Environmental Sciences Group. 2024 January 10;5(1):016-020. doi: 10.37871/jbres1867.
Forage Grass in Static Gas Exchange Chambers Deployed In Southern Amazon Influences Field Measurement of Soil N2O Emissions
Alexandre Ferreira do Nascimento1,2* and Anderson Ferreira2
2Embrapa Trigo, Rodovia BR 285, Km 294, Zona Rural, 99050-970, Passo Fundo, RS, Brazil
- Greenhouse gases
- Mitigation
- Pasture
- Livestock
Abstract
Static chambers are used to estimate the exchange of greenhouse gases between the soil and the atmosphere, but the presence of plants inside such chambers can alter gas fluxes. This study aimed to determine the influence of forage grass on N2O fluxes emanating from an oxisol in the southern Amazon region of Brazil. A randomized experiment comprising two treatments, namely static gas exchange Chambers with Grass (CWG) and Chambers with No Grass (CNG) with six replicates of each was performed to determine N2O fluxes over a period of one year. Soil N2O fluxes in the CWG were higher (19.08 µg N2O-N m-2 h-1) than those in the CNG (9.05 µg N2O-N m-2 h-1), most especially during the wet season. Cumulative N2O emissions were 1.60 and 0.72 kg N2O-N ha-1 for the CWG and CNG, respectively. The higher N2O estimates in the CWG may be attributed to the plant transpiration stream and/or to changes in soil attributes induced by the plants. Measurement of N2O emissions from a grass-covered oxisol inside gas exchange chambers may overestimate soil N2O flux in the tropical humid climate of the Southern Amazon.
Introduction
Soil is an important source of Nitrous Oxide (N2O), a potent gas impacting atmospheric radiation balance and ozone chemistry, having a warming potential 298 times greater than CO2 [1]. Field measurements of N2O emissions from soils are normally performed by collecting headspace air samples from chambers deployed on the soil surface in order to quantify soil-atmosphere gas exchange over time [2]. Although the methodology has improved during the last decade or so, the effects exerted by plants inside the chambers on the flux and cumulative emission of N2O remain unclear [3].
According to Chang C, et al. [4], although plants do not generate N2O within their tissues, the N2O formed from nitrogen (N) by various processes in the soil is absorbed by plant roots and subsequently released via the transpiration system [4]. Apparently, the amount of N2O released by plants may account for some 25 to 30 % of the total emission of this gas from the soil [5,6]. Considering that plant root exudates can affect N mineralization in soils [7,8], it is likely that the presence of living plants inside a gas exchange chamber would alter the flux of N2O from the soil. In this context, Vázquez E, et al. [9] observed high rates of ammonification in soil cultivated with Urochloa genotypes in tropical pastures, indicating that plants have a modulating effect on N mineralization. Along with chemical changes, the soil surrounding roots can undergo physical modifications [10,11], and these may also play important roles in the regulation of N processes in the soil [1].
In light of the above, the aim of this study was to determine the influence of forage grass on the N2O flux from an oxisol in the Southern Amazon region of Brazil. Our results will contribute to a better understanding of the variability of N2O fluxes at the soil–atmosphere interface. This knowledge is important because N2O is a potent greenhouse gas that is stimulated by agricultural activities, and reduction of such emissions could have a positive impact on global warming.
Material and Methods
Experiments were performed at the research farm of Embrapa Agrossilvipastoril located in Sinop, MT, Brazil (11°5´S, 55°30´W). According to the Köppen classification system, the climate of the region is tropical (Aw) and characterized by well-defined wet and dry seasons with mean temperatures in the range 24 to 34ºC and mean annual rainfall varying between 1700 and 2200 mm. The experimental area was of flat relief with a clayey textured soil, classified as Hapludox [12] or red-yellow latosol (oxisol) according to the Brazilian Soil Classification System [13], and containing 49 % clay, 16 % silt and 35 % sand. The chemical characteristics of the top 0 to 10 cm soil layer were: pH (pH measured in suspension solution after adding deionized water in a soil:water ratio of 1:2.5.) 5.4, 2.6 % total organic C, 0.2 % total organic N, 1.6 cmolc kg-1 total exchangeable bases and 42 % base saturation.
