Medicine Group. 2024 July 24;5(7):822-833. doi: 10.37871/jbres1960.
Crosstalk between Inflammatory Hypoxia and Gut Microbiota in Inflammatory Bowel Disease (IBD)
Boping Jing1 and Fei Gao2,3*
2Brown Center for Immunotherapy, School of Medicine, Indiana University, Indianapolis, IN 46202, USA
3Experimental and Developmental Therapeutics, Melvin and Bren Simon Comprehensive Cancer Center, Indiana University, Indianapolis, IN 46202, USA
- Inflammatory hypoxia
- Microbiota
- Inflammatory bowel disease
Abstract
In the healthy gut mucosa, epithelial cells lining the lumen experience low oxygen levels, typically less than 2%, referred to as physiological hypoxia or physioxia. This gradient is maintained by the diffusion of oxygen from blood vessels along the crypt-villus axis, creating varying degrees of hypoxia within the mucosal lining. Physiological hypoxia at the mucosal surface regulates innate immunity by enhancing epithelial barrier function and modulating resident immune cells. However, studies suggest that mucosal inflammatory diseases involve hypoxia, exacerbated by increased oxygen consumption due to inflammatory cell influx, termed inflammatory hypoxia. Additionally, endothelial dysfunction may induce microvascular occlusion and thrombosis, further exacerbating tissue hypoxia. The human Gastrointestinal (GI) tract harbors a diverse community of microorganisms known as gut microbiota, pivotal in intestinal immunity and metabolism. Crucially, the microbiota contributes to maintaining a physiologically low oxygen environment in the gut, crucial for mucosal defenses. Dysbiosis of gut microbiota has been implicated in the pathogenesis of various inflammatory diseases and infections. This review aims to consolidate our current knowledge of the intricate interplay between inflammatory hypoxia and gut microbiota, emphasizing the pivotal roles of gut microbiota in the pathological hypoxic conditions observed in Inflammatory Bowel Disease (IBD).
Introduction
The GI tract operates within a unique physiological framework characterized by low partial oxygen tensions, essential for maintaining the intestinal microecosystem and functions [1,2]. In both the healthy mucosa of the small and large intestine, lumen-apposed epithelia endure low partial pressure of Oxygen (pO2) conditions of <10 mmHg, defined as physiologic hypoxia. In contrast, oxygen-rich blood vessels reside in the subepithelium, with oxygen diffusing along the crypt-villus axis to adjacent venules. Consequently, GI tissues experience varying degrees of low oxygen levels [1,3-5].
The GI mucosa sustains controlled inflammation under physiologic hypoxia, regulating innate immunity by balancing resident immune cells and promoting epithelial barrier function [6-8]. Mucosal inflammatory diseases, characterized by inflammatory hypoxia, result from increased inflammatory cells and oxygen demands [9-11]. Conversely, conditions primarily driven by hypoxia may manifest secondary inflammatory changes [12,13]. Furthermore, hypoxia and inflammation exhibit interdependence [14]. During intestinal inflammation, lesions are profoundly hypoxic or anoxic, penetrating deep into mucosal tissue due to factors like increased oxygen consumption, edema, thrombosis, vasoconstriction, and vasculitis [15]. IBD, characterized by chronic GI tract inflammation, is notably associated with severe mucosal hypoxia [16,17]. Hyperbaric Oxygen Therapy (HBOT), which targets both tissue hypoxia and inflammation, has been evaluated in IBD by several studies [18-25].The gut microbiota, crucial for maintaining the intestinal hypoxic environment, plays a critical role in nutrient absorption, barrier integrity, and immune response modulation [26-28]. Dysbiosis of the gut microbiota is implicated in IBD pathogenesis [29]. This paper discusses the crosstalk between inflammatory hypoxia and gut microbiota in the context of IBD pathogenesis.
Physiological Hypoxia (Physioxia) in Gut
Tissues experience their own unique 'normoxia' under physiological conditions, known as 'physioxia', which is lower than atmospheric partial Oxygen pressure (pO2) but not indicative of oxygen insufficiency [30]. Conversely, in pathological states like inflammation and cancer, tissues exhibit markedly reduced pO2 levels, termed 'hypoxia', due to increased oxygen consumption or impaired tissue oxygenation [30]. Low oxygen tension serves as a potent signaling molecule that regulates stem cell proliferation and differentiation during early developmental stages [31]. Given that the gut epithelium constitutes a continuously renewing tissue, originating from stem cells within a progenitor pool throughout an organism's lifespan, maintaining a low oxygen microenvironment in the gut is crucial physiologically [32].
The unique structural characteristics of the GI tract, including an oxygen-poor lumen juxtaposed with a highly oxygenated subepithelial mucosa, create a steep oxygen gradient across the epithelial layer [1,33]. This feature of physiologic hypoxia is essential for preserving normal gut homeostasis [34].
At the cellular level within the intestine, Hypoxia-Inducible Factor (HIF) regulates genes that facilitate adaptation to low oxygen levels [35-41], playing critical roles in immune modulation and maintaining epithelial barrier function [42,43]. Key members of the HIF family, such as HIF1 and HIF2, exhibit differential regulation of hypoxia-induced gene expression across various cell types [44]. The expression of HIF1α and HIF1α-dependent genes is pivotal for maintaining gut physioxia and gastrointestinal homeostasis [45,46].
Oxygen Dysregulation and IBD
The GI tract experiences physiological hypoxia, a state associated with persistent low-grade inflammation. Within this context, the epithelial cell layer plays crucial roles in adapting to hypoxic stress and modulating immune responses to inflammation [1]. Reduced oxygen availability in the intestine has been implicated in the pathogenesis of IBD, where hypoxic injury contributes to recurrent epithelial cell damage [47,48]. Factors such as epithelial barrier compromise, bacterial infiltration, immune cell infiltration, vascular damage, and reduced blood flow (colonic ischemia or infarction) can precipitate a decline in oxygen levels, leading to inflammatory hypoxia [49-51]. Conversely, inflammatory hypoxia in the intestine may serve as an environmental trigger for the development of IBD [52].
HIFs can adapt cells to conditions of low oxygen tension and inflammation, and both HIF1α and HIF2α expression are markedly elevated in intestinal epithelial cells of IBD patients [47,52]. Specifically, HIF1α is focal in its expression (in epithelial cells, stromal fibroblasts, and myocytes) in both active ulcerative colitis (UC) and Crohn’s Disease (CD), whereas HIF2α is focal in UC and diffuse in CD, with no staining observed in normal intestinal mucosa for HIF2α [47]. HIF1α has been reported to have a protective role in colitis, reducing intestinal inflammation, whereas HIF2α exacerbates colitis and promotes massive intestinal inflammation [15,53]. However, conflicting findings suggest that HIF1α expression may augment inflammation in the proximal colon of sulindac-treated mice [54]. In a model of Dextran Sulfate Sodium (DSS)-induced colitis, HIF1a expression increases significantly in atrophic crypts during injury phase and subsequently decreases in hypertrophic crypts during the regeneration phase [55].
Interferon-γ (IFN-γ), a key cytokine in IBD pathogenesis, has been shown to increase HIF1α expression in a time- and dose-dependent manner, while not affecting the expression of HIF1β/ARNT [56,57]. However, another study indicates that although IFN-γ moderately increases HIF1α mRNA expression in intestinal epithelial cells, it attenuates overall HIF activity, selectively repressing HIF1β in a JAK-dependent manner [58].
