Biology Group 2025 February 28;6(2):204-211. doi: 10.37871/jbres2073.
Microbial Inoculant: A Potential and Sustainable way for Improvement and Production of Crops for Agricultural Sector Development
Vibha Bhardwaj*
- Bacterial culture
- Bacillus subtilis
- Foliar spray
- Biocontrol agent
- Biopesticide
- Biostimulant
- Pseudomonas sp.
Abstract
Introduction: Increasing demand for food due to rapidly increase in global population, it is necessary to meet the food requirements without degrading the environment. In cultivated zones, around highly populated areas, there is excessive use of fertilizers. There is a requirement to opt for natural or biological fertilizers to substitute the chemical fertilizers, due to the increasing demand for agriculture sustainability. Microbial Inoculants could be effective for crop production improvement without negative effects on the environment.
Objective: The present study focuses on the efficacy and utilization of Bacillus subtilis (ATCC 6633) and Pseudomonas sp. (ATCC 27853) as microbial inoculant to analyse the growth rate and of mustard (Brassica nigra) and carom seeds (Trachyspermum ammi) and compare with untreated seeds. Bacterial culture in the form of seed treatment and foliar spray were used as microbial Inoculant for the growth of plant.
Results and Discussion: The major seed treatment effect was observed with Bacillus subtilis that showed antifungal activity against phytopathogens. Initially, faster germination percentage was found with the carom seeds with seed treatment showed the best results, with 90% and 82% in 3 days compared to the untreated seeds and mustard seeds. Mustard Seeds treated with Bacillus subtilis showed faster plant growth rate after sowing in terms of increase in shoot length 11.6cm, 13.2 cm and root length 2.3cm, respectively, after 20 days. Spraying treatment resulted in shoot length 6.2 cm and root length 1.4cm, respectively, higher than compared to the control.
Conclusion: Bacillus subtilis ATCC 6633 and Pseudomonas sp. (ATCC 27853) microbial inoculum can be used as Microbial Inoculants, and they could act as an environmental-friendly and economical alternative to synthetic liquid fertilizer for promoting sustainable agriculture.
Abbreviations
SD: Standard Deviation; BS: Bacillus Subtilis; SA: Pseudomonas sp; sp: Species; RAK: Ras Al Khaimah
Introduction
From centuries, agriculture has been the backbone of human development, and with the increasing global population, the need for sustainable farming practices has never been more critical because of its economic, social, and environmental importance [1]. Additionally, the extreme use of chemical-based fertilizers, can be harmful for the environment and can lead to climate change. Therefore, the execution of agronomic practices that can reduce soil and environment pollution, as well as increase crop nutrition, yield and safety, to attain sustained growth and development. The inclusion of microorganisms into the soil, using Microbial Inoculants, is relevant in sustainable agriculture as a promising ecological and friendly substitute to boost plant growth and health, and to enhance soil quality [2,3].
Recently, microorganisms have become a sustainable source as bioinoculant and biostimulants for crop improvement and production [4]. The use of beneficial microorganisms for sustainable agriculture will be promising solution, particularly by using Bacillus subtilis and Pseudomonas. These significant bacteria can enhance crop production, improve soil health, and stimulate eco-friendly farming. Bacillus is a genus of concern as Microbial Inoculant [5], since its varied physiological diversity allows it to live in different habitats. Also, these bacteria are recognized for their action as biofertilizers, phytostimulants, and biological control agents since they produce various antibiotics [6,7]. There are various species of Bacillus, containing B. subtilis subs. Spizizenii and B. subtilis var. natto. The genus Bacillus is versatile for its application, which makes it an excellent candidate for the development of microbial inoculants [5].
Bacillus subtilis: Bacillus subtilis is a rod-shaped, gram-positive bacterium found in soil and the gastrointestinal tracts of humans and ruminants. Bacillus species is known for its ability to form endospore, which is a tough protective layer to withstand extreme environmental conditions. This resilience makes it an excellent candidate for use in agricultural applications [1] (Figure 1).
