Biology Group. 2024 July 18;5(7):779-786. doi: 10.37871/jbres1955.
Comparative Study of the Reactivity of Animal-Derived Blood on Medical Device Materials using Hematology and Platelet Activity Tests
Jeonghwa Kim1, Geonyong Kim1, Sekyung Kim2, Joonho Eom2 and Taewon Kim1*
2Department of Medical Device Research Division, National Institute of Food and Drug Safety Evaluation, Cheongju, Korea
- ISO 10993-4
- ASTM F2888
- Medical device materials
- Platelet activation, leukocyte activation
- Hematology
- Platelet count
- Leukocyte count
Abstract
In accordance with the requirements of ISO 10993-4, we established the feasibility of conditions for animal blood application in vitro to identify substances that induce thrombus formation and confirmed the differences in blood reactivity between animals necessary for interpreting in vivo results in this study. To this end, differences in the response of rabbit, pig, and monkey blood to each anticoagulant presented in ASTM F2888-19 were tested.
It was confirmed that the direct heparinization method had a higher test sensitivity than that of recalcified sodium citrate, thus distinguishing thrombotic substances regardless of the test species. Particularly in pig blood, platelet and white blood cell counts showed the greatest decrease after reactions with various test materials.
Expression of β-TG and PMN-elastase, indicators for platelet and leukocyte activities, was confirmed in pig blood when reacted with various test materials. A decrease in platelet count and significant increase in β-TG were observed in the black rubber test group, which was the positive candidate material. Conversely, a decreased leukocyte count was confirmed, but an increase in the expression of PMN-elastase was not observed. This result satisfied the ISO 10993-4 criteria for determining a positive thromboembolic substance.
This study provided additional information on the selection of anticoagulants for the design of trials and selection of animal species for evaluating thrombosis formation on medical device materials by using the methods described in ISO 10993-4 and ASTM F2888-19.
Introduction
The International Standard ISO 10993-4:2017 regulates the biological evaluation of the interaction of medical devices with blood. It categorizes tests into hemolysis and thrombosis to determine the risks arising from contact of medical devices with circulating blood. The in vitro thrombosis test consists of hematology, platelet activation, coagulation, and complement tests, and ASTM F2888 has been proposed as a practice standard for platelet activation and hematology tests [1,2].
ASTM F2888 aims to identify materials that cause clot formation through measurement of blood clots induced by contact of blood with materials and reduction of blood cell counts in residual blood [3]. The stipulated blood anticoagulant in the ASTM F2888-13 standard was 3.2% sodium citrate [4]. Lu et, al. [5] reported that the use of sodium citrate anticoagulants prevented tests from effectively differentiating platelet or leukocyte counts when blood and materials were in contact; thus, re-mineralized citrated blood or low-dose heparin were commonly used and reported to be able to better discriminate between the substances [6] Standard ASTM F2888 was revised in 2019 to reflect these anticoagulant amendments [3].
In ISO10993-4:2017, the following conditions must be satisfied in residual blood reacted with materials for the determination of hematological reactions: Decrease in the number of leukocytes or increase in the molecular marker, Polymorphonuclear Neutrophil (PMN)-elastase, as a sign of leukocyte activity; and a simultaneous decrease in platelet count in blood with an increase in the activating factors, β-Thromboglobulin (β-TG) and PF4, as a sign of platelet activity [2,8,9]. After platelet activation, alpha granules release large amounts of β-TG, inducing leukocyte recruitment, as measured using an Enzyme-Linked Immunosorbent Assay (ELISA) [10-14]. In addition to platelet activation, leukocyte activation is determined through the release of PMN-elastase, and this release from PMN granulocytes, particularly neutrophils, can be quantified with ELISA [15].
Due to differences in blood reactivity between species, human blood should be used if possible. In addition, except for cases where feasibility has been confirmed in vitro, it is usually specified that blood tests should start within 4 h of blood collection [2]. Due to such conditions, the test using human blood has limitations in confirming repeatability. For this reason, animal blood is used instead of human blood in many in vitro blood reaction studies [1].This study aimed to verify reactivity according to the application of blood anticoagulants proposed by Lu, et al. [5,6] and ASTM F2888 [3,4] in the blood of animals (rabbit, pig, and monkey) and confirm the effectiveness of animal blood used for hematology and platelet activation in vitro to identify substances that induced thrombus formation.