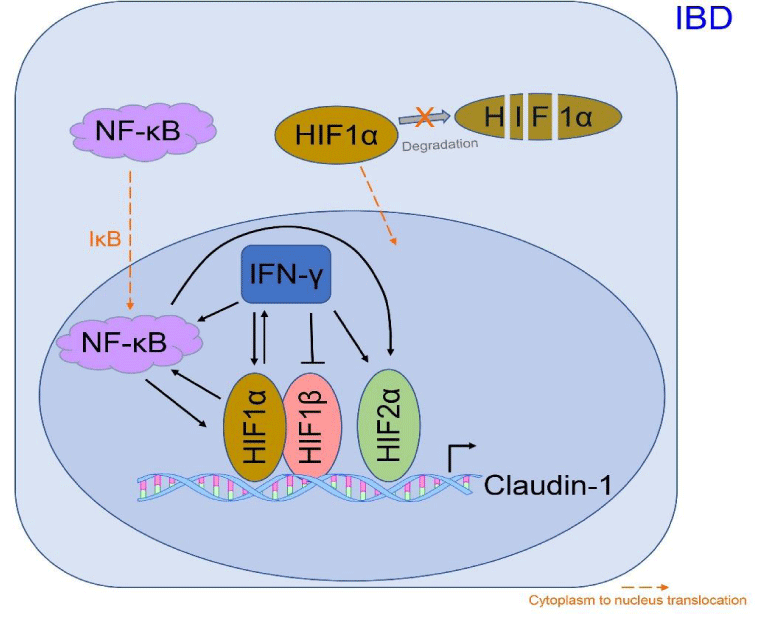
Hypoxia also involves nuclear factor-κB (NF-κB) as another transcription factor, and IKK/NF-κB signaling in intestinal epithelial cells is closely associated with maintaining gut epithelial integrity and immune homeostasis [59]. HIF1α has been shown to activate NF-κB, which in turn controls HIF1α transcription. IKK-β, a promoter of NF-κB, is critical for the optimal accumulation of HIF1α, but not HIF2α [60]. Additionally, the IFN-γ-induced increase in HIF1α is associated with NF-κB activation [57].
The claudin family of multigene transmembrane proteins, comprising more than 27 members, is predominantly located in intestinal epithelia and has been extensively investigated for its contributions to IBD pathogenesis [61]. Elevated expression of claudin-1 and claudin-2 is observed in active IBD and correlates positively with inflammatory activity [62,63]. Claudin-1, regulated by HIF1α, HIF2α, and HIF1β, serves as a target of HIF in the context of IBD [64].
Figure 1 illustrates the elevation of HIF1α, HIF2α, and HIF1β in IBD, with Claudin-1 identified as a prominent HIF target. The positive correlation between HIF1α and NF-κB plays a critical role in the pathogenesis of IBD, and IFN-γ activates both HIF1α and NF-κB pathways.
Gut Microbiota and Physioxia
The gut harbors a diverse array of microorganisms, including bacteria, yeast, viruses, and others, collectively known as gut microbiota, which serve crucial physiological roles such as regulating epithelial barrier function and immune homeostasis [65]. A healthy gut microbiota is characterized by a balanced composition of aerobic, facultative anaerobic, and obligate anaerobic bacteria. Particularly, obligate anaerobes predominate within the gut microbiota, actively reducing oxygen and nitrate levels to inhibit the proliferation of aerobic and facultative anaerobic species [66]. Both aerobic and facultative anaerobic bacteria contribute significantly to the establishment of an anaerobic environment [67]. Research on neonatal gut microbiota reveals that initial colonization by aerobic and facultative anaerobic bacteria consumes luminal oxygen, thereby facilitating the growth of obligate anaerobes [68,69]. Moreover, the microbiota in the distal gut, compared to the proximal gut, enhances microbial respiration and utilizes oxygen through processes such as lipid oxidation reactions, thereby reducing luminal oxygen availability [70].
Additionally, short-chain fatty acids, particularly butyrate, derived from microbial fermentation of dietary fibers, serve as a primary energy source for intestinal epithelial cells. During epithelial cell metabolism, butyrate enters mitochondrial fatty acid oxidation and undergoes Oxidative Phosphorylation (OXPHOS). This process consumes oxygen, leading to epithelial deoxygenation, which promotes the growth of beneficial anaerobic microbiota and reinforces epithelial barrier integrity [71-73]. Interestingly, recent studies highlight that butyrate stabilizes Hypoxia-Inducible Factor (HIF) in the anoxic gut lumen by inhibiting HIF Prolyl Hydroxylases (PHDs), enzymes responsible for HIF degradation under normoxic conditions [74-76].
Gut Microbiota and Inflammatory Hypoxia in IBD
The gut microbiota maintains a dynamic equilibrium critical for immune function, nutrient absorption, and maintaining a healthy epithelial barrier. Disruption of this balance can lead to the proliferation of opportunistic pathogens. For instance, under certain conditions, intestinal pathogens like C. rodentium can induce a glycolytic shift in intestinal epithelial cells, promoting epithelial re-oxygenation- a microenvironment conducive to pathogen growth [73]. Conversely, severe hypoxia in the gut due to inflammatory cell infiltration and elevated pro-inflammatory factors alters the gut microbiota composition, exacerbating hypoxic injury [77].
IBD is characterized by chronic immune responses triggered by microbial antigens [17,78]. Dysbiosis, observed in both CD and UC, varies in microbial alterations influenced by factors such as diet, ethnicity, age, and disease severity indices. Generally, studies indicate significant increases in facultative anaerobic bacteria like the Fusobacteria phylum and Enterobacteria group (especially Escherichia coli) in fecal samples of CD patients [79,80]. Conversely, obligate anaerobic bacteria such as Ruminococcus torques, Roseburia inulinivorans, Blautia faecis, Clostridium lavalense, and the Clostridium leptum group, including Faecalibacterium prausnitzii, are reduced in CD, despite an increase in the obligate anaerobic Fusobacteria phylum [81-85]. In UC, which exhibits a higher rate of bacterial translocation across the intestinal epithelium, there is an increase in aerotolerant bacteria like Desulfovibrio, while anaerobic bacteria such as the Clostridium coccoides group, Faecalibacterium prausnitzii, and Akkermansia muciniphila are decreased [85,86]. Notably, Faecalibacterium prausnitzii, an important butyrate producer in the gut, shows increased levels during UC remission periods [87]. Some researchers propose that the increased presence of facultatively anaerobic species and decreased obligately anaerobic organisms in IBD could stem from heightened oxygen concentrations in the gut lumen due to increased blood flow during inflammation [17] - a hypothesis conflicting with most studies that demonstrate severe hypoxia in IBD tissues. Measurements of colon and ileum blood flow in IBD patients indicate significant elevation in severe UC cases but a marked decrease in mild colitis (by 25-30%). Conversely, CD patients exhibit near-normal ileal blood flow in early exudative stages, which sharply decreases in late fibrosing stages (by 60-70%) [88]. Similar findings are observed in an animal model of chronic intestinal inflammation initiated by naïve T-lymphocyte subsets, mimicking IBD, which shows reduced blood flow during mild inflammation [89]. Moreover, intestinal fibrosis - a common complication in chronic IBD, especially CD - is influenced by hypoxia, impacting both intestinal inflammation and fibrosis progression [90,91]. Notably, controlling inflammation alone does not prevent fibrosis progression in CD, as gut microbiota also triggers pro-fibrotic processes alongside inflammatory responses [92-95].
In summary, during early severe stages of IBD, disruptions in the intestinal wall oxygen gradient due to epithelial barrier damage and increased blood flow lead to microbiota dysbiosis, including increased facultatively anaerobic species and pathogens. Facultatively anaerobic species consume oxygen, while pathogens invade damaged intestinal walls and exacerbate oxygen depletion. Bacterial metabolites further exacerbate inflammation, leading to immune cell infiltration and increased oxygen consumption. In later stages, microvascular occlusion and thrombosis further diminish blood flow, aggravating hypoxia (Figure 2).
Interestingly, research indicates that mucosal microbiota, enriched with aerotolerant and asaccharolytic bacteria, differs significantly from fecal microbiota in healthy human intestines [28]. Mucosal microbiota dysbiosis is closely associated with IBD pathology, with mucosal-associated bacteria reflecting clinical UC outcomes to some extent, as observed in colon mucosal biopsy studies [96].
Crosstalk Between Gut Microbiota and Inflammatory Hypoxia in IBD
Microbiota and HIF crosstalk has been demonstrated to defend tissue barrier, and depletion of the microbiota using antibiotics reduces HIF expression [72]. Hypoxia and HIF-1 are recognized as key regulators in the interplay between gut microbiota and host interactions, influencing inflammation and infectious conditions in both human and murine intestinal cells [42,97]. NF-kB is a validated therapeutic target in IBD, and certain "anti-inflammatory" gut bacteria such as Faecalibacterium prausnitzii A2-165 and other Firmicutes isolates produce peptides that inhibit NF-kB signaling both in vitro and in vivo, stabilizing the IKK complex [98,99]. Given that epithelial cell hypoxic responses are dependent on microbiota metabolites, questions persist regarding the interplay between microbiota and HIF-2α in the progression of IBD [72,100].