During the period November 2016 to October 2017, N2O fluxes were measured in 2 ha of pasture formed by forage grass [Brachiaria (Syn. Urochloa) brizantha cv. Marandu] and grazed at a stocking density intended to maintain a mean canopy height of 30 cm above the soil surface. The pasture received surface N fertilization (50 kg ha-1) twice during the experimental period, in November 2016 and March 2017. The randomized experimental design comprised two treatments, namely Chambers with Grass (CWG) and Chambers with No Grass (CNG), and incorporated six replicates of each [14].
The vented static chambers (non-flow-through; non-steady-state) comprised opaque rectangular PVC boxes (0.60 m long x 0.40 m wide x 0.095 m high) [2] and were deployed at random on grass covered areas or bare soil. Samplings of headspace gases were performed in the mornings (between 08h00 and 10h00) at 7 day intervals during the wet season (November 2016 to May 2017) when gas fluxes were high, and at 14 day intervals during the dry season (June to October 2017) when gas fluxes were low [15]. Gas samples were collected from the headspace of each chamber, with the aid of a 20cm3 syringe, at 0, 20, 40 and 60 min after deployment of the chamber [2]. The internal temperature of the chamber was monitored at the time of gas collection. Air samples were transferred from the syringes to 20 cm³ evacuated glass vials capped with gray butyl rubber septa, and subsequently analyzed on a model GC-2014 chromatograph (Shimadzu, Tokyo, Japan) equipped with an electrical conductivity detector. The amounts of N2O present were established from calibration curves constructed with N2O standards of three known concentrations analyzed using the same chromatographic equipment and parameters as the samples. The results were used to adjust the linear model by relating the variations of gas concentrations in the chamber headspace as a function of time (0, 20, 40, and 60 min). Soil N2O fluxes were calculated according to Eq. (1) proposed by Hutchinson and Livingston [16]:
where dC/dt is the change in gas concentration in the chamber as a function of time, V is the chamber volume (L), A is the area of the chamber (m²), m is the molecular weight (g) and vm is the molecular volume (L) of the gas. In the treatment with plants, V was adjusted by subtracting the volume occupied by the plant biomass, noting that tall grasses were folded to enable them to fit within the height of the chamber [3,17].
The N2O flux values were used to estimate cumulative N2O emissions during the study period according to the numerical integration method [18]. Values of N2O fluxes determined every 7 or 14 days throughout the evaluation year were expressed together with the Standard Error of the Mean (SEM) [19]. The Pearson correlation coefficient was used to determine the relationship between soil N2O fluxes recorded in the CWG and CNG during the evaluation period, and the best-fit linear regression equation was established in order to understand the difference between the two treatments. Cumulative emission data and mean N2O fluxes for the CWG and CNG obtained during the entire evaluation period were submitted to one-way analysis of variance (ANOVA) and the significance of the difference determined from the p value (α = 0.05). In order to verify the difference between N2O fluxes in the two treatments, the mean absolute error (MAE) and root mean squared error (RMSE) were calculated using Eqs. (2) and (3), respectively:
where y1 is the daily flux in the CNG, y2 is daily flux in the CWG and n is the number of sampling events. Values of MAE and RMSE near to zero mean that the difference between the treatments is small.