Materials and Methods
Medical device materials and reagents
Calcium chloride solution and heparin were purchased from Sigma-Aldrich (St. Louis, MO, USA) and EDTA from Bioneer (Daejeon, South Korea). High Density Polyethylene Film (HDPE) was purchased from Kemidas (Gyeonggi-do, South Korea). Black rubber and high-purity Tygon tubing were purchased from Saint-Gobain (Courbevoie, France). The β-TG and PMN-elastase ELISA kits were purchased from MyBioSource (San Diego, CA, USA).
Experimental animals and preparation of experimental blood
Rabbit (Oryctolagus cuniculus) blood from healthy adult donors was obtained from the Korea Testing Certification Institute (Cheongju, South Korea) with a protocol approved by their Institutional Review Board. Monkey (Rhesus macaque) and porcine (Jeju native black pig, Sus scrofa) blood were purchased from the Korea Research Institute of Bioscience and Biotechnology (Daejon, South Korea) and Cronex (Gyeonggi-do, South Korea), respectively. Circulating venous blood of the laboratory animals was anticoagulated with 3.2% 0.105 M sodium citrate (9:1 v/v blood:sodium citrate) or low concentrations of heparin (final concentration of approximately 1 U/mL), and differences on application of anticoagulants compared. Since blood reactivity may vary between individual animals within the same species, tests were conducted using three or more animals for each species. Blood samples were stored at room temperature (15-25°C) until testing. See table 1 for a summary of the experimental parameters used. The reaction with anticoagulant was initiated and completed within 2 and 4 h of blood collection, respectively.
| Table 1: Summary of experimental parameters. | |
| Parameters | Conditions |
| Species (strain, sex, age) | Rabbit (Oryctolagus cuniculus, Female, 16 weeks), Monkey (Rhesus macaque, Male, 5 months), Porcine (Sus scrofa, Female, 3 months) |
| Anticoagulant (final ratio with blood) | 3.2% sodium citrate (blood:sodium citrate solution volume ratio = 9:1), heparin (final concentration = 1 U/mL) |
| Test materials | HDPE, black rubber, latex rubber, PVC, TPU, TPE |
| HDPE: High Density Polyethylene Film; PVC: Poly Vinyl Chloride; TPU: Thermoplastic Polyurethane; TPE: Thermoplastic Elastome | |
Testing sodium citrate-anticoagulated blood with medical device materials
Testing of reactions with medical device materials (test materials, table 1) using sodium citrate-anticoagulated blood was performed according to the procedure described in Section 12 of the ASTM F2888-19 standard [3]. Before adding blood to the test material, it was recalcified with 2 M calcium chloride to achieve a final calcium concentration of 10 mM. Then, heparin stock solution (200 U/mL) was added to the blood to obtain a final concentration of 2 U/mL. After the test materials were exposed to an appropriate amount of blood (surface area of the material/blood: 12 cm2/1 mL), they were reacted in a shaking water bath (60 rev/min) at 37°C for 1 h.
Testing heparinized blood with medical device materials
Heparinized anticoagulated blood (final concentration of approximately 1 U/mL) was reacted with the test materials. Procedures and methods for material reactions were the same as those for sodium citrate-anticoagulated blood.
Measurement of the number of platelets and white blood cells in reaction-completed blood
After reactions with the test materials were completed, they were removed from the blood. EDTA solution was added to blood, adjusting the final concentration to 5 mm to prevent further reactions. Each blood sample was then transferred to a new polypropylene tube and placed on ice until platelet and leukocyte counts were measured using an automated hematology analyzer (HV950FS; Drew Scientific, Miami Lakes, FL, USA).
Enzyme-linked immunosorbent assay
The analyzed blood was centrifuged for 15 min at 3,000 rpm. After centrifugation, plasma was collected and stored at -80°C until analysis. The concentration of β-TG and PMN-elastase was determined using an ELISA kit according to the manufacturer’s instructions. The absorbance at 450 nm was determined using a spectrophotometer.
