Medicine Group 2025 January 06;6(1):020-022. doi: 10.37871/jbres2052.
Successful use of Filgotinib in the Treatment of Refractory Rheumatoid Arthritis-Associated Sweet Syndrome
Matteo Colina1*, Alessia Barisani2, Alberto Gualandi3, Francesca Poli3 and Gabriele Campana4
2Dermatology outpatient, AUSL Imola, Italy
3Section of Pathology, Department of Integrated Activities, AUSL Imola, Italy
4Alma Mater Studiorum, Department of Pharmacy and Biotechnology, University of Bologna, Bologna, Italy
Introduction
Filgotinib is a second-generation Janus Kinase (JAK) inhibitor with selective inhibitory activity for the subtype JAK1 used for the treatment of Rheumatoid Arthritis (RA). By inhibiting cytokines and blocking the activation of growth signals, it effectively reduces the expression of interleukins IL-6, IL-12, IL-3, and type II interferon [1]. Currently, corticosteroids are the first-line and typically effective therapy for treating Sweet Syndrome (SS), that may occur in association with RA. In cases where corticosteroid treatment fails, is contraindicated, or requires long-term use, second-line agents should be personalized, taking into account the patient's co-morbidities and medical history. Filgotinib is approved as monotherapy or in combination with methotrexate to treat moderate to severe RA in patients who have an inadequate response or intolerance to one or more Disease-Modifying Anti-Rheumatic Drugs (DMARDs) and Tumor Necrosis Factor inhibitors (TNFi) [2,3].
We described for the first time the successful use of filgotinib in a patient with a long-standing refractory rheumatoid arthritis-associated Sweet syndrome.
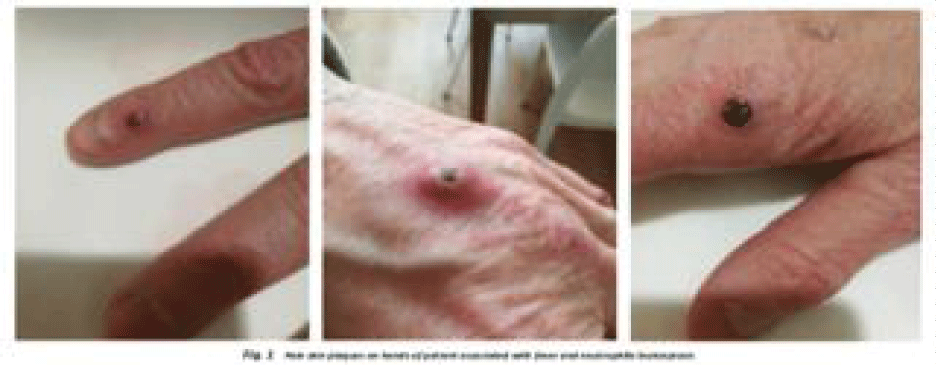
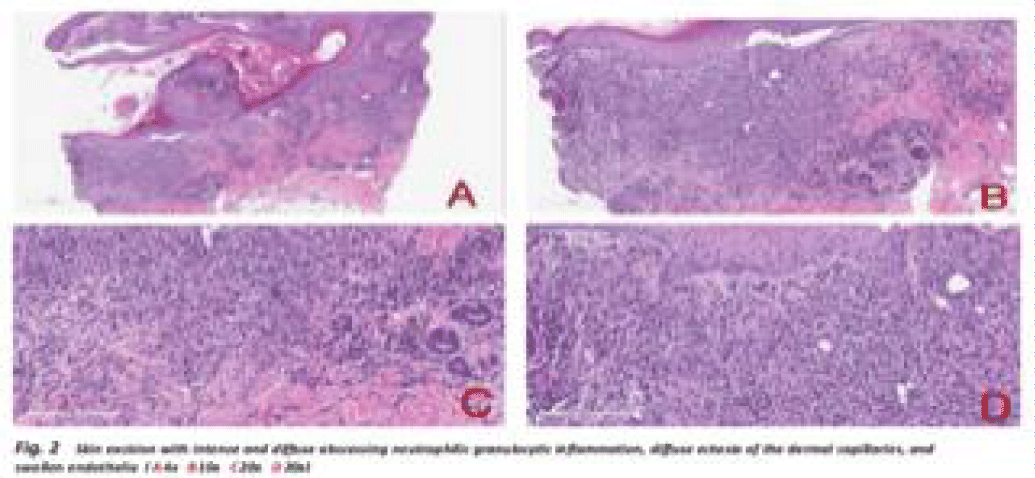
A 64-year-old woman, diagnosed with RA in 2013 initially responded well to treatment with hydroxychloroquine, methotrexate, and leflunomide, maintaining low disease activity or remission for five years without the need of corticosteroid treatment. However, due to hepatic intolerance and secondary ineffectiveness, the therapy had to be discontinued. In 2018, the patients was successful treated with tocilizumab until September 2021. A month later, the patient presented a flare-up of the RA that was treated with prednisone (10 mg/die). Additionally, the patient developed a rapid onset of painful infiltrated plaques, high-spiking fever, neutrophilic leukocytosis, and systemic symptoms. Macroscopic and microscopic features of inflammatory skin condition are shown in figures 1,2. Clinical observations, along with laboratory tests and a biopsy, confirmed the diagnosis of SS. We decided to initiate treatment with filgotinib (200 mg/day), leading to remission of osteoarticular, systemic, and cutaneous manifestations.