Pseudomonas: Pseudomonas is naturally important and discrete group of bacteria on the Earth. In the carbon and nitrogen cycles, they have an exceptionally significant role [8,9]. The plant growth is controlled by Pseudomonas species because of its various beneficial characteristics like the presence of siderophores, phosphorous solubilization, and antagonistic compounds secretion for several plant pathogens. The strains of Pseudomonas fluorescens correlated with the putida group functions as seed inoculants for enhanced crops yield [10,11].
Various Pseudomonas species flexibly grow on agro fields proving their stimulation, sustain ability, and remediating properties. Pseudomonas spp. are essential for establishing successively competent colonization by exchanges and uptake of nutrients [12]. Diverse strains of Pseudomonas display respective ecological characteristics, like quorum sensing mediation biofilm formation, antifungal metabolite production, chemo tactic mediation, synergistic attachment with the plant root system, and catabolism of numerous plant excretions [13]. Pseudomonas, species have several plants benefits and generally observed as plant elongation promoters (root and shoot), appending at different level from molecular.
There is a need for new methods development for commercial production of agricultural crops that will protect the crops from microbial pathogens and will enhance the yield and quality of the harvested crops. Alternatives there are requirement of more environmentally friendly, safer methods of plant protection by especially adopting biocontrol advances by utilizing beneficial microbes [14]. By adopting the use of microbes as Biological control agent, is an excellent approach to control the adverse effect of disease-causing microbes on plant health and productivity. Substantial effort has been placed on discovering microbial biocontrol agents that can inhibit phytopathogens, which are responsible for soilborne diseases, and parallelly to enhance agricultural productivity [15].
The present study focusses on the efficacy and utilization of Bacillus subtilis and Pseudomonas spp. as Microbial Inoculant/biopesticides to analyse the growth rate of mustard and carom plant and compare with untreated plant. Bacterial culture in the form of seed treatment and foliar spray were used as Microbial Inoculant for the growth of plant.
Material and Methods
Collection of sample
Mustard seed and Carom seeds were purchased from local market of Dahan area of Ras Al Khaimah, United Arab Emirates. Samples were kept and maintained in sterile bags at room temperature, until their use.
Chemicals
In the present research, chemicals used were of analytical grade and high purity from Himedia, Merck, and Honeywell. For investigation, the Standard kits and reagents were procured from USA and Germany.
Media used for study
Nutrient agar, nutrient broth was used for the analysis. Media and broth preparation was done according to the manufacturer’s manual procedures and experiments were performed in the microbiology division of Environment laboratory of Ras Al Khaimah Municipality, United Arab Emirates.
Pure bacterial culture
In the present examination, the bacterial strains used were Bacillus subtilis (ATCC 6633), Pseudomonas aeruginosa (ATCC 27853) obtained from the American Type Culture Collection (ATCC). The strains of bacteria were procured from LTA srl Italia. Pure strains of bacteria were preserved at 4°C on nutrient agar slants. Each strain’s triplicate was used for each sample.
Inoculums preparation for pure bacterial isolates
Inoculum of each of the bacterial pure culture isolates was prepared by inoculating a loopful of colony from each slant in nutrient broth and incubate overnight at 37°C. Preparation of inoculum according to Bhardwaj V [16].
Seed pretreatment and foliar spraying with microbial inoculum
Seed pretreatment process: Mustard (Brassica nigra) and carom seeds (Trachyspermum ammi) were procured from local market of Ras Al Khaimah. Washing with 70% alcohol and then three times with sterile distilled water and dried on 70 mm Whatman filter paper at room temperature disinfected the seeds. These dried seeds were distributed into 90 mm petri plates with different quantities of seeds per plate and treated with 1ml of appropriate concentrations of pure culture of bacteria inoculum (Bacillus subtilis and Pseudomonas sp.). Three replicates per treatment were taken and incubation for 24 h at room temperature in laminar air flow with closed petri plates. After the incubation period, bacterial inoculum was removed, and the seeds were kept for drying on 70 mm Whatman filter paper at room temperature. Seeds without any treatment as well as 0% treatment (only distilled water) served as a control for the seed treatment process. The dried seeds were transferred to a Petri plate containing a layer of sterilized cotton covered with sterile filter paper with the pore size equivalent to Whatman Grade 3 and moistened with 5 mL of sterile distilled water for germination and placed in environmental conditions with a temperature of 22-25ºC day/ 14-18ºC night. Observations were made 4 and 7 days after inoculation without uncovering the boxes, and a visible radicle length of at least 2mm was the criterion for germination [17]. The germination percentage (G%) was determined according to [18]. At 20 days, the length of shoot and the root were measured in each seedling.
