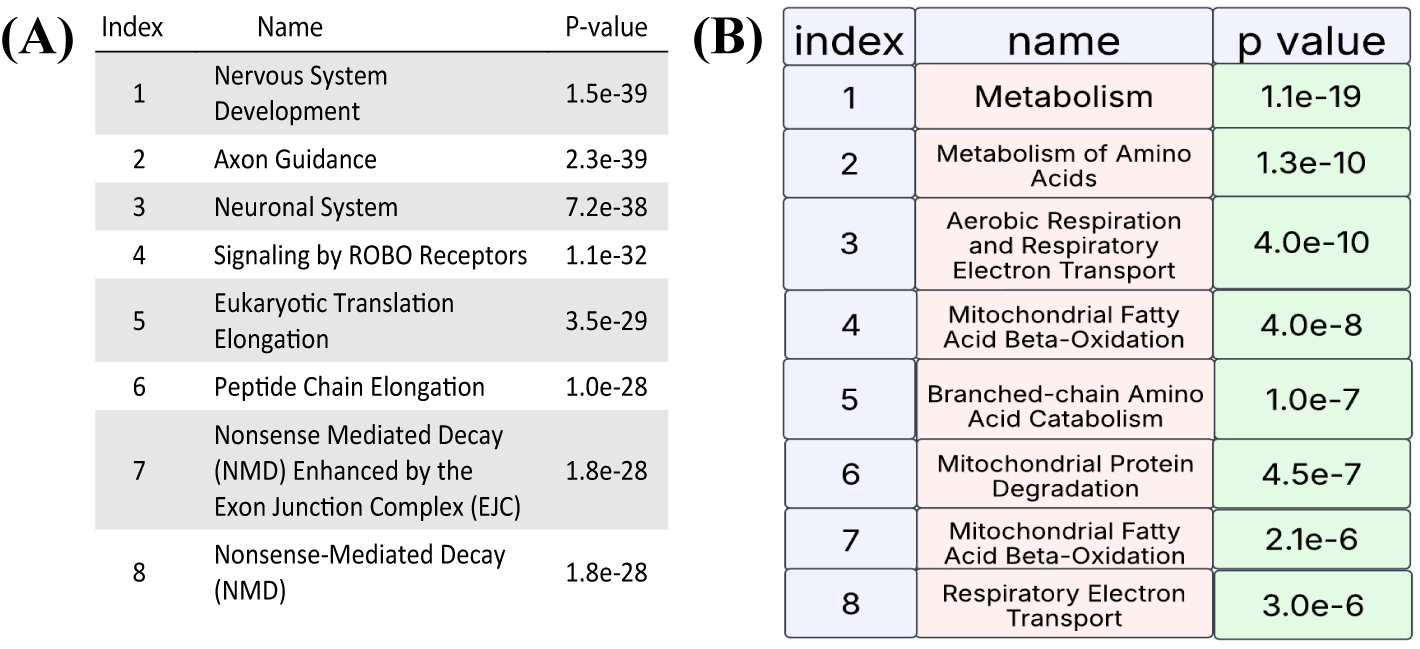
Complementary analysis using Enrichr-KG, which includes the Mammalian Phenotype (MP), Gene Ontology (GO) Biological Processes, and KEGG Pathway Browser categories, indicated significant enrichment in areas such as hyperactivity, abnormal CNS synaptic transmission, altered excitatory postsynaptic currents, attenuated long-term potentiation, nonsense-mediated mRNA decay, cytoplasmic translation, protein targeting to the membrane, ribosomal function, the oxytocin signaling pathway, and endocytosis [20] (Figure 4). Importantly, the terms identified across these various categories and through different analytical platforms have all been associated with the pathogenesis of schizophrenia in prior research, as well as with depression.
Pathways affected by the changed proteins
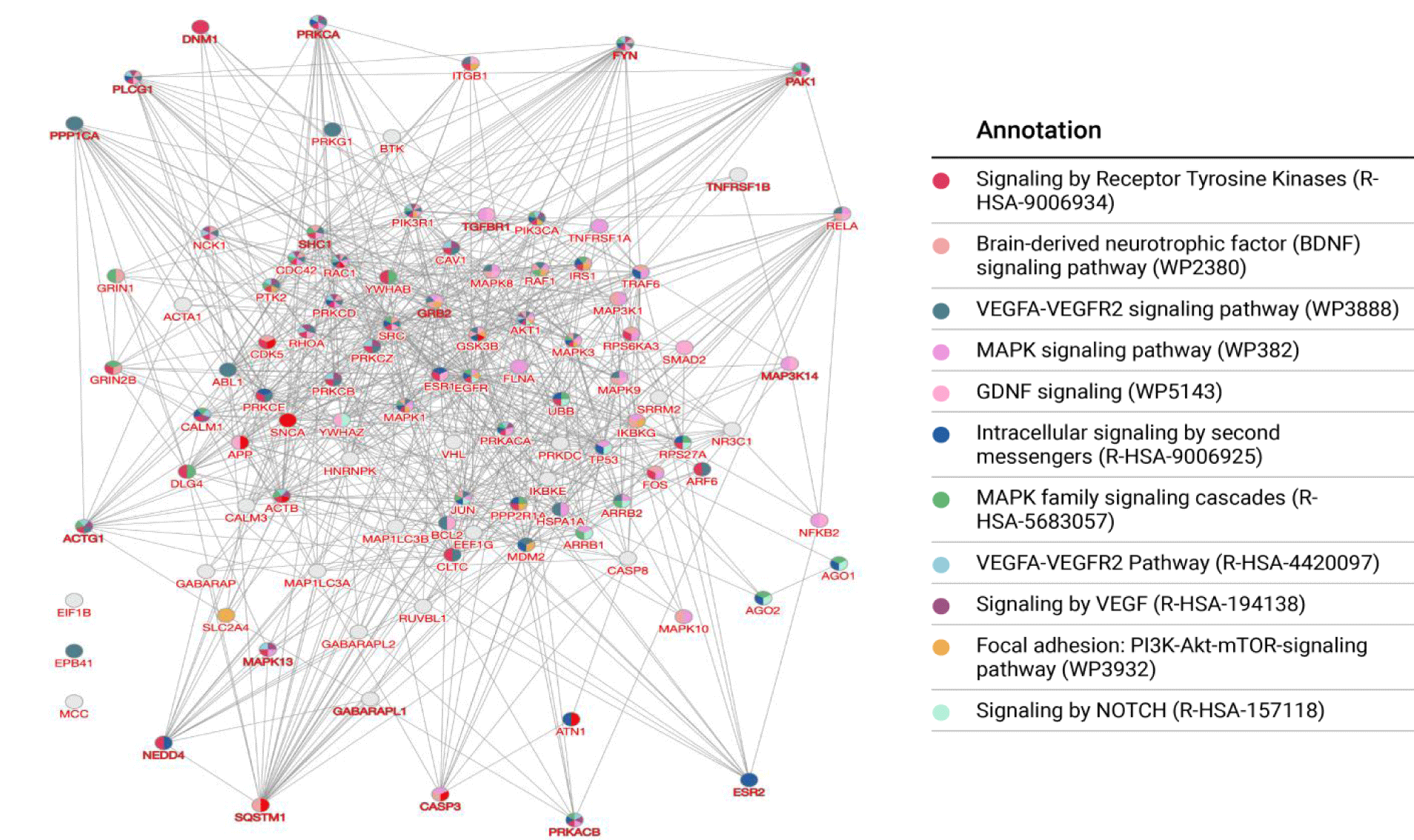
Specific pathways, such as Tyrosine Kinases (TrkB) and Brain-Derived Neurotrophic Factor (BDNF) [51], have been extensively studied in the context of schizophrenia pathogenesis, with numerous studies highlighting their roles in the disease. Additionally, signaling cascades related to the MAPK family [52], WNT signaling [53], VEGFA-VEGFR2 signaling [54], GDNF signaling [55], Notch [56] and the PI3K-Akt-mTOR [57] pathways have also been strongly associated with schizophrenia. Enrichment analysis of the synaptic proteins altered following ketamine infusion highlighted several of these pathways, with those mentioned above ranking at the top according to their P values (Figure 5).