Statistical analyses
Statistical analyses were performed using Student’s t-test and values indicated as mean ± Standard Deviation (SD). Significance was set at p < 0.05.
Results
Changes in leukocyte and platelet counts in monkey blood in contact with high density polyethylene film and black rubber
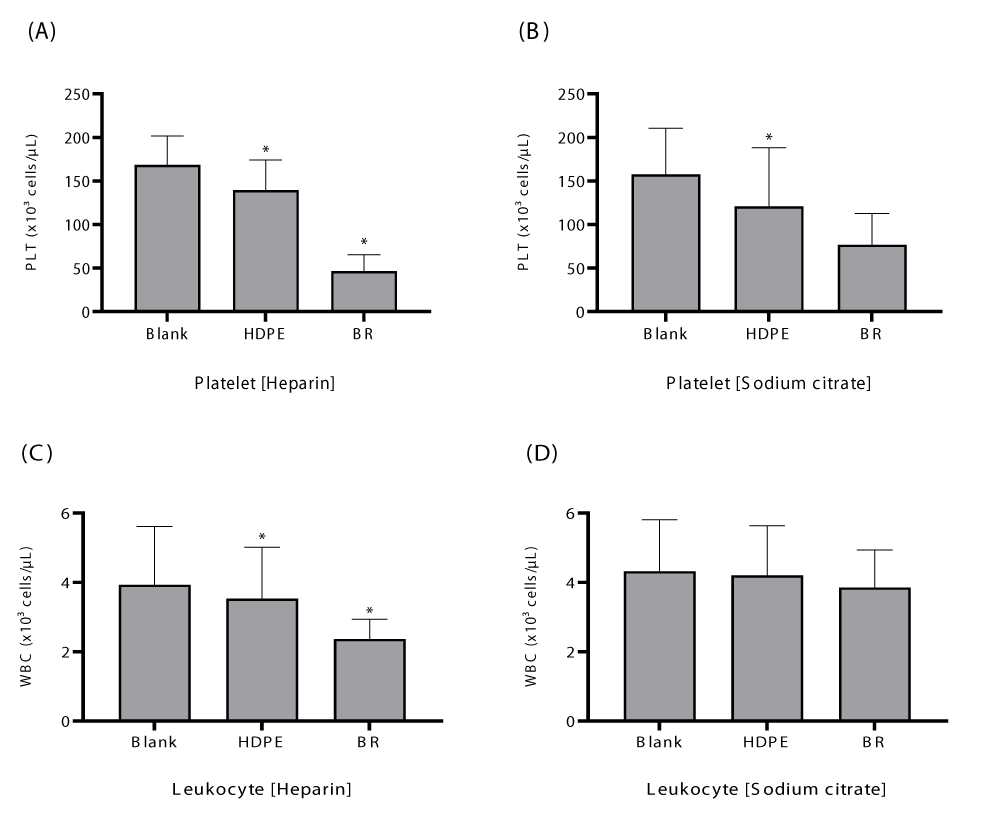
To determine the different effects of blood anticoagulants specified in ASTM F2888 (sodium citrate and heparin) on thrombogenesis in monkey blood, the anticoagulated blood was reacted with HDPE and black rubber, and changes in the number of leukocytes and platelets in the blood measured. In sodium citrate-anticoagulated blood, except for the HDPE test group, no significant changes in the number of platelets and white blood cells due to reaction with the test materials were observed (p < 0.05). In contrast, in heparinized blood, a significant decrease in the number of platelets and leukocytes due to reaction with the test materials was observed in all test groups (p < 0.05) (Figure 1).
Changes in leukocyte and platelet counts in rabbit blood in contact with high density polyethylene film and black rubber
To examine the difference in reactivity between sodium citrate and heparin anticoagulants in rabbit blood, the blood was reacted with test materials (HDPE and black rubber) and changes in leukocyte and platelet counts measured.
In sodium citrate anticoagulant-treated blood, no significant changes in platelet and leukocyte counts due to reaction with the test materials were observed, except for some test groups (the number of leukocytes in blood reacted with black rubber) (p < 0.05).