Results
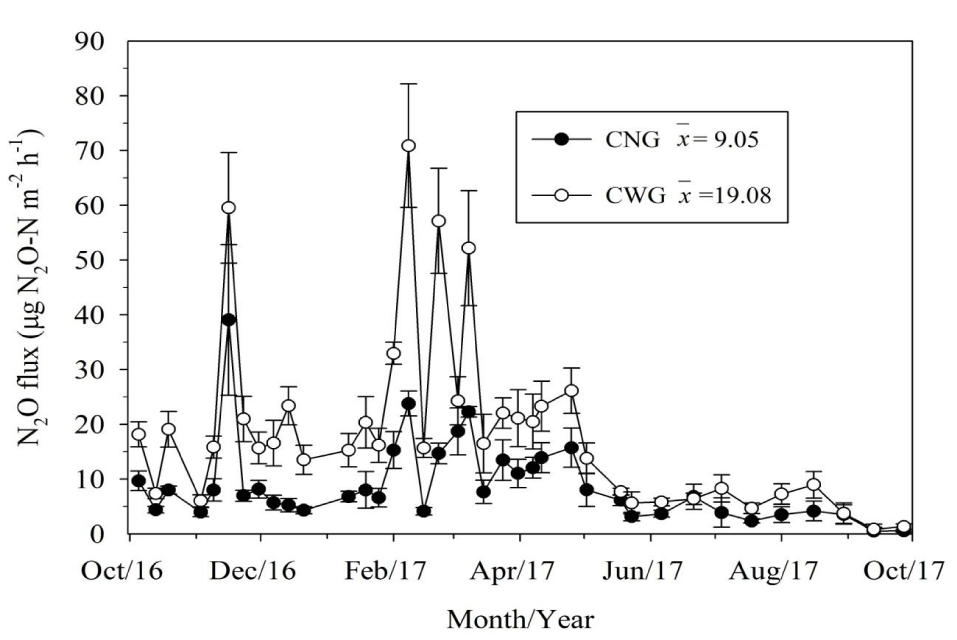
The N2O fluxes in the CWG were higher than those in their CNG counterparts on more than 80% of the sampling dates, most especially during the wet season (Figure 1). Throughout the dry season, the fortnightly N2O flux values recorded in the two treatments were similar, barring two instances in August 2017 when the values diverged, while the weekly N2O flux values registered during the wet season were dissimilar, except for two occasions (November 2016 and March 2017) when they converged.
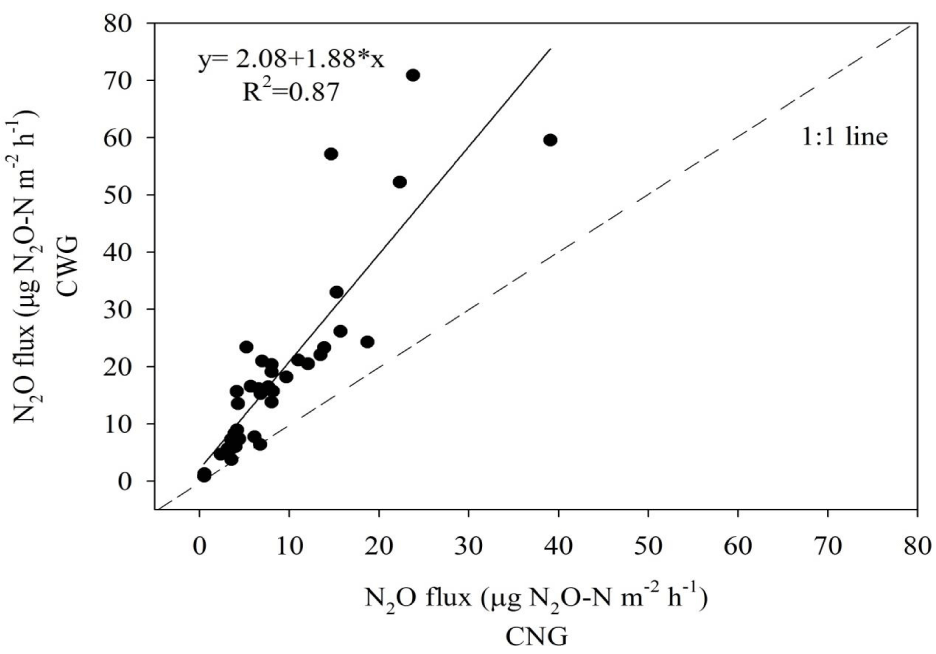
During the wet season, N2O fluxes ranged from 10 to around 70 µg N2O-N m-2 h-1 in the CWG and from 5 to around 40 µg N2O-N m-2 h-1 in the CNG, while in the dry season both chambers presented similar low N2O fluxes (< 10 µg N2O-N m-2 h-1). The mean N2O flux in the CWG (19.08 µg N2O-N m-2 h-1) was approximately double that in the CNG (9.05 µg N2O-N m-2 h-1), and a similar pattern was observed with regard to the cumulative N2O emissions, which were registered as 1.60 kg N2O-N ha-1 in the CWG and 0.72 kg N2O-N ha-1 in their CNG counterparts. The Person correlation coefficient indicated a very strong positive correlation between the N2O fluxes of the CWG and CNG. The slope of the regression line of best fit (1.88) demonstrated that the presence of plants inside the gas exchange chambers increased N2O flux considerably (Figure 2). Moreover, the MAE (14.34 N2O-N m-2 h-1) and RMSE (10.05 µg N2O-N m-2 h-1) values relating to N2O fluxes in the CWG and CNG verified that there were substantial differences between the two chambers.
Discussion
The principal aim of the present study was to determine the influence of forage grass on N2O fluxes emanating from an oxisol in a field in the Southern Amazon. All of the statistical tools applied to compare N2O fluxes in chambers with and without grass were consistent with the hypothesis that the inclusion of grass plants inside static chambers resulted in higher estimations of N2O emissions from the soil.