There are three distinct types of IFNs: type I (primarily IFN-α/β), type II (IFN-γ), and type III (IFN-λs). Type II IFN/IFN-γ, a pivotal cytokine in IBD pathogenesis, plays a crucial role in combating bacterial infections, while type I and type III IFNs are associated with antiviral defenses [56,101]. In a DSS model of acute intestinal injury lacking both type I and III IFN receptors, mice exhibited enhanced barrier disruption, extensive loss of goblet cells, and reduced epithelial cell proliferation, highlighting the role of these IFN types in mucosal barrier homeostasis in the gastrointestinal tract [101]. Immunoregulatory therapy involving type I IFN has been shown to inhibit IFN-γ and increase interleukin-10 (IL-10) production, emphasizing its role in modulating inflammatory responses [102]. HIF1α has been reported to suppress type I IFN, however, it serves as a key mediator of IFN-γ-dependent gene expression, leading to the release of inflammatory program [103,104].
Active IBD tissues are characterized by infiltration of innate immune cells (e.g., macrophages, dendritic cells) and adaptive immune cells (T and B cells). Effector CD4+ T cells play a pivotal role in pathogen defense, while regulatory T cells (Treg) are crucial in limiting excessive immune responses and CD4+ and CD8+ T cell activities [105]. Dysregulation of these T cell subsets contributes to IBD pathogenesis [106-109]. The gut microbiota directly influences inflammation in models of T cell transfer-induced colitis [110]. γδ T cells, which express γ and δ chains in their T Cell Receptors (TCRs), act as a bridge between innate and adaptive immune responses. In chronic IBD, Vδ2 T cells exhibit a proinflammatory profile [77,111]. In a model of hypoxia-induced intestinal injury, alterations in gut microbiota exacerbated intestinal damage, which was significantly ameliorated following antibiotic-mediated microbiota depletion. Additionally, microbiota-derived metabolites exacerbate hypoxic injury via γδ T cells, highlighting the intricate interactions between microbiota, inflammatory hypoxia, and immune cells in IBD [77]. These findings underscore the complex nature of these interactions and their potential implications for understanding and treating IBD.
Conclusions and Outlook
The influence of gut microbiota on various aspects of IBD has garnered significant attention, yet the dynamic mechanisms underlying its pathogenesis remain incompletely understood. Microbiota, along with environmental factors such as oxygen levels, blood flow, and immune responses, intricately regulate intestinal epithelial barrier function. Dysregulation or imbalance among these factors can lead to epithelial damage, exacerbating or triggering IBD.
Currently, many aspects of IBD pathophysiology remain elusive or controversial. For instance, while there is consensus on the disruption of the oxygen gradient across the intestinal wall in IBD, conflicting findings exist regarding luminal oxygen levels—some studies report increased oxygen due to enhanced blood flow, whereas others observe severe hypoxia within the intestinal wall alongside reduced vascular perfusion. We hypothesize that these discrepancies stem from differences in disease progression stages and the dynamic nature of IBD. Conducting longitudinal studies to track oxygen levels at various stages of IBD would help determine how luminal oxygen and tissue hypoxia evolve as the disease progresses and identify any stage-specific patterns, using consistent methods across studies.
Pharmacological interventions targeting HIFs, such as PHD inhibitors or HIF inhibitors, have shown promise in preclinical models of IBD [53,76,100,112,113]. However, the role of HIFs in IBD is complex: while they protect the epithelial barrier under physiological conditions, they can also increase barrier permeability and promote pro-inflammatory cytokine production under pathological conditions [112,114].
Blood flow measurements in IBD patients reveal distinct patterns between UC and CD, reflecting their different clinical manifestations-CD typically involves full-thickness inflammation, while UC primarily affects mucosal and submucosal layers. We propose that inflammatory hypoxia may play distinct roles in UC and CD, warranting further investigation.
Given the reported dysbiosis of gut microbiota in IBD, it is evident that UC and CD represent distinct disease entities with unique microbial perturbations. Variations in microbiota analyses across studies underscore the challenges in utilizing microbiota-directed therapies, such as antibiotics and fecal transplantation, in clinical practice [115]. Understanding the bidirectional relationship between mucosal hypoxic responses and microbiota dynamics requires more nuanced and condition-specific investigations.
HBOT has shown variable efficacy in CD and UC, highlighting the need for tailored approaches in clinical management [18, 21,22]. Dysbiosis-induced inflammatory cell infiltration further complicates the intestinal environment by consuming oxygen and altering microbial community dynamics. Novel therapeutic avenues targeting immunological pathways in IBD have been explored, yet current pharmacological treatments often exhibit limited efficacy and significant side effects. Promising therapeutic strategies targeting T cell effector cytokines and immune-regulating pathways require further refinement and evaluation in the context of precise IBD staging, integrating factors such as oxygen dynamics and microbiota composition [116-119]. Understanding how these treatments modify the oxygen gradient can inform better management strategies for IBD.
Conclusion
In conclusion, advancing our understanding of hypoxia and HIFs in the development and progression of IBD, alongside elucidating the complex interplay with gut microbiota, holds promise for developing more effective and safer therapies. Rigorous investigation into these interconnected mechanisms will pave the way for personalized treatments that improve outcomes for patients with IBD.
Acknowledgment
F.G. received support from the National Institutes of Health (NIH) grant R01 DK126827 (Tamas Ordog), a Pilot and Feasibility award from the Mayo Clinic Center for Cell Signaling in Gastroenterology (NIH P30 DK084567; Nicholas F. LaRusso), and American Neurogastroenterology and Motility Society (ANMS) Discovery Grants (2021).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zeitouni NE, Chotikatum S, von Köckritz-Blickwede M, Naim HY. The impact of hypoxia on intestinal epithelial cell functions: consequences for invasion by bacterial pathogens. Mol Cell Pediatr. 2016 Dec;3(1):14. doi: 10.1186/s40348-016-0041-y. Epub 2016 Mar 22. PMID: 27002817; PMCID: PMC4803720.
- Zheng L, Kelly CJ, Colgan SP. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A Review in the Theme: Cellular Responses to Hypoxia. Am J Physiol Cell Physiol. 2015 Sep 15;309(6):C350-60. doi: 10.1152/ajpcell.00191.2015. Epub 2015 Jul 15. PMID: 26179603; PMCID: PMC4572369.
- Shepherd AP. Metabolic control of intestinal oxygenation and blood flow. Fed Proc. 1982 Apr;41(6):2084-9. PMID: 7075783.
- Glover LE, Lee JS, Colgan SP. Oxygen metabolism and barrier regulation in the intestinal mucosa. J Clin Invest. 2016 Oct 3;126(10):3680-3688. doi: 10.1172/JCI84429. Epub 2016 Aug 8. PMID: 27500494; PMCID: PMC5096807.
- Muenchau S, Deutsch R, de Castro IJ, Hielscher T, Heber N, Niesler B, Lusic M, Stanifer ML, Boulant S. Hypoxic Environment Promotes Barrier Formation in Human Intestinal Epithelial Cells through Regulation of MicroRNA 320a Expression. Mol Cell Biol. 2019 Jun 27;39(14):e00553-18. doi: 10.1128/MCB.00553-18. PMID: 31061092; PMCID: PMC6597885.
- Biddlestone J, Bandarra D, Rocha S. The role of hypoxia in inflammatory disease (review). Int J Mol Med. 2015 Apr;35(4):859-69. doi: 10.3892/ijmm.2015.2079. Epub 2015 Jan 27. PMID: 25625467; PMCID: PMC4356629.
- Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 2010 May;7(5):281-7. doi: 10.1038/nrgastro.2010.39. Epub 2010 Apr 6. PMID: 20368740; PMCID: PMC4077542.
- Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011 Feb 17;364(7):656-65. doi: 10.1056/NEJMra0910283. PMID: 21323543; PMCID: PMC3930928.
- Hatoum OA, Binion DG, Gutterman DD. Paradox of simultaneous intestinal ischaemia and hyperaemia in inflammatory bowel disease. Eur J Clin Invest. 2005 Oct;35(10):599-609. doi: 10.1111/j.1365-2362.2005.01567.x. PMID: 16178878.