Location of changed proteins
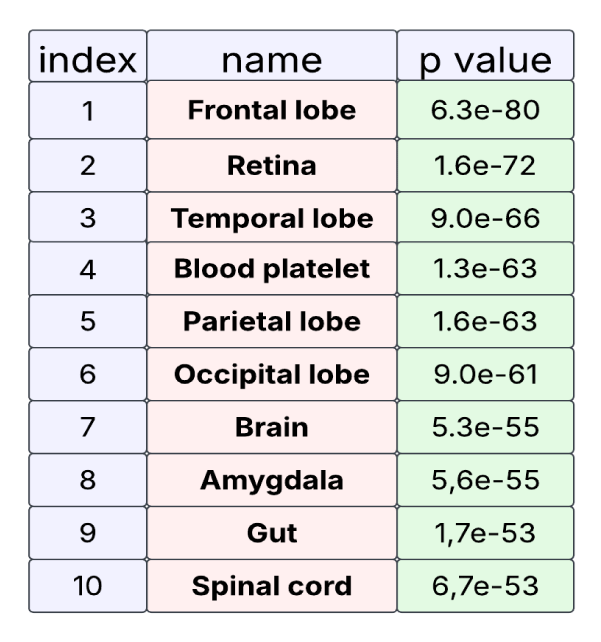
Our results from tissue enrichment analysis of the changed proteins revealed that the frontal lobes ranked highest, with a P value of 6.304e-80. This finding supports the long-standing view of schizophrenia as a syndrome of "hypofrontality," based on studies showing abnormalities in the structure and function of the frontal lobe [58]. Neuropsychological studies have demonstrated specific deficits in tasks such as working memory and executive control, which are mediated by the frontal lobes. Similarly, epilepsy, which also ranked highly in the analysis, is related to the frontal lobe [59].
Surprisingly, the retina ranked second highest after the frontal lobe (Figure 6). Studies suggest that changes in the retina are related to the pathology of schizophrenia [60]. Additionally, patients with schizophrenia have been shown to have measurable differences in the neural and vascular integrity of the retina. These differences in retinal vasculature are often attributed to the higher prevalence of diabetes and hypertension in these patients. The role of retinal features as adjunct outcomes in schizophrenia-especially in light of our findings linking changed proteins to the retina-requires further investigation.
Interestingly, blood platelets also ranked highly with a very strong P value. Studies have shown a relationship between blood platelets and schizophrenia [61]. For example, an elevated Platelet Count (PLTc) is associated with first-episode schizophrenia and adverse outcomes in individuals with precursory psychosis. Platelet parameters, specifically PLTc, are influenced by antipsychotic treatment and could potentially increase the risk of venous thromboembolism in patients with schizophrenia. Elevated PLTc levels and associated factors may impede symptom improvement by promoting inflammation. Given the ease of measuring PLTc and its clinical relevance, it warrants increased attention from psychiatrists, and our results support this relationship.
Aging
Recent studies have shown that schizophrenia is associated with an increased risk of developing multiple age-related diseases, including metabolic, respiratory, and cardiovascular diseases, as well as Alzheimer’s disease and related dementias [62]. This association leads to the hypothesis that schizophrenia may be accompanied by accelerated biological aging.
Research indicates that individuals with schizophrenia and older adults exhibit strikingly similar changes in gene activity within two types of cells. These findings suggest a shared biological basis for cognitive impairment in both schizophrenia and aging, pointing to new treatment strategies. One key strategy for alleviating aging-related conditions is addressing macromolecular dysfunction associated with protein aggregations, such as preventing the decline of proteasome activity, as indicated by our findings.
Huntington’s Disease (HD), an autosomal dominant neurodegenerative disorder, is characterized by the gradual onset and progression of motor, cognitive, and psychiatric symptoms that are directly related to aging conditions [63]. In fact, the symptoms of HD are so similar to those of schizophrenia that it is often misdiagnosed as such.
Our results support this connection; by searching through 178,975 human gene sets studied to date, EnrichR-RummaGEO identified the most relevant changes, with a P value of 2.32e-141, focusing on the study titled "RNA-Targeting CRISPR/Cas13d System, which alleviates disease-related phenotypes in preclinical models of Huntington’s disease." Notably, nearly 45% of the altered proteins identified in our results correspond to changes related to the study on HD.
Furthermore, when we examined the overall protein changes, in addition to the synaptic proteins in the brain after 2 hours of ketamine treatment, the highlighted changes primarily involved mitochondrial and metabolic processes, reflecting the main mechanisms underlying aging conditions (Figure 7).
Serine racemase
d-Serine is converted from l-serine by Serine Racemase (SR). SR deletion leads to adaptive NMDAR supersensitivity, as observed in genetically modified SR −/− mice [19,64]. Behaviorally, these mice show hyperactivity, impaired spatial memory, and elevated anxiety. Adults with schizophrenia typically have reduced serum d-serine levels, even though total serine levels are elevated. d-Serine has been effective as both monotherapy and adjunctive therapy in alleviating negative symptoms in treatment-refractory schizophrenia [19,65].