In heparinized blood, significant changes in platelet counts due to reaction with the test materials were observed in all test groups (p < 0.05); however, the leukocyte counts did not show significant changes in the HDPE and black rubber test groups compared to those of the blank control group (p < 0.05) (Figure 2).
Comparison of hematology and platelet activity responses towards contact with porcine blood and reference control materials
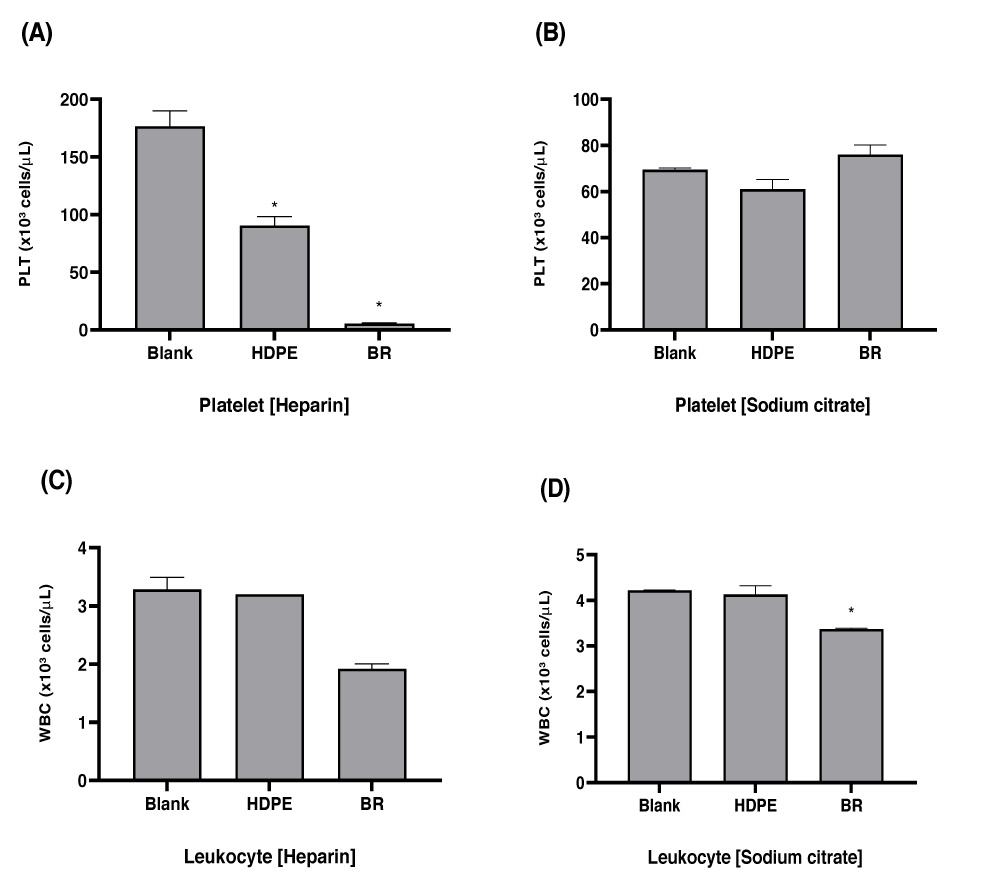
After heparinized anticoagulant-treated pig blood was reacted with the test materials, concentrations of PMN-elastase and β-TG, along with leukocyte and platelet counts in the blood, were measured.