Germination percentage = number of germinated seeds/ total number of seeds ×100 [19]
Foliar spraying process: Mustard (Brassica nigra) and carom seeds (Trachyspermum ammi) were germinated in a Petri plate containing a layer of sterilized cotton covered with sterile filter paper with the pore size equivalent to Whatman Grade 3 and moistened with 5 ml of sterile distilled water to obtain plantlets of two-leaf stage. The experiment consisted of control containing 0% extract concentration (distilled water) with three replicates per treatment. Similar experimental conditions were given as provided during seed treatment. Each plant received foliar spraying of bacterial inoculum (Bacillus subtilis and Pseudomonas spp.) every 4 days for a period of 20 days. Before the spraying process, the surface of inside petri plate was completely covered with aluminium foil to avoid leaching of spray into the cotton with filter paper that could be potentially taken up by the roots. Since the rate of absorption and cuticular penetration of the active biostimulants in the extract is dependent over the solar irradiance [20], the spraying of inoculum was acted during the noon time, because during that time the plant receives maximum possible sunlight and which resulting in wider stomatal openings. Higher resultant water pressure during noon allows greater penetration of the extracts into the leaf through the stomata. Water was given to all the plants, according to their requirement except after foliar spraying where they were not watered for 24h.
Determination of plant growth rate and productivity
Plant physiological growth analysis: The germination percentage rate of plant was evaluated in terms of an increase in shoot length and root length over a period of 20 days, from the day of sowing seeds in petri plate. The plant germination percentage was measured in control (untreated and 0% treatment) as well as, in all the treatments for every 5 days and a comparison was made to verify the treatment, which gave the maximum plant germination rate.
Statistical analysis: Experiments performed in triplicate. Data are expressed as mean. Standard Deviation (SD) was expressed as experimental error determination for triplicate samples results.
Results and Discussion
According to the research results, this is possibly the first report to study about mustard and carom seeds germination rate by using microorganisms as Microbial Inoculants, in Ras Al Khaimah, United Arab Emirates.
Seed treatment with microbial inoculum
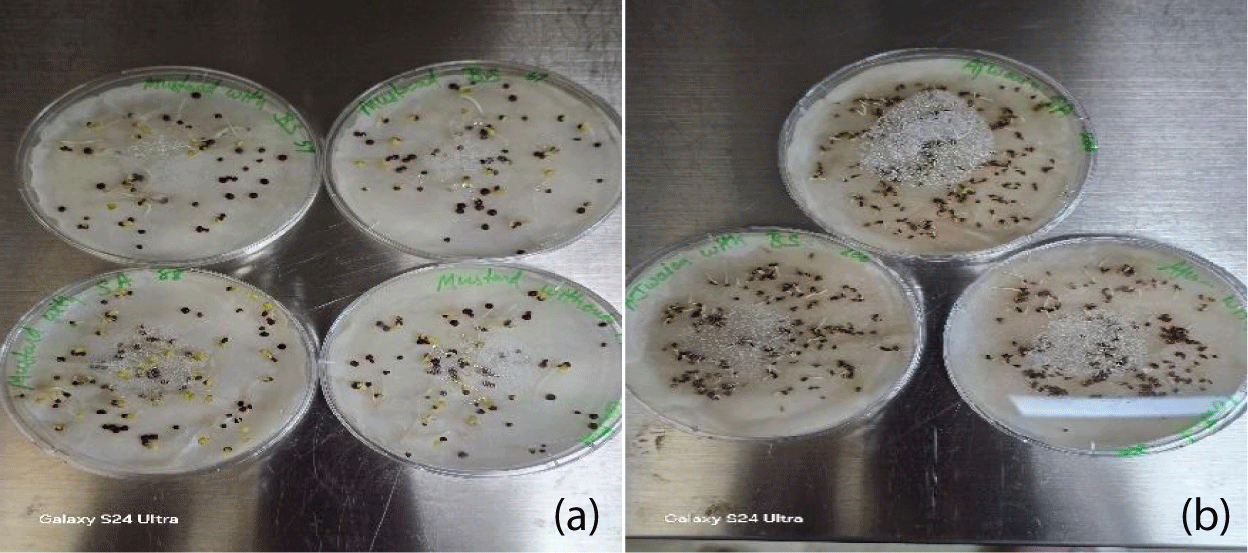
Determination of seed germination rate: The ability of a seed to germinate uniformly and rapidly at different environmental conditions is an essential characteristic required for most of the plants, including mustard and carom. Germination percentage gives the viability of the number of seeds germinated per treatment. The average percentage of seed germination in mustard is 40-67% figure 2a for an incubation period of 4-5 days (Table 1). Additionally, in case of carom seeds, the germination rate is 82-90% (Figure 2b).