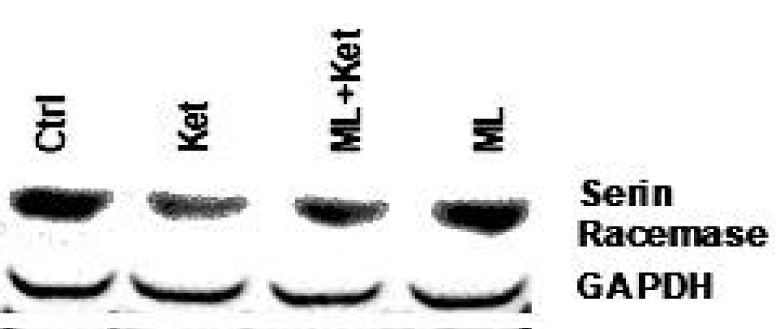
In our experiments using Western blot analysis, we demonstrated that, in addition to pTau and beta-amyloid, serine racemase levels also decreased dramatically when cells were exposed to NMDAR antagonists like ketamine or memantine in SH-SY5Y, T98G, and HepG2 cells. This decrease was prevented by proteasome inhibitors such as MG132 (Figure 8).
Additionally, we investigated how ion channel-specific binding sites modulate the effects of NMDAR antagonists on proteasome activity (Table 1). Ionotropic glutamate receptors, including NMDA, AMPA, and kainate receptors, are crucial for neural transmission. NMDARs are distinct due to their high selectivity for Ca2+ and specific ligand binding sites for glutamate, glycine, polyamines, channel blockers (e.g., ketamine, memantine, MK801), and allosteric modulators [20]. Our examination of how different NMDAR sites affect proteasome activity and p21 protein levels revealed that drugs binding to the channel region, glycine, or specific allosteric sites increased proteasomal chymotrypsin-like activity and reduced p21 levels (Table 1). This effect was not observed with polyamines, AMPA, or kainate receptors (Table 1). Interestingly, agonists on the glutamate receptor such as N-Methyl-D-Aspartic acid (NMDA), D-Aspartic acid, and DL-Glutamic acid monohydrate did not show a decrease in proteasome activity (Table 1).
| Table 1: Glutamate Receptors, used antagonist and agonist chemicals-binding sites and their effects on proteasome activity and p21 protein level. | |||
| Name | Receptor- binding site-function | Effect on chymotrypsin | Effect on p21 level |
| (+)-MK 801 maleate | NMDA - ion channel- antagonist | I | D |
| Memantine hydrochloride | NMDA- ion channel- antagonist | I | D |
| Ketamine hydrochloride | NMDA - ion channel-antagonist | I | D |
| (+/-)-1-(1,2-Diphenylethyl)piperidine maleate | NMDA- ion channel- antagonist | I | D |
| TCS 46b | NMDA- NR1A/NR2B selective- antagonist | I | D |
| Eliprodil | NMDA-NR2B selective-antagonist | I | D |
| D(-)-2-Amino-4-phosphonobutanoic acid (D-AP4) | NMDA- excitatory amino acid- antagonist | I | D |
| N20C hydrochloride | NMDA -channel- antagonist | I | D |
| O-Phospho-L-serine | NMDA -agonist (group III mGluR receptors (mGluR4, mGluR6, mGluR7, and mGluR8)-antagonist (weak antagonist for mGluR1 and a potent antagonist for mGluR2) | I | D |
| Felbamate | NMDA- glycine- antagonist | I | D |
| Gavestinel | NMDA- glycine- antagonist | I | D |
| L-701,324 | NMDA- glycine- antagonist | I | D |
| Mg++ | NMDA- Mg- antagonist | I-D | D-I |
| Arcaine sulfate | NMDA- polyamine- antagonist | NC | NC |
| Ifenprodil hemitartrate | NMDA- polyamine- antagonist | NC | NC |
| -(R)-3,4-DCPG | AMPA---- antagonist | NC | NC |
| gammaDGG | AMPA---- antagonist | NC | NC |
| Kynurenic acid | AMPA/Kainate---- antagonist | NC | NC |
| UBP 301 | Kainate----antagonist | NC | NC |
| ACPT-II | Metabotropic----antagonist | NC | NC |
| N-Methyl-D-Aspartic acid (NMDA) | NMDA- ionotropic- agonist | ND | I |
| D-Aspartic acid | NMDA-endogenous- agonist | NC | NC |
| DL-Glutamic acid monohydrate | NMDA-Glutamatergic---agonist | I | D |
| I, D, NC, and ND represent increase in chymotrypsin activity or p21 level, decrease in chymotrypsin activity or p21, no detectable chance, not determined, respectively | |||
Serine exists in two forms: l-serine and d-serine, each with similar chemical and physical properties. d-Serine binds more strongly than l-serine at the NMDAR subunit. O-Phospho-L-serine, the immediate precursor to L-serine in the serine synthesis pathway and a precursor of d-serine, acts as an agonist at the group III mGluR receptors (mGluR4, mGluR6, mGluR7, and mGluR8). It also serves as a weak antagonist for mGluR1 and a potent antagonist for mGluR2 [66]. We tested O-Phospho-L-serine for its effects on proteasome activity, and interestingly, it enhanced proteasome activity and increased the degradation of IDR-containing proteins.
Although we did not directly test d-serine activity, the results with O-Phospho-L-serine suggest that a decrease in serine racemase may lead to reduced levels of d-serine. This reduction is significant because d-serine acts as a potent neurotransmitter on glutamate receptors, potentially activating the proteasome and maintaining protein homeostasis in synapses. Without the effects of d-serine, there may be increased protein misfolding and aggregation, resulting in dysfunctional pathways and symptoms associated with schizophrenia.
Although serine racemase is an intriguing candidate gene, no clear conclusion has been reached regarding its variants being linked to schizophrenia [19,67].
Implications of Our Findings
- Our results clearly demonstrate that NMDAR antagonists cause dramatic changes in proteins, with the altered proteins showing the strongest enrichment for schizophrenia compared to other neurodegenerative and mental diseases, as indicated by the P value.
- The changes in proteins predominantly occur in the glutamatergic synapse, postsynaptic sites, and dendrites.
- These findings strongly support the glutamatergic hypothesis, which suggests that dysfunction in glutamatergic neurotransmission may play a role in the etiology of schizophrenia.