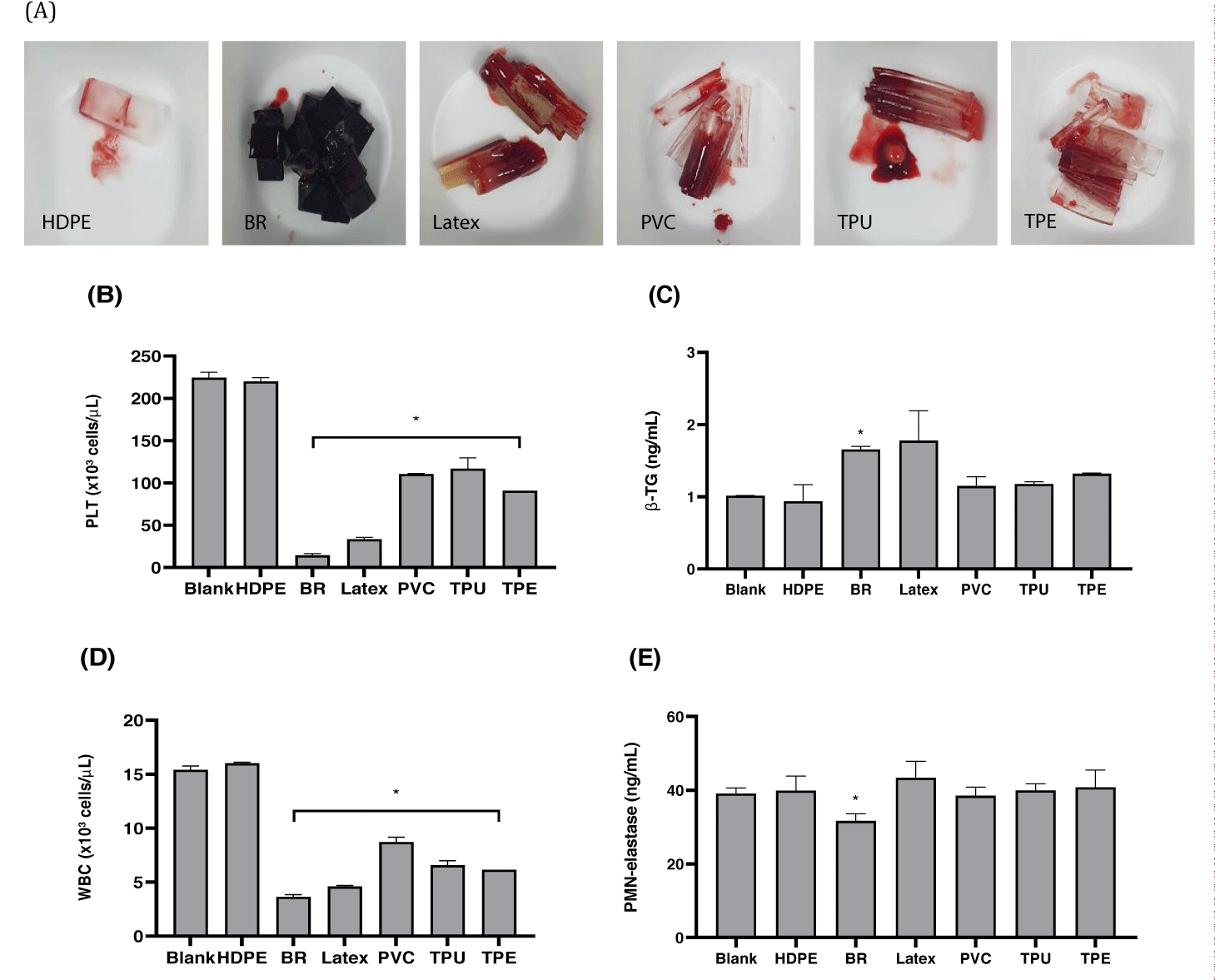
Blood reacted with black rubber, latex, PVC, TPU, and TPE, the positive reference materials recommended by the standard ASTM F2888, and showed significant reductions in platelet and leukocyte counts compared to those of the blank and negative control material, HDPE (p < 0.05). β-TG tended to increase in the black rubber and latex test groups, with a significant increase observed in the black rubber test group (p < 0.05). A significant decrease in PMN-elastase was observed in the black rubber test group, but no change in the other test groups (p < 0.05).