| Table 1: Germination percentage (G%) where BS (Bacillus subtilis) and SA (Pseudomonas spp.). Germination percentage (G%) = number of germinated seeds/ total number of seeds ×100. | |||
| Germination Percentage (G%) | |||
| Genotypes | Control | Mustard seeds (Brassica nigra) | Carom seeds (Trachyspermum ammi) |
| BS1 | 36.36% | 67.0% | 90% |
| BS2 | 24.00% | 52.6% | 90% |
| SA1 | 75.00% | 56.8% | 90% |
| SA2 | 61.00% | 40.00% | 82% |
Unfavorable conditions such as insufficient availability of seed nutrients, poor water supply and inadequate environmental conditions may result in increased seed dormancy and reduce the percentage of seed germination. Conventional method for improving the problem includes treating the seeds with chemicals, which causes a detrimental effect over the environment. Thus, to achieve maximal germination without deteriorating the environment, seed pretreatment with biostimulants could be used as an alternative, as suggested in the present study. It was also observed that the plantlets from the treated seeds were healthier than those from the untreated and control ones. During germination Barone V, et al. [21] and Kumar G, et al. [22] reported a 100% germination rate with 20% S. wightii extract for wheat. Similar to the above study, Garcia-Gonzalez J [23] also reported highest germination rate obtained with Acutodesmus extracts of 50% and 75% [24]. Also reported a 12-25% increase in germination percentage in seeds pretreated with marine algal extracts compared to the control (treated only with water). The enhanced germination percentage is due to the presence of carbohydrates, proteins and other microelements that acts as precursor of elicitor compounds accelerating the protrusion of radicle [25]. Also, seed treatment with microalgal extracts rich in phytohormones and plant growth promoters reduce the seed dormancy, promoting efficient germination.
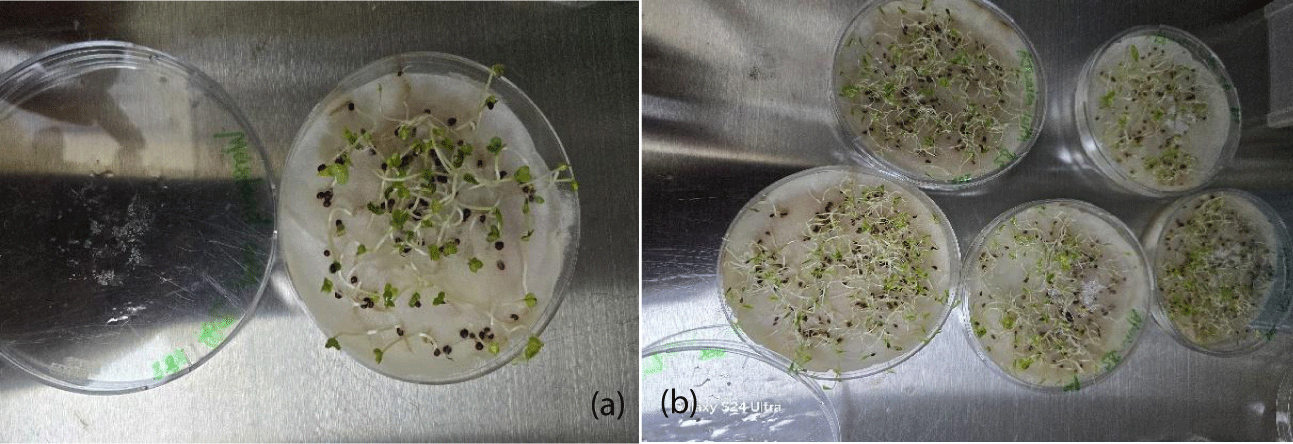
Plant growth rate analysis: Growth of plant was analyzed in terms of increase in shoot and root length. Table 2 shows the results of shoot length and root length obtained over a period of 20 days for all the treated plants. It was seen that the increase in shoot length in case of mustard seed up to 11.6 cm figure 3a but in case of carom seeds the shoot length increased maximum 10.2 cm (Figure 3b) [22]. Also reported that S. wightii extract concentrations greater than 20% resulted in shorter shoot lengths, smaller root lengths, lower lateral roots of wheat plants [25]. Reported that 1% extracts of U. lactuca and P. gymnospora resulted in highest germination rate as well as maximum plumule and radicle length of tomato plants [22]. Reported a shoot length of 25 cm and root length of 10 cm with S, wightii extracts after 25 days of sowing [25]. Reported a maximum radicle length of 7.3 m and plumule length of 8.3 m, with U. lactuca and P. gymnospora extracts respectively after 8 days of sowing with 1% cellular extracts.
| Table 2: Seed treatment root length and shoot length (cm) where BS (Bacillus subtilis) and SA (Pseudomonas spp.). | ||||||
| Averaged root length | Averaged shoot length | Averaged root length | Averaged shoot length | Averaged root length | Averaged shoot length | |
| BS1 | 1 | 6.3 | 1.2 | 11.6 | 1 | 9.7 |
| BS2 | 1.5 | 5.9 | 2.3 | 13.2 | 0.5 | 10.2 |
| SA1 | 2 | 5.1 | 1.4 | 7.2 | 1.5 | 8.6 |
| SA2 | 1.7 | 5.8 | 2 | 8.6 | 1 | 8.2 |
Foliar spraying of microbial inoculum
Plant growth rate analysis: Foliar spraying of microbial inoculum as biostimulants/Microbial Inoculant on plants is a common practice followed when plants are deprived of essential nutrients for their growth [26]. A plant can absorb nutrients through its leaves at a faster rate than its roots. Due to the opening and closing of stomatal pores of the leaves, water soluble microalgal cellular metabolites can also pass and disperse along the whole plant through translocation [20]. This process of cuticular absorption and penetration is governed by the laws of diffusion and depends on the availability of sunlight. During daytime, when the plant exposure to sunlight is the highest, application of foliar spray through the stomatal opening leads to a maximum plant nutrient uptake [27]. Growth of plant was analyzed in terms of changes in shoot length and root length as shown in table 3. Foliar spraying of higher concentrations often inhibited the metabolic process resulting in lesser productivity due to reduced plant nutrient uptake [28]. Similar results were obtained by Hernandez-Herrera RM [25] where foliar spraying of seaweed extracts greater than 180 mg/ml for tomato plants resulted in smaller shoot lengths.