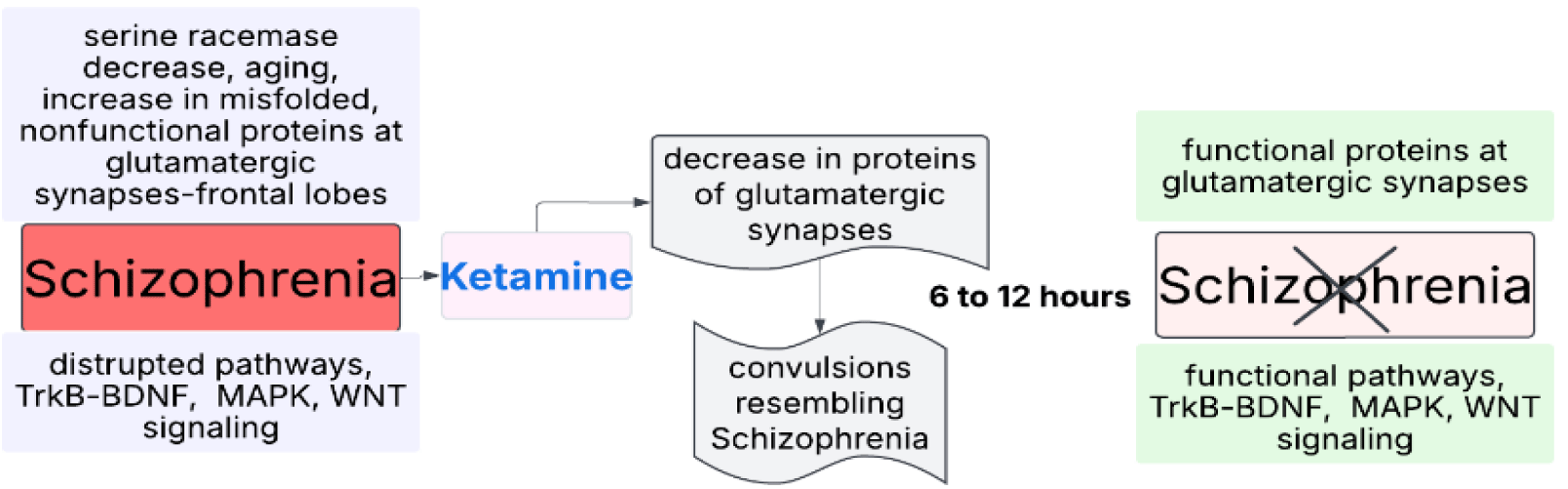
- NMDAR antagonists increase 20S proteasome activity starting from 0 to 5 minutes, reaching a maximum at 2 hours and lasting 4 to 6 hours. During this period, protein degradation is evident on Western blots. Results show that all decreased proteins return to normal levels within 6 to 12 hours, and at most within 24 hours. Therefore, protein-related studies not conducted within this period cannot provide definitive conclusions regarding the glutamatergic hypothesis related to NMDAR antagonists. Especially, mRNA-based studies may yield more complicated and confusing results. These conclusions may explain the inconclusive results regarding the glutamatergic hypothesis of schizophrenia obtained from studies conducted so far.
- The enrichment of changed proteins in functional groupings highlighted protein groups that, without exception, have been previously shown to play roles in the pathogenesis of schizophrenia. Notably, proteins from the HOMER, SHISA family, RAS oncogene family, and glutamate receptor family are the most studied and provide strong evidence for their involvement in schizophrenia pathogenesis.
- Pathways and biological processes related to the changed proteins were almost all previously shown to be involved in the pathogenesis of schizophrenia. In particular, Tyrosine Kinases (TrkB) and Brain-Derived Neurotrophic Factor (BDNF) have been considered main pathways in the pathogenesis of schizophrenia, with numerous studies indicating their roles in the disease.
- Schizophrenia has long been considered a syndrome of 'hypofrontality,' based on studies showing abnormalities in the structure and function of the frontal lobe. Our results strongly support this view, as tissue enrichment analysis of the changed proteins revealed that the frontal lobes ranked highest, with a P value of 6.304e-80. Additionally, the study's findings regarding the retina and blood platelets suggest that these two CNS-external fields may provide valuable information related to the pathogenesis, prognosis, and drug response in schizophrenia.
- Aging is a significant factor in the etiology of schizophrenia, similar to its role in other complex diseases like cancer. Misfolded and aggregated proteins due to oxidative stress are key pathological findings associated with aging and are common in various disorders, including schizophrenia. Our results indicate that changes in protein profiles, particularly those linked to Huntington’s disease, suggest a connection between aging and schizophrenia. Moreover, the enrichment of whole brain protein changes post-ketamine infusion highlights the involvement of mitochondria and other organelles, further supporting the relationship between aging and schizophrenia.
- According to our findings, d-serine likely plays a crucial role as a neurotransmitter at glutamate receptors, activating the proteasome and maintaining protein homeostasis in the synapse. A decrease in d-serine may lead to increased protein misfolding and aggregation, resulting in dysfunction in key pathways and the presentation of schizophrenia symptoms. Additionally, we found that Serine Racemase (SR) levels decrease within 2 hours in the cells studied, suggesting that changes in protein levels must occur before or simultaneously with the decline in d-serine. Therefore, the factors described above may not be directly affected by the decrease in d-serine due to reduced SR activity, indicating that these changes occur independently of SR.
The Therapeutic Potential of Ketamine and Other NMDAR Antagonists in Schizophrenia
Understanding the therapeutic potential of NMDAR antagonists, particularly ketamine, in schizophrenia involves examining their role in protein homeostasis, as demonstrated by our findings and their implications. NMDAR antagonists facilitate the degradation of dysfunctional, oxidized, unfolded, and misfolded proteins into amino acids, which are then recycled to synthesize new proteins that function properly within cellular pathways. Ketamine's effect in treating major depression serves as a notable example. Importantly, ketamine-induced changes in synaptic proteins are strongly correlated with major depression. However, as indicated above, the majority of altered proteins, related pathways, and their locations are more closely linked to the pathogenesis of schizophrenia than to depression. We propose that a similar mechanism occurs in schizophrenia-where defective proteins are degraded and replaced with functional ones within 6 to 12 hours after ketamine infusion-thereby restoring all proteins in the pathways to normal function (Figure 9).
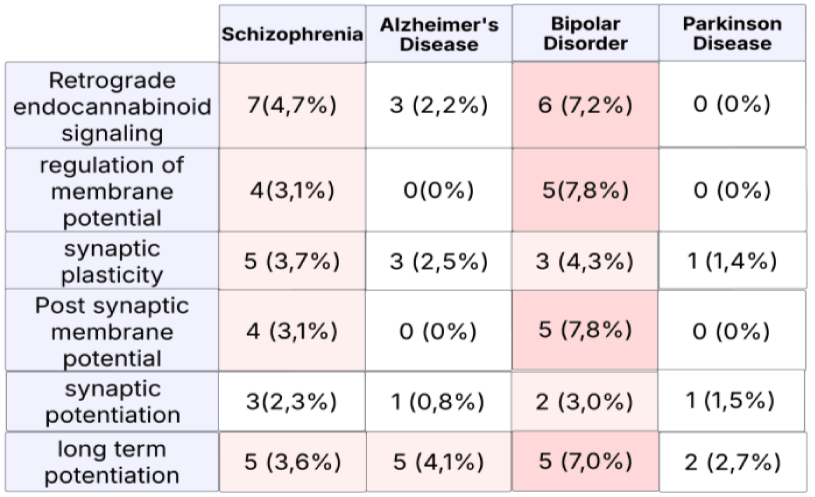
Our data elucidate how a single intravenous infusion of the NMDAR antagonist ketamine can rapidly alleviate symptoms of major depression. Additionally, the data show that alterations in various pathways related to synaptic plasticity and potentiation, affected by ketamine injection, may help explain the effects lasting up to two weeks in major depression (Figure 10). As seen in figure 10, changes in plasticity and potentiation related to bipolar disorders also appear in schizophrenia-related proteins but are not significantly affected in Alzheimer's and Parkinson's diseases. Notably, the roles of Retrograde Endocannabinoid Signaling in schizophrenia play a significant part in its pathogenesis and have been scrutinized in different studies [68]. Therefore, it would not be surprising to see the alleviation of schizophrenia symptoms extend beyond the initial protein changes after ketamine administration.