Comparison of changes in leukocyte and platelet counts after contact between test materials and animal blood
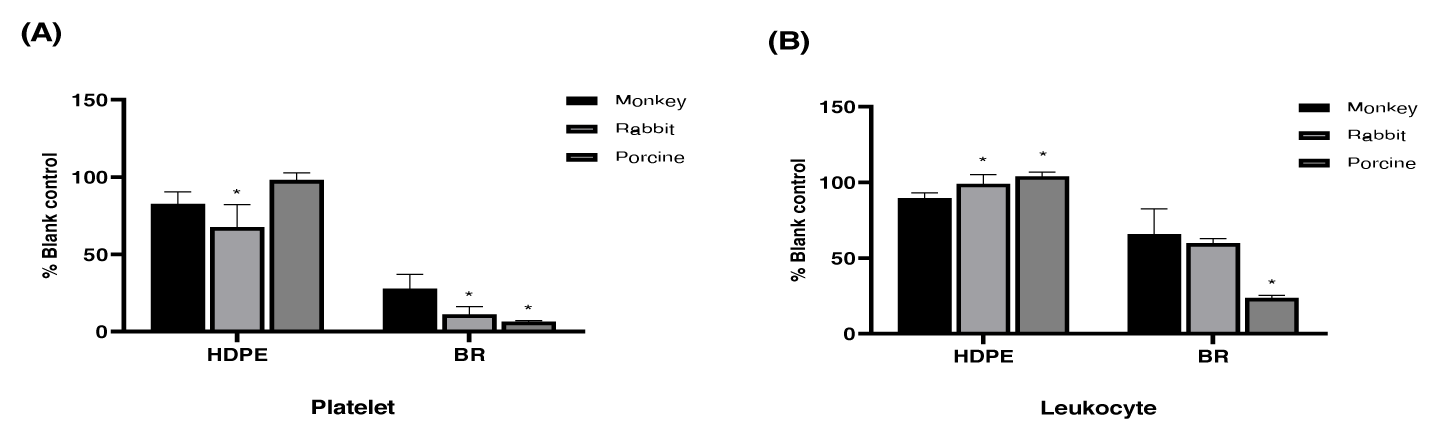
After exposing the test materials with heparinized anticoagulant rabbit, monkey, and pig blood, differences in the leukocyte and platelet counts were observed in each sample. The number of platelets in the blood that reacted with HDPE, a candidate negative control material, was in the range of 67-98% (monkey, 82%; rabbit, 67%; and pig, 98%; based on average values) compared to the blank control group. The leukocyte count in blood was in the range of 89-104% compared to the blank control group (monkey, 89%; rabbit, 99%; pig, 104%; based on the average values). The platelet count in blood reacted with black rubber, a positive control candidate, was 6-27% (monkey, 27%; rabbit, 11%; pig, 6%; based on the average values) and the leukocyte count in the range of 23-65% (monkey, 65%; rabbit, 59%; pig, 23%; based on average values). Compared to monkey blood, rabbit blood showed a significant decrease in platelet count (p < 0.05). Contrastingly, pig blood showed a significant decrease in platelet and leukocyte counts compared to those of monkey blood (p < 0.05).
Discussion
This study aimed to establish a hematological test method for the application of animal blood in evaluating thrombogenicity of medical device materials described in ISO 10993-4, the international standard for hemocompatibility assessment [2]. Since the medical device material acts as an activator of platelets and leukocytes [7], the test was conducted with reference to ASTM 2888-19, which enables quantitative confirmation of platelets and leukocytes in the blood [3]. For evaluating applicability and limitations of the standardized test method to animal blood, two anticoagulant conditions stipulated in the standard were applied to different types of animal blood, and the reactivity of negative and positive control test materials recommended in the standard were tested.
Two anticoagulation conditions were applied to negative and positive test controls in rabbit blood. In the case of sodium citrate anticoagulant, it was difficult to distinguish the negative and positive control groups because the difference in platelet and leukocyte counts was insignificant. Conversely, in the case of heparin anticoagulant, there was a large difference in the platelet and leukocyte counts between the negative and positive control groups (Figure 2).
Similar results were obtained for rabbit and monkey blood, the latter having similar properties and reactivity to that of human blood. Heparin anticoagulant showed greater differences in platelet and leukocyte counts than those of sodium citrate anticoagulant in monkey blood, indicating that it could discriminate between the negative and positive controls (Figure 3).