| Table 3: Foliar spray treatment root length and shoot length (cm) where BS (Bacillus subtilis) and SA (Pseudomonas spp.) | ||||||
| Root Length and Shoot Length (cm) | ||||||
| Genotypes | control | Mustard seeds (Brassica nigra) | Carom seeds (Trachyspermum ammi) | |||
| Averaged root length | Averaged shoot length | Averaged root length | Averaged shoot length | Averaged Root length | Averaged shoot length | |
| BS1 | 1.0 | 5.3 | 1.2 | 5.6 | 1.0 | 5.7 |
| BS2 | 1.5 | 5.9 | 2.3 | 6.2 | 0.5 | 6.0 |
| SA1 | 2.0 | 5.1 | 1.4 | 5.2 | 1.5 | 5.6 |
| SA2 | 1.7 | 4.8 | 2.0 | 4.6 | 1.0 | 5.1 |
Garcia-Gonzalez J, et al. [23] obtained similar results where tomato plants were sprayed with different concentrations of aqueous extracts of Acutodesmus dimorphus and obtained the highest productivity in case of 50% treatment resulting in 89cm shoot length, 390g fresh weight of tomato plants after 60 days. Also, Shaaban MM [29] reported that the foliar spraying of wheat plants with extracts of Chlorella vulgaris resulted in increased nutrient uptake, grain yield, fresh and dry weight by 60.7% compared to the control [30]. Elansary HO [31] reported that spraying 1 g L-1 of Scenedesmus spp. extract with 1 g L-1 chelated micronutrients resulted in the harvest of 8 g of dry matter accumulation in wheat plants after 66 days. Reported that foliar spray of 5 and 7 ml per L of A. Nodusum extracts has been reported to contain polysacharides, amino acids which act as elicitor for signaling pathways of phytohormones thus enhancing the leave numbers, root and shoot length as well as fresh and dry weight of mint and basil plants. Foliar spraying usually helps the plant in attaining the deficient nutrients like N, P, Fe, Mg, Ca and enable nutrient corrections. Hence, an optimum quantity of nutrients (appropriate concentrations) should be fed to the leaves without damaging them in order to get a healthy plant [28]. Also, foliar spraying depends on several other factors such as temperature, intensity of light, rate of application, humidity, etc. All these factors should be properly maintained to get a higher plant growth rate and productivity.
Conclusion
Seed treatment and foliar spray of microbial inoculum as Microbial Inoculant were found to have a positive influence over the seed germination and plant germination rate. Initially, 70% treatment of seeds with microbial inoculum showed maximum germination efficiency resulting in a shoot length of 11.6 cm and 9.2 cm. In case of foliar spray, maximal germination rate was observed initially with 90% resulting in total plant height of 6.2 cm in case of mustard and 6 cm in case of carom plant. Overall, seed treatment was found to be more effective compared to foliar spray. It was also observed that, similar to the synthetic fertilizers there exists a concentration cut off beyond which the extracts have an inhibitory effect. More studies are needed to gain insight into the actual cellular mechanism involved in stimulating plant growth. Microbial inoculum extract as Microbial Inoculant/biostimulants possess an extraordinary potential to revolutionize the agriculture sector, thereby reducing the harmful environmental and health issues associated with conventional chemical fertilizers. Microbial inoculants represent a promising and sustainable approach to improving crop production while maintaining soil health and ecological balance. Their integration into modern agricultural practices can significantly contribute to food security, environmental protection, and economic sustainability. By embracing microbial-based solutions, the agricultural sector can transition towards a more resilient and sustainable future. Research and development efforts should focus on improving formulation stability, field efficacy, and microbial strain adaptability to diverse environmental conditions. Government policies and agricultural extension programs must also play a vital role in promoting the use of microbial inoculants through subsidies, training, and incentives.
Acknowledgement
Authors would like to thank all individuals who provided their efforts for this research especially Aaesha Ahmed Alzaabi and Nizamudeen for their assistance during research work.
Ethics Approval and Consent to Participate
Ethics Approval and Consent to Participate
Consent for Publication
Not applicable.
Availability of Data and Materials
The relevant data and materials are available in the present study.
Competing Interest
The authors declare that they have no competing interests. All procedures followed were in accordance with the ethical standards (institutional and national).
Funding
Not applicable.
Authors’ Contributions
VB performed all the experiments. VB analysed the data and wrote the manuscript.
References
- Tsotetsi T, Nephali L, Malebe M, Tugizimana F. Bacillus for Plant Growth Promotion and Stress Resilience: What Have We Learned? Plants (Basel). 2022 Sep 22;11(19):2482. doi: 10.3390/plants11192482. PMID: 36235347; PMCID: PMC9571655.
- Sammauria S, Kumawat S, Kumawat P, Singh J, Jatwa TK. Microbial inoculants: Potential tool for sustainability of agricultural production systems. Arch Microbiol. 2020;202:677–693. doi: 10.1007/s00203-019-01795-w.
- Basu A, Prasad P, Das SN, Kalam S, Sayyed RZ Reddy MS, El Enshasy H. Plant growth Promoting Rhizobacteria (PGPR) as green microbial inoculants: Recent developments, constraints, and prospects. Sustainability. 2021;13:1140. doi: 10.3390/su13031140.