- Watts ER, Walmsley SR. Inflammation and Hypoxia: HIF and PHD Isoform Selectivity. Trends Mol Med. 2019 Jan;25(1):33-46. doi: 10.1016/j.molmed.2018.10.006. Epub 2018 Nov 12. PMID: 30442494.
- Campbell EL, Bruyninckx WJ, Kelly CJ, Glover LE, McNamee EN, Bowers BE, Bayless AJ, Scully M, Saeedi BJ, Golden-Mason L, Ehrentraut SF, Curtis VF, Burgess A, Garvey JF, Sorensen A, Nemenoff R, Jedlicka P, Taylor CT, Kominsky DJ, Colgan SP. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity. 2014 Jan 16;40(1):66-77. doi: 10.1016/j.immuni.2013.11.020. Epub 2014 Jan 9. PMID: 24412613; PMCID: PMC3951457.
- Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011 Nov 7;17(11):1391-401. doi: 10.1038/nm.2507. PMID: 22064429; PMCID: PMC3886192.
- Fröhlich S, Boylan J, McLoughlin P. Hypoxia-induced inflammation in the lung: a potential therapeutic target in acute lung injury? Am J Respir Cell Mol Biol. 2013 Mar;48(3):271-9. doi: 10.1165/rcmb.2012-0137TR. Epub 2012 Oct 18. PMID: 23087053.
- Taylor CT. Interdependent roles for hypoxia inducible factor and nuclear factor-kappaB in hypoxic inflammation. J Physiol. 2008 Sep 1;586(17):4055-9. doi: 10.1113/jphysiol.2008.157669. Epub 2008 Jul 3. PMID: 18599532; PMCID: PMC2652186.
- Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004 Oct;114(8):1098-106. doi: 10.1172/JCI21086. PMID: 15489957; PMCID: PMC522241.
- Cummins EP, Crean D. Hypoxia and inflammatory bowel disease. Microbes Infect. 2017 Mar;19(3):210-221. doi: 10.1016/j.micinf.2016.09.004. Epub 2016 Sep 20. PMID: 27664046.
- Rigottier-Gois L. Dysbiosis in inflammatory bowel diseases: the oxygen hypothesis. ISME J. 2013 Jul;7(7):1256-61. doi: 10.1038/ismej.2013.80. Epub 2013 May 16. PMID: 23677008; PMCID: PMC3695303.
- Dawra S, Manrai M, Kumar A, Kumar S. Hyperbaric oxygen therapy in inflammatory bowel disease: a systemic review and meta-analysis. Eur J Gastroenterol Hepatol. 2022 Mar 1;34(3):359-360. doi: 10.1097/MEG.0000000000002225. PMID: 35100179.
- Alenazi N, Alsaeed H, Alsulami A, Alanzi T. A Review of Hyperbaric Oxygen Therapy for Inflammatory Bowel Disease. Int J Gen Med. 2021 Oct 24;14:7099-7105. doi: 10.2147/IJGM.S336678. PMID: 34729019; PMCID: PMC8554584.
- McCurdy J, Siw KCK, Kandel R, Larrigan S, Rosenfeld G, Boet S. The Effectiveness and Safety of Hyperbaric Oxygen Therapy in Various Phenotypes of Inflammatory Bowel Disease: Systematic Review With Meta-analysis. Inflamm Bowel Dis. 2022 Mar 30;28(4):611-621. doi: 10.1093/ibd/izab098. PMID: 34003289.
- Singh AK, Jha DK, Jena A, Kumar-M P, Sebastian S, Sharma V. Hyperbaric oxygen therapy in inflammatory bowel disease: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2021 Dec 1;33(1S Suppl 1):e564-e573. doi: 10.1097/MEG.0000000000002164. PMID: 33905214.
- Wu X, Liang TY, Wang Z, Chen G. The role of hyperbaric oxygen therapy in inflammatory bowel disease: a narrative review. Med Gas Res. 2021 Apr-Jun;11(2):66-71. doi: 10.4103/2045-9912.311497. PMID: 33818446; PMCID: PMC8130665.
- Lansdorp CA, Buskens CJ, Gecse KB, D'Haens GRAM, van Hulst RA. Letter: off-label use of hyperbaric oxygen therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2020 Jul;52(1):215-216. doi: 10.1111/apt.15775. PMID: 32529770.
- Simsek M, de Boer NKH. Letter: off-label use of hyperbaric oxygen therapy in inflammatory bowel disease-Authors' reply. Aliment Pharmacol Ther. 2020 Jul;52(1):216-217. doi: 10.1111/apt.15806. PMID: 32529768.
- Dulai PS, Gleeson MW, Taylor D, Holubar SD, Buckey JC, Siegel CA. Systematic review: The safety and efficacy of hyperbaric oxygen therapy for inflammatory bowel disease. Aliment Pharmacol Ther. 2014 Jun;39(11):1266-75. doi: 10.1111/apt.12753. Epub 2014 Apr 16. PMID: 24738651.
- Gasaly N, de Vos P, Hermoso MA. Impact of Bacterial Metabolites on Gut Barrier Function and Host Immunity: A Focus on Bacterial Metabolism and Its Relevance for Intestinal Inflammation. Front Immunol. 2021 May 26;12:658354. doi: 10.3389/fimmu.2021.658354. PMID: 34122415; PMCID: PMC8187770.
- Han N, Pan Z, Liu G, Yang R, Yujing B. Hypoxia: The "Invisible Pusher" of Gut Microbiota. Front Microbiol. 2021 Jul 22;12:690600. doi: 10.3389/fmicb.2021.690600. PMID: 34367091; PMCID: PMC8339470.
- Albenberg L, Esipova TV, Judge CP, Bittinger K, Chen J, Laughlin A, Grunberg S, Baldassano RN, Lewis JD, Li H, Thom SR, Bushman FD, Vinogradov SA, Wu GD. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology. 2014 Nov;147(5):1055-63.e8. doi: 10.1053/j.gastro.2014.07.020. Epub 2014 Jul 18. PMID: 25046162; PMCID: PMC4252572.
- Qiu P, Ishimoto T, Fu L, Zhang J, Zhang Z, Liu Y. The Gut Microbiota in Inflammatory Bowel Disease. Front Cell Infect Microbiol. 2022 Feb 22;12:733992. doi: 10.3389/fcimb.2022.733992. PMID: 35273921; PMCID: PMC8902753.
- Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med. 2011 Jun;15(6):1239-53. doi: 10.1111/j.1582-4934.2011.01258.x. PMID: 21251211; PMCID: PMC4373326.
- Ma T, Grayson WL, Fröhlich M, Vunjak-Novakovic G. Hypoxia and stem cell-based engineering of mesenchymal tissues. Biotechnol Prog. 2009 Jan-Feb;25(1):32-42. doi: 10.1002/btpr.128. PMID: 19198002; PMCID: PMC2771546.
- de Santa Barbara P, van den Brink GR, Roberts DJ. Development and differentiation of the intestinal epithelium. Cell Mol Life Sci. 2003 Jul;60(7):1322-32. doi: 10.1007/s00018-003-2289-3. PMID: 12943221; PMCID: PMC2435618.
- Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. J Mol Med (Berl). 2007 Dec;85(12):1295-300. doi: 10.1007/s00109-007-0277-z. Epub 2007 Nov 20. PMID: 18026919.
- Taylor CT. Hypoxia in the Gut. Cell Mol Gastroenterol Hepatol. 2017 Sep 19;5(1):61-62. doi: 10.1016/j.jcmgh.2017.09.005. PMID: 29276750; PMCID: PMC5736870.
- Hasibeder W, Germann R, Sparr H, Haisjackl M, Friesenecker B, Luz G, Pernthaler H, Pfaller K, Maurer H, Ennemoser O. Vasomotion induces regular major oscillations in jejunal mucosal tissue oxygenation. Am J Physiol. 1994 Jun;266(6 Pt 1):G978-86. doi: 10.1152/ajpgi.1994.266.6.G978. PMID: 8023946.