Sodium citrate did not discriminate against thrombotic biomaterials in an anticoagulant sensitivity test to distinguish thrombogenic from thrombotic-resistant substances [5]. Although the recalcification and addition of heparin to sodium citrate anticoagulant, as described in the standard, involves an additional testing step, blood can be transported and stored more stably under these anticoagulant conditions compared to direct heparinization [2]. The use of heparin anticoagulants has been shown to discriminate materials better than sodium citrate anticoagulants [5]. This was reconfirmed in our study in which heparinized blood showed more sensitivity and distinguishing ability for reactivity against medical device materials, confirming effectiveness of its application in the blood of three animal species (rabbit, pig, and monkey).
Comparison of the two anticoagulant conditions in rabbit and monkey blood confirmed that heparin was a more sensitive anticoagulant than sodium citrate. The blood specified in the standard is only human blood, and physiological levels of blood ionized calcium may vary between species, so the amount of calcium chloride required to recalcify animal blood may differ from that of human blood [16,17]. Since Ca++ plays an important role in blood coagulation and platelet activation processes, it is important to appropriately recalcify blood to restore the ionized Calcium to a physiological level specific to animal species. Based on the results, a total of four commercial medical device materials were tested to confirm the activation of platelets and leukocytes using heparinized pig blood that was then quantified, including the concentrations of β-TG and PMN-elastase, the activity indicators of blood factors. To quantify the concentration of β-TG and PMN-elastase in pigs, an Enzyme-Linked Immunosorbent Assay (ELISA) analysis designed for in vitro quantitative measurement of pig serum, plasma, or cell culture supernatant and tissue was used.
The platelet and leukocyte counts exposed to all test materials, except for the negative control group, decreased in heparinized pig blood. However, the concentration of β-TG was higher in black rubber and latex than in HDPE, the negative control group. Conversely, the concentration of PMN-elastase did not show a significant increase between test materials.
Similar to PLT and WBC count-based thrombotic markers, both molecular biomarkers (β-TG and PMN-elastase) were able to differentiate between negative and positive controls under the test conditions of the current study.
When the differences in leukocyte and platelet counts by blood origin were compared, it was found that their decrease due to contact with test materials was greater in the blood of pigs and rabbits than in that of monkeys (Figure 4).
The results of this study confirmed that, regardless of species, the application of blood anticoagulants proposed in the ASTM F2888-19 standard can distinguish thrombotic substances in animal blood, and platelet and leukocyte counts showed similar trends in human blood and the three tested animal species (rabbit, pig, and monkey). This shows that the application of animal blood can be effective in hematology and platelet activation tests and provides further information on the applications of animal blood and selection of anticoagulants. However since blood coagulation and activity differ between animal species, different heparin concentration ranges may be needed for different animal species to achieve appropriate test sensitivity. There is also a need to additionally validate various molecular biomarkers in other animal species.
Conclusion
The results of this study confirmed that the direct heparinization method has higher test sensitivity than the method using re-mineralized sodium citrate, distinguishing thrombogenic substances in animal blood regardless of species. In particular, pig blood showed the most sensitive results. This study provides further information on the application of animal blood and selection of anticoagulants through validation of the evaluation method used for substance-induced thrombosis for blood in contact with medical device material described in ASTM F2888-19.
Acknowledgment
We thank to everyone who helped.
Funding
This research was supported by a grant (21174MFDS242) from Ministry of Food and Drug Safety in 2022.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Biological evaluation of medical devices - Part 1: Evaluation and testing within a risk management process. Arlington, VA: The International Organization for Standardization (ISO). 2018;10993-1.
- Biological evaluation of medical devices - Part 4: Selection of tests for interactions with blood. Arlington, VA: The International Organization for Standardization (ISO). 2017;10993-4:
- Standard practice for platelet leukocyte count-an in-vitro measure for hemocompatibility assessment of cardiovascular materials. West Conshohocken, PA: ASTM International. 2019;F2888-19.
- Standard test method for platelet leukocyte count-an in-vitro measure for hemocompatibility assessment of cardiovascular materials. West Conshohocken, PA: ASTM International. 2013;F2888-13
- Lu Q, Nehrer JM, Li J, Jamiolkowski MA, Rinaldi JE, Malinauskas RA. Platelet and leukocyte count assay for thrombogenicity screening of biomaterials and medical devices: Evaluation and improvement of ASTM F2888 test standard. J Biomed Mater Res B Appl Biomater. 2021 Dec;109(12):2259-2267. doi: 10.1002/jbm.b.34887. Epub 2021 Jun 9. PMID: 34106517; PMCID: PMC8490275.