Previous studies have shown that, in addition to soil emissions, the transpiration of plants present inside gas exchange chambers may contribute to N2O emissions [4-6]. When the emissions attributed to plant transpiration, which reportedly amount to 30% of total soil emissions [5,6], were subtracted from the cumulative N2O emission determined in the CWG, the result (1.12 kg N2O-N ha-1) was still 60% higher than the cumulative emission observed in the CNG (0.72 kg N2O-N ha-1). This calculation demonstrates that the plants inside the chambers may influence the formation of N2O in the soil, and that the excess emissions are not solely due to transpiration.
One hypothesis concerning the role of plant roots on the formation of N2O emissions relates to the manner in which they change the characteristics of the soil [10,11], thereby altering the processes of N2O formation. For example, the rise in N2O flux could be explained by an increase in N mineralization resulting from rhizodeposition and microbial activity [8,9], together with an increase in soil bulk density around the roots [10,11]. Increased NH4+ availability, caused by of N mineralization, and elevated soil bulk density in the rhizosphere, are ideal conditions for the formation of N2O under conditions of low soil redox potential (Eh) [1]. Reductions in Eh occur mainly after rainfall when water fills the soil pore spaces, a phenomenon that has been observed in pasture covered soil during the wet season in the southern Amazon [20].
It is likely that grass roots in the soil inside the CWG had opened interconnected macropores that facilitated the upward movement of N2O [21]. On the other hand, the bare soil inside the CNG possessed no interconnected macropores and, therefore, generated low N2O fluxes in comparison with those of the CWG. This explanation justifies the high Pearson correlation coefficient recorded despite the differences in N2O flux values between the two chambers, and suggests that the process of N2O formation is analogous in the CNG and CWG, although the presence of grass in the latter may have improved emission of the gas. If N2O had not been generated by the same processes in the soil of both chambers, it is likely that fluxes in the CNG would not have increased in step with those in the CWG and the Pearson correlation coefficient would not have been so strong. The observations above support the premise that the gas originated from analogous soil processes, regardless of the chamber, although the amounts were different.