- Cousins FL, Murray AA, Scanlon JP, Saunders PT. Hypoxyprobe™ reveals dynamic spatial and temporal changes in hypoxia in a mouse model of endometrial breakdown and repair. BMC Res Notes. 2016 Jan 19;9:30. doi: 10.1186/s13104-016-1842-8. PMID: 26780953; PMCID: PMC4717617.
- Rademakers SE, Lok J, van der Kogel AJ, Bussink J, Kaanders JH. Metabolic markers in relation to hypoxia; staining patterns and colocalization of pimonidazole, HIF-1α, CAIX, LDH-5, GLUT-1, MCT1 and MCT4. BMC Cancer. 2011 May 12;11:167. doi: 10.1186/1471-2407-11-167. PMID: 21569415; PMCID: PMC3115911.
- Cevallos SA, Lee JY, Tiffany CR, Byndloss AJ, Johnston L, Byndloss MX, Bäumler AJ. Increased Epithelial Oxygenation Links Colitis to an Expansion of Tumorigenic Bacteria. mBio. 2019 Oct 1;10(5):e02244-19. doi: 10.1128/mBio.02244-19. PMID: 31575772; PMCID: PMC6775460.
- He G, Shankar RA, Chzhan M, Samouilov A, Kuppusamy P, Zweier JL. Noninvasive measurement of anatomic structure and intraluminal oxygenation in the gastrointestinal tract of living mice with spatial and spectral EPR imaging. Proc Natl Acad Sci U S A. 1999 Apr 13;96(8):4586-91. doi: 10.1073/pnas.96.8.4586. PMID: 10200306; PMCID: PMC16376.
- Fisher EM, Khan M, Salisbury R, Kuppusamy P. Noninvasive monitoring of small intestinal oxygen in a rat model of chronic mesenteric ischemia. Cell Biochem Biophys. 2013 Nov;67(2):451-9. doi: 10.1007/s12013-013-9611-y. PMID: 23636684; PMCID: PMC3797231.
- Whitney AK, Campbell EL. Imaging Inflammatory Hypoxia in the Murine Gut. Methods Mol Biol. 2016;1422:115-26. doi: 10.1007/978-1-4939-3603-8_11. PMID: 27246027.
- Pral LP, Fachi JL, Corrêa RO, Colonna M, Vinolo MAR. Hypoxia and HIF-1 as key regulators of gut microbiota and host interactions. Trends Immunol. 2021 Jul;42(7):604-621. doi: 10.1016/j.it.2021.05.004. PMID: 34171295; PMCID: PMC8283795.
- Singhal R, Shah YM. Oxygen battle in the gut: Hypoxia and hypoxia-inducible factors in metabolic and inflammatory responses in the intestine. J Biol Chem. 2020 Jul 24;295(30):10493-10505. doi: 10.1074/jbc.REV120.011188. Epub 2020 Jun 5. PMID: 32503843; PMCID: PMC7383395.
- Fang HY, Hughes R, Murdoch C, Coffelt SB, Biswas SK, Harris AL, Johnson RS, Imityaz HZ, Simon MC, Fredlund E, Greten FR, Rius J, Lewis CE. Hypoxia-inducible factors 1 and 2 are important transcriptional effectors in primary macrophages experiencing hypoxia. Blood. 2009 Jul 23;114(4):844-59. doi: 10.1182/blood-2008-12-195941. Epub 2009 May 19. PMID: 19454749; PMCID: PMC2882173.
- Kumar T, Pandey R, Chauhan NS. Hypoxia Inducible Factor-1α: The Curator of Gut Homeostasis. Front Cell Infect Microbiol. 2020 May 15;10:227. doi: 10.3389/fcimb.2020.00227. PMID: 32500042; PMCID: PMC7242652.
- Koury J, Deitch EA, Homma H, Abungu B, Gangurde P, Condon MR, Lu Q, Xu DZ, Feinman R. Persistent HIF-1alpha activation in gut ischemia/reperfusion injury: potential role of bacteria and lipopolysaccharide. Shock. 2004 Sep;22(3):270-7. doi: 10.1097/01.shk.0000135256.67441.3f. PMID: 15316398.
- Giatromanolaki A, Sivridis E, Maltezos E, Papazoglou D, Simopoulos C, Gatter KC, Harris AL, Koukourakis MI. Hypoxia inducible factor 1alpha and 2alpha overexpression in inflammatory bowel disease. J Clin Pathol. 2003 Mar;56(3):209-13. doi: 10.1136/jcp.56.3.209. PMID: 12610101; PMCID: PMC1769899.
- Hatoum OA, Binion DG, Gutterman DD. Paradox of simultaneous intestinal ischaemia and hyperaemia in inflammatory bowel disease. Eur J Clin Invest. 2005 Oct;35(10):599-609. doi: 10.1111/j.1365-2362.2005.01567.x. PMID: 16178878.
- Goggins BJ, Minahan K, Sherwin S, Soh WS, Pryor J, Bruce J, Liu G, Mathe A, Knight D, Horvat JC, Walker MM, Keely S. Pharmacological HIF-1 stabilization promotes intestinal epithelial healing through regulation of α-integrin expression and function. Am J Physiol Gastrointest Liver Physiol. 2021 Apr 1;320(4):G420-G438. doi: 10.1152/ajpgi.00192.2020. Epub 2021 Jan 20. PMID: 33470153.
- Wakefield AJ, Sawyerr AM, Dhillon AP, Pittilo RM, Rowles PM, Lewis AA, Pounder RE. Pathogenesis of Crohn's disease: multifocal gastrointestinal infarction. Lancet. 1989 Nov 4;2(8671):1057-62. doi: 10.1016/s0140-6736(89)91078-7. PMID: 2572794.
- Piasecki C. Role of ischaemia in the initiation of peptic ulcer. Ann R Coll Surg Engl. 1977 Nov;59(6):476-8. PMID: 931328; PMCID: PMC2491821.
- Kim YI, Yi EJ, Kim YD, Lee AR, Chung J, Ha HC, Cho JM, Kim SR, Ko HJ, Cheon JH, Hong YR, Chang SY. Local Stabilization of Hypoxia-Inducible Factor-1α Controls Intestinal Inflammation via Enhanced Gut Barrier Function and Immune Regulation. Front Immunol. 2021 Jan 14;11:609689. doi: 10.3389/fimmu.2020.609689. PMID: 33519819; PMCID: PMC7840603.
- Solanki S, Devenport SN, Ramakrishnan SK, Shah YM. Temporal induction of intestinal epithelial hypoxia-inducible factor-2α is sufficient to drive colitis. Am J Physiol Gastrointest Liver Physiol. 2019 Aug 1;317(2):G98-G107. doi: 10.1152/ajpgi.00081.2019. Epub 2019 Jun 26. PMID: 31241981; PMCID: PMC6734372.
- Mladenova DN, Dahlstrom JE, Tran PN, Benthani F, Bean EG, Ng I, Pangon L, Currey N, Kohonen-Corish MR. HIF1α deficiency reduces inflammation in a mouse model of proximal colon cancer. Dis Model Mech. 2015 Sep;8(9):1093-103. doi: 10.1242/dmm.019000. Epub 2015 Jul 16. PMID: 26183215; PMCID: PMC4582097.
- Wang Y, Chiang IL, Ohara TE, Fujii S, Cheng J, Muegge BD, Ver Heul A, Han ND, Lu Q, Xiong S, Chen F, Lai CW, Janova H, Wu R, Whitehurst CE, VanDussen KL, Liu TC, Gordon JI, Sibley LD, Stappenbeck TS. Long-Term Culture Captures Injury-Repair Cycles of Colonic Stem Cells. Cell. 2019 Nov 14;179(5):1144-1159.e15. doi: 10.1016/j.cell.2019.10.015. Epub 2019 Nov 7. PMID: 31708126; PMCID: PMC6904908.
- Langer V, Vivi E, Regensburger D, Winkler TH, Waldner MJ, Rath T, Schmid B, Skottke L, Lee S, Jeon NL, Wohlfahrt T, Kramer V, Tripal P, Schumann M, Kersting S, Handtrack C, Geppert CI, Suchowski K, Adams RH, Becker C, Ramming A, Naschberger E, Britzen-Laurent N, Stürzl M. IFN-γ drives inflammatory bowel disease pathogenesis through VE-cadherin-directed vascular barrier disruption. J Clin Invest. 2019 Nov 1;129(11):4691-4707. doi: 10.1172/JCI124884. PMID: 31566580; PMCID: PMC6819119.