- Michalak I, Chojnacka K, Saeid A. Plant Growth Biostimulants, Dietary Feed Supplements and Cosmetics Formulated with Supercritical CO₂ Algal Extracts. Molecules. 2017 Jan 3;22(1):66. doi: 10.3390/molecules22010066. PMID: 28054954; PMCID: PMC6155630.
- Saxena AK, Kumar M, Chakdar H, Anuroopa N, Bagyaraj DJ. Bacillus species in soil as a natural resource for plant health and nutrition. J Appl Microbiol. 2020 Jun;128(6):1583-1594. doi: 10.1111/jam.14506. Epub 2019 Nov 21. PMID: 31705597.
- Kaspar F, Neubauer P, Gimpel M. Bioactive Secondary Metabolites from Bacillus subtilis: A Comprehensive Review. J Nat Prod. 2019 Jul 26;82(7):2038-2053. doi: 10.1021/acs.jnatprod.9b00110. Epub 2019 Jul 9. PMID: 31287310.
- Tran C, Cock IE, Chen X, Feng Y. Antimicrobial Bacillus: Metabolites and Their Mode of Action. Antibiotics (Basel). 2022 Jan 12;11(1):88. doi: 10.3390/antibiotics11010088. PMID: 35052965; PMCID: PMC8772736.
- Zhang L, Chen W, Jiang Q, Fei Z, Xiao M. Genome analysis of plant growth-promoting rhizobacterium Pseudomonas chlororaphis subsp. aurantiaca JD37 and insights from comparasion of genomics with three Pseudomonas strains. Microbiol Res. 2020 Aug;237:126483. doi: 10.1016/j.micres.2020.126483. Epub 2020 May 1. PMID: 32402945.
- Zhuang L, Li Y, Wang Z, Yu Y, Zhang N, Yang C, Zeng Q, Wang Q. Synthetic community with six Pseudomonas strains screened from garlic rhizosphere microbiome promotes plant growth. Microb Biotechnol. 2021 Mar;14(2):488-502. doi: 10.1111/1751-7915.13640. Epub 2020 Aug 6. PMID: 32762153; PMCID: PMC7936309.
- Yan Y, Yang J, Dou Y, Chen M, Ping S, Peng J, Lu W, Zhang W, Yao Z, Li H, Liu W, He S, Geng L, Zhang X, Yang F, Yu H, Zhan Y, Li D, Lin Z, Wang Y, Elmerich C, Lin M, Jin Q. Nitrogen fixation island and rhizosphere competence traits in the genome of root-associated Pseudomonas stutzeri A1501. Proc Natl Acad Sci U S A. 2008 May 27;105(21):7564-9. doi: 10.1073/pnas.0801093105. Epub 2008 May 21. PMID: 18495935; PMCID: PMC2396677.
- Costa-Gutierrez SB, Lami MJ, Santo MCC, Zenoff AM, Vincent PA, Molina-Henares MA, Espinosa-Urgel M, de Cristóbal RE. Plant growth promotion by Pseudomonas putida KT2440 under saline stress: role of eptA. Appl Microbiol Biotechnol. 2020 May;104(10):4577-4592. doi: 10.1007/s00253-020-10516-z. Epub 2020 Mar 27. PMID: 32221691.
- Sah S, Krishnani S, Singh R. Pseudomonas mediated nutritional and growth promotional activities for sustainable food security. Curr Res Microb Sci. 2021 Nov 24;2:100084. doi: 10.1016/j.crmicr.2021.100084. PMID: 34917993; PMCID: PMC8645841.
- Hashem A, Tabassum B, Fathi Abd Allah E. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J Biol Sci. 2019 Sep;26(6):1291-1297. doi: 10.1016/j.sjbs.2019.05.004. Epub 2019 May 20. PMID: 31516360; PMCID: PMC6734152.
- Warrior P. Living systems as natural crop-protection agents. Pest Manage Sci Formerly Pest Sci. 2000;56(8):681–687. doi: 10.1002/1526-4998(200008)56:8%3C681::AID-PS199%3E3.0.CO;2-S.