In addition to ketamine, other NMDAR antagonists like memantine may offer therapeutic benefits for schizophrenia. Memantine's advantage lies in its ability to deliver steady effects without inducing convulsion-like symptoms during initial exposure, as seen with ketamine. This approach might eliminate the need to supplement with d-serine if it acts as a proteasome activator, offering a potentially more streamlined treatment strategy.
Conclusion
In conclusion, our research highlights the therapeutic potential of NMDAR antagonists, particularly ketamine, in treating mental disorders such as schizophrenia. By enhancing proteasome activity, these antagonists facilitate the degradation of dysfunctional proteins in neuronal cells, thus correcting protein homeostasis. This process may not only alleviate disease symptoms but also underscores the broader implications for understanding the pathogenesis of schizophrenia. The rapid restoration of protein function following ketamine infusion suggests a promising mechanism that could extend to other neurodegenerative and psychiatric conditions.
References
- Chang HJ, Lane HY, Tsai GE. NMDA pathology and treatment of schizophrenia. Curr Pharm Des. 2014;20(32):5118-26. doi: 10.2174/1381612819666140110121908. PMID: 24410561.
- Lai WS, Chang CY, Wong WR, Pei JC, Chen YS, Hung WL. Assessing schizophrenia-relevant cognitive and social deficits in mice: a selection of mouse behavioral tasks and potential therapeutic compounds. Curr Pharm Des. 2014;20(32):5139-50. doi: 10.2174/1381612819666140110122750. PMID: 24410559.
- Lin CH, Huang YJ, Lin CJ, Lane HY, Tsai GE. NMDA neurotransmission dysfunction in mild cognitive impairment and Alzheimer's disease. Curr Pharm Des. 2014;20(32):5169-79. doi: 10.2174/1381612819666140110115603. PMID: 24410566.
- Panizzutti R, Scoriels L, Avellar M. The co-agonist site of NMDA-glutamate receptors: a novel therapeutic target for age-related cognitive decline. Curr Pharm Des. 2014;20(32):5160-8. doi: 10.2174/1381612819666140110121139. PMID: 24410562.
- Schmitt A, Falkai P, Papiol S. Neurodevelopmental disturbances in schizophrenia: evidence from genetic and environmental factors. J Neural Transm (Vienna). 2023 Mar;130(3):195-205. doi: 10.1007/s00702-022-02567-5. Epub 2022 Nov 12. PMID: 36370183; PMCID: PMC9660136.
- Schmitt A, Malchow B, Hasan A, Falkai P. The impact of environmental factors in severe psychiatric disorders. Front Neurosci. 2014 Feb 11;8:19. doi: 10.3389/fnins.2014.00019. PMID: 24574956; PMCID: PMC3920481.
- Gilmour G, Dix S, Fellini L, Gastambide F, Plath N, Steckler T, Talpos J, Tricklebank M. NMDA receptors, cognition and schizophrenia--testing the validity of the NMDA receptor hypofunction hypothesis. Neuropharmacology. 2012 Mar;62(3):1401-12. doi: 10.1016/j.neuropharm.2011.03.015. Epub 2011 Mar 21. PMID: 21420987.
- Reiner A, Levitz J. Glutamatergic Signaling in the Central Nervous System: Ionotropic and Metabotropic Receptors in Concert. Neuron. 2018 Jun 27;98(6):1080-1098. doi: 10.1016/j.neuron.2018.05.018. PMID: 29953871; PMCID: PMC6484838.
- Frohlich J, Van Horn JD. Reviewing the ketamine model for schizophrenia. J Psychopharmacol. 2014 Apr;28(4):287-302. doi: 10.1177/0269881113512909. Epub 2013 Nov 20. PMID: 24257811; PMCID: PMC4133098.
- Beck K, Hindley G, Borgan F, Ginestet C, McCutcheon R, Brugger S, Driesen N, Ranganathan M, D'Souza DC, Taylor M, Krystal JH, Howes OD. Association of Ketamine With Psychiatric Symptoms and Implications for Its Therapeutic Use and for Understanding Schizophrenia: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020 May 1;3(5):e204693. doi: 10.1001/jamanetworkopen.2020.4693. PMID: 32437573; PMCID: PMC7243091.
- Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012 Jan;37(1):4-15. doi: 10.1038/npp.2011.181. Epub 2011 Sep 28. PMID: 21956446; PMCID: PMC3238069.
- Zhang T, Liu C, Zhong N, Wang Y, Huang Y, Zhang X. Advances in the Treatment of Cognitive Impairment in Schizophrenia: Targeting NMDA Receptor Pathways. Int J Mol Sci. 2024 Oct 3;25(19):10668. doi: 10.3390/ijms251910668. PMID: 39408997; PMCID: PMC11477438.
- Hu W, MacDonald ML, Elswick DE, Sweet RA. The glutamate hypothesis of schizophrenia: evidence from human brain tissue studies. Ann N Y Acad Sci. 2015 Mar;1338(1):38-57. doi: 10.1111/nyas.12547. Epub 2014 Oct 14. PMID: 25315318; PMCID: PMC4363164.
- Balu DT, Basu AC, Corradi JP, Cacace AM, Coyle JT. The NMDA receptor co-agonists, D-serine and glycine, regulate neuronal dendritic architecture in the somatosensory cortex. Neurobiol Dis. 2012 Feb;45(2):671-82. doi: 10.1016/j.nbd.2011.10.006. Epub 2011 Oct 17. PMID: 22024716; PMCID: PMC3259183.
- Forrest MP, Parnell E, Penzes P. Dendritic structural plasticity and neuropsychiatric disease. Nat Rev Neurosci. 2018 Mar 16;19(4):215-234. doi: 10.1038/nrn.2018.16. PMID: 29545546; PMCID: PMC6442683.
- Puhl MD, Mintzopoulos D, Jensen JE, Gillis TE, Konopaske GT, Kaufman MJ, Coyle JT. In vivo magnetic resonance studies reveal neuroanatomical and neurochemical abnormalities in the serine racemase knockout mouse model of schizophrenia. Neurobiol Dis. 2015 Jan;73:269-74. doi: 10.1016/j.nbd.2014.10.009. Epub 2014 Oct 23. PMID: 25461193; PMCID: PMC4408217.
- Balu DT. The NMDA Receptor and Schizophrenia: From Pathophysiology to Treatment. Adv Pharmacol. 2016;76:351-82. doi: 10.1016/bs.apha.2016.01.006. Epub 2016 Mar 4. PMID: 27288082; PMCID: PMC5518924.