It would be interesting to investigate the influence of the plant transpiration stream on the upward movement of N2O formed in deeper soil horizons. Nevertheless, the amount of N2O emitted in the CWG in the present study cannot be explained by this pathway alone because N2O formation in deep soil horizons is lower than that in surface soil [22]. However, to confirm the hypothesis outlined above, it is imperative to investigate N2O production in the soil profile.
Considerable research effort has been devoted to the development and application of static gas exchange chambers but the results obtained have been somewhat discrepant because some studies have included plants while others have not [2]. Moreover, a number of researchers have opted to assess fluxes in chambers containing plants that were subsequently removed after reaching a certain height [3]. Although chambers with plants may lead to the overestimation of gas emissions from the soil, it is likely that chambers without plants cannot accurately represent the situation in the field [2].
Conclusion
In the humid climate of the Southern Amazon, N2O emissions from oxisol in static gas exchange chambers containing tropical forage grass are considerably higher than those in chambers that do not contain grass. Such overestimation of N2O flux may be attributed to the plant transpiration stream and/or to changes in soil attributes induced by the plants. Our study has provided a number of constructive pointers for future research.
Acknowledgement
This study was partially funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de Mato Grosso (FAPEMAT).
Conflicts of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Author contributions
Conceptualization, Formal analysis, Investigation, Resources, Writing – original draft Writing – review & editing – Nascimento, A.F.; Ferreira, A.; Funding acquisition, Methodology, Project administration, Supervision - Nascimento, A.F.
References
- Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister-Boltenstern S. Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philos Trans R Soc Lond B Biol Sci. 2013 May 27;368(1621):20130122. doi: 10.1098/rstb.2013.0122. PMID: 23713120; PMCID: PMC3682742.
- Parkin TB, Venterea RT. Chamber-based trace gas flux measurements. In: Follett, R.F. ed. USDA-ARS GRACEnet project protocols: Sampling protocols. USDA-ARS, Fort Collins, CO, USA. 2010;p.1-39.3.
- Collier SM, Dean AP, Oates LG, Ruark MD, Jackson RD. Does Plant Biomass Manipulation in Static Chambers Affect Nitrous Oxide Emissions from Soils? J Environ Qual. 2016 Mar;45(2):751-6. doi: 10.2134/jeq2015.07.0377. PMID: 27065424.
- Chang C, Janzen HH, Nakonechny EM, Cho CM. Nitrous oxide emission through plants. Soil Science Society of American Journal. 1998;35:35-38. doi: 10.2136/sssaj1998.03615995006200010005x.
- Zou J, Huang Y, Sun W, Zheng X. Contribution of plants to N2O emissions in soil-winter wheat ecosystem: pot and field experiments. Plant Soil. 2005;269:205-211. doi: 10.1007/s11104-004-0484-0.
- Lenhart K, Behrendt T, Greiner S, Steinkamp J, Well R, Giesemann A, Keppler F. Nitrous oxide effluxes from plants as a potentially important source to the atmosphere. New Phytol. 2019 Feb;221(3):1398-1408. doi: 10.1111/nph.15455. Epub 2018 Oct 10. PMID: 30303249.
- Kuzyakov Y, Cheng W. Photosynthesis controls of rhizosphere respiration and organic matter decomposition. Soil Biology & Biochemistry. 2001;33:1915-1925. doi: 10.1016/S0038-0717(01)00117-1.
- Shahzad T, Chenu C, Genet P, Barot S, Perveen N, Mougin C, Fontaine S. Contribution of exudates, arbuscular mycorrhizal fungi and litter depositions to the rhizosphere priming effect induced by grassland species. Soil Biology and Biochemistry. 2015;80:146-155. doi: 10.1016/j.soilbio.2014.09.023.
- Vázquez E, Teutscherova N, Dannenmann M, Töchterle P, Butterbach-Bahl K, Pulleman M, Arango J. Gross nitrogen transformations in tropical pasture soils as affected by Urochloa genotypes differing in biological nitrification inhibition (BNI) capacity. Soil Biology & Biochemistry. 2020;151: 108058. doi: 10.1016/j.soilbio.2020.108058.