- Yang S, Yu M, Sun L, Xiao W, Yang X, Sun L, Zhang C, Ma Y, Yang H, Liu Y, Lu D, Teitelbaum DH, Yang H. Interferon-γ-induced intestinal epithelial barrier dysfunction by NF-κB/HIF-1α pathway. J Interferon Cytokine Res. 2014 Mar;34(3):195-203. doi: 10.1089/jir.2013.0044. Epub 2013 Nov 15. PMID: 24237301.
- Glover LE, Irizarry K, Scully M, Campbell EL, Bowers BE, Aherne CM, Kominsky DJ, MacManus CF, Colgan SP. IFN-γ attenuates hypoxia-inducible factor (HIF) activity in intestinal epithelial cells through transcriptional repression of HIF-1β. J Immunol. 2011 Feb 1;186(3):1790-8. doi: 10.4049/jimmunol.1001442. Epub 2011 Jan 3. PMID: 21199896; PMCID: PMC4040106.
- Pasparakis M. IKK/NF-kappaB signaling in intestinal epithelial cells controls immune homeostasis in the gut. Mucosal Immunol. 2008 Nov;1 Suppl 1:S54-7. doi: 10.1038/mi.2008.53. PMID: 19079232.
- Lakhdari O, Tap J, Béguet-Crespel F, Le Roux K, de Wouters T, Cultrone A, Nepelska M, Lefèvre F, Doré J, Blottière HM. Identification of NF-κB modulation capabilities within human intestinal commensal bacteria. J Biomed Biotechnol. 2011;2011:282356. doi: 10.1155/2011/282356. Epub 2011 Jun 14. PMID: 21765633; PMCID: PMC3134244.
- Zhu L, Han J, Li L, Wang Y, Li Y, Zhang S. Claudin Family Participates in the Pathogenesis of Inflammatory Bowel Diseases and Colitis-Associated Colorectal Cancer. Front Immunol. 2019 Jun 27;10:1441. doi: 10.3389/fimmu.2019.01441. PMID: 31316506; PMCID: PMC6610251.
- Weber CR, Nalle SC, Tretiakova M, Rubin DT, Turner JR. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab Invest. 2008 Oct;88(10):1110-20. doi: 10.1038/labinvest.2008.78. Epub 2008 Aug 18. PMID: 18711353; PMCID: PMC2586671.
- Kim TI. The Role of Barrier Dysfunction and Change of Claudin Expression in Inflammatory Bowel Disease. Gut Liver. 2015 Nov 23;9(6):699-700. doi: 10.5009/gnl15430. PMID: 26503569; PMCID: PMC4625696.
- Saeedi BJ, Kao DJ, Kitzenberg DA, Dobrinskikh E, Schwisow KD, Masterson JC, Kendrick AA, Kelly CJ, Bayless AJ, Kominsky DJ, Campbell EL, Kuhn KA, Furuta GT, Colgan SP, Glover LE. HIF-dependent regulation of claudin-1 is central to intestinal epithelial tight junction integrity. Mol Biol Cell. 2015 Jun 15;26(12):2252-62. doi: 10.1091/mbc.E14-07-1194. Epub 2015 Apr 22. PMID: 25904334; PMCID: PMC4462943.
- Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, Taylor TD, Itoh K, Kikuchi J, Morita H, Hattori M, Ohno H. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011 Jan 27;469(7331):543-7. doi: 10.1038/nature09646. PMID: 21270894.
- Vacca I. Microbiome: The microbiota maintains oxygen balance in the gut. Nat Rev Microbiol. 2017 Oct;15(10):574-575. doi: 10.1038/nrmicro.2017.112. Epub 2017 Sep 4. PMID: 28867820.
- Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007 Aug 21;104(34):13780-5. doi: 10.1073/pnas.0706625104. Epub 2007 Aug 15. PMID: 17699621; PMCID: PMC1959459.
- Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl. 2003 Sep;91(441):48-55. doi: 10.1111/j.1651-2227.2003.tb00646.x. PMID: 14599042.
- Favier CF, Vaughan EE, De Vos WM, Akkermans AD. Molecular monitoring of succession of bacterial communities in human neonates. Appl Environ Microbiol. 2002 Jan;68(1):219-26. doi: 10.1128/AEM.68.1.219-226.2002. PMID: 11772630; PMCID: PMC126580.
- Friedman ES, Bittinger K, Esipova TV, Hou L, Chau L, Jiang J, Mesaros C, Lund PJ, Liang X, FitzGerald GA, Goulian M, Lee D, Garcia BA, Blair IA, Vinogradov SA, Wu GD. Microbes vs. chemistry in the origin of the anaerobic gut lumen. Proc Natl Acad Sci U S A. 2018 Apr 17;115(16):4170-4175. doi: 10.1073/pnas.1718635115. Epub 2018 Apr 2. Erratum in: Proc Natl Acad Sci U S A. 2022 Jun 14;119(24):e2207826119. doi: 10.1073/pnas.2207826119. PMID: 29610310; PMCID: PMC5910840.
- Kaiko GE, Ryu SH, Koues OI, Collins PL, Solnica-Krezel L, Pearce EJ, Pearce EL, Oltz EM, Stappenbeck TS. The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell. 2016 Jun 16;165(7):1708-1720. doi: 10.1016/j.cell.2016.05.018. Epub 2016 Jun 2. Erratum in: Cell. 2016 Nov 3;167(4):1137. doi: 10.1016/j.cell.2016.10.034. PMID: 27264604; PMCID: PMC5026192.
- Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, Weir TL, Ehrentraut SF, Pickel C, Kuhn KA, Lanis JM, Nguyen V, Taylor CT, Colgan SP. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe. 2015 May 13;17(5):662-71. doi: 10.1016/j.chom.2015.03.005. Epub 2015 Apr 9. PMID: 25865369; PMCID: PMC4433427.
- Li S, Lu CW, Diem EC, Li W, Guderian M, Lindenberg M, Kruse F, Buettner M, Floess S, Winny MR, Geffers R, Richnow HH, Abraham WR, Grassl GA, Lochner M. Acetyl-CoA-Carboxylase 1-mediated de novo fatty acid synthesis sustains Lgr5+ intestinal stem cell function. Nat Commun. 2022 Jul 9;13(1):3998. doi: 10.1038/s41467-022-31725-2. PMID: 35810180; PMCID: PMC9271096.
- Wang RX, Henen MA, Lee JS, Vögeli B, Colgan SP. Microbiota-derived butyrate is an endogenous HIF prolyl hydroxylase inhibitor. Gut Microbes. 2021 Jan-Dec;13(1):1938380. doi: 10.1080/19490976.2021.1938380. PMID: 34190032; PMCID: PMC8253137.
- Olcina MM, Giaccia AJ. Reducing radiation-induced gastrointestinal toxicity - the role of the PHD/HIF axis. J Clin Invest. 2016 Oct 3;126(10):3708-3715. doi: 10.1172/JCI84432. Epub 2016 Aug 22. PMID: 27548524; PMCID: PMC5096800.
- Strowitzki MJ, Kimmer G, Wehrmann J, Ritter AS, Radhakrishnan P, Opitz VM, Tuffs C, Biller M, Kugler J, Keppler U, Harnoss JM, Klose J, Schmidt T, Blanco A, Taylor CT, Schneider M. Inhibition of HIF-prolyl hydroxylases improves healing of intestinal anastomoses. JCI Insight. 2021 Mar 30;6(8):e139191. doi: 10.1172/jci.insight.139191. PMID: 33784253; PMCID: PMC8119215.
- Li Y, Wang Y, Shi F, Zhang X, Zhang Y, Bi K, Chen X, Li L, Diao H. Phospholipid metabolites of the gut microbiota promote hypoxia-induced intestinal injury via CD1d-dependent γδ T cells. Gut Microbes. 2022 Jan-Dec;14(1):2096994. doi: 10.1080/19490976.2022.2096994. PMID: 35898110; PMCID: PMC9336479.
- Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008 Feb;134(2):577-94. doi: 10.1053/j.gastro.2007.11.059. PMID: 18242222.