- Okubo R, Okada M, Motomura E. Dysfunction of the NMDA Receptor in the Pathophysiology of Schizophrenia and/or the Pathomechanisms of Treatment-Resistant Schizophrenia. Biomolecules. 2024 Sep 6;14(9):1128. doi: 10.3390/biom14091128. PMID: 39334894; PMCID: PMC11430065.
- Boks MP, Rietkerk T, van de Beek MH, Sommer IE, de Koning TJ, Kahn RS. Reviewing the role of the genes G72 and DAAO in glutamate neurotransmission in schizophrenia. Eur Neuropsychopharmacol. 2007 Sep;17(9):567-72. doi: 10.1016/j.euroneuro.2006.12.003. Epub 2007 Jan 23. PMID: 17250995.
- Sahin F, Gunel A, Atasoy BT, Guler U, Salih B, Kuzu I, Taspinar M, Cinar O, Kahveci S. Enhancing proteasome activity by NMDAR antagonists explains their therapeutic effect in neurodegenerative and mental diseases. Sci Rep. 2025 Jan 13;15(1):1165. doi: 10.1038/s41598-024-84479-w. PMID: 39805913; PMCID: PMC11729902.
- Sanghai N, Tranmer GK. Biochemical and Molecular Pathways in Neurodegenerative Diseases: An Integrated View. Cells. 2023 Sep 20;12(18):2318. doi: 10.3390/cells12182318. PMID: 37759540; PMCID: PMC10527779.
- Ciurea AV, Mohan AG, Covache-Busuioc RA, Costin HP, Glavan LA, Corlatescu AD, Saceleanu VM. Unraveling Molecular and Genetic Insights into Neurodegenerative Diseases: Advances in Understanding Alzheimer's, Parkinson's, and Huntington's Diseases and Amyotrophic Lateral Sclerosis. Int J Mol Sci. 2023 Jun 28;24(13):10809. doi: 10.3390/ijms241310809. PMID: 37445986; PMCID: PMC10341997.
- Tampi RR, van Dyck CH. Memantine: efficacy and safety in mild-to-severe Alzheimer's disease. Neuropsychiatr Dis Treat. 2007 Apr;3(2):245-58. doi: 10.2147/nedt.2007.3.2.245. PMID: 19300557; PMCID: PMC2654628.
- Krystal JH, Kavalali ET, Monteggia LM. Ketamine and rapid antidepressant action: new treatments and novel synaptic signaling mechanisms. Neuropsychopharmacology. 2024 Jan;49(1):41-50. doi: 10.1038/s41386-023-01629-w. Epub 2023 Jul 24. PMID: 37488280; PMCID: PMC10700627.
- Moraes BJ, Coelho P, Fão L, Ferreira IL, Rego AC. Modified Glutamatergic Postsynapse in Neurodegenerative Disorders. Neuroscience. 2021 Feb 1;454:116-139. doi: 10.1016/j.neuroscience.2019.12.002. Epub 2019 Dec 27. PMID: 31887357.
- Ryan TJ, Grant SG. The origin and evolution of synapses. Nat Rev Neurosci. 2009 Oct;10(10):701-12. doi: 10.1038/nrn2717. Epub 2009 Sep 9. Erratum in: Nat Rev Neurosci. 2009 Nov;10(11):829. PMID: 19738623.
- Tu CH, MacDonald I, Chen YH. The Effects of Acupuncture on Glutamatergic Neurotransmission in Depression, Anxiety, Schizophrenia, and Alzheimer's Disease: A Review of the Literature. Front Psychiatry. 2019 Feb 12;10:14. doi: 10.3389/fpsyt.2019.00014. PMID: 30809158; PMCID: PMC6379324.
- Reiner A, Levitz J. Glutamatergic Signaling in the Central Nervous System: Ionotropic and Metabotropic Receptors in Concert. Neuron. 2018 Jun 27;98(6):1080-1098. doi: 10.1016/j.neuron.2018.05.018. PMID: 29953871; PMCID: PMC6484838.
- Chang PK, Verbich D, McKinney RA. AMPA receptors as drug targets in neurological disease--advantages, caveats, and future outlook. Eur J Neurosci. 2012 Jun;35(12):1908-16. doi: 10.1111/j.1460-9568.2012.08165.x. PMID: 22708602.
- Borsellino P, Krider RI, Chea D, Grinnell R, Vida TA. Ketamine and the Disinhibition Hypothesis: Neurotrophic Factor-Mediated Treatment of Depression. Pharmaceuticals (Basel). 2023 May 12;16(5):742. doi: 10.3390/ph16050742. PMID: 37242525; PMCID: PMC10221455.
- Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017 Jan 4;45(D1):D353-D361. doi: 10.1093/nar/gkw1092. Epub 2016 Nov 28. PMID: 27899662; PMCID: PMC5210567.
- English JA, Fan Y, Föcking M, Lopez LM, Hryniewiecka M, Wynne K, Dicker P, Matigian N, Cagney G, Mackay-Sim A, Cotter DR. Reduced protein synthesis in schizophrenia patient-derived olfactory cells. Transl Psychiatry. 2015 Oct 20;5(10):e663. doi: 10.1038/tp.2015.119. PMID: 26485547; PMCID: PMC4930119.
- Sabaie H, Gharesouran J, Asadi MR, Farhang S, Ahangar NK, Brand S, Arsang-Jang S, Dastar S, Taheri M, Rezazadeh M. Downregulation of miR-185 is a common pathogenic event in 22q11.2 deletion syndrome-related and idiopathic schizophrenia. Metab Brain Dis. 2022 Apr;37(4):1175-1184. doi: 10.1007/s11011-022-00918-5. Epub 2022 Jan 25. PMID: 35075501.
- Ikenouchi J, Umeda M. FRMD4A regulates epithelial polarity by connecting Arf6 activation with the PAR complex. Proc Natl Acad Sci U S A. 2010 Jan 12;107(2):748-53. doi: 10.1073/pnas.0908423107. Epub 2009 Dec 22. PMID: 20080746; PMCID: PMC2818900.
- Accogli A, Eric Jarvis G, Schiavetto A, Lai L, Amirali EL, Jimenez Cruz DA, Rivière JB, Trakadis Y. Psychiatric features and variable neurodevelopment outcome in four females with IQSEC2 spectrum disorder. J Genet. 2020;99:47. PMID: 32529990.
- Park SJ, Jeong J, Park YU, Park KS, Lee H, Lee N, Kim SM, Kuroda K, Nguyen MD, Kaibuchi K, Park SK. Disrupted-in-schizophrenia-1 (DISC1) Regulates Endoplasmic Reticulum Calcium Dynamics. Sci Rep. 2015 Mar 3;5:8694. doi: 10.1038/srep08694. PMID: 25732993; PMCID: PMC4346799.