- Lu Q, Nehrer J, Malinauskas R. Platelet and leukocyte counts as hemocompatibility indicators for biomaterials: An evaluation of ASTM standard test method F2888-13. Paper presented at: Transactions of the 39th Annual Society for Biomaterials. Charlotte, NC: Omnipress. 2015;57.
- Jaffer IH, Fredenburgh JC, Hirsh J, Weitz JI. Medical device-induced thrombosis: what causes it and how can we prevent it? J Thromb Haemost. 2015 Jun;13 Suppl 1:S72-81. doi: 10.1111/jth.12961. PMID: 26149053.
- Kerry PJ, Curtis AD. Standardization of beta-thromboglobulin (beta-TG) and platelet factor 4 (PF4): a collaborative study to establish international standards for beta-TG and PF4. Thromb Haemost. 1985 Feb 18;53(1):51-5. PMID: 2581331.
- Swars H, Hafner G, Weilemann LS, Ehrenthal W, Schinzel H, Prellwitz W, Meyer J. Acute dialysis: PMN-elastase as a new parameter for controlling individual anticoagulation with low molecular weight heparin (Fragmin). Intensive Care Med. 1991;17(1):52-6. doi: 10.1007/BF01708410. PMID: 1645379.
- Brandt E, Petersen F, Ludwig A, Ehlert JE, Bock L, Flad HD. The beta-thromboglobulins and platelet factor 4: blood platelet-derived CXC chemokines with divergent roles in early neutrophil regulation. J Leukoc Biol. 2000 Apr;67(4):471-8. doi: 10.1002/jlb.67.4.471. PMID: 10770278.
- Fröhlich E. Action of Nanoparticles on Platelet Activation and Plasmatic Coagulation. Curr Med Chem. 2016;23(5):408-30. doi: 10.2174/0929867323666160106151428. PMID: 26063498; PMCID: PMC5403968.
- Mayer A, Vadon M, Rinner B, Novak A, Wintersteiger R, Fröhlich E. The role of nanoparticle size in hemocompatibility. Toxicology. 2009 Apr 28;258(2-3):139-47. doi: 10.1016/j.tox.2009.01.015. Epub 2009 Jan 22. PMID: 19428933.
- Teligui L, Dalmayrac E, Corbeau JJ, Bouquet E, Godon A, Denommé AS, Binuani P, Verron L, Boer C, Baufreton C. Ex vivo simulation of cardiopulmonary bypass with human blood for hemocompatibility testing. Perfusion. 2016 Jul;31(5):376-83. doi: 10.1177/0267659115599454. Epub 2015 Aug 4. PMID: 26243277.
- Stoll H, Steinle H, Stang K, Kunnakattu S, Scheideler L, Neumann B, Kurz J, Degenkolbe I, Perle N, Schlensak C, Wendel HP, Avci-Adali M. Generation of Large-Scale DNA Hydrogels with Excellent Blood and Cell Compatibility. Macromol Biosci. 2017 Apr;17(4). doi: 10.1002/mabi.201600252. Epub 2016 Oct 19. PMID: 27758025.
- Zimmermann AK, Weber N, Aebert H, Ziemer G, Wendel HP. Effect of biopassive and bioactive surface-coatings on the hemocompatibility of membrane oxygenators. J Biomed Mater Res B Appl Biomater. 2007 Feb;80(2):433-9. doi: 10.1002/jbm.b.30614. PMID: 16850460.
- Warren HB, Lausen NC, Segre GV, el-Hajj G, Brown EM. Regulation of calciotropic hormones in vivo in the New Zealand white rabbit. Endocrinology. 1989 Nov;125(5):2683-90. doi: 10.1210/endo-125-5-2683. PMID: 2507295.
- Harcourt-Brown F. Clinical pathology. Textbook of Rabbit Medicine. 2002;140-164.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.