- Seksik P, Rigottier-Gois L, Gramet G, Sutren M, Pochart P, Marteau P, Jian R, Doré J. Alterations of the dominant faecal bacterial groups in patients with Crohn's disease of the colon. Gut. 2003 Feb;52(2):237-42. doi: 10.1136/gut.52.2.237. PMID: 12524406; PMCID: PMC1774977.
- Martin HM, Campbell BJ, Hart CA, Mpofu C, Nayar M, Singh R, Englyst H, Williams HF, Rhodes JM. Enhanced Escherichia coli adherence and invasion in Crohn's disease and colon cancer. Gastroenterology. 2004 Jul;127(1):80-93. doi: 10.1053/j.gastro.2004.03.054. PMID: 15236175.
- Sokol H, Seksik P, Rigottier-Gois L, Lay C, Lepage P, Podglajen I, Marteau P, Doré J. Specificities of the fecal microbiota in inflammatory bowel disease. Inflamm Bowel Dis. 2006 Feb;12(2):106-11. doi: 10.1097/01.MIB.0000200323.38139.c6. PMID: 16432374.
- Swidsinski A, Loening-Baucke V, Theissig F, Engelhardt H, Bengmark S, Koch S, Lochs H, Dörffel Y. Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut. 2007 Mar;56(3):343-50. doi: 10.1136/gut.2006.098160. Epub 2006 Aug 14. PMID: 16908512; PMCID: PMC1856798.
- Fujimoto T, Imaeda H, Takahashi K, Kasumi E, Bamba S, Fujiyama Y, Andoh A. Decreased abundance of Faecalibacterium prausnitzii in the gut microbiota of Crohn's disease. J Gastroenterol Hepatol. 2013 Apr;28(4):613-9. doi: 10.1111/jgh.12073. PMID: 23216550.
- Takahashi K, Nishida A, Fujimoto T, Fujii M, Shioya M, Imaeda H, Inatomi O, Bamba S, Sugimoto M, Andoh A. Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn's Disease. Digestion. 2016;93(1):59-65. doi: 10.1159/000441768. Epub 2016 Jan 14. Erratum in: Digestion. 2016;93(2):174. doi: 10.1159/000444541. PMID: 26789999.
- Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018 Feb;11(1):1-10. doi: 10.1007/s12328-017-0813-5. Epub 2017 Dec 29. PMID: 29285689.
- Kushkevych I, Dordević D, Vítězová M. Possible synergy effect of hydrogen sulfide and acetate produced by sulfate-reducing bacteria on inflammatory bowel disease development. J Adv Res. 2020 Mar 24;27:71-78. doi: 10.1016/j.jare.2020.03.007. PMID: 33318867; PMCID: PMC7728581.
- Varela E, Manichanh C, Gallart M, Torrejón A, Borruel N, Casellas F, Guarner F, Antolin M. Colonisation by Faecalibacterium prausnitzii and maintenance of clinical remission in patients with ulcerative colitis. Aliment Pharmacol Ther. 2013 Jul;38(2):151-61. doi: 10.1111/apt.12365. Epub 2013 Jun 3. PMID: 23725320.
- Hultén L, Lindhagen J, Lundgren O, Fasth S, Ahrén C. Regional intestinal blood flow in ulcerative colitis and Crohn's disease. Gastroenterology. 1977 Mar;72(3):388-96. PMID: 832785.
- Harris NR, Carter PR, Lee S, Watts MN, Zhang S, Grisham MB. Association between blood flow and inflammatory state in a T-cell transfer model of inflammatory bowel disease in mice. Inflamm Bowel Dis. 2010 May;16(5):776-82. doi: 10.1002/ibd.21126. PMID: 19821506; PMCID: PMC2856723.
- D'Alessio S, Ungaro F, Noviello D, Lovisa S, Peyrin-Biroulet L, Danese S. Revisiting fibrosis in inflammatory bowel disease: the gut thickens. Nat Rev Gastroenterol Hepatol. 2022 Mar;19(3):169-184. doi: 10.1038/s41575-021-00543-0. Epub 2021 Dec 7. PMID: 34876680.
- Simmen S, Maane M, Rogler S, Baebler K, Lang S, Cosin-Roger J, Atrott K, Frey-Wagner I, Spielmann P, Wenger RH, Weder B, Zeitz J, Vavricka SR, Rogler G, de Vallière C, Hausmann M, Ruiz PA. Hypoxia Reduces the Transcription of Fibrotic Markers in the Intestinal Mucosa. Inflamm Intest Dis. 2021 May;6(2):87-100. doi: 10.1159/000513061. Epub 2021 Mar 29. PMID: 34124180; PMCID: PMC8160569.
- Johnson LA, Luke A, Sauder K, Moons DS, Horowitz JC, Higgins PD. Intestinal fibrosis is reduced by early elimination of inflammation in a mouse model of IBD: impact of a "Top-Down" approach to intestinal fibrosis in mice. Inflamm Bowel Dis. 2012 Mar;18(3):460-71. doi: 10.1002/ibd.21812. Epub 2011 Jul 14. PMID: 21761511; PMCID: PMC3206985.
- Cosnes J, Nion-Larmurier I, Beaugerie L, Afchain P, Tiret E, Gendre JP. Impact of the increasing use of immunosuppressants in Crohn's disease on the need for intestinal surgery. Gut. 2005 Feb;54(2):237-41. doi: 10.1136/gut.2004.045294. Erratum in: Gut. 2005 May;54(5):734. PMID: 15647188; PMCID: PMC1774826.
- Bazett M, Honeyman L, Stefanov AN, Pope CE, Hoffman LR, Haston CK. Cystic fibrosis mouse model-dependent intestinal structure and gut microbiome. Mamm Genome. 2015 Jun;26(5-6):222-34. doi: 10.1007/s00335-015-9560-4. Epub 2015 Feb 27. PMID: 25721416; PMCID: PMC4629800.
- Rieder F. The gut microbiome in intestinal fibrosis: environmental protector or provocateur? Sci Transl Med. 2013 Jun 19;5(190):190ps10. doi: 10.1126/scitranslmed.3004731. PMID: 23785034; PMCID: PMC3823049.
- Nishihara Y, Ogino H, Tanaka M, Ihara E, Fukaura K, Nishioka K, Chinen T, Tanaka Y, Nakayama J, Kang D, Ogawa Y. Mucosa-associated gut microbiota reflects clinical course of ulcerative colitis. Sci Rep. 2021 Jul 2;11(1):13743. doi: 10.1038/s41598-021-92870-0. PMID: 34215773; PMCID: PMC8253849.
- Fachi JL, Felipe JS, Pral LP, da Silva BK, Corrêa RO, de Andrade MCP, da Fonseca DM, Basso PJ, Câmara NOS, de Sales E Souza ÉL, Dos Santos Martins F, Guima SES, Thomas AM, Setubal JC, Magalhães YT, Forti FL, Candreva T, Rodrigues HG, de Jesus MB, Consonni SR, Farias ADS, Varga-Weisz P, Vinolo MAR. Butyrate Protects Mice from Clostridium difficile-Induced Colitis through an HIF-1-Dependent Mechanism. Cell Rep. 2019 Apr 16;27(3):750-761.e7. doi: 10.1016/j.celrep.2019.03.054. PMID: 30995474.
- Quévrain E, Maubert MA, Michon C, Chain F, Marquant R, Tailhades J, Miquel S, Carlier L, Bermúdez-Humarán LG, Pigneur B, Lequin O, Kharrat P, Thomas G, Rainteau D, Aubry C, Breyner N, Afonso C, Lavielle S, Grill JP, Chassaing G, Chatel JM, Trugnan G, Xavier R, Langella P, Sokol H, Seksik P. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn's disease. Gut. 2016 Mar;65(3):415-425. doi: 10.1136/gutjnl-2014-307649. Epub 2015 Jun 4. PMID: 26045134; PMCID: PMC5136800.
- Giri R, Hoedt EC, Khushi S, Salim AA, Bergot AS, Schreiber V, Thomas R, McGuckin MA, Florin TH, Morrison M, Capon RJ, Ó Cuív P, Begun J. Secreted NF-κB suppressive microbial metabolites modulate gut inflammation. Cell Rep. 2022 Apr 12;39(2):110646. doi: 10.1016/j.celrep.2022.110646. PMID: 35417687.