- Hashimoto R, Ohi K, Okada T, Yasuda Y, Yamamori H, Hori H, Hikita T, Taya S, Saitoh O, Kosuga A, Tatsumi M, Kamijima K, Kaibuchi K, Takeda M, Kunugi H. Association analysis between schizophrenia and the AP-3 complex genes. Neurosci Res. 2009 Sep;65(1):113-5. doi: 10.1016/j.neures.2009.05.008. Epub 2009 May 27. PMID: 19481122.
- Gardiner EJ, Cairns MJ, Liu B, Beveridge NJ, Carr V, Kelly B, Scott RJ, Tooney PA. Gene expression analysis reveals schizophrenia-associated dysregulation of immune pathways in peripheral blood mononuclear cells. J Psychiatr Res. 2013 Apr;47(4):425-37. doi: 10.1016/j.jpsychires.2012.11.007. Epub 2012 Dec 4. PMID: 23218666; PMCID: PMC7094548.
- Andrade A, Brennecke A, Mallat S, Brown J, Gomez-Rivadeneira J, Czepiel N, Londrigan L. Genetic Associations between Voltage-Gated Calcium Channels and Psychiatric Disorders. Int J Mol Sci. 2019 Jul 19;20(14):3537. doi: 10.3390/ijms20143537. PMID: 31331039; PMCID: PMC6679227.
- Chen Y, Gu Y, Wang B, Wei A, Dong N, Jiang Y, Liu X, Zhu L, Zhu F, Tan T, Jing Z, Mao F, Zhang Y, Yao J, Yang Y, Wang H, Wu H, Li H, Zheng C, Duan X, Huo J, Wu X, Hu S, Zhao A, Li Z, Cheng X, Qin Y, Song Q, Zhan S, Qu Q, Guan F, Xu H, Kang X, Wang C. Synaptotagmin-11 deficiency mediates schizophrenia-like behaviors in mice via dopamine over-transmission. Nat Commun. 2024 Dec 4;15(1):10571. doi: 10.1038/s41467-024-54604-4. PMID: 39632880; PMCID: PMC11618495.
- Vieira N, Rito T, Correia-Neves M, Sousa N. Sorting Out Sorting Nexins Functions in the Nervous System in Health and Disease. Mol Neurobiol. 2021 Aug;58(8):4070-4106. doi: 10.1007/s12035-021-02388-9. Epub 2021 May 1. PMID: 33931804; PMCID: PMC8280035.
- Bruce HA, Kochunov P, Paciga SA, Hyde CL, Chen X, Xie Z, Zhang B, Xi HS, O'Donnell P, Whelan C, Schubert CR, Bellon A, Ament SA, Shukla DK, Du X, Rowland LM, O'Neill H, Hong LE. Potassium channel gene associations with joint processing speed and white matter impairments in schizophrenia. Genes Brain Behav. 2017 Jun;16(5):515-521. doi: 10.1111/gbb.12372. Epub 2017 Mar 13. PMID: 28188958; PMCID: PMC5457349.
- Hawi Z, Tong J, Dark C, Yates H, Johnson B, Bellgrove MA. The role of cadherin genes in five major psychiatric disorders: A literature update. Am J Med Genet B Neuropsychiatr Genet. 2018 Mar;177(2):168-180. doi: 10.1002/ajmg.b.32592. Epub 2017 Sep 18. PMID: 28921840.
- Andrews JL, Zalesky A, Nair S, Sullivan RP, Green MJ, Pantelis C, Newell KA, Fernandez F. Genetic and Epigenetic Regulation in Lingo-1: Effects on Cognitive Function and White Matter Microstructure in a Case-Control Study for Schizophrenia. Int J Mol Sci. 2023 Oct 26;24(21):15624. doi: 10.3390/ijms242115624. PMID: 37958608; PMCID: PMC10648795.
- McCutcheon RA, Krystal JH, Howes OD. Dopamine and glutamate in schizophrenia: biology, symptoms and treatment. World Psychiatry. 2020 Feb;19(1):15-33. doi: 10.1002/wps.20693. PMID: 31922684; PMCID: PMC6953551.
- Kruse AO, Bustillo JR. Glutamatergic dysfunction in Schizophrenia. Transl Psychiatry. 2022 Dec 3;12(1):500. doi: 10.1038/s41398-022-02253-w. PMID: 36463316; PMCID: PMC9719533.
- Li W, Su X, Chen T, Li Z, Yang Y, Zhang L, Liu Q, Shao M, Zhang Y, Ding M, Lu Y, Yu H, Fan X, Song M, Lv L. Solute Carrier Family 1 (SLC1A1) Contributes to Susceptibility and Psychopathology Symptoms of Schizophrenia in the Han Chinese Population. Front Psychiatry. 2020 Sep 23;11:559210. doi: 10.3389/fpsyt.2020.559210. PMID: 33173509; PMCID: PMC7538510.
- Wong AH, Trakalo J, Likhodi O, Yusuf M, Macedo A, Azevedo MH, Klempan T, Pato MT, Honer WG, Pato CN, Van Tol HH, Kennedy JL. Association between schizophrenia and the syntaxin 1A gene. Biol Psychiatry. 2004 Jul 1;56(1):24-9. doi: 10.1016/j.biopsych.2004.03.008. PMID: 15219469.
- de Jonge JC, Vinkers CH, Hulshoff Pol HE, Marsman A. GABAergic Mechanisms in Schizophrenia: Linking Postmortem and In Vivo Studies. Front Psychiatry. 2017 Aug 11;8:118. doi: 10.3389/fpsyt.2017.00118. PMID: 28848455; PMCID: PMC5554536.
- Spellmann I, Rujescu D, Musil R, Mayr A, Giegling I, Genius J, Zill P, Dehning S, Opgen-Rhein M, Cerovecki A, Hartmann AM, Schäfer M, Bondy B, Müller N, Möller HJ, Riedel M. Homer-1 polymorphisms are associated with psychopathology and response to treatment in schizophrenic patients. J Psychiatr Res. 2011 Feb;45(2):234-41. doi: 10.1016/j.jpsychires.2010.06.004. Epub 2010 Jul 3. PMID: 20598711.
- Gören JL. Brain-derived neurotrophic factor and schizophrenia. Ment Health Clin. 2016 Nov 3;6(6):285-288. doi: 10.9740/mhc.2016.11.285. PMID: 29955483; PMCID: PMC6007539.
- Deane AR, Potemkin N, Ward RD. Mitogen-activated protein kinase (MAPK) signalling corresponds with distinct behavioural profiles in a rat model of maternal immune activation. Behav Brain Res. 2021 Jan 1;396:112876. doi: 10.1016/j.bbr.2020.112876. Epub 2020 Aug 23. PMID: 32846206.
- Hoseth EZ, Krull F, Dieset I, Mørch RH, Hope S, Gardsjord ES, Steen NE, Melle I, Brattbakk HR, Steen VM, Aukrust P, Djurovic S, Andreassen OA, Ueland T. Exploring the Wnt signaling pathway in schizophrenia and bipolar disorder. Transl Psychiatry. 2018 Mar 6;8(1):55. doi: 10.1038/s41398-018-0102-1. PMID: 29507296; PMCID: PMC5838215.