- Helliwell JR, Sturrock CJ, Mairhofer S, Craigon J, Ashton RW, Miller AJ, Whalley WR, Mooney SJ. The emergent rhizosphere: imaging the development of the porous architecture at the root-soil interface. Scientific Reports. 2017;7:1-10. doi: 10.1038/s41598-017-14904-w.
- Lucas M, Schlüter S, Vogel HJ, Vetterlein D. Roots compact the surrounding soil depending on the structures they encounter. Scientific Reports. 2019;9:1-13. doi: 10.1038/s41598-019-52665-w.
- United States Department of Agriculture. Soil taxonomy: A basic system of soil classification for making and interpreting soil surveys. 2nd edition. Natural Resources Conservation Service, U.S. Department of Agriculture Handbook, Washington DC, USA; 1999.
- Santos HG, Jacomine PKT, Anjos LHC, Oliveira VA, Lumbreras JF, Coelho MR, Almeida JA, Araujo Filho JC, Oliveira JB, Cunha TJF. Brazilian Soil Classification System. Embrapa, Brasília, DF, Brazil. 2018.
- Ferreira DF, Cargnelutti Filho A, Lúcio AD. Procedimentos estatísticos em planejamentos experimentais com restrição na casualização. Boletim Informativo Sociedade Brasileira de Ciência do Solo. Sociedade Brasileira de Ciência do Solo, Campinas, SP, Brazil. 2012;p.16-19.
- Nascimento AF, Rodrigues RAR. Sampling frequency to estimate cumulative nitrous oxide emissions from the soil. Pesquisa Agropecuária Brasileira. 2019;54:1-5. doi: 10.1590/S1678-3921.pab2019.v54.00211.
- Hutchinson GL, Livingston GP. Use of chamber systems to measure trace gas fluxes. In: Harper LA, Mosier AR, Duxbury JM, Rolston chair ED, editors. Agricultural ecosystem effects on trace gases and global climate change. ASA, CSSA and SSSA, Madison, WI, USA. 1993;p.63-78.
- Alexander JR, Venterea RT, Baker JM, Coulter JA. Kura clover living mulch: Spring management effects on nitrogen. Agronomy. 2019;69:1-14. doi: 10.3390/agronomy9020069.
- Parkin TB, Kaspar TC. Temporal variability of soil carbon dioxide flux. Soil Science Society of American Journal. 2004;68:1234-1241. doi: 10.2136/sssaj2004.1234.
- Alfaro MA, Giltrap D, Topp CFE, Klein CAM. How to report your experimental data. In: Klein CAM, Harvey MJ, editors. Nitrous oxide chamber methodology guidelines - Version 1.1. Global Research Alliance, New Zealand. 2015;p.122-130.
- Nascimento AF, Oliveira CM, Pedreira BC, Pereira DH, Rodrigues RRA. Nitrous oxide emissions and forage accumulation in the Brazilian Amazon forage‐livestock systems submitted to N input strategies. Grassland Science. 2021;67: 63-72. doi: 10.1111/grs.12287.
- Teramoto S, Takayasu S, Kitomi Y, Arai-Sanoh Y, Tanabata T, Uga Y. High-throughput three-dimensional visualization of root system architecture of rice using X-ray computed tomography. Plant Methods. 2020 May 11;16:66. doi: 10.1186/s13007-020-00612-6. PMID: 32426023; PMCID: PMC7216661.
- Wang Y, Li X, Dong W, Wu D, Hu C, Zhang Y, Luo Y. Depth-dependent greenhouse gas production and consumption in an upland cropping system in northern China. Geoderma. 2018;319:100-112. doi: 10.1016/j.geoderma.2018.01.001.
Content Alerts
SignUp to our
Content alerts.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.