- Ramakrishnan SK, Shah YM. Role of Intestinal HIF-2α in Health and Disease. Annu Rev Physiol. 2016;78:301-25. doi: 10.1146/annurev-physiol-021115-105202. Epub 2015 Nov 19. PMID: 26667076; PMCID: PMC4809193.
- McElrath C, Espinosa V, Lin JD, Peng J, Sridhar R, Dutta O, Tseng HC, Smirnov SV, Risman H, Sandoval MJ, Davra V, Chang YJ, Pollack BP, Birge RB, Galan M, Rivera A, Durbin JE, Kotenko SV. Critical role of interferons in gastrointestinal injury repair. Nat Commun. 2021 May 11;12(1):2624. doi: 10.1038/s41467-021-22928-0. PMID: 33976143; PMCID: PMC8113246.
- Nikolaus S, Rutgeerts P, Fedorak R, Steinhart AH, Wild GE, Theuer D, Möhrle J, Schreiber S. Interferon beta-1a in ulcerative colitis: a placebo controlled, randomised, dose escalating study. Gut. 2003 Sep;52(9):1286-90. doi: 10.1136/gut.52.9.1286. Erratum in: Gut. 2003 Nov;52(11):1657. PMID: 12912859; PMCID: PMC1773804.
- Peng T, Du SY, Son M, Diamond B. HIF-1α is a negative regulator of interferon regulatory factors: Implications for interferon production by hypoxic monocytes. Proc Natl Acad Sci U S A. 2021 Jun 29;118(26):e2106017118. doi: 10.1073/pnas.2106017118. PMID: 34108245; PMCID: PMC8256008.
- Braverman J, Sogi KM, Benjamin D, Nomura DK, Stanley SA. HIF-1α Is an Essential Mediator of IFN-γ-Dependent Immunity to Mycobacterium tuberculosis. J Immunol. 2016 Aug 15;197(4):1287-97. doi: 10.4049/jimmunol.1600266. Epub 2016 Jul 18. PMID: 27430718; PMCID: PMC4976004.
- Janikashvili N, Chikovani T, Audia S, Bonnotte B, Larmonier N. T Lymphocyte Plasticity in Autoimmunity and Cancer. Biomed Res Int. 2015;2015:540750. doi: 10.1155/2015/540750. Epub 2015 Oct 25. PMID: 26587539; PMCID: PMC4637466.
- Larmonier CB, Shehab KW, Ghishan FK, Kiela PR. T Lymphocyte Dynamics in Inflammatory Bowel Diseases: Role of the Microbiome. Biomed Res Int. 2015;2015:504638. doi: 10.1155/2015/504638. Epub 2015 Oct 25. PMID: 26583115; PMCID: PMC4637034.
- Rosati E, Rios Martini G, Pogorelyy MV, Minervina AA, Degenhardt F, Wendorff M, Sari S, Mayr G, Fazio A, Dowds CM, Hauser C, Tran F, von Schönfels W, Pochhammer J, Salnikova MA, Jaeckel C, Gigla JB, Sabet SS, Hübenthal M, Schiminsky E, Schreiber S, Rosenstiel PC, Scheffold A, Thomas PG, Lieb W, Bokemeyer B, Witte M, Aden K, Hendricks A, Schafmayer C, Egberts JH, Mamedov IZ, Bacher P, Franke A. A novel unconventional T cell population enriched in Crohn's disease. Gut. 2022 Nov;71(11):2194-2204. doi: 10.1136/gutjnl-2021-325373. Epub 2022 Mar 9. PMID: 35264446; PMCID: PMC9554086.
- Ray K. Deciphering the role of CD8+ T cells in IBD: from single-cell analysis to biomarkers. Nat Rev Gastroenterol Hepatol. 2020 Oct;17(10):595. doi: 10.1038/s41575-020-00362-9. PMID: 32884120.
- Casalegno Garduño R, Däbritz J. New Insights on CD8+ T Cells in Inflammatory Bowel Disease and Therapeutic Approaches. Front Immunol. 2021 Oct 11;12:738762. doi: 10.3389/fimmu.2021.738762. PMID: 34707610; PMCID: PMC8542854.
- Gronbach K, Flade I, Holst O, Lindner B, Ruscheweyh HJ, Wittmann A, Menz S, Schwiertz A, Adam P, Stecher B, Josenhans C, Suerbaum S, Gruber AD, Kulik A, Huson D, Autenrieth IB, Frick JS. Endotoxicity of lipopolysaccharide as a determinant of T-cell-mediated colitis induction in mice. Gastroenterology. 2014 Mar;146(3):765-75. doi: 10.1053/j.gastro.2013.11.033. Epub 2013 Nov 21. PMID: 24269927.
- Lo Presti E, Mocciaro F, Mitri RD, Corsale AM, Di Simone M, Vieni S, Scibetta N, Unti E, Dieli F, Meraviglia S. Analysis of colon-infiltrating γδ T cells in chronic inflammatory bowel disease and in colitis-associated cancer. J Leukoc Biol. 2020 Aug;108(2):749-760. doi: 10.1002/JLB.5MA0320-201RR. Epub 2020 Mar 23. PMID: 32202356.
- Bakshi HA, Mishra V, Satija S, Mehta M, Hakkim FL, Kesharwani P, Dua K, Chellappan DK, Charbe NB, Shrivastava G, Rajeshkumar S, Aljabali AA, Al-Trad B, Pabreja K, Tambuwala MM. Dynamics of Prolyl Hydroxylases Levels During Disease Progression in Experimental Colitis. Inflammation. 2019 Dec;42(6):2032-2036. doi: 10.1007/s10753-019-01065-3. PMID: 31377947; PMCID: PMC6856031.
- Marks E, Goggins BJ, Cardona J, Cole S, Minahan K, Mateer S, Walker MM, Shalwitz R, Keely S. Oral delivery of prolyl hydroxylase inhibitor: AKB-4924 promotes localized mucosal healing in a mouse model of colitis. Inflamm Bowel Dis. 2015 Feb;21(2):267-75. doi: 10.1097/MIB.0000000000000277. PMID: 25545377.
- Taylor CT, Dzus AL, Colgan SP. Autocrine regulation of epithelial permeability by hypoxia: role for polarized release of tumor necrosis factor alpha. Gastroenterology. 1998 Apr;114(4):657-68. doi: 10.1016/s0016-5085(98)70579-7. PMID: 9516386.
- Podlesny D, Durdevic M, Paramsothy S, Kaakoush NO, Högenauer C, Gorkiewicz G, Walter J, Fricke WF. Identification of clinical and ecological determinants of strain engraftment after fecal microbiota transplantation using metagenomics. Cell Rep Med. 2022 Aug 16;3(8):100711. doi: 10.1016/j.xcrm.2022.100711. Epub 2022 Aug 4. PMID: 35931074; PMCID: PMC9418803.
- Catalan-Serra I, Brenna Ø. Immunotherapy in inflammatory bowel disease: Novel and emerging treatments. Hum Vaccin Immunother. 2018;14(11):2597-2611. doi: 10.1080/21645515.2018.1461297. Epub 2018 May 22. PMID: 29624476; PMCID: PMC6314405.
- Chen ML, Sundrud MS. Cytokine Networks and T-Cell Subsets in Inflammatory Bowel Diseases. Inflamm Bowel Dis. 2016 May;22(5):1157-67. doi: 10.1097/MIB.0000000000000714. PMID: 26863267; PMCID: PMC4838490.
- Meredith J, Henderson P, Wilson DC, Van Limbergen J, Wine E, Russell RK. Withdrawal of Combination Immunotherapy in Paediatric Inflammatory Bowel Disease-An International Survey of Practice. J Pediatr Gastroenterol Nutr. 2021 Jul 1;73(1):54-60. doi: 10.1097/MPG.0000000000003098. PMID: 33661242.
- Xiong JQ, Fu YF, Qiu JH, Liao WD, Luo LY, Chen SH. Global research trends of immunotherapy and biotherapy for inflammatory bowel disease: a bibliometric analysis from 2002 to 2021. Biomed Eng Online. 2022 Jun 27;21(1):42. doi: 10.1186/s12938-022-01011-9. PMID: 35761289; PMCID: PMC9238098.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.