The patient is currently continuing on filgotinib 200 mg daily, resulting in sustained remission of both cutaneous and joint disease.
To our knowledge, a prior case of rheumatoid arthritis-associated Sweet syndrome was positively treated with the oral administration of 2 mg of baricitinib [4]. Despite, recent comparative analyses have validated both baricitinib and filgotinib as effective treatment options for RA, filgotinib has demonstrated a superior efficacy and safety profile compared to baricitinib [5]. In this study, we report the first successful use of filgotinib in the management of Sweet syndrome.
Funding Information
No specific funding was received to carry out the work described in this article.
Conflict of Interest
All authors declared no conflicts of interest.
Acknowledgements
The authors thank Dr. Daniela Bencardino for medical writing and editorial assistance.
References
- Namour F, Anderson K, Nelson C, Tasset C. Filgotinib: A Clinical Pharmacology Review. Clin Pharmacokinet. 2022 Jun;61(6):819-832. doi: 10.1007/s40262-022-01129-y. Epub 2022 May 31. PMID: 35637376; PMCID: PMC9249714.
- Joshi TP, Friske SK, Hsiou DA, Duvic M. New Practical Aspects of Sweet Syndrome. Am J Clin Dermatol. 2022 May;23(3):301-318. doi: 10.1007/s40257-022-00673-4. Epub 2022 Feb 14. PMID: 35157247; PMCID: PMC8853033.
- Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, McInnes IB, Sepriano A, van Vollenhoven RF, de Wit M, Aletaha D, Aringer M, Askling J, Balsa A, Boers M, den Broeder AA, Buch MH, Buttgereit F, Caporali R, Cardiel MH, De Cock D, Codreanu C, Cutolo M, Edwards CJ, van Eijk-Hustings Y, Emery P, Finckh A, Gossec L, Gottenberg JE, Hetland ML, Huizinga TWJ, Koloumas M, Li Z, Mariette X, Müller-Ladner U, Mysler EF, da Silva JAP, Poór G, Pope JE, Rubbert-Roth A, Ruyssen-Witrand A, Saag KG, Strangfeld A, Takeuchi T, Voshaar M, Westhovens R, van der Heijde D. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020 Jun;79(6):685-699. doi: 10.1136/annrheumdis-2019-216655. Epub 2020 Jan 22. PMID: 31969328.
- Nousari Y, Wu BC, Valenzuela G. Successful use of baricitinib in the treatment of refractory rheumatoid arthritis-associated Sweet syndrome. Clin Exp Dermatol. 2021 Oct;46(7):1330-1332. doi: 10.1111/ced.14712. Epub 2021 Jun 4. PMID: 33914946.
- Lee YH, Song GG. Comparative efficacy and safety of tofacitinib, baricitinib, upadacitinib, and filgotinib in active rheumatoid arthritis refractory to biologic disease-modifying antirheumatic drugs. Z Rheumatol. 2021 May;80(4):379-392. English. doi: 10.1007/s00393-020-00796-1. PMID: 32367211.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.