- Casas BS, Vitória G, Prieto CP, Casas M, Chacón C, Uhrig M, Ezquer F, Ezquer M, Rehen SK, Palma V. Schizophrenia-derived hiPSC brain microvascular endothelial-like cells show impairments in angiogenesis and blood-brain barrier function. Mol Psychiatry. 2022 Sep;27(9):3708-3718. doi: 10.1038/s41380-022-01653-0. Epub 2022 Jun 15. PMID: 35705634.
- Mätlik K, Garton DR, Montaño-Rodríguez AR, Olfat S, Eren F, Casserly L, Damdimopoulos A, Panhelainen A, Porokuokka LL, Kopra JJ, Turconi G, Schweizer N, Bereczki E, Piehl F, Engberg G, Cervenka S, Piepponen TP, Zhang FP, Sipilä P, Jakobsson J, Sellgren CM, Erhardt S, Andressoo JO. Elevated endogenous GDNF induces altered dopamine signalling in mice and correlates with clinical severity in schizophrenia. Mol Psychiatry. 2022 Aug;27(8):3247-3261. doi: 10.1038/s41380-022-01554-2. Epub 2022 May 26. PMID: 35618883; PMCID: PMC9708553.
- Hoseth EZ, Krull F, Dieset I, Mørch RH, Hope S, Gardsjord ES, Steen NE, Melle I, Brattbakk HR, Steen VM, Aukrust P, Djurovic S, Andreassen OA, Ueland T. Attenuated Notch signaling in schizophrenia and bipolar disorder. Sci Rep. 2018 Mar 28;8(1):5349. doi: 10.1038/s41598-018-23703-w. PMID: 29593239; PMCID: PMC5871764.
- Chen Y, Guan W, Wang ML, Lin XY. PI3K-AKT/mTOR Signaling in Psychiatric Disorders: A Valuable Target to Stimulate or Suppress? Int J Neuropsychopharmacol. 2024 Feb 1;27(2):pyae010. doi: 10.1093/ijnp/pyae010. PMID: 38365306; PMCID: PMC10888523.
- Hill K, Mann L, Laws KR, Stephenson CM, Nimmo-Smith I, McKenna PJ. Hypofrontality in schizophrenia: a meta-analysis of functional imaging studies. Acta Psychiatr Scand. 2004 Oct;110(4):243-56. doi: 10.1111/j.1600-0447.2004.00376.x. PMID: 15352925.
- Mula M, Schmitz B. Depression in epilepsy: mechanisms and therapeutic approach. Ther Adv Neurol Disord. 2009 Sep;2(5):337-44. doi: 10.1177/1756285609337340. PMID: 21180624; PMCID: PMC3002598.
- Komatsu H, Onoguchi G, Silverstein SM, Jerotic S, Sakuma A, Kanahara N, Kakuto Y, Ono T, Yabana T, Nakazawa T, Tomita H. Retina as a potential biomarker in schizophrenia spectrum disorders: a systematic review and meta-analysis of optical coherence tomography and electroretinography. Mol Psychiatry. 2024 Feb;29(2):464-482. doi: 10.1038/s41380-023-02340-4. Epub 2023 Dec 11. PMID: 38081943; PMCID: PMC11116118.
- Asor E, Ben-Shachar D. Platelets: A possible glance into brain biological processes in schizophrenia. World J Psychiatry. 2012 Dec 22;2(6):124-33. doi: 10.5498/wjp.v2.i6.124. PMID: 24175178; PMCID: PMC3782191.
- Ling E, Nemesh J, Goldman M, Kamitaki N, Reed N, Handsaker RE, Genovese G, Vogelgsang JS, Gerges S, Kashin S, Ghosh S, Esposito JM, Morris K, Meyer D, Lutservitz A, Mullally CD, Wysoker A, Spina L, Neumann A, Hogan M, Ichihara K, Berretta S, McCarroll SA. A concerted neuron-astrocyte program declines in ageing and schizophrenia. Nature. 2024 Mar;627(8004):604-611. doi: 10.1038/s41586-024-07109-5. Epub 2024 Mar 6. PMID: 38448582; PMCID: PMC10954558.
- Oh YM, Lee SW, Kim WK, Chen S, Church VA, Cates K, Li T, Zhang B, Dolle RE, Dahiya S, Pak SC, Silverman GA, Perlmutter DH, Yoo AS. Age-related Huntington's disease progression modeled in directly reprogrammed patient-derived striatal neurons highlights impaired autophagy. Nat Neurosci. 2022 Nov;25(11):1420-1433. doi: 10.1038/s41593-022-01185-4. Epub 2022 Oct 27. PMID: 36303071; PMCID: PMC10162007.
- Labrie V, Fukumura R, Rastogi A, Fick LJ, Wang W, Boutros PC, Kennedy JL, Semeralul MO, Lee FH, Baker GB, Belsham DD, Barger SW, Gondo Y, Wong AH, Roder JC. Serine racemase is associated with schizophrenia susceptibility in humans and in a mouse model. Hum Mol Genet. 2009 Sep 1;18(17):3227-43. doi: 10.1093/hmg/ddp261. Epub 2009 May 30. PMID: 19483194; PMCID: PMC2722985.
- Lin H, Jacobi AA, Anderson SA, Lynch DR. D-Serine and Serine Racemase Are Associated with PSD-95 and Glutamatergic Synapse Stability. Front Cell Neurosci. 2016 Feb 25;10:34. doi: 10.3389/fncel.2016.00034. PMID: 26941605; PMCID: PMC4766304.
- Kang HJ, Wilkins AD, Lichtarge O, Wensel TG. Determinants of endogenous ligand specificity divergence among metabotropic glutamate receptors. J Biol Chem. 2015 Jan 30;290(5):2870-8. doi: 10.1074/jbc.M114.622233. Epub 2014 Dec 17. PMID: 25519912; PMCID: PMC4317032.
- Goltsov AY, Loseva JG, Andreeva TV, Grigorenko AP, Abramova LI, Kaleda VG, Orlova VA, Moliaka YK, Rogaev EI. Polymorphism in the 5'-promoter region of serine racemase gene in schizophrenia. Mol Psychiatry. 2006 Apr;11(4):325-6. doi: 10.1038/sj.mp.4001801. PMID: 16446740.
- Minichino A, Senior M, Brondino N, Zhang SH, Godwlewska BR, Burnet PWJ, Cipriani A, Lennox BR. Measuring Disturbance of the Endocannabinoid System in Psychosis: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2019 Sep 1;76(9):914-923. doi: 10.1001/jamapsychiatry.2019.0970. Erratum in: JAMA Psychiatry. 2021 Jan 1;78(1):112. doi: 10.1001/jamapsychiatry.2020.3882. PMID: 31166595; PMCID: PMC6552109.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.