- Cazorla FM, Romero D, Pérez-García A, Lugtenberg BJ, Vicente Ad, Bloemberg G. Isolation and characterization of antagonistic Bacillus subtilis strains from the avocado rhizoplane displaying biocontrol activity. J Appl Microbiol. 2007 Nov;103(5):1950-9. doi: 10.1111/j.1365-2672.2007.03433.x. PMID: 17953605.
- Bhardwaj V. Antimicrobial Potential of Cocos nucifera (Coconut) Oil on Bacterial Isolates. Adv Exp Med Biol. 2023 Aug 19. doi: 10.1007/5584_2023_786. Epub ahead of print. PMID: 37594604.
- Marti L. Effect of saninity and temperature on seed germination of linonium mansaltrarum. 2010. doi: 10.1139/cjb-2012-0157.
- Araya E, Gómez L, Hidalgo N, Valverde R. Efecto de la luz y del ácido giberélico sobre la germinación in vitro de (Alunus Acuminata). Agron. Costarric. 2000;24:75-80.
- Viteri ML, Ghezán G, Iglesias D. Tomato and lettuce production marketing and comsumption. Estud Socioeconómico De Los Sist: INTA Balcarce Argentina. 2013;14:185-190. doi: 10.3390/su152115406.
- Ronga D, Biazzi E, Parati K, Carminati D, Carminati E, Tava A. Microalgal biostimulants and biofertilisers in crop productions. Agron J. 2019;9(4):146-163. doi: 10.3390/agronomy9040192.
- Barone V, Baglieri A, Stevanato P, Broccanello C, Bertoldo G, Bertaggia M, Fornasier F. Root morphological and molecular responses induced by microalgae extracts in sugar beet (Beta vulgaris L.). J Appl Phycol. 2018;30(2):1061-1071. doi: 10.1007/s10811-017-1283-3.
- Kumar G, Sahoo D, Effect of seaweed liquid extract on growth and yield of Triticum aestivum var Pusa Gold. J Appl. Phycol. 2011;23(2):251-255. doi: 10.1007/s10811-011-9660-9.
- Garcia-Gonzalez J, Sommerfeld M. Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J Appl Phycol. 2016;28:1051-1061. doi: 10.1007/s10811-015-0625-2. Epub 2015 May 29. PMID: 27057088; PMCID: PMC4789255.
- Ibrahim WM. Potential impact of marine algal extracts on the growth and metabolic activities of salinity stressed wheat seedlings. J Appl Sci. 2016;16(8):388-394. doi: 10.3923/jas.2016.388.394.
- Hernandez-Herrera RM, Santacruz-Ruvalcaba F, Ruiz-Lopez MA, Norrie J, Hernandez-Carmona G. Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.). J Appl Phycol. 2014;26(1):619–628. doi: 10.1007/s10811-013-0078-4.
- Win TT, Barone GD, Secundo F, Fu P. Algal biofertilizers and plant growth stimulants for sustainable agriculture. Ind Biotechnol. 2018;14(4):203-211. doi: 10.1089/ind.2018.0010.
- Battacharyya D, Zamani Babgohari M, Rathor P, Prithiviraj B. (2015). Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015;196:39-48. doi: 10.1016/j.scienta.2015.09.012.
- Plaza BM, Gomez-Serrano C, Acien-Fernandez FG, Jimenez-Becker S. Effect of microalgae hydrolysate foliar application (Arthrospira platensis and Scenedesmus sp.) on Petunia x hybrida growth. J Appl Phycol. 2018;30(4):2359-2365. doi: 10.1007/s10811-018-1427-0.
- Shaaban MM. Green microalgae water extract as foliar feeding to wheat plants. Pak J Biol Sci. 2001;4(6):628-632. doi: 10.3923/pjbs.2001.628.632.
- Shaaban MM, El-Saady AKM, El-Sayed AEB. Green microalgae water extract and micronutrients foliar application as promoters to nutrient balance and growth of wheat plants. J Am Sci. 2010;6(9):631-636. doi: 10.3923/pjbs.2001.628.632.
- Elansary HO, Yessoufou K, Shokralla S, Mahmoud EA, Skalicka-Woźniak K. Enhancing mint and basil oil composition and antibacterial activity using seaweed extracts. Ind. Crops Prod. 2016;92:50-56. doi: 10.1016/j.indcrop.2016.07.048.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.